Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Flexion Therapeutics Inc | flxn-8k_20210511.htm |

Interim Data from the First-in-Human Phase 1 Trial of FX201, An Intra-Articular, Helper-Dependent Adenoviral Gene Therapy for Osteoarthritis – Safety, Tolerability, Biodistribution, and Preliminary Evaluation of Clinical Activity in 5 Patients Scott Kelley1, Alan Kivitz2, Becca Senter1, David Golod1, Amy Cinar1, Emily Walsh Martin1, Won Hong1 1Flexion Therapeutics, Inc, Burlington, MA. 2Altoona Clinical Research, Duncansville, PA. Exhibit 99.1
Background: Gene Therapy is Well-Suited to Treatment of OA Osteoarthritis (OA) is a disabling, degenerative disease characterized by progressive pathological joint tissue changes including cartilage loss, bony outgrowths, thickening of subchondral bone, and synovial inflammation Interleukin-1 (IL-1) is believed to be a chief orchestrator of increased inflammation, pain and potentially disease progression Gene therapy offers singular duration of therapeutic effect Disease progression in OA is slow Gene therapy has the potential to maintain expression for years Targeting the joint compartment brings several advantages Local delivery to key cell types involved in pathogenesis and progression of OA Reduced dose of viral particles by orders of magnitude compared to systemic delivery Limits systemic exposure, potentially reducing safety liabilities Evans et al., Human Gene Therapy 2018
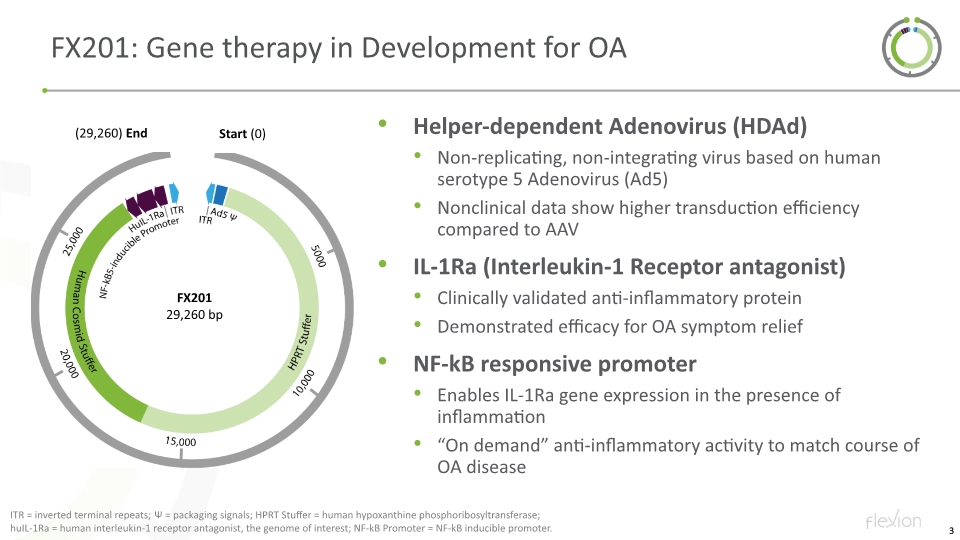
FX201: Gene therapy in Development for OA Helper-dependent Adenovirus (HDAd) Non-replicating, non-integrating virus based on human serotype 5 Adenovirus (Ad5) Nonclinical data show higher transduction efficiency compared to AAV IL-1Ra (Interleukin-1 Receptor antagonist) Clinically validated anti-inflammatory protein Demonstrated efficacy for OA symptom relief NF-kB responsive promoter Enables IL-1Ra gene expression in the presence of inflammation “On demand” anti-inflammatory activity to match course of OA disease ITR = inverted terminal repeats; Ψ = packaging signals; HPRT Stuffer = human hypoxanthine phosphoribosyltransferase; huIL-1Ra = human interleukin-1 receptor antagonist, the genome of interest; NF-kB Promoter = NF-kB inducible promoter.
