Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Inhibrx, Inc. | axoxfordthirdamendmentxjun.htm |
| EX-99.1 - EX-99.1 - Inhibrx, Inc. | june2021chondropr.htm |
| EX-10.1 - EX-10.1 - Inhibrx, Inc. | exhibit101thirdamendmentto.htm |

Outcomes Focused Innovation Driven June 2021

2 This presentation contains forward-looking statements. In some cases, you can identify forward-looking statements by the words “will,” “expect,” “intend,” “plan,” “objective,” “believe,” “estimate,” “potential,” “continue” and “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. These statements are based on management’s current beliefs and expectations. These statements include but are not limited to statements regarding Inhibrx, Inc.’s (the “Company”) business strategy, the Company’s plans to develop and commercialize its product candidates, the safety and efficacy of the Company’s product candidates, the Company’s plans and expected timing with respect to clinical trials and regulatory filings and approvals, and the size and growth potential of the markets for the Company’s product candidates. These statements involve substantial known and unknown risks, uncertainties and other factors that may cause the Company’s actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward- looking statements. Additional information regarding the Company’s risks and uncertainties are described in its Registration Statement on Form S-1 filed with the Securities and Exchange Commission (“SEC”) on August 12, 2020 in the section titled “Risk Factors,” and in other filings the Company may make with the SEC from time to time. The Company may not actually achieve the plans, intentions or expectations disclosed in its forward-looking statements, and you should not place undue reliance on the Company’s forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements the Company makes. The forward-looking statements in this presentation represent the Company’s views as of the date of this presentation. The Company anticipates that subsequent events and developments will cause