Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Viracta Therapeutics, Inc. | d31397d8k.htm |
| EX-99.1 - EX-99.1 - Viracta Therapeutics, Inc. | d31397dex991.htm |
Exhibit 99.2
DESCRIPTION OF VIRACTA’S BUSINESS
Overview
Viracta Therapeutics, Inc. (“Viracta”) is a clinical-stage, precision oncology company focused on advancing new medicines for the treatment of virus-associated malignancies. The association of viruses and cancer has been well characterized, and Viracta’s lead program is focused on cancers associated with the Epstein-Barr virus (“EBV”). EBV has been recognized as a Group 1 human carcinogen by the World Health Organization. Despite the association of EBV with cancer, attempts to develop vaccines have not proven successful. EBV enters periods of latency during which most viral genes are epigenetically suppressed, which allows an infected cell to not be killed by the virus should it enter a lytic replication cycle. Likewise, the latently infected cell can evade the body’s immune surveillance mechanisms. In some stages of latency, no viral proteins are expressed on the cell surface, making it difficult to develop broadly effective immunotherapies. There are over 300,000 new cases of EBV-associated cancers each year with regard to lymphoma, nasopharyngeal carcinoma (“NPC”) and gastric carcinoma (“GC”), and there are currently no approved therapies for these cancers, which are responsible for over 180,000 deaths each year. Viracta’s novel synthetic lethality approach targets the EBV genome to enable the killing of the tumor cells by inducing the expression of certain viral kinase genes which in-turn activate an antiviral drug. The activated antiviral drug disrupts the DNA replication cycle of the target cells resulting in chain termination and killing of the tumor cells by inducing apoptosis, also known as programmed cell death. This synthetic lethality approach may also be applicable to other cancers associated with the herpes family of viruses, to which EBV belongs, such as glioblastoma associated with cytomegalovirus (“CMV”), Kaposi’s sarcoma with Kapois’s sarcoma virus (“KSV”), and gastrointestinal carcinomas with Human Herpesvirus 6 (“HHV6”).
Viracta’s lead product candidate is an all-oral combination of nanatinostat, Viracta’s proprietary investigational drug, and valganciclovir. Viracta has recently completed enrollment in the Phase 2 portion of its ongoing Phase 1b/2 clinical trial for the treatment of EBV+ lymphoma and expects updated data from this trial in the second half of 2021. Viracta also plans to initiate its global pivotal trial in the second quarter of 2021. In addition, Viracta expects the clearance of a U.S. Investigational New Drug application for the treatment of EBV-associated solid tumors in mid-2021 and expects to initiate a global Phase 1b/2 clinical trial the second half of 2021. Viracta has received Fast Track Designation by the U.S. Food and Drug Administration (the “FDA”) for the treatment of relapsed or refractory EBV+ lymphoid malignancies, in addition to orphan drug designations for the treatment of post-transplant lymphoproliferative disorders (“PTLD”), plasmablastic lymphoma, and T-cell lymphomas.
The Viracta team possesses deep, cross-functional experience and longstanding commitment to developing new medicines to benefit patients. Collectively, the Viracta management team has extensive industry experience in both private and publicly-traded companies, having co-founded companies, such as Hybritech, Inc. and IDEC Corporation, raised substantial debt and equity capital, and discovered and developed oncology treatments at companies such as Novartis, Janssen and MorphoSys.
Strategy
Viracta’s strategy is to build a leading precision oncology company focused on virus-associated malignancies. While the association between viruses and cancer has been well characterized, there are currently no approved therapies that specifically target virus-harboring cancer cells. Viracta’s lead program targets an area of high unmet medical need with no approved therapies. Viracta’s current pipeline is derived from its ability to leverage its synthetic lethality and biomarker-driven approach. Viracta prioritizes virus-associated targets that it believes have the potential to change the paradigm of treatment in virus-associated cancers.
