Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - RAPT Therapeutics, Inc. | d181504dex991.htm |
| 8-K - 8-K - RAPT Therapeutics, Inc. | d181504d8k.htm |

Transforming the Treatment of Cancer and Inflammation RPT193 Topline Results June 14, 2021 Exhbit 99.2

Legal Disclaimers Statements in this Presentation that are not statements of historical fact are forward-looking statements. Such forward-looking statements include, without limitation, statements regarding RAPT Therapeutics, Inc.’s (the "Company," "we," or "us") research and clinical development plans, including the planned inititation of a Phase 2b dose-ranging study of RPT193 in patients with moderate-to-severe atopic dermatitis and a planned Phase 2a trial in patients with asthma, interpretations of the topline results from the Phase 1b clinical trial of RPT193; current and future drug candidates; business strategy and plans; regulatory pathways; and our ability to complete certain milestones. Words such as "believe," "anticipate," "plan," "expect," "will," "may," "upcoming," "milestone," "potential," "target" or the negative of these terms or similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. These forward-looking statements are based on the current beliefs of the Company's management with respect to future events and trends and are subject to known and unknown risks and uncertainties, including those described in the “Risk Factors” section of our most recent Form 10-Q filed with the Securities and Exchange Commission, that may cause our actual performance or achievements to be materially different from any future performance or achievements expressed or implied by the forward-looking statements in this Presentation. These forward-looking statements should not be taken as forecasts or promises nor should they be taken as implying any indication, assurance or guarantee that any assumptions on which such forward-looking statements have been made are correct or exhaustive or, in the case of such assumptions, fully stated in the Presentation. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this Presentation is given. Although we believe that the beliefs and assumptions reflected in the forward-looking statements are reasonable, we cannot guarantee future performance or achievements. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this Presentation. This Presentation discusses drug candidates that are under clinical study and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of any drug candidates for any use for which such drug candidates are being studied.
Summary Topline data from a placebo-controlled double-blinded Phase 1b trial examining 400 mg oral RPT193 as monotherapy for 4 weeks in 31 patients with moderate-to-severe atopic dermatitis* Efficacy: RPT193 demonstrates clear improvement over placebo on all key exploratory endpoints At Day 29: EASI [36.3% vs. 17.0%], EASI-50 [42.9% vs. 10.0%], vIGA 0/1 [4.8% vs. 0.0%], and pruritis NRS-4 [45.0% vs. 22.2%] Further improvement observed during the 2-week follow up period to Day 43: EASI [53.2% vs. 9.6%]†, EASI-50 [61.9% vs. 20.0%]†, and vIGA 0/1 [14.3% vs. 0.0%] Safety: Overall safety profile to date suggests a well-tolerated oral drug that would not require laboratory safety monitoring No SAEs reported; all AEs reported were mild or moderate in intensity The clear clinical benefit combined with the favorable safety profile and oral convenience would support positioning ahead of approved and late-stage therapies A 16-week Phase 2b dose-ranging study in patients with moderate-to-severe AD will be initiated * Initial topline data and analyses are being reported today. Additional analyses are ongoing and full datasets will be incorporated as part of a future publication or at a medical conference. † Difference between RPT193 and placebo on the EASI and EASI-50 score was statistically significant at Day 43 (p < 0.05). No other endpoints or timepoints achieved statistical significance.
