Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Inspire Medical Systems, Inc. | insp-20210607.htm |

1 Inspire Medical Systems, Inc. June 2021 NYSE: INSP

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Disclaimer This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts are forward- looking statements. In some cases, you can identify forward-looking statements by terms such as ‘‘may,’’ ‘‘will,’’ ‘‘should,’’ ‘‘expect,’’ ‘‘plan,’’ ‘‘anticipate,’’ ‘‘could,’’ “future,” “outlook,” ‘‘intend,’’ ‘‘target,’’ ‘‘project,’’ ‘‘contemplate,’’ ‘‘believe,’’ ‘‘estimate,’’ ‘‘predict,’’ ‘‘potential,’’ ‘‘continue,’’ or the negative of these terms or other similar expressions, although not all forward-looking statements contain these words. The forward-looking statements in this presentation relate to, among other things, statements regarding the impact of the COVID-19 pandemic on our business operations, financial results and financial condition, investments in our business, our growth strategies, our expectation that a substantial portion of postponed Inspire therapy procedures will be rescheduled, the activity of our commercial team once circumstances allow, full year 2021 financial and operational outlook, and positive insurance coverage of Inspire therapy and improvements in market access, clinical data growth, product development, indication expansion, market development, and prior authorization approvals. These forward-looking statements are based on management’s current expectations and involve known and unknown risks and uncertainties that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, estimates regarding the annual total addressable market for our Inspire therapy in the U.S. and our market opportunity outside the U.S.; future results of operations, financial position, research and development costs, capital requirements and our needs for additional financing; commercial success and market acceptance of our Inspire therapy; the impact of the ongoing and global COVID- 19 pandemic; general and international economic, political, and other risks, including currency and stock market fluctuations and the uncertain economic environment; our ability to achieve and maintain adequate levels of coverage or reimbursement for our Inspire system or any future products we may seek to commercialize; competitive companies and technologies in our industry; our ability to enhance our Inspire system, expand our indications and develop and commercialize additional products; our business model and strategic plans for our products, technologies and business, including our implementation thereof; our ability to accurately forecast customer demand for our Inspire system and manage our inventory; our dependence on third-party suppliers, contract manufacturers and shipping carriers; consolidation in the healthcare industry; our ability to expand, manage and maintain our direct sales and marketing organization, and to market and sell our Inspire system in markets outside of the U.S.; risks associated with international operations; our ability to manage our growth; our ability to increase the number of active medical centers implanting Inspire therapy; our ability to hire and retain our senior management and other highly qualified personnel; risk of product liability claims; risks related to information technology and cybersecurity; risk of damage to or interruptions at our facilities; our ability to commercialize or obtain regulatory approvals for our Inspire therapy and system, or the effect of delays in commercializing or obtaining regulatory approvals; FDA or other U.S. or foreign regulatory actions affecting us or the healthcare industry generally, including healthcare reform measures in the U.S. and international markets; the timing or likelihood of regulatory filings and approvals; risks related to our debt and capital structure; our ability to establish and maintain intellectual property protection for our Inspire therapy and system or avoid claims of infringement; tax risks; risks that we may be deemed an investment company under the Investment Company Act of 1940; regulatory risks; the volatility of the trading price of our common stock; and our expectations about market trends. Other important factors that could cause actual results, performance or achievements to differ materially from those contemplated in this presentation can be found under the captions “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Annual Report on Form 10-K for the year ended December 31, 2020, as updated in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2021, and as such factors may be updated from time to time in our other filings with the SEC, which are accessible on the SEC’s website at www.sec.gov and the Investors page of our website at www.inspiresleep.com. These and other important factors could cause actual results to differ materially from those indicated by the forward-looking statements made in this presentation. Any such forward-looking statements represent management’s estimates as of the date of this presentation. While we may elect to update such forward-looking statements at some point in the future, unless required by applicable law, we disclaim any obligation to do so, even if subsequent events cause our views to change. Thus, one should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation. This presentation contains trademarks, trade names and service marks of other companies, which are the property of their respective owners. We do not intend our use or display of other parties' trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties. 2

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL 3

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Continued Momentum in 2021 FY 2021 Guidance revised post-Q1: Revenue: $192M - $196M, representing 66% - 70% growth from FY 2020 revenue of $115.4M Gross margin: 84% - 85% Territories: 8 to 9 new territories per quarter U.S. centers: 36 to 40 new centers per quarter In 2021, focus is on continuing expansion by improving capacity and conversion: Increasing capacity by adding territories and centers (hospitals and ASC networks) Entered into a network agreement with CHS, a publicly held hospital system with 84 hospitals and about 30 ASCs, for over 110 locations across 16 states USPI recently entered a joint venture which increased the number of USPI locations from over 300 to over 500 potentials locations. Inspire has opened about 20 USPI centers to-date. New direct-to-consumer TV campaigns are driving more website visitors Increasing and improving connections with potential patients with continued expansion of the Advisor Care Program (call center) to new and existing centers to capture most in-bound phone calls 4

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Continued Momentum in 2021 (continued) Strong enthusiasm and acceptance of the FDA-approved two-incision implanting approach Over 90% of Inspire procedures now utilize this technique which significantly reduces surgical time Recent Anthem coverage policy -- approvals now occurring in just days for new Anthem patients and efficient approval of previous denials Approximately 50 new ENT surgeons recently completed the inaugural Inspire Fellows Program and are joining new and existing centers Resuming and increasing the number of Sleep Innovation Center events at headquarters which educates and strengthens relationships with sleep docs Planning and training has begun with Japan LifeLine Co., with first implants anticipated later in 2021 Netherlands reimbursement update – Reimbursement authorities met this month and agreed that: No longer required for individual patients to be reviewed by a multidisciplinary physician committee prior to implant, thereby removing a significant patient access bottleneck. Existing AHI limit of 20-50 will be lifted, and patients with AHI from 15-65 are now eligible Restriction on opening new centers also lifted, with an expectation of additional centers being trained by the end of the year (currently only two centers in the Netherlands). 5
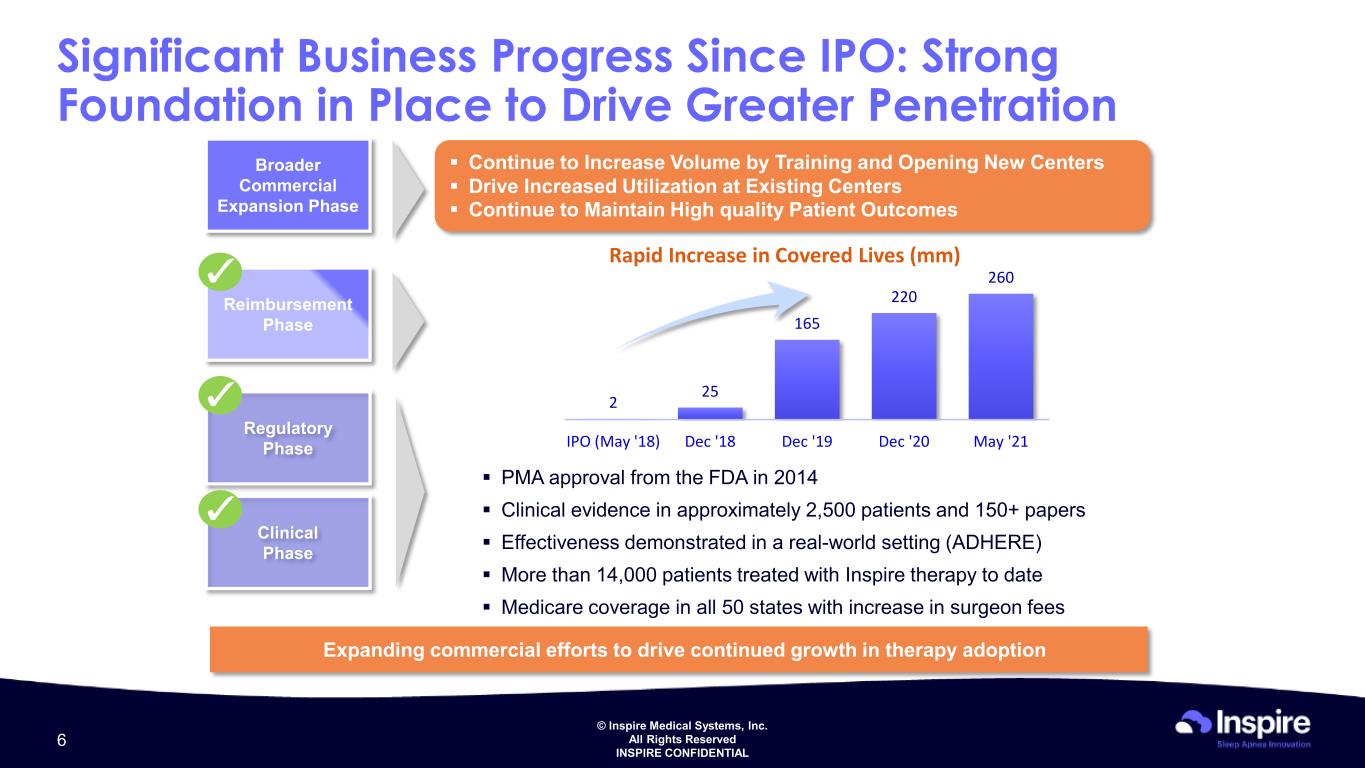
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Significant Business Progress Since IPO: Strong Foundation in Place to Drive Greater Penetration Clinical Phase Regulatory Phase Reimbursement Phase 1 Broader Commercial Expansion Phase PMA approval from the FDA in 2014 Clinical evidence in approximately 2,500 patients and 150+ papers Effectiveness demonstrated in a real-world setting (ADHERE) More than 14,000 patients treated with Inspire therapy to date Medicare coverage in all 50 states with increase in surgeon fees Rapid Increase in Covered Lives (mm) Expanding commercial efforts to drive continued growth in therapy adoption Continue to Increase Volume by Training and Opening New Centers Drive Increased Utilization at Existing Centers Continue to Maintain High quality Patient Outcomes 2 25 165 220 260 IPO (May '18) Dec '18 Dec '19 Dec '20 May '21 6
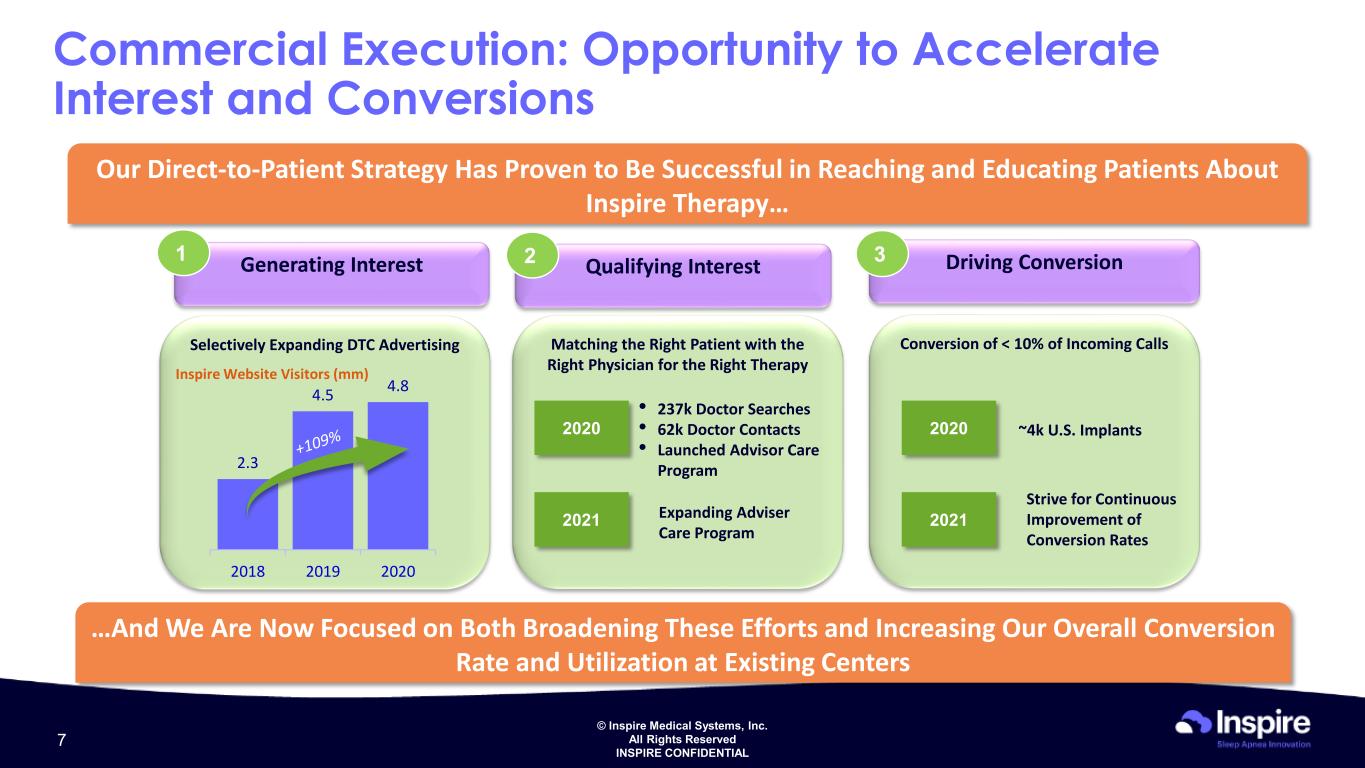
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Qualifying Interest Driving ConversionGenerating Interest Selectively Expanding DTC Advertising Commercial Execution: Opportunity to Accelerate Interest and Conversions Our Direct-to-Patient Strategy Has Proven to Be Successful in Reaching and Educating Patients About Inspire Therapy… Inspire Website Visitors (mm) …And We Are Now Focused on Both Broadening These Efforts and Increasing Our Overall Conversion Rate and Utilization at Existing Centers 1 Matching the Right Patient with the Right Physician for the Right Therapy Conversion of < 10% of Incoming Calls 2 3 • 237k Doctor Searches • 62k Doctor Contacts • Launched Advisor Care Program 2020 2021 Expanding Adviser Care Program ~4k U.S. Implants2020 2021 Strive for Continuous Improvement of Conversion Rates 2.3 4.5 4.8 2018 2019 2020 7
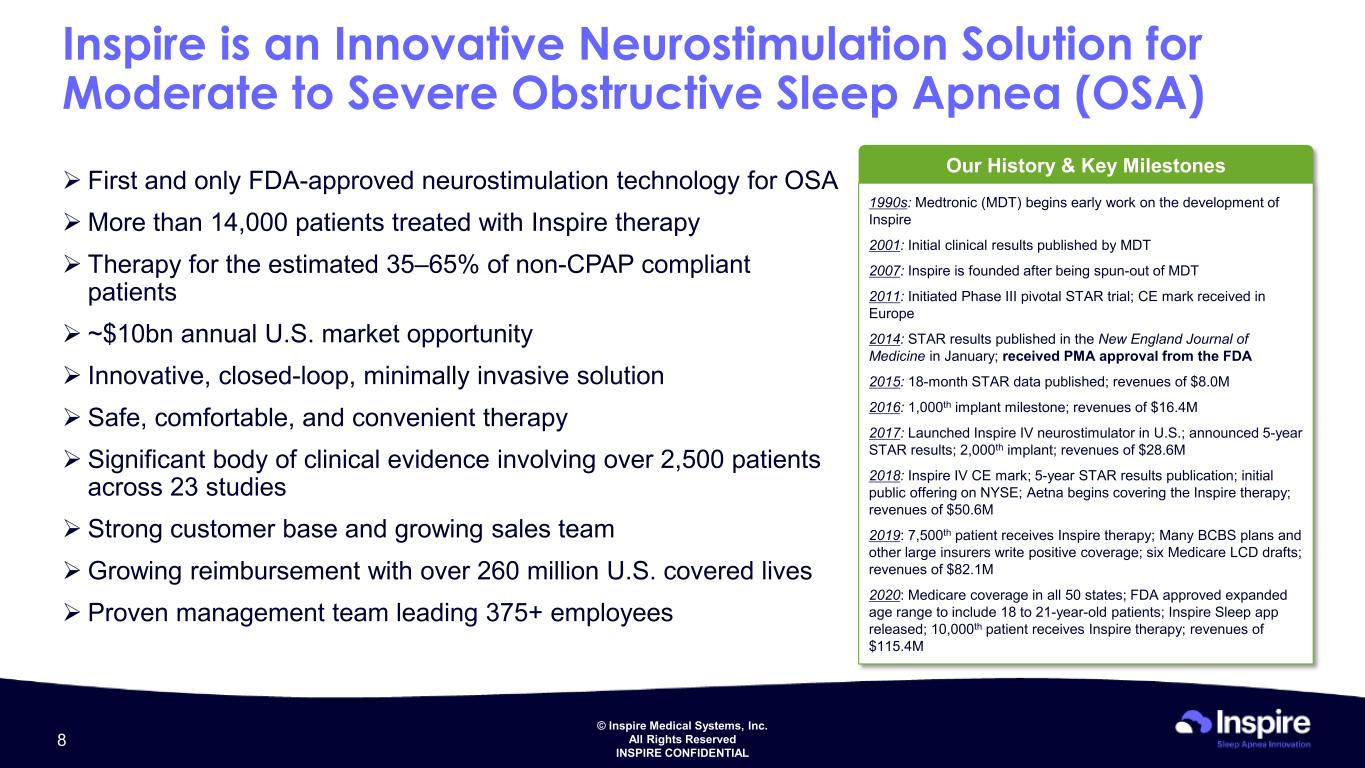
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Inspire is an Innovative Neurostimulation Solution for Moderate to Severe Obstructive Sleep Apnea (OSA) First and only FDA-approved neurostimulation technology for OSA More than 14,000 patients treated with Inspire therapy Therapy for the estimated 35–65% of non-CPAP compliant patients ~$10bn annual U.S. market opportunity Innovative, closed-loop, minimally invasive solution Safe, comfortable, and convenient therapy Significant body of clinical evidence involving over 2,500 patients across 23 studies Strong customer base and growing sales team Growing reimbursement with over 260 million U.S. covered lives Proven management team leading 375+ employees Our History & Key Milestones 1990s: Medtronic (MDT) begins early work on the development of Inspire 2001: Initial clinical results published by MDT 2007: Inspire is founded after being spun-out of MDT 2011: Initiated Phase III pivotal STAR trial; CE mark received in Europe 2014: STAR results published in the New England Journal of Medicine in January; received PMA approval from the FDA 2015: 18-month STAR data published; revenues of $8.0M 2016: 1,000th implant milestone; revenues of $16.4M 2017: Launched Inspire IV neurostimulator in U.S.; announced 5-year STAR results; 2,000th implant; revenues of $28.6M 2018: Inspire IV CE mark; 5-year STAR results publication; initial public offering on NYSE; Aetna begins covering the Inspire therapy; revenues of $50.6M 2019: 7,500th patient receives Inspire therapy; Many BCBS plans and other large insurers write positive coverage; six Medicare LCD drafts; revenues of $82.1M 2020: Medicare coverage in all 50 states; FDA approved expanded age range to include 18 to 21-year-old patients; Inspire Sleep app released; 10,000th patient receives Inspire therapy; revenues of $115.4M 8

