Attached files
| file | filename |
|---|---|
| 8-K - 8-K - MIMEDX GROUP, INC. | d143041d8k.htm |
| EX-99.2 - EX-99.2 - MIMEDX GROUP, INC. | d143041dex992.htm |

Advancing regenerative Medicine treatment through placental science Bank of America Securities 2021 Virtual Healthcare Conference May 13, 2021 Exhibit 99.1
Disclaimer & Cautionary Statements Important Cautionary Statement This presentation includes forward-looking statements. Forward-looking statements are subject to risks and uncertainties, and the Company cautions investors against placing undue reliance on such statements. Actual results may differ materially from those set forth in the forward-looking statements. Such forward-looking statements include statements regarding: the Company’s plans to review and analyze the results of its plantar fasciitis, Achilles tendonitis, and knee osteoarthritis clinical trials, and to announce top-line data in Q3 2021, plans for meetings with the FDA, and planned submissions to the FDA, and their timing, and potential FDA approvals, and potential product launch; the results of a clinical trial or trials may have little or no statistical value, or may fail to demonstrate that the product is safe or effective, any meeting with the FDA depends on successful clinical trial results, the availability of such a meeting and its timing is outside of the Company’s control, and the Company may change its plans due to unforeseen circumstances, to conduct additional analyses, or for other reasons, and delay or alter the timeline for future trials, analyses, or public announcements; plans for expansion outside of the U.S., and the potential to expand the Company’s portfolio of products through licensing transactions or additional clinical research; the process of obtaining regulatory clearances or approvals to market a biological product or medical device from the FDA or similar regulatory authorities outside of the U.S. is costly and time consuming, and such clearances or approvals may not be granted on a timely basis, or at all, and the ability to obtain the rights to market additional, suitable products depends on negotiations with third parties which may not be forthcoming; the effectiveness of amniotic tissue as a therapy