its views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company has no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing the Company’s views as of any date subsequent to the date of this presentation. The investigational product candidates discussed in this presentation have not been approved or licensed by the U.S. Food and Drug Administration or by any other regulatory authority, and they are not commercially available in any market. This presentation also contains estimates and other statistical data made by independent parties and by the Company relating to market size and growth and other data about its industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of the Company’s future performance and the future performance of the markets in which it operates are necessarily subject to a high degree of uncertainty and risk. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy securities. Presentation disclaimer
3 Inhibrx at a glance + Experienced leadership team + Proven innovation and execution VALIDATION FROM INDUSTRY LEADING PARTNERS All platforms and programs developed in-house with strong patent protection Inhibrx’s modular sdAb platform + Ability to precision engineer to specific target biology + Smaller than conventional antibodies + Antibody-like PK profile + Readily manufactured at high yields using standard processes

4 Why invest in Inhibrx? Backed by solid institutional investor base with substantial internal ownership Four differentiated clinical programs with value-creating readouts in 2H 2021/2022 KEY FINANCIAL HIGHLIGHTS* 37.8M $108.0M 41.9M Robust emerging pre-clinical pipeline Potential to reach financial sustainability with minimal dilution $20M/Qtr Cash Average burn rate Common stock outstanding Fully diluted outstanding *As of 3/31/2021
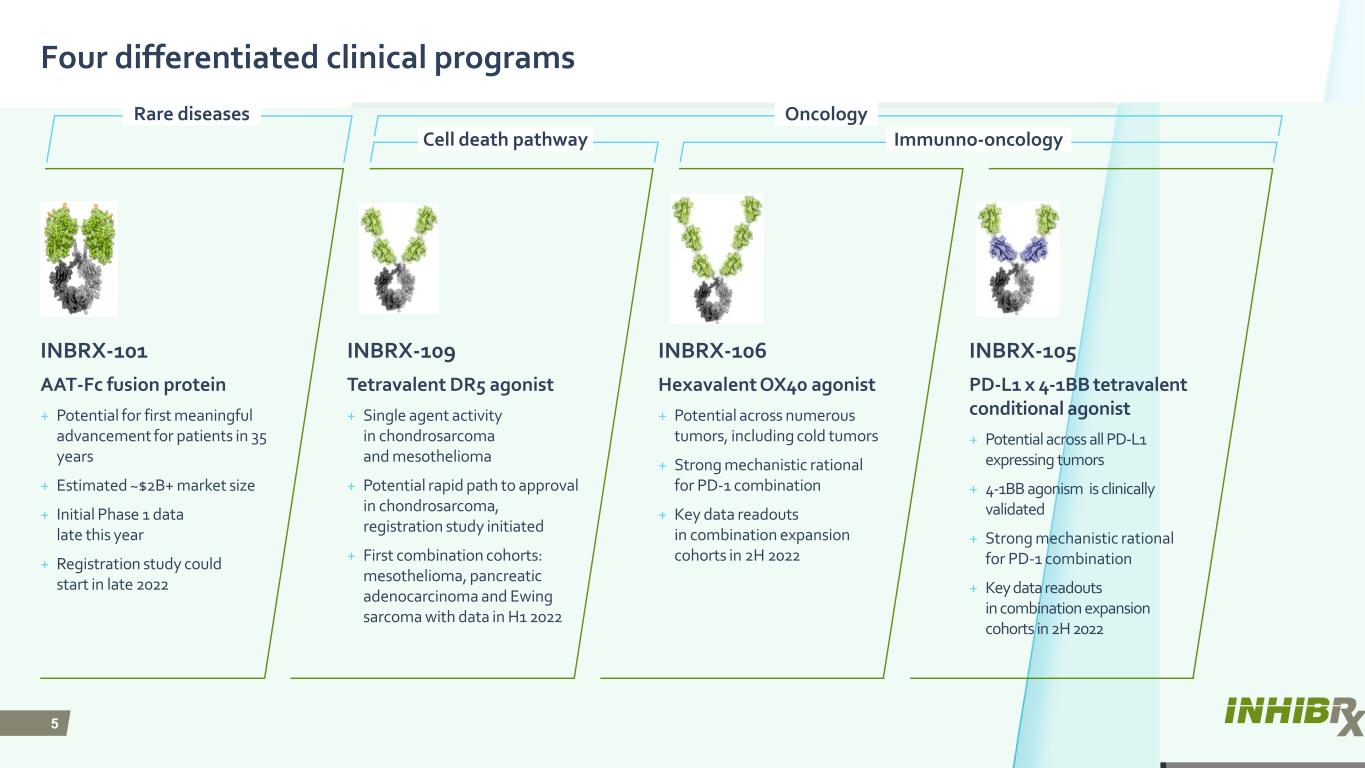
