Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Harmony Biosciences Holdings, Inc. | d501073dex991.htm |
| 8-K - 8-K - Harmony Biosciences Holdings, Inc. | d501073d8k.htm |

Exhibit 99.2 Harmony Biosciences Q1 2021 Financial and Business Update May 11, 2021Exhibit 99.2 Harmony Biosciences Q1 2021 Financial and Business Update May 11, 2021

Legal Disclaimer This presentation includes forward‐looking statements within the meaning of the Private Securities Reform Act of 1995. All statements other than statements of historical facts contained in these materials or elsewhere, including statements regarding Harmony Biosciences Holdings, Inc.’s (the “Company”) future financial position, business strategy and plans and objectives of management for future operations, should be considered forward-looking statements. Forward-looking statements use words like “believes,” “plans,” “expects,” “intends,” “will,” “would,” “anticipates,” “estimates,” and similar words or expressions in discussions of the Company’s future operations, financial performance or the Company’s strategies. These statements are based on current expectations or objectives that are inherently uncertain, especially in light of the Company’s limited operating history. These and other important factors discussed under the caption “Risk Factors” in the Company’s Annual Report on Form 10-K filed with the U.S. Securities and Exchange Commission (the “SEC”) on March 25, 2021 and its other filings with the SEC could cause actual results to differ materially and adversely from those indicated by the forward-looking statements made in this presentation. While the Company may elect to update such forward-looking statements at some point in the future, it disclaims any obligation to do so, even if subsequent events cause its views to change. This presentation includes information related to market opportunity as well as cost and other estimates obtained from internal analyses and external sources. The internal analyses are based upon management’s understanding of market and industry conditions and have not been verified by independent sources. Similarly, the externally sourced information has been obtained from sources the Company believes to be reliable, but the accuracy and completeness of such information cannot be assured. Neither the company, nor any of its respective officers, directors, managers, employees, agents, or representatives, (i) make any representations or warranties, express or implied, with respect to any of the information contained herein, including the accuracy or completeness of this presentation or any other written or oral information made available to any interested party or its advisor (and any liability therefore is expressly disclaimed), (ii) have any liability from the use of the information, including with respect to any forward-looking statements, or (iii) undertake to update any of the information contained herein or provide additional information as a result of new information or future events or developments. 2Legal Disclaimer This presentation includes forward‐looking statements within the meaning of the Private Securities Reform Act of 1995. All statements other than statements of historical facts contained in these materials or elsewhere, including statements regarding Harmony Biosciences Holdings, Inc.’s (the “Company”) future financial position, business strategy and plans and objectives of management for future operations, should be considered forward-looking statements. Forward-looking statements use words like “believes,” “plans,” “expects,” “intends,” “will,” “would,” “anticipates,” “estimates,” and similar words or expressions in discussions of the Company’s future operations, financial performance or the Company’s strategies. These statements are based on current expectations or objectives that are inherently uncertain, especially in light of the Company’s limited operating history. These and other important factors discussed under the caption “Risk Factors” in the Company’s Annual Report on Form 10-K filed with the U.S. Securities and Exchange Commission (the “SEC”) on March 25, 2021 and its other filings with the SEC could cause actual results to differ materially and adversely from those indicated by the forward-looking statements made in this presentation. While the Company may elect to update such forward-looking statements at some point in the future, it disclaims any obligation to do so, even if subsequent events cause its views to change. This presentation includes information related to market opportunity as well as cost and other estimates obtained from internal analyses and external sources. The internal analyses are based upon management’s understanding of market and industry conditions and have not been verified by independent sources. Similarly, the externally sourced information has been obtained from sources the Company believes to be reliable, but the accuracy and completeness of such information cannot be assured. Neither the company, nor any of its respective officers, directors, managers, employees, agents, or representatives, (i) make any representations or warranties, express or implied, with respect to any of the information contained herein, including the accuracy or completeness of this presentation or any other written or oral information made available to any interested party or its advisor (and any liability therefore is expressly disclaimed), (ii) have any liability from the use of the information, including with respect to any forward-looking statements, or (iii) undertake to update any of the information contained herein or provide additional information as a result of new information or future events or developments. 2