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Strong Management Team Tim Herbert President, CEO & Founder 30+ Years of Experience Rick Buchholz Chief Financial Officer 25+ Years of Experience Randy Ban Chief Commercial Officer 25+ Years of Experience Phil Ebeling Chief Operating Officer 20+ Years of Experience 9 Bryan Phillips SVP, General Counsel & Chief Compliance Officer 20+ Years of Experience
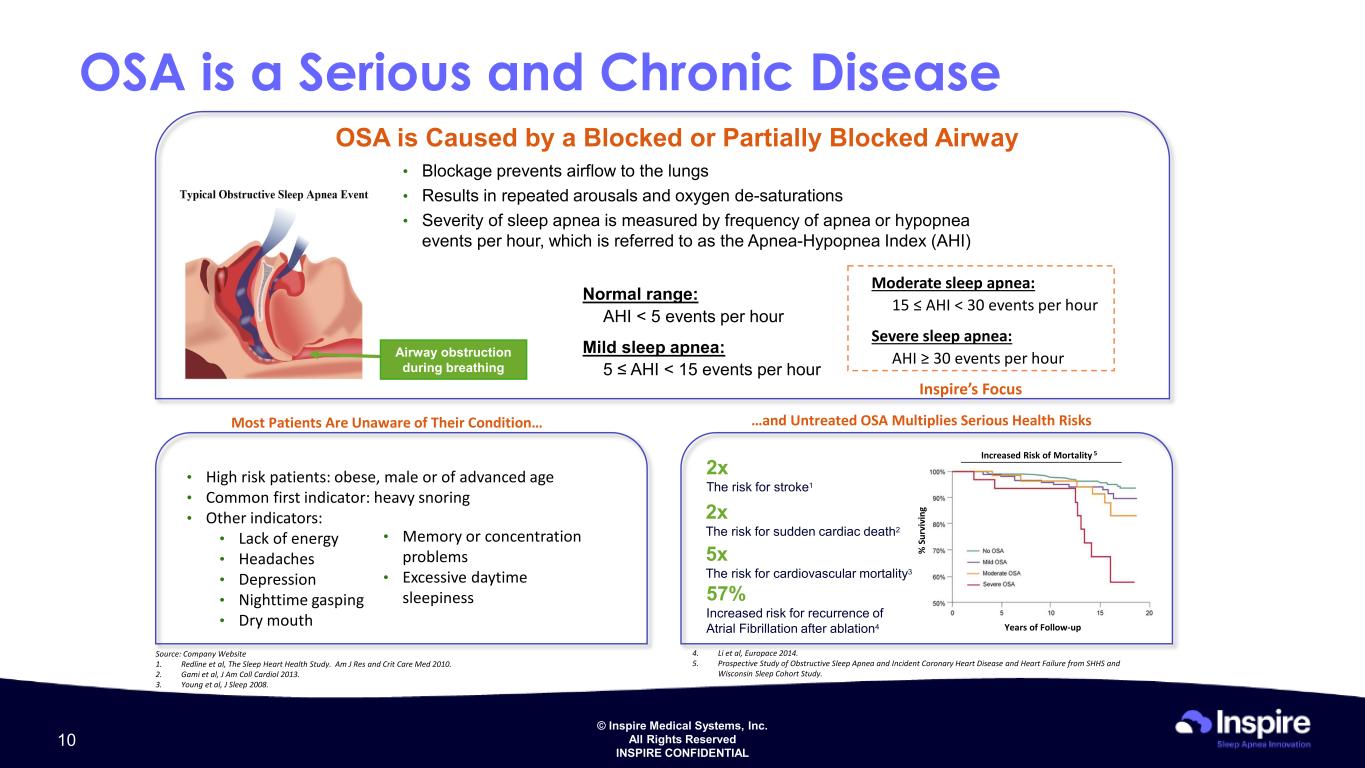
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL OSA is a Serious and Chronic Disease OSA is Caused by a Blocked or Partially Blocked Airway • Blockage prevents airflow to the lungs • Results in repeated arousals and oxygen de-saturations • Severity of sleep apnea is measured by frequency of apnea or hypopnea events per hour, which is referred to as the Apnea-Hypopnea Index (AHI) Airway obstruction during breathing Normal range: AHI < 5 events per hour Mild sleep apnea: 5 ≤ AHI < 15 events per hour Moderate sleep apnea: 15 ≤ AHI < 30 events per hour Severe sleep apnea: AHI ≥ 30 events per hour Inspire’s Focus Most Patients Are Unaware of Their Condition… …and Untreated OSA Multiplies Serious Health Risks • High risk patients: obese, male or of advanced age • Common first indicator: heavy snoring • Other indicators: • Lack of energy • Headaches • Depression • Nighttime gasping • Dry mouth • Memory or concentration problems • Excessive daytime sleepiness Source: Company Website 1. Redline et al, The Sleep Heart Health Study. Am J Res and Crit Care Med 2010. 2. Gami et al, J Am Coll Cardiol 2013. 3. Young et al, J Sleep 2008. 2x The risk for stroke1 2x The risk for sudden cardiac death2 57% Increased risk for recurrence of Atrial Fibrillation after ablation4 Years of Follow-up % S ur vi vi ng Increased Risk of Mortality 5 4. Li et al, Europace 2014. 5. Prospective Study of Obstructive Sleep Apnea and Incident Coronary Heart Disease and Heart Failure from SHHS and Wisconsin Sleep Cohort Study. 5x The risk for cardiovascular mortality3 10
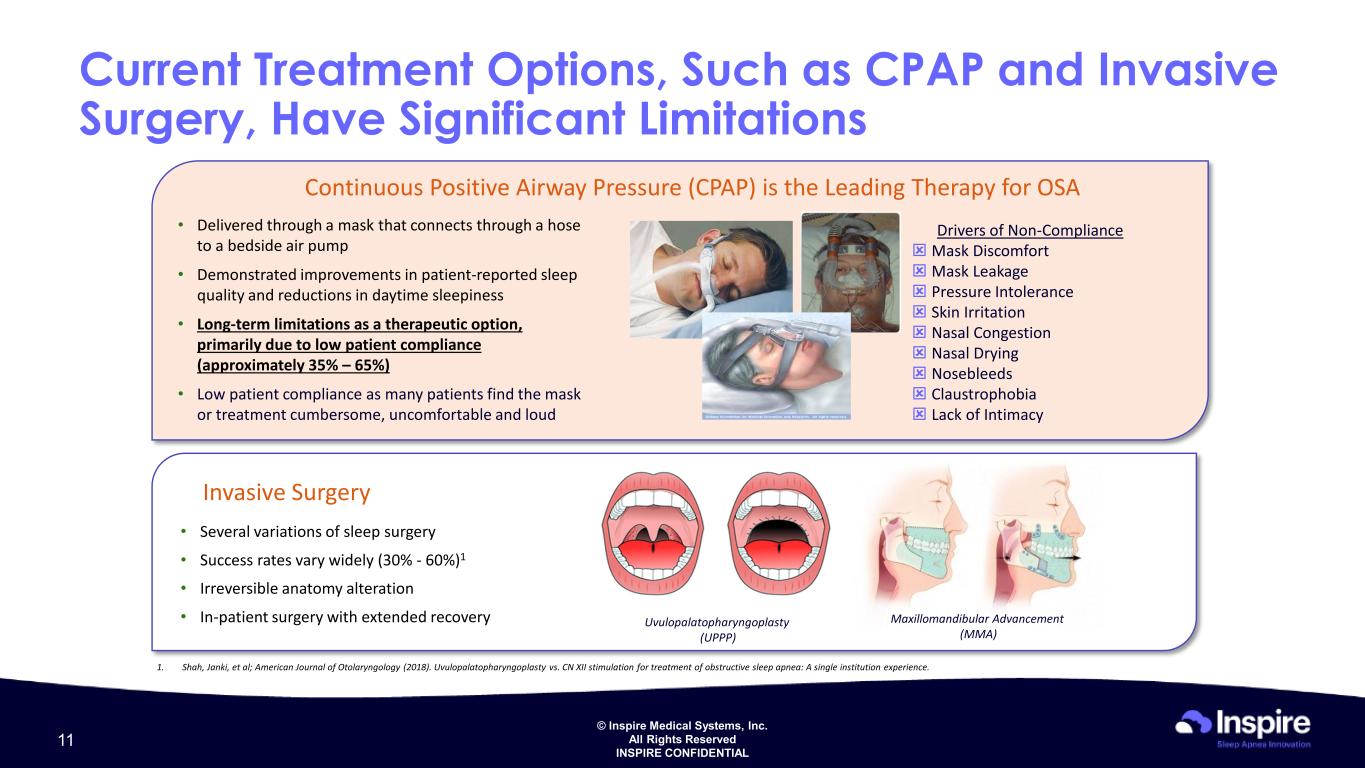
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Current Treatment Options, Such as CPAP and Invasive Surgery, Have Significant Limitations Ina Uvulopalatopharyngoplasty (UPPP) Maxillomandibular Advancement (MMA) • Several variations of sleep surgery • Success rates vary widely (30% - 60%)1 • Irreversible anatomy alteration • In-patient surgery with extended recovery Invasive Surgery Ina Continuous Positive Airway Pressure (CPAP) is the Leading Therapy for OSA 1. Shah, Janki, et al; American Journal of Otolaryngology (2018). Uvulopalatopharyngoplasty vs. CN XII stimulation for treatment of obstructive sleep apnea: A single institution experience. Drivers of Non-Compliance Mask Discomfort Mask Leakage Pressure Intolerance Skin Irritation Nasal Congestion Nasal Drying Nosebleeds Claustrophobia Lack of Intimacy • Delivered through a mask that connects through a hose to a bedside air pump • Demonstrated improvements in patient-reported sleep quality and reductions in daytime sleepiness • Long-term limitations as a therapeutic option, primarily due to low patient compliance (approximately 35% – 65%) • Low patient compliance as many patients find the mask or treatment cumbersome, uncomfortable and loud 11
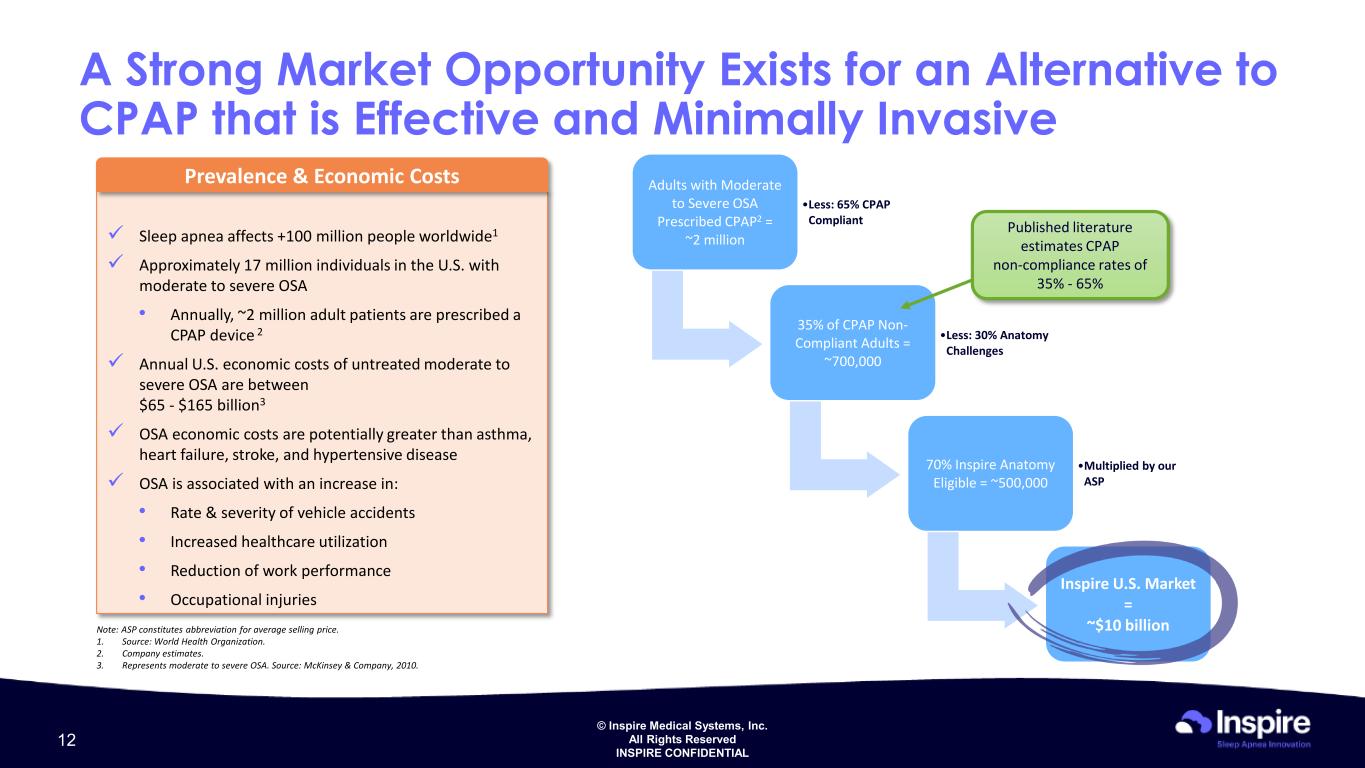
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL A Strong Market Opportunity Exists for an Alternative to CPAP that is Effective and Minimally Invasive Sleep apnea affects +100 million people worldwide1 Approximately 17 million individuals in the U.S. with moderate to severe OSA • Annually, ~2 million adult patients are prescribed a CPAP device 2 Annual U.S. economic costs of untreated moderate to severe OSA are between $65 - $165 billion3 OSA economic costs are potentially greater than asthma, heart failure, stroke, and hypertensive disease OSA is associated with an increase in: • Rate & severity of vehicle accidents • Increased healthcare utilization • Reduction of work performance • Occupational injuries Prevalence & Economic Costs Note: ASP constitutes abbreviation for average selling price. 1. Source: World Health Organization. 2. Company estimates. 3. Represents moderate to severe OSA. Source: McKinsey & Company, 2010. Adults with Moderate to Severe OSA Prescribed CPAP2 = ~2 million •Less: 65% CPAP Compliant 35% of CPAP Non- Compliant Adults = ~700,000 •Less: 30% Anatomy Challenges 70% Inspire Anatomy Eligible = ~500,000 •Multiplied by our ASP Inspire U.S. Market = ~$10 billion Published literature estimates CPAP non-compliance rates of 35% - 65% 12
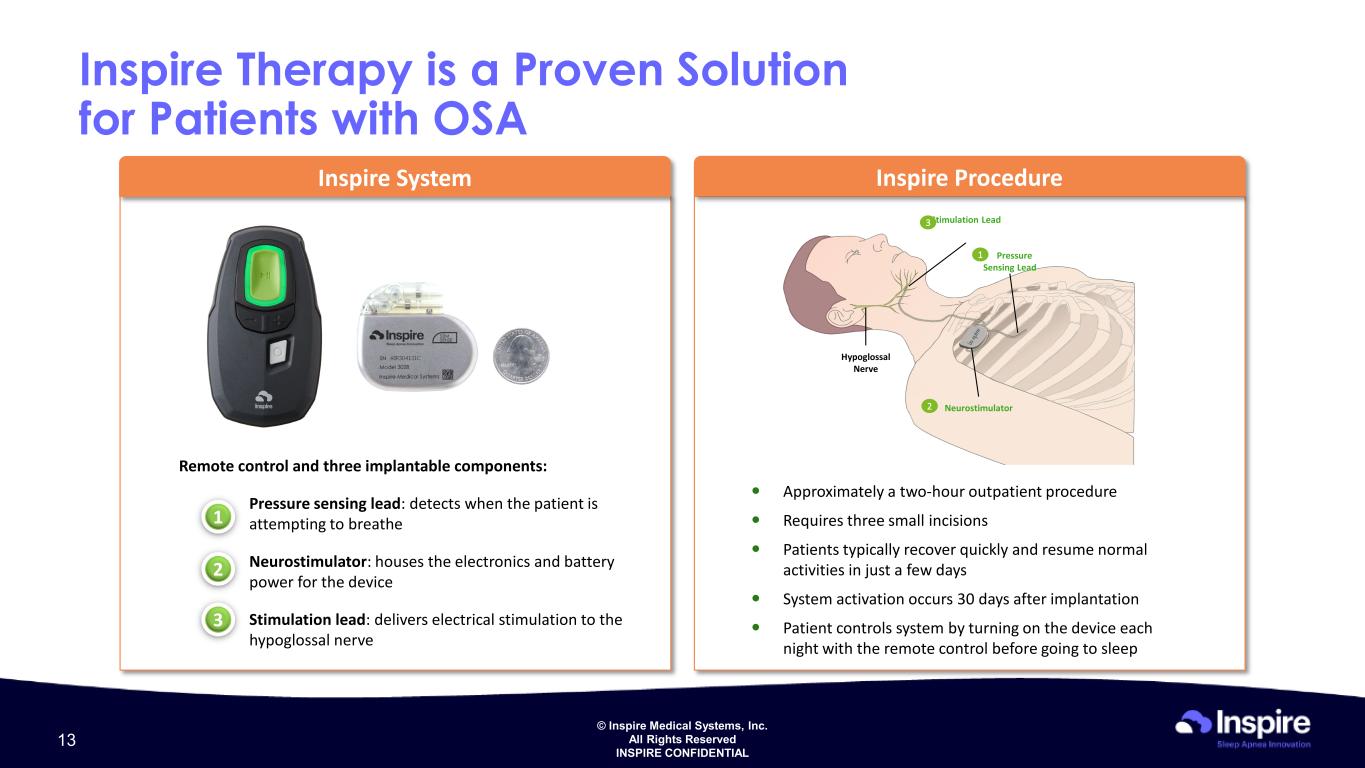
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Inspire Therapy is a Proven Solution for Patients with OSA Inspire System 1 2 3 Inspire Procedure Approximately a two-hour outpatient procedure Requires three small incisions Patients typically recover quickly and resume normal activities in just a few days System activation occurs 30 days after implantation Patient controls system by turning on the device each night with the remote control before going to sleep 2 1 Hypoglossal Nerve Neurostimulator Stimulation Lead Pressure Sensing Lead 3 Remote control and three implantable components: Pressure sensing lead: detects when the patient is attempting to breathe Neurostimulator: houses the electronics and battery power for the device Stimulation lead: delivers electrical stimulation to the hypoglossal nerve 13
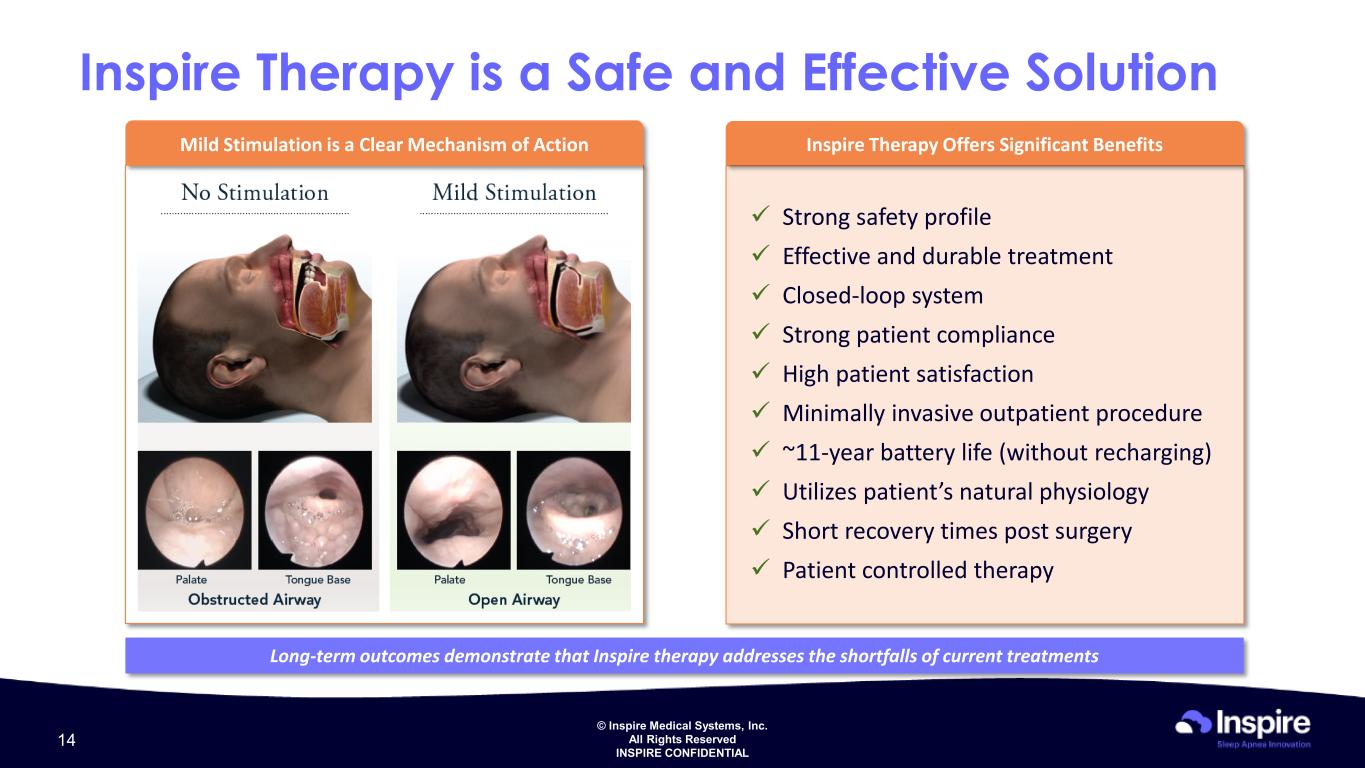
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Inspire Therapy is a Safe and Effective Solution Mild Stimulation is a Clear Mechanism of Action Strong safety profile Effective and durable treatment Closed-loop system Strong patient compliance High patient satisfaction Minimally invasive outpatient procedure ~11-year battery life (without recharging) Utilizes patient’s natural physiology Short recovery times post surgery Patient controlled therapy Inspire Therapy Offers Significant Benefits Long-term outcomes demonstrate that Inspire therapy addresses the shortfalls of current treatments 14
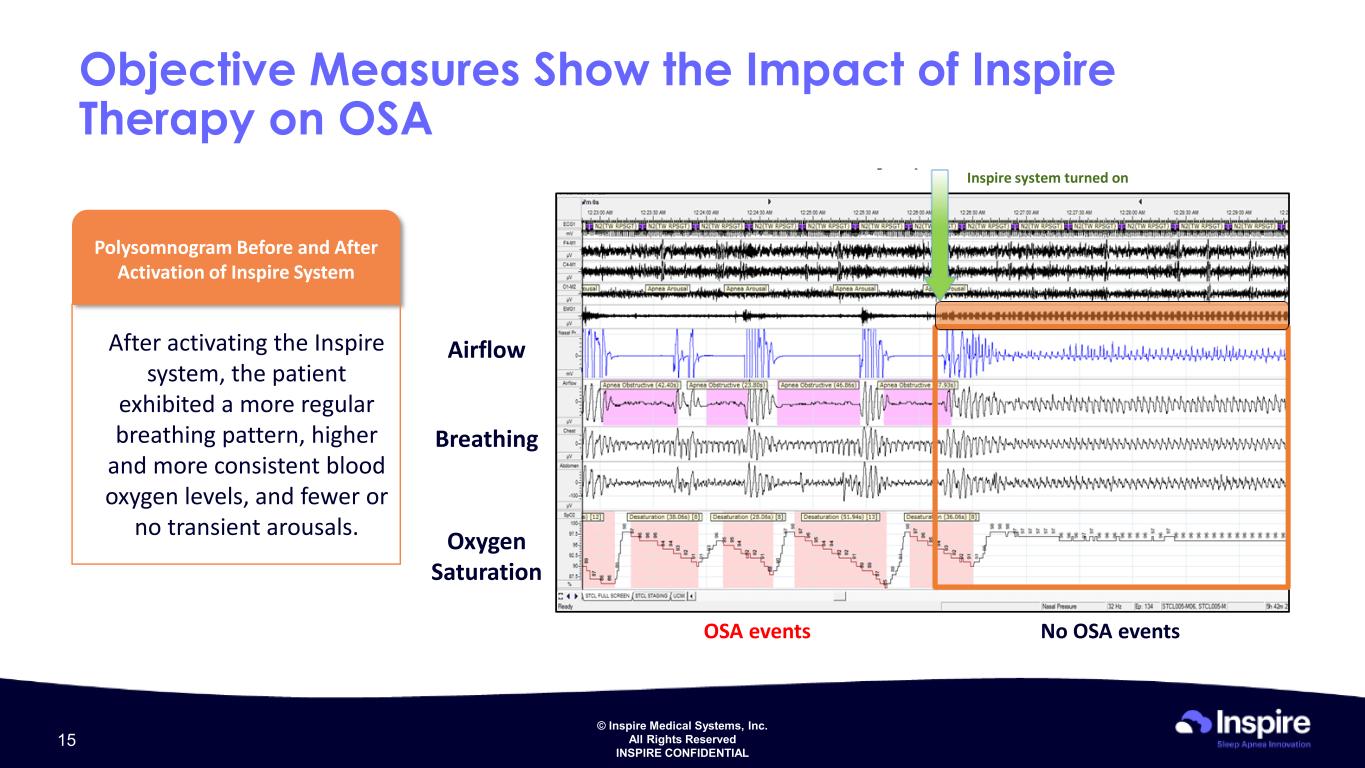
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Objective Measures Show the Impact of Inspire Therapy on OSA Inspire system turned on Airflow Breathing Oxygen Saturation No OSA eventsOSA events Polysomnogram Before and After Activation of Inspire System After activating the Inspire system, the patient exhibited a more regular breathing pattern, higher and more consistent blood oxygen levels, and fewer or no transient arousals. 15