5 Four differentiated clinical programs INBRX-109 Tetravalent DR5 agonist + Single agent activity in chondrosarcoma and mesothelioma + Potential rapid path to approval in chondrosarcoma, registration study initiated + First combination cohorts: mesothelioma, pancreatic adenocarcinoma and Ewing sarcoma with data in H1 2022 INBRX-106 Hexavalent OX40 agonist + Potential across numerous tumors, including cold tumors + Strong mechanistic rational for PD-1 combination + Key data readouts in combination expansion cohorts in 2H 2022 INBRX-105 PD-L1 x 4-1BB tetravalent conditional agonist + Potential across all PD-L1 expressing tumors + 4-1BB agonism is clinically validated + Strong mechanistic rational for PD-1 combination + Key data readouts in combination expansion cohorts in 2H 2022 INBRX-101 AAT-Fc fusion protein + Potential for first meaningful advancement for patients in 35 years + Estimated ~$2B+ market size + Initial Phase 1 data late this year + Registration study could start in late 2022 Oncology Cell death pathway Immunno-oncology Rare diseases

INBRX-101 Recombinant Alpha-1 Antitrypsin Fc-fusion Protein AAT Fc
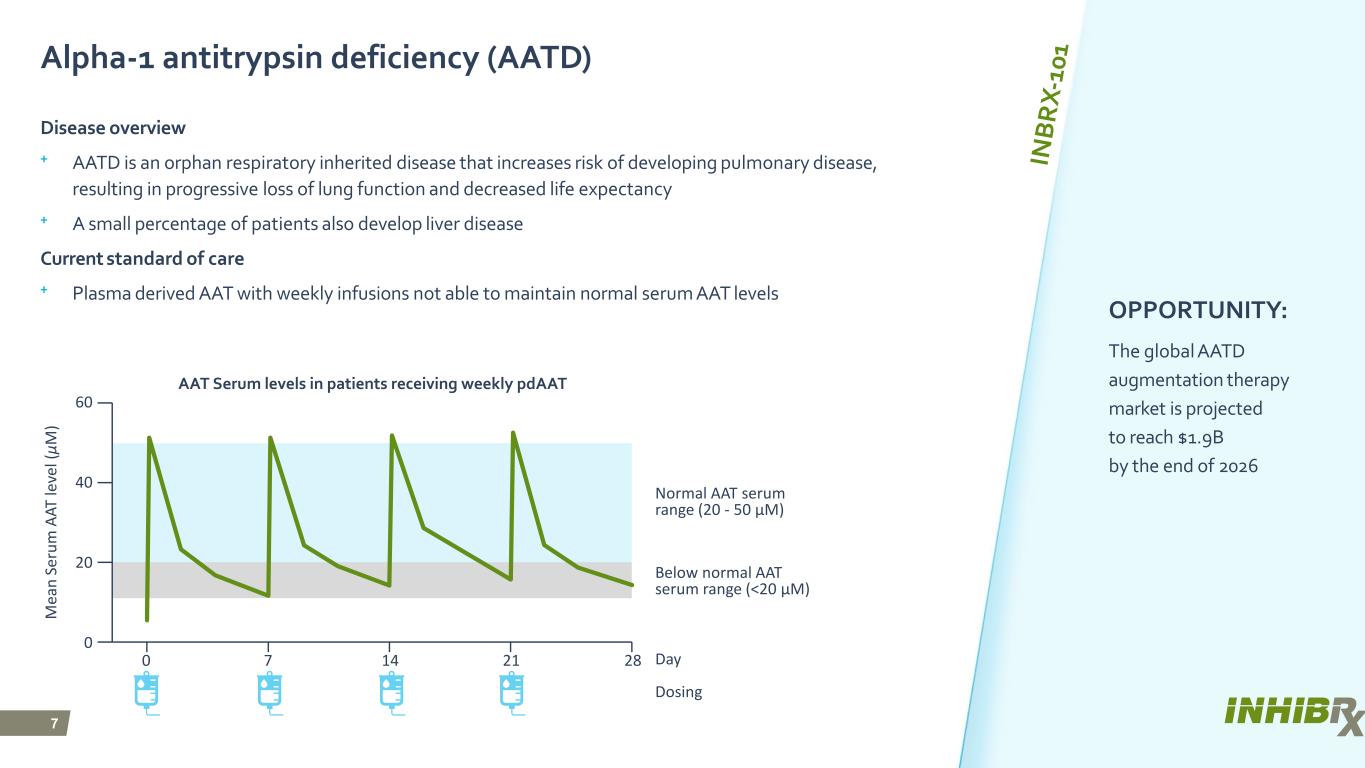
7 Alpha-1 antitrypsin deficiency (AATD) Disease overview ⁺ AATD is an orphan respiratory inherited disease that increases risk of developing pulmonary disease, resulting in progressive loss of lung function and decreased life expectancy ⁺ A small percentage of patients also develop liver disease Current standard of care ⁺ Plasma derived AAT with weekly infusions not able to maintain normal serum AAT levels OPPORTUNITY: The global AATD augmentation therapy market is projected to reach $1.9B by the end of 2026 AAT Serum levels in patients receiving weekly pdAAT 60 M ea n Se ru m A AT le ve l ( μM ) 40 20 0 0 7 14 21 28 Day Normal AAT serum range (20 - 50 μM) Below normal AAT serum range (<20 μM) Dosing
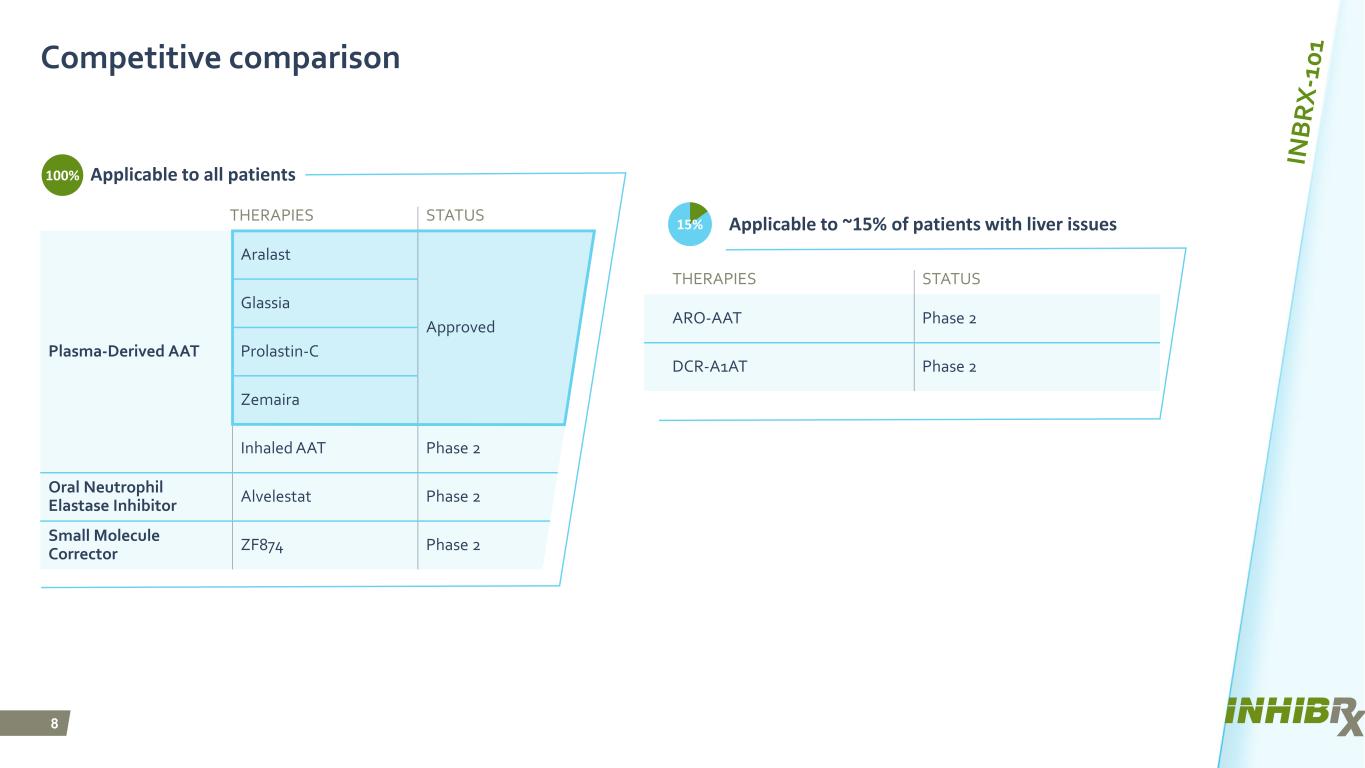
8 THERAPIES STATUS Plasma-Derived AAT Aralast Approved Glassia Prolastin-C Zemaira Inhaled AAT Phase 2 Oral Neutrophil Elastase Inhibitor Alvelestat Phase 2 Small Molecule Corrector ZF874 Phase 2 Competitive comparison Applicable to all patients THERAPIES STATUS ARO-AAT Phase 2 DCR-A1AT Phase 2 Applicable to ~15% of patients with liver issues15% 100%