Harmony’s Strategy for Growth Expand Optimize Acquire ® WAKIX Clinical New Commercial Utility of Assets Launch WAKIX 3Harmony’s Strategy for Growth Expand Optimize Acquire ® WAKIX Clinical New Commercial Utility of Assets Launch WAKIX 3

Q1 2021 WAKIX Revenue Performance Continued Growth with Q1 Revenue of $59.7M 1Q21 1Q21 $59.7 $56.3 1Q20 4Q20 1Q21 vs. vs. 4Q20 1Q20 $45.6 $19.8 $56.3 $59.7 6% 201% $38.0 Strong Revenue Growth in Q1 2021 $19.8 ▪ Over 200% growth Q1 2021 vs. Q1 2020 ▪ Continued sequential quarter over $6.0 quarter growth from Q4 2020 to Q1 2021 Q4 2019 Q1 2020 Q2 2020 Q3 2020 Q4 2020 Q1 2021 WAKIX Net Revenue ($m) 4Q1 2021 WAKIX Revenue Performance Continued Growth with Q1 Revenue of $59.7M 1Q21 1Q21 $59.7 $56.3 1Q20 4Q20 1Q21 vs. vs. 4Q20 1Q20 $45.6 $19.8 $56.3 $59.7 6% 201% $38.0 Strong Revenue Growth in Q1 2021 $19.8 ▪ Over 200% growth Q1 2021 vs. Q1 2020 ▪ Continued sequential quarter over $6.0 quarter growth from Q4 2020 to Q1 2021 Q4 2019 Q1 2020 Q2 2020 Q3 2020 Q4 2020 Q1 2021 WAKIX Net Revenue ($m) 4

Driving Growth Through Our Launch For WAKIX Q1 2021 Performance ~2,800 Average # of Patient Outreach WAKIX Patients Programs & Support Expanded Virtual Engagement Programs Unique HCP Prescribers >2,700 Since Launch Healthcare Professional Educational Initiatives U.S. Covered Lives With Formulary Access ~80% Managed Care Education & Outreach 5Driving Growth Through Our Launch For WAKIX Q1 2021 Performance ~2,800 Average # of Patient Outreach WAKIX Patients Programs & Support Expanded Virtual Engagement Programs Unique HCP Prescribers >2,700 Since Launch Healthcare Professional Educational Initiatives U.S. Covered Lives With Formulary Access ~80% Managed Care Education & Outreach 5

Core Attributes of WAKIX Product Profile Align with Existing Unmet Needs in Narcolepsy Top Unmet Needs in Narcolepsy * WAKIX (pitolisant) (cited by patients and HCPs) Need for non-scheduled treatment options First and only FDA approved (low/no abuse potential) non-scheduled treatment option for narcolepsy Need for more tolerable treatment Established Safety Profile regimens No Boxed Warning, no REMS Program Need for more effective treatment options Statistically significant reduction in EDS and cataplexy demonstrated in two Phase III trials Novel MOAs beyond currently available First-in-class molecule with a novel MOA; therapies needed H R antagonist/inverse agonist; works 3 through histamine Need for less frequently dosed products; Convenient, once daily dosing in the need for once-daily options morning upon wakening * Based on FDA approved product labeling Source: Harmony ATU, July 2018 (n=286); Versta Research, Know Narcolepsy Survey (“Know Narcolepsy”), October 2018 6 In descending order of importance as stated by combined HCP and patient audienceCore Attributes of WAKIX Product Profile Align with Existing Unmet Needs in Narcolepsy Top Unmet Needs in Narcolepsy * WAKIX (pitolisant) (cited by patients and HCPs) Need for non-scheduled treatment options First and only FDA approved (low/no abuse potential) non-scheduled treatment option for narcolepsy Need for more tolerable treatment Established Safety Profile regimens No Boxed Warning, no REMS Program Need for more effective treatment options Statistically significant reduction in EDS and cataplexy demonstrated in two Phase III trials Novel MOAs beyond currently available First-in-class molecule with a novel MOA; therapies needed H R antagonist/inverse agonist; works 3 through histamine Need for less frequently dosed products; Convenient, once daily dosing in the need for once-daily options morning upon wakening * Based on FDA approved product labeling Source: Harmony ATU, July 2018 (n=286); Versta Research, Know Narcolepsy Survey (“Know Narcolepsy”), October 2018 6 In descending order of importance as stated by combined HCP and patient audience