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Clinical Evidence 16

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Significant Body of Clinical Evidence Evaluating Inspire in more than 2,500 Patients, in 150+ papers 1. Due to the inclusion of certain patients in multiple studies, some studies are not shown in the table because they do not add any incremental patients to the overall total. 2. Includes Thomas Jefferson University Hospital (TJUH) & University of Pittsburgh Medical Center (UPMC); University Hospitals – Cleveland; Non-Academic Hospital in San Diego; and University of Pennsylvania. Clinical Studies Patients Evaluated Co m pa ny S po ns or ed Stimulation Therapy for Apnea Reduction (STAR) 126 German Post-Market Study 60 ADHERE Patient Registry 1,017 Pediatric / Down Syndrome 26 In de pe nd en t Inspire vs. traditional sleep surgery (Cleveland Clinic, Thomas Jefferson, UPenn) 248 Independent Studies in Single Centers 2 150 Independent Studies of Specific Populations 418 German and French Experience (Munich, Lubeck, Bordeaux) 143 Total Patients Evaluated Above 2,188 17
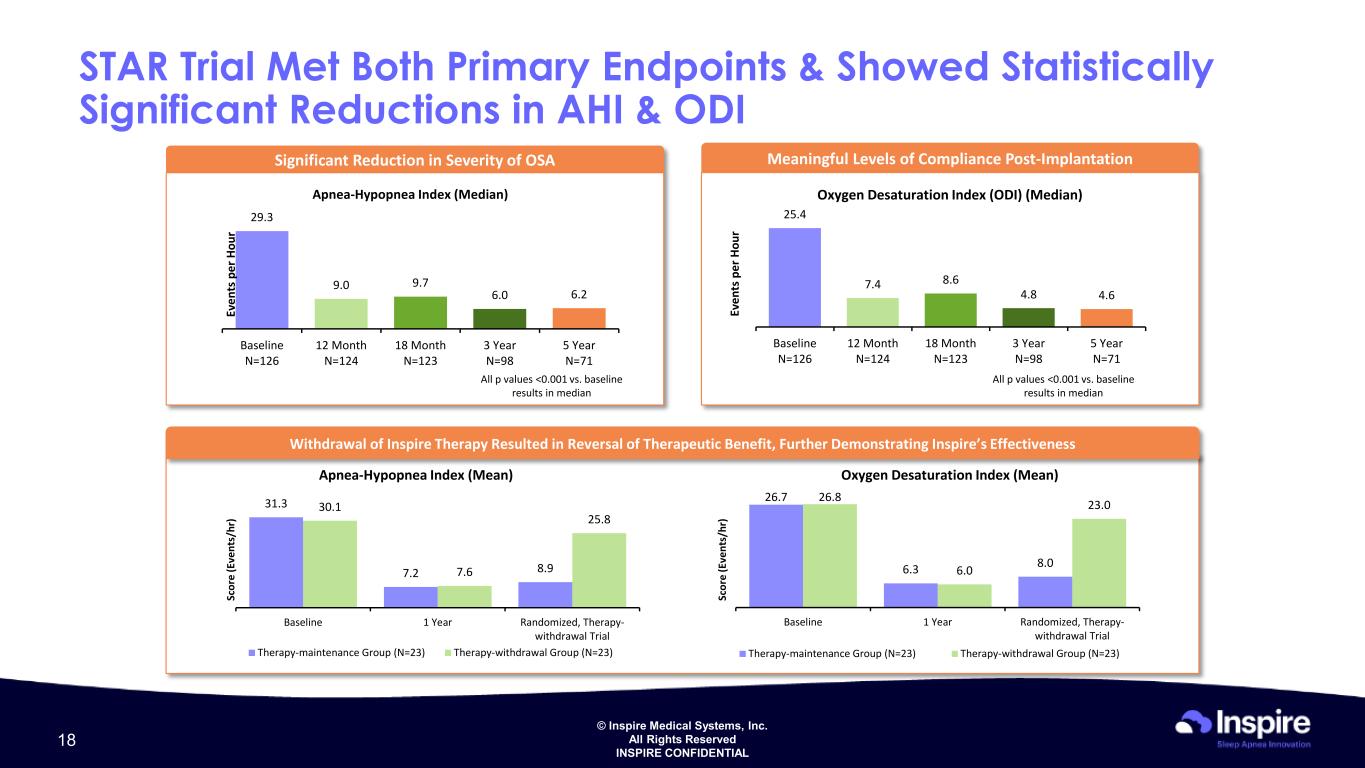
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL STAR Trial Met Both Primary Endpoints & Showed Statistically Significant Reductions in AHI & ODI Significant Reduction in Severity of OSA Apnea-Hypopnea Index (Median) 29.3 9.0 9.7 6.0 6.2 Baseline N=126 12 Month N=124 18 Month N=123 3 Year N=98 5 Year N=71 All p values <0.001 vs. baseline results in median Ev en ts p er H ou r Meaningful Levels of Compliance Post-Implantation Oxygen Desaturation Index (ODI) (Median) 25.4 7.4 8.6 4.8 4.6 Baseline N=126 12 Month N=124 18 Month N=123 3 Year N=98 5 Year N=71 All p values <0.001 vs. baseline results in median Ev en ts p er H ou r Withdrawal of Inspire Therapy Resulted in Reversal of Therapeutic Benefit, Further Demonstrating Inspire’s Effectiveness 31.3 7.2 8.9 30.1 7.6 25.8 Baseline 1 Year Randomized, Therapy- withdrawal Trial Therapy-maintenance Group (N=23) Therapy-withdrawal Group (N=23) Sc or e (E ve nt s/ hr ) 26.7 6.3 8.0 26.8 6.0 23.0 Baseline 1 Year Randomized, Therapy- withdrawal Trial Therapy-maintenance Group (N=23) Therapy-withdrawal Group (N=23) Sc or e (E ve nt s/ hr ) Apnea-Hypopnea Index (Mean) Oxygen Desaturation Index (Mean) 18
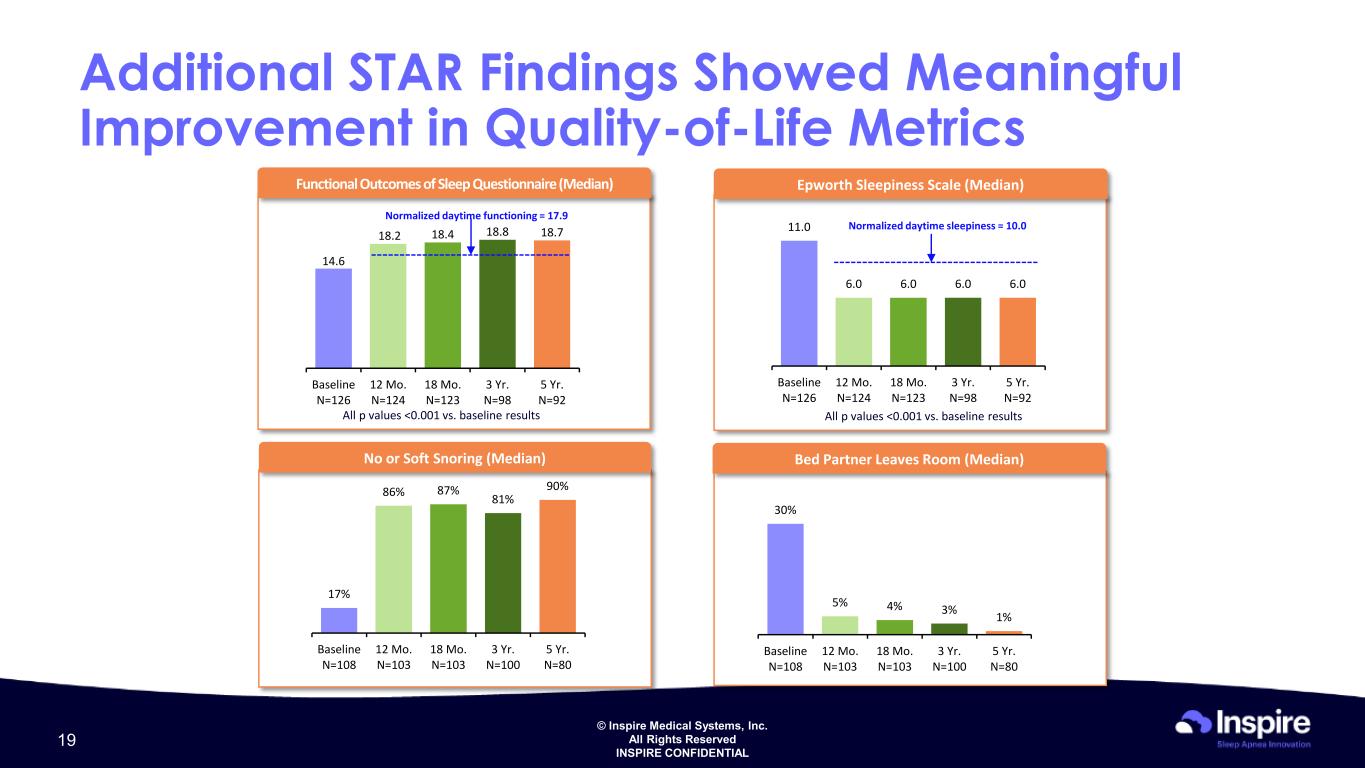
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL 30% 5% 4% 3% 1% Baseline N=108 12 Mo. N=103 18 Mo. N=103 3 Yr. N=100 5 Yr. N=80 17% 86% 87% 81% 90% Baseline N=108 12 Mo. N=103 18 Mo. N=103 3 Yr. N=100 5 Yr. N=80 Additional STAR Findings Showed Meaningful Improvement in Quality-of-Life Metrics Functional Outcomes of Sleep Questionnaire (Median) 14.6 18.2 18.4 18.8 18.7 Baseline N=126 12 Mo. N=124 18 Mo. N=123 3 Yr. N=98 5 Yr. N=92 All p values <0.001 vs. baseline results 11.0 6.0 6.0 6.0 6.0 Baseline N=126 12 Mo. N=124 18 Mo. N=123 3 Yr. N=98 5 Yr. N=92 Epworth Sleepiness Scale (Median) Normalized daytime sleepiness = 10.0 Normalized daytime functioning = 17.9 No or Soft Snoring (Median) Bed Partner Leaves Room (Median) All p values <0.001 vs. baseline results 19
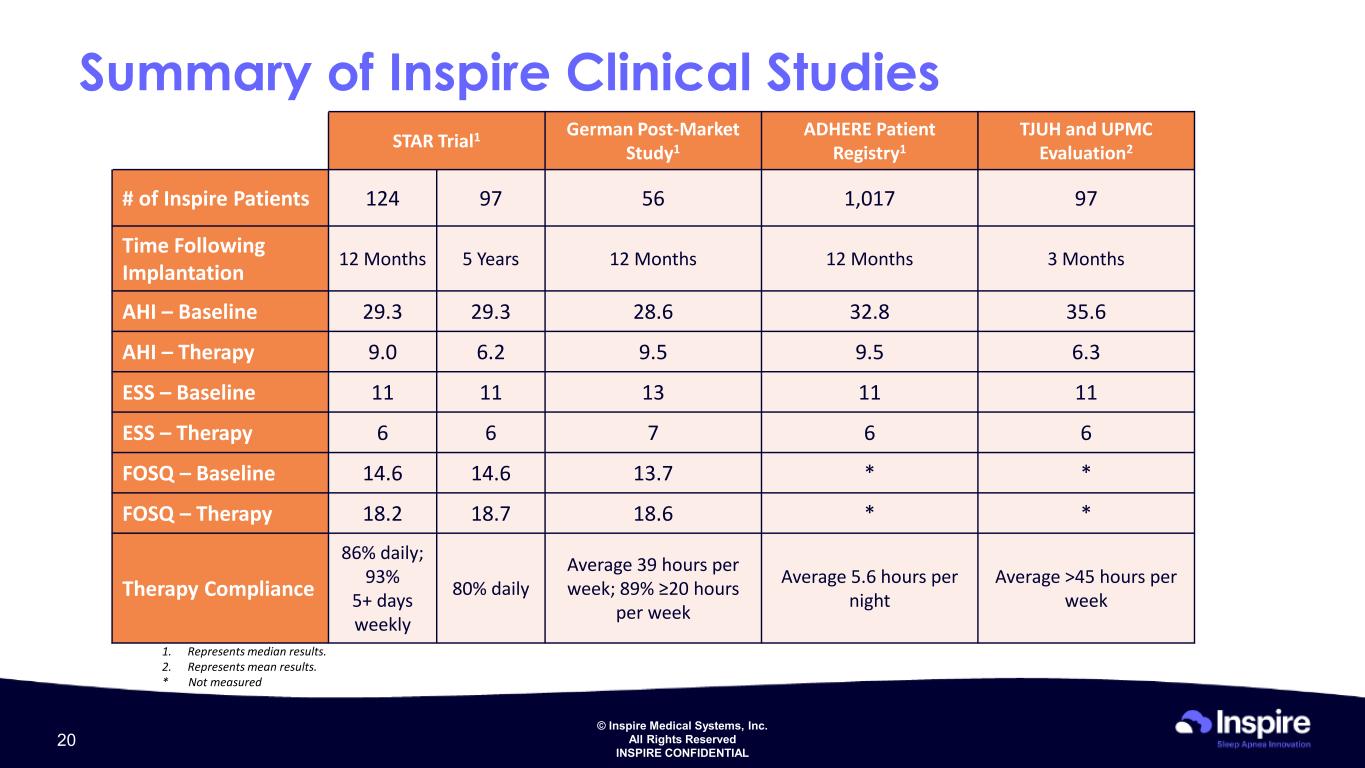
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Summary of Inspire Clinical Studies STAR Trial1 German Post-Market Study1 ADHERE Patient Registry1 TJUH and UPMC Evaluation2 # of Inspire Patients 124 97 56 1,017 97 Time Following Implantation 12 Months 5 Years 12 Months 12 Months 3 Months AHI – Baseline 29.3 29.3 28.6 32.8 35.6 AHI – Therapy 9.0 6.2 9.5 9.5 6.3 ESS – Baseline 11 11 13 11 11 ESS – Therapy 6 6 7 6 6 FOSQ – Baseline 14.6 14.6 13.7 * * FOSQ – Therapy 18.2 18.7 18.6 * * Therapy Compliance 86% daily; 93% 5+ days weekly 80% daily Average 39 hours per week; 89% ≥20 hours per week Average 5.6 hours per night Average >45 hours per week 1. Represents median results. 2. Represents mean results. * Not measured 20
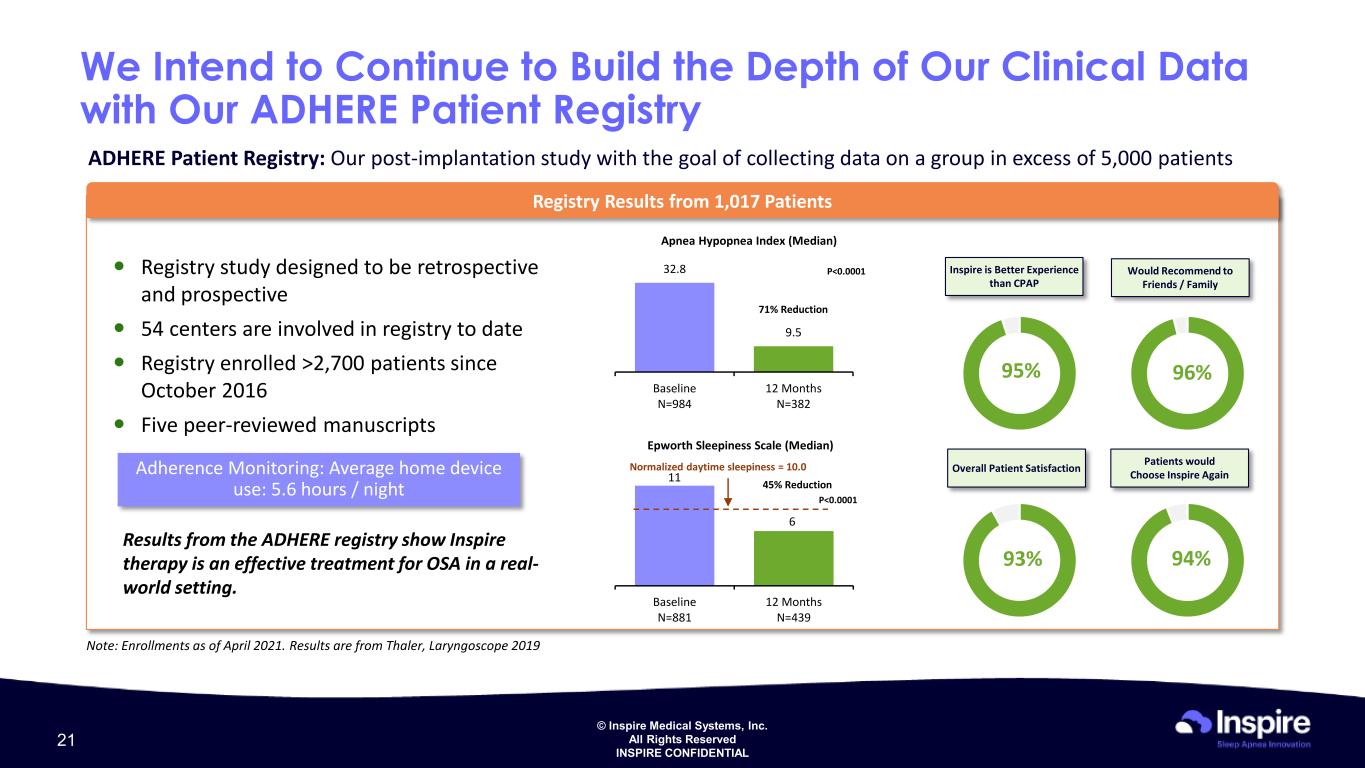
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL We Intend to Continue to Build the Depth of Our Clinical Data with Our ADHERE Patient Registry Registry Results from 1,017 Patients Registry study designed to be retrospective and prospective 54 centers are involved in registry to date Registry enrolled >2,700 patients since October 2016 Five peer-reviewed manuscripts Patients would Choose Inspire Again Apnea Hypopnea Index (Median) P<0.000132.8 9.5 Baseline N=984 12 Months N=382 71% Reduction 45% Reduction P<0.0001 Results from the ADHERE registry show Inspire therapy is an effective treatment for OSA in a real- world setting. ADHERE Patient Registry: Our post-implantation study with the goal of collecting data on a group in excess of 5,000 patients Overall Patient Satisfaction Would Recommend to Friends / Family Adherence Monitoring: Average home device use: 5.6 hours / night Epworth Sleepiness Scale (Median) 11 6 Baseline N=881 12 Months N=439 Normalized daytime sleepiness = 10.0 Note: Enrollments as of April 2021. Results are from Thaler, Laryngoscope 2019 Inspire is Better Experience than CPAP 95% 96% 93% 94% 21