Atopic Dermatitis Represents a Major Market Opportunity Atopic dermatitis (AD) is a common disease affecting ~19M adults and ~10M children in the US $24B market by 2029* While the injectable Dupixent® (dupilumab) provides an important option, a well-tolerated, effective, oral drug that does not require laboratory safety monitoring remains a high unmet need in these patients RPT193, a highly potent and selective oral C-C chemokine receptor type 4 (CCR4) antagonist, has the potential to address this need Novel mechanism of action – upstream of current agents targeting cytokines and the JAK pathway – designed to target the specific type of inflammation that drives allergic disease, without broadly suppressing the immune response * Decision Resources Guide May 2021; EU, US, and Japan market
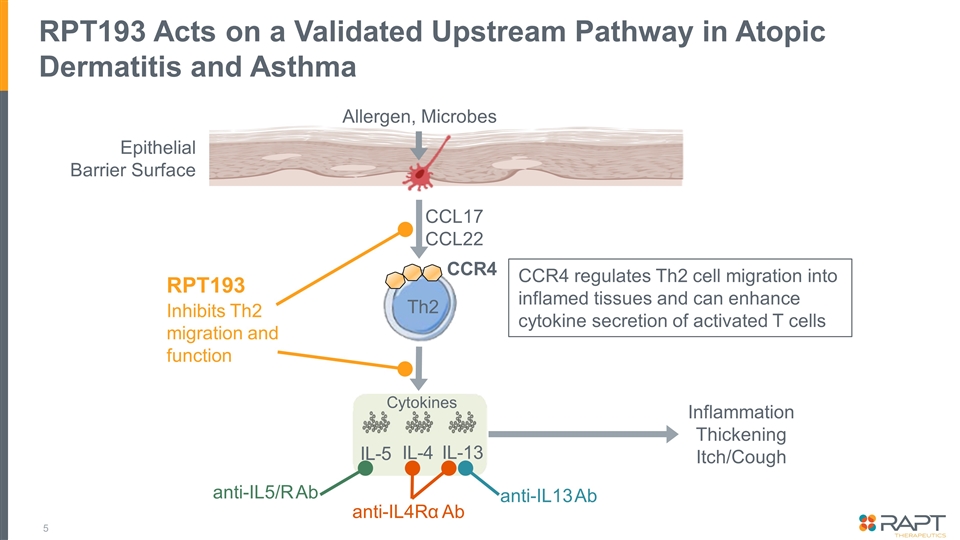
RPT193 Acts on a Validated Upstream Pathway in Atopic Dermatitis and Asthma Epithelial Barrier Surface CCR4 Th2 CCL17 CCL22 anti-IL4Rα Ab anti-IL5/R Ab anti-IL13 Ab IL-5 IL-4 IL-13 Inflammation Thickening Itch/Cough RPT193 Inhibits Th2 migration and function Cytokines Allergen, Microbes CCR4 regulates Th2 cell migration into inflamed tissues and can enhance cytokine secretion of activated T cells