FX201 First in Human Phase 1 Trial: Single Ascending Dose Escalation in Knee OA Patients NCT04119687: Patients and Methods Adult patients with moderate-to-severe knee OA (symptoms and radiographic) and prior failure of ≥2 other OA treatments were enrolled in the low-dose cohort FX201 administered to index knee via ultrasound-guided intra-articular injection 5 patients in initial low dose cohort were each treated with a local dose of 1.4E10 genome copies Assessments Safety and tolerability Systemic vector biodistribution and shedding Clinical activity of FX201 measured Change in pain (WOMAC-A) Change in function (KOOS Function in daily living) Clinical importance of pain relief assessed using the IMMPACT criteria to characterize moderate (threshold ≥30%) and substantial (threshold ≥50%) improvement (Responder) IMMPACT = Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials; KOOS = Knee Injury and Osteoarthritis Outcome Score; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index
FX201 Safety and Biodistribution in Low-dose Cohort 5 patients dosed in low-dose cohort All patients remain in study 38-56+ weeks following treatment Safety: 4 patients experienced 15 AEs to date 2 patients experienced 5 index-knee AEs probably or possibly related to study medication Onset within the first 4 weeks following administration AEs include knee pain, swelling and effusion; maximum severity = Grade 2 (knee pain) AEs resolved with rest, ice, and/or joint aspiration Biodistribution: Samples from blood, urine, and skin at injection site Samples at 4 hours post-injection, Day 4 and Weeks 1, 2, 3, and 4 No systemic biodistribution in plasma or shedding in urine or at skin site observed in any patient AE = Adverse Event
