Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Sigilon Therapeutics, Inc. | tmb-20210510x8k.htm |
| EX-99.1 - EX-99.1 - Sigilon Therapeutics, Inc. | tmb-20210510xex99d1.htm |
Exhibit 99.2
| Advancing Potential Functional Cures for Patients With Chronic Diseases ©2021 Sigilon Therapeutics, Inc. May 2021 |
| Disclaimer This presentation has been prepared by Sigilon Therapeutics, Inc. (“we,” “us,” “our,” “Sigilon” or the “Company”) and is made for informational purposes only and not for any other purpose. Certain information contained in this presentation and statements made orally during this presentation relate to or are based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and Sigilon makes no representation as to the adequacy, fairness, accuracy or completeness of any information obtained from third-party sources. While the Company believes its internal research is reliable, such research has not been verified by any independent source. This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation are forward- looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements include, but are not limited to, statements concerning: our current cash runway; the initiation, timing, progress and results of our research and development programs, preclinical studies and clinical trials, including the timing of our clinical trials for SIG-001 for the treatment of Hemophilia A and the submission of INDs or CTAs for our other product candidates; our ability to advance any product candidates that we may develop and successfully complete any clinical studies, including the manufacture of any such product candidates; our ability to leverage our initial programs to develop additional product candidates using our SLTx platform; and our ability to successfully scale our manufacturing capabilities. Any forward-looking statements represent the Company’s views only as of today and should not be relied upon as representing its views as of any subsequent date. The Company explicitly disclaims any obligation to update any forward-looking statements. The Company’s business is subject to substantial risks and uncertainties. Applicable