HCP Insights Demonstrate Future Growth Opportunity for WAKIX in Adult Narcolepsy Key Findings from HCP Market Research: 95% ~90% 84% Significant unmet need WAKIX is effective for Expecting to prescribe the and WAKIX offers a treatment of EDS and same or increase their unique treatment option 90% effective for use of WAKIX in more for patients cataplexy patients in the future ▪ WAKIX is being well received by patients ▪ WAKIX is appropriate for the vast majority of narcolepsy patients ▪ Patient opportunity increased since the approval for the cataplexy indication Demonstrates the overall benefit/risk profile, broad clinical utility to narcolepsy patients Source: Harmony Market Research conducted with 50 narcolepsy treating HCPs, April 2021 (n=50) 7HCP Insights Demonstrate Future Growth Opportunity for WAKIX in Adult Narcolepsy Key Findings from HCP Market Research: 95% ~90% 84% Significant unmet need WAKIX is effective for Expecting to prescribe the and WAKIX offers a treatment of EDS and same or increase their unique treatment option 90% effective for use of WAKIX in more for patients cataplexy patients in the future ▪ WAKIX is being well received by patients ▪ WAKIX is appropriate for the vast majority of narcolepsy patients ▪ Patient opportunity increased since the approval for the cataplexy indication Demonstrates the overall benefit/risk profile, broad clinical utility to narcolepsy patients Source: Harmony Market Research conducted with 50 narcolepsy treating HCPs, April 2021 (n=50) 7
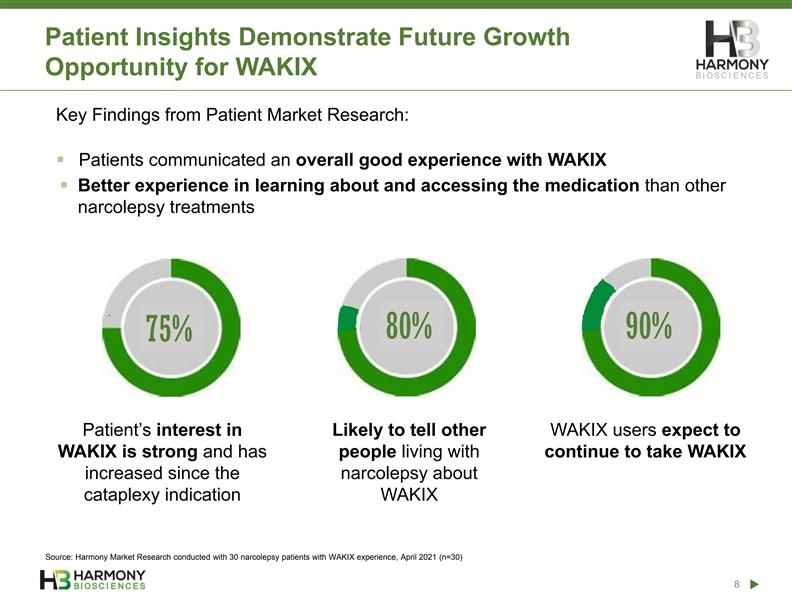
Patient Insights Demonstrate Future Growth Opportunity for WAKIX Key Findings from Patient Market Research: ▪ Patients communicated an overall good experience with WAKIX ▪ Better experience in learning about and accessing the medication than other narcolepsy treatments 80% 90% 75% Patient’s interest in Likely to tell other WAKIX users expect to WAKIX is strong and has people living with continue to take WAKIX increased since the narcolepsy about cataplexy indication WAKIX Source: Harmony Market Research conducted with 30 narcolepsy patients with WAKIX experience, April 2021 (n=30) 8Patient Insights Demonstrate Future Growth Opportunity for WAKIX Key Findings from Patient Market Research: ▪ Patients communicated an overall good experience with WAKIX ▪ Better experience in learning about and accessing the medication than other narcolepsy treatments 80% 90% 75% Patient’s interest in Likely to tell other WAKIX users expect to WAKIX is strong and has people living with continue to take WAKIX increased since the narcolepsy about cataplexy indication WAKIX Source: Harmony Market Research conducted with 30 narcolepsy patients with WAKIX experience, April 2021 (n=30) 8
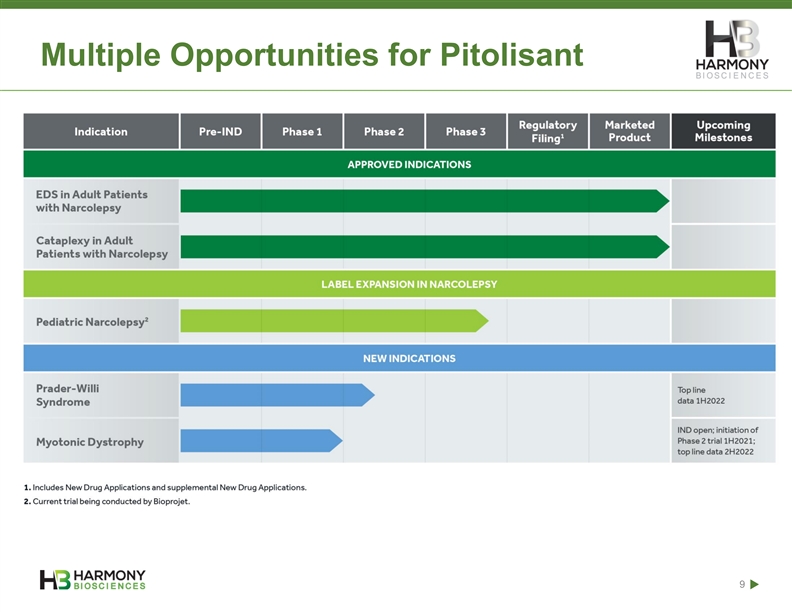
Multiple Opportunities for Pitolisant 9Multiple Opportunities for Pitolisant 9