9 Our solution Potential to extend the dosing range from weekly to every three weeks, while maintaining patients in the normal range of AAT exposure Engineered to maximize the functional activity of AAT, particularly in the lung Recombinant manufacturing provides lower cost of goods, unlimited supply and no pathogen risk INBRX-101 proposed standard of care
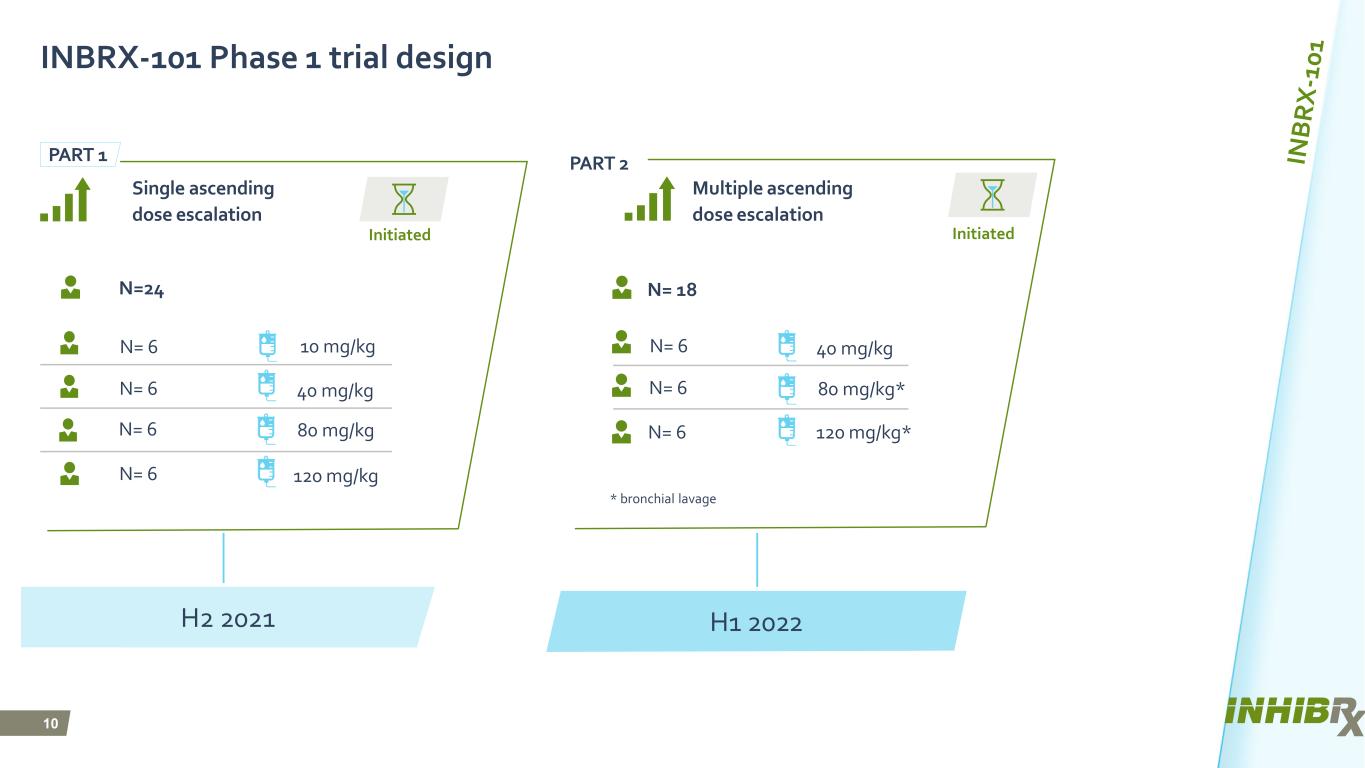
10 Initiated H2 2021 H1 2022 INBRX-101 Phase 1 trial design Multiple ascending dose escalation N= 18N=24 Initiated * bronchial lavage N= 6 10 mg/kg N= 6 40 mg/kg N= 6 120 mg/kg N= 6 80 mg/kg Single ascending dose escalation N= 6 40 mg/kg N= 6 80 mg/kg* N= 6 120 mg/kg* PART 1 PART 2
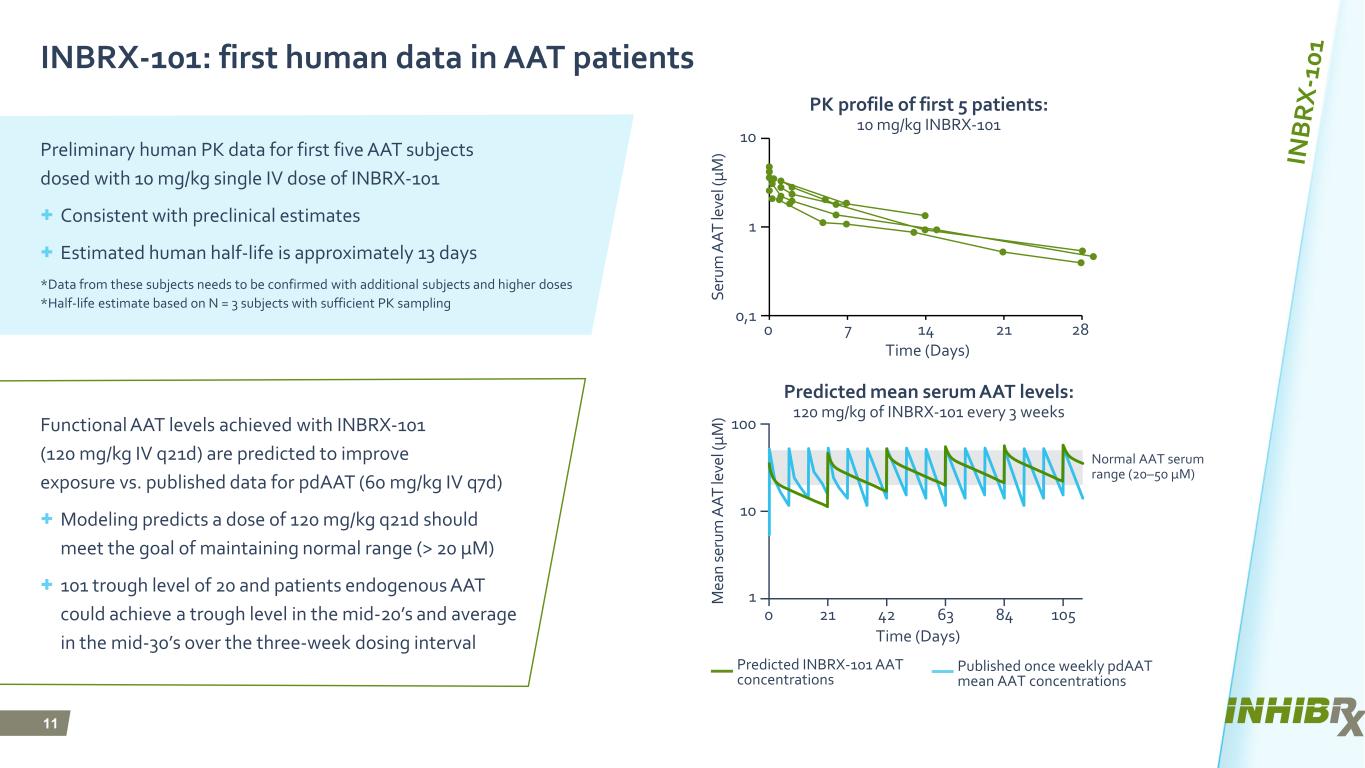
11 INBRX-101: first human data in AAT patients Preliminary human PK data for first five AAT subjects dosed with 10 mg/kg single IV dose of INBRX-101 + Consistent with preclinical estimates + Estimated human half-life is approximately 13 days *Data from these subjects needs to be confirmed with additional subjects and higher doses *Half-life estimate based on N = 3 subjects with sufficient PK sampling Functional AAT levels achieved with INBRX-101 (120 mg/kg IV q21d) are predicted to improve exposure vs. published data for pdAAT (60 mg/kg IV q7d) + Modeling predicts a dose of 120 mg/kg q21d should meet the goal of maintaining normal range (> 20 μM) + 101 trough level of 20 and patients endogenous AAT could achieve a trough level in the mid-20’s and average in the mid-30’s over the three-week dosing interval PK profile of first 5 patients: 10 mg/kg INBRX-101 10 Se ru m A A T le ve l ( μ M ) Time (Days) 0 1 0,1 7 14 21 28 Predicted mean serum AAT levels: 120 mg/kg of INBRX-101 every 3 weeks M ea n se ru m A A T le ve l ( μ M ) Time (Days) 0 21 42 63 84 105 Normal AAT serum range (20–50 μM) Predicted INBRX-101 AAT concentrations Published once weekly pdAAT mean AAT concentrations 100 10 1

INBRX-109 Tetravalent DR5 Agonist
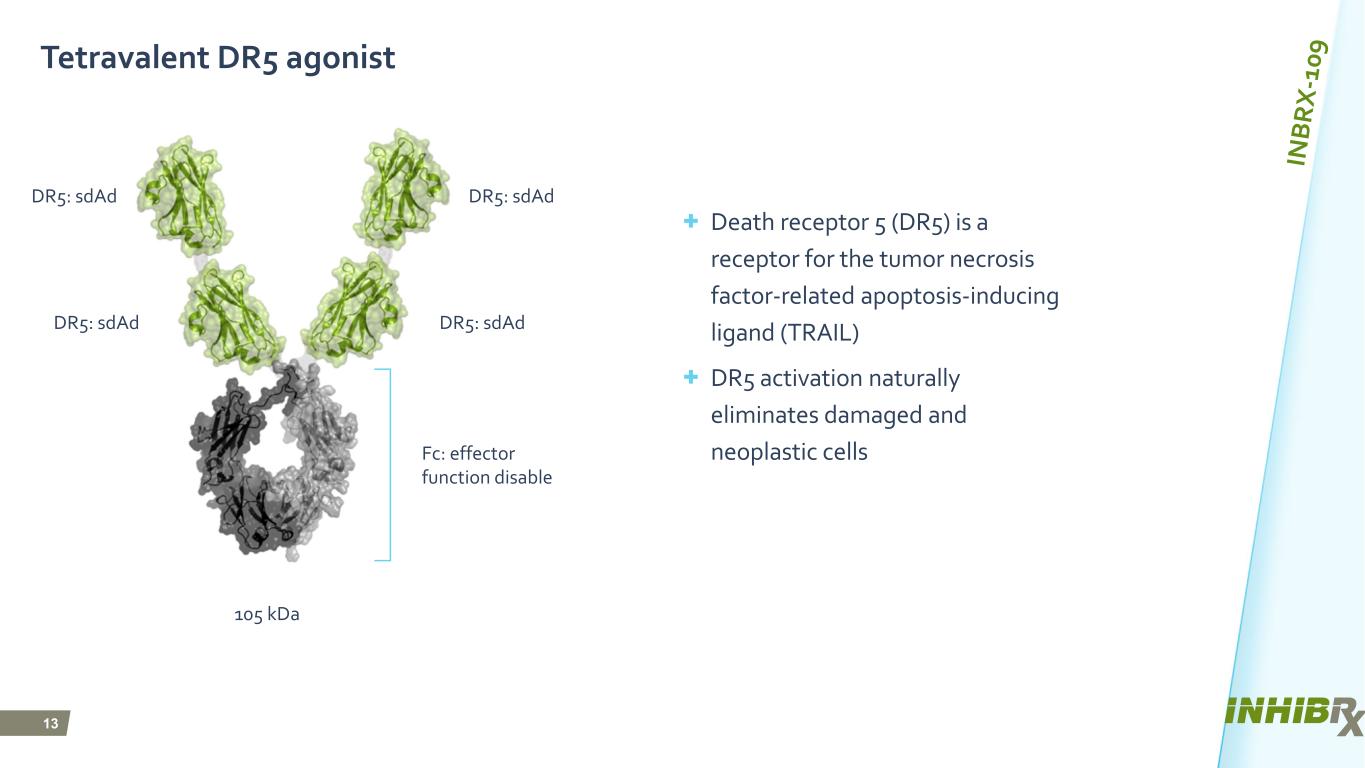