Key elements of Viracta’s strategy are as follows:
| • | Rapidly advance Viracta’s lead product candidate, nanatinostat in combination with valganciclovir , through clinical development, initially in EBV+ lymphomas; |
| • | Expand development of nanatinostat in combination with valganciclovir or variants thereof in other EBV+ malignancies, beginning with solid tumors harboring the EBV genome; |
| • | Leverage its synthetic lethality approach and evaluate other malignancies associated with other viruses in the herpes family; |
| • | Evaluate opportunities to combine nanatinostat in combination with valganciclovir or nanatinostat alone with other therapeutic modalities; and |
| • | Evaluate other opportunities to expand Viracta’s pipeline through licensing, acquisitions and/or partnerships. |
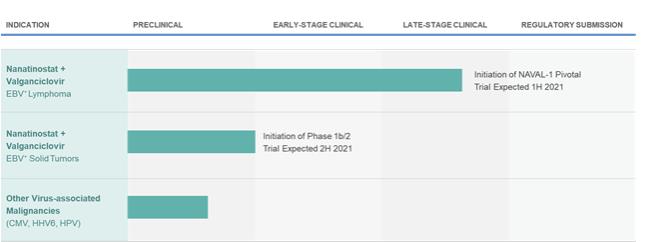
Pipeline
The following figure summarizes Viracta’s current development programs:
Figure 1: Viracta’s Development Pipeline
Scientific Background
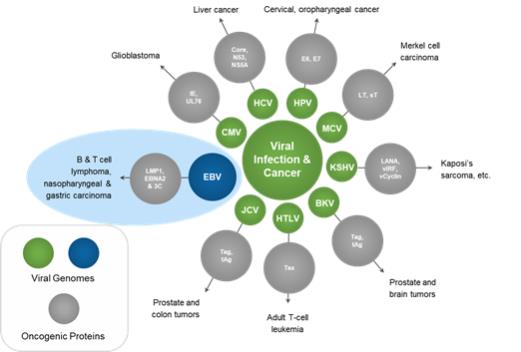
Overview of Virus-associated Malignancies
The association of viruses and cancer has been well characterized. Despite knowing of EBV’s association with cancer for over half a century, attempts to develop vaccines have not proven successful. The figure below illustrates the association of a number of viruses with various cancers.
Figure 2: Viral Infection and Cancer
EBV, a member of the g-herpesvirus family, was the first virus directly implicated in the development of a human tumor and is formally classified as a Group 1 human carcinogen by the WHO. Primary infection with EBV typically occurs in childhood, occurring first in the nasopharynx and is generally asymptomatic; however, infection later in life may manifest as infectious mononucleosis. Once infected, individuals remain life-long carriers of the virus, with more than 90% of the world’s population asymptomatically infected with EBV. The EBV genome can be detected in approximately one out of one million circulating B lymphocytes.
EBV Latency
EBV enters periods of latency during which most viral genes are epigenetically suppressed, as depicted in the figure below. In some stages, no viral proteins are expressed on the cell surface, making it difficult to develop broadly effective immunotherapies.
Figure 3: EBV Latency in Cells
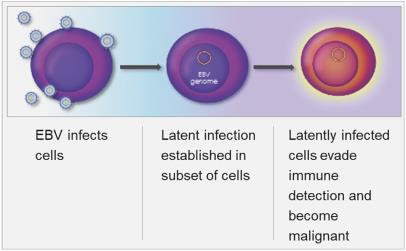
Latent infection and intermittent reactivation are two important characteristics of the EBV lifecycle. The maintenance of latent EBV infection requires the expression of a small subset of genes, and specific expression patterns (Types I – III) of these genes are associated with specific EBV-driven malignancies. EBV has been shown to infect B-cells, T-cells, T/NK-cells and epithelial cells, though its greatest predilection is for B-cells. EBV has been associated with a wide spectrum of human malignancies, with B-cell lymphomas being the most common, and include EBV+ diffuse large B cell lymphoma, not otherwise specified (“EBV+ DLBCL, NOS”), Burkitt’s lymphoma (“BL”), PTLD, and lymphomas associated with congenital and acquired immunodeficiencies, including HIV-related lymphomas.
