Attached files
| file | filename |
|---|---|
| 8-K - 8-K - MEI Pharma, Inc. | d153791d8k.htm |

Post-ASCO 2021 Investor & Analyst Event: Zandelisib in Focus NASDAQ: MEIP June 10, 2021 Exhibit 99.1

Forward-Looking Statements This presentation contains, and our officers and representatives may from time to time make, statements that are “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Examples of forward-looking statements include, among others, statements regarding our development strategy; potential advantages of our product candidates; the initiation and completion of preclinical and clinical studies and the reporting of the results thereof; the timing of regulatory submissions and actions; the sufficiency of our existing cash; and all other statements relating to our plans, objectives, expectations and beliefs regarding future performance, operations, financial condition and other future events (including assumptions underlying or relating to any of the foregoing). These forward-looking statements rely on a number of assumptions concerning future events and are subject to a number of risks, uncertainties, and other factors, many of which are outside of our control. Important factors that could cause our actual results and financial condition to differ materially from those indicated in forward-looking statements include, among others: uncertainties relating to the initiation and completion of preclinical and clinical studies; whether preclinical and clinical study results will validate and support the safety and efficacy of our product candidates; the outcome of regulatory reviews of our product candidates; varying interpretation of research and development and market data; the impact of the COVID-19 pandemic on our industry and individual companies, including on our counterparties, the supply chain, the execution of our clinical development programs, our access to financing and the allocation of government resources; risks and uncertainties relating to intellectual property and the other factors discussed under the caption “Item 1A. Risk Factors” in our most recent annual report on Form 10-K and our most recent quarterly report on Form 10-Q. Any forward-looking statement made by us in this presentation is based only on information currently available to us and speaks only as of the date on which it is made. In addition, we operate in a highly competitive and rapidly changing environment, and new risks may arise. Accordingly, you should not place any reliance on forward-looking statements as a prediction of actual results. We disclaim any intention to, and undertake no obligation to, update or revise any forward-looking statement. You are urged to carefully review and consider the various disclosures in our most recent annual report on Form 10-K, our most recent Form 10-Q and our other public filings with the SEC since the filing of our most recent annual report.