for any particular indication or condition; the results of a clinical trial or trials may have little or no statistical value, or may fail to demonstrate that the product is safe or effective; expected spending on research and development in 2021, which depends in part on the results of pending clinical trials; and the Company’s long-term strategy for value creation, expectations of future growth, the status of its pipeline products, expectations for future indications or products; such expectations depend upon most or all of the above factors. Additional forward-looking statements may be identified by words such as "believe," "expect," "may," "plan," "potential," "will," "preliminary," and similar expressions, and are based on management's current beliefs and expectations. The Company describes additional risks and uncertainties in the Risk Factors section of its most recent annual report and quarterly reports filed with the Securities and Exchange Commission. Any forward-looking statements speak only as of the date of this presentation and the Company assumes no obligation to update any forward-looking statement.
INDUSTRY LEADER IN UTILIZING AMNIOTIC TISSUE AS A PLATFORM FOR REGENERATIVE MEDICINE Distinct drivers of significant shareholder value with current and future growth potential Base Business Advanced Wound Care Promising Late-Stage Pipeline Musculoskeletal
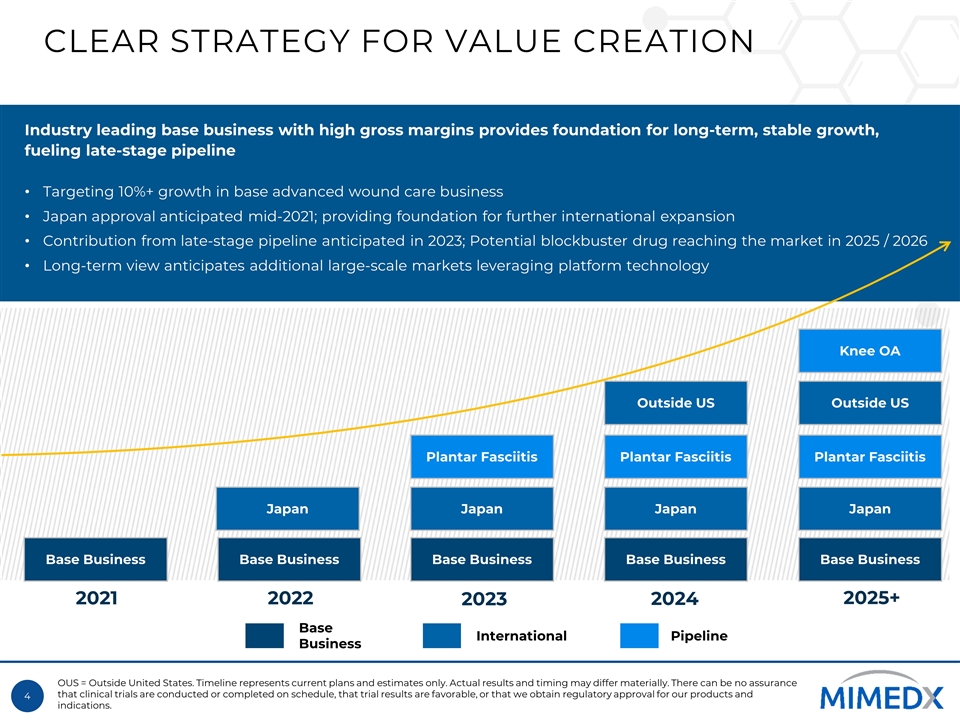
CLEAR STRATEGY FOR VALUE CREATION 2021 2022 2023 2024 Industry leading base business with high gross margins provides foundation for long-term, stable growth, fueling late-stage pipeline Targeting 10%+ growth in base advanced wound care business Japan approval anticipated mid-2021; providing foundation for further international expansion Contribution from late-stage pipeline anticipated in 2023; Potential blockbuster drug reaching the market in 2025 / 2026 Long-term view anticipates additional large-scale markets leveraging platform technology OUS = Outside United States. Timeline represents current plans and estimates only. Actual results and timing may differ materially. There can be no assurance that clinical trials are conducted or completed on schedule, that trial results are favorable, or that we obtain regulatory approval for our products and indications. Japan Base Business Base Business Plantar Fasciitis Japan Base Business Plantar Fasciitis Japan Outside US Base Business Plantar Fasciitis Japan Outside US Base Business Knee OA 2025+ Base Business International Pipeline
