Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Lipocine Inc. | tm2119156d1_8k.htm |

Enabling Oral Drug Delivery to Improve Patient Compliance June 2021 CORPORATE PRESENTATION Exhibit 99.1

Forward - Looking Statements This presentation contains forward - looking statements about Lipocine Inc . (the “Company”) . These forward - looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 . These forward - looking statements relate to the Company’s products and product candidates, FDA’s approval of TLANDO Œ , the expected timing of Phase 3 trials for TLANDO XR and LPCN 1107 and Phase 2 studies for LPCN 1144 , LPCN 1148 and LPCN 1154 , clinical and regulatory processes and objectives, potential benefits of the Company’s product candidates, intellectual property and related matters, all of which involve known and unknown risks and uncertainties . Actual results may differ materially from the forward - looking statements discussed in this presentation . Accordingly, the Company cautions investors not to place undue reliance on the forward - looking statements contained in, or made in connection with, this presentation . Several factors may affect the initiation and completion of clinical trials and studies, the potential advantages of the Company’s product candidates and the Company’s capital needs . The forward - looking statements contained in this presentation are qualified by the detailed discussion of risks and uncertainties set forth in the Company’s annual report on Form 10 - K and other periodic reports filed by the Company with the Securities and Exchange Commission, all of which can be obtained on the Company’s website at www . lipocine . com or on the