MEI Pharma: Who We Are Clinical Development Company Building a Leading Oncology Franchise with 4 Clinical-Stage Programs: Focus On HemOnc Zandelisib, Potential Best-in-Class PI3Kδ Inhibitor in Phase 2 Study Intended to Support Accelerated Approval Applications with U.S. FDA Well Capitalized with $164.6 Million* * As of March 31, 2021, MEI had $164.6 million in cash, cash equivalents, and short-term investments with no outstanding debt.
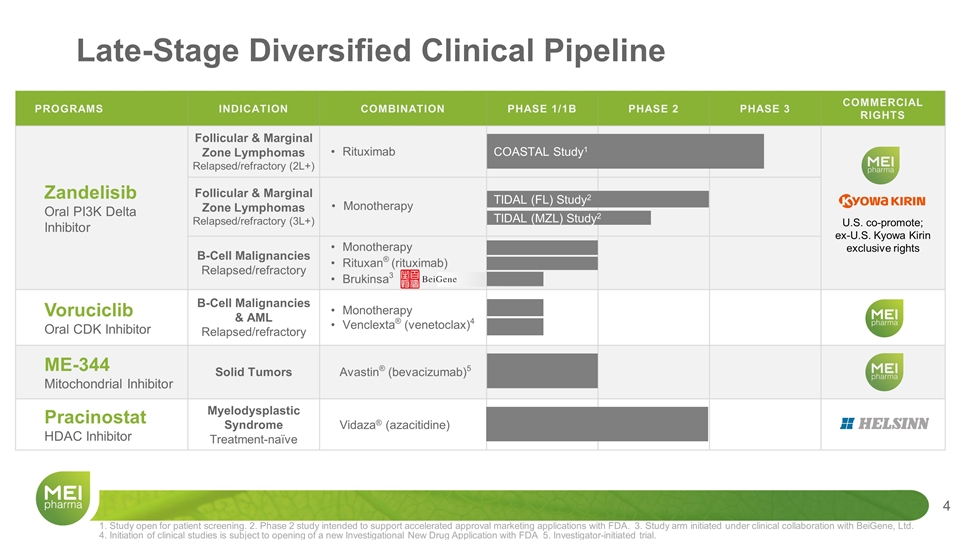
PROGRAMS INDICATION COMBINATION PHASE 1/1B PHASE 2 PHASE 3 COMMERCIAL RIGHTS Zandelisib Oral PI3K Delta Inhibitor Follicular & Marginal Zone Lymphomas Relapsed/refractory (2L+) Rituximab U.S. co-promote; ex-U.S. Kyowa Kirin exclusive rights Zandelisib Oral P13K Delta Inhibitor Follicular & Marginal Zone Lymphomas Relapsed/refractory (3L+) Monotherapy U.S. co-promote; ex-U.S. Kyowa Kirin exclusive rights B-Cell Malignancies Relapsed/refractory Monotherapy Rituxan® (rituximab) Brukinsa3 Voruciclib Oral CDK Inhibitor B-Cell Malignancies & AML Relapsed/refractory Monotherapy Venclexta® (venetoclax)4 ME-344 Mitochondrial Inhibitor Solid Tumors Avastin® (bevacizumab)5 Pracinostat HDAC Inhibitor Myelodysplastic Syndrome Treatment-naïve Vidaza® (azacitidine) Late-Stage Diversified Clinical Pipeline TIDAL (FL) Study2 1. Study open for patient screening. 2. Phase 2 study intended to support accelerated approval marketing applications with FDA. 3. Study arm initiated under clinical collaboration with BeiGene, Ltd. 4. Initiation of clinical studies is subject to opening of a new Investigational New Drug Application with FDA 5. Investigator-initiated trial. COASTAL Study1 TIDAL (MZL) Study2

Zandelisib Topline TIDAL Study Data on Track to be Reported in the Fourth Quarter of CY2021 Enrollment Complete in Follicular Lymphoma Primary Efficacy Population The complete Phase 2 TIDAL study data are intended to be submitted to FDA to support accelerated approval applications

Zandelisib: Data Driven Advancement in the Treatment of B-cell Malignancies
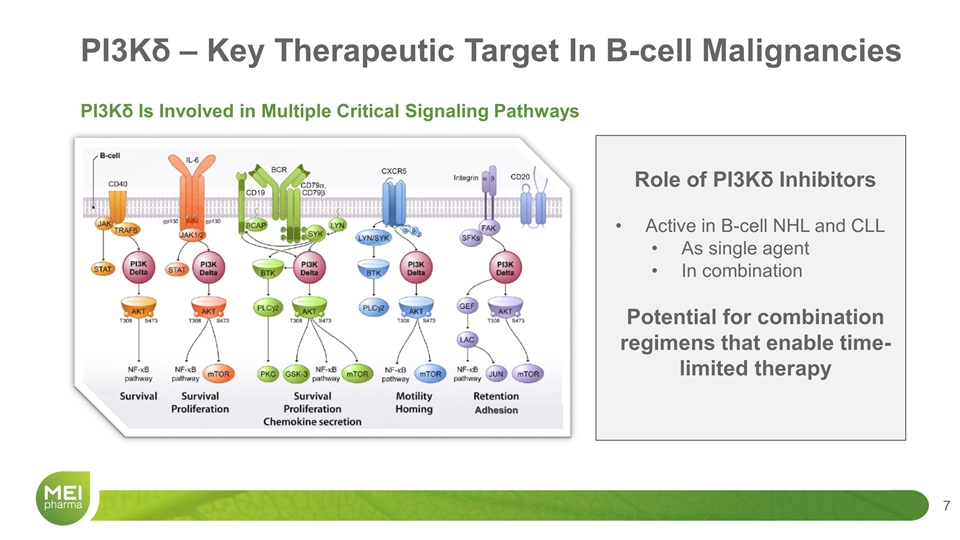
PI3Kδ – Key Therapeutic Target In B-cell Malignancies PI3Kδ Is Involved in Multiple Critical Signaling Pathways Role of PI3Kδ Inhibitors Active in B-cell NHL and CLL As single agent In combination Potential for combination regimens that enable time-limited therapy Adhesion
