Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Novan, Inc. | a06112021exhibit991.htm |
| 8-K - 8-K - Novan, Inc. | novn-061120218k.htm |

Nitric Oxide-Based Medicine novan.com NASDAQ: NOVN SB206: B-SIMPLE4 Top-Line Results June 11, 2021 Exhibit 99.2

Forward-Looking Statements This presentation contains forward-looking statements including, but not limited to, statements related to the potential therapeutic value of our NITRICIL™ platform technology, our pharmaceutical development of nitric oxide-releasing product candidates, including SB206, the potential timing of an NDA submission, the commercial prospects of SB206, our expected cash runway and our intention to partner with third parties. These forward-looking statements are included throughout this presentation. We have used the words “anticipate,” “assume,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “future,” “will,” “seek,” “foreseeable,” “targeted” and similar terms and phrases to identify forward- looking statements in this presentation. Forward-looking statements are subject to a number of risks and uncertainties that could cause actual results to differ materially from our expectations, including, but not limited to, risks related to the regulatory approval process, which is lengthy, time-consuming and inherently unpredictable, including the risk that the FDA will not agree with our approach a potential NDA submission, our product candidates may not be approved or that additional studies may be required for approval or other delays may occur, that we may not have sufficient quantities of drug substance and/or drug product to support regulatory submissions and that we may not obtain funding sufficient to complete the regulatory or development process; our limited experience as a company in obtaining regulatory approvals and commercializing pharmaceutical products; our ability to obtain additional funding or enter into strategic or other business relationships necessary or useful for the further development or, following regulatory approval, commercialization of our product candidates; our ability to enter into arrangements with third parties to support our development or commercialization efforts on terms that are acceptable to us or at all; risks and uncertainties in the clinical development process, including, among others, length, expense, ability to enroll patients, potential for delays or other impacts, whether as a result of the COVID-19 pandemic or other factors, and that results of earlier research and preclinical or clinical trials may not be predictive of results, conclusions or interpretations of later research activities or additional trials; any operational or other disruptions as a result of the COVID-19 pandemic; risks related to the manufacture of raw materials, including our active pharmaceutical ingredient and drug product components utilized in clinical trial materials, including supply chain disruptions or delays, failure to transfer technology and processes to third parties effectively or failure of those third parties (or us in connection with the upfit of our new facility) to obtain approval of and maintain compliance with the FDA or comparable regulatory authorities; our reliance on arrangements with third parties to support our operations and development efforts and the risk that such parties will not successfully carry out their contractual duties or meet expected deadlines; volatility in the price of our common stock; and other risks and uncertainties described in our annual report filed with the SEC on Form 10-K for the twelve months ended December 31, 2020, and in our subsequent filings with the SEC. These forward-looking statements speak only as of the date of this presentation, and Novan disclaims any intent or obligation to update these forward-looking statements to reflect events or circumstances after the date of such statements, except as may be required by law. 2

Agenda 3 • B-SIMPLE4: study design and demographics • Top-line efficacy: B-SIMPLE4 primary and secondary results • SB206 and molluscum indication: B-SIMPLE4 and B-SIMPLE2 • Molluscum market overview and commercial strategy

Treatment 12 Weeks Follow-up at Week 24 4 B-SIMPLE4 Pivotal Phase 3 Study Design in Molluscum Study Design Based on Learnings from B-SIMPLE1 and B-SIMPLE2: One and two-subject household stratification Inflammation of molluscum lesions added as a stratification factor1 Additional patient and caregiver training and retention efforts 1. As measured by BOTE (beginning of the end). Multi-center, double-blind, randomized, vehicle-controlled study Randomization891 Subjects (Aged 6-mos and older) Primary endpoint: Complete clearance of all treatable molluscum lesions Commenced Study in September 2020 With Last Week-12 Visit April 2021

Study Demographics and Disposition1 5 The B-SIMPLE4 pivotal trial was balanced across treatment arms and consistent with B-SIMPLE2 1. This table will be updated post completion of Week 24 data analysis and study closeout. 2. Intent-to-Treat Population (ITT): consists of all subjects who were randomized. SB206 Vehicle ITT Population2 444 447 Completed the 12 Week Treatment Period 394 400 Week 12 Premature Discontinuation 11.3% 10.5% Number of Sites (n=55) % Dermatologists 58.6% 59.3% Age (years) Mean 6.6 6.5 % Ages 2 to 17 95.0% 96.0% Gender Female 51.4% 47.7% Male 48.6% 52.3% Baseline Lesion Count Mean (Median) 23.1 (18.5) 20.5 (15.0)

