Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Terns Pharmaceuticals, Inc. | d158977dex991.htm |
| 8-K - 8-K - Terns Pharmaceuticals, Inc. | d158977d8k.htm |
Exhibit 99.2

TERNS PHARMACEUTICALS LIFT Study Topline Results A 12-Week Phase 2a Trial of TERN-101 in NASH Patients June 14th, 2021 NASDAQ: TERN

Forward-Looking Statements This presentation contains forward-looking statements about Terns Pharmaceuticals, Inc. (the “Company,” “we,” “us,” or “our”) and its industry that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this presentation, including statements regarding the Company’s strategy, future financial condition, future operations, projected costs, prospects, plans, objectives of management and expected market growth or statements regarding the therapeutic potential of TERN-101, the clinical utility of the data from and the endpoints used in the Phase 2a LIFT Study of TERN-101 and potential clinical development plans for TERN-101 and the Company’s other product candidates such as TERN-501, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “design,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “positioned,” “potential,” “predict,” “seek,” “should,” “target,” “will,” “would” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. The Company has based these forward-looking statements largely on its current expectations, estimates, forecasts and projections about future events and financial trends that it believes may affect its financial condition, results of operations, business strategy and financial needs. In light of the significant uncertainties in these forwardlooking statements, you should not rely upon forward-looking statements as predictions of future events. Although the Company believes that it has a reasonable basis for each forward-looking statement contained in this presentation, it cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur at all. These risks are not exhaustive. For a detailed discussion of the risk factors that could affect our actual results, please refer to the risk factors identified in our SEC reports, including but not limited to our Annual Report on Form 10-Kforthe year ended December 31, 2020 and our Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2021. New risk factors emerge from time to time and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward-looking statements. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation. This presentation discusses product candidates that are investigational only and have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of these product candidates for the use for which such product candidates are being studied. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. Acknowledgements ,fUFT Terns would like to acknowledge and thank the patients, investigators, and LIFT study team,

TfUFT Terns would like to acknowledge and thank theerns Pipeline: Designed to Address Multifaceted Nature of NASH Combining candidateswith complementary mechanisms to maximize NASH response rates patients, investigators, and LIT study team,
3LIFT 3 Important Firsts for NASH Treatment First FXR agonist trial to demonstrate no discontinuations due to AEs, including pruritus - TERN-101 was generally well-tolerated with similar incidence of AEs across treatment groups - No treatment-related SAEs First 12-week controlled trial in NASH to show significant improvements in cT1 - cT1 is an imaging marker of liver inflammation and fibrosis linked to clinical outcomes1 - Also observed improvements in PDFF and liver enzymes First NASH trial of an FXR agonist (TERN-101) in combination with a THR-p agonist (TERN-501) planned for 1H22 initiation - TERN-501 Phase 1 MAD portion started in June 2021 with data expected in 2H 2021 AE = adverse event. SAE = severe adverse event. FXR = farnesoid X receptor. NASH = nonalcoholic steatohepatitis. THR=thyroid hormone receptor 1. Liver International. 2020;40:3071-3082
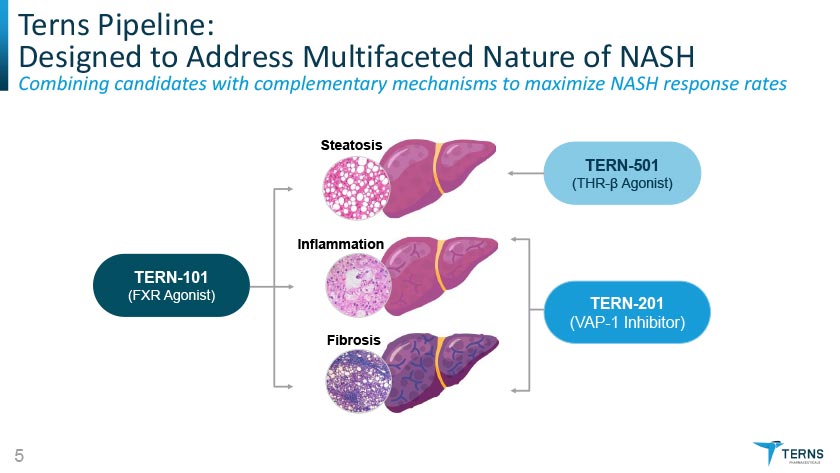
Terns Pipeline: Designed to Address Multifaceted Nature of NASH Combining candidates with complementary mechanisms to maximize NASH respo nse rates
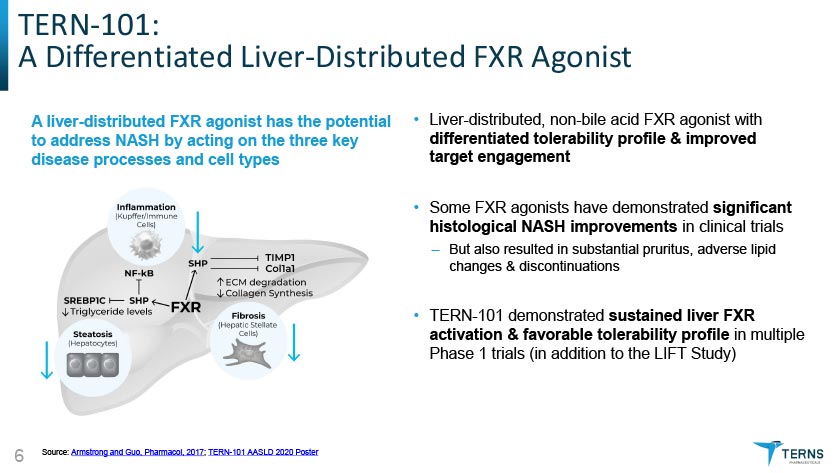
TERN-101: A Differentiated Liver-Distributed FXR Agonist
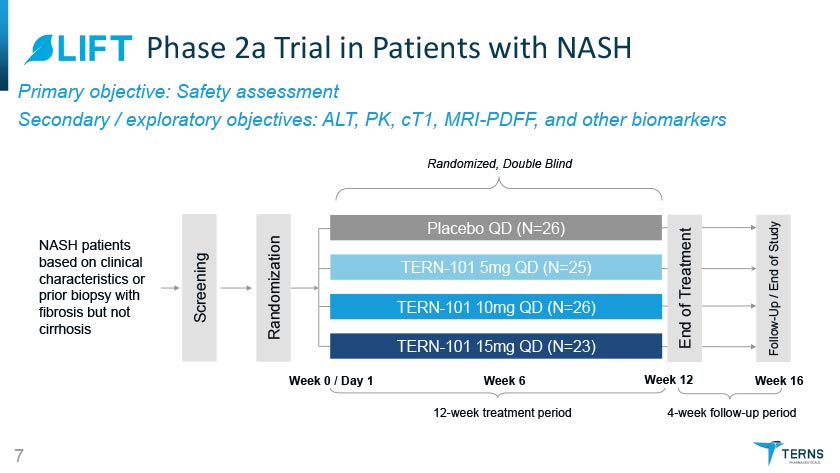
^LIFT Phase 2a Trial in Patients with NASH Primary objective: Safety assessment Secondary /exploratory objectives: ALT, PK, cT1, MRI-PDFF, and other biomarkers Randomized, Double Blind NAH patients based on clinical characteristics or prior biopsy with fibrosis but not cirrhosis
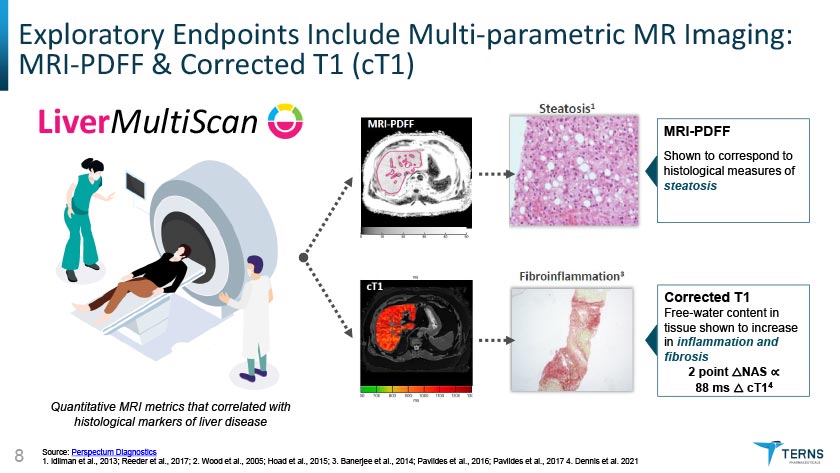
Exploratory Endpoints Include Multi-parametric MR Imaging: MRI-PDFF & Corrected T1 (cT1)
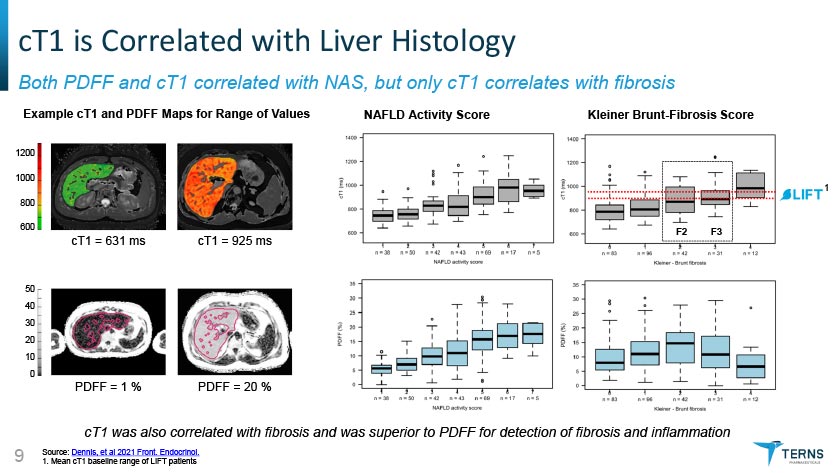