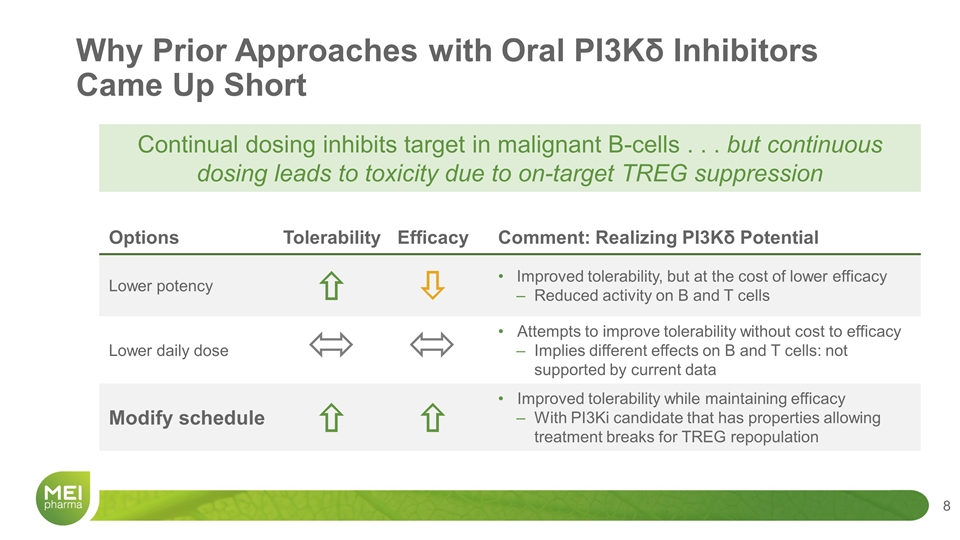
Options Tolerability Efficacy Comment: Realizing PI3Kδ Potential Lower potency ñ ò Improved tolerability, but at the cost of lower efficacy Reduced activity on B and T cells Lower daily dose Attempts to improve tolerability without cost to efficacy Implies different effects on B and T cells: not supported by current data Modify schedule ñ ñ Improved tolerability while maintaining efficacy With PI3Ki candidate that has properties allowing treatment breaks for TREG repopulation Why Prior Approaches with Oral PI3Kδ Inhibitors Came Up Short Continual dosing inhibits target in malignant B-cells . . . but continuous dosing leads to toxicity due to on-target TREG suppression
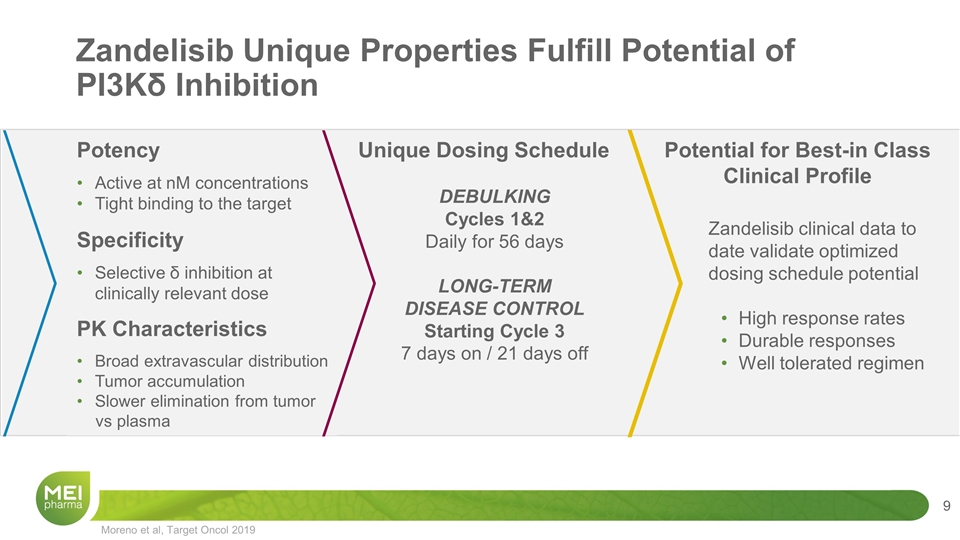
Zandelisib Unique Properties Fulfill Potential of PI3Kδ Inhibition DEBULKING Cycles 1&2 Daily for 56 days LONG-TERM DISEASE CONTROL Starting Cycle 3 7 days on / 21 days off Unique Dosing Schedule Zandelisib clinical data to date validate optimized dosing schedule potential High response rates Durable responses Well tolerated regimen Potential for Best-in Class Clinical Profile Potency Active at nM concentrations Tight binding to the target Specificity Selective δ inhibition at clinically relevant dose PK Characteristics Broad extravascular distribution Tumor accumulation Slower elimination from tumor vs plasma Moreno et al, Target Oncol 2019
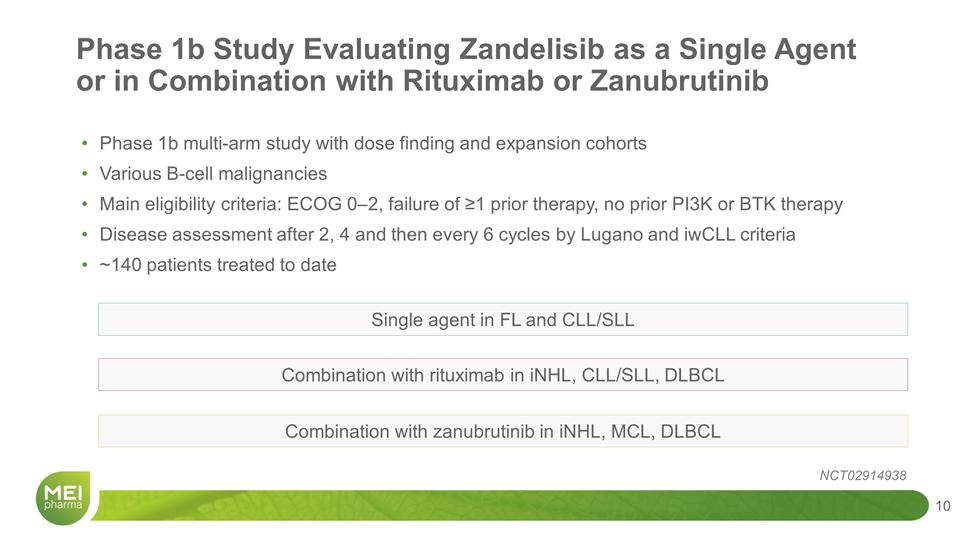
Phase 1b multi-arm study with dose finding and expansion cohorts Various B-cell malignancies Main eligibility criteria: ECOG 0‒2, failure of ≥1 prior therapy, no prior PI3K or BTK therapy Disease assessment after 2, 4 and then every 6 cycles by Lugano and iwCLL criteria ~140 patients treated to date Phase 1b Study Evaluating Zandelisib as a Single Agent or in Combination with Rituximab or Zanubrutinib NCT02914938 Single agent in FL and CLL/SLL Combination with rituximab in iNHL, CLL/SLL, DLBCL Combination with zanubrutinib in iNHL, MCL, DLBCL

Efficacy and Safety of the PI3Kδ Inhibitor Zandelisib (ME-401) on an Intermittent Schedule (IS) in Patients with Relapsed/Refractory Follicular Lymphoma (FL) with Progression of Disease within 24 Months of First-Line Chemoimmunotherapy (POD24) John M. Pagel1, Nishitha M. Reddy2, Deepa Jagadeesh3, Anastasios Stathis4, Adam S. Asch5, Huda S. Salman6, Vaishalee P. Kenkre7, Jacob D. Soumerai8, Judith Llorin-Sangalang9, Igor Gorbatchevsky9, Joanne Li9, Andrew D. Zelenetz10 1Swedish Cancer Institute, Seattle, WA; 2Vanderbilt University Medical Center, Vanderbilt-Ingram Cancer Center, Nashville, TN; 