SEC website at www . sec . gov . The forward - looking statements contained in this document represent the Company’s estimates and assumptions only as of the date of this presentation and the Company undertakes no duty or obligation to update or revise publicly any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations .
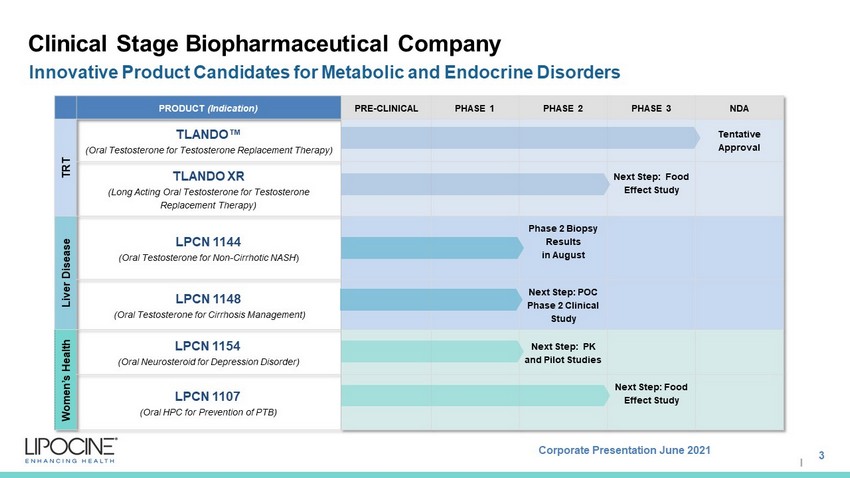
Corporate Presentation June 2021 I Clinical Stage Biopharmaceutical Company Innovative Product Candidates for Metabolic and Endocrine Disorders PRODUCT (Indication) PRE - CLINICAL PHASE 1 PHASE 2 PHASE 3 NDA TRT TLANDO Œ (Oral Testosterone for Testosterone Replacement Therapy) Tentative Approval TLANDO XR (Long Acting Oral Testosterone for Testosterone Replacement Therapy ) Next Step: Food Effect Study Liver Disease LPCN 1144 (Oral Testosterone for Non - Cirrhotic NASH ) Phase 2 Biopsy Results in August LPCN 1148 (Oral Testosterone for Cirrhosis Management) Next Step: POC Phase 2 Clinical Study Women’s Health LPCN 1154 (Oral Neurosteroid for Depression Disorder) Next Step: PK and Pilot Studies LPCN 1107 (Oral HPC for Prevention of PTB) Next Step: Food Effect Study 3

TLANDO ΠThe Convenient Oral TRT without Titration Requirement TLANDO XR Once Daily Oral TRT
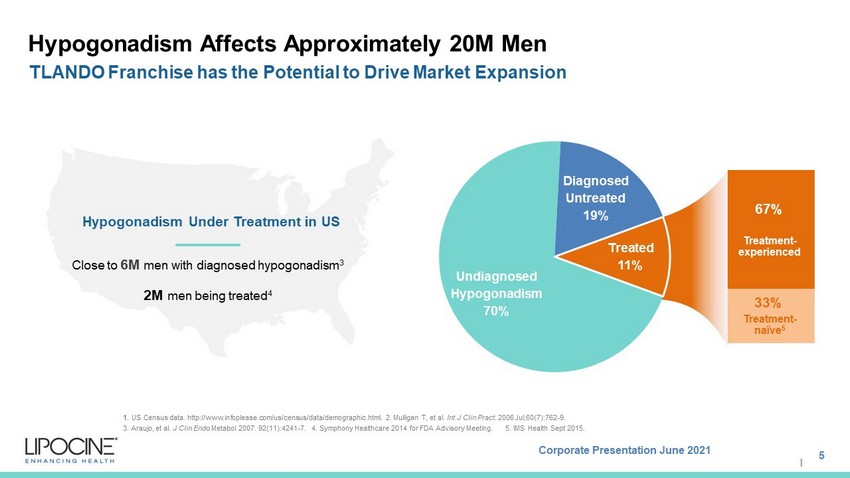
Corporate Presentation June 2021 I Hypogonadism Affects Approximately 20M Men 5 TLANDO Franchise has the Potential to Drive Market Expansion 1. US Census data. http://www.infoplease.com/us/census/data/demographic.html. 2. Mulligan T, et al. Int J Clin Pract . 2006 Jul;60(7):762 - 9. 3. Araujo, et al. J Clin Endo Metabol 2007. 92(11):4241 - 7. 4. Symphony Healthcare 2014 for FDA Advisory Meeting. 5. IMS Health Sept 2015. Undiagnosed Hypogonadism 70% Diagnosed Untreated 19% 67% 33% Treated 11% Treatment - naïve 5 Treatment - experienced Hypogonadism Under Treatment in US Close to 6M men with diagnosed hypogonadism 3 2M men being treated 4
