Attached files
| file | filename |
|---|---|
| 8-K - 8-K - C4 Therapeutics, Inc. | cccc-8k_20210607.htm |
| EX-99.1 - EX-99.1 - C4 Therapeutics, Inc. | cccc-ex991_8.htm |

CFT8919 Pre-Clinical Data Investor Call June 7, 2021 Exhibit 99.2

Forward-looking Statements The following presentation contains forward-looking statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “goal,” “intend,” “look forward to,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” and similar expressions. These forward-looking statements include, but are not limited to, statements regarding the therapeutic potential of C4 Therapeutics, Inc.’s technology and products. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. Among the factors that could cause actual results to differ materially from those described or projected herein include uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals, as well as the fact that the product candidates that we are developing or may develop may not demonstrate success in clinical trials. Prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. C4 Therapeutics, Inc. undertakes no obligation to update or revise the information contained in this presentation, whether as a result of new information, future events or circumstances or otherwise. Intellectual Property C4 Therapeutics, Inc. owns various registered and unregistered trademarks in the U.S. and internationally, including, without limitation, C4 THERAPEUTICS, our housemark logo, the name of our TORPEDO platform, and the names of our BIDAC and MONODAC degrader products. All trademarks or trade names referred to in this presentation that we do not own are the property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the symbols ® and ™, but those references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. Forward-looking Statements and Intellectual Property 2
Today’s Agenda 3

What You Will Hear Today 4 CFT8919 is an orally bioavailable, selective, allosteric degrader of EGFR L858R Active in vitro and in vivo in models with secondary EGFR mutations Demonstrates intracranial activity indicating potential to prevent or treat brain metastases in patients with EGFR L858R-driven tumors 25-45% of mutant EGFR NSCLC is driven by L858R activating mutation; these patients are not adequately addressed with current EGFR therapies