3Cleveland Clinic, Taussig Cancer Institute, Cleveland, OH; 4Oncology Institute of Southern Switzerland, Bellinzona, Switzerland; 5University of Oklahoma Health Sciences Center, Stephenson Cancer Center, Oklahoma City, OK; 6Stony Brook University School of Medicine, Stony Brook, NY; 7University of Wisconsin Carbone Cancer Center, Madison, WI; 8Massachusetts General Hospital Cancer Center, Boston, MA; 9MEI Pharma, Inc., San Diego, CA; 10Memorial Sloan Kettering Cancer Center, New York, NY
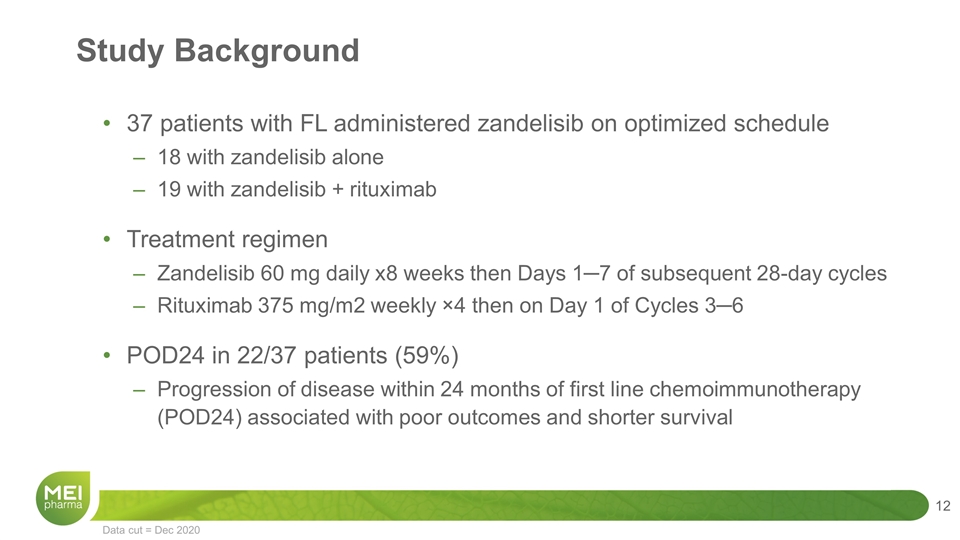
Study Background 37 patients with FL administered zandelisib on optimized schedule 18 with zandelisib alone 19 with zandelisib + rituximab Treatment regimen Zandelisib 60 mg daily x8 weeks then Days 1─7 of subsequent 28-day cycles Rituximab 375 mg/m2 weekly ×4 then on Day 1 of Cycles 3─6 POD24 in 22/37 patients (59%) Progression of disease within 24 months of first line chemoimmunotherapy (POD24) associated with poor outcomes and shorter survival Data cut = Dec 2020
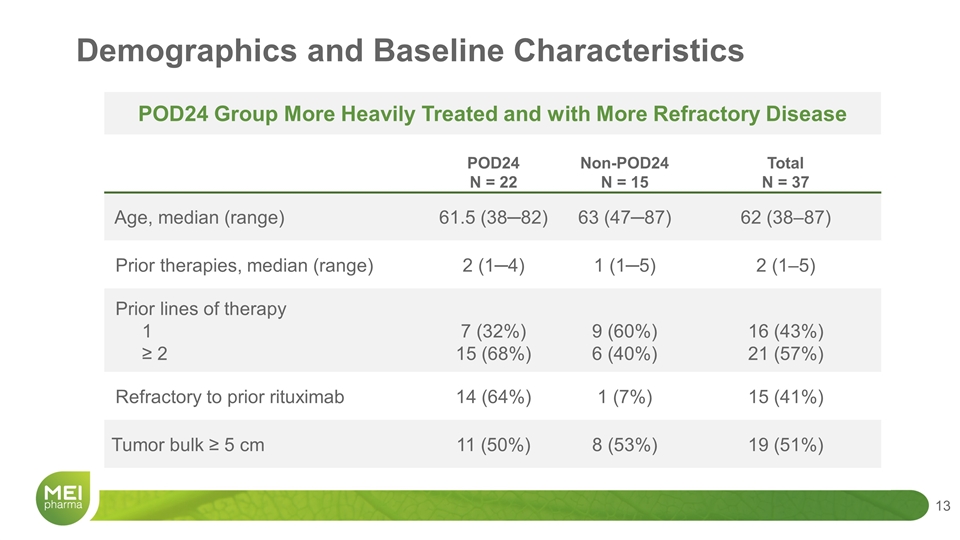
Demographics and Baseline Characteristics POD24 N = 22 Non-POD24 N = 15 Total N = 37 Age, median (range) 61.5 (38─82) 63 (47─87) 62 (38–87) Prior therapies, median (range) 2 (1─4) 1 (1─5) 2 (1–5) Prior lines of therapy 1 ≥ 2 7 (32%) 15 (68%) 9 (60%) 6 (40%) 16 (43%) 21 (57%) Refractory to prior rituximab 14 (64%) 1 (7%) 15 (41%) Tumor bulk ≥ 5 cm 11 (50%) 8 (53%) 19 (51%) POD24 Group More Heavily Treated and with More Refractory Disease
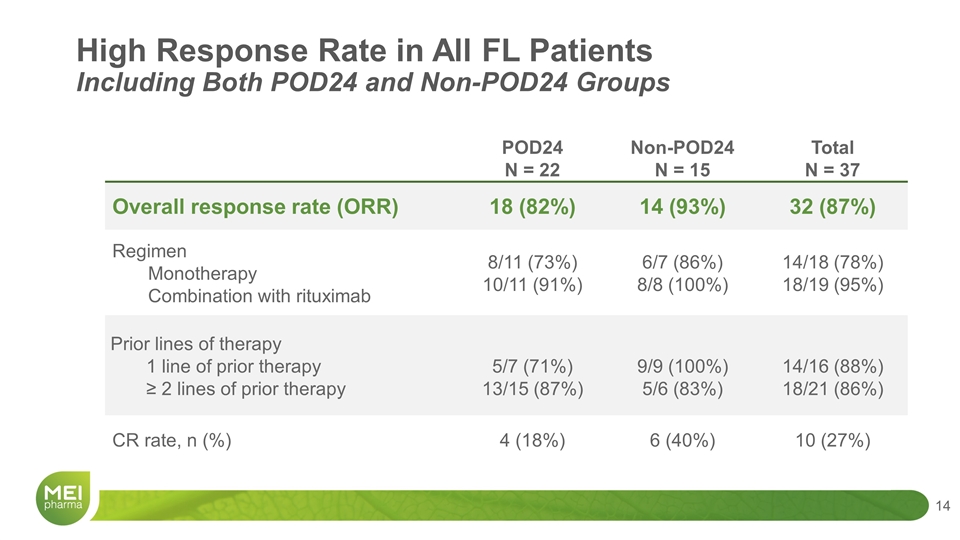
High Response Rate in All FL Patients Including Both POD24 and Non-POD24 Groups POD24 N = 22 Non-POD24 N = 15 Total N = 37 Overall response rate (ORR) 18 (82%) 14 (93%) 32 (87%) Regimen Monotherapy Combination with rituximab 8/11 (73%) 10/11 (91%) 6/7 (86%) 8/8 (100%) 14/18 (78%) 18/19 (95%) Prior lines of therapy 1 line of prior therapy ≥ 2 lines of prior therapy 5/7 (71%) 13/15 (87%) 9/9 (100%) 5/6 (83%) 14/16 (88%) 18/21 (86%) CR rate, n (%) 4 (18%) 6 (40%) 10 (27%)
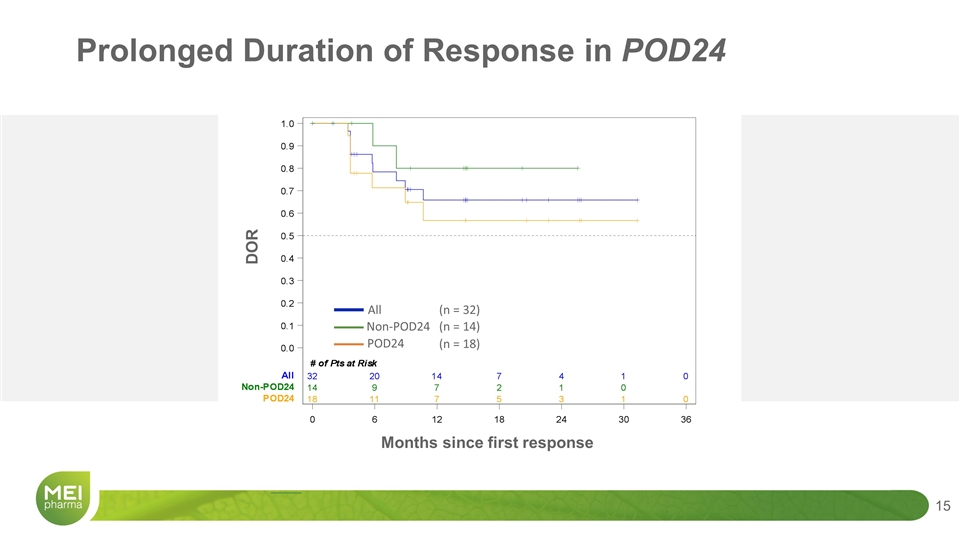
DOR POD24 Non-POD24 (n = 18) (n = 14) All (n = 32) Months since first response Prolonged Duration of Response in POD24
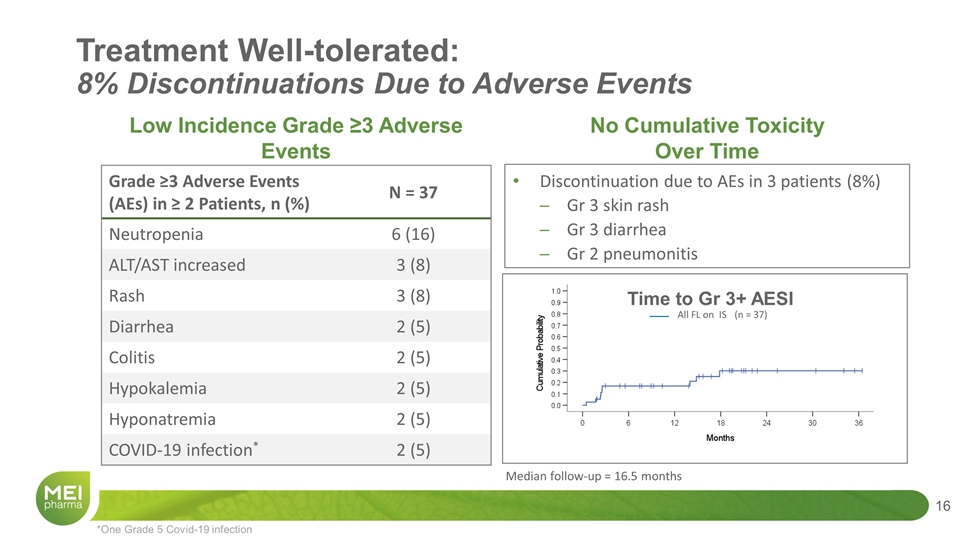
No Cumulative Toxicity Over Time Grade ≥3 Adverse Events (AEs) in ≥ 2 Patients, n (%) N = 37 Neutropenia 6 (16) ALT/AST increased 3 (8) Rash 3 (8) Diarrhea 2 (5) Colitis 2 (5) Hypokalemia 2 (5) Hyponatremia 2 (5) COVID-19 infection* 2 (5) Discontinuation due to AEs in 3 patients (8%) Gr 3 skin rash Gr 3 diarrhea Gr 2 pneumonitis *One Grade 5 Covid-19 infection Low Incidence Grade ≥3 Adverse Events Treatment Well-tolerated: 8% Discontinuations Due to Adverse Events All FL on IS (n = 37) Time to Gr 3+ AESI Median follow-up = 16.5 months