cT1 is Correlated with Liver Histology Both PDFF and cT1 correlated with NAS, but only cT1 correlates with fibrosis cT1 was also correlated with fibrosis and was superior to PDFF for detection of fibrosis and inflammation Source: Dennis, et al 2021 Front. Endocrinol. 1. Mean cT1 baseline range of LIFT patients
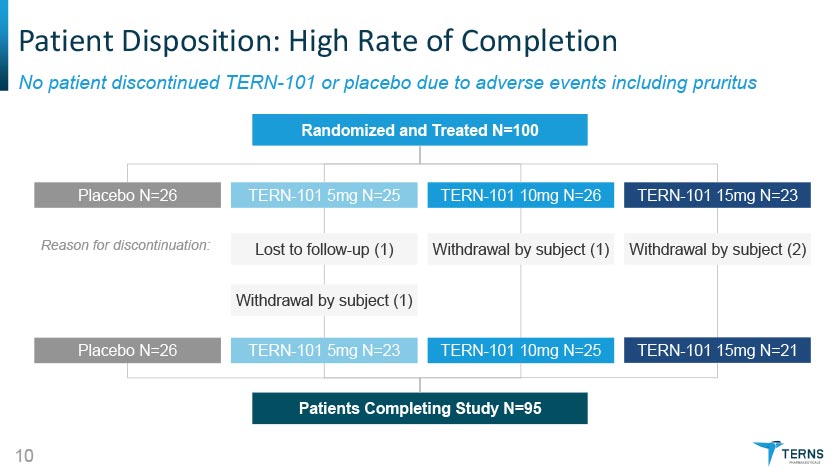
Patient Disposition: High Rate of Completion No patient discontinued TERN-101 or placebo due to adverse events including pruritus
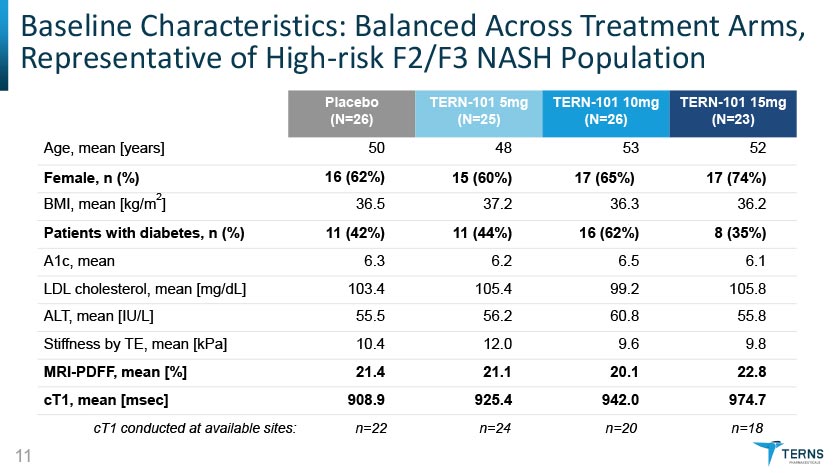
Baseline Characteristics: Balanced Across Treatment Arms Representative of High-risk F2/F3 NASH Population
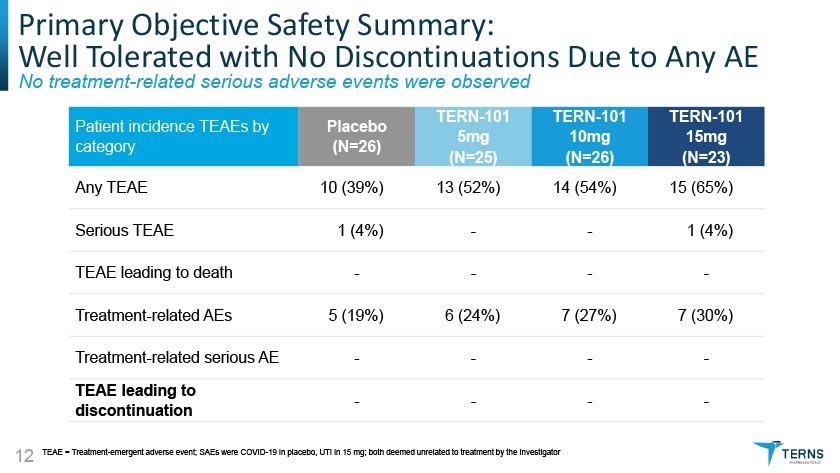
Primary Objective Safety Summary: Well Tolerated with No Discontinuations Due to Any AE No treatment-related serious adverse events were observed
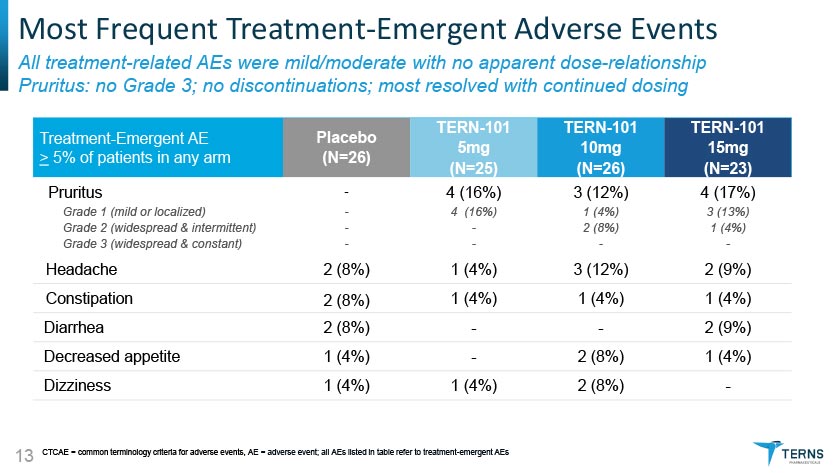
Most Frequent Treatment-Emergent Adverse Events All treatment-related AEs were mild/moderate with no apparent dose-relationship Pruritus: no Grade 3; no discontinuations; most resolved with continued dosing
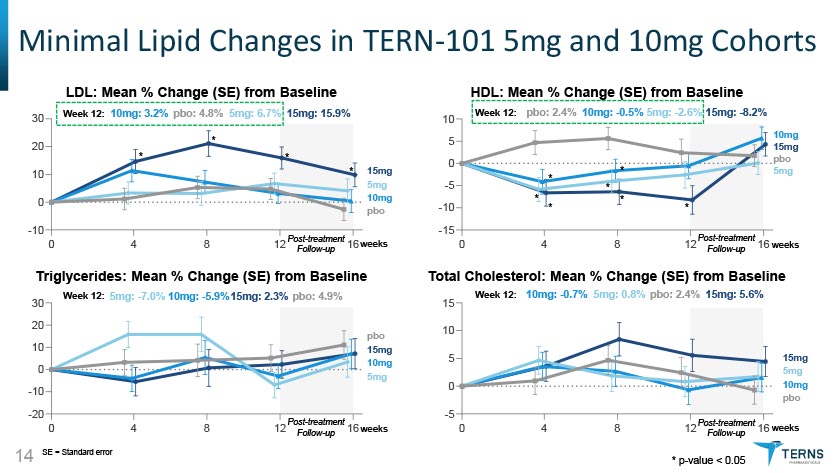
Minimal Lipid Changes in TERN-101 5mg and 10mg Cohorts
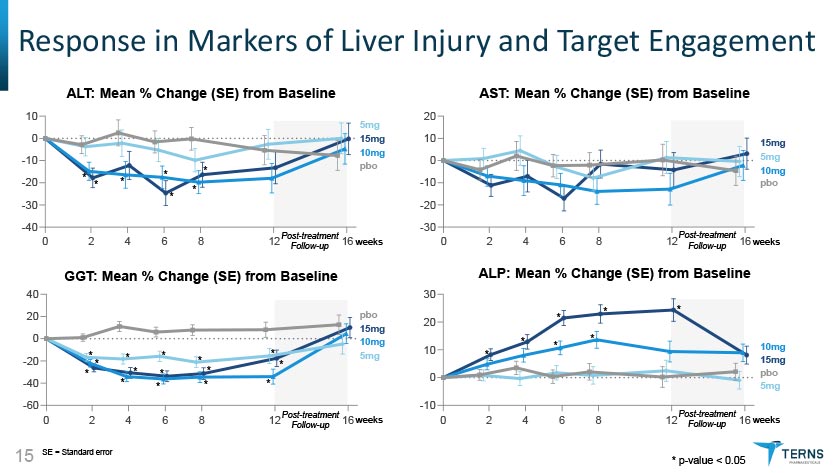
ALT: Mean % Change (SE) from Baseline AST: Mean % Change (SE) from Baseline GGT: Mean % Change (SE) from Baseline SE = Standard error 15 ALP: Mean % Change (SE) from Baseline
