Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Vir Biotechnology, Inc. | d129136d8k.htm |
| EX-99.1 - EX-99.1 - Vir Biotechnology, Inc. | d129136dex991.htm |

Vir Biotechnology, Inc. A WORLD WITHOUT INFECTIOUS DISEASE May 26, 2021 Exhibit 99.2

Legal disclaimers © 2021 Vir Biotechnology, Inc. Forward-Looking Statements Statements in this presentation that are not statements of historical fact are forward-looking statements. Such forward-looking statements include, without limitation, statements regarding the expected success, cost, and timing of our research and clinical development plans and clinical trials, our goals with respect to the prophylaxis or treatment of COVID-19, HBV, influenza A and HIV, our objectives, strategy, technology platform and clinical trial designs, the potential benefits of our collaborations, and our ability to complete certain milestones. Words such as “believe,” “anticipate,” “plan,” “expect,” “intend,” “will,” “may,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. These forward-looking statements are based on the beliefs of the management of Vir Biotechnology, Inc. (the “Company”) as well as assumptions made by and information currently available to the Company. Such statements reflect the current views of the Company with respect to future events and are subject to known and unknown risks, including business, regulatory, economic and competitive risks, uncertainties, contingencies and assumptions about the Company, including, without limitation, risks inherent in developing the Company’s products and technologies, future results from the Company’s ongoing and planned clinical trials such as unexpected data or clinical site activation rates or clinical trial enrollment rates that are lower than expected, difficulties arising from our collaborations, challenges in accessing adequate manufacturing capacity, the Company’s ability to obtain adequate financing to fund its planned clinical trials and other expenses, statements related to regulatory authorizations and approvals, trends in the industry, changes in the competitive landscape, delays or disruptions due to the COVID-19 pandemic, the legal and regulatory framework for the industry, unexpected litigation or disputes and future expenditures. In light of these risks and uncertainties, the events or circumstances referred to in the forward-looking statements may not occur. The actual results may vary from the anticipated results and the variations may be material. Other factors that may cause the Company’s actual results to differ from current expectations are discussed in the Company’s filings with the U.S. Securities and Exchange Commission, including the section titled “Risk Factors” contained therein. These forward-looking statements should not be taken as forecasts or promises nor should they be taken as implying any indication, assurance or guarantee that the assumptions on which such forward-looking statements have been made are correct or exhaustive or, in the case of the assumptions, fully stated in this presentation. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this presentation is given. Except as required by law, the Company undertakes no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise. The Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995 for all forward-looking statements. This presentation discusses product candidates that are under clinical study and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of these product candidates for the use for which such product candidates are being studied. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products.

© 2021 Vir Biotechnology, Inc. Taking on the most serious infectious diseases Products and product candidates with demonstrated clinical impact Applying learnings to broad ID pipeline Potential significant near-term value drivers
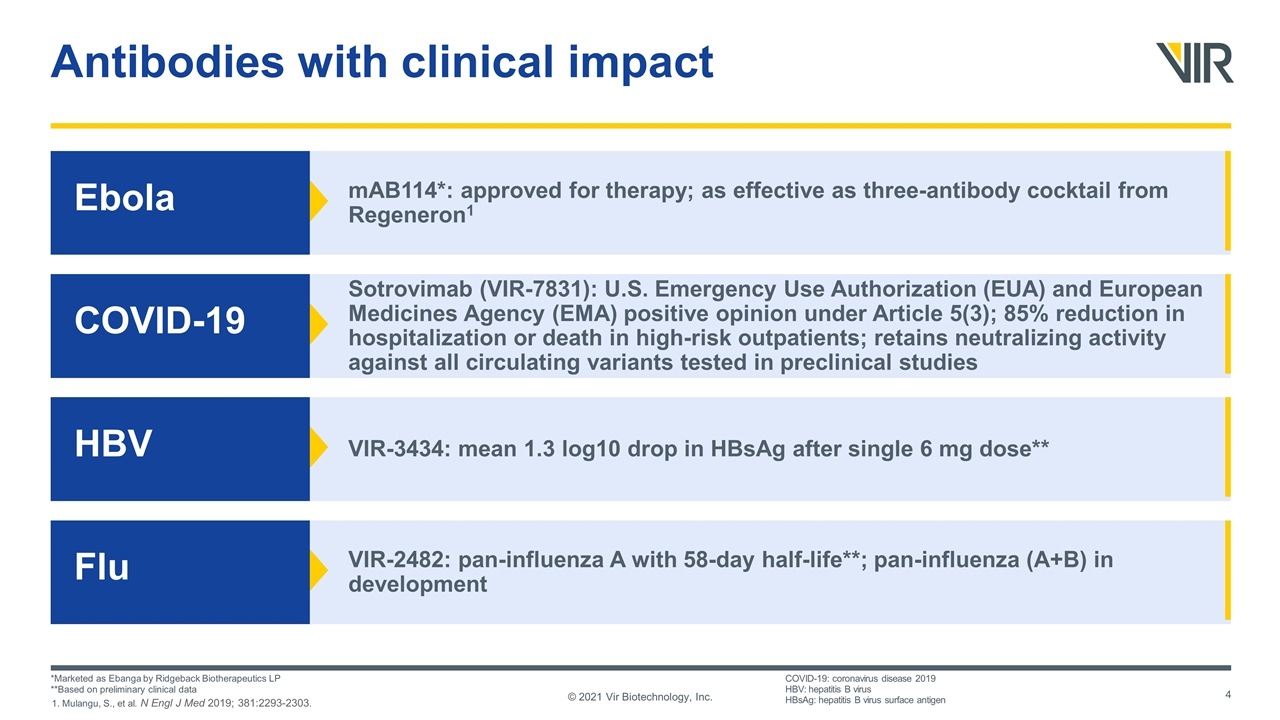
mAB114*: approved for therapy; as effective as three-antibody cocktail from Regeneron1 Antibodies with clinical impact © 2021 Vir Biotechnology, Inc. Ebola Sotrovimab (VIR-7831): U.S. Emergency Use Authorization (EUA) and European Medicines Agency (EMA) positive opinion under Article 5(3); 85% reduction in hospitalization or death in high-risk outpatients; retains neutralizing activity against all circulating variants tested in preclinical studies COVID-19 VIR-3434: mean 1.3 log10 drop in HBsAg after single 6 mg dose** HBV VIR-2482: pan-influenza A with 58-day half-life**; pan-influenza (A+B) in development Flu *Marketed as Ebanga by Ridgeback Biotherapeutics LP **Based on preliminary clinical data COVID-19: coronavirus disease 2019 HBV: hepatitis B virus HBsAg: hepatitis B virus surface antigen 1. Mulangu, S., et al. N Engl J Med 2019; 381:2293-2303.