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Reimbursement 22

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL AMA Grants Approval for New, Category I Procedure Codes • Hypoglossal Nerve Stim will no longer share Procedure Codes with Vagus Nerve Stim for Epilepsy • No more ‘New Technology’ Cat III codes for Inspire (T-Codes) effective January 1, 2022 • High potential that Physician payment for Inspire’s procedure will increase with new codes • ‘RUC’ survey will be performed to evaluate physician ‘work’ and expenses for our codes • Medicare makes the call on how many ‘RVUs’ will be assigned: RVU (Relative Value Units) proposal from Medicare publishes in mid-year 2021 • Hospital payment for Inspire will not change: New code maps to same Facility payment bucket (APC) as old codes for both hospital and ambulatory surgical centers (ASC) • Hospital: 2021 National Average Medicare payment of $29,455; commercial insurance is ≈ 1.4x Medicare • ASC: 2021 National Average Medicare payment of $24,299; commercial insurance is ≈1.9x Medicare The AAO-HNS physician society applied for a comprehensive, dedicated new CPT Code set for the hypoglossal nerve stimulation implant. These new codes will have payment rates measured only for Inspire and not blended, becoming effective January 1, 2022 23
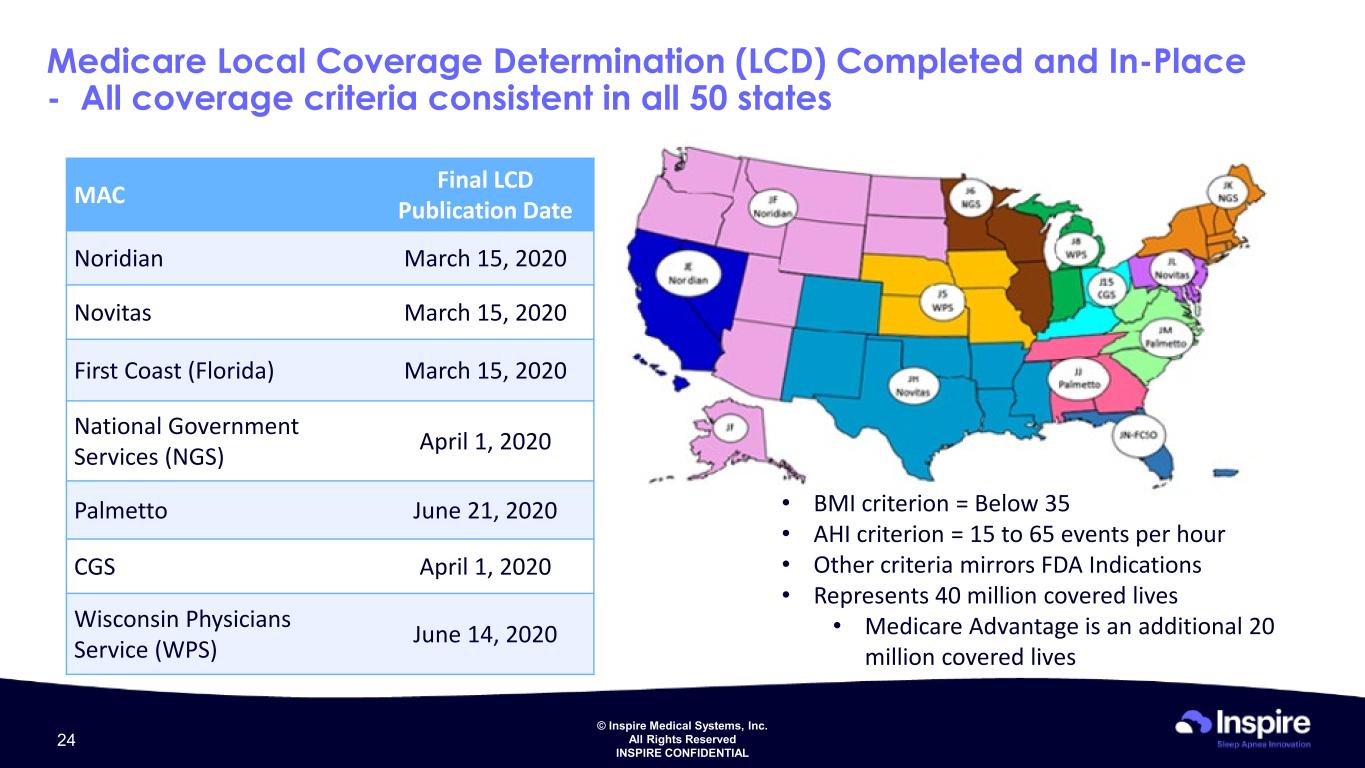
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Medicare Local Coverage Determination (LCD) Completed and In-Place - All coverage criteria consistent in all 50 states MAC Final LCD Publication Date Noridian March 15, 2020 Novitas March 15, 2020 First Coast (Florida) March 15, 2020 National Government Services (NGS) April 1, 2020 Palmetto June 21, 2020 CGS April 1, 2020 Wisconsin Physicians Service (WPS) June 14, 2020 • BMI criterion = Below 35 • AHI criterion = 15 to 65 events per hour • Other criteria mirrors FDA Indications • Represents 40 million covered lives • Medicare Advantage is an additional 20 million covered lives 24

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Product Development and Digital Health 25

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Inspire Digital Health Definition Connected tools for Sleep Docs, Surgeons, and Patients that improve outcomes and reduce work. • Collaborative patient screening • Seamless, personalized care • Efficient therapy management 26

