Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Quantum-Si Inc | tm2118908d5_8k.htm |
Exhibit 99.1

Quantum - Si Investor Presentation

Forward - Looking Statements This presentation includes “forward - looking statements” within the meaning of the “safe harbor” provisions of the United States Private Securities Litigation Reform Act of 1995. Actual results of Quantum - Si Incorporated (the “Company”) may differ from its expectations, estimates, and projections and, consequently, you should not rely on these forward - looking statements as predictions of future events. Words such as “expect,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believes,” “predicts,” “potential,” “continue,” and similar expressions (or the negative versions of such words or expressions) are inte nde d to identify such forward - looking statements. These forward - looking statements include, without limitation, the Company’s expectations with r espect to future performance, development of products and services, potential regulatory approvals, the size and potential growth of cu rrent or future markets for the Company’s future products and services, or the Company’s plans, expectations or future operations, fin anc ial position, revenues, costs or expenses. These forward - looking statements involve significant risks and uncertainties that could c ause the actual results to differ materially from those discussed in the forward - looking statements. Most of these factors are outside the Company’s control and are difficult to predict. Factors that may cause such differences include, but are not limited to: the impact of COVID - 19 on the Company’s business; the inability to maintain the listing of the Company’s shares of Class A common stock on The Nasdaq Stock Market; the ability to recognize the anticipated benefits of the Company’s recently - completed business combination, which may be affected by, among other things, competition and the ability of the Company to grow and manage growth profitably and retain its key employees; changes in applicable laws or regulations; the Company’s ability to raise financing in the future; the success, cost and tim ing of the Company’s product development activities; the potential attributes and benefits of the Company’s products and services; the