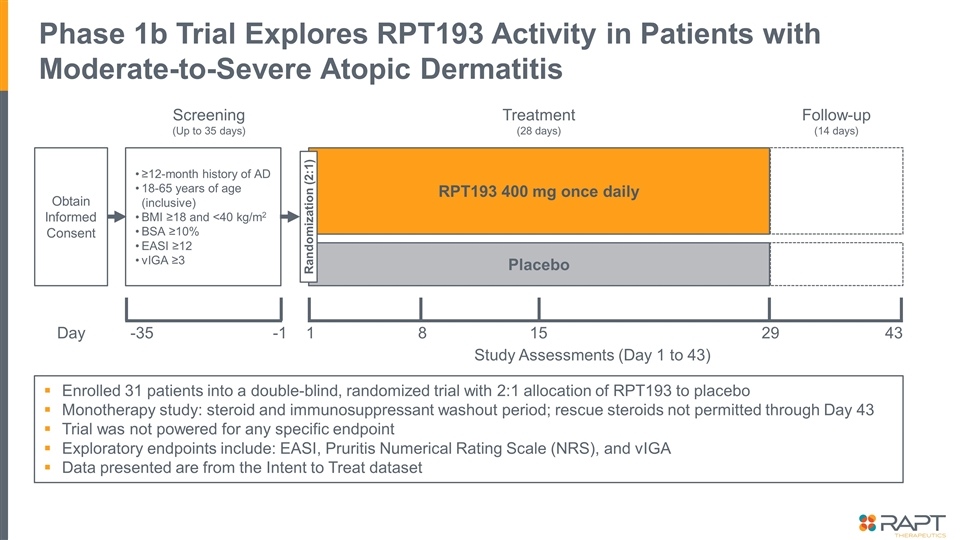
Enrolled 31 patients into a double-blind, randomized trial with 2:1 allocation of RPT193 to placebo Monotherapy study: steroid and immunosuppressant washout period; rescue steroids not permitted through Day 43 Trial was not powered for any specific endpoint Exploratory endpoints include: EASI, Pruritis Numerical Rating Scale (NRS), and vIGA Data presented are from the Intent to Treat dataset Phase 1b Trial Explores RPT193 Activity in Patients with Moderate-to-Severe Atopic Dermatitis Obtain Informed Consent ≥12-month history of AD 18-65 years of age (inclusive) BMI ≥18 and <40 kg/m2 BSA ≥10% EASI ≥12 vIGA ≥3 Screening (Up to 35 days) Day -35 -1 RPT193 400 mg once daily Placebo Randomization (2:1) Treatment (28 days) Follow-up (14 days) 1 29 8 15 43 Study Assessments (Day 1 to 43)
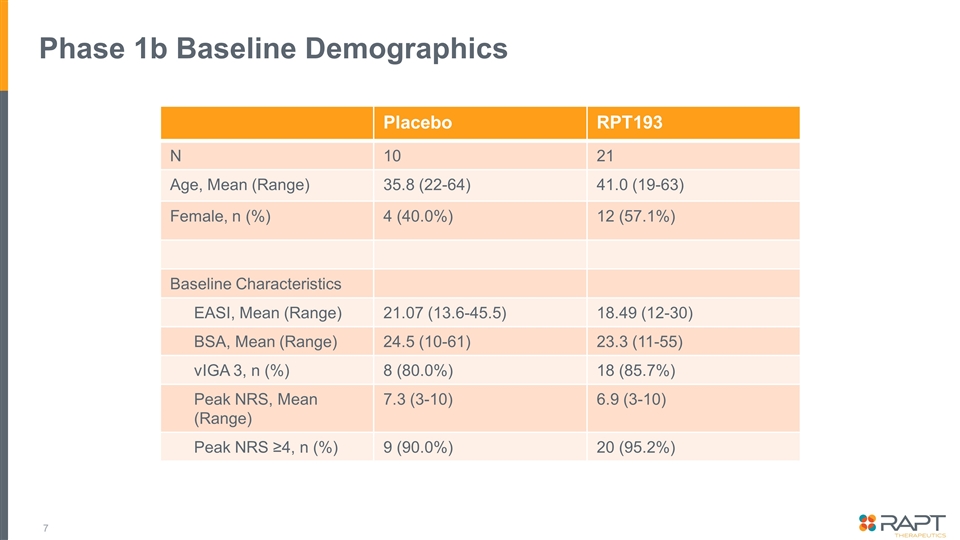
Phase 1b Baseline Demographics Placebo RPT193 N 10 21 Age, Mean (Range) 35.8 (22-64) 41.0 (19-63) Female, n (%) 4 (40.0%) 12 (57.1%) Baseline Characteristics EASI, Mean (Range) 21.07 (13.6-45.5) 18.49 (12-30) BSA, Mean (Range) 24.5 (10-61) 23.3 (11-55) vIGA 3, n (%) 8 (80.0%) 18 (85.7%) Peak NRS, Mean (Range) 7.3 (3-10) 6.9 (3-10) Peak NRS ≥4, n (%) 9 (90.0%) 20 (95.2%)
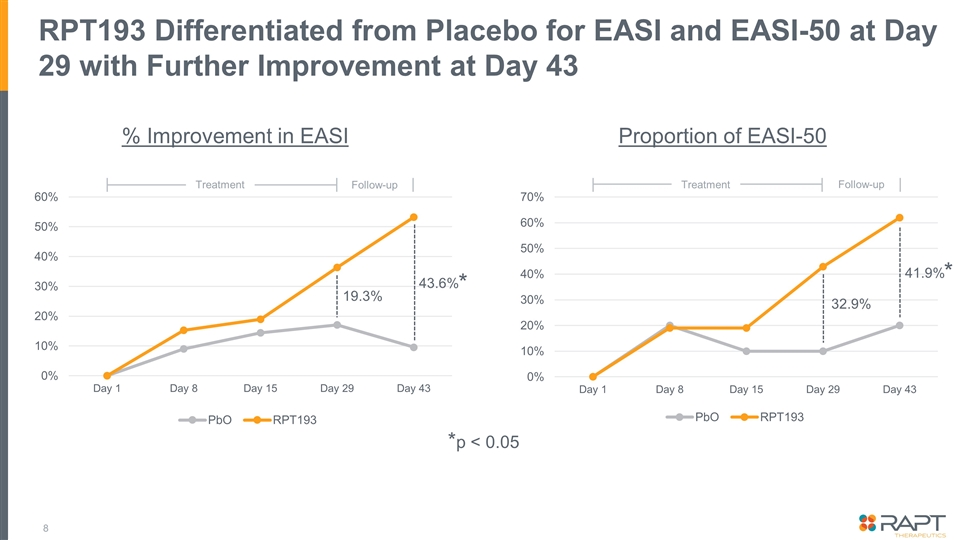