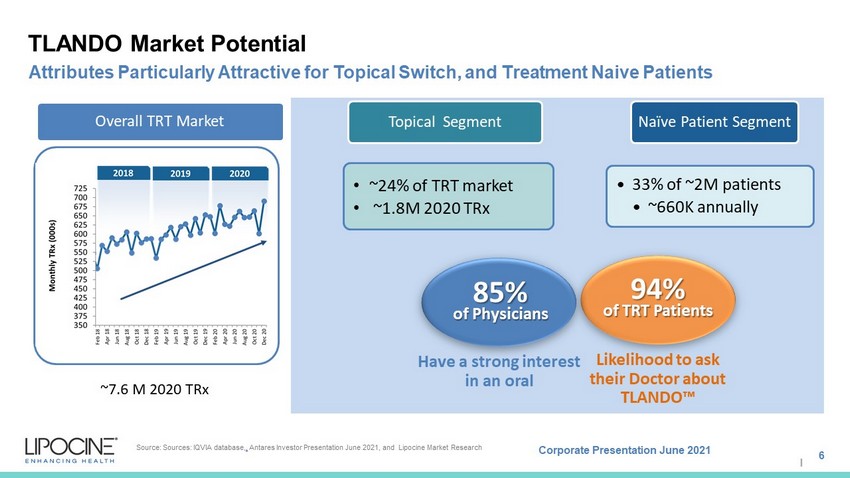
Corporate Presentation June 2021 I Overall TRT Market • ~24% of TRT market • ~1.8M 2020 TRx Topical Segment • 33% of ~2M patients • ~660K annually Naïve Patient Segment TLANDO Market Potential 6 Attributes Particularly Attractive for Topical Switch, and Treatment Naive Patients Source: Sources: IQVIA database, . Antares Investor Presentation June 2021, and Lipocine Market Research Likelihood to ask their Doctor about TLANDO Ρ 94% of TRT Patients Have a strong interest in an oral 85% of Physicians ~7.6 M 2020 TRx

Corporate Presentation June 2021 I f TLANDO Œ Attributes* Convenient Oral Route No inadvertent transference or pulmonary oil micro embolism risks Single strength and dose TRT without titration requirement Enables selection of an effective dose at the start of therapy without delay No “efficacy gap” upon switching from other TRTs No additional pharmacy and clinic copays to reach efficacious dose No dose adjustment clinic and pharmacy visits No dose adjustment invasive samplings No titration decision errors Bioequivalent exposure in low/med/high fat food Not known to produce hepatic adverse events associated with 17 - methylated testosterone 7 Physician Research: Physicians View No Titration Product as Positive ▪ Cited “easy/less titration” as an important advantage of TLANDO Œ ▪ Finding the adequate TRT dose through titration is burdensome for physicians and patients *pending final approval