Pre-clinical data suggests CFT8919 has path to registration in EGFR patients who develop resistance to osimertinib Sources: Li, K et al. Oncol Rep 37, 1347–1358 (2017);Jin Y. et al. Scientific Reports 6:31636 (2016); Soria, J.-C. et al. NEJM 378, 113–125 (2018)
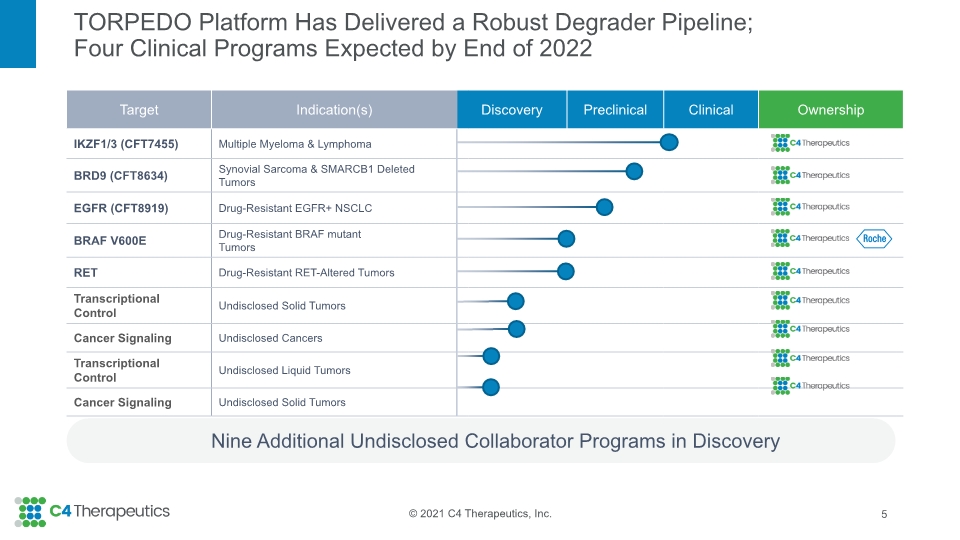
TORPEDO Platform Has Delivered a Robust Degrader Pipeline; Four Clinical Programs Expected by End of 2022 Nine Additional Undisclosed Collaborator Programs in Discovery 5
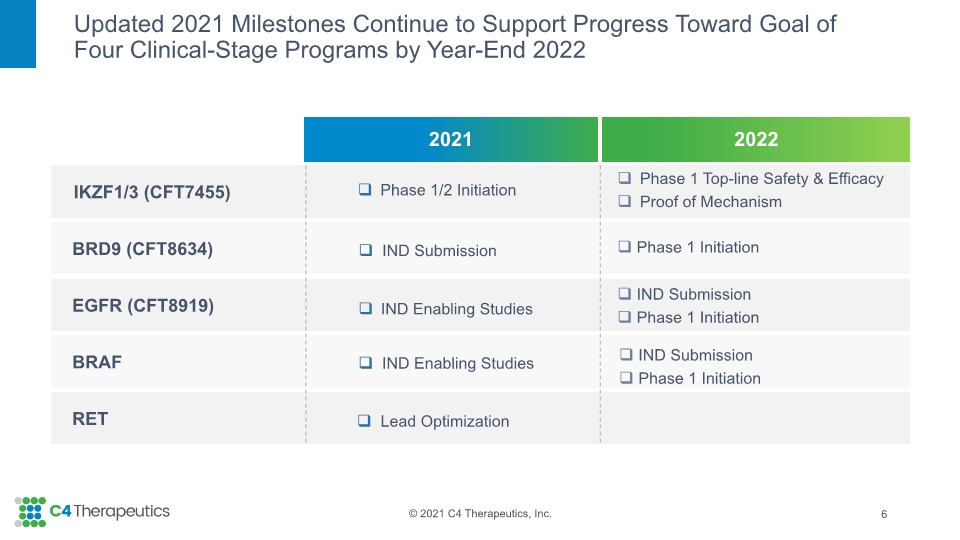
Updated 2021 Milestones Continue to Support Progress Toward Goal of Four Clinical-Stage Programs by Year-End 2022 6 Phase 1/2 Initiation IND Submission Lead Optimization IND Enabling Studies IND Enabling Studies Phase 1 Top-line Safety & Efficacy Proof of Mechanism Phase 1 Initiation IND Submission Phase 1 Initiation IND Submission Phase 1 Initiation

Preclinical Evaluation of CFT8919 as a Mutant Selective Degrader of EGFR with L858R Activating Mutations for the Treatment of Non-Small Cell Lung Cancer
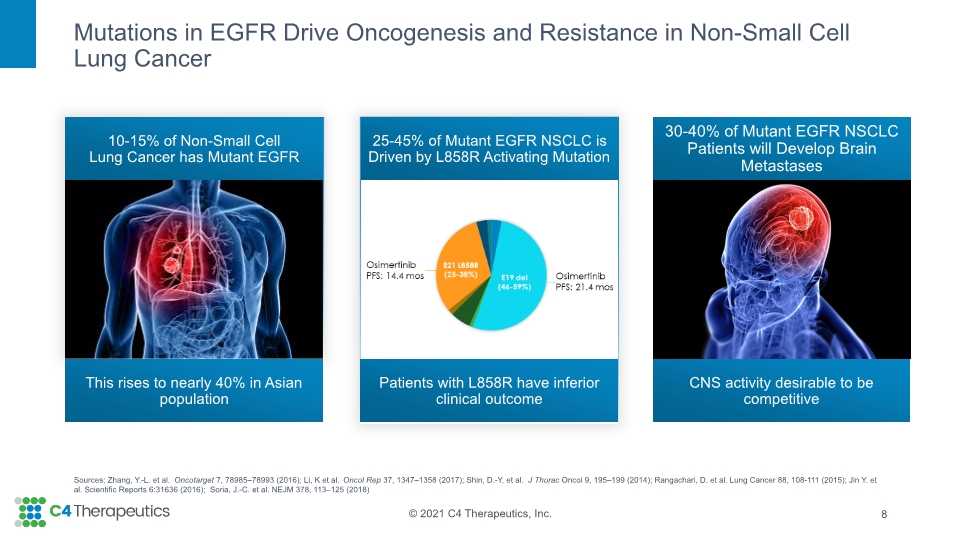