Com pany’s ability to obtain and maintain regulatory approval for its products, and any related restrictions and limitations of any appr ove d product; the Company’s ability to identify, in - license or acquire additional technology; the Company’s ability to maintain its existing license, manufacture and supply agreements; the Company’s ability to compete with other companies currently marketing or engaged in t he development of products and services that the Company is developing; the size and growth potential of the markets for the Company’s future products and services, and its ability to serve those markets, either alone or in partnership with others; the pricing of the Company’s products and services following anticipated commercial launch; the Company’s estimates regarding future expenses, future rev enu e, capital requirements and needs for additional financing; the Company’s financial performance; and other risks and uncertaint ies indicated from time to time in the proxy statement/prospectus relating to the business combination, including those under “Risk Factor s” therein, and in the Company’s other filings with the SEC. The Company cautions that the foregoing list of factors is not exclusive. The Company cautions readers not to place undue reliance upon any forward - looking statements, which speak only as of the date made. The Company does not undertake or accept any obligation or undertaking to release publicly any updates or revisions to any forward - looking statements to reflect any change in its expectations or any change in events, conditions, or circumstances on which any such statement is based.


3 What if we could understand how disease is progressing in real - time and provide immediate guidance on personalized treatment?

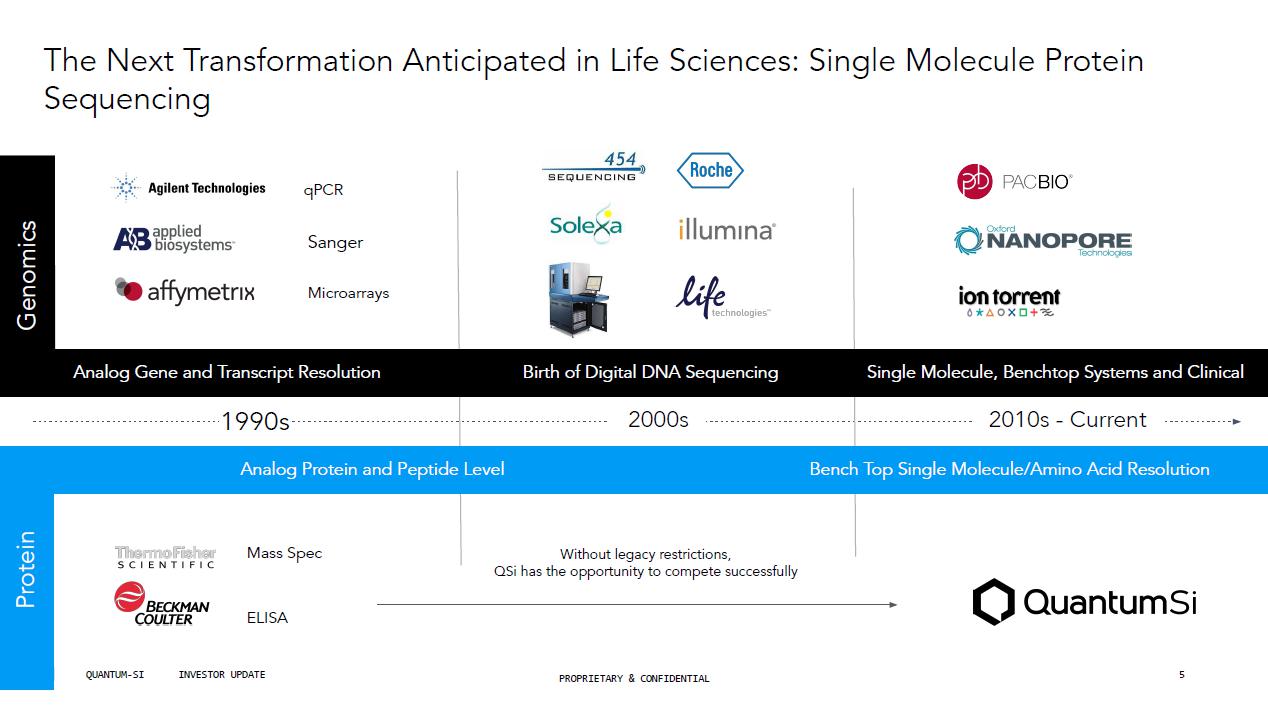
The Next Transformation Anticipated in Life Sciences: Single Molecule Protein Sequencing qPCR Sanger Mic r oarrays Mass Spec ELISA Genomics P r otein 2000s Analog Gene and Transcript Resolution Birth of Digital DNA Sequencing Analog Protein and Peptide Level Single Molecule, Benchtop Systems and Clinical 2010s - Current Bench Top Single Molecule/Amino Acid Resolution Without legacy restrictions, QSi has the opportunity to compete successfully 1990s
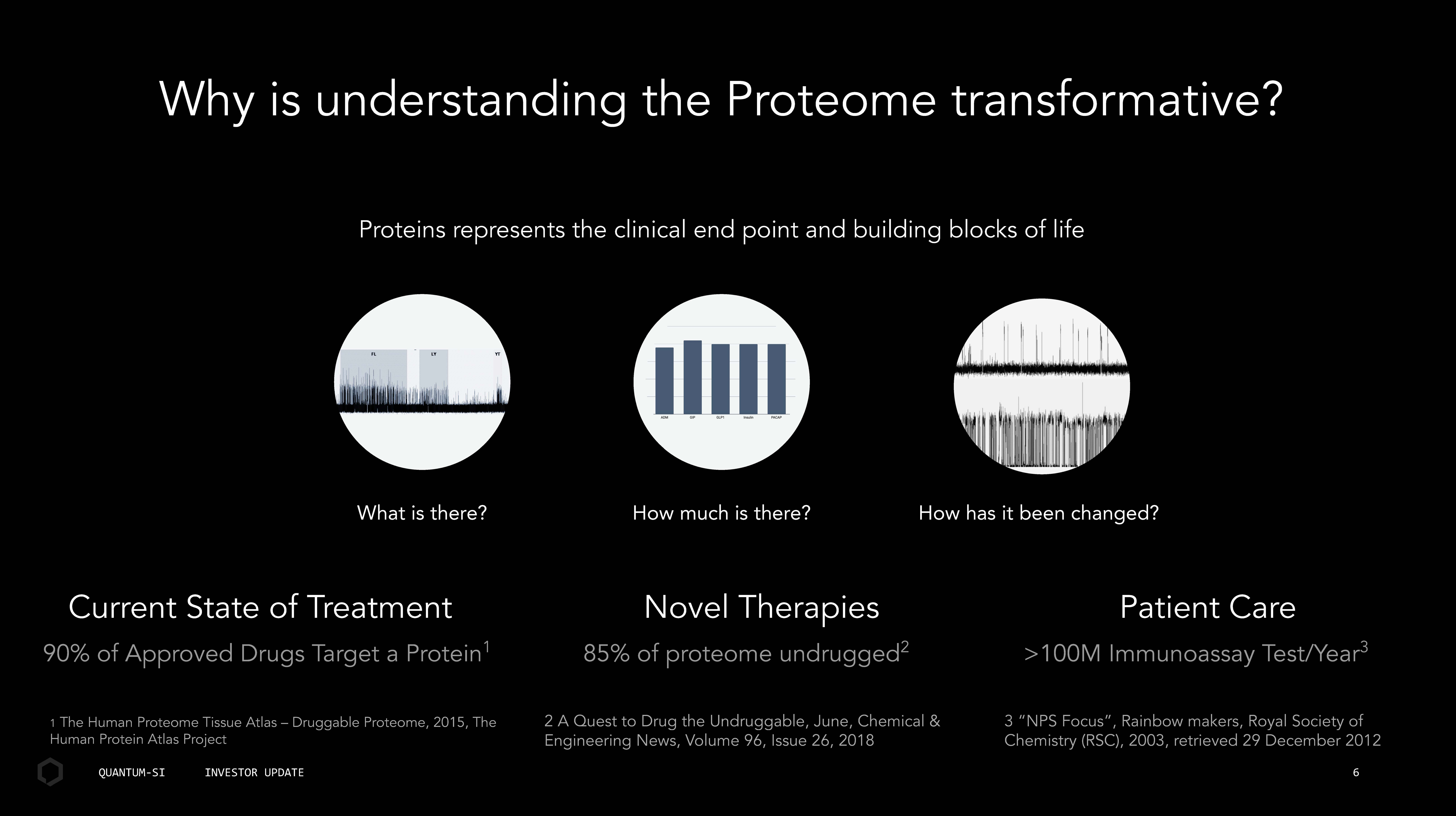