B-SIMPLE4: ITT Population1 6 Positive study with robust clinical evidence of efficacy Primary Endpoint: Complete Clearance of All Lesions at Week 12 Secondary Endpoint: Proportion Achieving a Lesion Count of 0 or 1 at Week 12 SB206 (N=444) B-SIMPLE4 19.6% 11.6% Secondary Endpoint: Complete Clearance of All Lesions at Week 8 Secondary Endpoint: Proportion Achieving >90% Clearance of Lesions at Week 12 p=0.0014 43.0% 32.4% 19.7% p<0.0001 43.5% 24.6% p<0.0001 p<0.000123.9% Vehicle (N=447) p-value 1. Intent-to-Treat Population (ITT): consists of all subjects who were randomized.

Two Phase 3 Trials: Complete Clearance at Week 121 7 Approximately a 31% increase in treatment difference from B-SIMPLE2 to B-SIMPLE4 1. Intent-to-Treat Population (ITT): consists of all subjects who were randomized. 20.3% 19.7% 30.0% 32.4% 0.0% 5.0% 10.0% 15.0% 20.0% 25.0% 30.0% 35.0% 40.0% B-SIMPLE2 B-SIMPLE4 Vehicle SB206 p<0.0001 12.7% (N=355) (N=891) % o f p at ie nt s w ith c om pl et e cl ea ra nc e of a ll tr ea ta bl e m ol lu sc um le si on s at W ee k 12 9.7%

Summary of Adverse Events During 12-Week Treatment1 8 B-SIMPLE4 B-SIMPLE2 SB206 (N=444) Vehicle (N=447) SB206 (N=237) Vehicle (N=117) Subjects with at least one Treatment Emergent Adverse Event (TEAE) 189 (42.6%) 103 (23.0%) 120 (50.6%) 29 (24.8%) Subjects with at least one Serious TEAE 0 1 (0.2%) 1 (0.4%) 1 (0.9%) Subjects with at least one TEAE leading to Study Drug Discontinuation 17 (3.8%) 3 (0.7%) 17 (7.2%) 1 (0.9%) Maximum Severity Mild 106 (23.9%) 75 (16.8%) 51 (21.5%) 18 (15.4%) Moderate 79 (17.8%) 26 (5.8%) 64 (27.0%) 10 (8.5%) Severe 4 (0.9%) 2 (0.4%) 5 (2.1%) 1 (0.9%) 1. Safety Population consists of all patients who received drug any time during the treatment phase of the study. Note: results provided through Week 12 visit, final safety profile will be updated through Week 24 and is targeted to be released in 3Q 2021.
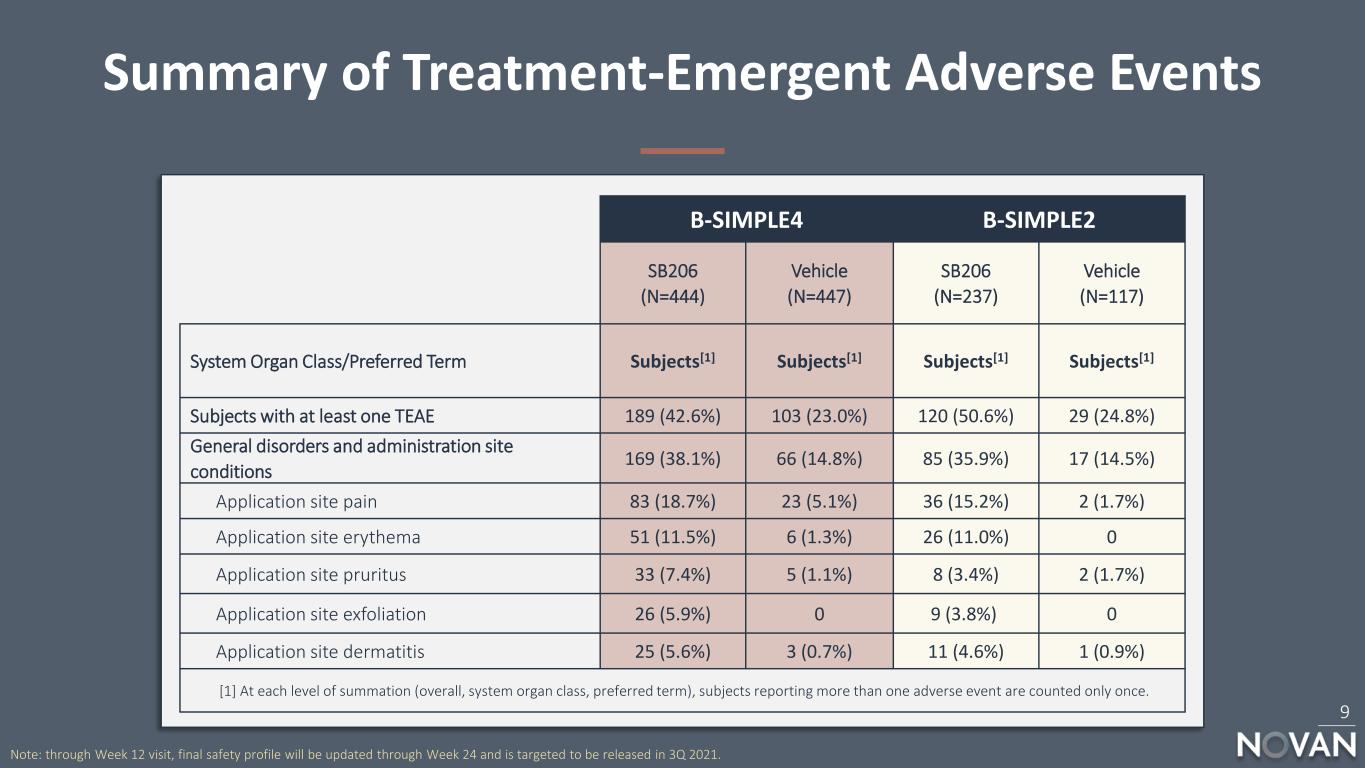
Summary of Treatment-Emergent Adverse Events 9 Note: through Week 12 visit, final safety profile will be updated through Week 24 and is targeted to be released in 3Q 2021. B-SIMPLE4 B-SIMPLE2 SB206 (N=444) Vehicle (N=447) SB206 (N=237) Vehicle (N=117) System Organ Class/Preferred Term Subjects[1] Subjects[1] Subjects[1] Subjects[1] Subjects with at least one TEAE 189 (42.6%) 103 (23.0%) 120 (50.6%) 29 (24.8%) General disorders and administration site conditions 169 (38.1%) 66 (14.8%) 85 (35.9%) 17 (14.5%) Application site pain 83 (18.7%) 23 (5.1%) 36 (15.2%) 2 (1.7%) Application site erythema 51 (11.5%) 6 (1.3%) 26 (11.0%) 0 Application site pruritus 33 (7.4%) 5 (1.1%) 8 (3.4%) 2 (1.7%) Application site exfoliation 26 (5.9%) 0 9 (3.8%) 0 Application site dermatitis 25 (5.6%) 3 (0.7%) 11 (4.6%) 1 (0.9%) [1] At each level of summation (overall, system organ class, preferred term), subjects reporting more than one adverse event are counted only once.