WAKIX: Effective in Patients with High Burden of Narcolepsy Symptoms ¾ Post-hoc analyses of pooled data from pivotal randomized controlled trials (HARMONY 1 and HARMONY CTP) ¾ Assessed response to WAKIX compared to placebo in patients who had a high burden of EDS (ESS ≥ 16) or cataplexy (weekly rate of cataplexy attacks ≥ 15) at baseline Epworth Sleepiness Scale (ESS) Scores Weekly Rate of Cataplexy (WRC) WAKIX was significantly more effective on WAKIX was significantly more effective on improving reducing the WRC compared with placebo; EDS compared with placebo; mean change in ESS mean change in WRC from baseline to end from baseline to end of treatment was -6.1 for WAKIX of treatment was -14.5 for WAKIX compared compared with -2.4 for placebo (p<0.001) with -0.1 for placebo (p<0.004) Davis et al. Sleep Medicine; 2021 10WAKIX: Effective in Patients with High Burden of Narcolepsy Symptoms ¾ Post-hoc analyses of pooled data from pivotal randomized controlled trials (HARMONY 1 and HARMONY CTP) ¾ Assessed response to WAKIX compared to placebo in patients who had a high burden of EDS (ESS ≥ 16) or cataplexy (weekly rate of cataplexy attacks ≥ 15) at baseline Epworth Sleepiness Scale (ESS) Scores Weekly Rate of Cataplexy (WRC) WAKIX was significantly more effective on WAKIX was significantly more effective on improving reducing the WRC compared with placebo; EDS compared with placebo; mean change in ESS mean change in WRC from baseline to end from baseline to end of treatment was -6.1 for WAKIX of treatment was -14.5 for WAKIX compared compared with -2.4 for placebo (p<0.001) with -0.1 for placebo (p<0.004) Davis et al. Sleep Medicine; 2021 10