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Foundational Customer Needs Opportunities to Create Brand Preference 27 ENT Surgeon • Strong, Predictable Patient Outcomes • Speed and Ease of Implant Procedure • Strong, Predictable Patient Outcomes • Speed and Ease of Patient Management • Outcomes – Feel Better / Reduce Risk • Comfort • Convenient / Easy to use Sleep MD Sleep Apnea Patients
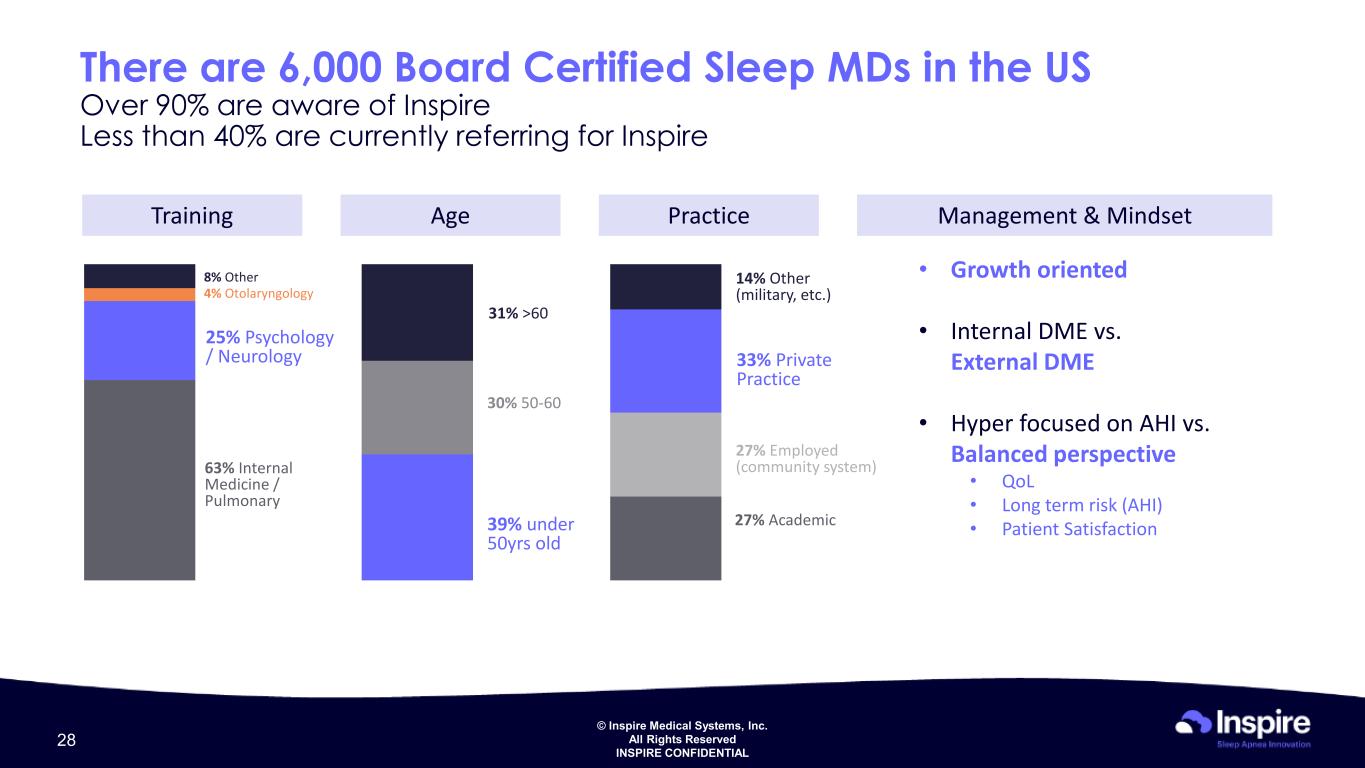
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL There are 6,000 Board Certified Sleep MDs in the US Over 90% are aware of Inspire Less than 40% are currently referring for Inspire Training Age Practice Management & Mindset 63% Internal Medicine / Pulmonary 25% Psychology / Neurology 4% Otolaryngology 8% Other 39% under 50yrs old 30% 50-60 31% >60 27% Academic 33% Private Practice 27% Employed (community system) 14% Other (military, etc.) • Growth oriented • Internal DME vs. External DME • Hyper focused on AHI vs. Balanced perspective • QoL • Long term risk (AHI) • Patient Satisfaction 28
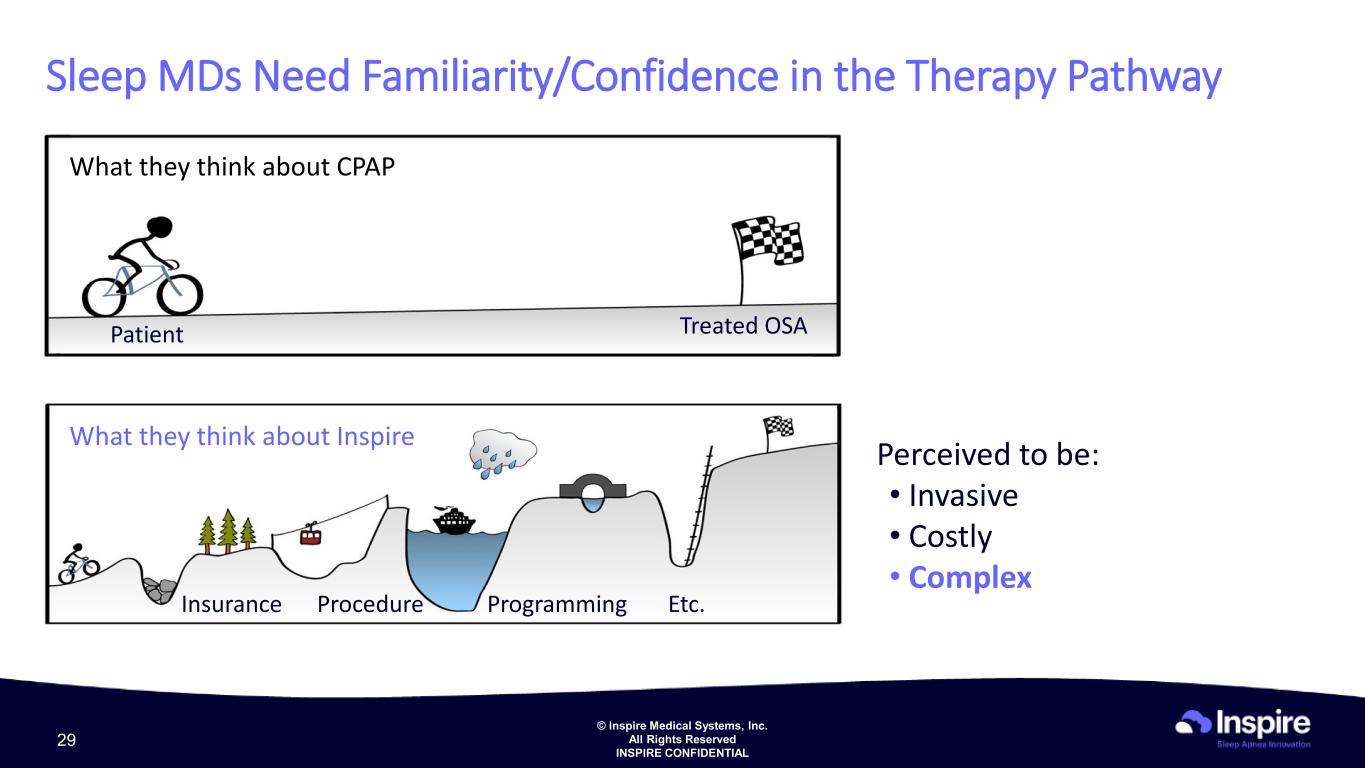
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL What they think about CPAP What they think about Inspire Patient Treated OSA Perceived to be: • Invasive • Costly • Complex Sleep MDs Need Familiarity/Confidence in the Therapy Pathway Insurance Procedure Programming Etc. 29
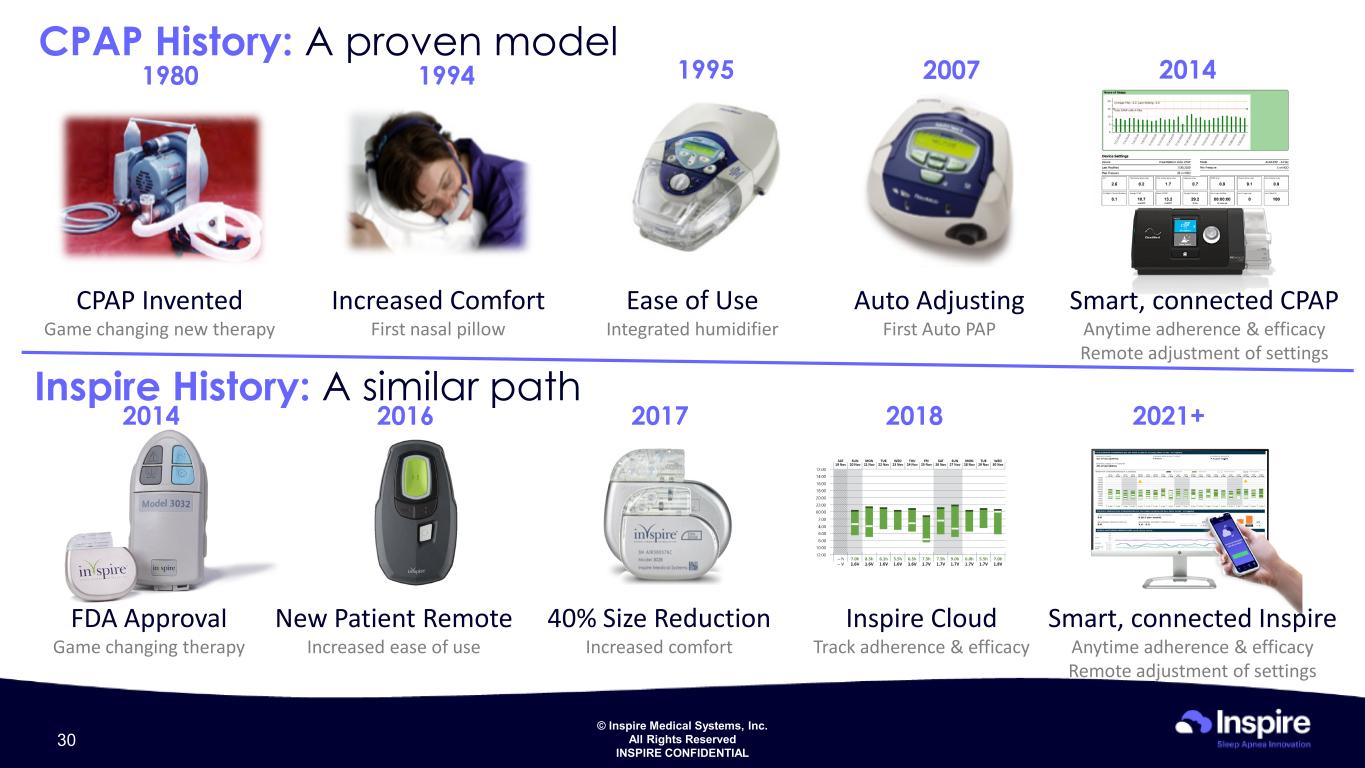
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL CPAP History: A proven model CPAP Invented Game changing new therapy Increased Comfort First nasal pillow Ease of Use Integrated humidifier Auto Adjusting First Auto PAP Smart, connected CPAP Anytime adherence & efficacy Remote adjustment of settings 2007 2014199519941980 Inspire History: A similar path FDA Approval Game changing therapy 40% Size Reduction Increased comfort New Patient Remote Increased ease of use Inspire Cloud Track adherence & efficacy 2018 2021+2016 20172014 Smart, connected Inspire Anytime adherence & efficacy Remote adjustment of settings 30
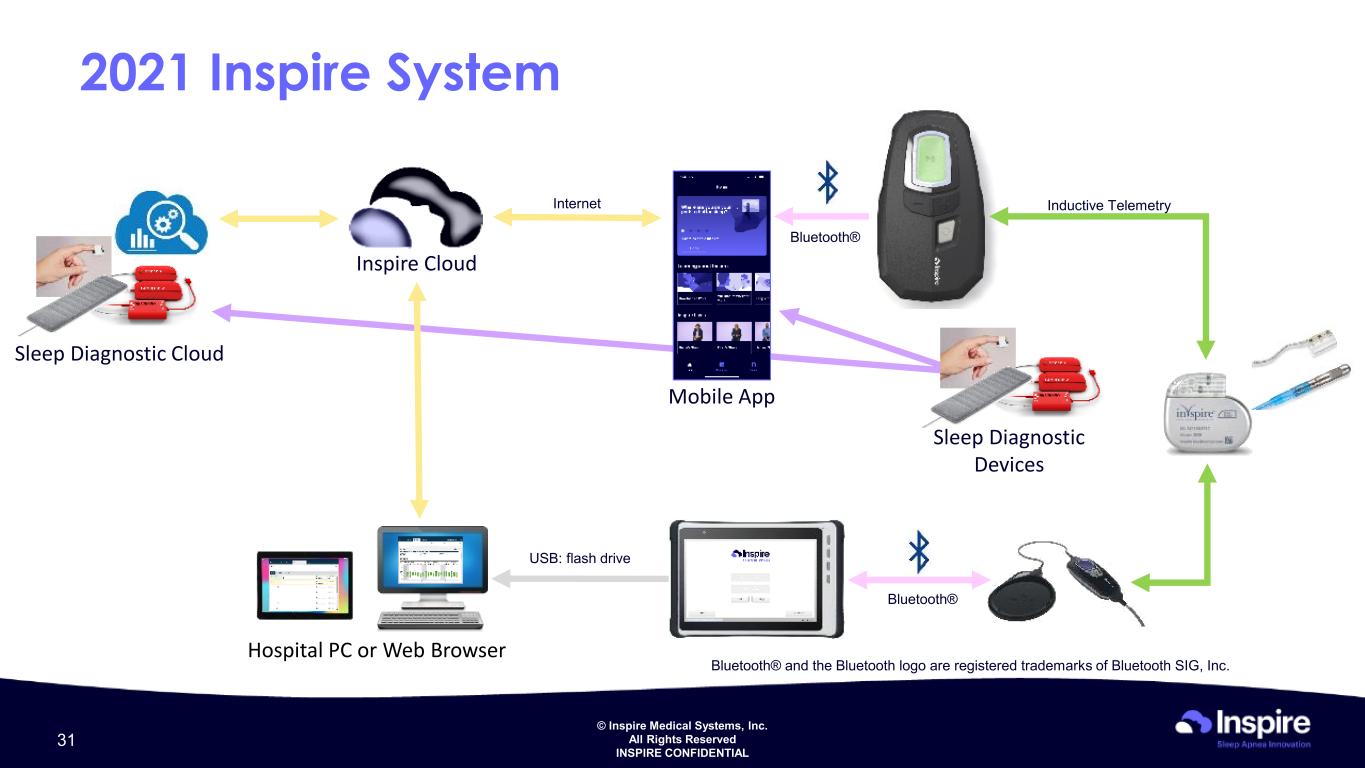