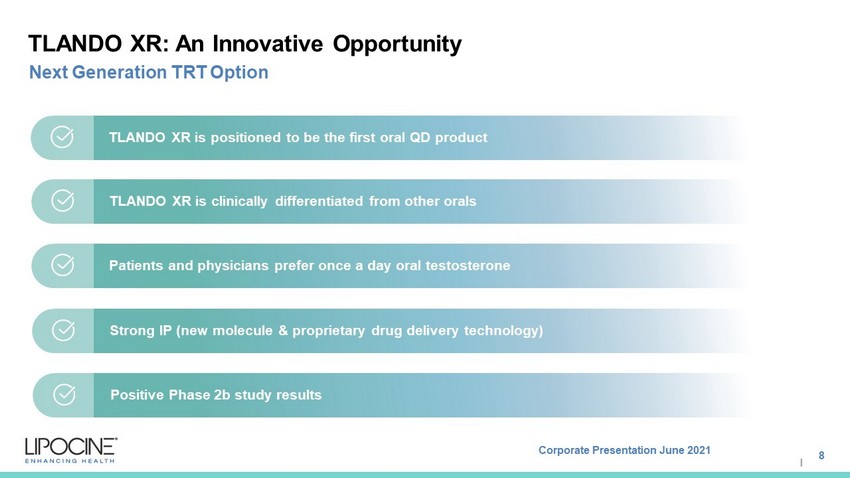
Corporate Presentation June 2021 I TLANDO XR: An Innovative Opportunity 8 Next Generation TRT Option TLANDO XR is positioned to be the first oral QD product TLANDO XR is clinically differentiated from other orals Patients and physicians prefer once a day oral testosterone Strong IP (new molecule & proprietary drug delivery technology) Positive Phase 2b study results

LPCN 1144 for Non - Cirrhotic NASH Currently No Approved Treatment
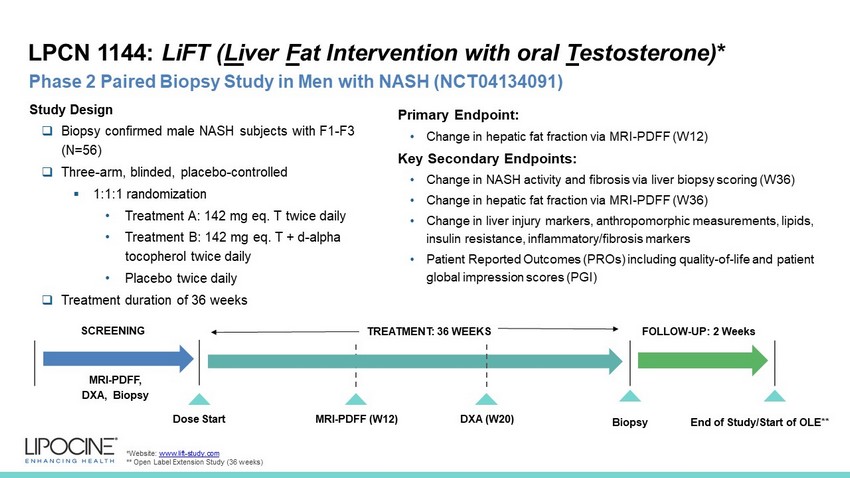
LPCN 1144: LiFT ( Li ver F at Intervention with oral T estosterone) * 10 Phase 2 Paired Biopsy Study in Men with NASH (NCT04134091) *Website: www.lift - study.com ** Open Label Extension Study (36 weeks) SCREENING MRI - PDFF (W12) Dose Start MRI - PDFF, DXA, Biopsy Study Design □ Biopsy confirmed male NASH subjects with F1 - F3 (N=56) □ Three - arm, blinded, placebo - controlled ▪ 1:1:1 randomization • Treatment A: 142 mg eq. T twice daily • Treatment B: 142 mg eq. T + d - alpha tocopherol twice daily • Placebo twice daily □ Treatment duration of 36 weeks 12 Weeks TREATMENT: 36 WEEKS Primary Endpoint: • Change in hepatic fat fraction via MRI - PDFF (W12) Key Secondary Endpoints: • Change in NASH activity and fibrosis via liver biopsy scoring (W36) • Change in hepatic fat fraction via MRI - PDFF (W36) • Change in liver injury markers, anthropomorphic measurements, lipids, insulin resistance, inflammatory/fibrosis markers • Patient Reported Outcomes (PROs) including quality - of - life and patient global impression scores (PGI) Biopsy FOLLOW - UP: 2 Weeks End of Study/Start of OLE** DXA (W20)
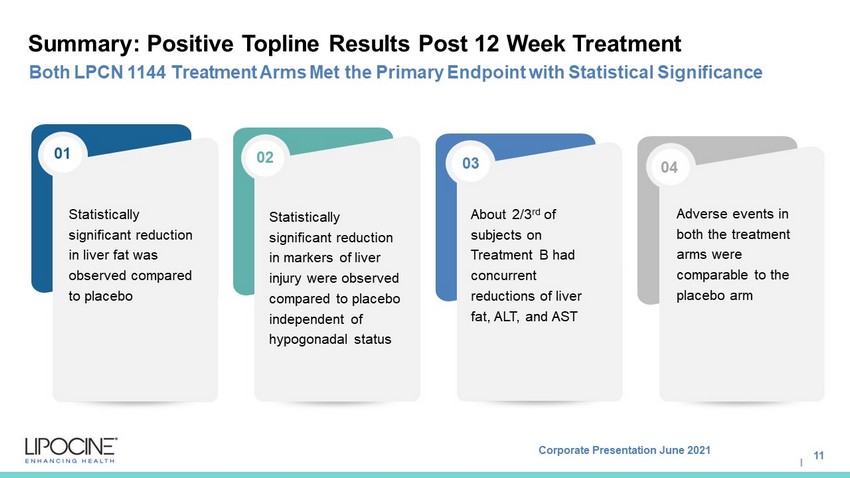