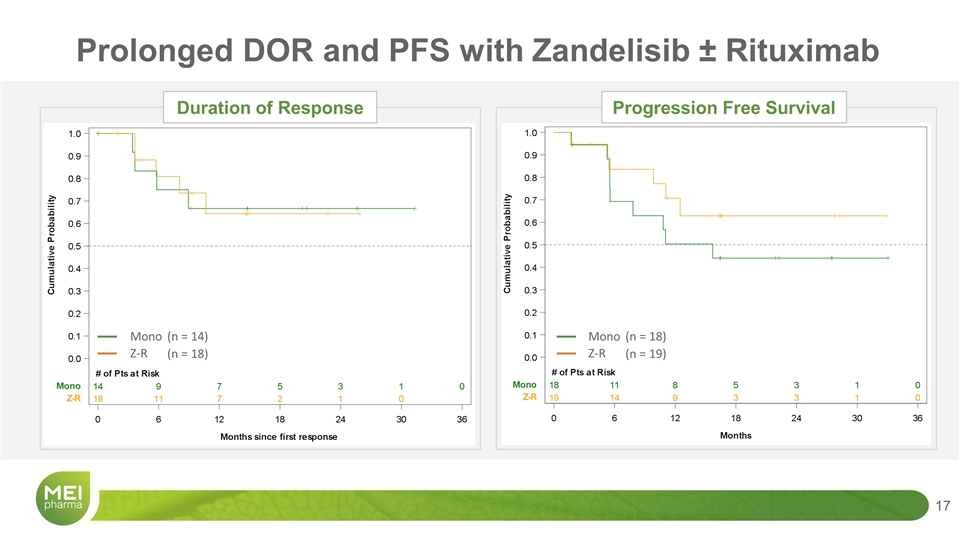
Z-R Mono (n = 18) (n = 14) Z-R Mono (n = 19) (n = 18) Prolonged DOR and PFS with Zandelisib ± Rituximab Duration of Response Progression Free Survival
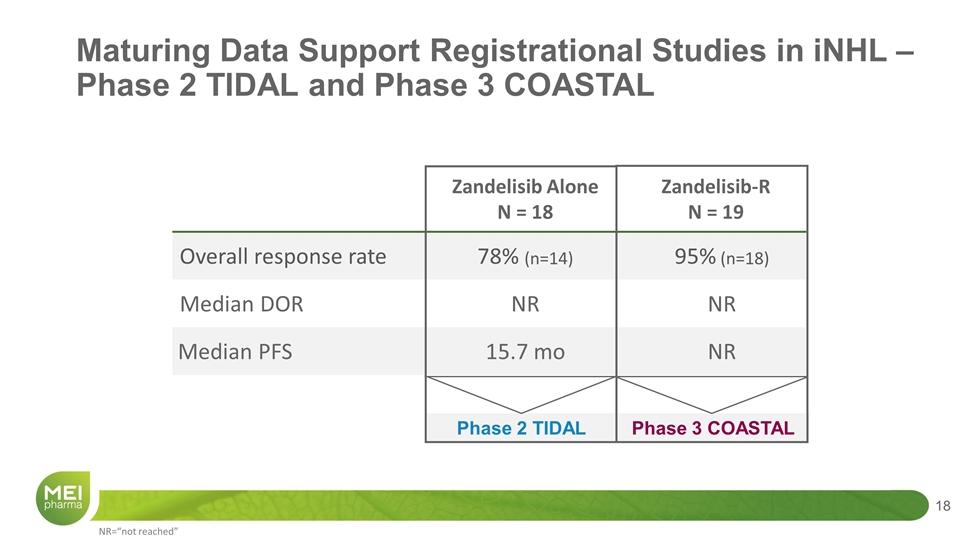
Maturing Data Support Registrational Studies in iNHL – Phase 2 TIDAL and Phase 3 COASTAL Zandelisib Alone N = 18 Zandelisib-R N = 19 Overall response rate 78% (n=14) 95% (n=18) Median DOR NR NR Median PFS 15.7 mo NR Phase 2 TIDAL Phase 3 COASTAL NR=“not reached”
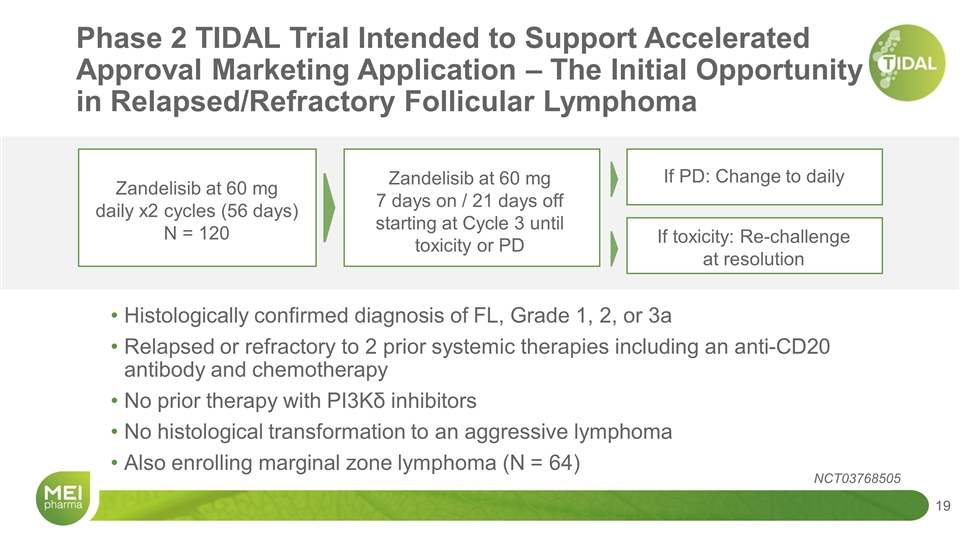
Zandelisib at 60 mg daily x2 cycles (56 days) N = 120 Zandelisib at 60 mg 7 days on / 21 days off starting at Cycle 3 until toxicity or PD If toxicity: Re-challenge at resolution Histologically confirmed diagnosis of FL, Grade 1, 2, or 3a Relapsed or refractory to 2 prior systemic therapies including an anti-CD20 antibody and chemotherapy No prior therapy with PI3Kδ inhibitors No histological transformation to an aggressive lymphoma Also enrolling marginal zone lymphoma (N = 64) If PD: Change to daily Phase 2 TIDAL Trial Intended to Support Accelerated Approval Marketing Application – The Initial Opportunity in Relapsed/Refractory Follicular Lymphoma NCT03768505
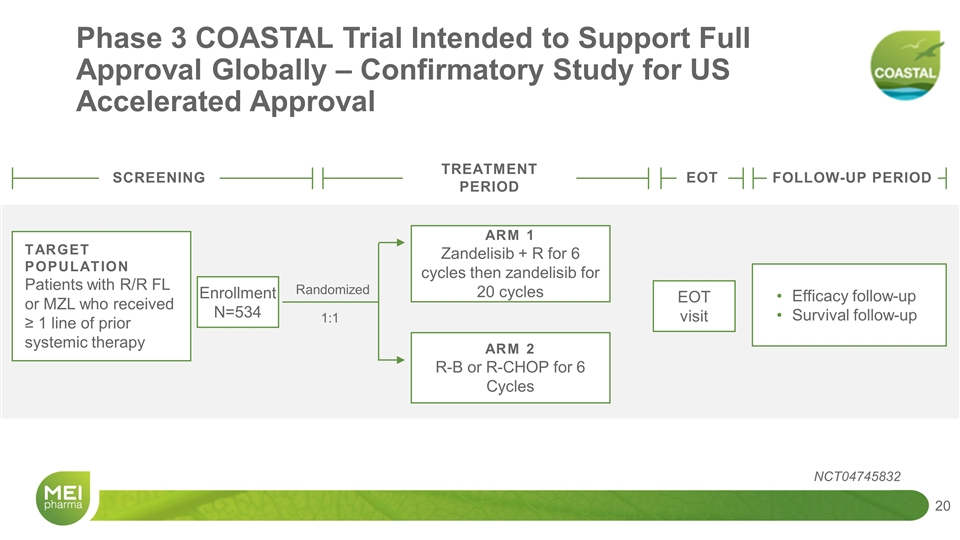
Phase 3 COASTAL Trial Intended to Support Full Approval Globally – Confirmatory Study for US Accelerated Approval SCREENING EOT FOLLOW-UP PERIOD TARGET POPULATION Patients with R/R FL or MZL who received ≥ 1 line of prior systemic therapy Enrollment N=534 Randomized 1:1 ARM 1 Zandelisib + R for 6 cycles then zandelisib for 20 cycles ARM 2 R-B or R-CHOP for 6 Cycles EOT visit Efficacy follow-up Survival follow-up TREATMENT PERIOD NCT04745832
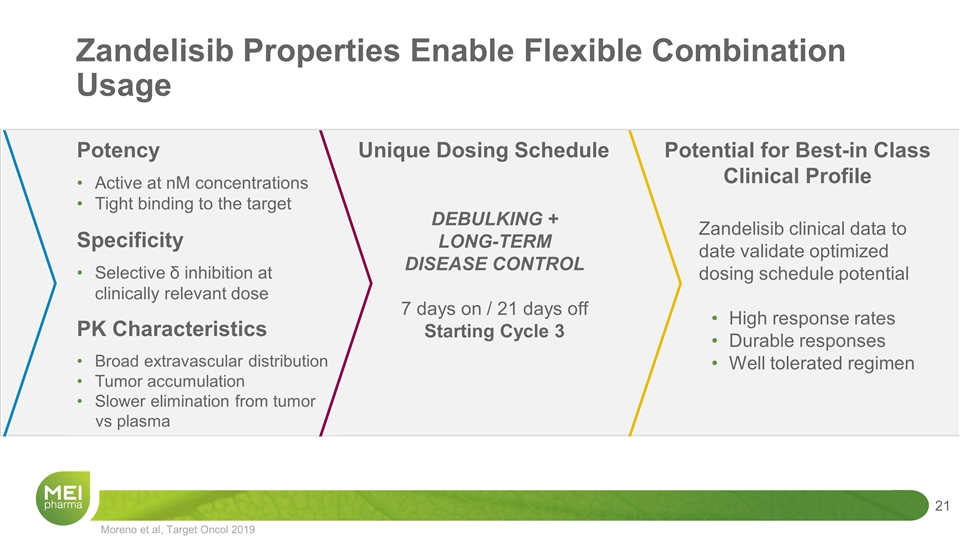
Moreno et al, Target Oncol 2019 Zandelisib Properties Enable Flexible Combination Usage DEBULKING + LONG-TERM DISEASE CONTROL 7 days on / 21 days off Starting Cycle 3 Unique Dosing Schedule Zandelisib clinical data to date validate optimized dosing schedule potential High response rates Durable responses Well tolerated regimen Potential for Best-in Class Clinical Profile Potency Active at nM concentrations Tight binding to the target Specificity Selective δ inhibition at clinically relevant dose PK Characteristics Broad extravascular distribution Tumor accumulation Slower elimination from tumor vs plasma