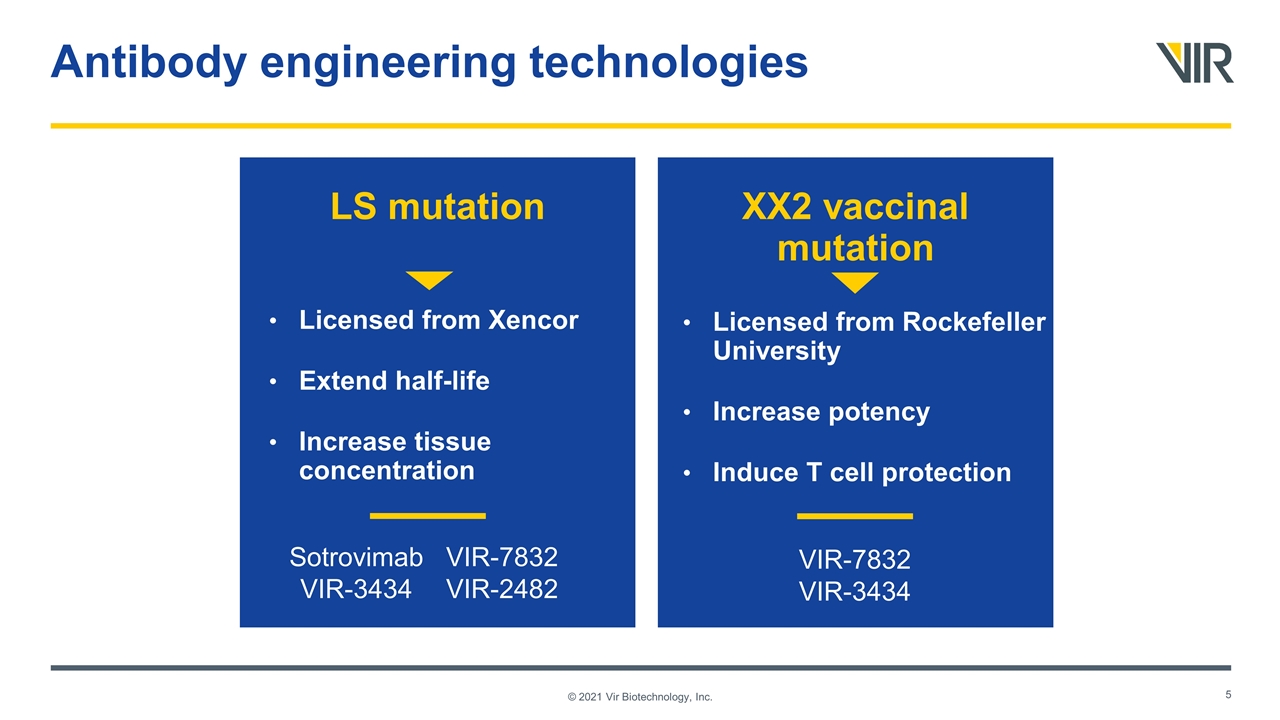
Antibody engineering technologies © 2021 Vir Biotechnology, Inc. XX2 vaccinal mutation LS mutation Sotrovimab VIR-3434 VIR-7832 VIR-2482 VIR-7832 VIR-3434 Licensed from Rockefeller University Increase potency Induce T cell protection Licensed from Xencor Extend half-life Increase tissue concentration
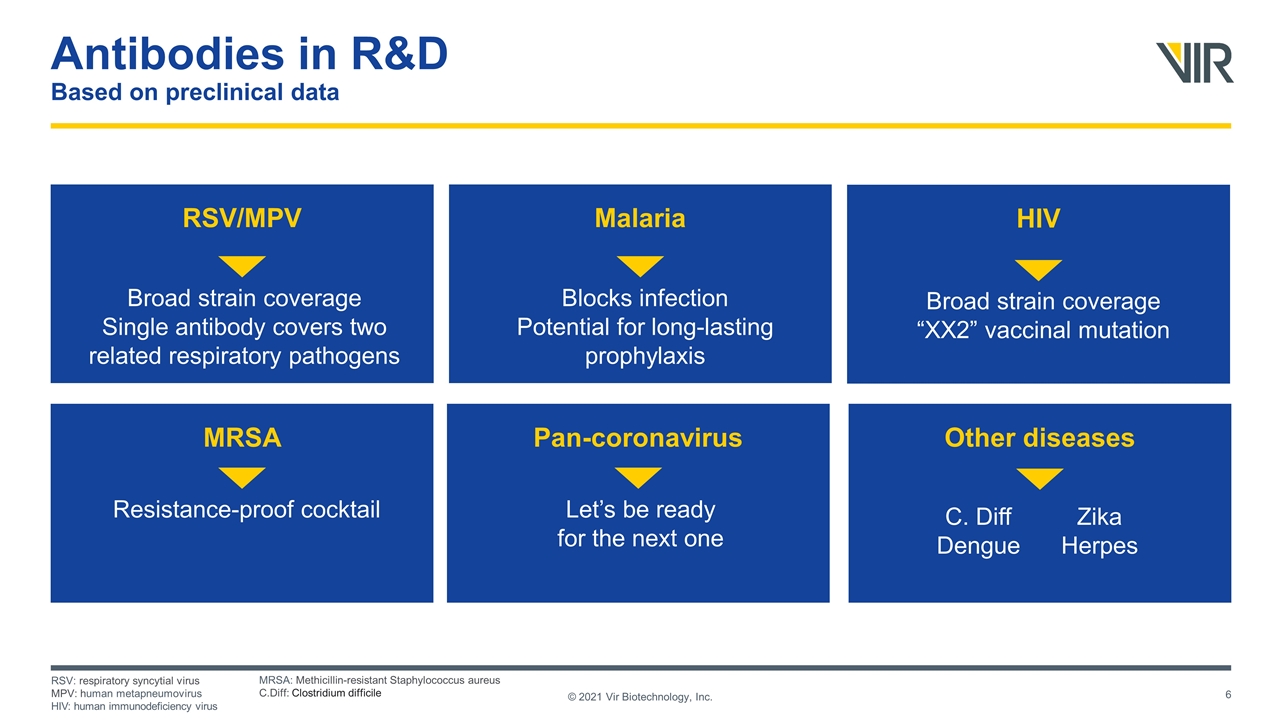
Antibodies in R&D Based on preclinical data © 2021 Vir Biotechnology, Inc. RSV/MPV Malaria Other diseases Pan-coronavirus HIV MRSA Broad strain coverage Single antibody covers two related respiratory pathogens Blocks infection Potential for long-lasting prophylaxis C. Diff Dengue Let’s be ready for the next one Broad strain coverage “XX2” vaccinal mutation Resistance-proof cocktail Zika Herpes RSV: respiratory syncytial virus MPV: human metapneumovirus HIV: human immunodeficiency virus MRSA: Methicillin-resistant Staphylococcus aureus C.Diff: Clostridium difficile
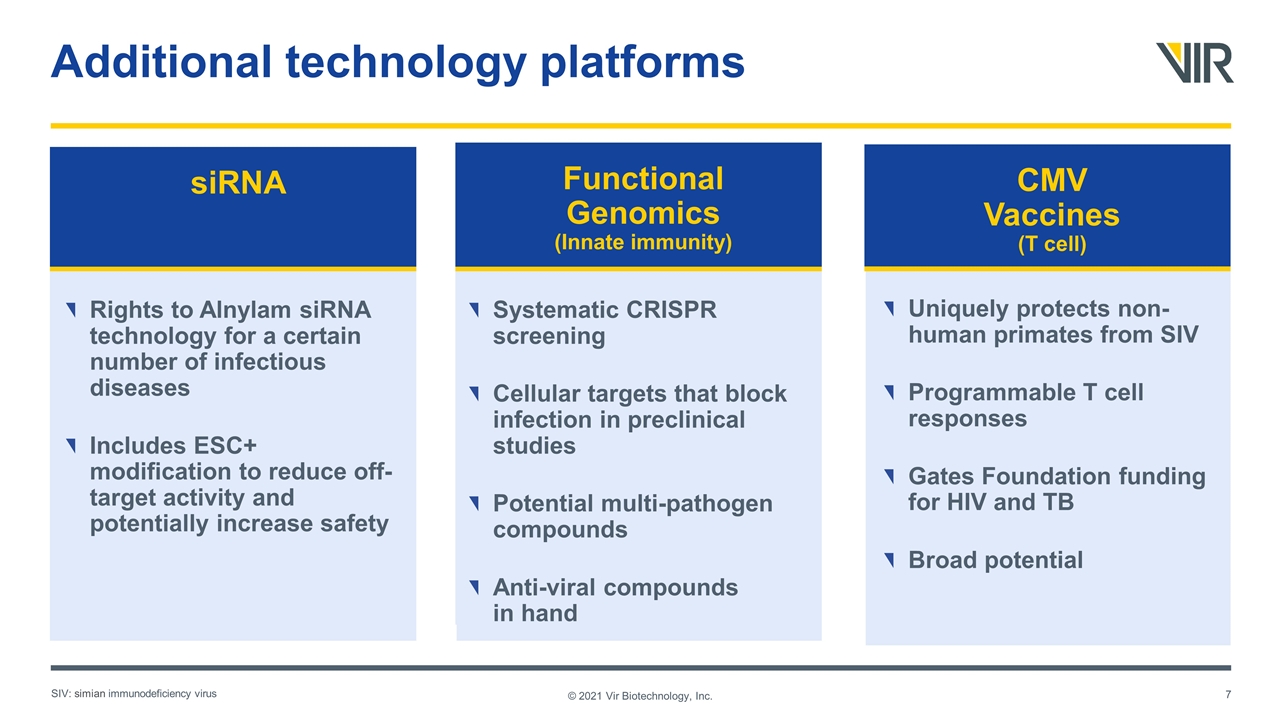
Additional technology platforms © 2021 Vir Biotechnology, Inc. siRNA Rights to Alnylam siRNA technology for a certain number of infectious diseases Includes ESC+ modification to reduce off-target activity and potentially increase safety CMV Vaccines (T cell) Systematic CRISPR screening