Mechanism of Action of Nanatinostat in Combination with Valganciclovir
Viracta’s lead product candidate utilizes a combination of Viracta’s oral proprietary epigenetic drug, nanatinostat, in combination with the oral antiviral drug valganciclovir. Nanatinostat targets the EBV genome and induces the expression of certain viral kinase genes. Valganciclovir is converted to ganciclovir in the gut, and these viral genes then activate ganciclovir, which disrupts the DNA replication cycle, leading to DNA chain termination and killing of the tumor cells by inducing apoptosis. This type of killing can be considered a form of synthetic lethality, where neither drug alone would be as effective in killing the tumor cells, but together the two drugs are lethal to the tumor cells.
Epigenetic Re-Programming
EBV gene products can drive aberrant cell proliferation, inhibit programmed cell death and other mechanisms that promote formation of cancer. Viruses and cancers have also evolved mechanisms to evade immune detection by up-regulating immunosuppressive signals, including the induction of PD-L1. Due to these pleiotropic effects, so-called molecularly targeted approaches that target only one oncogenic activity have shown limited ability to impact these cancers.
Epigenetics refers to mechanisms that control which genes or gene programs are turned on or which ones are silenced. Chemical modification of gene promoter regions via methylation, or acetylation or deacetylation of histones around which DNA is coiled, are major mechanisms by which gene expression is controlled.
EBV-Associated Lymphomas
EBV-associated lymphomas are a heterogeneous group of malignancies that harbor latent EBV within the tumor cells. Within a specific histologic subtype of lymphoma, the frequency of EBV positivity may vary considerably. EBV is associated with approximately 5% of DLBCL in Western countries and approximately 10%-15% in Asia and South America, whereas approximately 30% of peripheral T cell lymphoma (“PTCL”) and HL within North America are EBV+, and endemic BL are EBV+ in approximately 95% of the cases. The risk for developing an EBV+ lymphoma has been observed to be higher in the setting of immunodeficiency, including in patients with HIV, congenital immunodeficiencies and post-transplant immunosuppression. EBV-related lymphomas express a limited number of viral genes, with latency expression patterns associated with specific lymphoma subtypes. The limited expression of EBV genes may allow for persistence of the viral DNA in cells by restricting the visibility of the virus to the immune system. The observation that lymphomas in patients with impaired immune function are more likely to express a greater number of viral genes is supportive of this concept. EBV-related lymphoproliferative disease in immunosuppressed patients may respond to therapies that improve immune function, such as reduced immunosuppression (e.g., transplant patients) or adoptive immunotherapy. In contrast, EBV-related lymphomas that arise in immunocompetent patients generally express fewer viral genes and proteins, and as a result are less prone to immune attack.
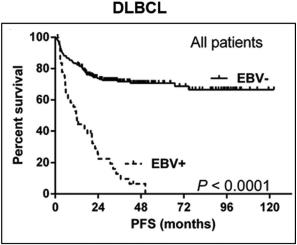
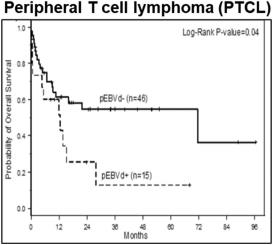
While outcomes vary based on the specific malignancy, EBV-associated lymphomas (e.g., DLBCL, HL, BL, and a number of T-cell lymphomas) often present a treatment challenge as clinical outcomes such as progression free survival and overall survival are worse following standard of care regimens as compared to those for EBV-negative patients. The presence of EBV in lymphomas is therefore generally considered to be a poor prognostic indicator. As shown in the figure below, across a series of patients with DLBCL, those who were EBV+ generally presented with more aggressive disease, had lower response rates to first-line therapy, and poorer progression free survival and overall survival. In a meta-analysis evaluating survival outcomes across several lymphoma types (HL, DLBCL, T/NKCL, PTCL), EBV+ disease was associated with significantly worse overall survival.
Figure 4: Outcomes in EBV-negative vs EBV+ Lymphomas
|
|
Source: Lu TX, et al. Sci Rep 5,12168, 2015; Haverkos BM et al. Int J Cancer. 2017
EBV-positivity in tumor cells can be detected using in situ hybridization for EBV-encoded RNA (EBER-ISH). This is a clinical laboratory test that detects the presence of EBV in the cancer cell. This test is currently most often used to assist in the diagnosis of lymphoma subtypes. Because there has been a lack of actionable targeted therapies in past, EBER-ISH has not been routinely used to guide course of treatment.