Initial Results of the Combination of PI3Kδ Inhibitor Zandelisib (ME-401) and the BTK Inhibitor Zanubrutinib in Patients with Relapsed or Refractory (R/R) B-cell Malignancies Jacob D. Soumerai1, Deepa Jagadeesh2, Huda S. Salman3, Izidore S. Lossos4, Nishitha M. Reddy5, Adam S. Asch6, Vaishalee P. Kenkre7, John M. Pagel8, Daniel O. Persky9, Farrukh T. Awan10, Catherine S. Diefenbach11, Judith Llorin-Sangalang12, Igor Gorbatchevsky12, Wenying Huang12, Andrew D. Zelenetz13 1Massachusetts General Hospital Cancer Center, Boston, MA; 2Cleveland Clinic, Taussig Cancer Institute, Cleveland, OH; 3Stony Brook University School of Medicine, Stony Brook, NY; 4University of Miami Health System, Sylvester Comprehensive Cancer Center, Miami, FL; 5Vanderbilt University Medical Center, Vanderbilt-Ingram Cancer Center, Nashville, TN; 6University of Oklahoma Health Sciences Center, Stephenson Cancer Center, Oklahoma City, OK; 7University of Wisconsin Carbone Cancer Center, Madison, WI; 8Swedish Cancer Institute, Seattle, WA; 9University of Arizona, Tucson, AZ; 10UT Southwestern Medical Center, Dallas, TX; 11NYU Langone Health, Perlmutter Cancer Center, New York, NY; 12MEI Pharma, Inc., San Diego, CA; 13Memorial Sloan Kettering Cancer Center, New York, NY
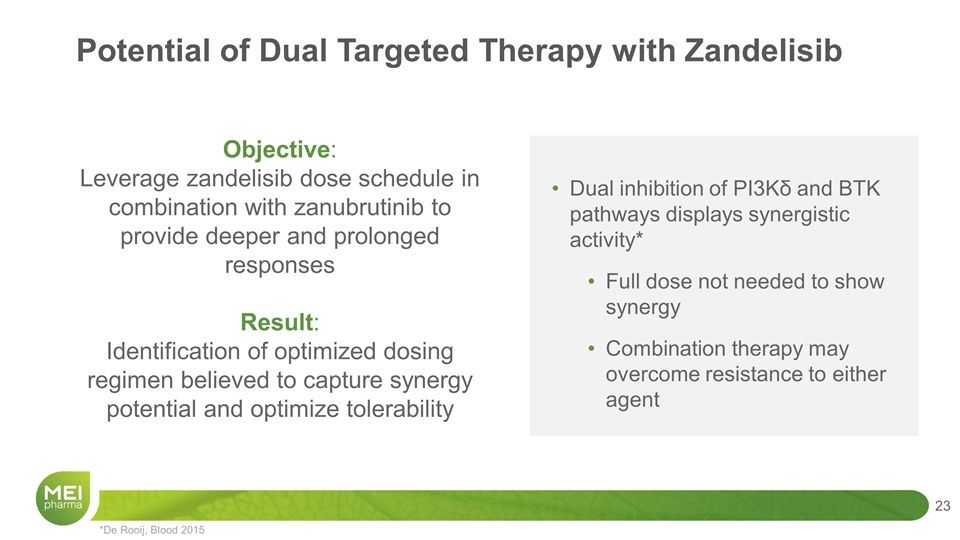
Dual inhibition of PI3Kδ and BTK pathways displays synergistic activity* Full dose not needed to show synergy Combination therapy may overcome resistance to either agent *De Rooij, Blood 2015 Objective: Leverage zandelisib dose schedule in combination with zanubrutinib to provide deeper and prolonged responses Result: Identification of optimized dosing regimen believed to capture synergy potential and optimize tolerability Potential of Dual Targeted Therapy with Zandelisib
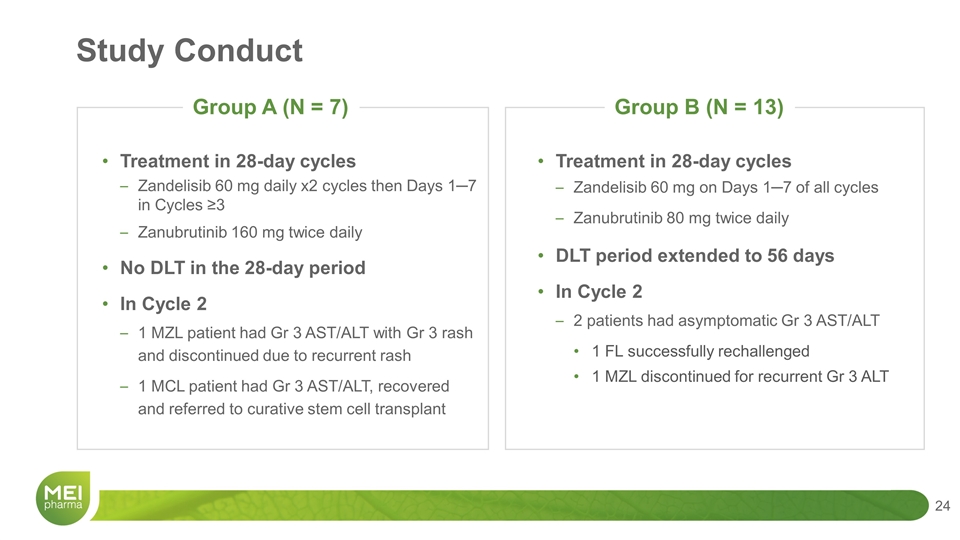
Treatment in 28-day cycles Zandelisib 60 mg daily x2 cycles then Days 1─7 in Cycles ≥3 Zanubrutinib 160 mg twice daily No DLT in the 28-day period In Cycle 2 1 MZL patient had Gr 3 AST/ALT with Gr 3 rash and discontinued due to recurrent rash 1 MCL patient had Gr 3 AST/ALT, recovered and referred to curative stem cell transplant Treatment in 28-day cycles Zandelisib 60 mg on Days 1─7 of all cycles Zanubrutinib 80 mg twice daily DLT period extended to 56 days In Cycle 2 2 patients had asymptomatic Gr 3 AST/ALT 1 FL successfully rechallenged 1 MZL discontinued for recurrent Gr 3 ALT Study Conduct Group A (N = 7) Group B (N = 13)
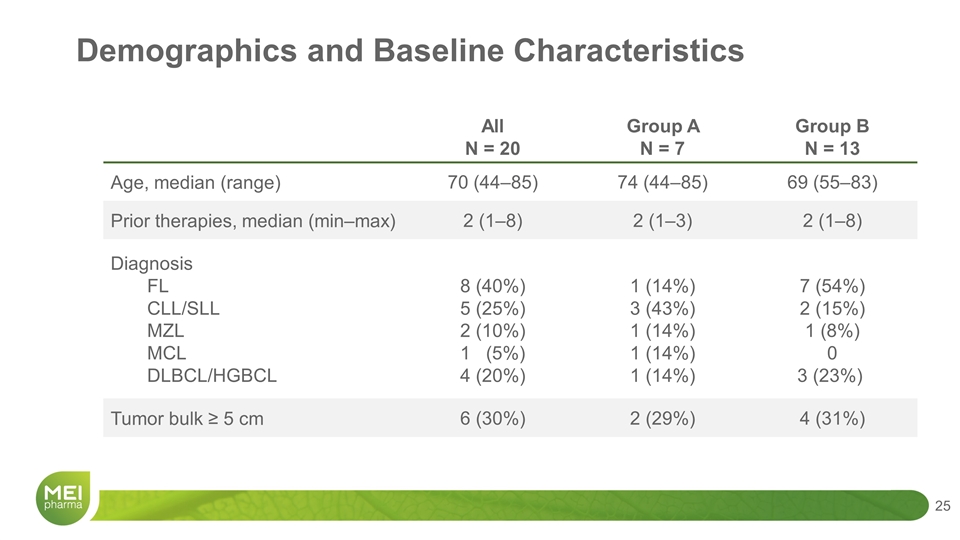
All N = 20 Group A N = 7 Group B N = 13 Age, median (range) 70 (44–85) 74 (44–85) 69 (55–83) Prior therapies, median (min–max) 2 (1–8) 2 (1–3) 2 (1–8) Diagnosis FL CLL/SLL MZL MCL DLBCL/HGBCL 8 (40%) 5 (25%) 2 (10%) 1 (5%) 4 (20%) 1 (14%) 3 (43%) 1 (14%) 1 (14%) 1 (14%) 7 (54%) 2 (15%) 1 (8%) 0 3 (23%) Tumor bulk ≥ 5 cm 6 (30%) 2 (29%) 4 (31%) Demographics and Baseline Characteristics