Mutations in EGFR Drive Oncogenesis and Resistance in Non-Small Cell Lung Cancer 8 10-15% of Non-Small Cell Lung Cancer has Mutant EGFR 30-40% of Mutant EGFR NSCLC Patients will Develop Brain Metastases 25-45% of Mutant EGFR NSCLC is Driven by L858R Activating Mutation This rises to nearly 40% in Asian population CNS activity desirable to be competitive Sources: Zhang, Y.-L. et al. Oncotarget 7, 78985–78993 (2016); Li, K et al. Oncol Rep 37, 1347–1358 (2017); Shin, D.-Y. et al. J Thorac Oncol 9, 195–199 (2014); Rangachari, D. et al. Lung Cancer 88, 108-111 (2015); Jin Y. et al. Scientific Reports 6:31636 (2016); Soria, J.-C. et al. NEJM 378, 113–125 (2018) Patients with L858R have inferior clinical outcome
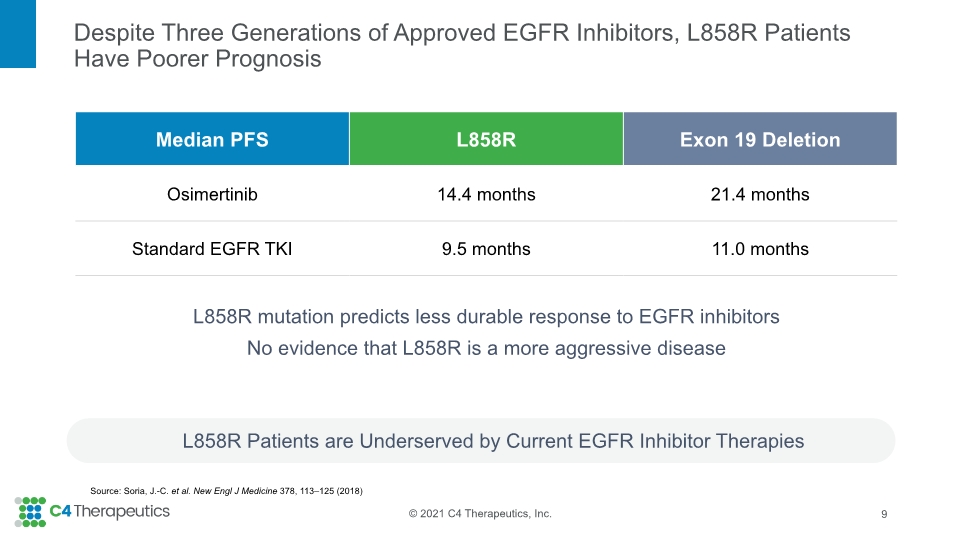
L858R mutation predicts less durable response to EGFR inhibitors No evidence that L858R is a more aggressive disease Despite Three Generations of Approved EGFR Inhibitors, L858R Patients Have Poorer Prognosis L858R Patients are Underserved by Current EGFR Inhibitor Therapies 9 Source: Soria, J.-C. et al. New Engl J Medicine 378, 113–125 (2018)
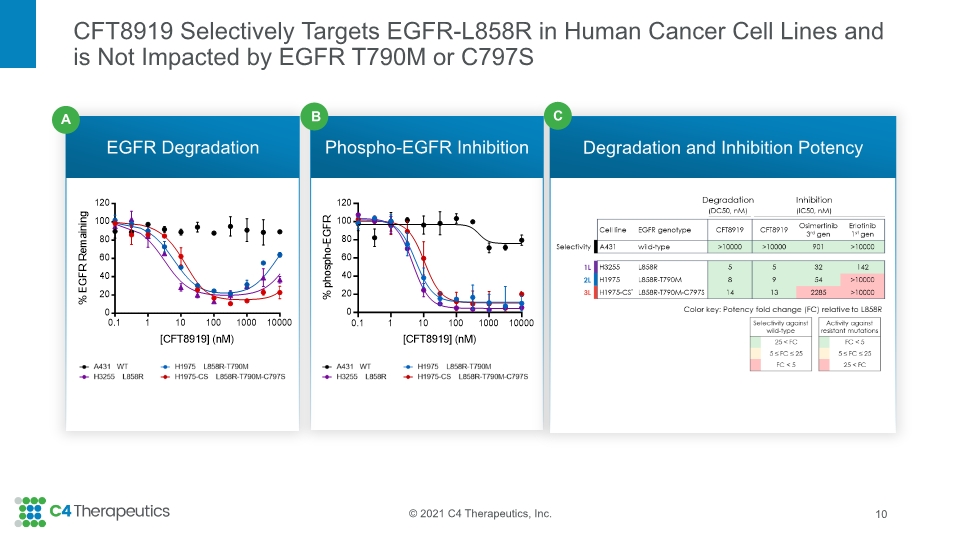
CFT8919 Selectively Targets EGFR-L858R in Human Cancer Cell Lines and is Not Impacted by EGFR T790M or C797S EGFR Degradation Phospho-EGFR Inhibition Degradation and Inhibition Potency 10 C B A