Current State of Treatment 90 of Approved Drugs Target a Protein 1 Novel Therapies of proteome undrugged 2 Why is understanding the Proteome transformative? Proteins represents the clinical end point and uilding locks of life What is there? How much is there? How has it been changed? Patient Care 100M Immunoassay Test/ ear 1 The uman Proteome Tissue Atlas Drugga le Proteome, 201 , The uman Protein Atlas Pro ect 2 A Quest to Drug the ndrugga le, une, Chemical Engineering News, olume 9 , Issue 2 , 201 NPS ocus , Rain ow makers, Royal Society of Chemistry RSC , 200 , retrieved 29 Decem er 2012

End - to - End Protein Analysis Platform Car on Universal sample preparation for both protein and A Platinum Single Molecule Analysis Cloud Secure data storage and analysis wor ows Sample Prep Sequencing Analysis
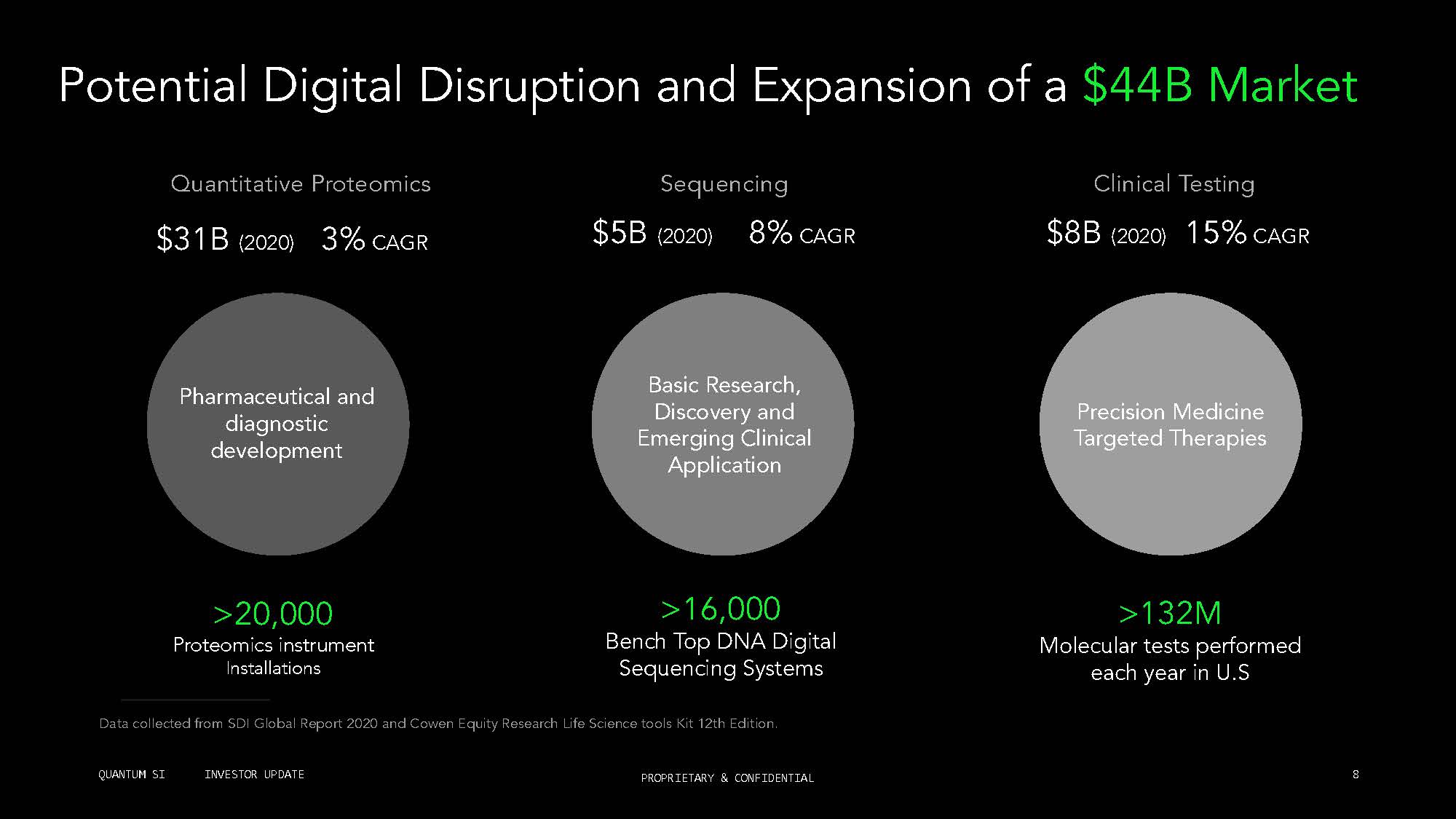
1 2M Molecular tests performed each year in S Clinical Testing 20,000 Proteomics instrument Installations 1 ,000 Bench Top DNA Digital Sequencing Systems Potential Digital Disruption and Expansion of a $44B Market Quantitative Proteomics $ 1B 2020 CAGR Sequencing $ B 2020 CAGR $ B 2020 1 