Cellular targets that block infection in preclinical studies Potential multi-pathogen compounds Anti-viral compounds in hand Functional Genomics (Innate immunity) Uniquely protects non-human primates from SIV Programmable T cell responses Gates Foundation funding for HIV and TB Broad potential SIV: simian immunodeficiency virus
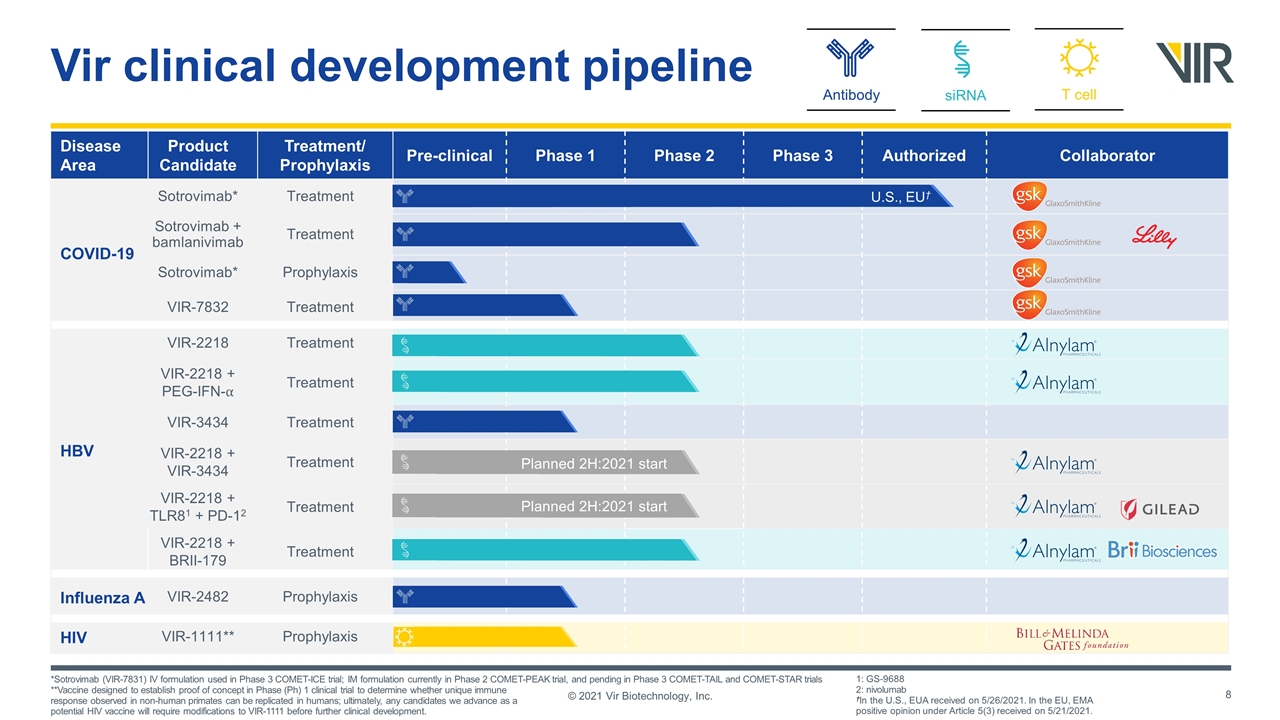
Disease Area Product Candidate Treatment/ Prophylaxis Pre-clinical Phase 1 Phase 2 Phase 3 Authorized Collaborator COVID-19 Sotrovimab* Treatment Sotrovimab + bamlanivimab Treatment Sotrovimab* Prophylaxis VIR-7832 Treatment HBV VIR-2218 Treatment VIR-2218 + PEG-IFN-⍺ Treatment VIR-3434 Treatment VIR-2218 + VIR-3434 Treatment VIR-2218 + TLR81 + PD-12 Treatment VIR-2218 + BRII-179 Treatment Influenza A VIR-2482 Prophylaxis HIV VIR-1111** Prophylaxis siRNA T cell Antibody Vir clinical development pipeline Planned 2H:2021 start Planned 2H:2021 start *Sotrovimab (VIR-7831) IV formulation used in Phase 3 COMET-ICE trial; IM formulation currently in Phase 2 COMET-PEAK trial, and pending in Phase 3 COMET-TAIL and COMET-STAR trials **Vaccine designed to establish proof of concept in Phase (Ph) 1 clinical trial to determine whether unique immune response observed in non-human primates can be replicated in humans; ultimately, any candidates we advance as a potential HIV vaccine will require modifications to VIR-1111 before further clinical development. 