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL 2021 Inspire System Bluetooth® USB: flash drive Hospital PC or Web Browser Inductive Telemetry Bluetooth® Inspire Cloud Internet Mobile App Sleep Diagnostic Cloud Sleep Diagnostic Devices 31 Bluetooth® and the Bluetooth logo are registered trademarks of Bluetooth SIG, Inc.
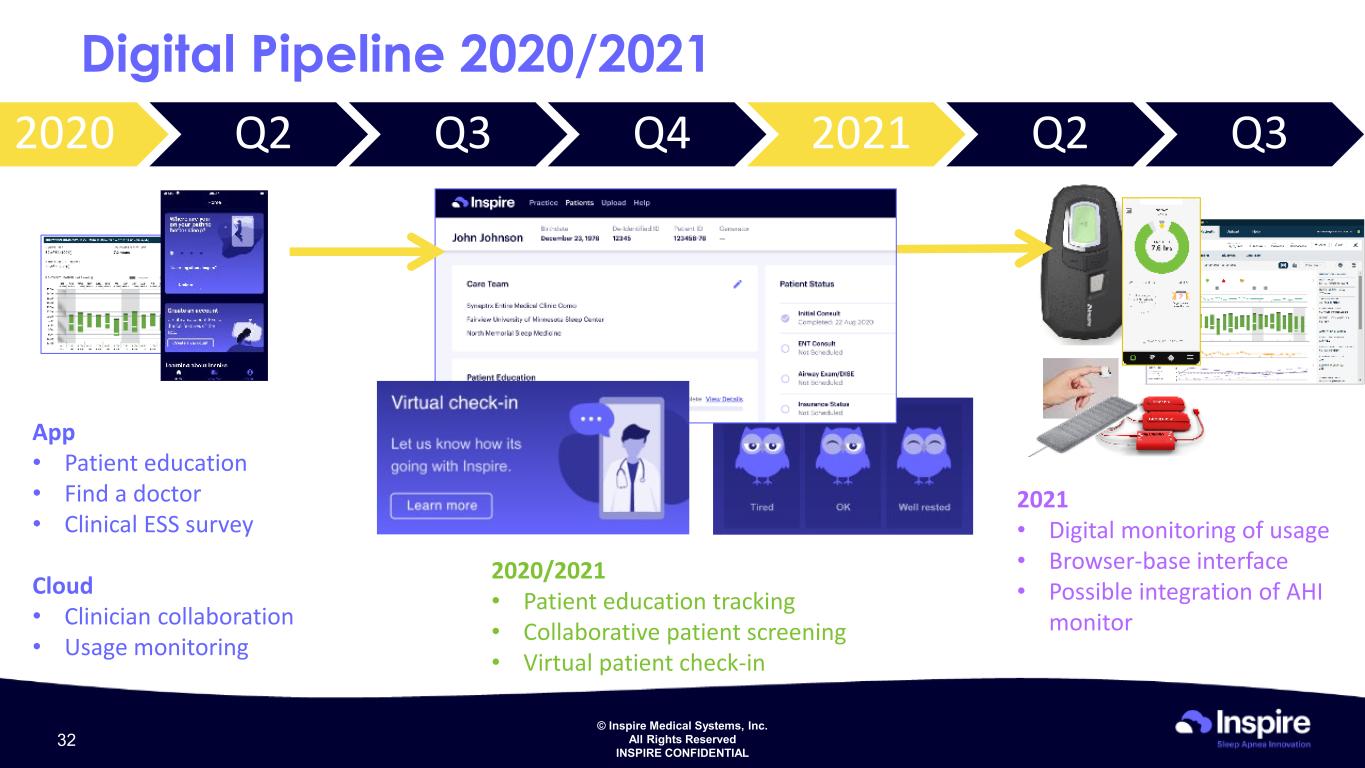
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL 2020 Q2 Q3 Q4 2021 Q2 Q3 Digital Pipeline 2020/2021 2020/2021 • Patient education tracking • Collaborative patient screening • Virtual patient check-in 2021 • Digital monitoring of usage • Browser-base interface • Possible integration of AHI monitor App • Patient education • Find a doctor • Clinical ESS survey Cloud • Clinician collaboration • Usage monitoring 32
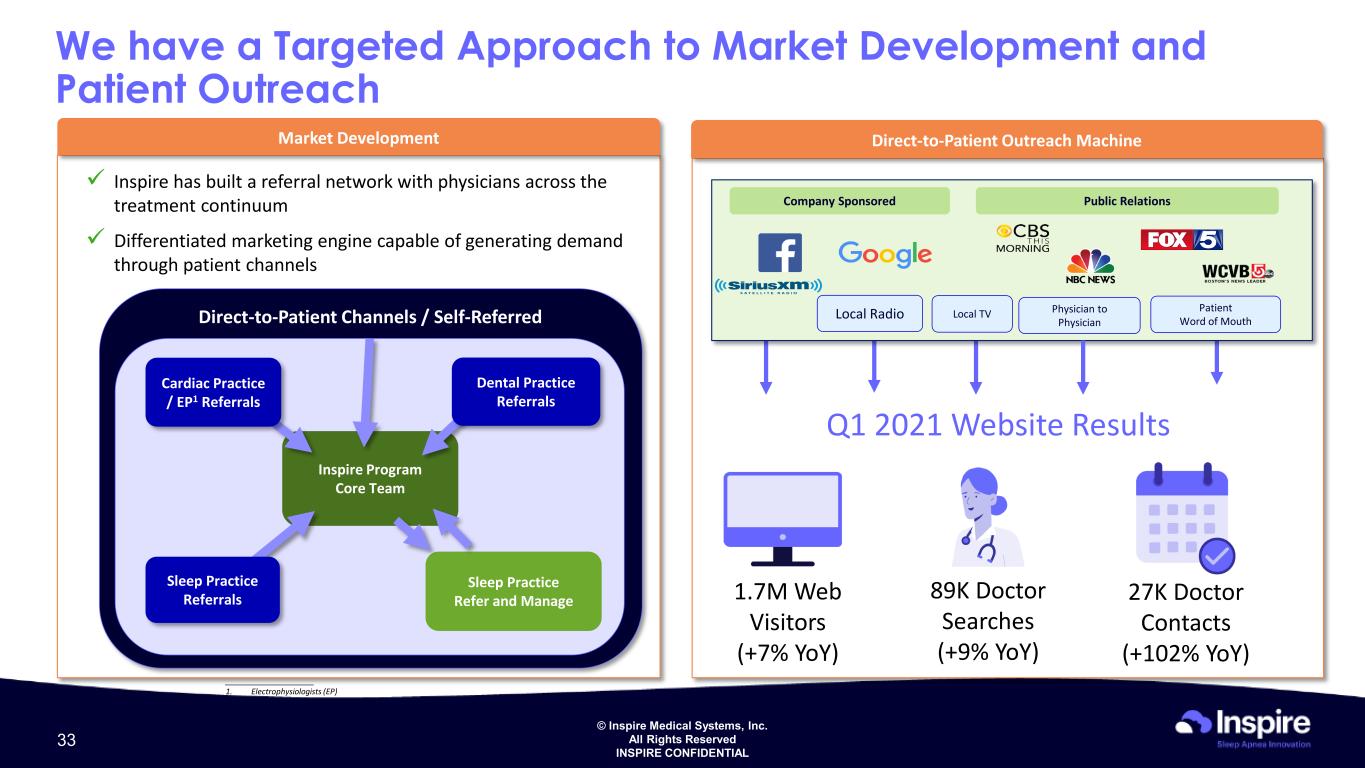
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Market Development Direct-to-Patient Channels / Self-Referred Inspire Program Core Team Sleep Practice Refer and Manage Sleep Practice Referrals Dental Practice Referrals Cardiac Practice / EP1 Referrals Direct-to-Patient Outreach Machine Inspire has built a referral network with physicians across the treatment continuum Differentiated marketing engine capable of generating demand through patient channels ____________________ 1. Electrophysiologists (EP) We have a Targeted Approach to Market Development and Patient Outreach 89K Doctor Searches (+9% YoY) 27K Doctor Contacts (+102% YoY) 1.7M Web Visitors (+7% YoY) Q1 2021 Website Results Physician to Physician Patient Word of Mouth Company Sponsored Public Relations Local Radio Local TV 33
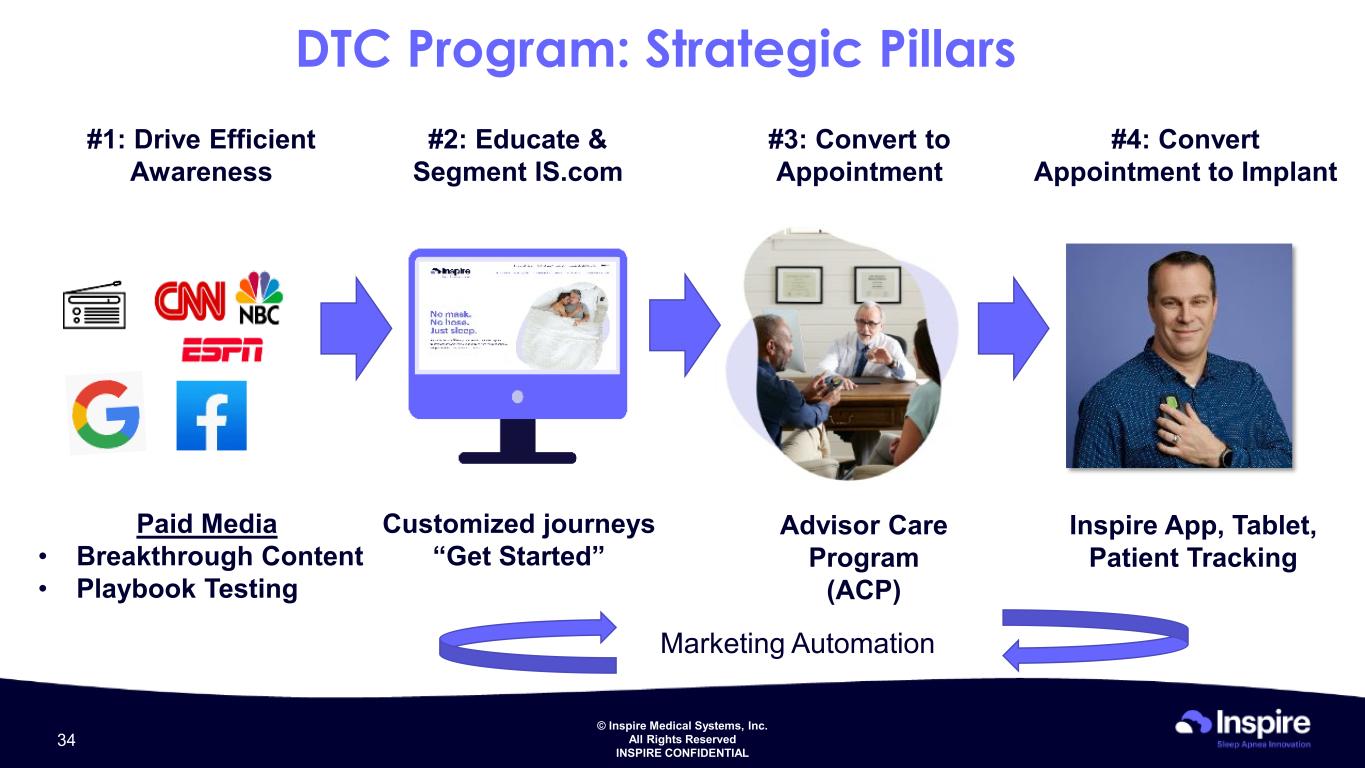
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL DTC Program: Strategic Pillars #3: Convert to Appointment #1: Drive Efficient Awareness #2: Educate & Segment IS.com #4: Convert Appointment to Implant Paid Media • Breakthrough Content • Playbook Testing Customized journeys “Get Started” Advisor Care Program (ACP) Marketing Automation Inspire App, Tablet, Patient Tracking 34