13 Tetravalent DR5 agonist + Death receptor 5 (DR5) is a receptor for the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) + DR5 activation naturally eliminates damaged and neoplastic cells DR5: sdAd DR5: sdAdDR5: sdAd DR5: sdAd 105 kDa Fc: effector function disable

14 INBRX-109 is a best-in-class DR5 agonist CANDIDATE VALENCY SIZE (KDA) STATUS INBRX-109 Tetravalent 105 Phase II TAS-266 Tetravalent 60 Discontinued1 Eftozanermin alpha (TRAIL-Fc fusion) Hexavalent 167 Phase I GEN10292 Dodecavalent 150 ka (2x mAbs) Phase I IGM-8444 Decavalent3 ~950 Phase I Dulanermin (recombinant TRAIL) Trivalent 150 Discontinued Tigatuzumab Bivalent 150 Discontinued LBY-135 Discontinued Conatumumab Discontinued Drozitumab Discontinued Lexatumumab Discontinued 1. Hepatoxicity – likely ADA hyper-crosslinking 2. Two hexamerizing non-competing mAbs 3. Size and rigidity of IgM may prevent effective clustering of DR5

15 Fast to market opportunity ~1,400 cases per year Incidence rate of ~1 in 200,000 persons per year USA Worldwide Chondrosarcoma *The figures on this slide represent market research estimates. No approved therapeutic for the treatment of chondrosarcoma Therapeutic IPI-926 (HH) Control arm Placebo Subject number 100 (2:1) Placebo arm PFS 2.9 months Therapeutic Regorafenib Control arm Placebo Subject number 46 (2:1) Placebo arm PFS 2 months PFS from placebo-controlled studies
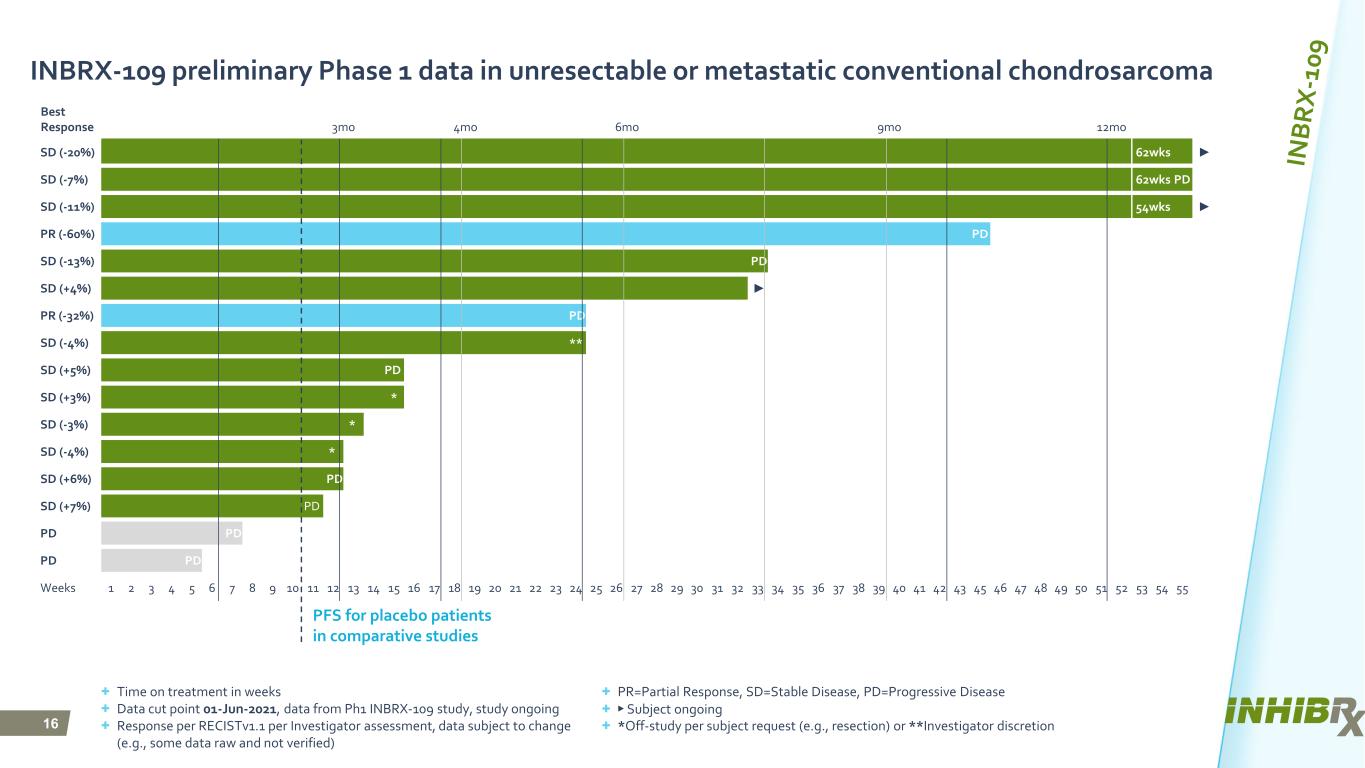
16 Best Response 3mo 4mo 6mo 9mo 12mo SD (-20%) 62wks ► SD (-7%) 62wks PD SD (-11%) 54wks ► PR (-60%) PD SD (-13%) PD SD (+4%) ► PR (-32%) PD SD (-4%) ** SD (+5%) PD SD (+3%) * SD (-3%) * SD (-4%) * SD (+6%) PD SD (+7%) PD PD PD PD PD Weeks 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 45 46 47 48 49 50 51 52 53 54 55 INBRX-109 preliminary Phase 1 data in unresectable or metastatic conventional chondrosarcoma + Time on treatment in weeks + Data cut point 01-Jun-2021, data from Ph1 INBRX-109 study, study ongoing + Response per RECISTv1.1 per Investigator assessment, data subject to change (e.g., some data raw and not verified) PFS for placebo patients in comparative studies + PR=Partial Response, SD=Stable Disease, PD=Progressive Disease + ► Subject ongoing + *Off-study per subject request (e.g., resection) or **Investigator discretion