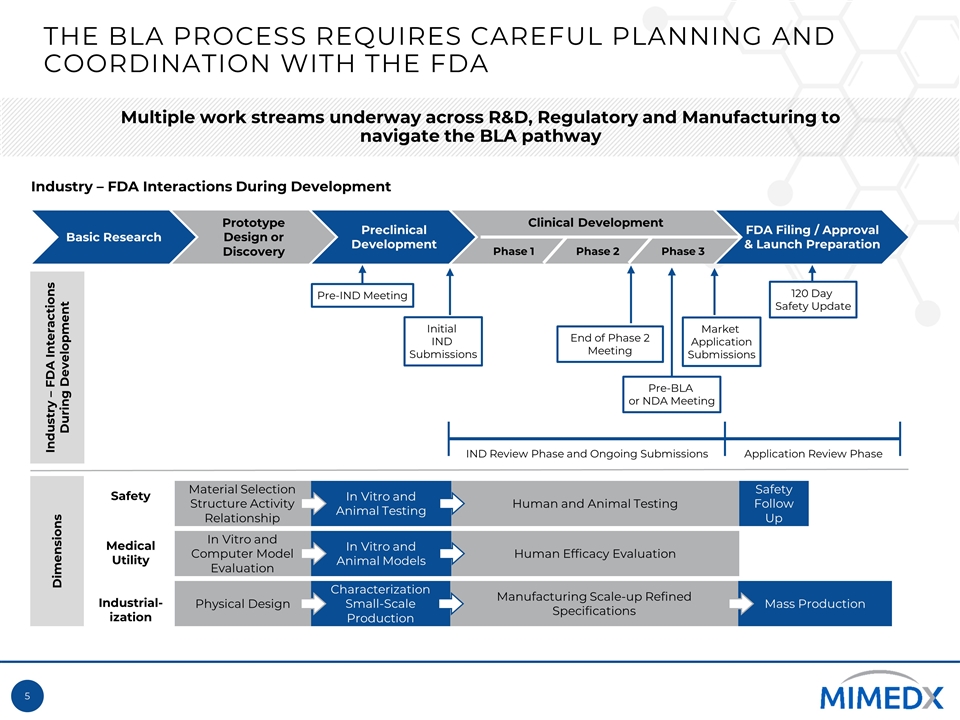
Initial IND Submissions End of Phase 2 Meeting Pre-BLA or NDA Meeting Market Application Submissions Basic Research Prototype Design or Discovery Preclinical Development Clinical Development FDA Filing / Approval & Launch Preparation Phase 1 Phase 3 Phase 2 IND Review Phase and Ongoing Submissions Application Review Phase Dimensions Safety Medical Utility Industrial-ization Material Selection Structure Activity Relationship In Vitro and Animal Testing Human and Animal Testing Safety Follow Up In Vitro and Computer Model Evaluation In Vitro and Animal Models Human Efficacy Evaluation Physical Design Characterization Small-Scale Production Manufacturing Scale-up Refined Specifications Mass Production Industry – FDA Interactions During Development The BLA Process requires careful planning and coordination with the FDA Multiple work streams underway across R&D, Regulatory and Manufacturing to navigate the BLA pathway Pre-IND Meeting 120 Day Safety Update Industry – FDA Interactions During Development
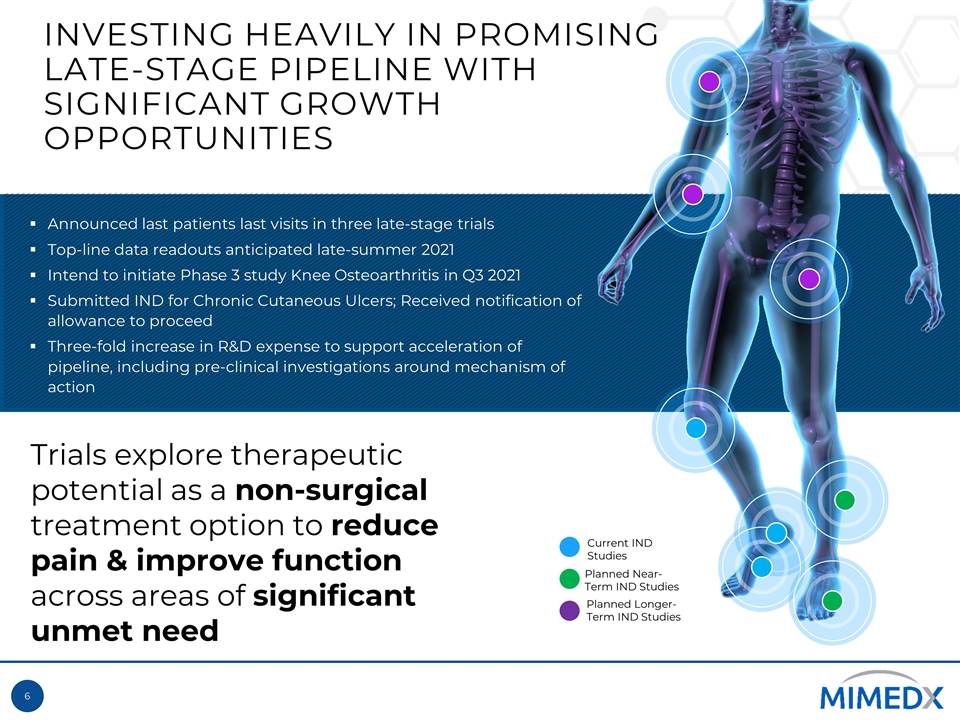
Current IND Studies Planned Near-Term IND Studies INVESTING HEAVILY IN PROMISING LATE-STAGE PIPELINE WITH SIGNIFICANT GROWTH OPPORTUNITIES Announced last patients last visits in three late-stage trials Top-line data readouts anticipated late-summer 2021 Intend to initiate Phase 3 study Knee Osteoarthritis in Q3 2021 Submitted IND for Chronic Cutaneous Ulcers; Received notification of allowance to proceed Three-fold increase in R&D expense to support acceleration of pipeline, including pre-clinical investigations around mechanism of action Planned Longer-Term IND Studies Trials explore therapeutic potential as a non-surgical treatment option to reduce pain & improve function across areas of significant unmet need
FROM FOUNDATION TO TRANSFORMATION Positioning for pipeline acceleration Focusing capital on strategic initiatives Investing in base business for growth