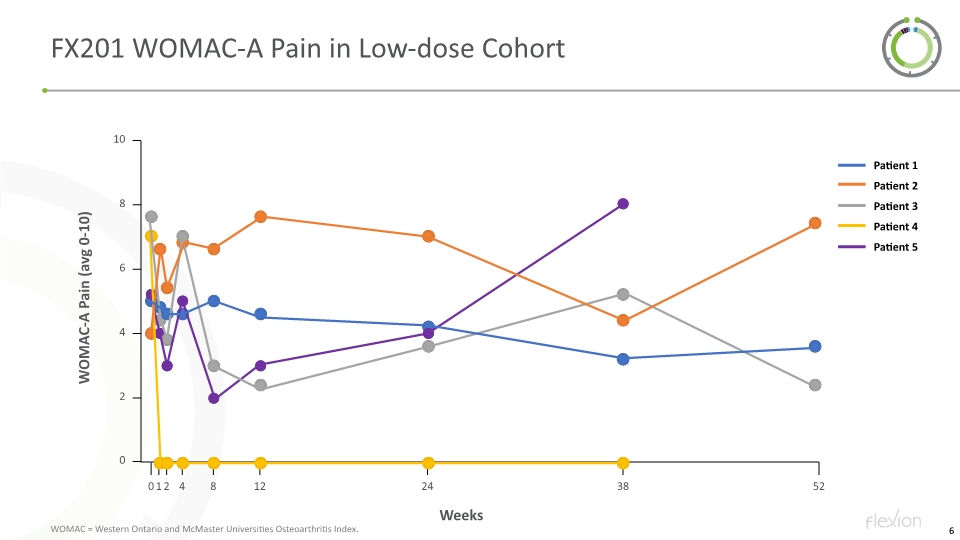
FX201 WOMAC-A Pain in Low-dose Cohort WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
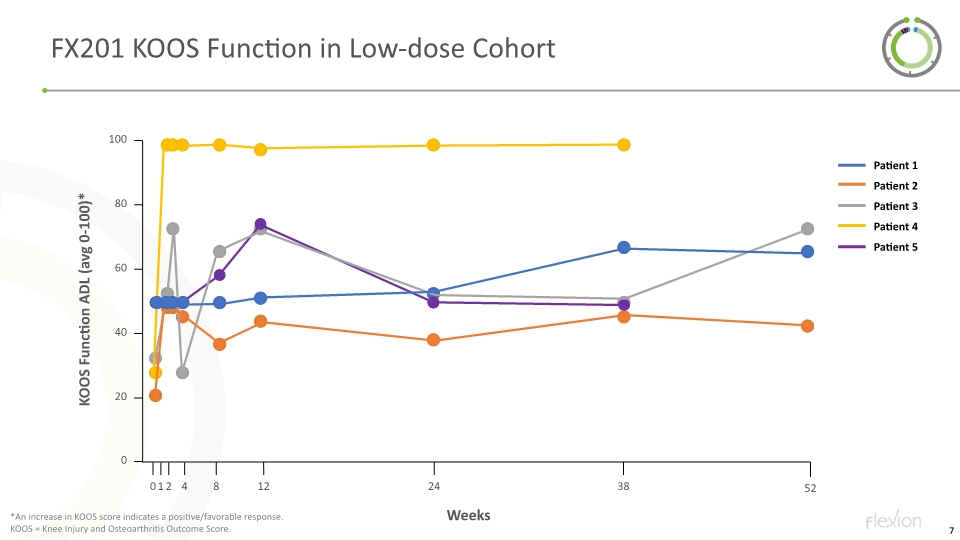
FX201 KOOS Function in Low-dose Cohort *An increase in KOOS score indicates a positive/favorable response. KOOS = Knee Injury and Osteoarthritis Outcome Score. Patient 1 Patient 2 Patient 3 Patient 4 Patient 5 52
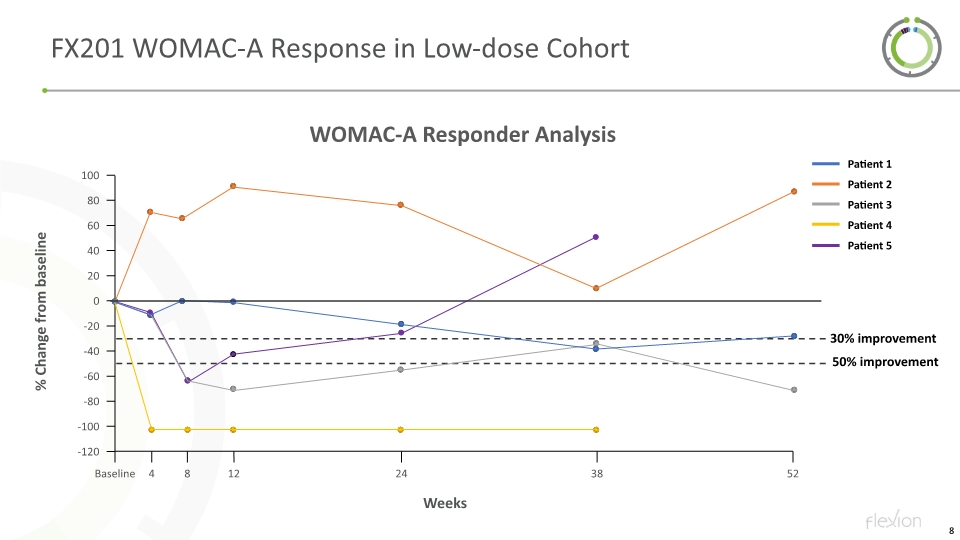
FX201 WOMAC-A Response in Low-dose Cohort Weeks
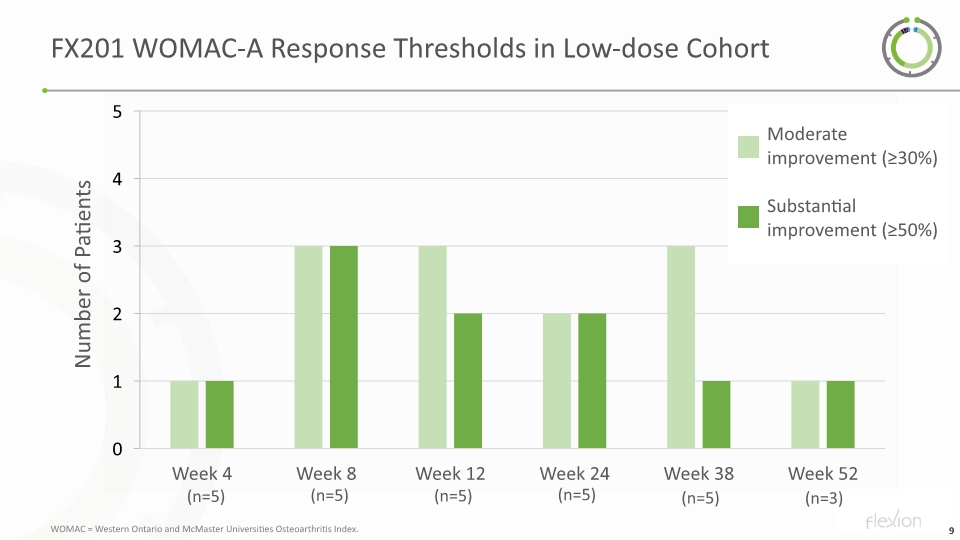
FX201 WOMAC-A Response Thresholds in Low-dose Cohort Number of Patients Moderate improvement (≥30%) Substantial improvement (≥50%) (n=5) (n=5) (n=5) (n=5) (n=5) (n=3) WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.

FX201 First-in-Human Phase 1 Trial: Discussion and Future Direction Initial clinical experience with FX201 in knee OA Intra-articular treatment with FX201 in patients with knee OA generally well tolerated Substantial improvement in WOMAC-A pain intensity observed at 38-52 weeks following single administration NCT04119687 Study Progress Synovial fluid assessments are underway to evaluate for presence of FX201 DNA and expression of FX201-driven IL-1Ra mRNA Expect additional data readouts by end 2021 Early experience shows promise of FX201 to provide important and durable clinical benefit in knee OA