1: GS-9688 2: nivolumab †In the U.S., EUA received on 5/26/2021. In the EU, EMA positive opinion under Article 5(3) received on 5/21/2021. © 2021 Vir Biotechnology, Inc. U.S., EU†

Vir Biotechnology, Inc. COVID-19
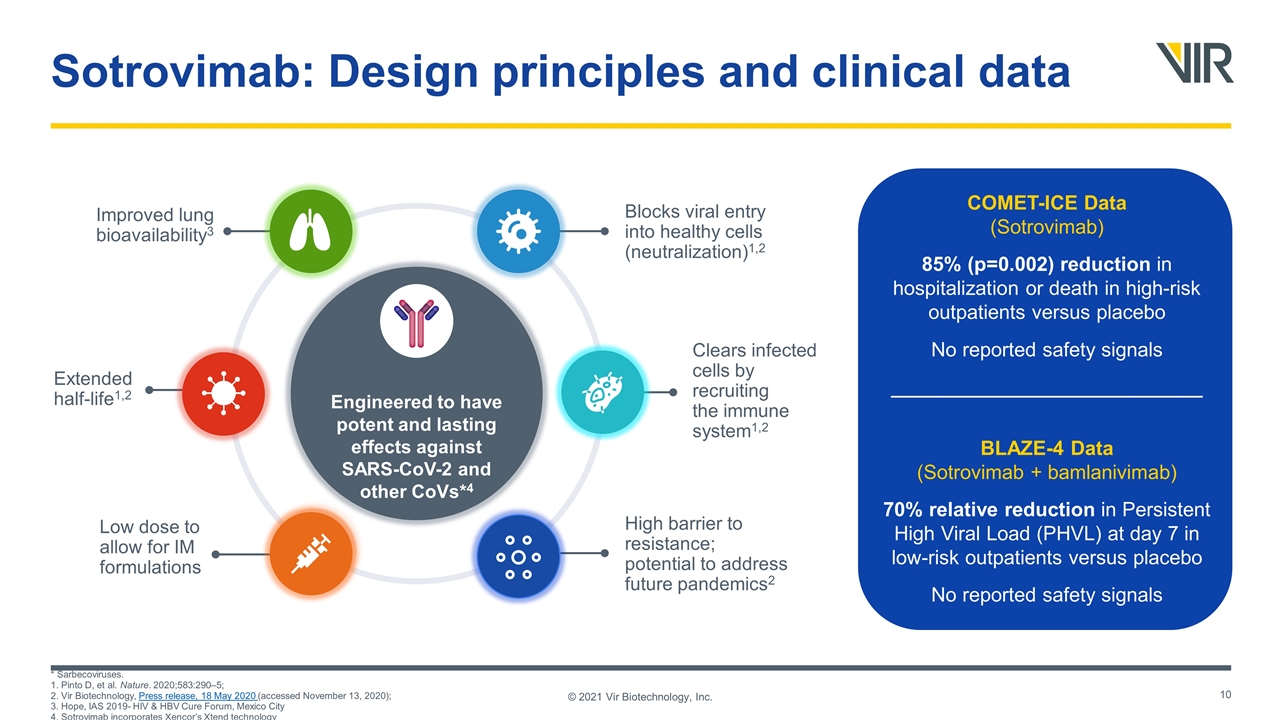
* Sarbecoviruses. 1. Pinto D, et al. Nature. 2020;583:290–5; 2. Vir Biotechnology, Press release, 18 May 2020 (accessed November 13, 2020); 3. Hope, IAS 2019- HIV & HBV Cure Forum, Mexico City 4. Sotrovimab incorporates Xencor’s Xtend technology © 2021 Vir Biotechnology, Inc. Sotrovimab: Design principles and clinical data COMET-ICE Data (Sotrovimab) 85% (p=0.002) reduction in hospitalization or death in high-risk outpatients versus placebo No reported safety signals ____________________________ BLAZE-4 Data (Sotrovimab + bamlanivimab) 70% relative reduction in Persistent High Viral Load (PHVL) at day 7 in low-risk outpatients versus placebo No reported safety signals Engineered to have potent and lasting effects against SARS-CoV-2 and other CoVs*4 High barrier to resistance; potential to address future pandemics2 Improved lung bioavailability3 Blocks viral entry into healthy cells (neutralization)1,2 Clears infected cells by recruiting the immune system1,2 Extended half-life1,2 Low dose to allow for IM formulations