WAKIX: Onset of Effect Beginning at Week 2 ¾ Post-hoc analyses of pooled data from pivotal randomized controlled trials (HARMONY 1 and HARMONY CTP) ¾ Assessed time-to-onset of response for WAKIX compared to placebo from baseline to end of trial Conclusions: ¾ WAKIX was effective for improvement in EDS and reduction in cataplexy beginning at week 2 after dosing ¾ Clinical response at the end of the study was more robust when patients were titrated up to the 35.6 mg dose compared to 17.8 mg dose Davis et al. AAN Annual Meeting, April 17 – 22, 2021 11WAKIX: Onset of Effect Beginning at Week 2 ¾ Post-hoc analyses of pooled data from pivotal randomized controlled trials (HARMONY 1 and HARMONY CTP) ¾ Assessed time-to-onset of response for WAKIX compared to placebo from baseline to end of trial Conclusions: ¾ WAKIX was effective for improvement in EDS and reduction in cataplexy beginning at week 2 after dosing ¾ Clinical response at the end of the study was more robust when patients were titrated up to the 35.6 mg dose compared to 17.8 mg dose Davis et al. AAN Annual Meeting, April 17 – 22, 2021 11

Q1 2021 Financial Summary (in millions, USD) Three Months Ended March 31, 2021 2020 Net Product Revenues $ 59.7 $ 19.8 Cost of Product Sold 10.4 3.5 Total Operating Expenses $ 34.7 $ 26.0 R&D Expense 4.7 3.4 S&M Expense 15.5 13.3 G&A Expense 14.5 9.3 Net Income (Loss) $ 7.4 $ (38.6) Cash & cash equivalents $ 141.2 12Q1 2021 Financial Summary (in millions, USD) Three Months Ended March 31, 2021 2020 Net Product Revenues $ 59.7 $ 19.8 Cost of Product Sold 10.4 3.5 Total Operating Expenses $ 34.7 $ 26.0 R&D Expense 4.7 3.4 S&M Expense 15.5 13.3 G&A Expense 14.5 9.3 Net Income (Loss) $ 7.4 $ (38.6) Cash & cash equivalents $ 141.2 12

GAAP vs Non-GAAP Reconciliation (in millions, USD) Three Months Ended March 31, 2021 2020 GAAP reported net income (loss) $ 7.4 $ (38.6) Interest expense / income 7.1 6.4 Taxes Depreciation 0.1 0.1 Amortization 4.6 1.8 EBITDA 19.2 (30.4) Stock-based compensation expense 3.3 0.4 Loss on debt extinguishment 22.6 Warrant expense 1.1 Non-GAAP adjusted net income (loss) 22.4 (6.2) Accumulation of yield on preferred stock (10.4) Non-GAAP adjusted net income (loss) available to $ 22.4 $ (16.7) common stockholders GAAP reported net loss per diluted share $ 0.13 $ (6.30) Non-GAAP adjusted net income (loss) per diluted share $ 0.38 $ (2.14) Weighted average number of shares of common stock used in 58,805,285 7,790,667 non-GAAP diluted per share Totals may not foot due to rounding 13GAAP vs Non-GAAP Reconciliation (in millions, USD) Three Months Ended March 31, 2021 2020 GAAP reported net income (loss) $ 7.4 $ (38.6) Interest expense / income 7.1 6.4 Taxes Depreciation 0.1 0.1 Amortization 4.6 1.8 EBITDA 19.2 (30.4) Stock-based compensation expense 3.3 0.4 Loss on debt extinguishment 22.6 Warrant expense 1.1 Non-GAAP adjusted net income (loss) 22.4 (6.2) Accumulation of yield on preferred stock (10.4) Non-GAAP adjusted net income (loss) available to $ 22.4 $ (16.7) common stockholders GAAP reported net loss per diluted share $ 0.13 $ (6.30) Non-GAAP adjusted net income (loss) per diluted share $ 0.38 $ (2.14) Weighted average number of shares of common stock used in 58,805,285 7,790,667 non-GAAP diluted per share Totals may not foot due to rounding 13

Thank You May 11, 2021Thank You May 11, 2021