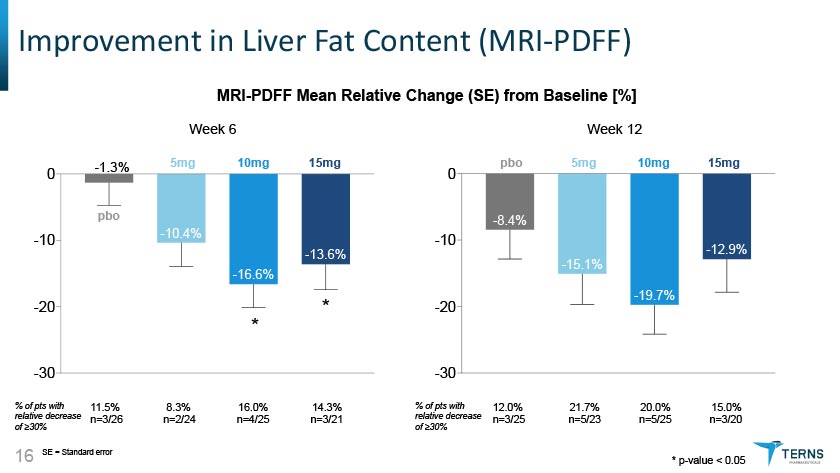
Improvement in Liver Fat Content (MRI-PDFF) MRI-PDFF Mean Relative Change (SE) from Baseline [%] Week 12
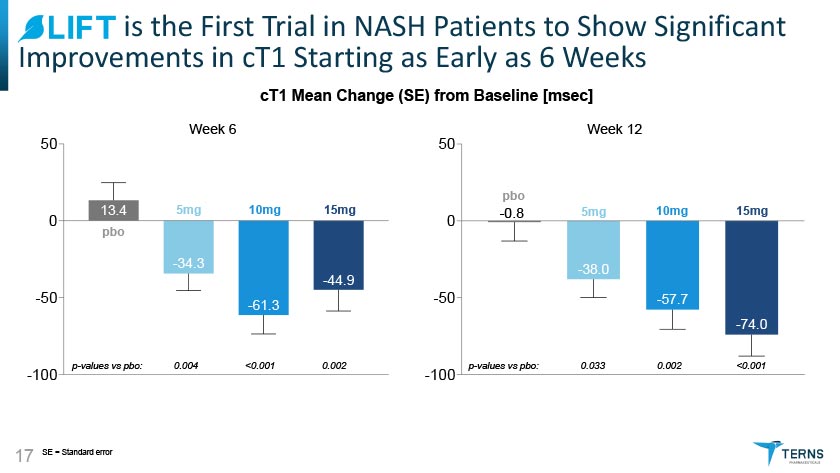
^LIFT is the First Trial in NASH Patients to Show Significant Improvements in cT1 Starting as Early as 6 Weeks cT1 Mean Change (SE) from Baseline [msec] TERNS PHARMACEUTICALS
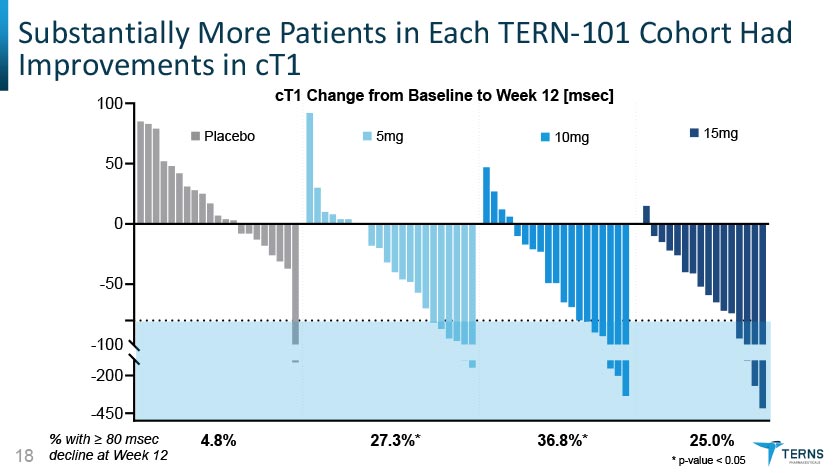
Substantially More Patients in Each TERN-101 Cohort Had Improvements in cT1 % with > 80 msec 18 decline at Week 12
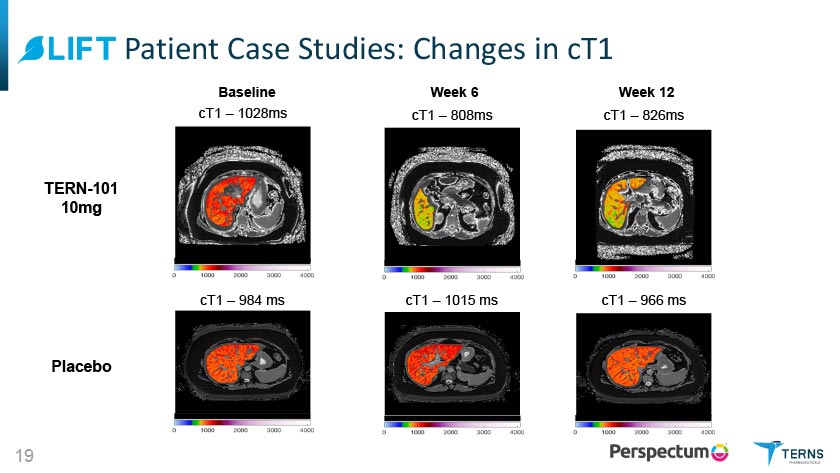
^LIFT Patient Case Studies: Changes in cT1
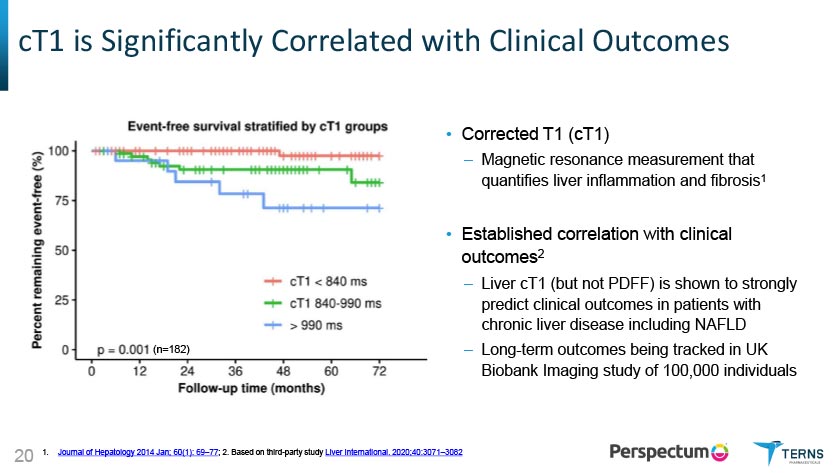
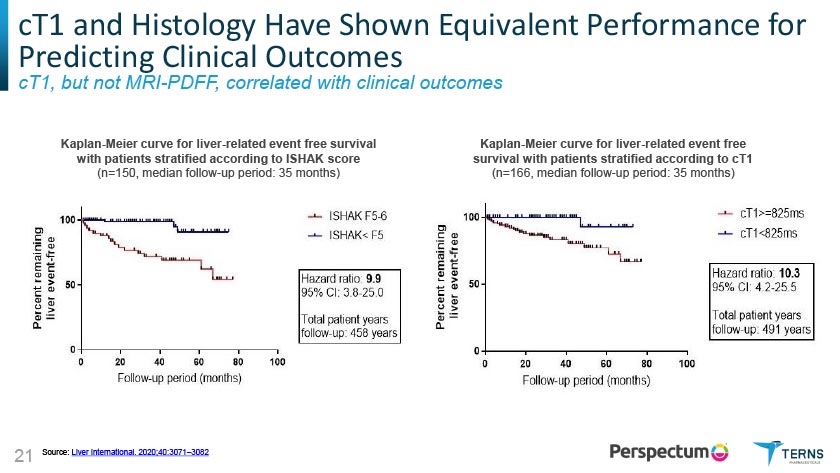
cT1 is Significantly Correlated with Clinical Outcomes cT1 and Histology Have Shown Equivalent Performance for Predicting Clinical Outcomes cT1, but not MRI-PDFF, correlated with clinical outcomes
cT1 Results in Context of Late-Stage NASH Investigational Products TERN-101 cT1 changes comparable to late-stage development candidates
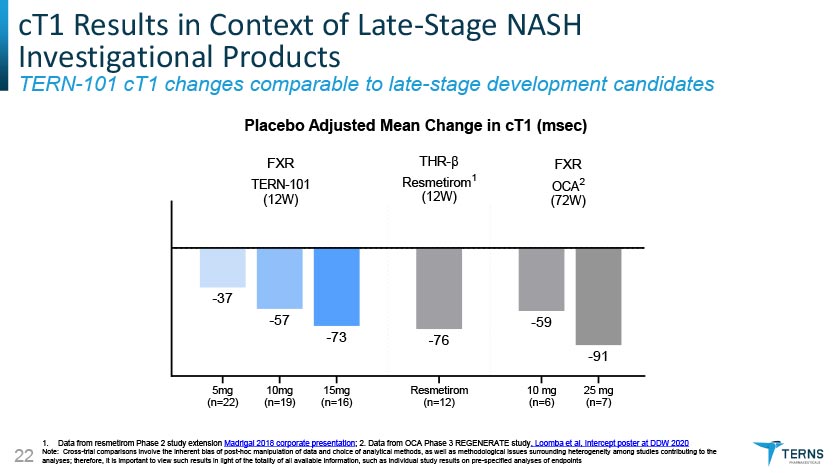
cT1 Results in Context of Late-Stage NASH Investigational Products TERN-101 cT1 changes comparable to late-stage development candidates