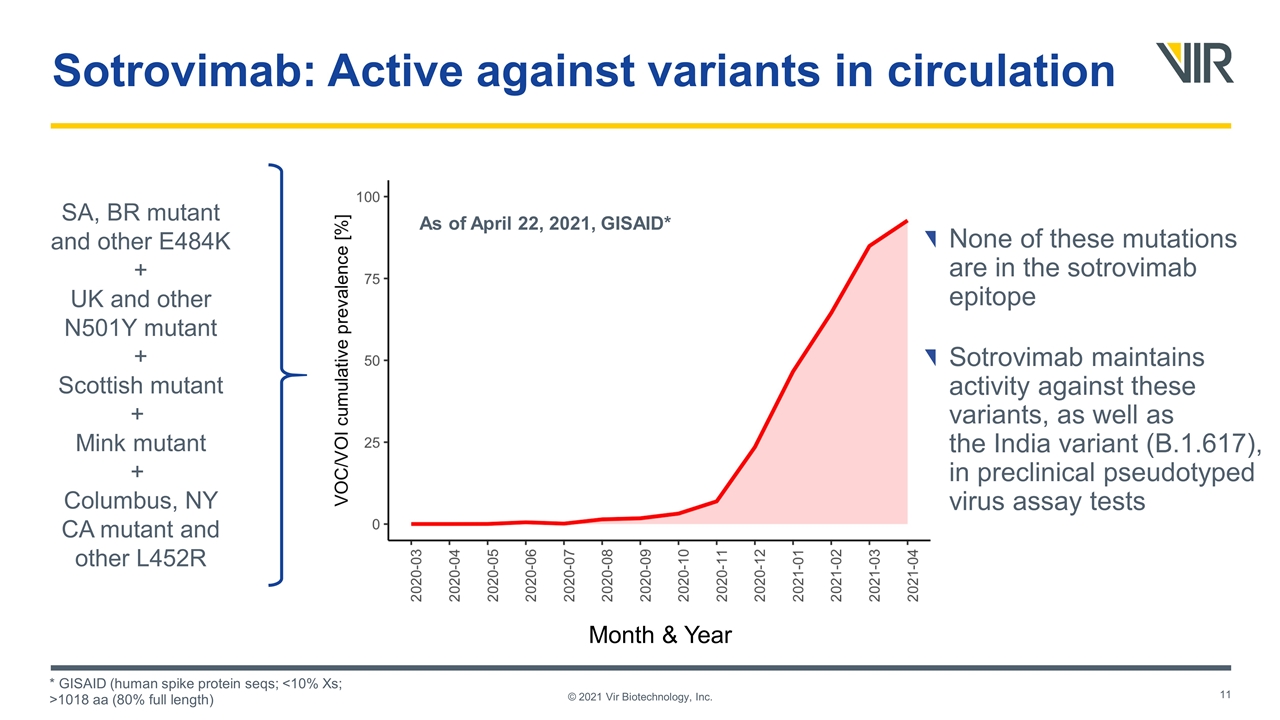
Sotrovimab: Active against variants in circulation SA, BR mutant and other E484K + UK and other N501Y mutant + Scottish mutant + Mink mutant + Columbus, NY CA mutant and other L452R Month & Year * GISAID (human spike protein seqs; <10% Xs; >1018 aa (80% full length) © 2021 Vir Biotechnology, Inc. As of April 22, 2021, GISAID* None of these mutations are in the sotrovimab epitope Sotrovimab maintains activity against these variants, as well as the India variant (B.1.617), in preclinical pseudotyped virus assay tests
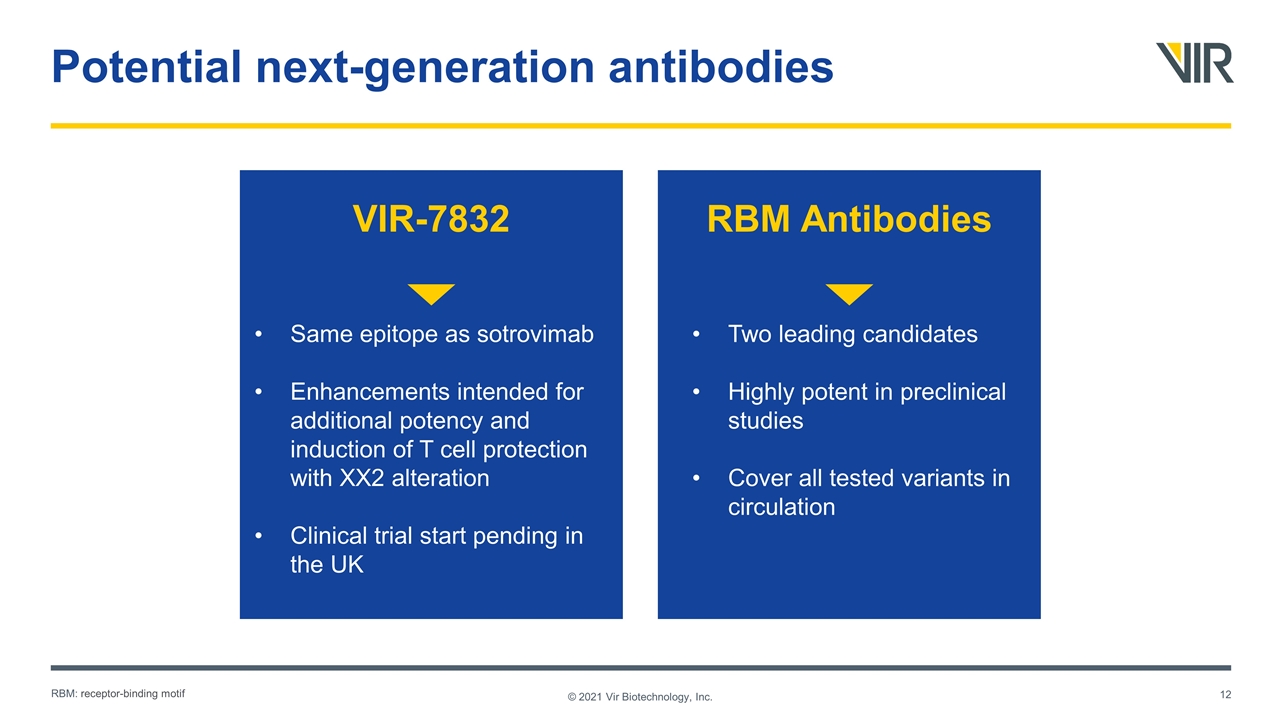
Potential next-generation antibodies © 2021 Vir Biotechnology, Inc. RBM Antibodies VIR-7832 Two leading candidates Highly potent in preclinical studies Cover all tested variants in circulation Same epitope as sotrovimab Enhancements intended for additional potency and induction of T cell protection with XX2 alteration Clinical trial start pending in the UK RBM: receptor-binding motif
Vir Biotechnology, Inc. Hepatitis B (HBV)
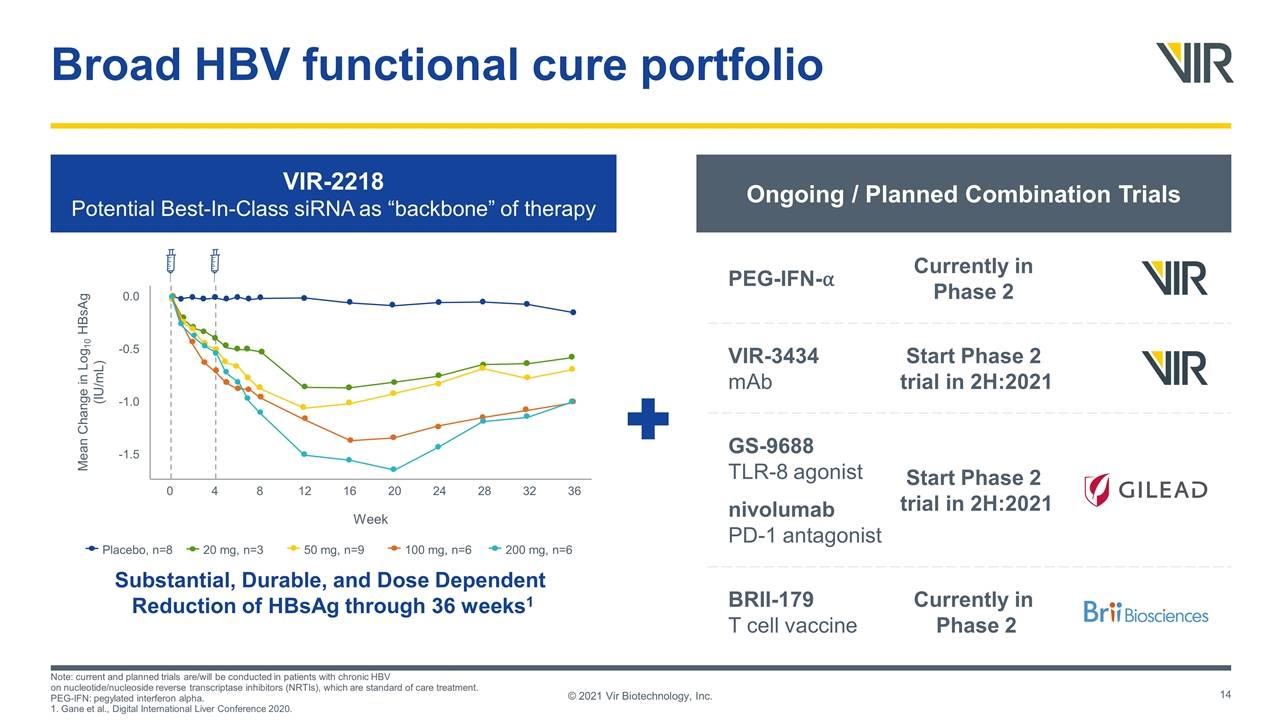