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Advisor Care Program (ACP) Connecting Qualified Patients Directly to our Centers 35 Patient calls number on MD Listing Call connected to Inspire Advisor Inspire Advisor calls clinic; Connects patient with LIVE scheduler Turning Patient Interest into Actionable Steps Toward an Inspire Implant
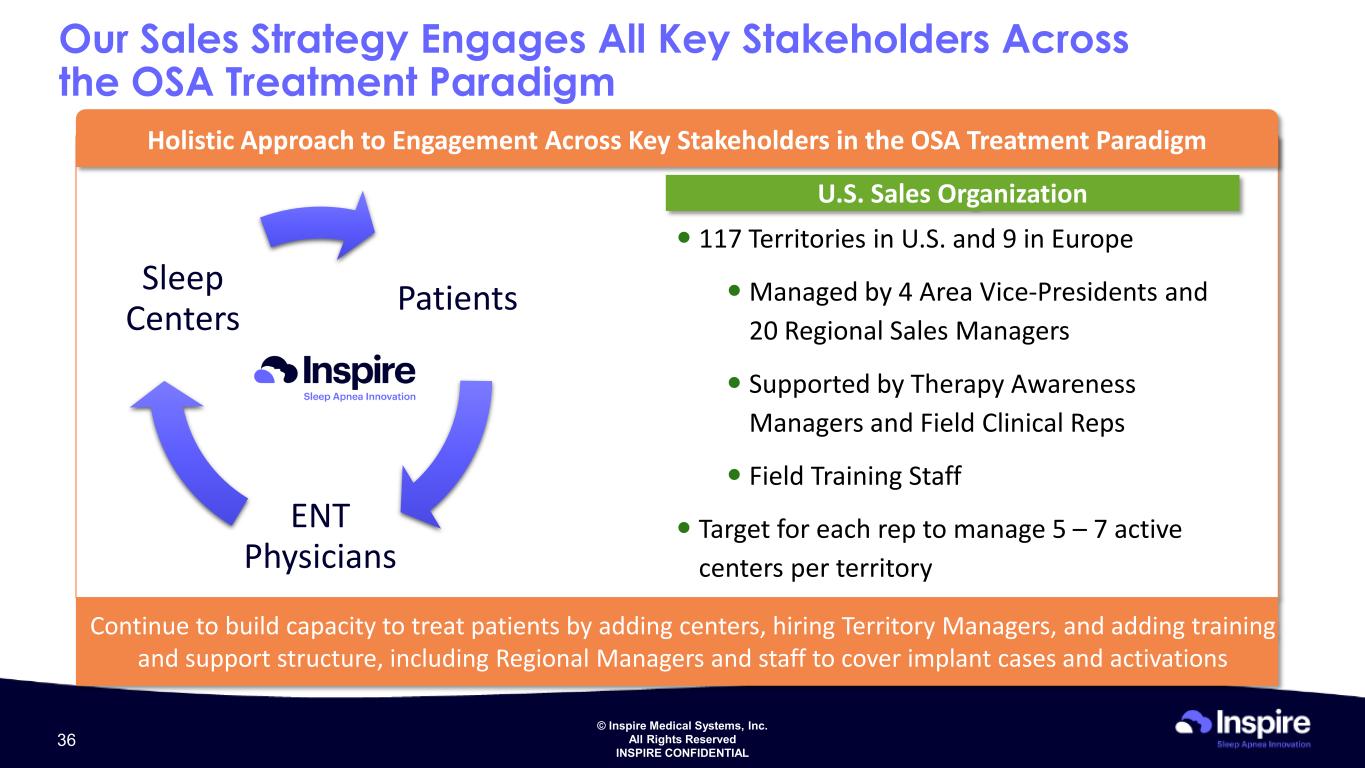
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Holistic Approach to Engagement Across Key Stakeholders in the OSA Treatment Paradigm U.S. Sales Organization 117 Territories in U.S. and 9 in Europe Managed by 4 Area Vice-Presidents and 20 Regional Sales Managers Supported by Therapy Awareness Managers and Field Clinical Reps Field Training Staff Target for each rep to manage 5 – 7 active centers per territory Continue to build capacity to treat patients by adding centers, hiring Territory Managers, and adding training and support structure, including Regional Managers and staff to cover implant cases and activations Our Sales Strategy Engages All Key Stakeholders Across the OSA Treatment Paradigm Patients ENT Physicians Sleep Centers 36
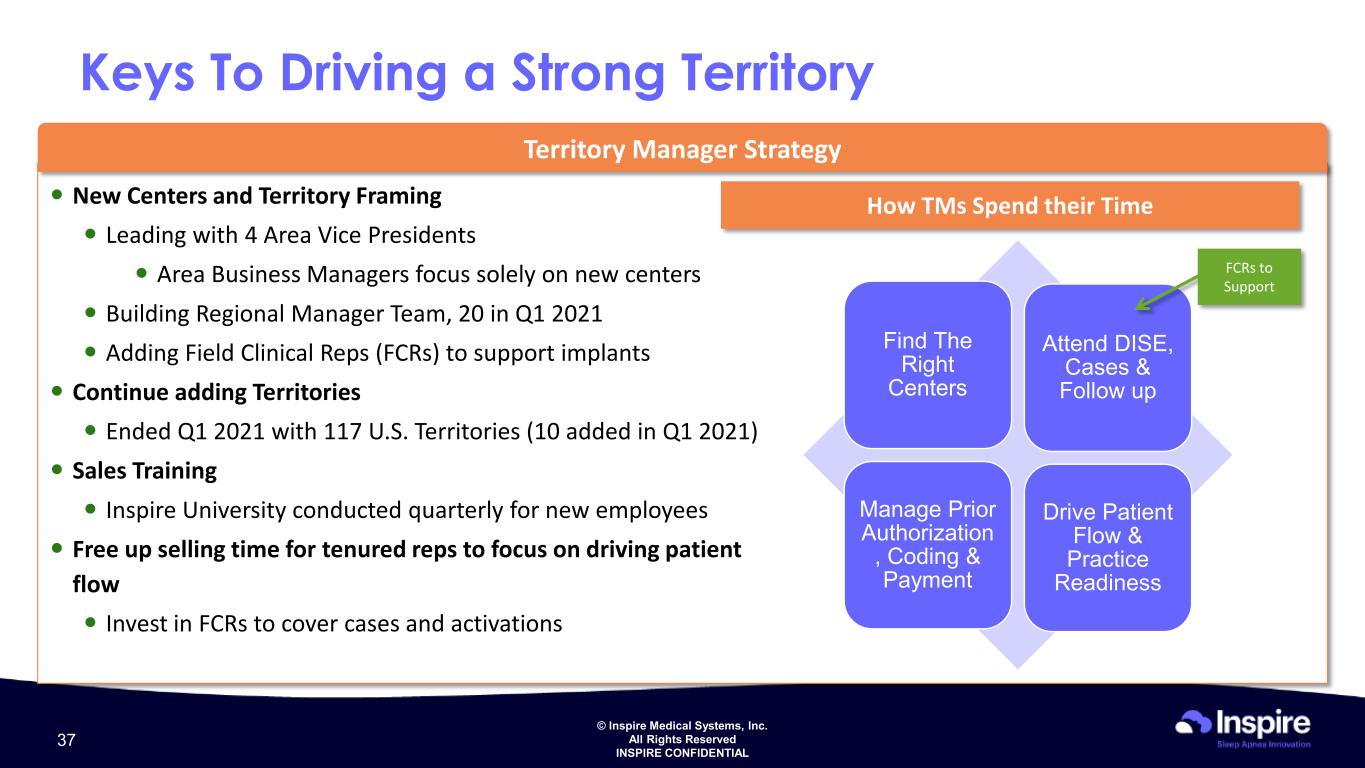
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Territory Manager Strategy Keys To Driving a Strong Territory How TMs Spend their Time Find The Right Centers Attend DISE, Cases & Follow up Drive Patient Flow & Practice Readiness New Centers and Territory Framing Leading with 4 Area Vice Presidents Area Business Managers focus solely on new centers Building Regional Manager Team, 20 in Q1 2021 Adding Field Clinical Reps (FCRs) to support implants Continue adding Territories Ended Q1 2021 with 117 U.S. Territories (10 added in Q1 2021) Sales Training Inspire University conducted quarterly for new employees Free up selling time for tenured reps to focus on driving patient flow Invest in FCRs to cover cases and activations Find The Right Centers Attend DISE, Cases & Foll w up Manage Prior Authoriz tion , Coding & Payment Drive Patient Flow & Practice Readiness FCRs to Support 37

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL KEEPING CONTROL WHILE GROWING FAST Focus on the rapid scaling of commercialization while ensuring proper training to maintain control of high-quality therapy outcomes The Great Balancing Act 38