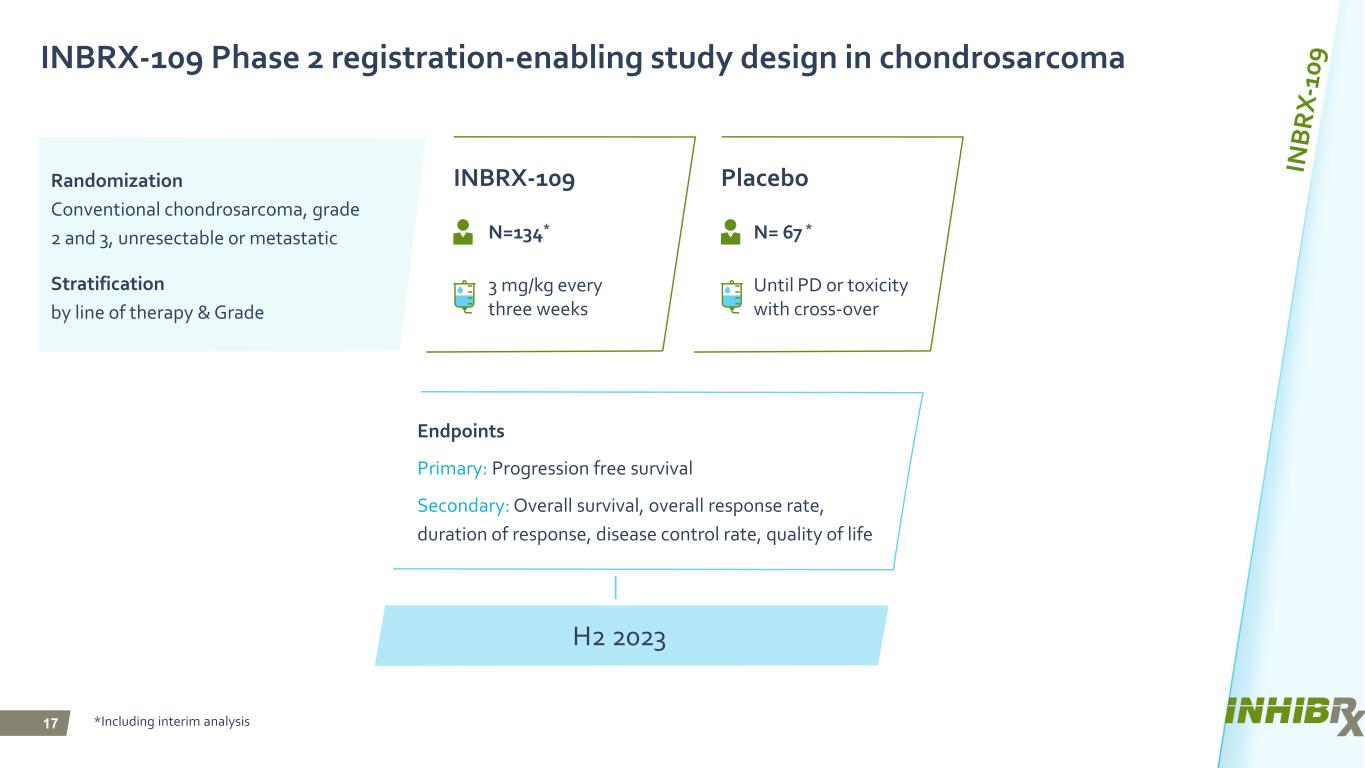
17 Endpoints Primary: Progression free survival Secondary: Overall survival, overall response rate, duration of response, disease control rate, quality of life INBRX-109 Phase 2 registration-enabling study design in chondrosarcoma Randomization Conventional chondrosarcoma, grade 2 and 3, unresectable or metastatic Stratification by line of therapy & Grade INBRX-109 Placebo Until PD or toxicity with cross-over *Including interim analysis N=134* N= 67 * 3 mg/kg every three weeks H2 2023
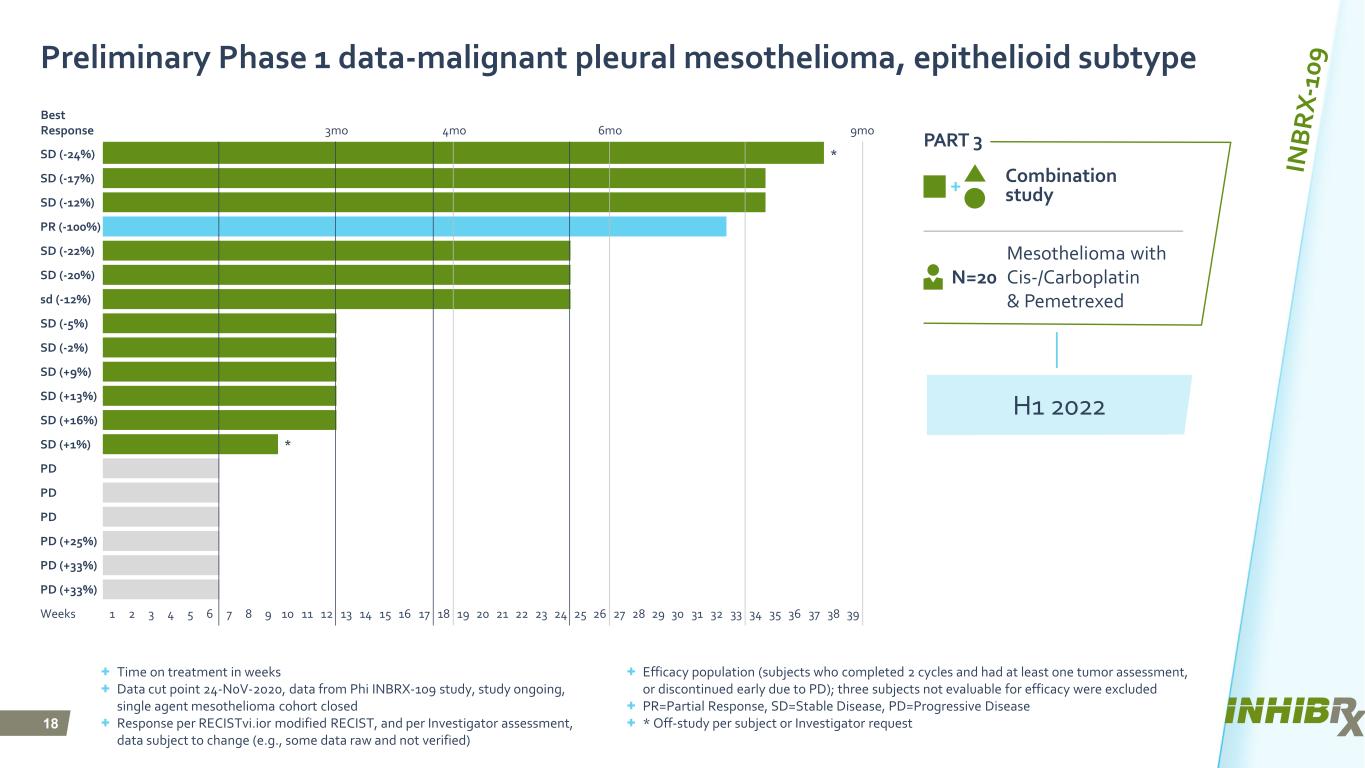
18 Best Response 3mo 4mo 6mo 9mo SD (-24%) * SD (-17%) SD (-12%) PR (-100%) SD (-22%) SD (-20%) sd (-12%) SD (-5%) SD (-2%) SD (+9%) SD (+13%) SD (+16%) SD (+1%) * PD PD PD PD (+25%) PD (+33%) PD (+33%) Weeks 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 Combination study Mesothelioma with Cis-/Carboplatin & Pemetrexed N=20 + Time on treatment in weeks + Data cut point 24-N0V-2020, data from Phi INBRX-109 study, study ongoing, single agent mesothelioma cohort closed + Response per RECISTvi.ior modified RECIST, and per Investigator assessment, data subject to change (e.g., some data raw and not verified) Preliminary Phase 1 data-malignant pleural mesothelioma, epithelioid subtype H1 2022 PART 3 + Efficacy population (subjects who completed 2 cycles and had at least one tumor assessment, or discontinued early due to PD); three subjects not evaluable for efficacy were excluded + PR=Partial Response, SD=Stable Disease, PD=Progressive Disease + * Off-study per subject or Investigator request
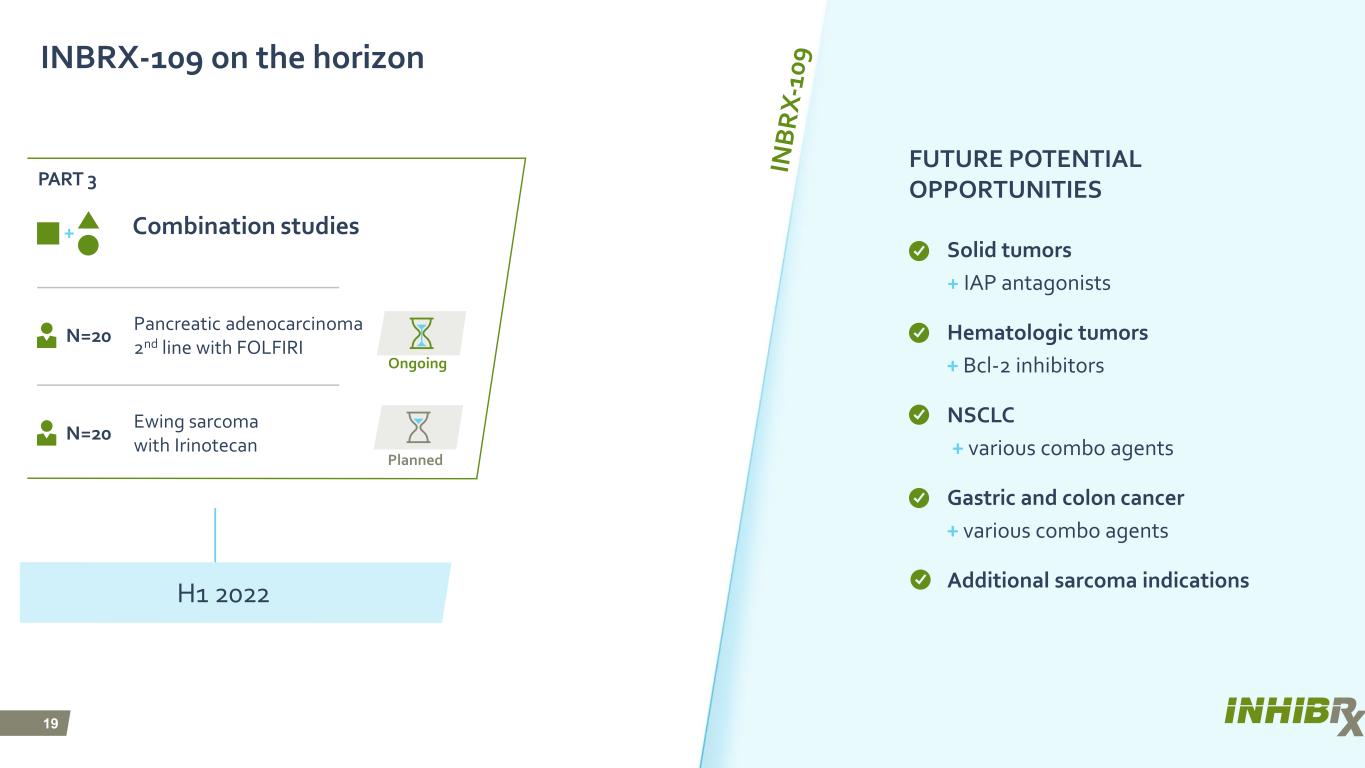
19 Planned Ongoing FUTURE POTENTIAL OPPORTUNITIES Solid tumors + IAP antagonists Hematologic tumors + Bcl-2 inhibitors NSCLC + various combo agents Gastric and colon cancer + various combo agents Additional sarcoma indications INBRX-109 on the horizon Combination studies Pancreatic adenocarcinoma 2nd line with FOLFIRI N=20 H1 2022 Ewing sarcoma with Irinotecan N=20 PART 3