Broad HBV functional cure portfolio © 2021 Vir Biotechnology, Inc. VIR-2218 Potential Best-In-Class siRNA as “backbone” of therapy Substantial, Durable, and Dose Dependent Reduction of HBsAg through 36 weeks1 Ongoing / Planned Combination Trials Placebo, n=8 20 mg, n=3 50 mg, n=9 100 mg, n=6 200 mg, n=6 Week 0 4 8 12 16 20 24 28 32 36 -1.5 -1.0 -0.5 0.0 Mean Change in Log10 HBsAg (IU/mL) Currently in Phase 2 PEG-IFN-⍺ Start Phase 2 trial in 2H:2021 VIR-3434 mAb Start Phase 2 trial in 2H:2021 GS-9688 TLR-8 agonist nivolumab PD-1 antagonist Currently in Phase 2 BRII-179 T cell vaccine Note: current and planned trials are/will be conducted in patients with chronic HBV on nucleotide/nucleoside reverse transcriptase inhibitors (NRTIs), which are standard of care treatment. PEG-IFN: pegylated interferon alpha. 1. Gane et al., Digital International Liver Conference 2020.
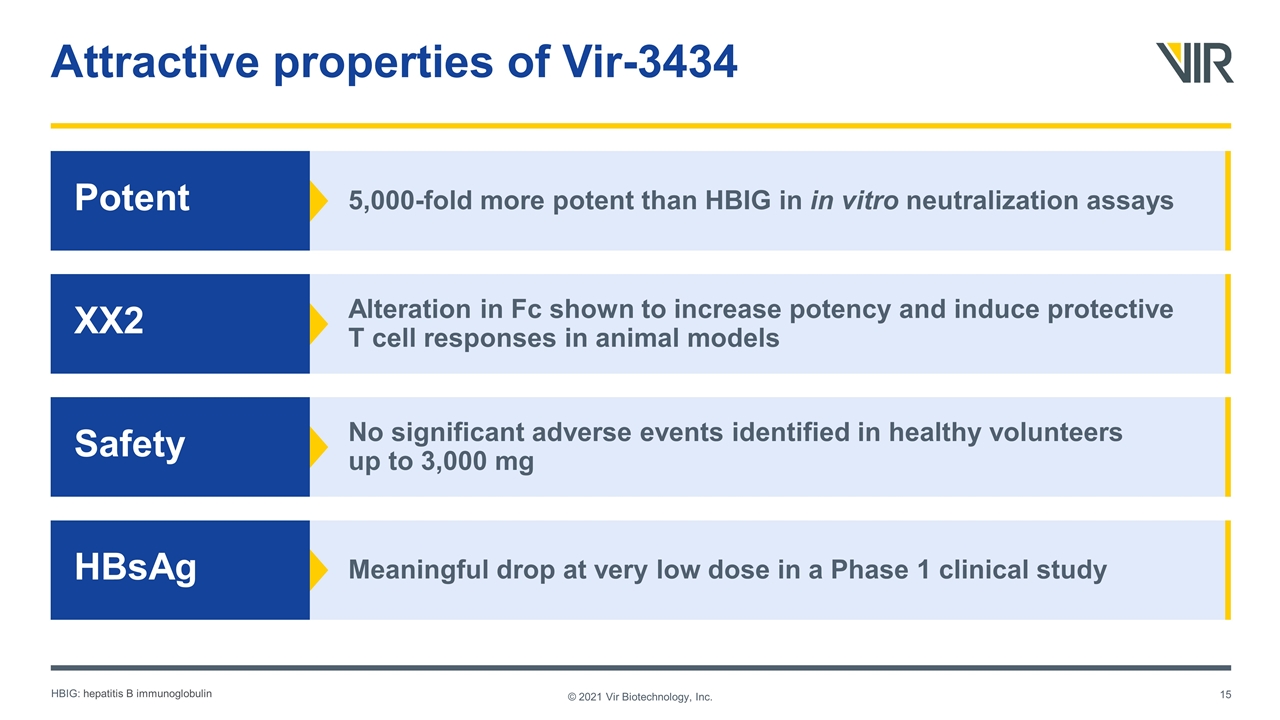
5,000-fold more potent than HBIG in in vitro neutralization assays Attractive properties of Vir-3434 © 2021 Vir Biotechnology, Inc. Potent Alteration in Fc shown to increase potency and induce protective T cell responses in animal models XX2 No significant adverse events identified in healthy volunteers up to 3,000 mg Safety Meaningful drop at very low dose in a Phase 1 clinical study HBsAg HBIG: hepatitis B immunoglobulin
