Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Aclaris Therapeutics, Inc. | acrs-20210608xex99d2.htm |
| 8-K - 8-K - Aclaris Therapeutics, Inc. | acrs-20210608x8k.htm |
Exhibit 99.1
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2021 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0589 06/21) © Copyright 2021 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0602 06/21) ATI-1777-AD-201 (Investigational Compound) Preliminary Topline Data June 8, 2021 EMPOWERING PATIENTS THROUGH KINOME INNOVATION |
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2021 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0602 06/21) Any statements contained in this presentation that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements may be identified by words such as “believe,” “expect,” “may,” “plan,” “potential,” “will,” and similar expressions, and are based on Aclaris' current beliefs and expectations. These forward-looking statements include expectations regarding ATI-1777 as a potential treatment for moderate to severe atopic dermatitis and the clinical development of ATI-1777. These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements. Risks and uncertainties that may cause actual results to differ materially include uncertainties inherent in the conduct of clinical trials, risks and uncertainties associated with preliminary trial results varying from final results, Aclaris' reliance on third parties over which it may not always have full control, the uncertainty regarding the COVID-19 pandemic including its impact on the timing of Aclaris’ regulatory and research and development activities, and other risks and uncertainties that are described in the Risk Factors section of Aclaris’ Annual Report on Form 10-K for the year ended December 31, 2020 and other filings Aclaris makes with the U.S. Securities and Exchange Commission from time to time. These documents are available under the “SEC Filings” page of the “Investors” section of Aclaris' website at http://www.aclaristx.com. Any forward-looking statements speak only as of the date of this presentation and are based on information available to Aclaris as of the date of this presentation, and Aclaris assumes no obligation to, and does not intend to, update any forward-looking statements, whether as a result of new information, future events or otherwise This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Cautionary Note Regarding Forward-Looking Statements 2 |
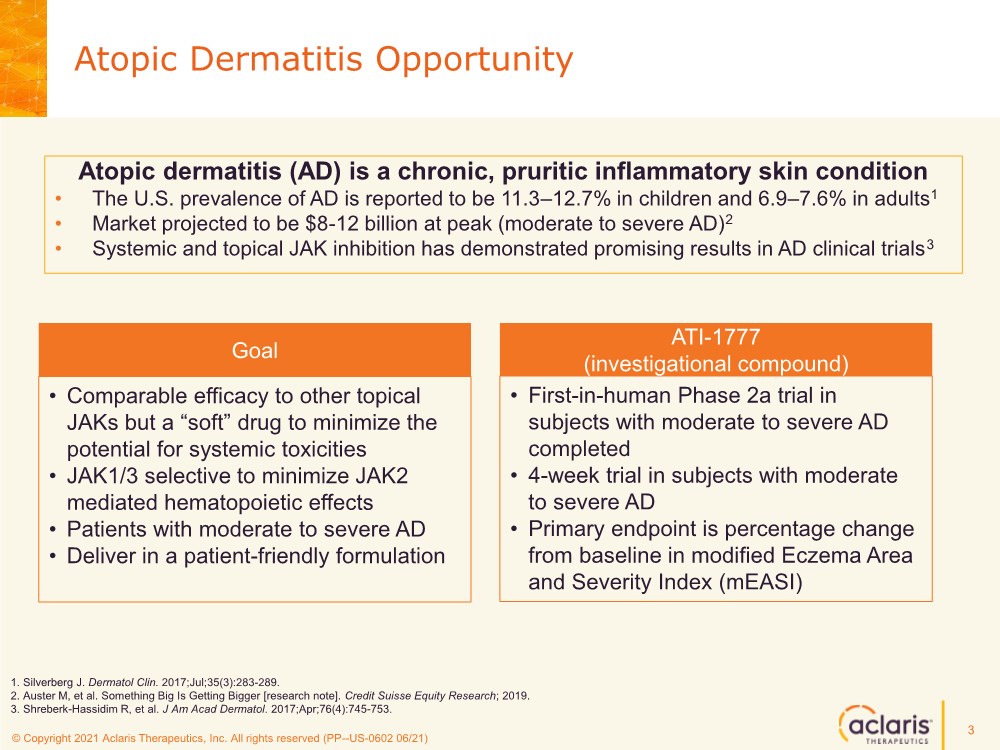
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2021 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0602 06/21) Atopic dermatitis (AD) is a chronic, pruritic inflammatory skin condition • The U.S. prevalence of AD is reported to be 11.3–12.7% in children and 6.9–7.6% in adults1 • Market projected to be $8-12 billion at peak (moderate to severe AD)2 • Systemic and topical JAK inhibition has demonstrated promising results in AD clinical