RPT193 Differentiated from Placebo for EASI and EASI-50 at Day 29 with Further Improvement at Day 43 % Improvement in EASI Proportion of EASI-50 19.3% 43.6% 32.9% 41.9% Follow-up Treatment Follow-up Treatment *p < 0.05 * *
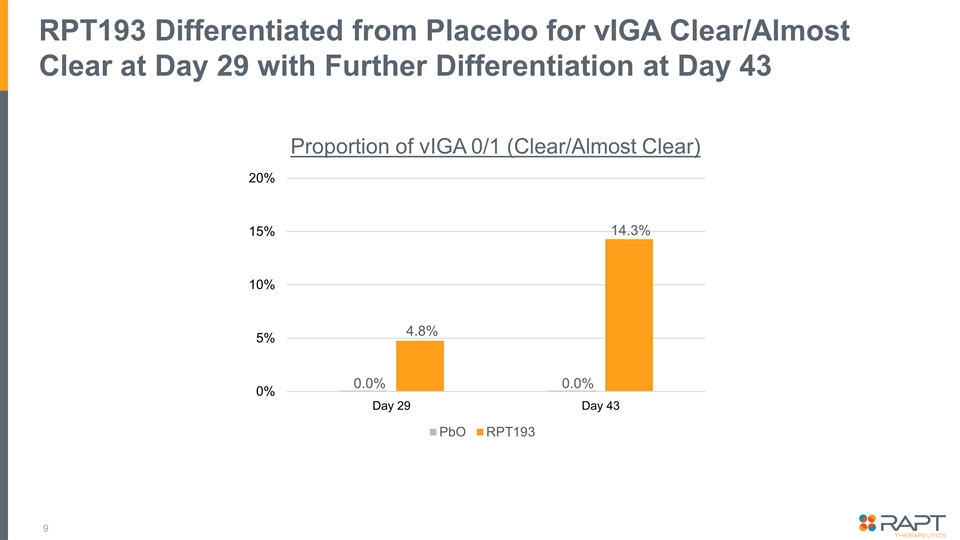
RPT193 Differentiated from Placebo for vIGA Clear/Almost Clear at Day 29 with Further Differentiation at Day 43 0.0% 0.0% 4.8% 14.3% Proportion of vIGA 0/1 (Clear/Almost Clear)
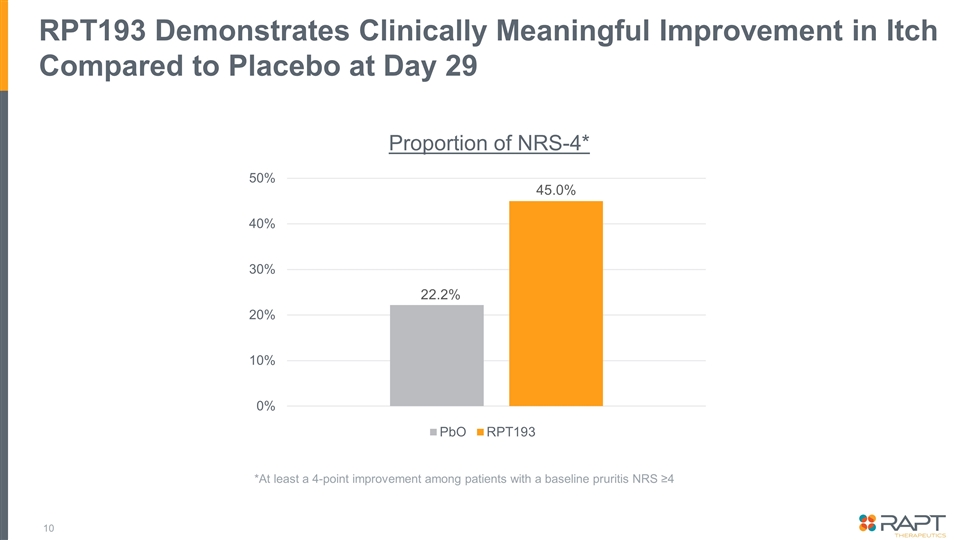
RPT193 Demonstrates Clinically Meaningful Improvement in Itch Compared to Placebo at Day 29 Proportion of NRS-4* 22.2% 45.0% *At least a 4-point improvement among patients with a baseline pruritis NRS ≥4

Safety No SAEs reported All AEs reported were mild or moderate in intensity No clinically significant safety laboratory abnormalities observed Overall safety profile to date suggests a well-tolerated oral drug that should not require laboratory safety monitoring
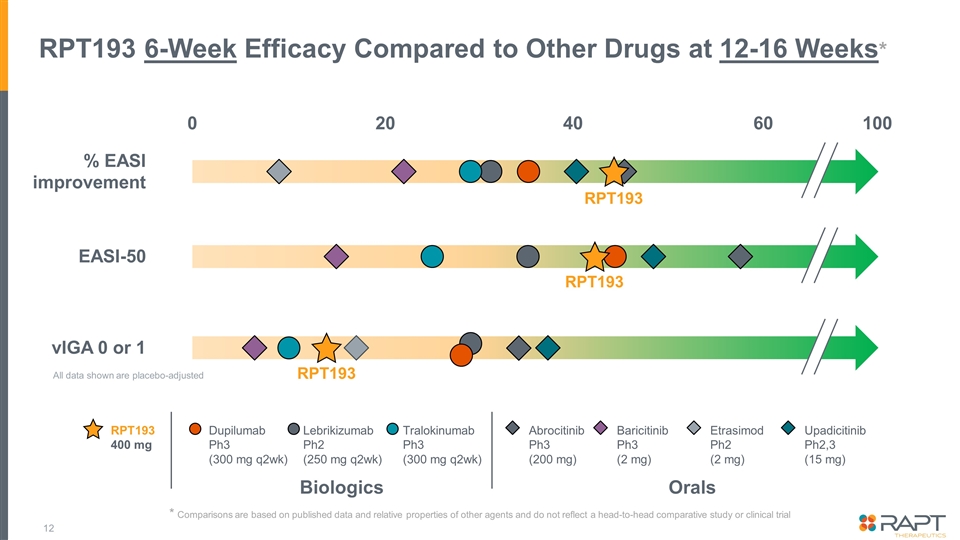
RPT193 6-Week Efficacy Compared to Other Drugs at 12-16 Weeks* RPT193 RPT193 400 mg Biologics Orals Dupilumab Ph3 (300 mg q2wk) Lebrikizumab Ph2 (250 mg q2wk) Tralokinumab Ph3 (300 mg q2wk) Abrocitinib Ph3 (200 mg) Baricitinib Ph3 (2 mg) Etrasimod Ph2 (2 