Corporate Presentation June 2021 I Leveraging the largest, multi - modal proprietary clinical database of its kind to expand a robust pipeline of predictive algorithms with regulatory grade evidence. Developing novel transformative algorithms to transform healthcare. Commercially validated across 3 business models Experienced team Summary: Positive Topline Results Post 12 Week Treatment 11 Both LPCN 1144 Treatment A rms M et the Primary E ndpoint with Statistical S ignificance Statistically significant reduction in liver fat was observed compared to placebo Statistically significant reduction in markers of liver injury were observed compared to placebo independent of hypogonadal status About 2/3 rd of subjects on Treatment B had concurrent reductions of liver fat, ALT, and AST Adverse events in both the treatment arms were comparable to the placebo arm 01 02 03 04
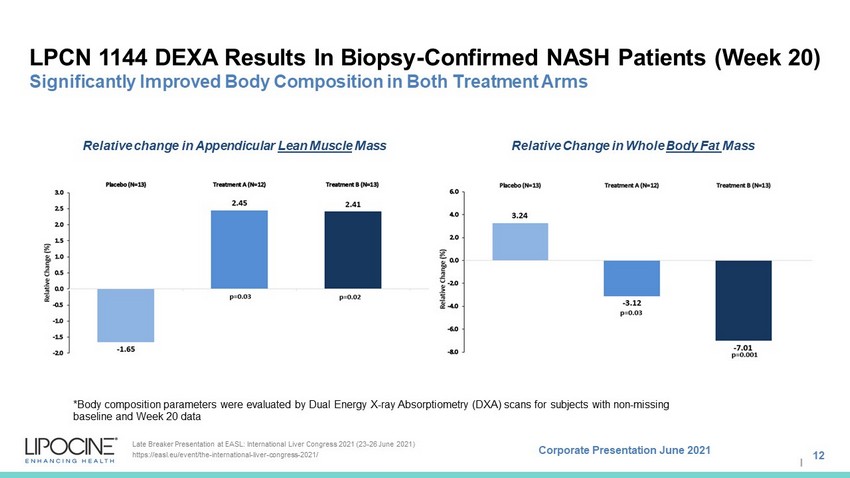
Corporate Presentation June 2021 I LPCN 1144 DEXA Results In Biopsy - Confirmed NASH Patients (Week 20) Significantly Improved Body Composition in Both Treatment Arms 12 Late Breaker Presentation at EASL: International Liver Congress 2021 (23 - 26 June 2021) https://easl.eu/event/the - international - liver - congress - 2021/ Relative Change in Whole Body Fat Mass Relative change in Appendicular Lean Muscle Mass *Body composition parameters were evaluated by Dual Energy X - ray Absorptiometry (DXA) scans for subjects with non - missing baseline and Week 20 data

LPCN 1148 for the Management of Liver Cirrhosis (an End Stage Disease)
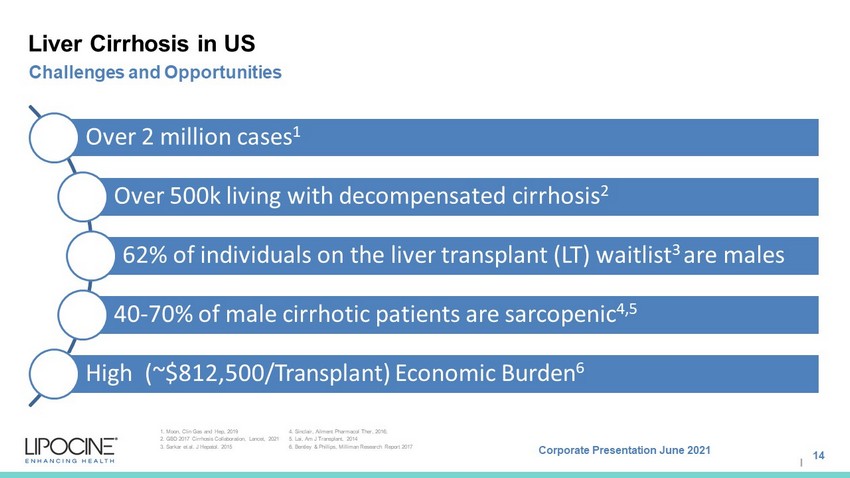