trials3 Atopic Dermatitis Opportunity 3 • Comparable efficacy to other topical JAKs but a “soft” drug to minimize the potential for systemic toxicities • JAK1/3 selective to minimize JAK2 mediated hematopoietic effects • Patients with moderate to severe AD • Deliver in a patient-friendly formulation Goal • First-in-human Phase 2a trial in subjects with moderate to severe AD completed • 4-week trial in subjects with moderate to severe AD • Primary endpoint is percentage change from baseline in modified Eczema Area and Severity Index (mEASI) ATI-1777 (investigational compound) 1. Silverberg J. Dermatol Clin. 2017;Jul;35(3):283-289. 2. Auster M, et al. Something Big Is Getting Bigger [research note]. Credit Suisse Equity Research; 2019. 3. Shreberk-Hassidim R, et al. J Am Acad Dermatol. 2017;Apr;76(4):745-753. |
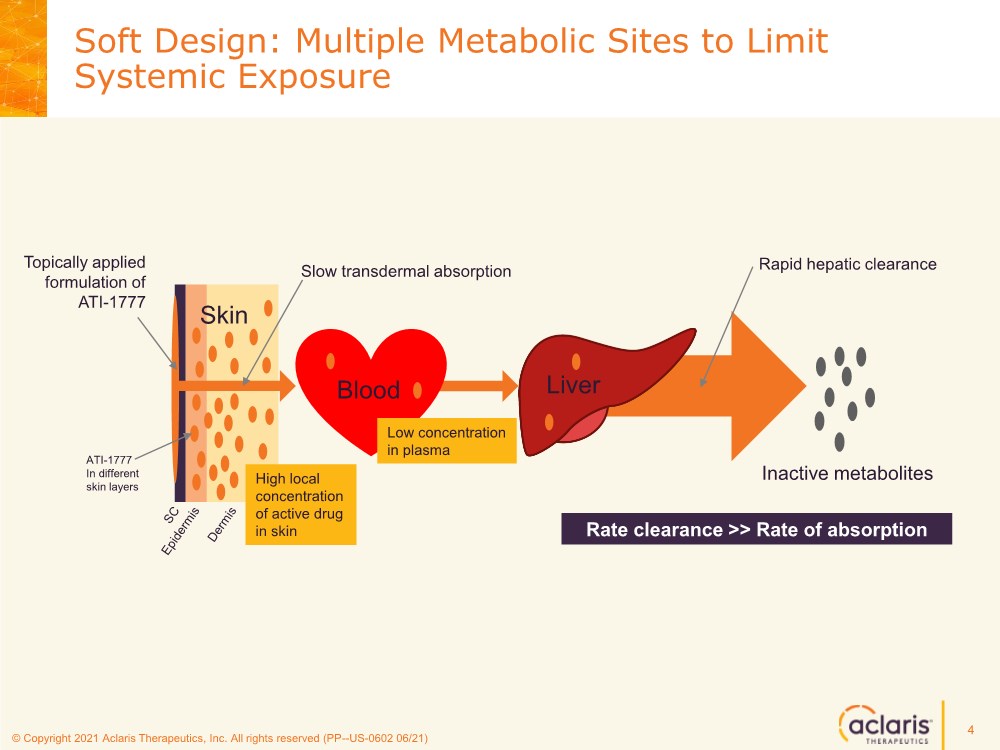
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2021 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0602 06/21) Soft Design: Multiple Metabolic Sites to Limit Systemic Exposure 4 Topically applied formulation of ATI-1777 Slow transdermal absorption Skin Blood Liver Rapid hepatic clearance Inactive metabolites High local concentration of active drug in skin Low concentration in plasma ATI-1777 In different skin layers Rate clearance >> Rate of absorption |
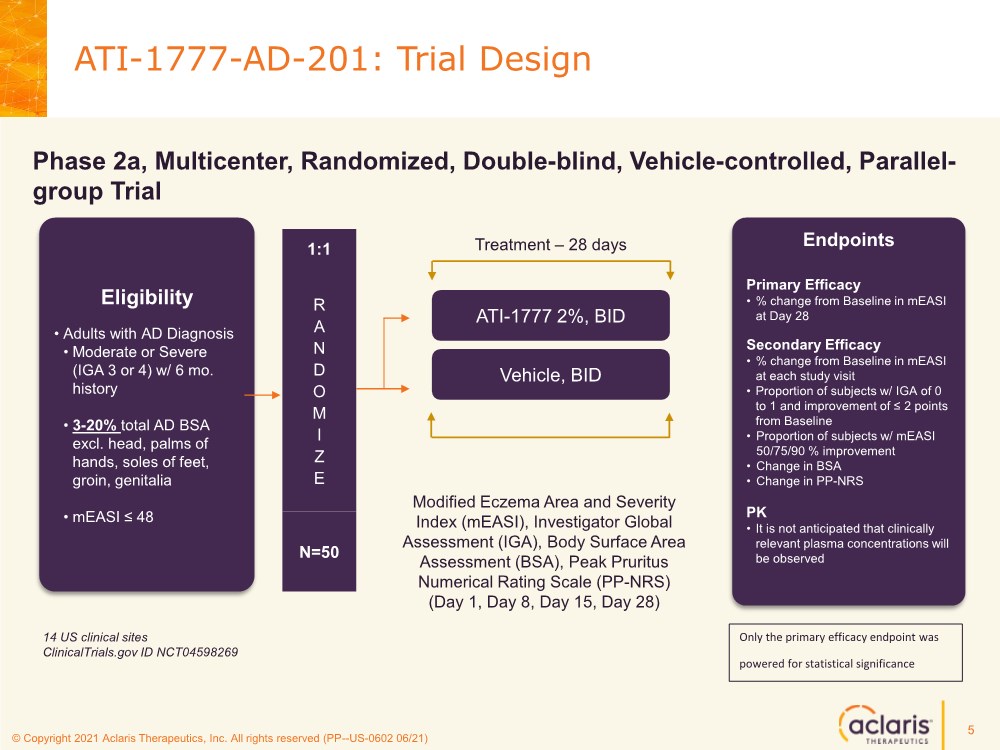