risks and uncertainties include, among others, that we have incurred significant losses since inception and our need for additional funding; the SLTx platform consists of novel technologies that are not yet clinically validated for human therapeutic use; that we do not have any results from the testing of any of our product candidates in clinical trials and any favorable preclinical results are not predictive of results that may be observed in clinical trials; we may be unable to obtain and maintain patent protection and other intellectual property rights for SIG-001 or any other product candidates and for our SLTx platform, or the scope of the patent and other intellectual property protection obtained may not be sufficiently broad; that a pandemic, epidemic, or outbreak of an infectious disease, such the COVID-19 pandemic, may materially and adversely affect our business and our financial results and could cause a disruption to the development or supply of SIG-001 or any other product candidates; and other risks and uncertainties identified under the heading “Risk Factors” and in our Annual Report on Form 10-K for the year ended December 31, 2020, our Quarterly Report on Form 10-Q for the period ended March 31, 2021 and in any subsequent filings with the Securities and Exchange Commission. 1 Non-Confidential |
| SHIELDED LIVING THERAPEUTICS PLATFORM Company Overview Our mission is to develop functional cures for patients living with a wide range of chronic diseases • Designed to overcome significant limitations of cell and gene therapies and drawbacks of current biologic-based therapies • Potential benefits include: ✓ Safety – no interference with patient’s DNA ✓ Durability ✓ Ability to redose and retrieve ✓ Controllable dosing ✓ Broad patient eligibility ✓ No immunosuppression required ✓ Modularity – efficient development and manufacturing • $372 million in funding to date • Completed IPO in December with $144.9 million in gross proceeds (priced at $18) • Current cash runway into Q4 2022 with IPO proceeds •Dosing in FIH trial in Hem A started in 4Q ‘20 •Regulatory agencies have acknowledged the potential to leverage CMC and non-clinical data across pipeline •Pipeline with 4 IND filings expected within next 2 years STRONG FINANCIAL POSITION CLEARLY DEFINED REGULATORY PATH 2 |