Corporate Presentation June 2021 I Liver Cirrhosis in US Over 2 million cases 1 Over 500k living with decompensated cirrhosis 2 62% of individuals on the liver transplant (LT) waitlist 3 are males 40 - 70% of male cirrhotic patients are sarcopenic 4,5 High ( ~$812,500/Transplant) Economic Burden 6 14 Challenges and Opportunities 1. Moon, Clin Gas and Hep, 2019 2. GBD 2017 Cirrhosis Collaboration, Lancet, 2021 3. Sarkar et al. J Hepatol. 2015 4. Sinclair, Ailment Pharmacol Ther , 2016; 5. Lai, Am J Transplant, 2014 6. Bentley & Phillips, Milliman Research Report 2017
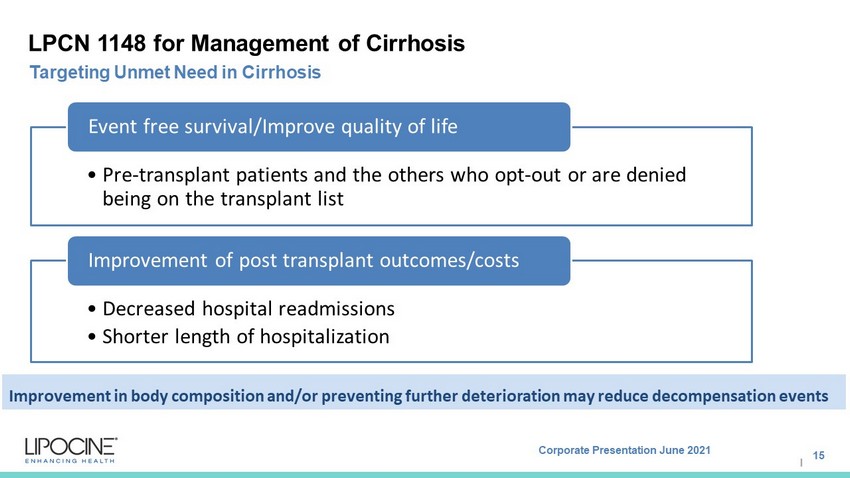
Corporate Presentation June 2021 I LPCN 1148 for Management of Cirrhosis • Pre - transplant patients and the others who opt - out or are denied being on the transplant list Event free survival/Improve quality of life • Decreased hospital readmissions • Shorter length of hospitalization Improvement of post transplant outcomes/costs 15 Targeting Unmet Need in Cirrhosis Improvement in body composition and/or preventing further deterioration may reduce decompensation events
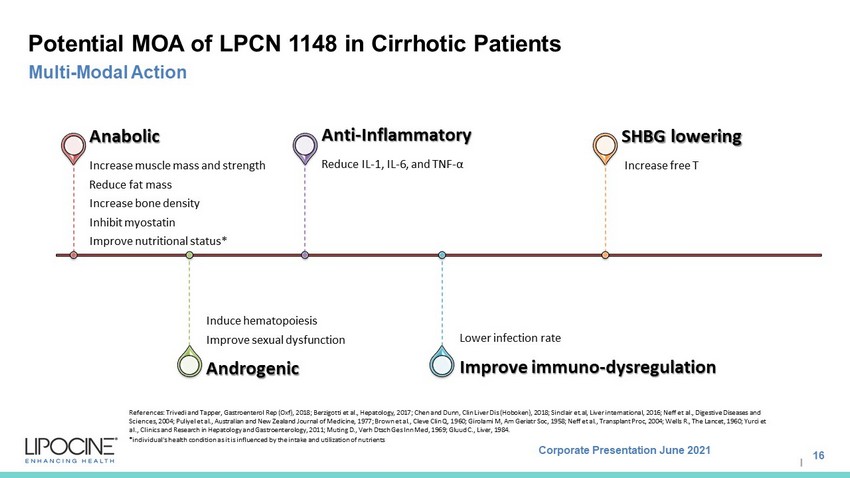
Corporate Presentation June 2021 I Potential MOA of LPCN 1148 in Cirrhotic Patients 16 Multi - Modal Action Increase muscle mass and strength Reduce fat mass Increase bone density Inhibit myostatin Improve nutritional status* Anabolic Induce hematopoiesis Improve sexual dysfunction Androgenic Reduce IL - 1, IL - 6, and TNF - α Anti - Inflammatory Lower infection rate Improve immuno - dysregulation Increase free T SHBG lowering References: Trivedi and Tapper, Gastroenterol Rep ( Oxf ), 2018; Berzigotti et al., Hepatology, 2017; Chen and Dunn, Clin Liver Dis (Hoboken), 2018; Sinclair et.al, Liver international, 2016; Neff et al., Digestive Diseases