INBRX-106 Hexavalent OX40 Agonist
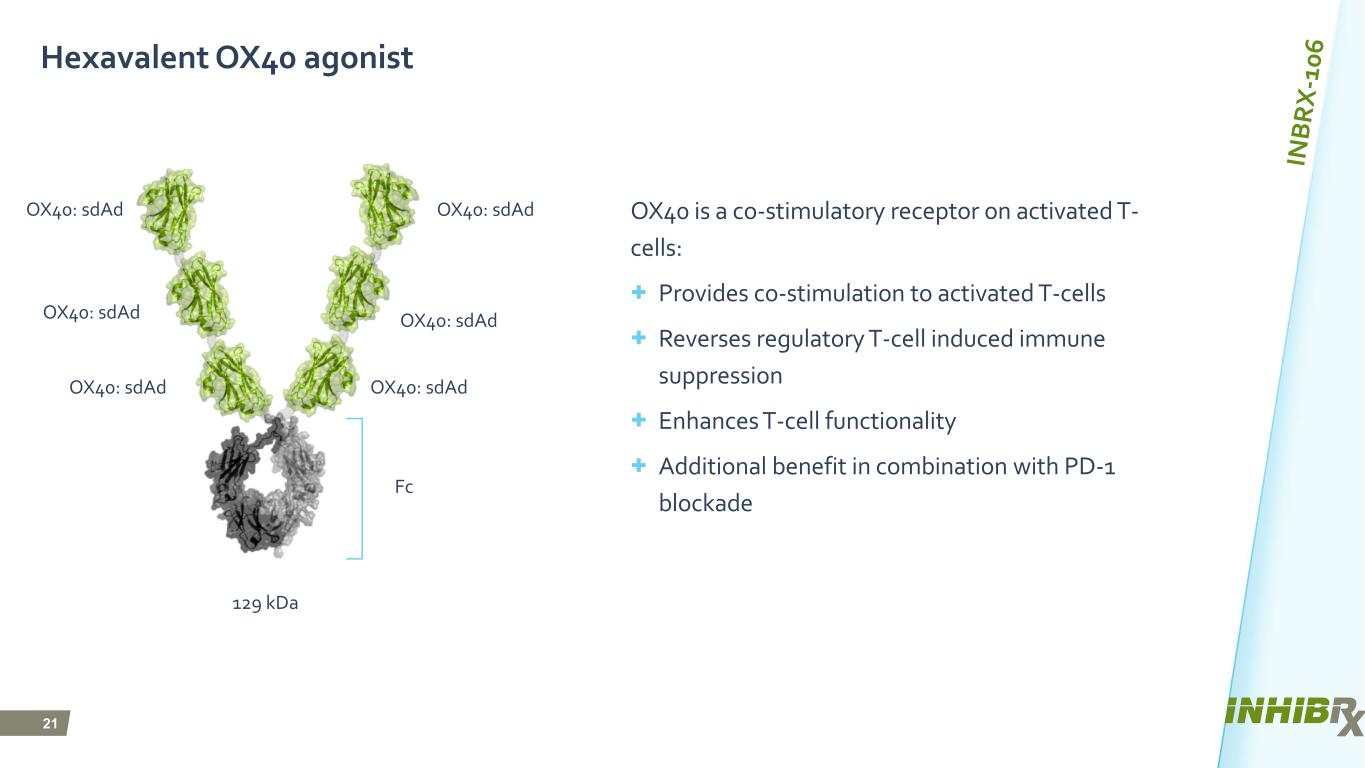
21 Hexavalent OX40 agonist OX40 is a co-stimulatory receptor on activated T- cells: + Provides co-stimulation to activated T-cells + Reverses regulatory T-cell induced immune suppression + Enhances T-cell functionality + Additional benefit in combination with PD-1 blockade T T T T T T T T T OX40: sdAd OX40: sdAd OX40: sdAdOX40: sdAd OX40: sdAd OX40: sdAd Fc 129 kDa

22 INBRX-106 is a best-in-class OX40 agonist CANDIDATES VALENCY ISOTYPE LIGAND BLOCKING STATUS INBRX-106 Hex- IgG1 N Ph 1 (2019) MOXR-0916 Bi- IgG1 Y Discontinued GSK-3174998 Ph 1 (MM, 2019) BMS-986178 Ph 1 (2016) INCAGN-1949 Ph 1 (2016) ABBV-368 Ph 1 (HNSCC, 2020) IBI-101 Ph 1 (2018) MEDI-0562 Discontinued PF-04518600 Bi- IgG2 Y Discontinued BGB-A445 Bi- IgG1 N Ph 1 (2020)

23 INBRX-106 Phase 1 trial design No pre-screening, all-comers Dose optimization study PlannedInitiatedInitiatedCompleteSingle agent dose escalation Dose escalation with Keytruda™ Single agent dose expansion N=80N= 24 N= 13N=20 N=20 PD-L1+NSCLC N=20 PD-L1+NSCLC N=20 PD-L1+Basket N=20 PD-L1+NSCLC, CPI naїve PART 1 PART 2 PART 3 PART 4 + Well tolerated with mild or moderate immune-related toxicities + Patients were not pre-screened for PD-L1 positivity + Longest duration as of June 1, 2021= 63 weeks + Greatest reduction in tumor volume= 23%(by RECIST v1.1) + Maximum administered dose was 3 mg/kg and no MTD reached H2 2021 H2 2022 Dose expansion with Keytruda™

INBRX-105 PD-L1 x 4-1BB Multispecific
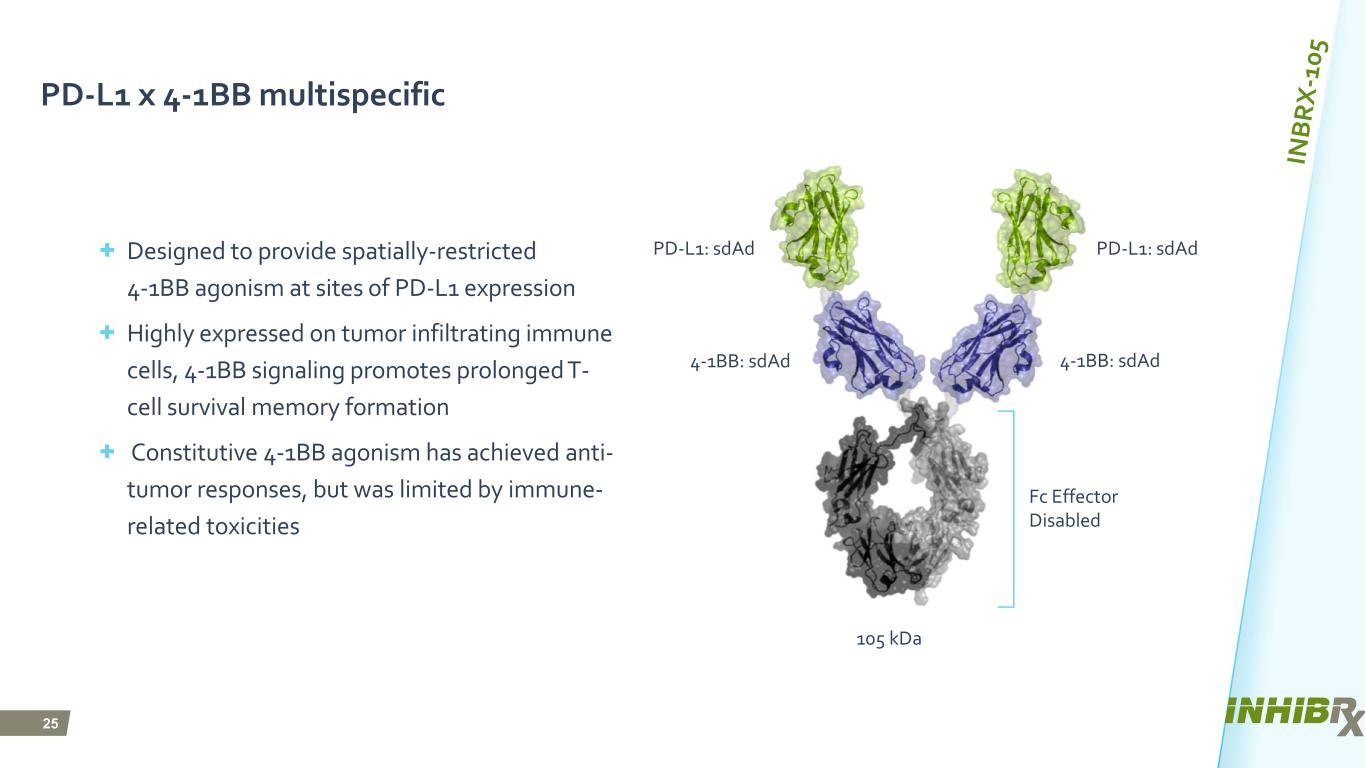
25 PD-L1 x 4-1BB multispecific + Designed to provide spatially-restricted 4-1BB agonism at sites of PD-L1 expression + Highly expressed on tumor infiltrating immune cells, 4-1BB signaling promotes prolonged T- cell survival memory formation + Constitutive 4-1BB agonism has achieved anti- tumor responses, but was limited by immune- related toxicities PD-L1: sdAd 4-1BB: sdAd Fc Effector Disabled PD-L1: sdAd 4-1BB: sdAd 105 kDa