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2021 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0602 06/21) ATI-1777-AD-201: Trial Design Eligibility • Adults with AD Diagnosis • Moderate or Severe (IGA 3 or 4) w/ 6 mo. history • 3-20% total AD BSA excl. head, palms of hands, soles of feet, groin, genitalia • mEASI ≤ 48 R A N D O M I Z E 1:1 N=50 ATI-1777 2%, BID Vehicle, BID Phase 2a, Multicenter, Randomized, Double-blind, Vehicle-controlled, Parallel- group Trial Endpoints Primary Efficacy •% change from Baseline in mEASI at Day 28 Secondary Efficacy •% change from Baseline in mEASI at each study visit • Proportion of subjects w/ IGA of 0 to 1 and improvement of ≤ 2 points from Baseline • Proportion of subjects w/ mEASI 50/75/90 % improvement • Change in BSA • Change in PP-NRS PK • It is not anticipated that clinically relevant plasma concentrations will be observed Treatment – 28 days Modified Eczema Area and Severity Index (mEASI), Investigator Global Assessment (IGA), Body Surface Area Assessment (BSA), Peak Pruritus Numerical Rating Scale (PP-NRS) (Day 1, Day 8, Day 15, Day 28) 14 US clinical sites ClinicalTrials.gov ID NCT04598269 5 Only the primary efficacy endpoint was powered for statistical significance |
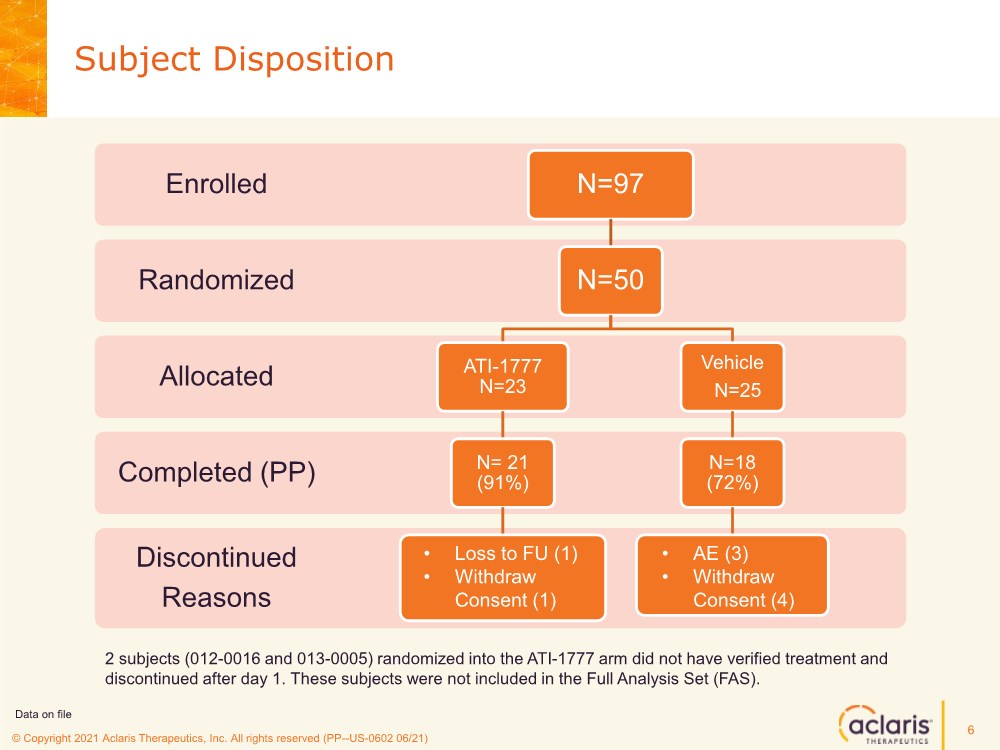
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2021 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0602 06/21) Subject Disposition 6 Discontinued Reasons Completed (PP) Allocated Randomized Enrolled N=97 N=50 ATI-1777 N=23 N= 21 (91%) Vehicle N=25 N=18 (72%) • Loss to FU (1) • Withdraw Consent (1) • AE (3) • Withdraw Consent (4) 2 subjects (012-0016 and 013-0005) randomized into the ATI-1777 arm did not have verified treatment and discontinued after day 1. These subjects were not included in the Full Analysis Set (FAS). Data on file |
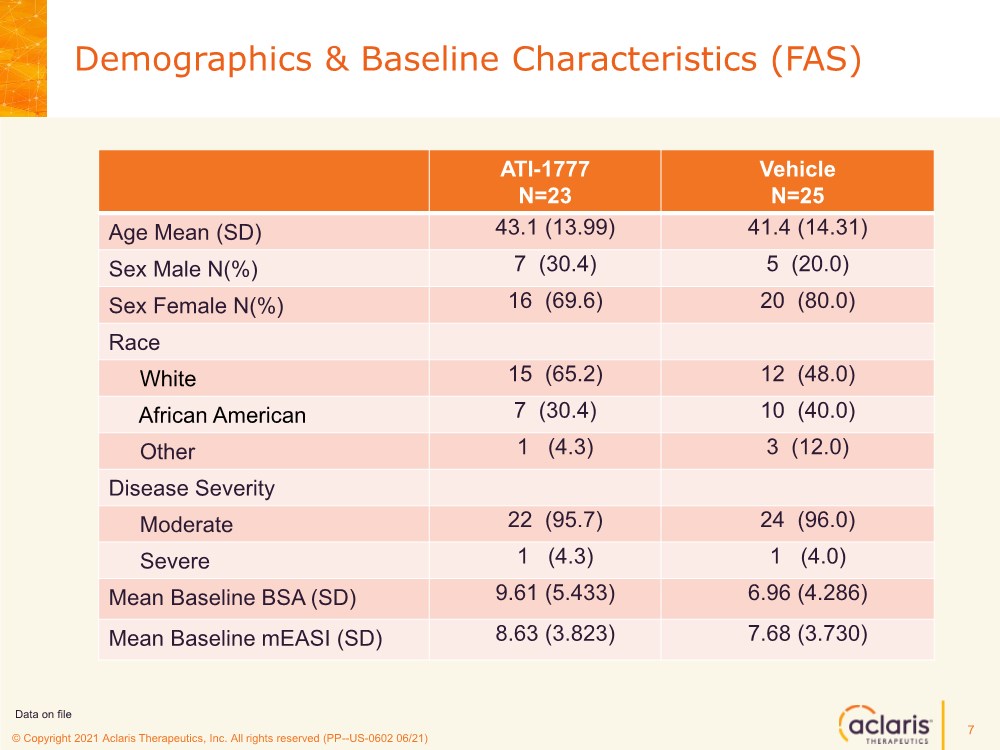
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2021 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0602 06/21) Demographics & Baseline Characteristics (FAS) 7 ATI-1777 N=23 Vehicle N=25 Age Mean (SD) 43.1 (13.99) 41.4 (14.31) Sex Male N(%) 7 (30.4) 5 (20.0) Sex Female N(%) 16 (69.6) 20 (80.0) Race White 15 (65.2) 12 (48.0) African American 7 (30.4) 10 (40.0) Other 1 (4.3) 3 (12.0) Disease Severity Moderate 22 (95.7) 24 (96.0) Severe 1 (4.3) 1 (4.0) Mean Baseline BSA (SD) 9.61 (5.433) 6.96 (4.286) Mean Baseline mEASI (SD) 8.63 (3.823) 7.68 (3.730) Data on file |
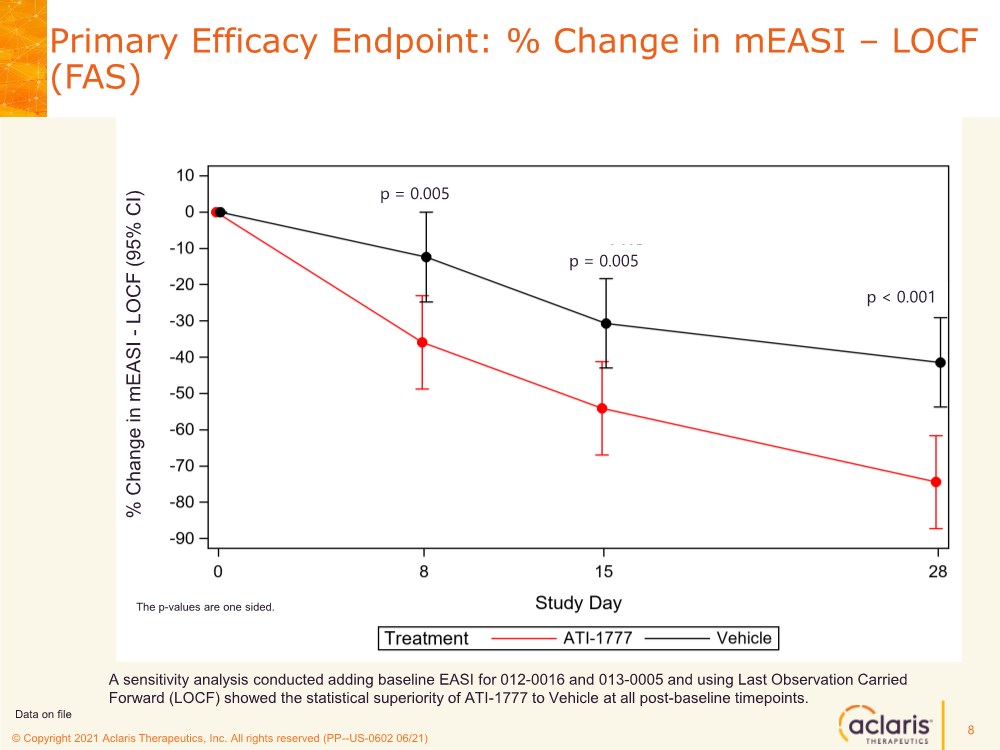
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2021 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0602 06/21) Primary Efficacy Endpoint: % Change in mEASI – LOCF (FAS) 8 A sensitivity analysis conducted adding baseline EASI for 012-0016 and 013-0005 and using Last Observation Carried Forward (LOCF) showed the statistical superiority of ATI-1777 to Vehicle at all post-baseline timepoints. Data on file p = 0.005 p = 0.005 p < 0.001 % Change in mEASI - LOCF (95% CI) The p-values are one sided. |
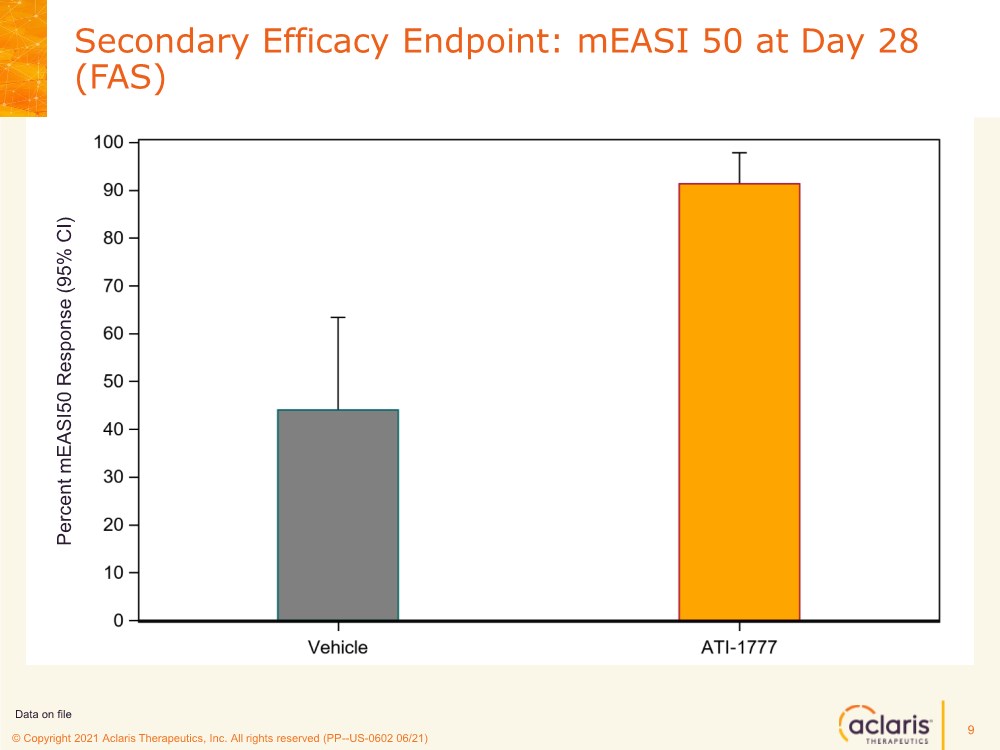