Histopathological detection of EBV is included in the NCCN guidelines, which comment on the importance of determining EBV positivity utilizing either EBER-ISH and/or LMP-1 immunohistochemistry (“IHC”) in B and T cell lymphomas. Additionally, protocols established by the American College of Pathology for both Hodgkin and non-Hodgkin lymphoma include determination of EBV status. These laboratory developed tests are widely available in pathology labs, however the frequency at which the testing is performed varies by treatment center and disease type and is lower in community treatment centers compared to academic institutions. This is likely primarily due to the absence of an available EBV targeted therapy, which impacts the evaluation of treatment options for these patients.
Several antiviral drugs have been tested in the clinic against EBV-related lymphomas but have not shown activity as single agent treatments. Antivirals that are prodrugs, such as ganciclovir, acyclovir and valganciclovir (an oral prodrug of ganciclovir) are specific for the viral kinases and not the cellular kinases, and, therefore, require the presence of functional viral kinases, such as thymidine kinase (TK) and protein kinase (PK) for activation and are ineffective at eliminating EBV-associated tumors because these kinase enzymes are not being produced in the latent state. To overcome this barrier, Viracta utilizes its proprietary epigenetic drug candidate, nanatinostat, to induce the expression of these viral kinase genes. Once induced, the viral kinases will phosphorylate and activate the the antiviral prodrug in the tumor cell, resulting in the killing of the tumor cell. Viracta has determined that for EBV-associated malignancies, oral valganciclovir, which is converted to ganciclovir in the gut, is its preferred antiviral drug.
Nanatinostat belongs to a class of drugs known as histone deacetylase (“HDAC”) inhibitors. HDAC inhibitors have been explored in the past, at high doses, as anti-tumor tumor agents. They were shown to have, in general, limited activity and in general, the efficacy they did exhibit was compromised by their adverse event profile. Nanatinostat is a highly potent class I HDAC inhibitor, specifically targeting those few HDAC isozymes required for silencing of EBV in tumors. In contrast to the way in which other HDAC inhibitors have been tested in oncology, Viracta has used nanatinostat at low, epigenetically-active doses to re-activate the latent EBV gene expression, thereby lowering and in some instances, avoiding, certain issues common to all other HDAC inhibitors.
HDAC Enzymes and Inhibitors
Currently, 18 HDAC enzymes have been identified in mammalian cells, varying in function, localization, and substrates. They are subdivided into four main classes based on homology to yeast proteins. Three of the four classes (Classes I, II, and IV) are zinc (Zn2+) dependent enzymes and are inhibited by the pan-HDAC inhibitors.
Epigenetic modulation of gene expression is an essential biological process, with chromatin structure and gene transcription tightly regulated by the acetylation state of histones in the nucleosome. Histone acetylation is a balance of the actions of histone acetyltransferases (“HATs”) and HDACs, with acetylation of histones generally associated with induction of gene transcription and deacetylation with decreases in transcription. Though a seemingly simple concept, it belies the complexity of the effects of HDAC inhibition on chromatin structure. Exposure to HDAC inhibitors rapidly leads to compensating changes in histone methylation and changes in expression of histone modulators. In tumor cells, the most commonly reported effects of HDAC inhibitors relate to induction of apoptosis, though they have also been shown to interfere with cell growth and differentiation and inhibit angiogenesis. In addition, HDAC inhibitors have been shown to modulate immune responses which, in turn, affect many diverse cellular functions.
To date, more than 15 HDAC inhibitors have been tested in preclinical and clinical studies. HDAC inhibitors are broadly classified into four main groups based on their structure: hydroxamic acids, cyclic peptides/depsipeptides, benzamides, and short-chain fatty acids. A common mechanism of action of these drugs is to bind a critical Zn2+ ion required for catalytic function of the HDAC enzyme.