CAGR Pharmaceutical and diagnostic development Basic Research, iscovery and Emerging Clinical Application Precision Medicine Targeted Therapies Data collected from SDI Glo al Report 2020 and Cowen Equity Research Life Science tools Kit 12th Edition
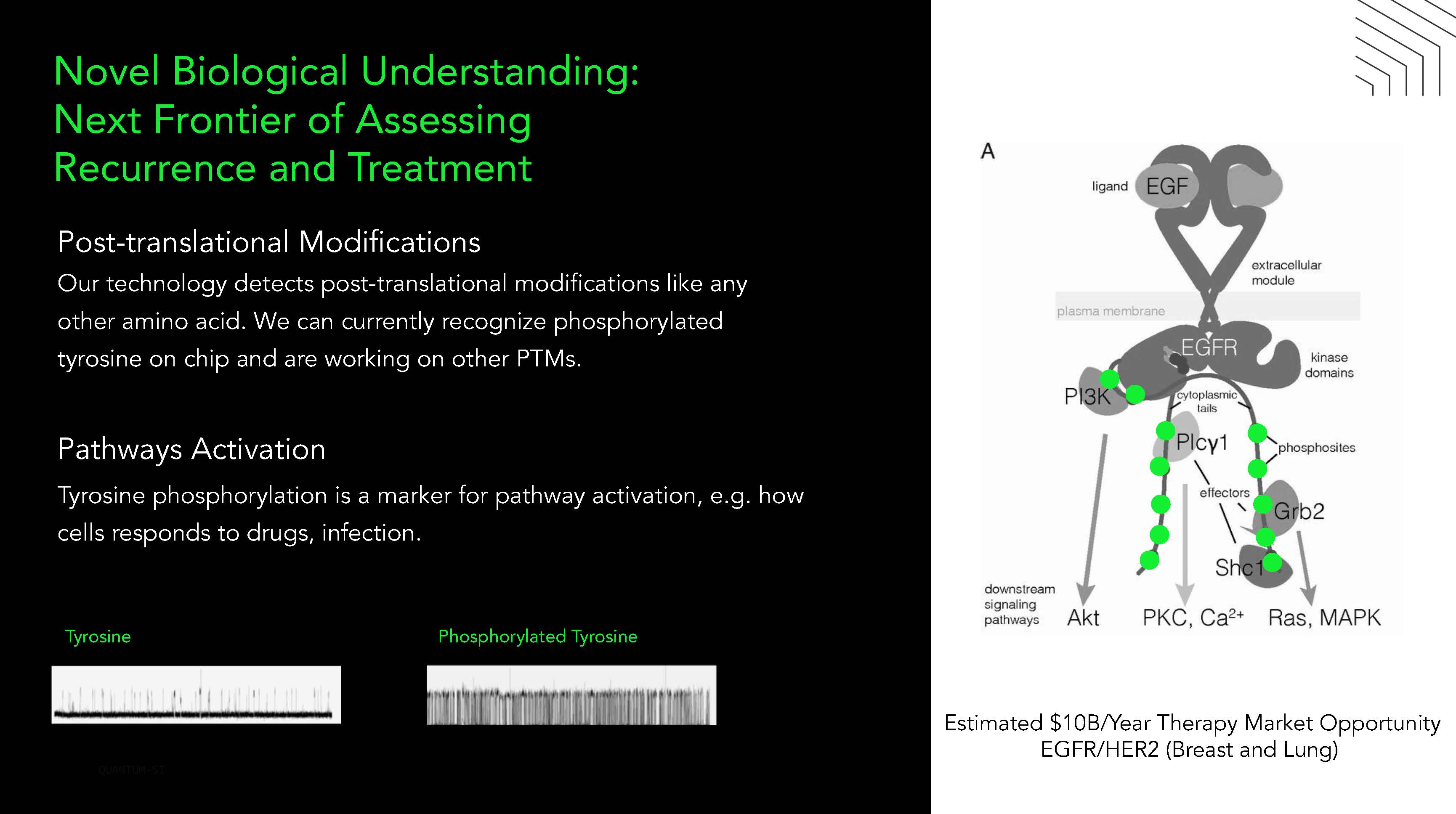
9 ovel Biological Understanding: ext Frontier of Assessing Recurrence and Treatment Phosphorylated Tyrosine T y r osine Post - translational Modiications Our technology detects post - translational modiications like any other amino acid We can currently recognize phosphorylated tyrosine on chip and are working on other PTMs Pathways Activation Tyrosine phosphorylation is a marker for pathway activation, e g how cells responds to drugs, infection Estimated $10B/ ear Therapy Market Opportunity EG R/ ER2 Breast and Lung

Mass Spec igital A Sequencing Single Molecule A Sequencing Quantum - Si Quantitative Analysis Protein Quantitative Analysis DNA Benchtop System Single Molecule And inally delivering on what we elieve the market demands
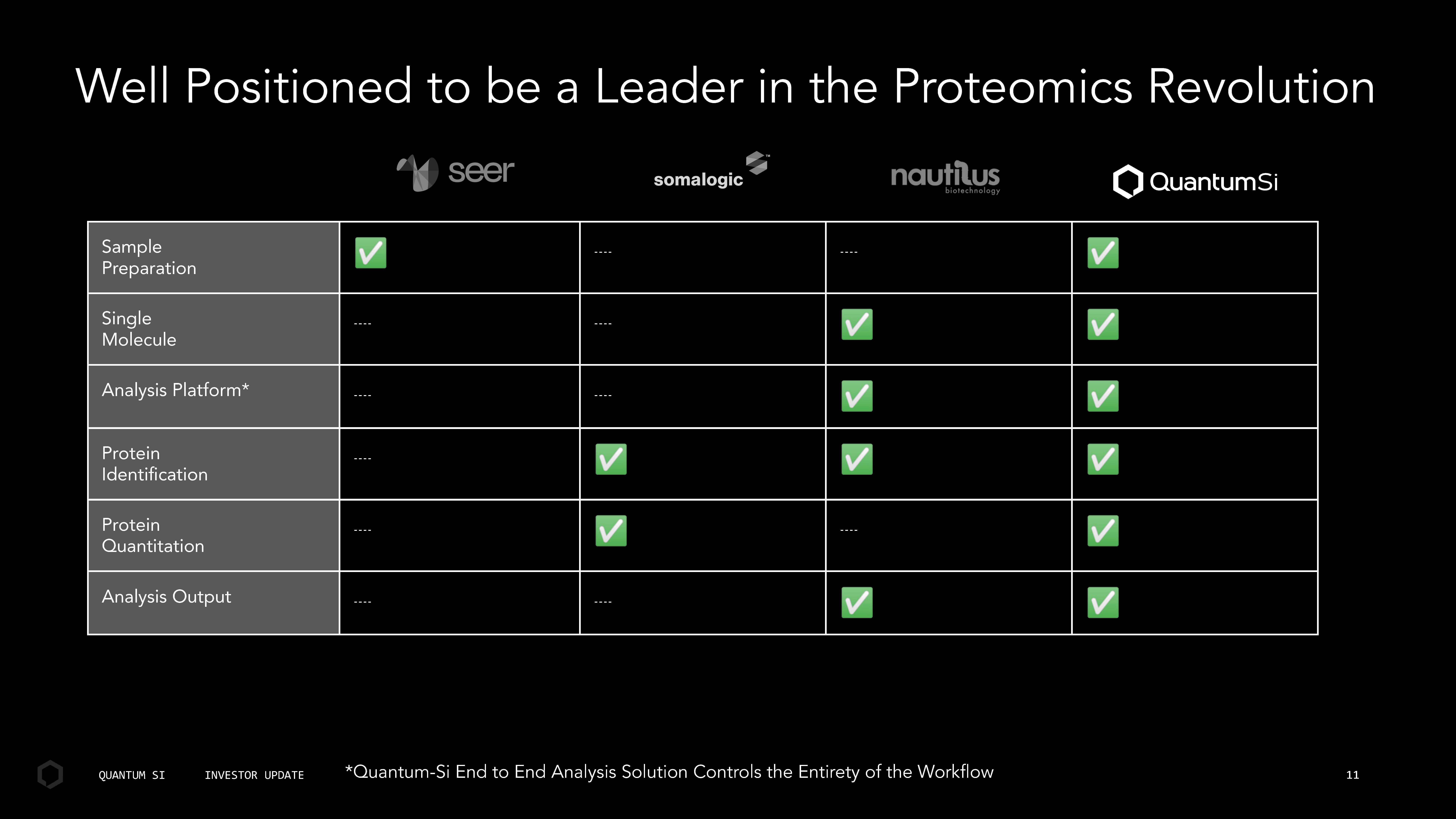