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Financials 39
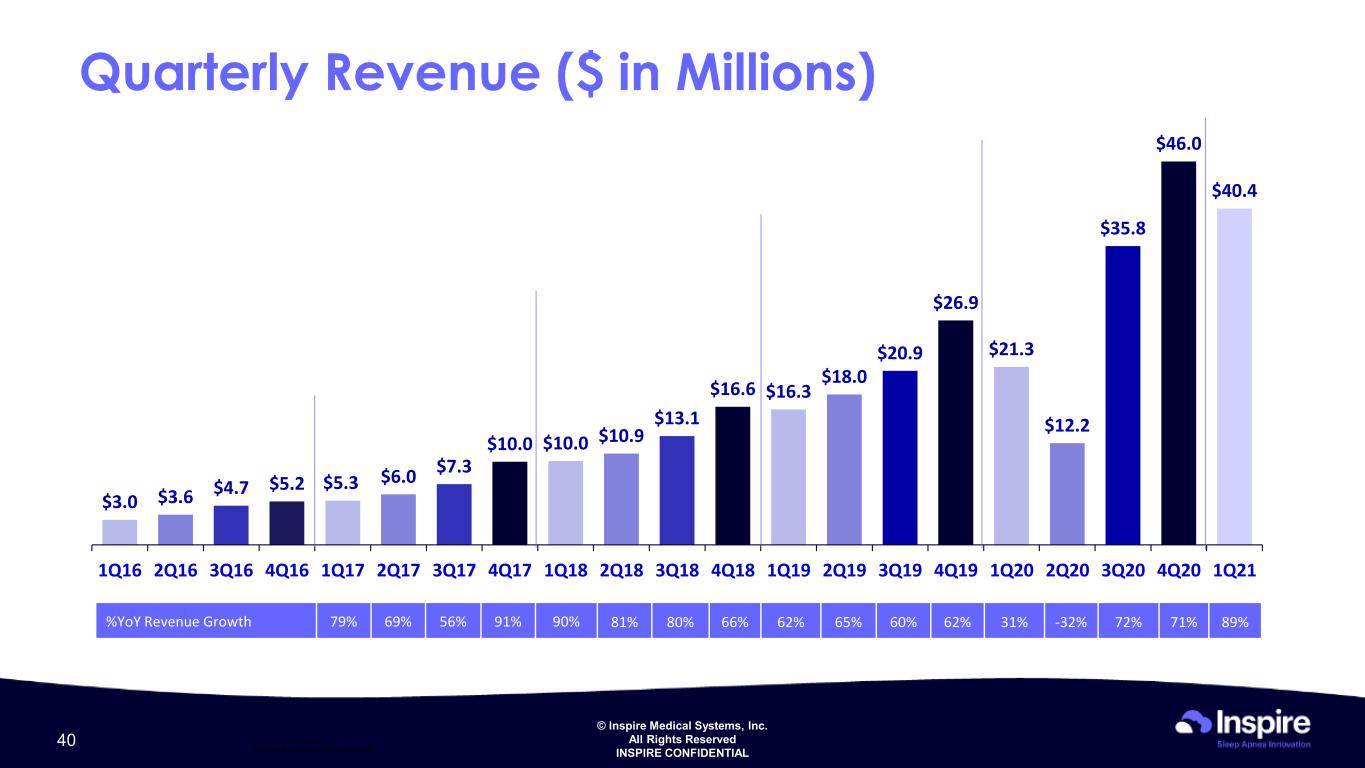
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL $3.0 $3.6 $4.7 $5.2 $5.3 $6.0 $7.3 $10.0 $10.0 $10.9 $13.1 $16.6 $16.3 $18.0 $20.9 $26.9 $21.3 $12.2 $35.8 $46.0 $40.4 1Q16 2Q16 3Q16 4Q16 1Q17 2Q17 3Q17 4Q17 1Q18 2Q18 3Q18 4Q18 1Q19 2Q19 3Q19 4Q19 1Q20 2Q20 3Q20 4Q20 1Q21 ____________________ Quarterly amounts are unaudited Quarterly Revenue ($ in Millions) 40 %YoY Revenue Growth 79% 69% 56% 91% 90% 81% 80% 66% 62% 65% 60% 62% 31% -32% 72% 71% 89%
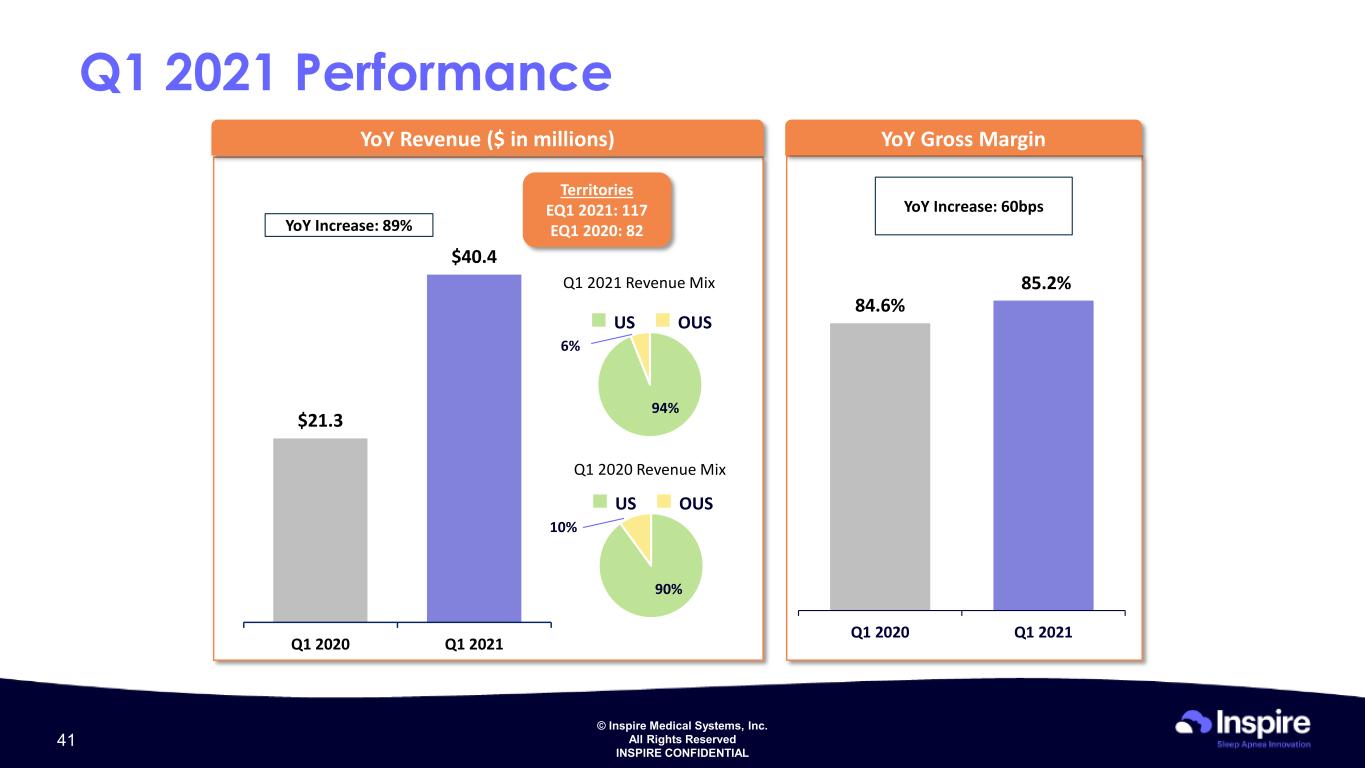
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL $21.3 $40.4 Q1 2020 Q1 2021 YoY Revenue ($ in millions) YoY Increase: 89% YoY Gross Margin YoY Increase: 60bps Q1 2021 Performance 84.6% 85.2% Q1 2020 Q1 2021 Territories EQ1 2021: 117 EQ1 2020: 82 90% 10% Q1 2020 Revenue Mix US OUS 94% 6% Q1 2021 Revenue Mix US OUS 41
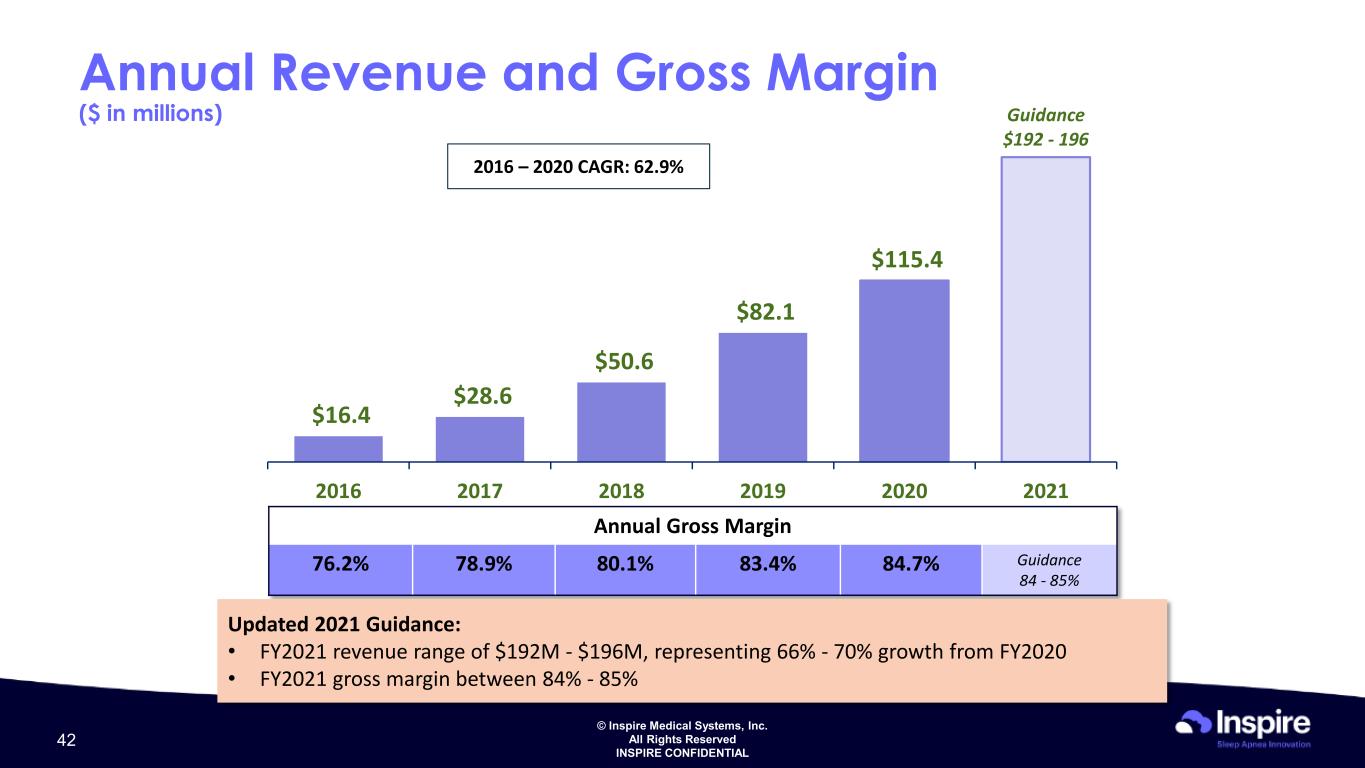
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL $16.4 $28.6 $50.6 $82.1 $115.4 Guidance $192 - 196 2016 2017 2018 2019 2020 2021 2016 – 2020 CAGR: 62.9% Annual Revenue and Gross Margin ($ in millions) Annual Gross Margin 76.2% 78.9% 80.1% 83.4% 84.7% Guidance 84 - 85% Updated 2021 Guidance: • FY2021 revenue range of $192M - $196M, representing 66% - 70% growth from FY2020 • FY2021 gross margin between 84% - 85% 42

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Our Growth Strategies Ensure strong and consistent patient outcomes globally through planned and controlled expansion and robust physician training Promote awareness among patients, ENT physicians, sleep centers, and referring physicians Expand U.S. sales and marketing organization to drive adoption of our Inspire therapy Leverage final Medicare LCDs and 260 million covered lives while continuing with prior authorization model Invest in research and development to drive innovation and expand indications Further penetrate existing and expand into new international markets 43
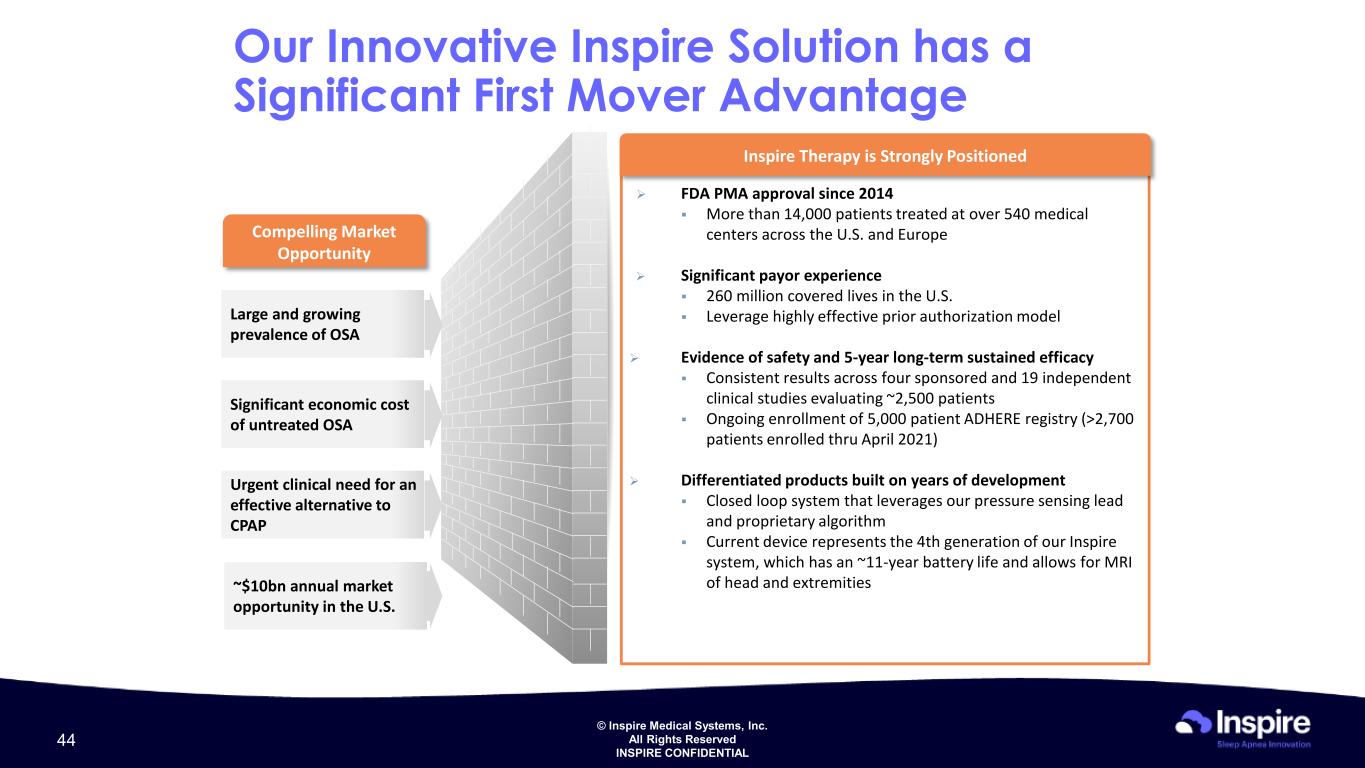
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Large and growing prevalence of OSA Significant economic cost of untreated OSA Urgent clinical need for an effective alternative to CPAP ~$10bn annual market opportunity in the U.S. Compelling Market Opportunity Our Innovative Inspire Solution has a Significant First Mover Advantage FDA PMA approval since 2014 More than 14,000 patients treated at over 540 medical centers across the U.S. and Europe Significant payor experience 260 million covered lives in the U.S. Leverage highly effective prior authorization model Evidence of safety and 5-year long-term sustained efficacy Consistent results across four sponsored and 19 independent clinical studies evaluating ~2,500 patients Ongoing enrollment of 5,000 patient ADHERE registry (>2,700 patients enrolled thru April 2021) Differentiated products built on years of development Closed loop system that leverages our pressure sensing lead and proprietary algorithm Current device represents the 4th generation of our Inspire system, which has an ~11-year battery life and allows for MRI of head and extremities Inspire Therapy is Strongly Positioned 44