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2021 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0602 06/21) Secondary Efficacy Endpoint: mEASI 50 at Day 28 (FAS) 9 Data on file Percent mEASI50 Response (95% CI) |
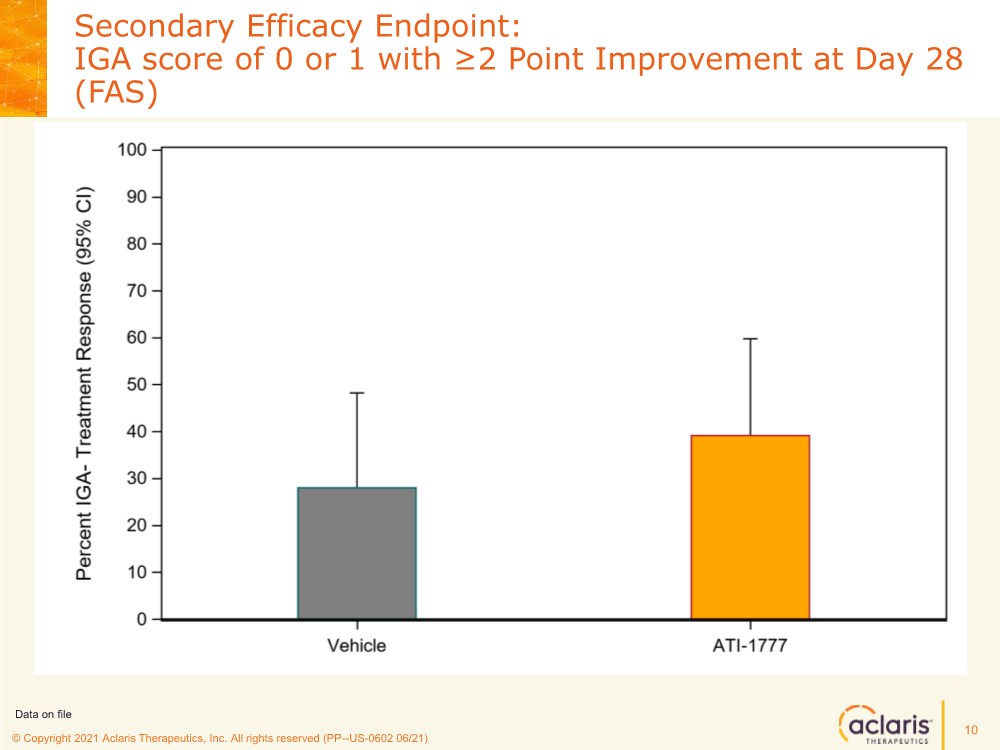
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2021 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0602 06/21) Secondary Efficacy Endpoint: IGA score of 0 or 1 with ≥2 Point Improvement at Day 28 (FAS) 10 Data on file |
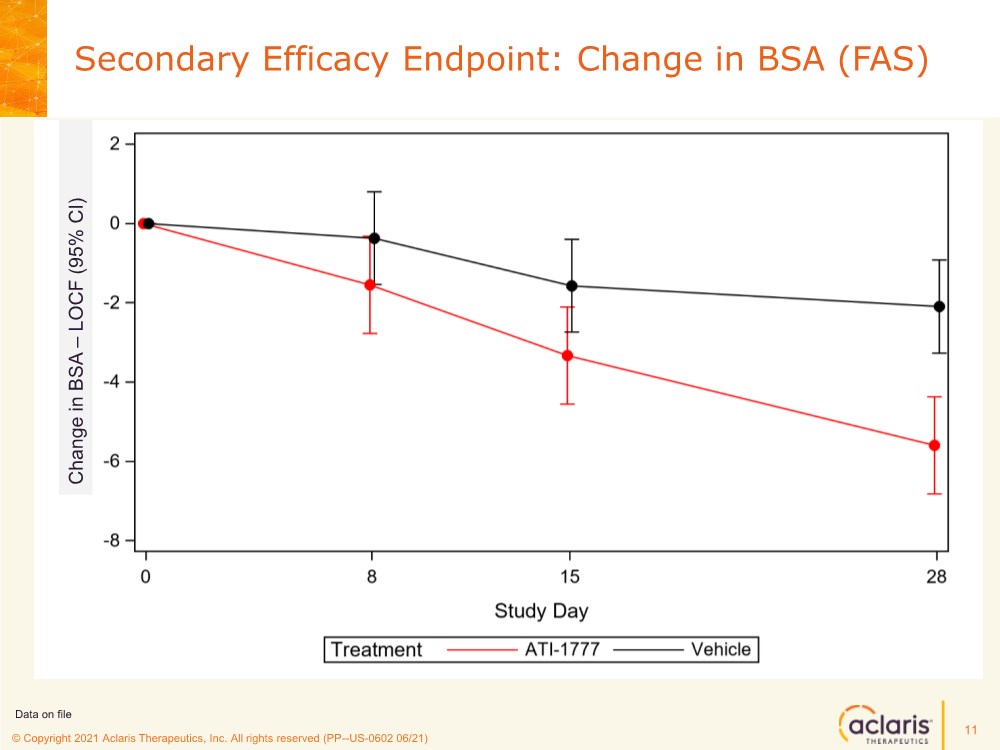
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2021 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0602 06/21) Secondary Efficacy Endpoint: Change in BSA (FAS) 11 Data on file Change in BSA – LOCF (95% CI) |
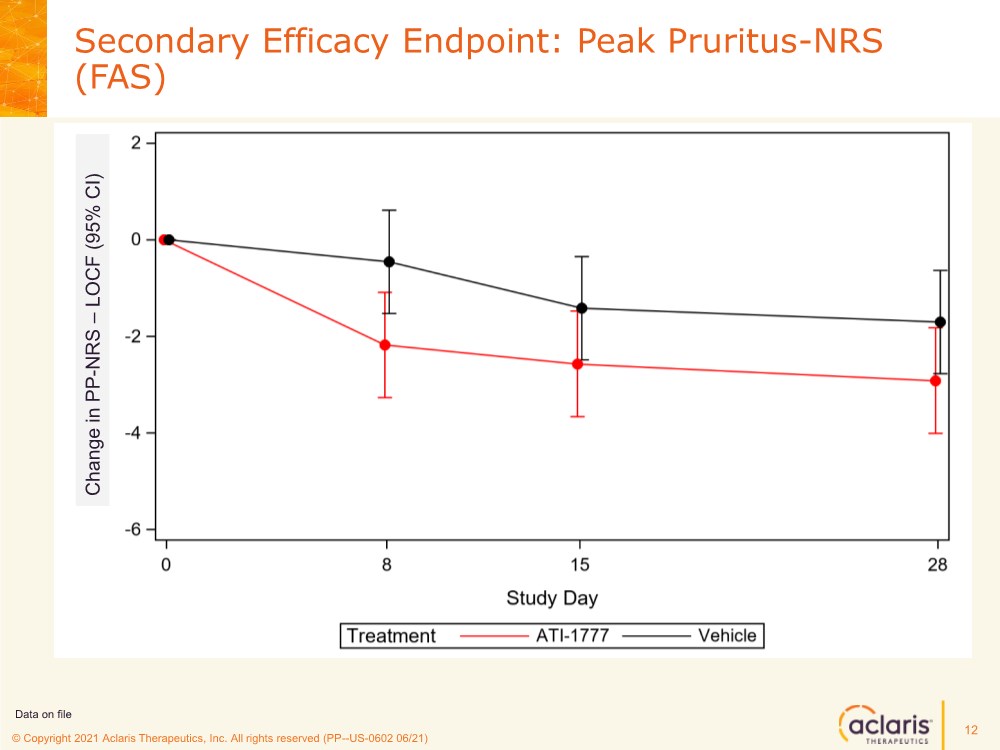
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2021 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0602 06/21) Secondary Efficacy Endpoint: Peak Pruritus-NRS (FAS) 12 Data on file Change in PP - NRS – LOCF (95% CI) |
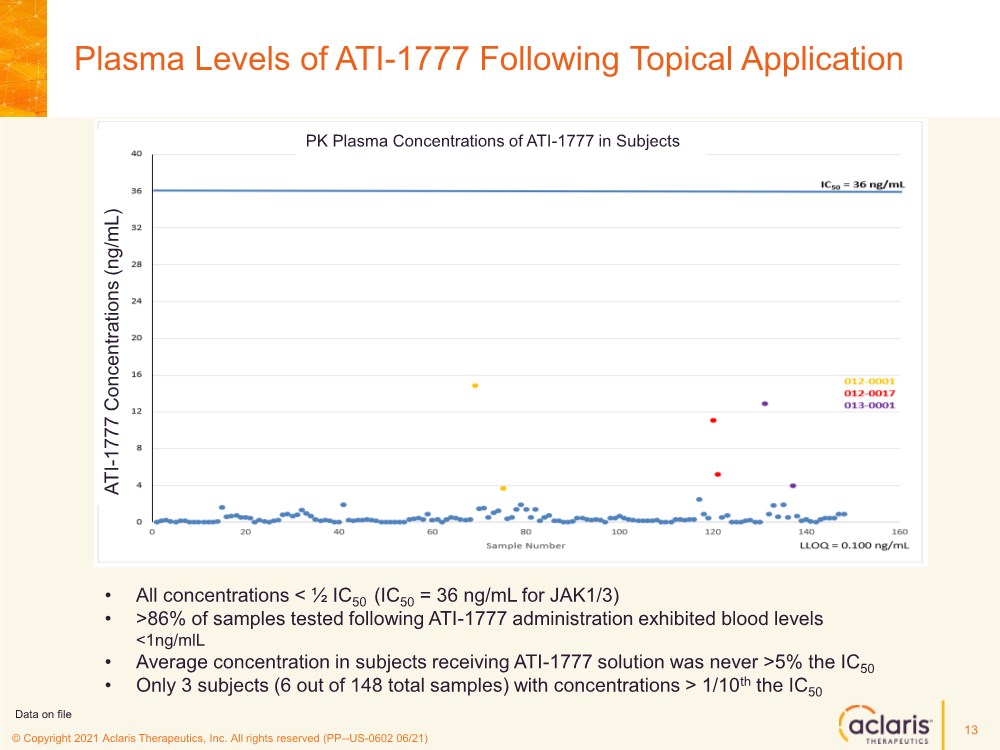
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2021 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0602 06/21) Plasma Levels of ATI-1777 Following Topical Application 13 • All concentrations < ½ IC50 (IC50 = 36 ng/mL for JAK1/3) • >86% of samples tested following ATI-1777 administration exhibited blood levels <1ng/mlL • Average concentration in subjects receiving ATI-1777 solution was never >5% the IC50 • Only 3 subjects (6 out of 148 total samples) with concentrations > 1/10th the IC50 PK Plasma Concentrations of ATI-1777 in Subjects Data on file ATI - 1777 Concentrations (ng/mL) |