Sample P r eparatio n Single Molecul e Analysis Platform* Protein Identiication Protein Quantitatio n Analysis Output Well Positioned to e a Leader in the Proteomics Revolution *Quantum - Si End to End Analysis Solution Controls the Entirety of the Workflow
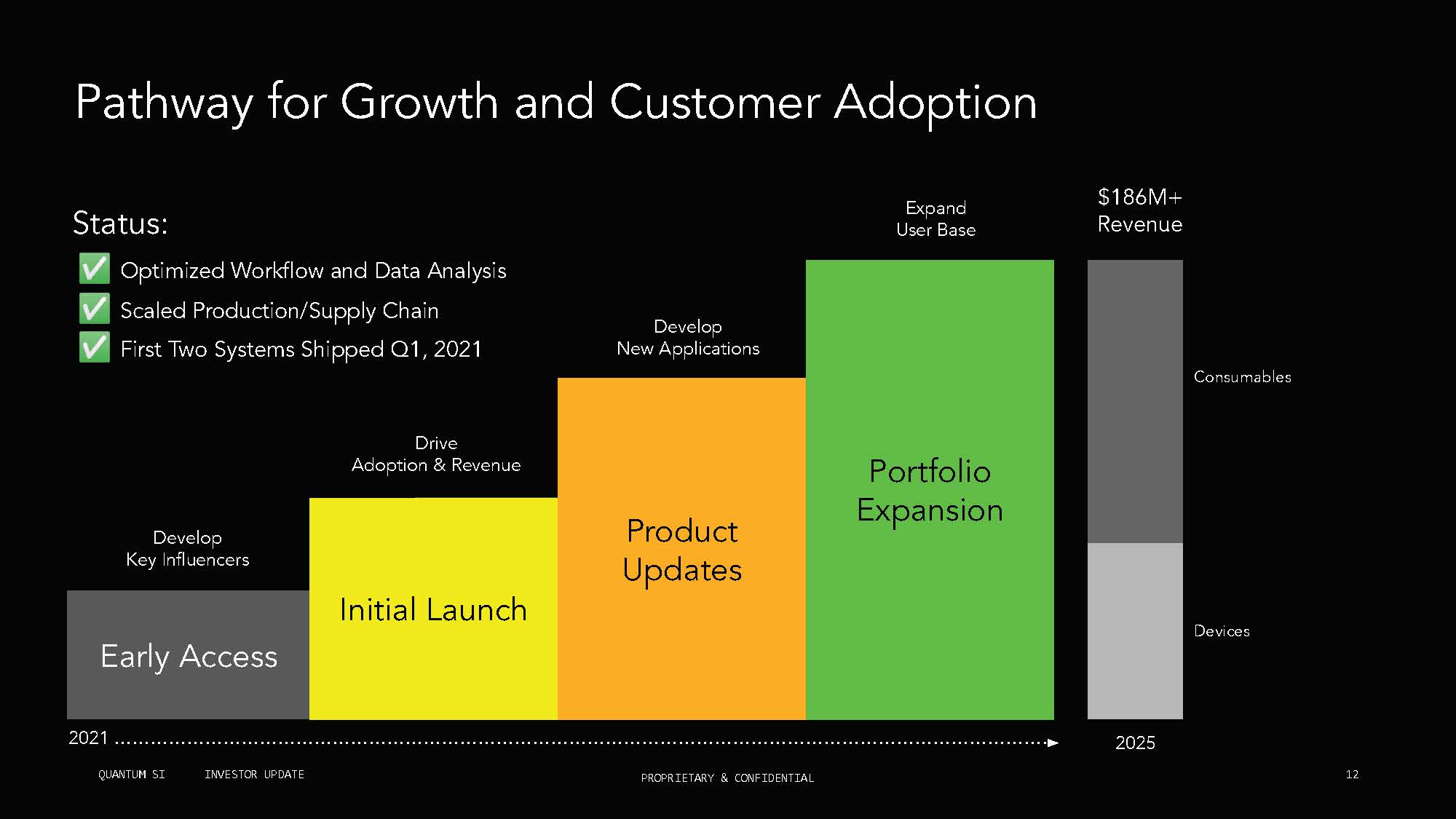
Pathway for Growth and Customer Adoption Drive Adoption Revenue Expand ser Base $1 M+ Revenue 2021 202 Develop New Applications Optimized Workflow and Data Analysis Scaled Production/Supply Chain irst Two Systems Shipped Q1, 2021 Early Access Develop Key Influencers Initial Launch Product pdates Portfolio Expansion Status: Consuma les Devices
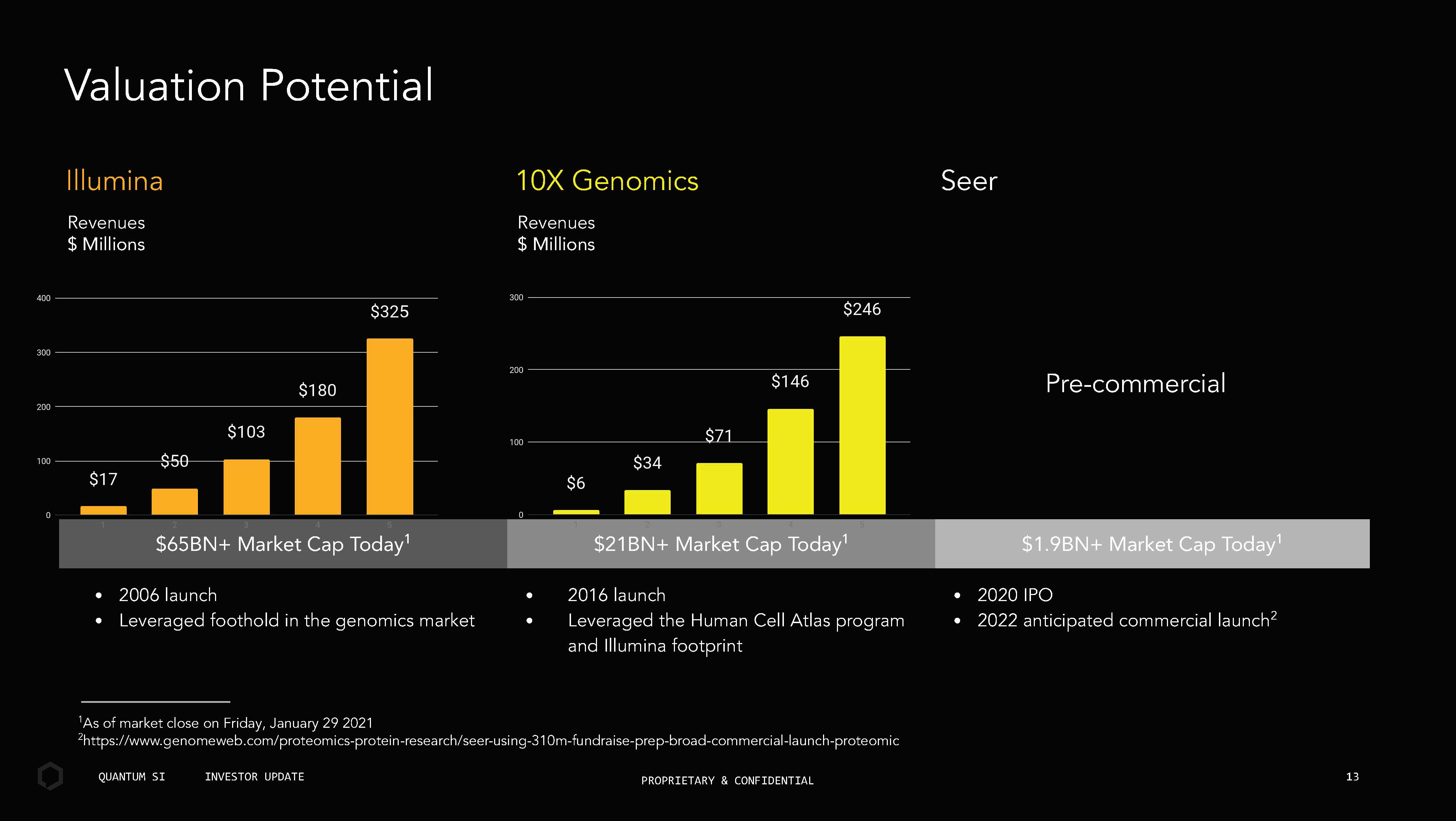
3 aluation Potential • 200 launch • Leveraged foothold in the genomics market 201 launch Leveraged the uman Cell Atlas program and Illumina footprint • 2020 IPO • 2022 anticipated commercial launch 2 P r e - comme r cial B Mar et Cap Today B Mar et Cap Today B Mar et Cap Today Seer Illumina Revenues Millions 10X Genomics Revenues Millions 1 As of market close on riday, anuary 29 2021 2 https://www genomewe com/proteomics - protein - research/seer - using - 10m - fundraise - prep - road - commercial - launch - proteomic

Meet the Team ONAT AN ROT BERG Executive Chai r man O N S T ARK Chief Executive Oficer CLA DIA DRA TON Chief inancial Oficer M A TT D ER Chief Business Oficer MIKE MCKENNA Chief Operations Oficer TODD REARICK Chief Technology Oficer C RISTIAN LAPOINTE General Counsel BRIAN REED ead of Resear ch AISAL A MAD ead of Optical Devices GERARD SC MID ead of Chip Manufacturing KIEREN PATEL ead of Product and Marketing LINDSA T OMPSON ead of People MEL DA E ead of Softwa r e MIKE ERRIGNO ead of ardware 120 Employees

• Commercializing single - molecule protein sequencing platform scaled y a irst of its kind semiconductor chip with over 0 1 issued and pending patents • Positioned for massive market expansion and to digitize a $44B 2 legacy proteomics research and diagnostic markets • Leveraging decades of advancement in digital DNA sequencing, QSi is poised to ecome the market leader in single molecule protein sequencing 1 Over 100 issued patents and 4 0 pending patent applications across 12 patent families 2 Data collected from SDI Glo al Report 2020 and Cowen Equity Research Life Science tools Kit 12th Edition Pioneering Next Generation Single Molecule Sequencing