3LIFT 3 Important Firsts for NASH Treatment First FXR agonist trial to demonstrate no discontinuations due to AEs, including pruritus TERN-101 was generally well-tolerated with similar incidence of AEs across treatment groups No treatment-related SAEs First 12-week controlled trial in NASH to show significant improvements in cT1 cT1 is an imaging marker of liver inflammation and fibrosis linked to clinical outcomes1 Also observed improvements in PDFF and liver enzymes First NASH trial of an FXR agonist (TERN-101) in combination with a THR-p agonist (TERN-501) planned for 1H22 initiation - TERN-501 Phase 1 MAD portion started in June 2021 with data expected in 2H 2021 AE = adverse event. SAE = severe adverse event. FXR = farnesoid X receptor. NASH = nonalcoholic steatohepatitis. THR=thyroid hormone receptor 1. Liver International. 2020;40:3071-3082
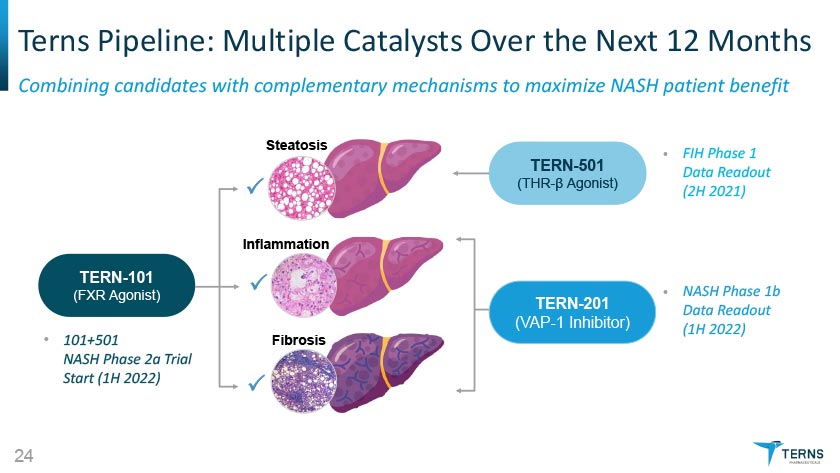
Terns Pipeline: Multiple Catalysts Over the Next 12 Months Combining candidates with complementary mechanisms to maximize NASH patient benefit

Appendix

Key Completed and Upcoming Milestones Multiple clinical milestones in 2021/2022 in preparation for combo trials
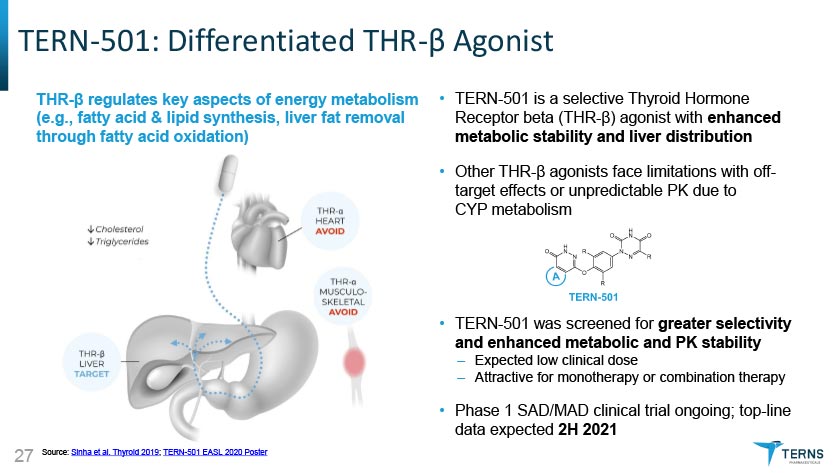