Several HDAC inhibitors have demonstrated clinical antitumor activity when dosed at or near the maximum tolerated dose, with four currently approved by the FDA for oncology indications. These are vorinostat for the treatment of cutaneous T cell lymphoma, romidepsin for the treatment of cutaneous T-cell lymphoma, belinostat for the treatment of peripheral T-cell lymphoma, and panobinostat for the treatment of multiple myeloma. Common side effects seen with these HDAC inhibitors include thrombocytopenia, neutropenia, anemia, fatigue and diarrhea. While HDAC inhibitors have displayed some antitumor activity as single agents, data suggest that the HDAC inhibitors may have a greater role as part of combination regimens.
Rationale for the Combination of HDAC Inhibitors and Antivirals for the Treatment of EBV-Associated Lymphomas
Induction of the viral enzymes TK and PK within EBV+ lymphomas results in the sensitization of tumor cells to antiviral agents such as ganciclovir. This approach is also predicted to have high tumor specificity as only EBV-containing replicating cells would be targeted and neighboring EBV-negative non-replicating cells would be unaffected. The potential for this approach was initially demonstrated in a patient who had developed an EBV+ immunoblastic lymphoma in a donor lung four months after transplantation. Based on previous work that had shown that butyrate congeners could induce EBV lytic phase genes (including viral TK13,14) a cell line derived from the patient’s tumor was exposed to arginine butyrate, a pan-HDAC inhibitor, which induced EBV TK transcription and in combination with ganciclovir inhibited cell proliferation and resulted in cell death. Arginine butyrate was added to the patient’s existing treatment with ganciclovir with no apparent additional toxicity. Though the patient succumbed to a systemic Aspergillus infection that had preceded arginine butyrate therapy, subsequent pathologic examination of the tumor showed substantial necrosis compared to pre-therapy histology. However, a definitive causal relationship between arginine butyrate/ganciclovir therapy and antitumor effect was confounded by prior treatment with chemotherapy.
Additional clinical support was demonstrated in a Phase 1/2 investigator sponsored trial that combined the HDAC inhibitor arginine butyrate with intravenous ganciclovir in 15 patients with recurrent EBV+ lymphomas. Arginine butyrate was administered as a continuous intravenous infusion daily on an escalating dose schedule within each patient for 21 days of a 28-day treatment course, combined with a fixed daily dose of ganciclovir that was not interrupted. Eleven patients received at least one full cycle of therapy, and all 15 patients were evaluable for response. Antitumor activity was reported in 10 patients, with 4 complete responses and 6 partial responses.
Development Programs
Viracta’s Combination Approach
The use of nanatinostat in combination with valganciclovir is a novel therapeutic approach to target EBV+ malignancies, using a low-dose of Viracta’s oral proprietary epigenetic drug, nanatinostat, to target the EBV genome and induce the expression of certain viral kinase genes. These viral genes then activate the antiviral prodrug, valganciclovir, which is converted to ganciclovir in the gut, and disrupts the DNA replication cycle. This disruption then leads to chain termination and killing of the tumor cells by inducing apoptosis. This type of killing can be considered a form of synthetic lethality, where neither drug alone would be as effective in killing the tumor cells, but together the two drugs are lethal to the tumor cells.
Nanatinostat is a hydroxamic acid-based Class 1 HDAC inhibitor and is a more than 2,000 times more potent inducer of the latent EBV genes targeted by this approach than arginine butyrate, the first generation HDAC inhibitor previously investigated in an academic setting. Additionally, the class specificity of nanatinostat is critical for both the targeted efficacy and safety of the approach. As a class, HDACs control the acetylation status of a range of histone and non-histone proteins, giving these enzymes a key role in a number of vital cellular processes. HDAC inhibition alters gene expression at many levels, including gene transcription, RNA expression, and signal transduction pathways. Cellular effects include control of the cell cycle, cell differentiation and senescence, inflammation, angiogenesis and apoptosis. Nanatinostat has demonstrated pleiotropic activity both in vitro and in vivo across a range of human cancers in preclinical studies.
The initial clinical trial of nanatinostat was a single-agent Phase 1 clinical trial, wherein nanatinostat was administered as a single-agent at oral doses ranging from 5 to 160 mg daily in 39 patients with refractory solid tumors. The maximum tolerated dose (“MTD”) for once daily continuous dosing of nanatinostat as a single agent was established as 80 mg, with a maximum acceptable dose for once daily continuous dosing of 40 mg. This program was beneficial in establishing the initial safety profile and tolerability of nanatinostat before it was acquired by Viracta for the development of nanatinostat in combination with valganciclovir.