and Sciences, 2004; Puliyel et al., Australian and New Zealand Journal of Medicine, 1977; Brown et al., Cleve Clin Q, 1960; Girolami M, Am Geriatr Soc, 1958; Neff et al., Transplant Proc, 2004; Wells R., The Lancet, 1960; Yurci et al., Clinics and Research in Hepatology and Gastroenterology, 2011; Muting D., Verh Dtsch Ges Inn Med, 1969; Gluud C., Liver, 1984. *individual's health condition as it is influenced by the intake and utilization of nutrients
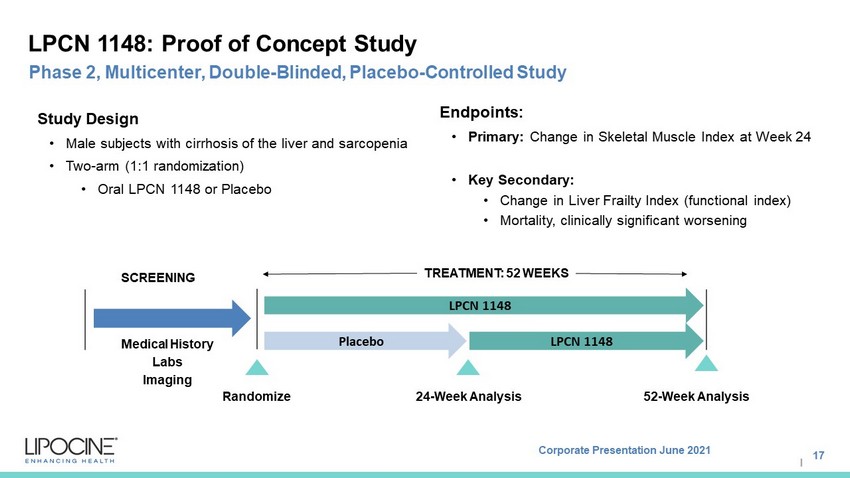
Corporate Presentation June 2021 I LPCN 1148: Proof of Concept Study 17 Phase 2, Multicenter, Double - Blinded, Placebo - Controlled Study Study Design • Male subjects with cirrhosis of the liver and sarcopenia • Two - arm (1:1 randomization) • Oral LPCN 1148 or Placebo Endpoints: • Primary: Change in Skeletal Muscle Index at Week 24 • Key Secondary: • Change in Liver Frailty Index (functional index) • Mortality, clinically significant worsening LPCN 1148 SCREENING 24 - Week Analysis Randomize Medical History Labs Imaging TREATMENT: 52 WEEKS 52 - Week Analysis Placebo LPCN 1148

LPCN 1154 Oral Neuro - Steroid for Depression Disorder
Corporate Presentation June 2021 I Postpartum Depression (PPD) ~ 1 in 7 women suffer from PPD after giving birth Only 50% (~400,000) of patients are currently diagnosed and treated in US Negative impact on maternal and infant outcomes Negative impact on spouse 19 A Major Depressive Episode Within Weeks of Delivery https://www.postpartumdepression.org/resources/statistics/ Evins GG et al. Am J Obstet Gynecol. 2000;182(5):1080 - 1082 Moore Simas et al. Curr Med Res and Opin . 2018; 35(3):383 - 393 Goodman J. J Adv Nurs . 2004;45(1):26 - 35.