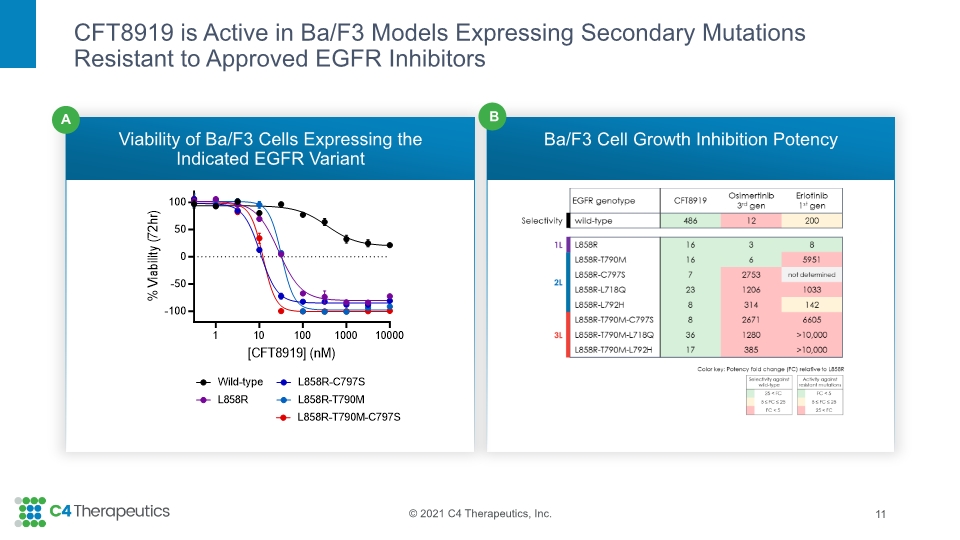
CFT8919 is Active in Ba/F3 Models Expressing Secondary Mutations Resistant to Approved EGFR Inhibitors Viability of Ba/F3 Cells Expressing the Indicated EGFR Variant Ba/F3 Cell Growth Inhibition Potency 11 B A
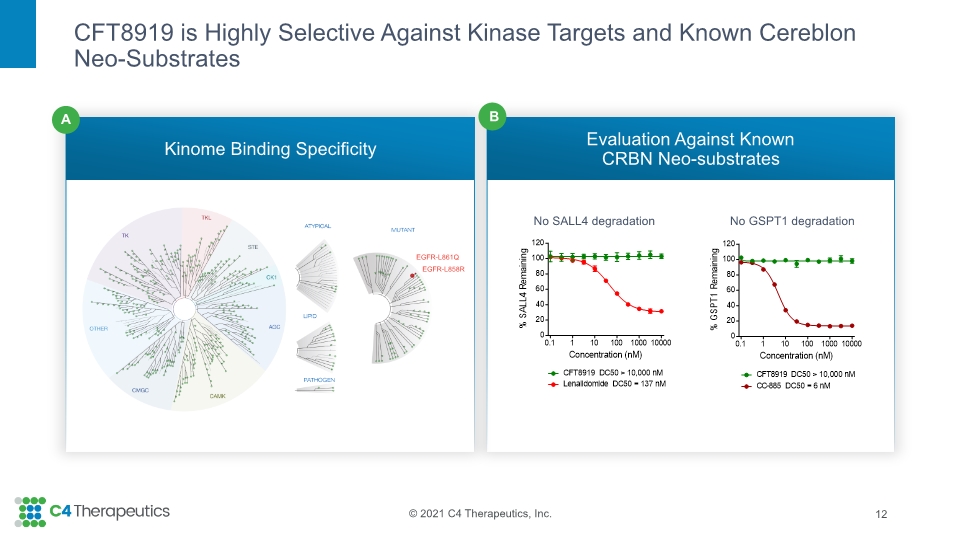
CFT8919 is Highly Selective Against Kinase Targets and Known Cereblon Neo-Substrates Kinome Binding Specificity Evaluation Against Known CRBN Neo-substrates 12 No SALL4 degradation No GSPT1 degradation B A
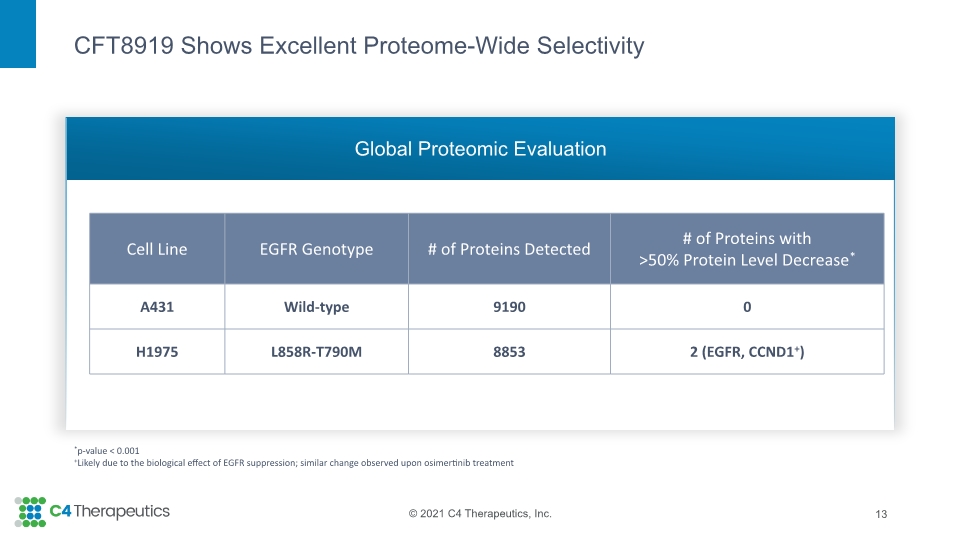
CFT8919 Shows Excellent Proteome-Wide Selectivity Global Proteomic Evaluation 13 *p-value < 0.001 +Likely due to the biological effect of EGFR suppression; similar change observed upon osimertinib treatment
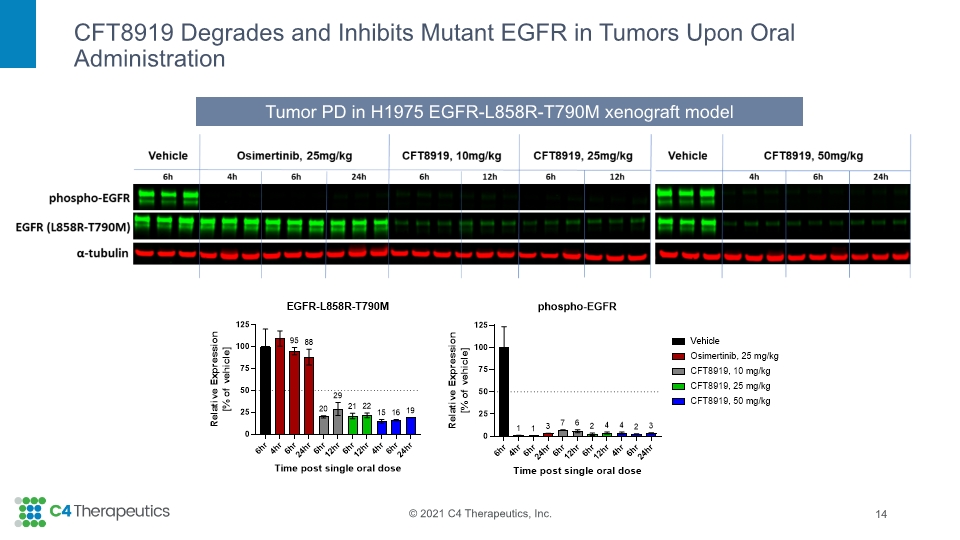
CFT8919 Degrades and Inhibits Mutant EGFR in Tumors Upon Oral Administration 14 Tumor PD in H1975 EGFR-L858R-T790M xenograft model
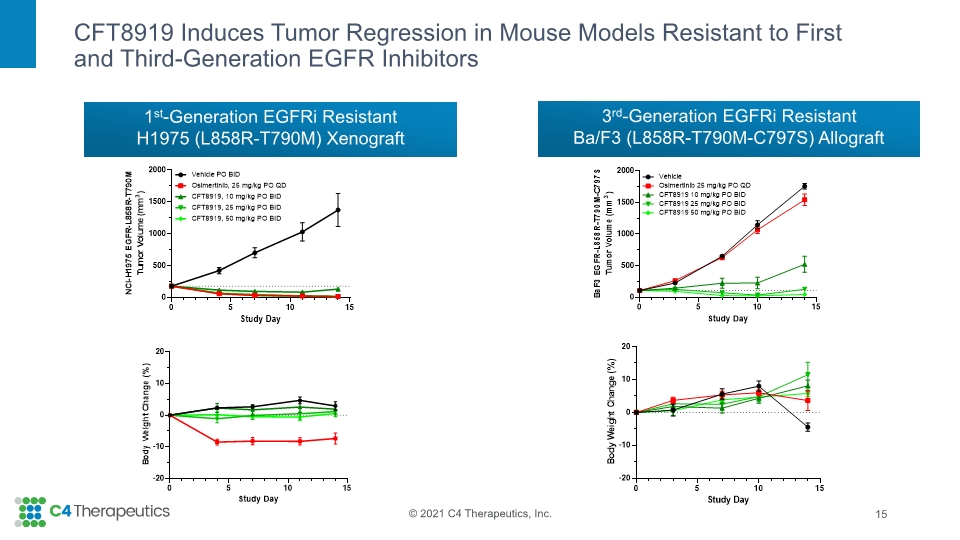
CFT8919 Induces Tumor Regression in Mouse Models Resistant to First and Third-Generation EGFR Inhibitors 15 3rd-Generation EGFRi Resistant Ba/F3 (L858R-T790M-C797S) Allograft 1st-Generation EGFRi Resistant H1975 (L858R-T790M) Xenograft