Nanatinostat induces the expression of EBV kinase genes to activate ganciclovir, rather than acting directly as a cytotoxic agent. In clinical trials, nanatinostat has been active below the MTD and has been observed to be non-immunosuppressive. In 46 patients with R/R EBV+ lymphomas in the ongoing Phase 1b/2 trial, the recommended Phase 2 dose regimen of nanatinostat 20 mg p.o. daily for four days/week, i.e., 4 days on, 3 days off, with valganciclovir 900 mg p.o. daily has been generally well-tolerated with the most common grade 3/4 adverse events being reversible cytopenias.
Nanatinostat in Combination with Valganciclovir in Relapsed/Refractory EBV+ Lymphoma
Phase 1b/2 Clinical Trial in Relapsed/Refractory EBV+ Lymphomas
In 2018, Viracta initiated a Phase 1b/2 dose escalation and expansion trial to evaluate the safety and preliminary efficacy and define a recommended Phase 2 dose (RP2D) of nanatinostat in combination with valganciclovir in patients with relapsed/refractory EBV+ lymphomas who had received one or more prior therapies.
The trial is being conducted at approximately 20 centers in the US and 6 centers in Brazil.
Figure 5: Design of Phase 1b/2 Trial in R/R EBV+ Lymphomas

Twenty-five patients were enrolled in the Phase 1b portion of the trial, and the RP2D was determined to be nanatinostat 20 mg p.o. daily for four days/week, i.e., 4 days on, 3 days off, with valganciclovir 900 mg p.o. daily. In December 2020, the initial results were presented from the Phase 2 portion of the trial, which showed that nanatinostat in combination with valganciclovir demonstrated encouraging efficacy in multiple subtypes, including in HDAC inhibitor-refractory disease.
Figure 6: Summary of Efficacy per Subtype in Phase 1b/2 Trial in R/R EBV+ Lymphomas, Presented at ASH 2020
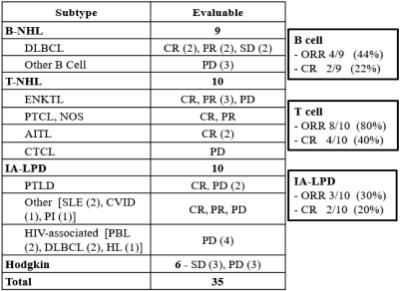
* CR: complete response; PR: partial response; PD: progressive disease; ORR: objective response rate: B-NHL: B-cell non-Hodgkin lymphomas; DLBCL: diffuse large B-cell lymphoma; T-NHL: T-cell non-Hodgkin lymphomas; ENKTL: extranodal NK/T cell lymphoma; PTCL, NOS: peripheral T-cell lymphoma not otherwise specified; AITL: angioimmunoblastic T-cell lymphoma; CTCL: cutaneous T-cell lymphoma; IA-LPD: Immunodeficiency-associated lymphoproliferative disorder; PTLD: post-transplant lymphoproliferative disorder; SLE: systemic lupus erythematosus; CVID: common variable immunodeficiency disorder; PI: primary immunodeficiency; PBL: Plasmablastic lymphoma; HL: Hodgkins lymphoma.
In 46 patients evaluable for safety, the RP2D was generally well-tolerated with the most common grade 3/4 adverse events being reversible cytopenias. Serious adverse events (SAEs) occurred in 14 patients (30%); six in the Phase 2 cohort. SAEs occurring in ³2 patients were febrile neutropenia and pneumonia (both n=2). No study drug related deaths occurred in the treatment period.