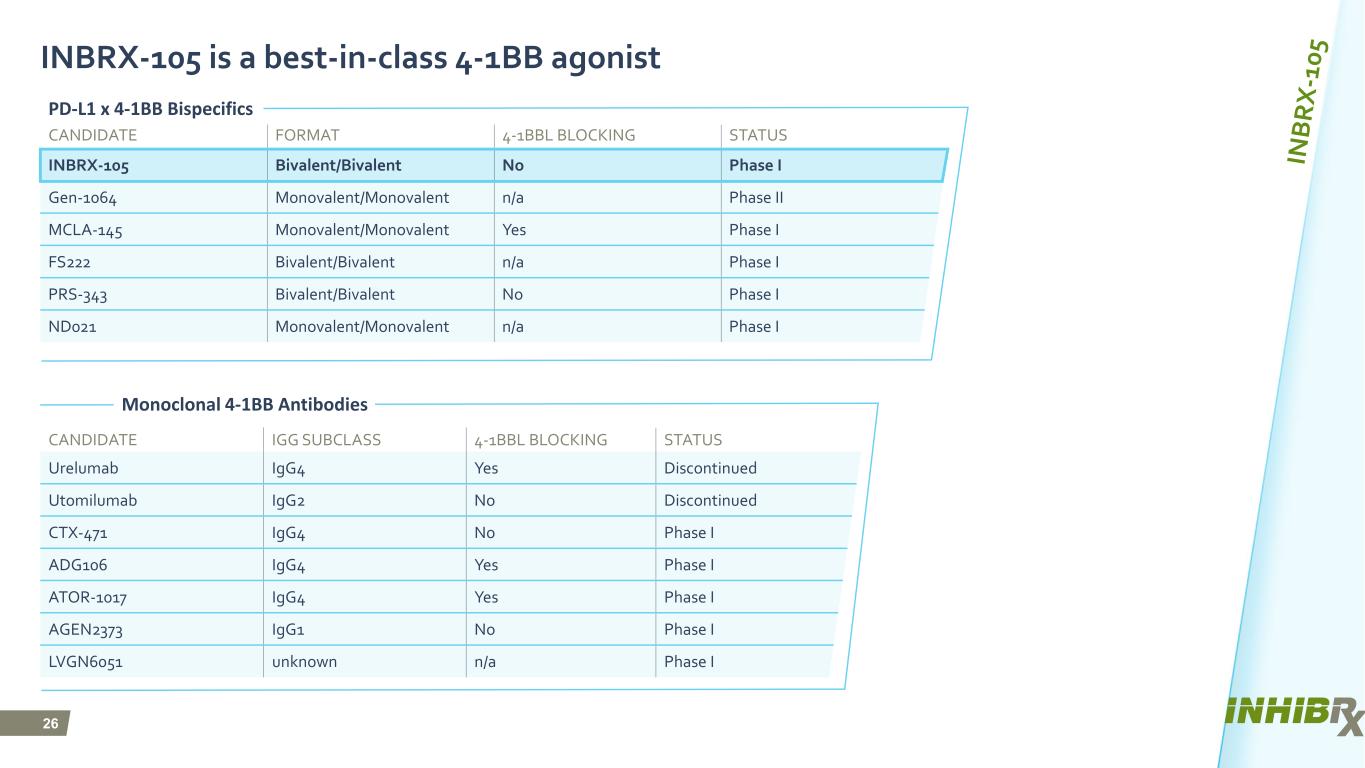
26 INBRX-105 is a best-in-class 4-1BB agonist CANDIDATE FORMAT 4-1BBL BLOCKING STATUS INBRX-105 Bivalent/Bivalent No Phase I Gen-1064 Monovalent/Monovalent n/a Phase II MCLA-145 Monovalent/Monovalent Yes Phase I FS222 Bivalent/Bivalent n/a Phase I PRS-343 Bivalent/Bivalent No Phase I ND021 Monovalent/Monovalent n/a Phase I CANDIDATE IGG SUBCLASS 4-1BBL BLOCKING STATUS Urelumab IgG4 Yes Discontinued Utomilumab IgG2 No Discontinued CTX-471 IgG4 No Phase I ADG106 IgG4 Yes Phase I ATOR-1017 IgG4 Yes Phase I AGEN2373 IgG1 No Phase I LVGN6051 unknown n/a Phase I PD-L1 x 4-1BB Bispecifics Monoclonal 4-1BB Antibodies
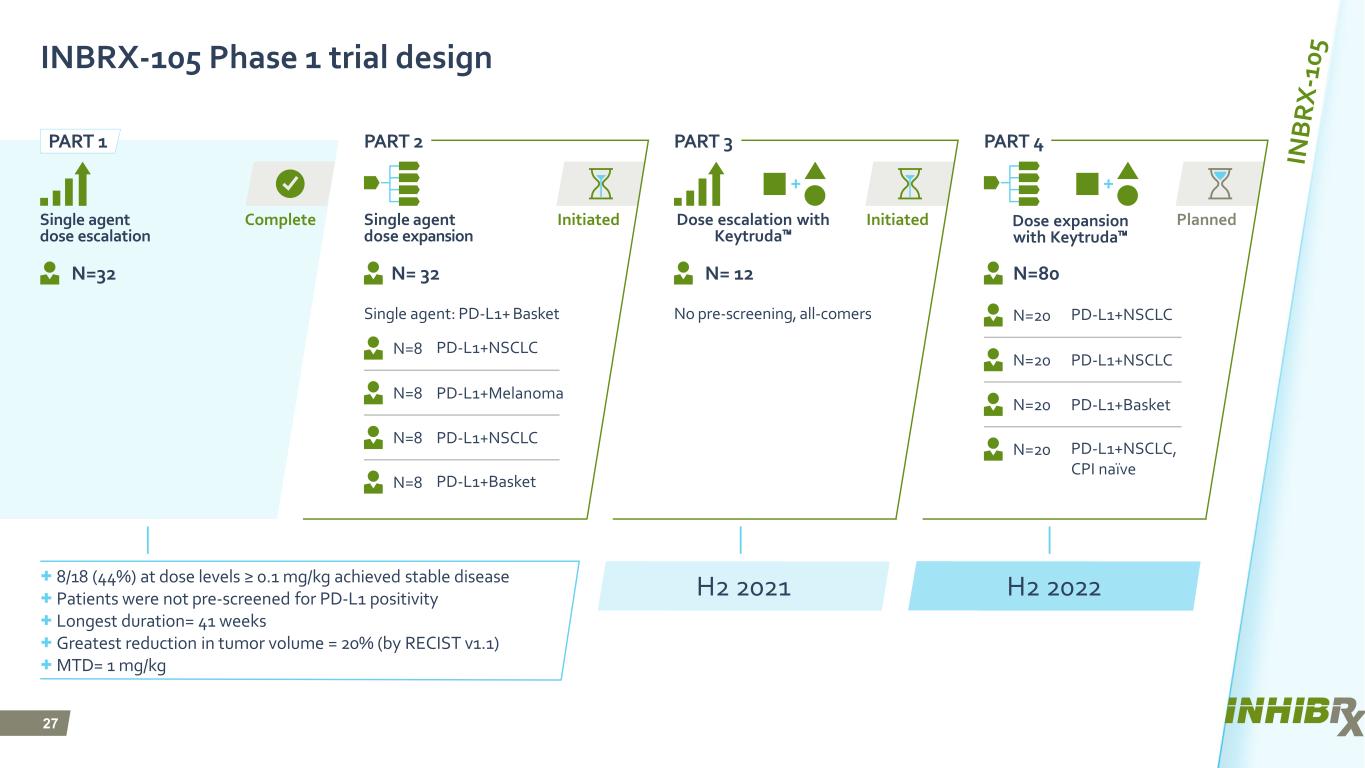
27 PlannedInitiatedInitiatedComplete INBRX-105 Phase 1 trial design Single agent: PD-L1+ Basket Single agent dose escalation Single agent dose expansion N=80N= 32 N= 12N=32 No pre-screening, all-comers + 8/18 (44%) at dose levels ≥ 0.1 mg/kg achieved stable disease + Patients were not pre-screened for PD-L1 positivity + Longest duration= 41 weeks + Greatest reduction in tumor volume = 20% (by RECIST v1.1) + MTD= 1 mg/kg N=20 PD-L1+NSCLC N=20 PD-L1+NSCLC N=20 PD-L1+Basket N=20 PD-L1+NSCLC, CPI naїve N=8 PD-L1+NSCLC N=8 PD-L1+Melanoma N=8 PD-L1+NSCLC N=8 PD-L1+Basket H2 2021 H2 2022 PART 1 PART 2 PART 3 PART 4 Dose expansion with Keytruda™ Dose escalation with Keytruda™
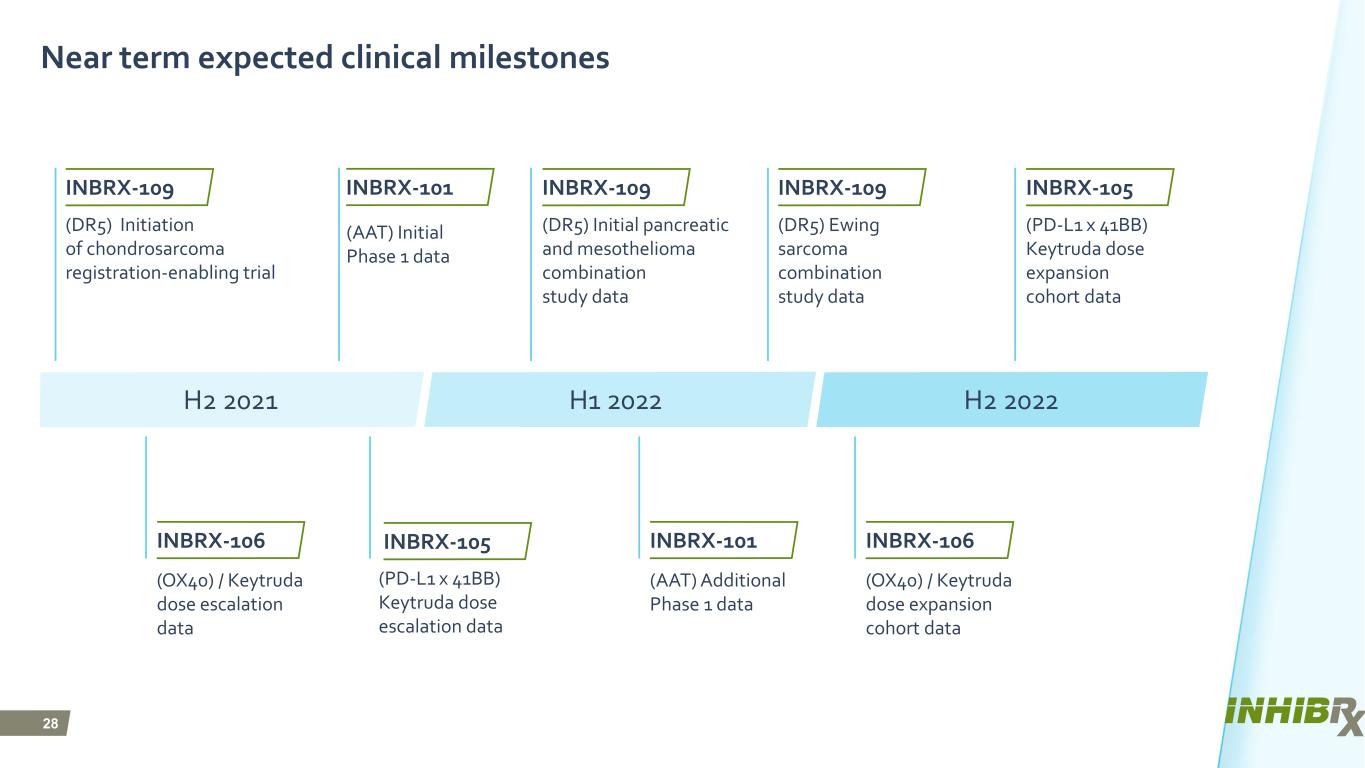
28 Near term expected clinical milestones H2 2021 H1 2022 H2 2022 INBRX-109 (OX40) / Keytruda dose escalation data (OX40) / Keytruda dose expansion cohort data (AAT) Initial Phase 1 data (AAT) Additional Phase 1 data (DR5) Initiation of chondrosarcoma registration-enabling trial (PD-L1 x 41BB) Keytruda dose escalation data (DR5) Initial pancreatic and mesothelioma combination study data (PD-L1 x 41BB) Keytruda dose expansion cohort data (DR5) Ewing sarcoma combination study data INBRX-105 INBRX-109 INBRX-109 INBRX-105 INBRX-106 INBRX-101 INBRX-101 INBRX-106

11025 N. Torrey Pines Rd Ste 200 La Jolla, CA 92037 www.inhibrx.com