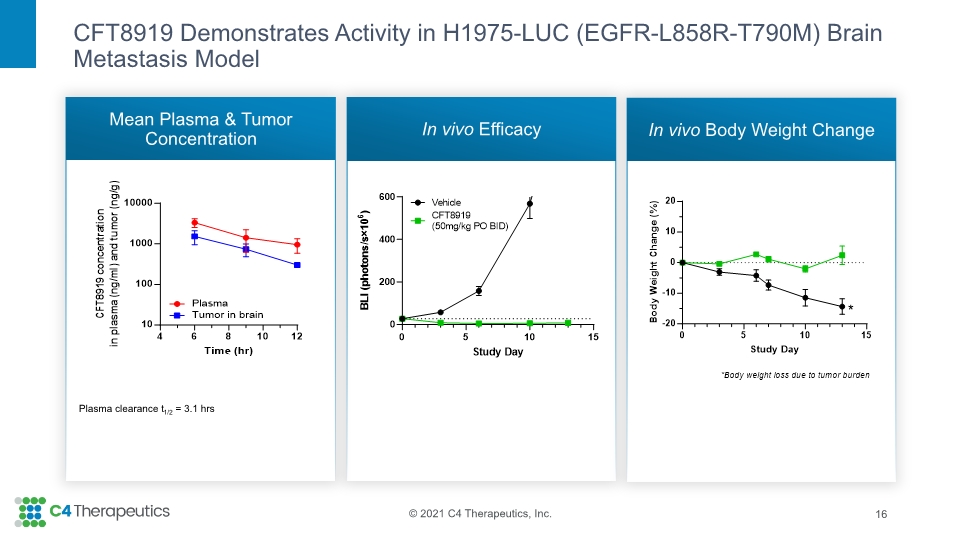
CFT8919 Demonstrates Activity in H1975-LUC (EGFR-L858R-T790M) Brain Metastasis Model Mean Plasma & Tumor Concentration In vivo Efficacy In vivo Body Weight Change 16 Plasma clearance t1/2 = 3.1 hrs
Active in vitro and in vivo in models with secondary mutations (such as T790M, C797S, T790M-C797S) that cause acquired resistance to 1st-, 2nd-, and 3rd-generation EGFR inhibitors Demonstrates intracranial activity indicating potential to prevent or treat brain metastases in patients with EGFR L858R-driven tumors Clinical evaluation is warranted in patients with EGFR L858R driven NSCLC who have progressed on prior EGFR inhibitors By binding to an allosteric EGFR site, CFT8919 may combine with approved EGFR inhibitors which bind to the EGFR active site Pre-clinical profile highlight potential for single agent activity in the front-line setting CFT8919 is a Potent, Oral, Allosteric, Mutant-selective Degrader of EGFR L858R 17 IND Submission Expected mid-2022 with Potential Phase 1 Trial Initiation by YE 2022

Q&A Session

Thank You