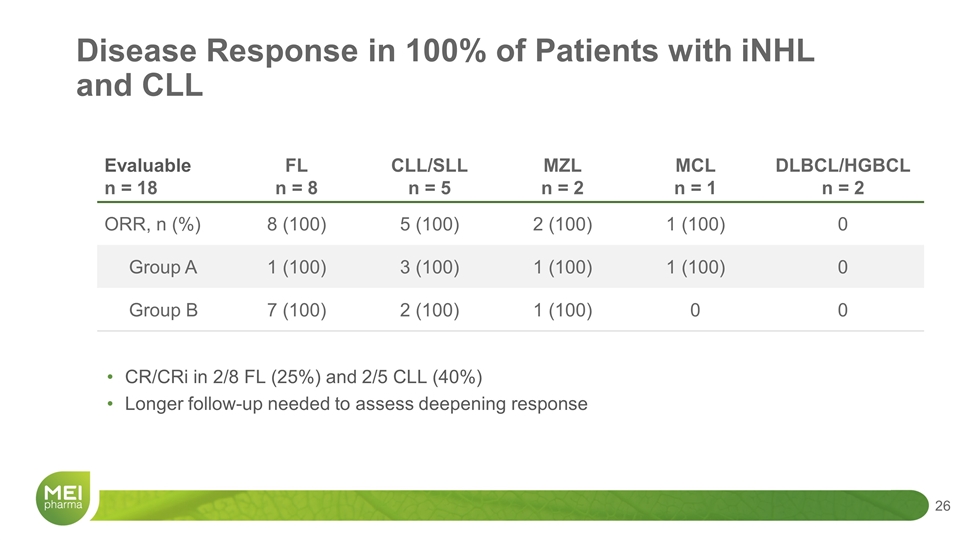
CR/CRi in 2/8 FL (25%) and 2/5 CLL (40%) Longer follow-up needed to assess deepening response Evaluable n = 18 FL n = 8 CLL/SLL n = 5 MZL n = 2 MCL n = 1 DLBCL/HGBCL n = 2 ORR, n (%) 8 (100) 5 (100) 2 (100) 1 (100) 0 Group A 1 (100) 3 (100) 1 (100) 1 (100) 0 Group B 7 (100) 2 (100) 1 (100) 0 0 Disease Response in 100% of Patients with iNHL and CLL
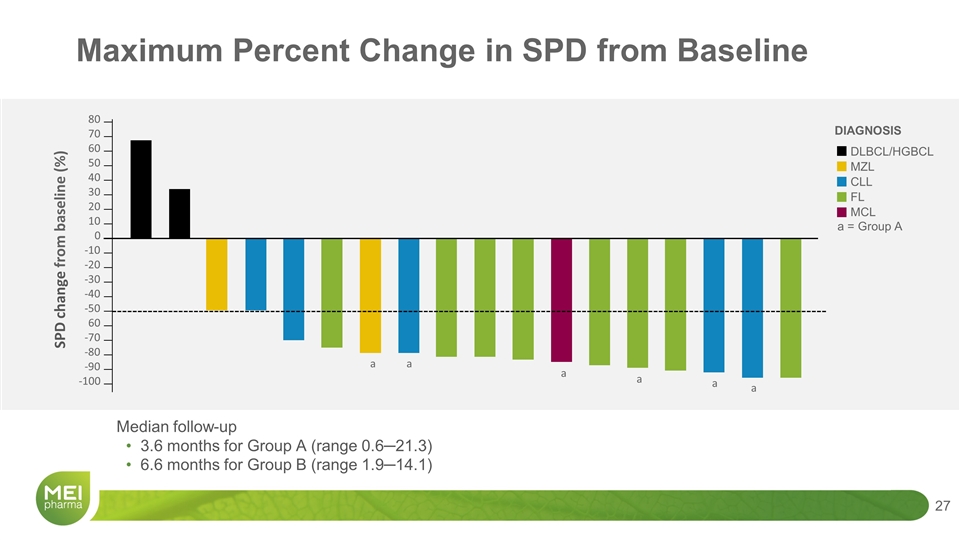
Median follow-up 3.6 months for Group A (range 0.6─21.3) 6.6 months for Group B (range 1.9─14.1) DIAGNOSIS DLBCL/HGBCL MZL CLL FL MCL 80 70 60 50 40 30 20 10 0 -10 -20 -30 -40 -50 60 -70 -80 -90 -100 SPD change from baseline (%) a = Group A a a a a a a Maximum Percent Change in SPD from Baseline
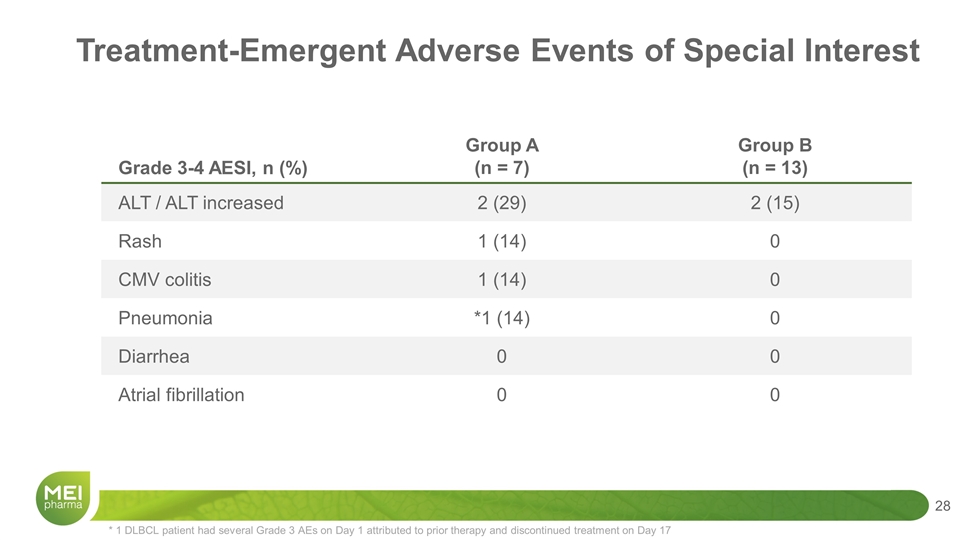
* 1 DLBCL patient had several Grade 3 AEs on Day 1 attributed to prior therapy and discontinued treatment on Day 17 Grade 3-4 AESI, n (%) Group A (n = 7) Group B (n = 13) ALT / ALT increased 2 (29) 2 (15) Rash 1 (14) 0 CMV colitis 1 (14) 0 Pneumonia *1 (14) 0 Diarrhea 0 0 Atrial fibrillation 0 0 Treatment-Emergent Adverse Events of Special Interest

Expanding Zandelisib Development Activities to Explore Full Potential Zandelisib Single Agent Ph 2 Study TIDAL in 3L+ FL and MZL Ph 2 study K02 in 3L+ in iNHL (Japan) Zandelisib + Rituximab Ph 3 Study COATSAL in 2L+ FL and MZL Other Zandelisib Combinations + Zanubrutinib in FL and MCL in 2L+ + R-CHOP in DLBCL in 1L + Ven-R in CLL
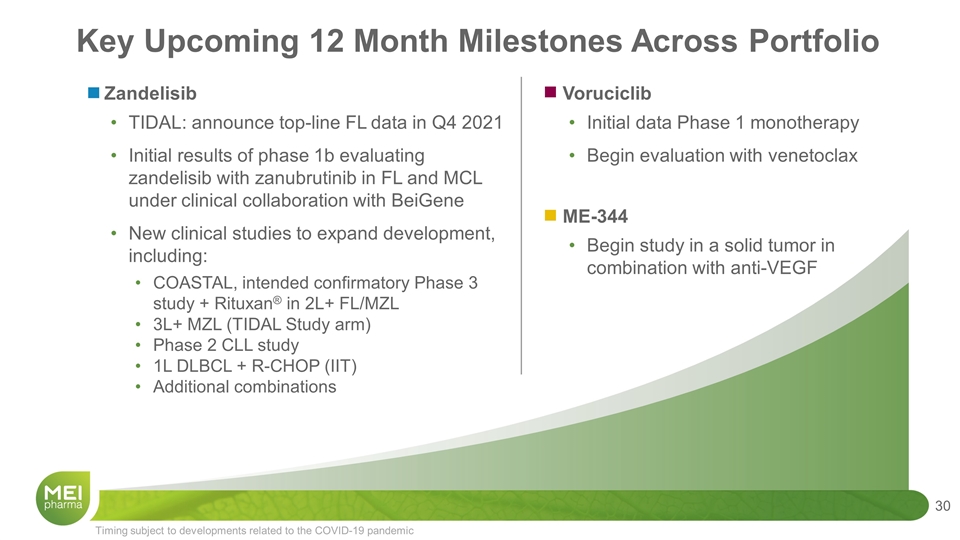
Key Upcoming 12 Month Milestones Across Portfolio Zandelisib TIDAL: announce top-line FL data in Q4 2021 Initial results of phase 1b evaluating zandelisib with zanubrutinib in FL and MCL under clinical collaboration with BeiGene New clinical studies to expand development, including: COASTAL, intended confirmatory Phase 3 study + Rituxan® in 2L+ FL/MZL 3L+ MZL (TIDAL Study arm) Phase 2 CLL study 1L DLBCL + R-CHOP (IIT) Additional combinations Timing subject to developments related to the COVID-19 pandemic Voruciclib Initial data Phase 1 monotherapy Begin evaluation with venetoclax ME-344 Begin study in a solid tumor in combination with anti-VEGF

PI3Ki in Lymphoma Deepa Jagadeesh, MD, MPH Cleveland Clinic June 10, 2021
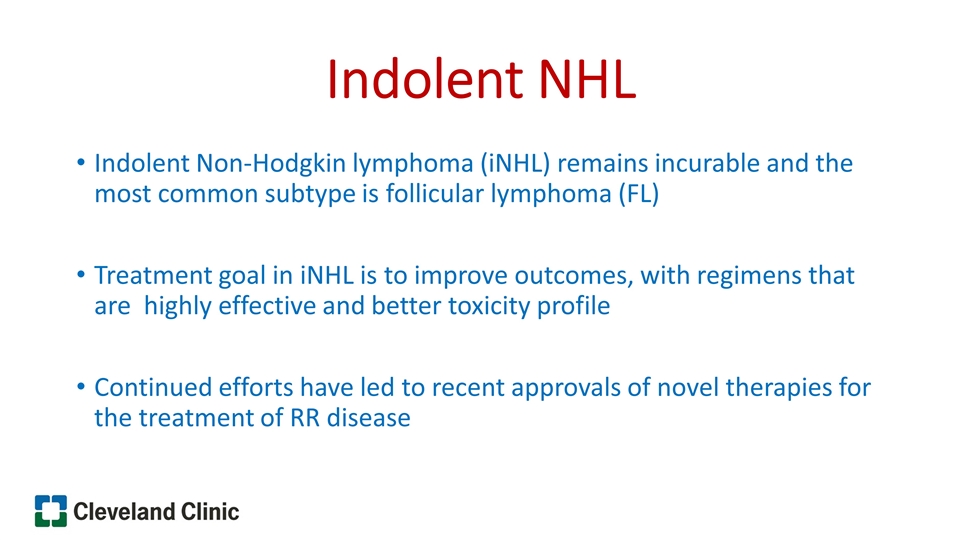
Indolent NHL Indolent Non-Hodgkin lymphoma (iNHL) remains incurable and the most common subtype is follicular lymphoma (FL) Treatment goal in iNHL is to improve outcomes, with regimens that are highly effective and better toxicity profile Continued efforts have led to recent approvals of novel therapies for the treatment of RR disease