© 2021 Vir Biotechnology, Inc. VIR-3434 Phase 1 clinical data First blinded cohort of eight patients with HBsAg levels less than 1,000 IU/ml Two received placebo Six received a single 6 mg dose of VIR-3434 Six of eight patients achieved a mean reduction of 1.3 log10 IU/mL HBsAg by day eight, the day when nadir was achieved in most patients Additional clinical data expected in 2Q:2021
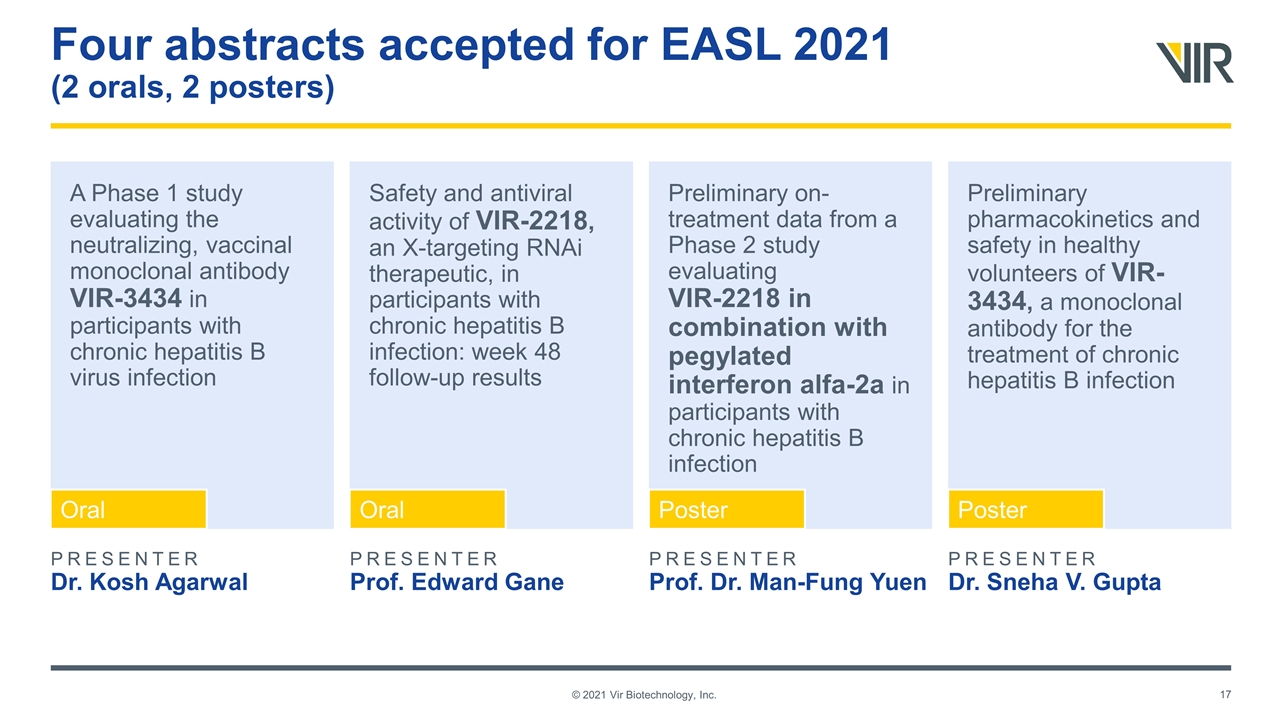
A Phase 1 study evaluating the neutralizing, vaccinal monoclonal antibody VIR-3434 in participants with chronic hepatitis B virus infection Safety and antiviral activity of VIR-2218, an X-targeting RNAi therapeutic, in participants with chronic hepatitis B infection: week 48 follow-up results Preliminary on-treatment data from a Phase 2 study evaluating VIR-2218 in combination with pegylated interferon alfa-2a in participants with chronic hepatitis B infection Preliminary pharmacokinetics and safety in healthy volunteers of VIR-3434, a monoclonal antibody for the treatment of chronic hepatitis B infection Four abstracts accepted for EASL 2021 (2 orals, 2 posters) © 2021 Vir Biotechnology, Inc. PRESENTER Dr. Kosh Agarwal PRESENTER Prof. Edward Gane PRESENTER Prof. Dr. Man-Fung Yuen PRESENTER Dr. Sneha V. Gupta Oral Oral Poster Poster © 2021 Vir Biotechnology, Inc.