mg) Upadicitinib Ph2,3 (15 mg) % EASI improvement EASI-50 vIGA 0 or 1 100 RPT193 RPT193 0 40 20 60 All data shown are placebo-adjusted * Comparisons are based on published data and relative properties of other agents and do not reflect a head-to-head comparative study or clinical trial
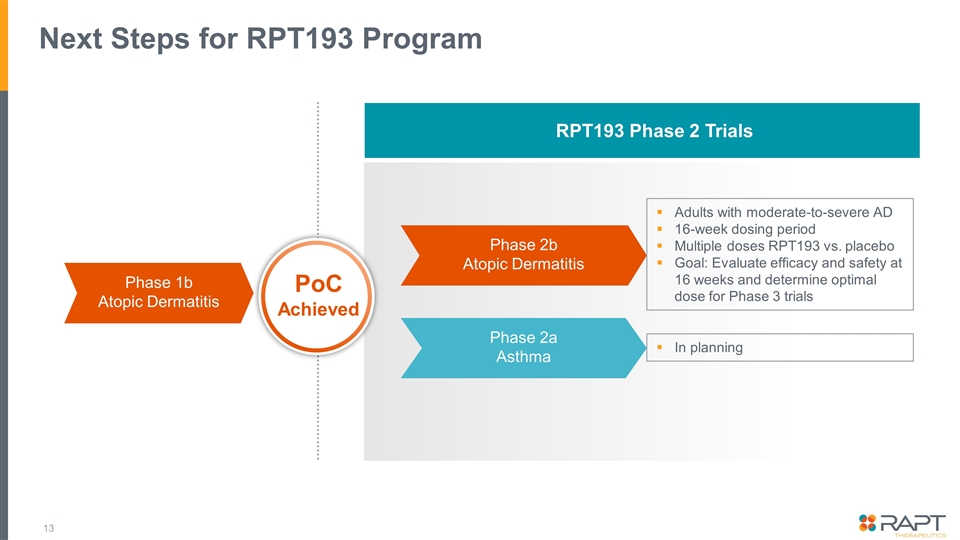
Next Steps for RPT193 Program RPT193 Phase 2 Trials Phase 1b Atopic Dermatitis Phase 2b Atopic Dermatitis Phase 2a Asthma PoC Achieved Adults with moderate-to-severe AD 16-week dosing period Multiple doses RPT193 vs. placebo Goal: Evaluate efficacy and safety at 16 weeks and determine optimal dose for Phase 3 trials In planning

Conclusion Data from this Phase 1b study in patients with atopic dermatitis demonstrate clear benefit in key exploratory clinical endpoints including EASI and vIGA Continued deepening of responses through the 2-week follow-up period suggests higher levels of efficacy could be achieved in longer studies Profile suggests an effective, well-tolerated oral molecule without the need for laboratory safety monitoring, with potential positioning ahead of injectables Next step: 16-week Phase 2b study in patients with moderate-to-severe AD Planning a Phase 2a study in patients with asthma

Thank You