Figure 7: Grade 3/4 Treatment-emergent AEs in ³2 Patients from the Phase 1b/2 Trial in R/R EBV+ Lymphomas Data Presented at ASH 2020
| Overall (n=46) |
Phase 2 (n=21) | |||||||||||
| All |
G3 |
G4 |
All |
G3 |
G4 | |||||||
| Thrombocytopenia |
18 (39%) | 6 (13%) | 5 (11%) | 5 (24%) | 1 (5%) | 2 (10%) | ||||||
| Nausea |
17 (37%) | 2 (4%) | — | 7 (33%) | 2 (10%) | — | ||||||
| Neutropenia |
15 (33%) | 4 (9%) | 9 (20%) | 6 (29%) | 2 (10%) | 4 (19%) | ||||||
| Anemia |
14 (30%) | 9 (20%) | 1 (2%) | 5 (24%) | 4 (19%) | 1 (5%) | ||||||
| Lymphopenia |
10 (22%) | 4 (9%) | 4 (9%) | 4 (19%) | 2 (10%) | 1 (5%) | ||||||
| Leukopenia |
9 (20%) | 2 (4%) | 3 (7%) | 4 (19%) | 1 (5%) | 1 (5%) | ||||||
| Acute kidney injury |
6 (13%) | 2 (4%) | 2 (4%) | 1 (5%) | — | 1 (5%) | ||||||
| Febrile neutropenia |
5 (11%) | 4 (9%) | 1 (2%) | 3 (14%) | 2 (10%) | 1 (5%) | ||||||
| Hypokalemia |
5 (11%) | 2 (4%) | — | 3 (14%) | 1 (5%) | — | ||||||
| Urinary tract infection |
5 (11%) | 2 (4%) | — | 2 (10%) | 1 (5%) | — | ||||||
| Hypertension |
3 (7%) | 2 (4%) | — | — | — | — | ||||||
| Pneumonia |
3 (7%) | 1 (2%) | 1 (2%) | 2 (10%) | 1 (5%) | — | ||||||
Pivotal Trial of Nanatinostat in Combination with Valganciclovir in Relapsed/refractory EBV+ Lymphomas
Viracta plans to initiate NAVAL-1 (Nanatinostat in Combination with Valganciclovir), a multinational, multicenter, open-label Phase 2 basket design trial. The trial will include multiple subtype-specific cohorts of R/R EBV+ lymphoma patients designed to evaluate the anti-tumor activity of the combination treatment of nanatinostat with valganciclovir and is anticipated to enroll up to 140 patients. The primary endpoint of the trial is objective tumor response rate as assessed by an independent review committee, while secondary endpoints include duration of response, survival outcomes, and the safety profile of the combined treatment. If successful, Viracta believes this trial could support multiple U.S. New Drug Application filings across various EBV+ lymphoma subtypes.
Nanatinostat in Combination with Valganciclovir in EBV+ Solid Tumors
Viracta intends to investigate nanatinostat in combination with valganciclovir in solid tumors. The annual incidence of NPC and GC is estimated to be approximately 100,000 cases for each of the two malignancy types. Although some treatment options are available, there remains a high unmet need, particularly in patients with relapsed/refractory disease. Beyond NPC and GC, Viracta believes EBV-associated breast cancer, especially triple-negative, cervical, and other head & neck cancers are potential areas for further investigation.
High tumor concentrations of nanatinostat have been demonstrated in murine xenograft mouse models of colorectal cancer. As shown in the figure below, the combination of the class I HDAC inhibitor romidepsin with ganciclovir in human tumor murine xenograft mouse models of EBV+ NPC and GC has demonstrated a greater effect upon tumor growth than the administration of either agent alone, providing the preclinical proof of concept for combining an HDAC inhibitor with an antiviral agent in solid tumors.
Figure 8: Murine Model of EBV+ NPV and GC
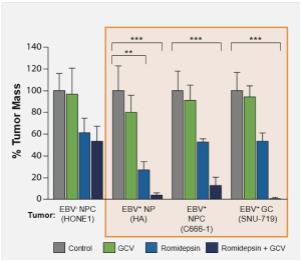
Source: Hui KW, et al. Int J Cancer:138.125-136 (2016)
Viracta expects the clearance of a U.S. Investigational New Drug application for the treatment of EBV-associated solid tumors in mid-2021 and expects to initiate a global Phase 1b/2 clinical trial the second half of 2021.
Other Preclinical Programs
Viracta also plans to explore the application of its synthetic lethality approach in other herpes family virus-associated malignancies, such as glioblastomas associated with CMV.