Current Treatments Treatment regimens used in FL includes Chemoimmunotherapy Single agent rituximab BR – bendamustine+ rituximab RR – rituximab + Revlimid PI3K inihibitors FDA approved – Idelalisib, Duvelisib, copanlisib, and umbralisib In clinical trials – Zandelisib (ME-401) EZH2 inhibitor Tazemetostat CAR T cell therapy Axicabtagene ciloeucel (YESCARTA) - anti-CD19 CAR T cell
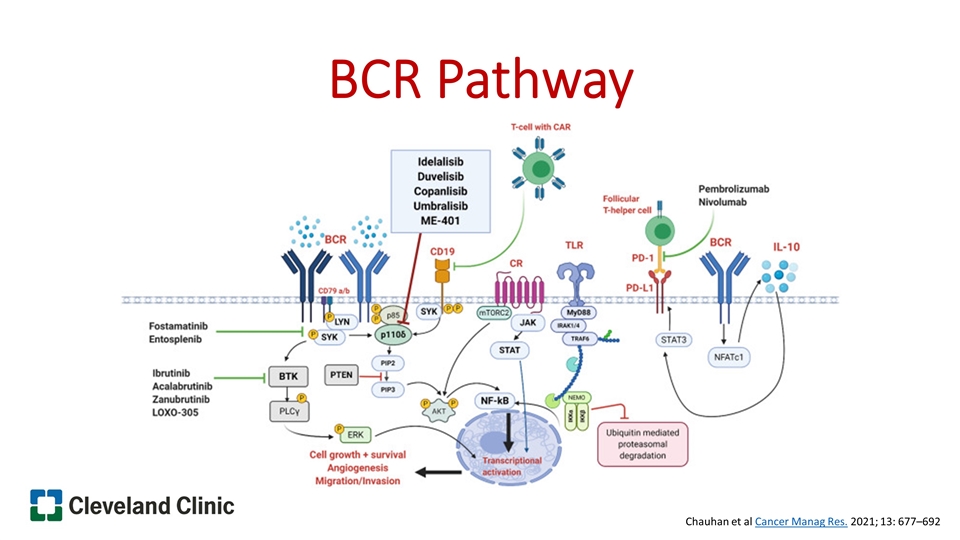
BCR Pathway Chauhan et al Cancer Manag Res. 2021; 13: 677–692
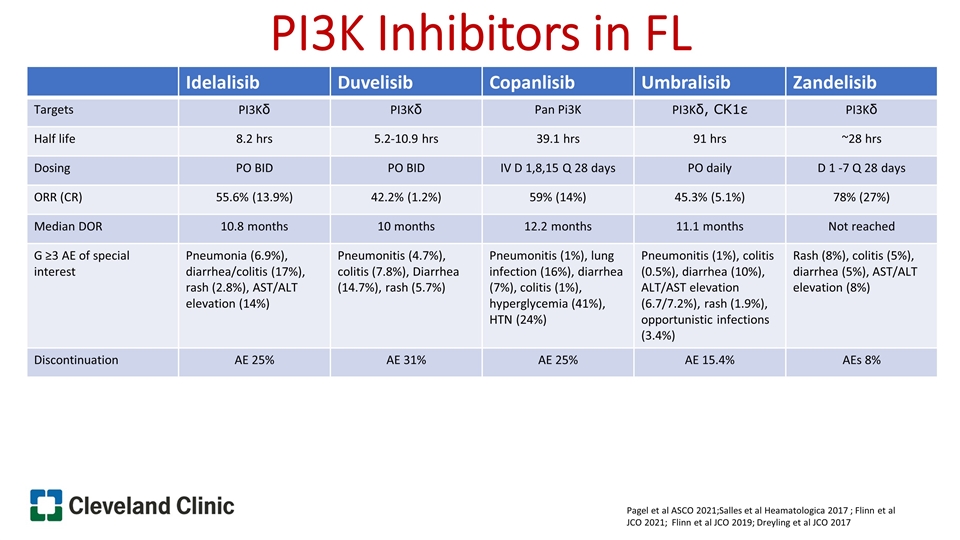
PI3K Inhibitors in FL Pagel et al ASCO 2021;Salles et al Heamatologica 2017 ; Flinn et al JCO 2021; Flinn et al JCO 2019; Dreyling et al JCO 2017 Idelalisib Duvelisib Copanlisib Umbralisib Zandelisib Targets PI3Kδ PI3Kδ Pan Pi3K PI3Kδ, CK1ε PI3Kδ Half life 8.2 hrs 5.2-10.9 hrs 39.1 hrs 91 hrs ~28 hrs Dosing PO BID PO BID IV D 1,8,15 Q 28 days PO daily D 1 -7 Q 28 days ORR (CR) 55.6% (13.9%) 42.2% (1.2%) 59% (14%) 45.3% (5.1%) 78% (27%) Median DOR 10.8 months 10 months 12.2 months 11.1 months Not reached G ≥3 AE of special interest Pneumonia (6.9%), diarrhea/colitis (17%), rash (2.8%), AST/ALT elevation (14%) Pneumonitis (4.7%), colitis (7.8%), Diarrhea (14.7%), rash (5.7%) Pneumonitis (1%), lung infection (16%), diarrhea (7%), colitis (1%), hyperglycemia (41%), HTN (24%) Pneumonitis (1%), colitis (0.5%), diarrhea (10%), ALT/AST elevation (6.7/7.2%), rash (1.9%), opportunistic infections (3.4%) Rash (8%), colitis (5%), diarrhea (5%), AST/ALT elevation (8%) Discontinuation AE 25% AE 31% AE 25% AE 15.4% AEs 8%
Zandelisib Potent, highly selective oral PI3Kδ inhibitor Long half life and sustained high tumor concentration of the drug permits intermittent schedule (IS) administration IS decreased the incidence of AE without compromising efficacy Preliminary data shows promising clinical activity with single agent and in combination with rituximab and BTKi Zanubrutinib in iNHL and CLL/SLL
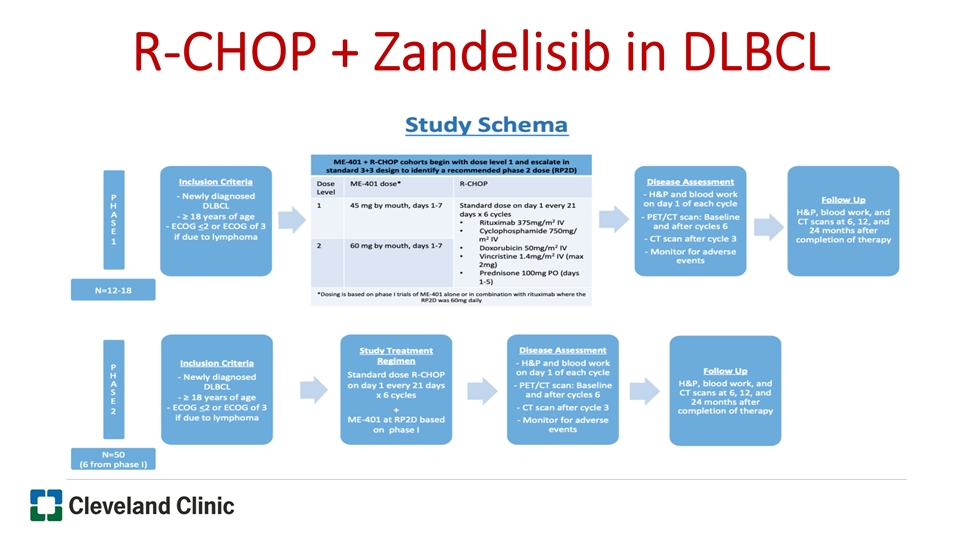
R-CHOP + Zandelisib in DLBCL
Conclusions Treatment landscape of FL and other iNHL is rapidly evolving with addition of newer treatment modalities Unanswered questions remain as to the optimal sequencing of current treatments Future research – integrate novel agents in the upfront setting, combination of novel agents, feasibility of time limited treatment Emerging promising treatment include newer generation CAR T cell therapies (dual and multi target CAR T) and bispecific antibodies

Thank you

Q&A

Post-ASCO 2021 Investor & Analyst Event: Zandelisib in Focus NASDAQ: MEIP June 10, 2021