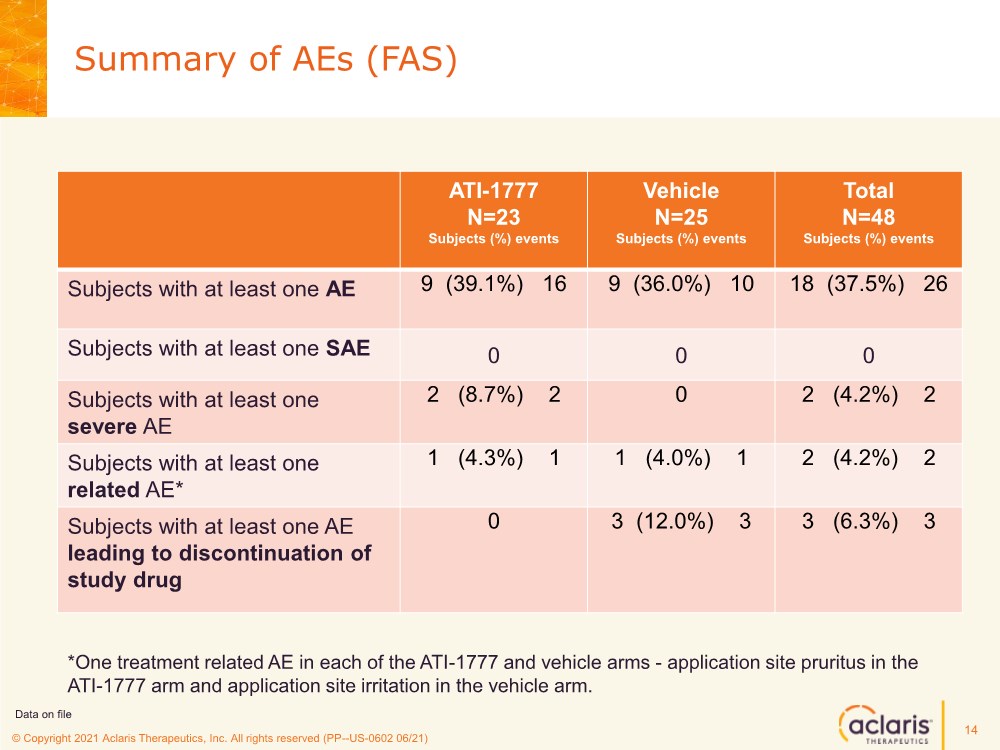
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2021 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0602 06/21) Summary of AEs (FAS) 14 ATI-1777 N=23 Subjects (%) events Vehicle N=25 Subjects (%) events Total N=48 Subjects (%) events Subjects with at least one AE 9 (39.1%) 16 9 (36.0%) 10 18 (37.5%) 26 Subjects with at least one SAE 0 0 0 Subjects with at least one severe AE 2 (8.7%) 2 0 2 (4.2%) 2 Subjects with at least one related AE* 1 (4.3%) 1 1 (4.0%) 1 2 (4.2%) 2 Subjects with at least one AE leading to discontinuation of study drug 0 3 (12.0%) 3 3 (6.3%) 3 Data on file *One treatment related AE in each of the ATI-1777 and vehicle arms - application site pruritus in the ATI-1777 arm and application site irritation in the vehicle arm. |
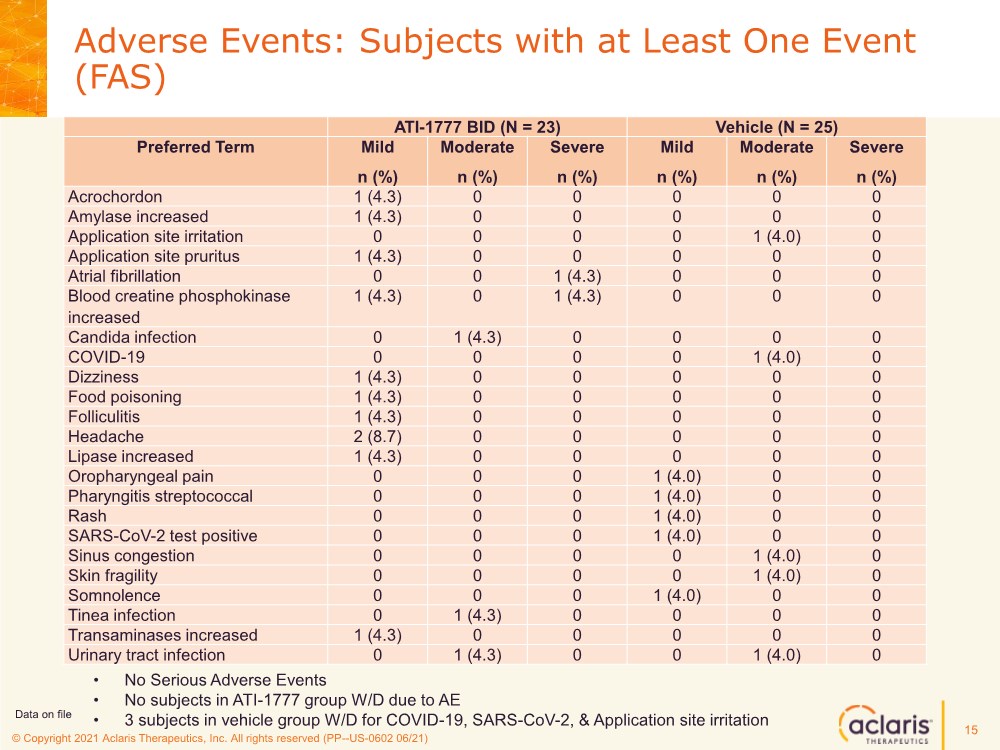
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2021 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0602 06/21) • No Serious Adverse Events • No subjects in ATI-1777 group W/D due to AE • 3 subjects in vehicle group W/D for COVID-19, SARS-CoV-2, & Application site irritation Adverse Events: Subjects with at Least One Event (FAS) ATI-1777 BID (N = 23) Vehicle (N = 25) Preferred Term Mild n (%) Moderate n (%) Severe n (%) Mild n (%) Moderate n (%) Severe n (%) Acrochordon 1 (4.3) 0 0 0 0 0 Amylase increased 1 (4.3) 0 0 0 0 0 Application site irritation 0 0 0 0 1 (4.0) 0 Application site pruritus 1 (4.3) 0 0 0 0 0 Atrial fibrillation 0 0 1 (4.3) 0 0 0 Blood creatine phosphokinase increased 1 (4.3) 0 1 (4.3) 0 0 0 Candida infection 0 1 (4.3) 0 0 0 0 COVID-19 0 0 0 0 1 (4.0) 0 Dizziness 1 (4.3) 0 0 0 0 0 Food poisoning 1 (4.3) 0 0 0 0 0 Folliculitis 1 (4.3) 0 0 0 0 0 Headache 2 (8.7) 0 0 0 0 0 Lipase increased 1 (4.3) 0 0 0 0 0 Oropharyngeal pain 0 0 0 1 (4.0) 0 0 Pharyngitis streptococcal 0 0 0 1 (4.0) 0 0 Rash 0 0 0 1 (4.0) 0 0 SARS-CoV-2 test positive 0 0 0 1 (4.0) 0 0 Sinus congestion 0 0 0 0 1 (4.0) 0 Skin fragility 0 0 0 0 1 (4.0) 0 Somnolence 0 0 0 1 (4.0) 0 0 Tinea infection 0 1 (4.3) 0 0 0 0 Transaminases increased 1 (4.3) 0 0 0 0 0 Urinary tract infection 0 1 (4.3) 0 0 1 (4.0) 0 Data on file 15 |
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2021 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0602 06/21) • Positive Proof of Concept First in Human Study ✓Moderate to Severe Atopic Dermatitis • Traditionally the domain of systemic therapy ✓Rapid and continuing improvement over 4 weeks ✓PK supports tissue specific approach ✓Generally well tolerated • Potential positioning in moderate to severe atopic dermatitis ✓Monotherapy ✓Combination therapy with biologics to potentially drive improved efficacy1 Conclusions 16 Data on file 1. Reich, Teixeira, Bruin-Weller, Bieber, Lancet 397, Issue 10290, P2169-2181, June 5, 2021 |