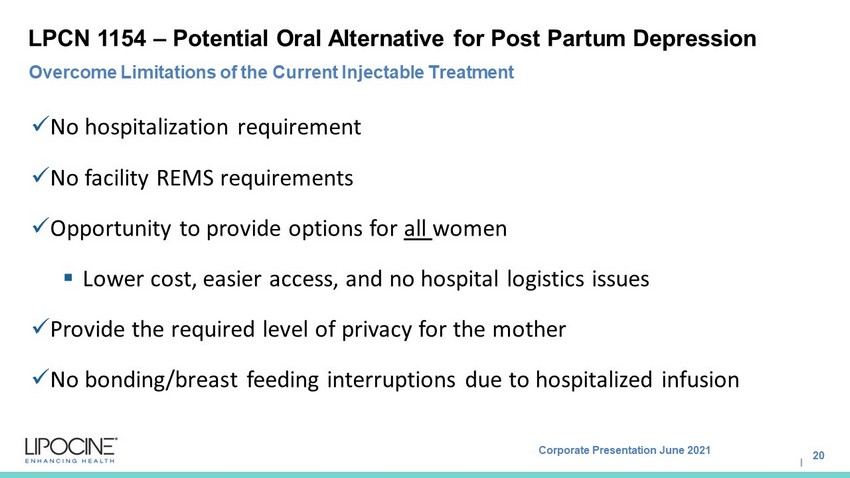
Corporate Presentation June 2021 I LPCN 1154 – Potential Oral Alternative for Post Partum Depression x No hospitalization requirement x No facility REMS requirements x Opportunity to provide options for all women ▪ Lower cost, easier access, and no hospital logistics issues x Provide the required level of privacy for the mother x No bonding/breast feeding interruptions due to hospitalized infusion 20 Overcome Limitations of the Current Injectable Treatment

LPCN 1154 : Next Steps 21 Proof of Concept Clinical Studies Received IND clearance for Phase 2 study • Phase 2 – Pilot POC Study • Objective: Safety and efficacy in PPD patients • Projected 1 st patient dosed 4Q 2021 - PK study in July 2021 Objective: Dose proportionality Projected top - line results in 3Q 2021

Corporate Presentation June 2021 I Upcoming Milestones Near Term Value Drivers Event Expected Timing LPCN 1144 LiFT Biopsy Results August 2021 LPCN 1154 POC Studies PK results : Q3 2021 P2 patient Dosing : Q4 2021 LPCN 1148 First Patient Dosed 2H 2021 TLANDO ΠFinal FDA Approval Decision March 2022 22
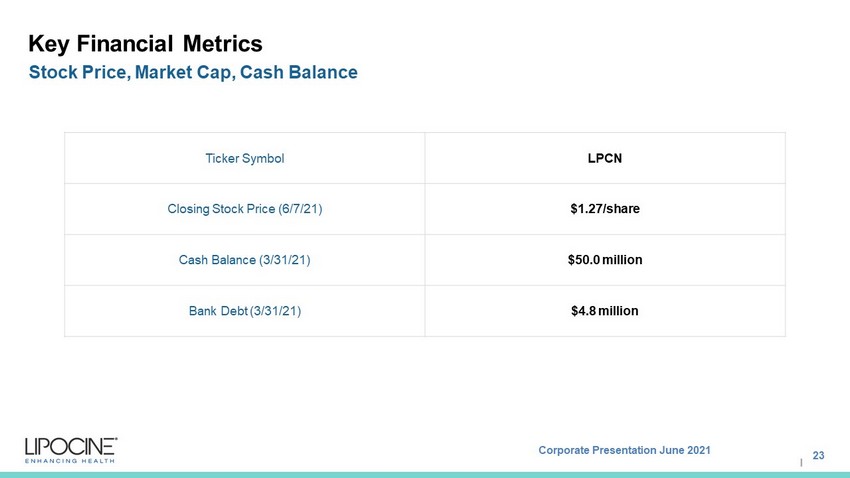
Corporate Presentation June 2021 I Key Financial Metrics 23 Stock Price, Market Cap, Cash Balance Ticker Symbol LPCN Closing Stock Price (6/7/21) $1.27/share Cash Balance (3/31/21) $50.0 million Bank Debt (3/31/21) $4.8 million