TERN-501: Differentiated THR-0 AgonistTHR-p regulates key aspects of energy metabolism (e.g., fatty acid & lipid synthesis, liver fat removal through fatty acid oxidation) • TERN-501 is a selective Thyroid Hormone Receptor beta (THR-p) agonist with enhanced metabolic stability and liver distribution Other THR-p agonists face limitations with off- target effects or unpredictable PK due to CYP metabolism TERN-501 was screened for greater selectivity and enhanced metabolic and PK stability - Expected low clinical dose - Attractive for monotherapy or combination therapy • Phase 1 SAD/MAD
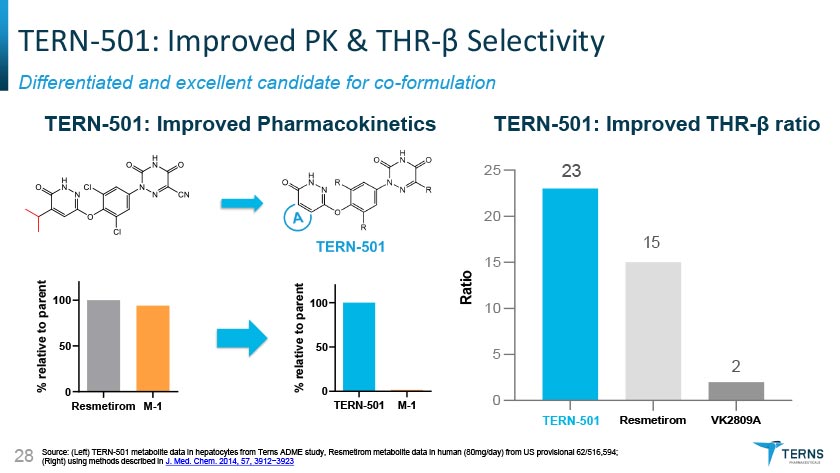
TERN-501: Improved PK & THR-0 Selectivity Differentiated and excellent candidate for co-formulation TERN-501: Improved Pharmacokinetics
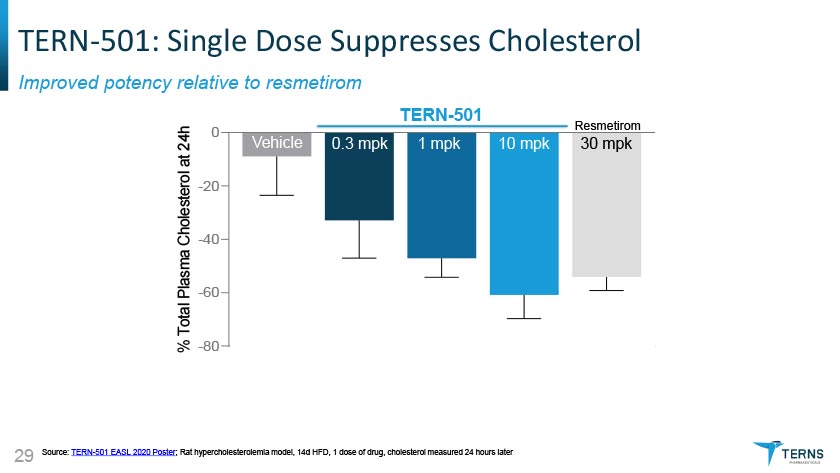
TERN-501: Single Dose Suppresses Cholesterol Improved potency relative to resmetirom
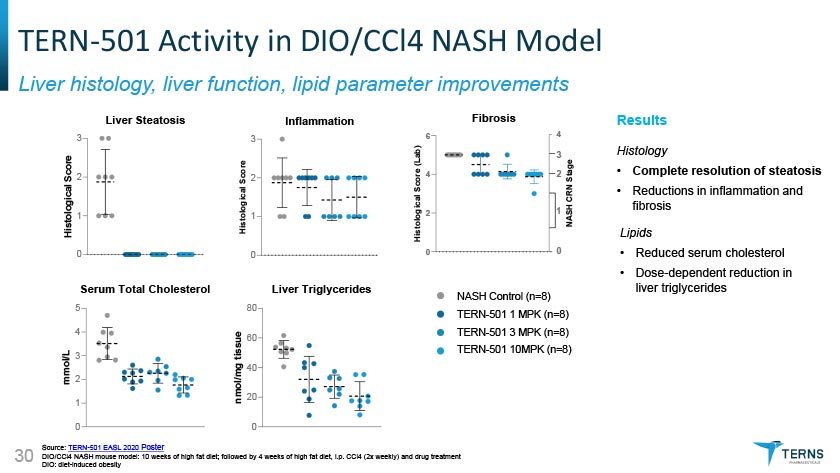
I TERN-501 Activity in DIO/CCI4 NASH Model I Liver histology, liver function, lipid parameter improvements
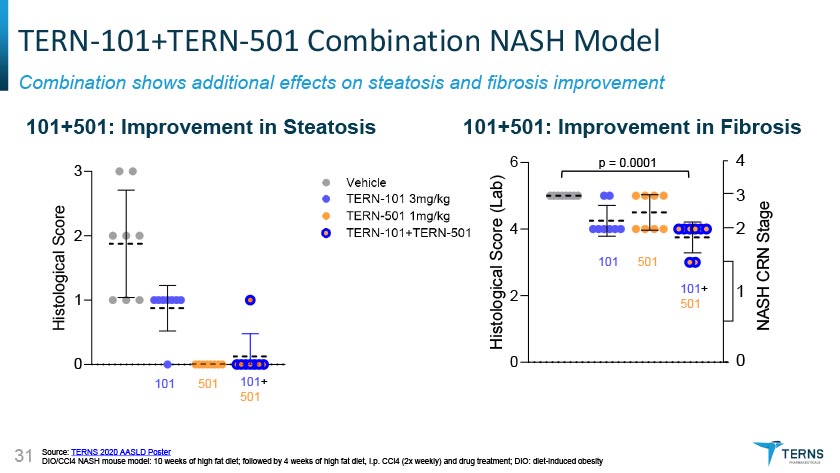
TERN-101+TERN-501 Combination NASH Model Combination shows additional effects on steatosis and fibrosis improvement