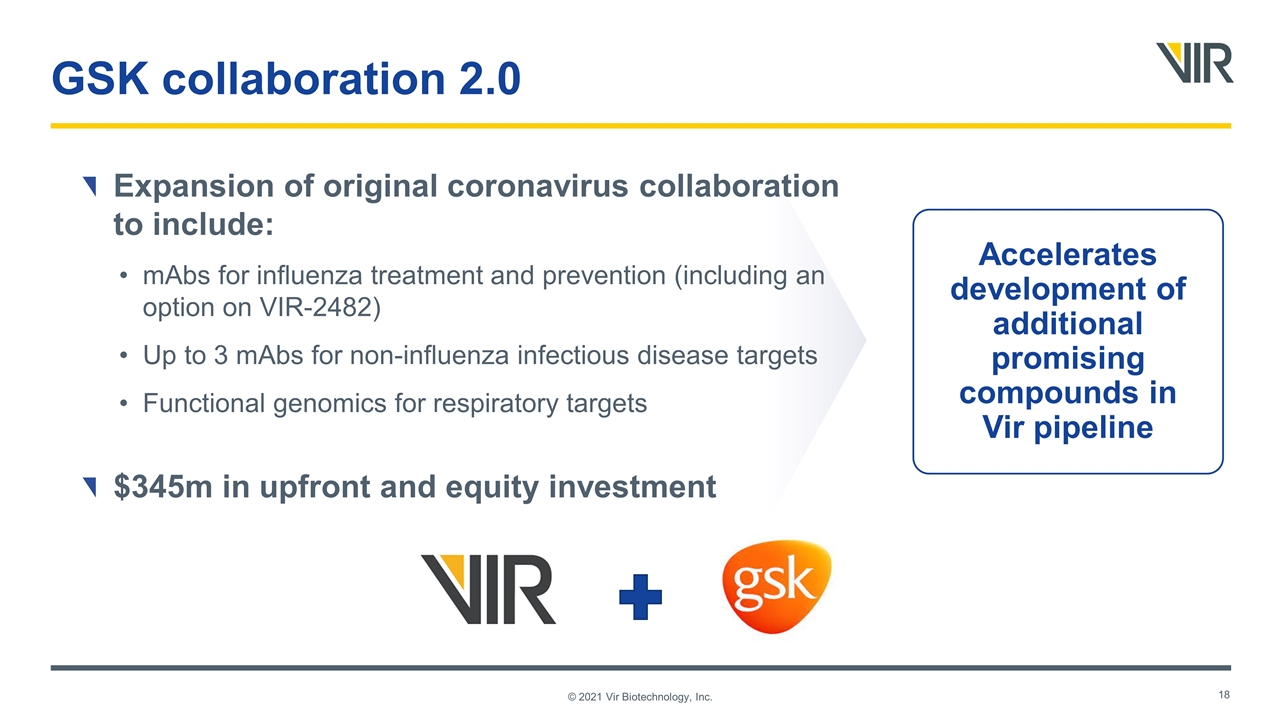
GSK collaboration 2.0 Expansion of original coronavirus collaboration to include: mAbs for influenza treatment and prevention (including an option on VIR-2482) Up to 3 mAbs for non-influenza infectious disease targets Functional genomics for respiratory targets $345m in upfront and equity investment Accelerates development of additional promising compounds in Vir pipeline © 2021 Vir Biotechnology, Inc.
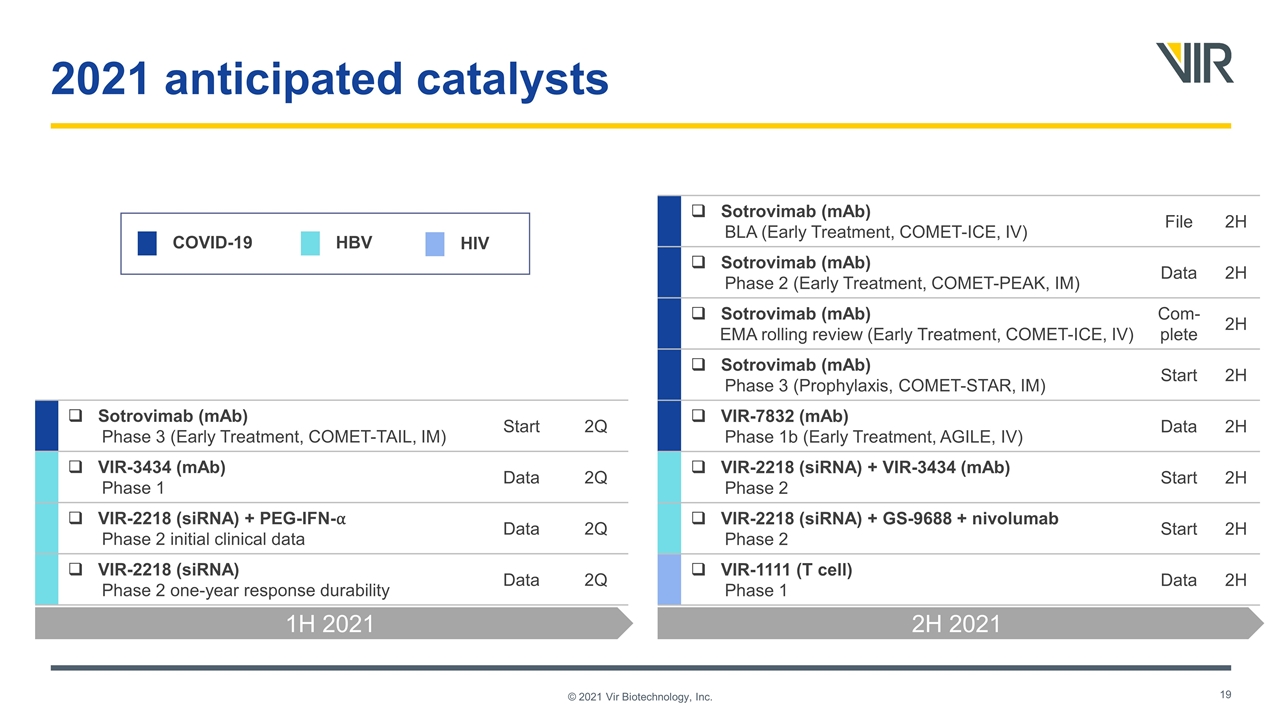
© 2021 Vir Biotechnology, Inc. 2021 anticipated catalysts Sotrovimab (mAb) Phase 3 (Early Treatment, COMET-TAIL, IM) Start 2Q VIR-3434 (mAb) Phase 1 Data 2Q VIR-2218 (siRNA) + PEG-IFN-⍺ Phase 2 initial clinical data Data 2Q VIR-2218 (siRNA) Phase 2 one-year response durability Data 2Q 1H 2021 2H 2021 COVID-19 HBV HIV Sotrovimab (mAb) BLA (Early Treatment, COMET-ICE, IV) File 2H Sotrovimab (mAb) Phase 2 (Early Treatment, COMET-PEAK, IM) Data 2H Sotrovimab (mAb) EMA rolling review (Early Treatment, COMET-ICE, IV) Com- plete 2H Sotrovimab (mAb) Phase 3 (Prophylaxis, COMET-STAR, IM) Start 2H VIR-7832 (mAb) Phase 1b (Early Treatment, AGILE, IV) Data 2H VIR-2218 (siRNA) + VIR-3434 (mAb) Phase 2 Start 2H VIR-2218 (siRNA) + GS-9688 + nivolumab Phase 2 Start 2H VIR-1111 (T cell) Phase 1 Data 2H