SB206: Summary of B-SIMPLE Results 10 B-SIMPLE4 was a positive Phase 3 study with robust clinical data SB206 demonstrates clinical evidence of efficacy B-SIMPLE2 will be included as the confirmatory trial per FDA guidance received in April 2020 We have confidence to move forward with NDA preparation and potential submission ✓ ✓ ✓ ✓

SB206: Next Steps 11 All safety data including the prospectively planned safety evaluation through Week 24, targeted to be available in 3Q 2021 Intend to utilize B-SIMPLE4 as the pivotal Phase 3 trial and B-SIMPLE2 as the confirmatory trial for New Drug Application ("NDA") submission1 Potential NDA submission remains consistent with previous timelines and targeted no later than 3Q 2022 1. Subject to discussions with the U.S. Food and Drug Administration (FDA).

Molluscum: The Unmet Medical Need 12 No FDA-Approved Treatments ~3.4 million prevalence in the U.S.1 ~1.3 million diagnosed per annum1 ~70% of patients below the age of 10 Average time to resolution 13 Months SB206: Potential to be the First FDA Approved Patient or Caregiver Administered Topical Therapy for Molluscum Contagiosum 1. Syneos Health Consulting Primary (n=40 Pediatricians, n=39 Dermatologists, n=4 Pediatric Dermatologists) and Secondary Research (2019).

SB206 Commercialization Strategy 13 Evaluating All Strategic Options Goal: Maximize Value Novan Commercializes Secure Commercialization Partner Co-Commercialize with Partner

THANK YOU!!! 14 Employees Patients Investigators Partners InvestorsCROs

Q&A 15

Nitric Oxide-Based Medicine Investor Relations JTC Team 833.475.8247 novn@jtcir.com novan.com NASDAQ: NOVN