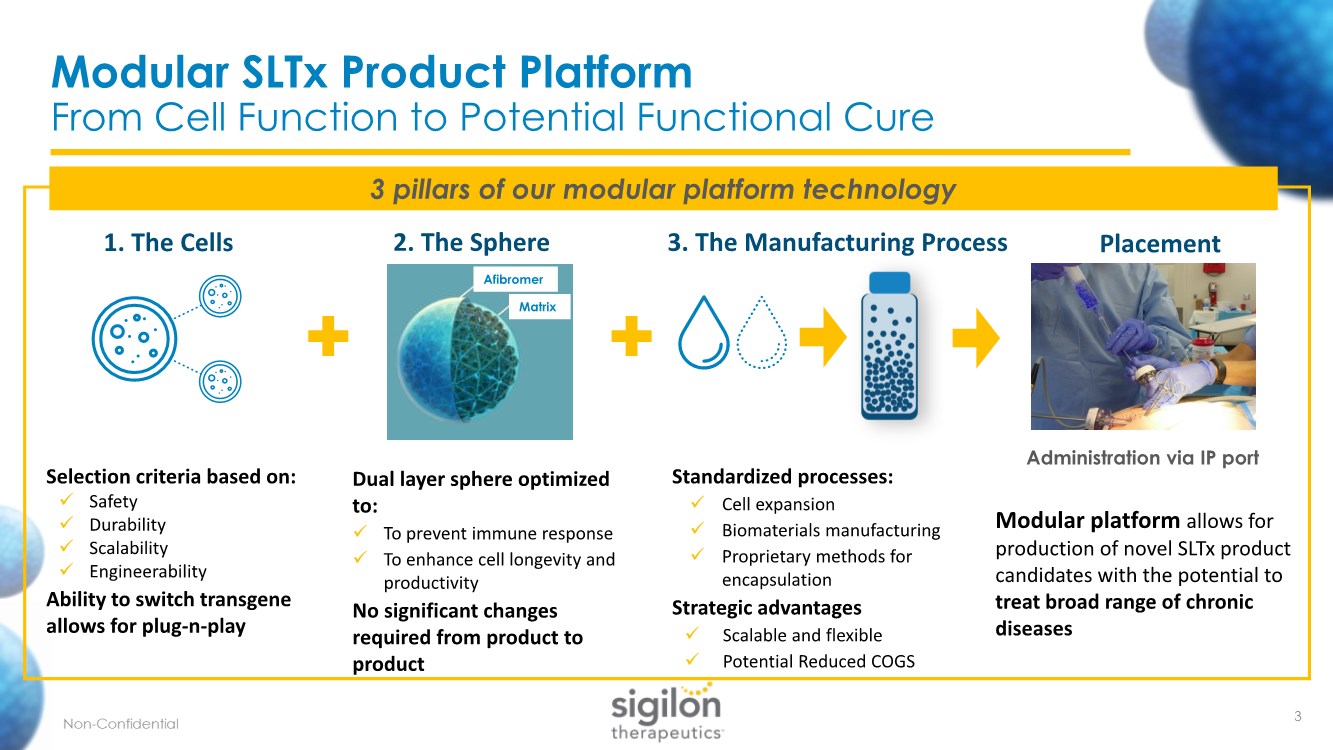
| + + 1. The Cells Selection criteria based on: ✓ Safety ✓ Durability ✓ Scalability ✓ Engineerability Ability to switch transgene allows for plug-n-play 3 pillars of our modular platform technology Modular SLTx Product Platform From Cell Function to Potential Functional Cure 2. The Sphere 3. The Manufacturing Process Dual layer sphere optimized to: ✓ To prevent immune response ✓ To enhance cell longevity and productivity No significant changes required from product to product Standardized processes: ✓ Cell expansion ✓ Biomaterials manufacturing ✓ Proprietary methods for encapsulation Strategic advantages ✓ Scalable and flexible ✓ Potential Reduced COGS Modular platform allows for production of novel SLTx product candidates with the potential to treat broad range of chronic diseases Placement 3 Afibromer Matrix Administration via IP port |
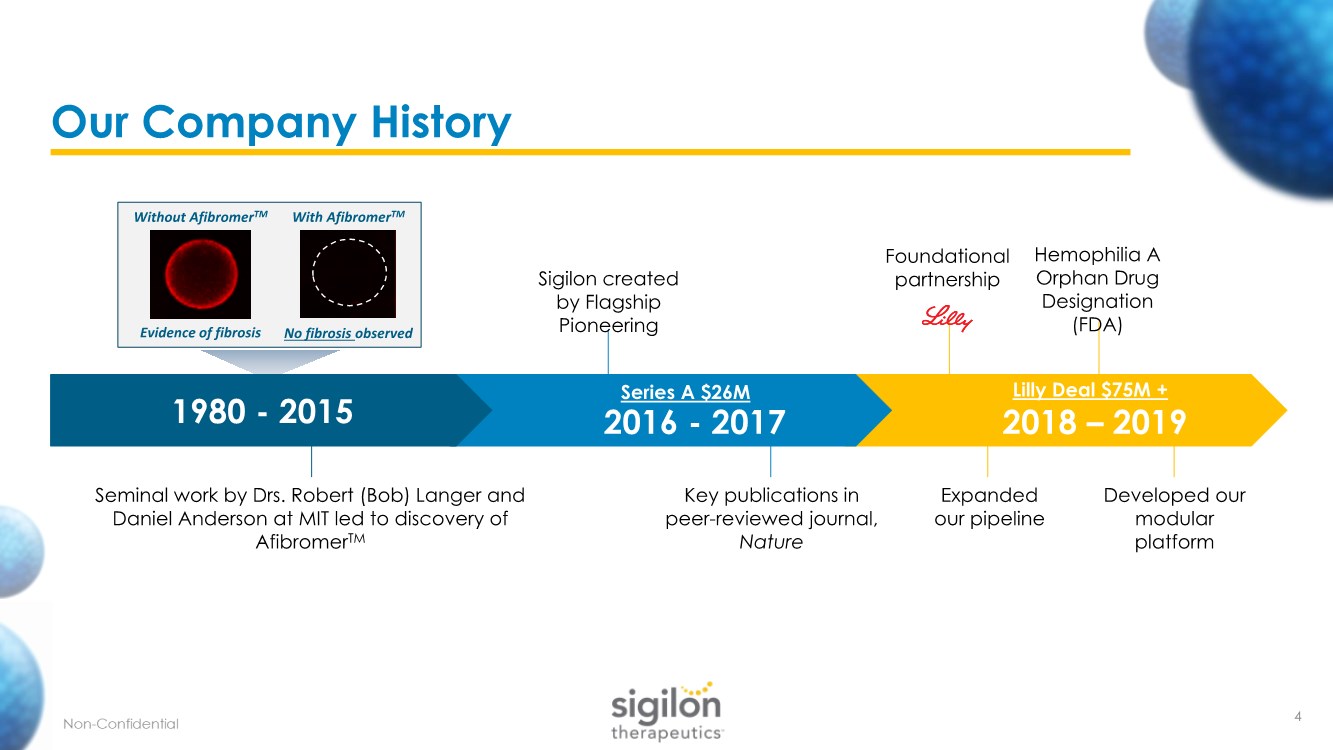
| Our Company History 2018 – 2019 2016 - 2017 1980 - 2015 Sigilon created by Flagship Pioneering Foundational partnership Developed our modular platform Hemophilia A Orphan Drug Designation (FDA) Key publications in peer-reviewed journal, Nature Seminal work by Drs. Robert (Bob) Langer and Daniel Anderson at MIT led to discovery of AfibromerTM Evidence of fibrosis No fibrosis observed Series A $26M Lilly Deal $75M + With AfibromerTM Without AfibromerTM Expanded our pipeline 4 |
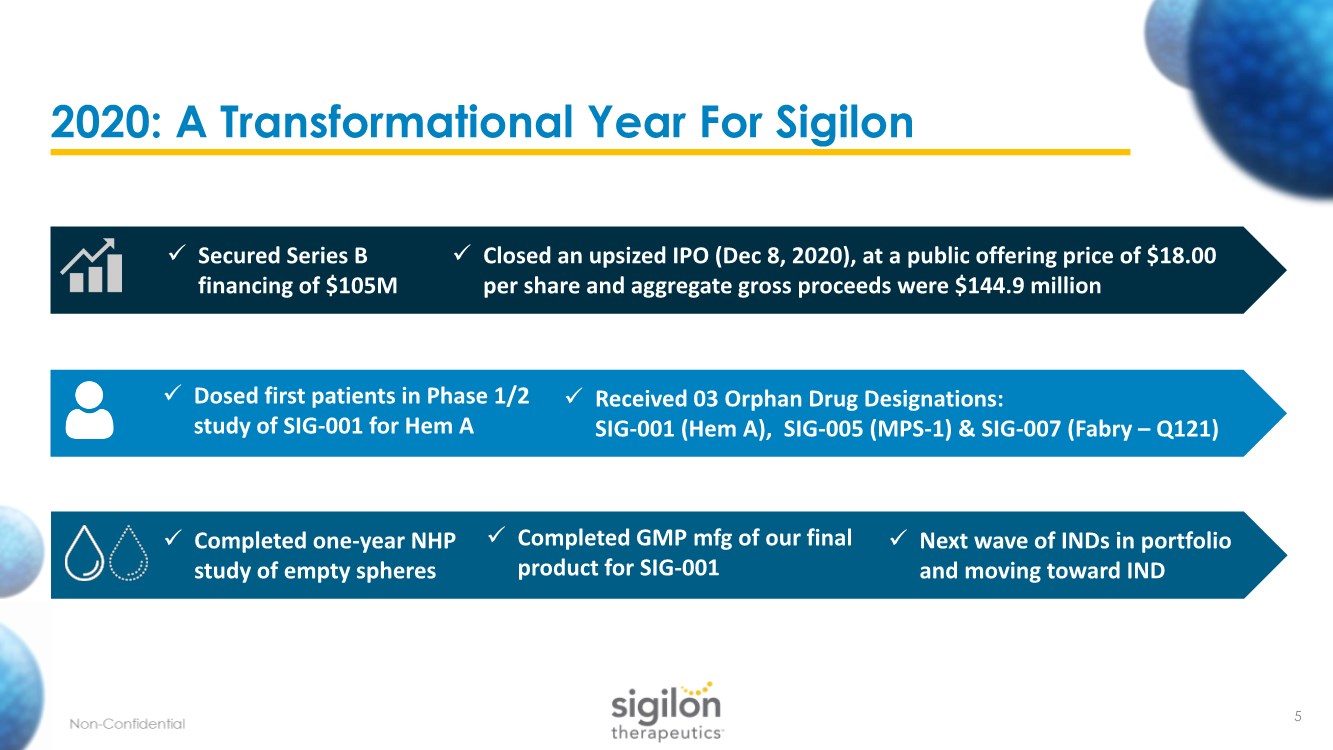
| 2020: A Transformational Year For Sigilon ✓ Secured Series B financing of $105M ✓ Closed an upsized IPO (Dec 8, 2020), at a public offering price of $18.00 per share and aggregate gross proceeds were $144.9 million ✓ Dosed first patients in Phase 1/2 study of SIG-001 for Hem A ✓ Received 03 Orphan Drug Designations: SIG-001 (Hem A), SIG-005 (MPS-1) & SIG-007 (Fabry – Q121) ✓ Completed one-year NHP study of empty spheres ✓ Completed GMP mfg of our final product for SIG-001 ✓ Next wave of INDs in portfolio and moving toward IND 5 |
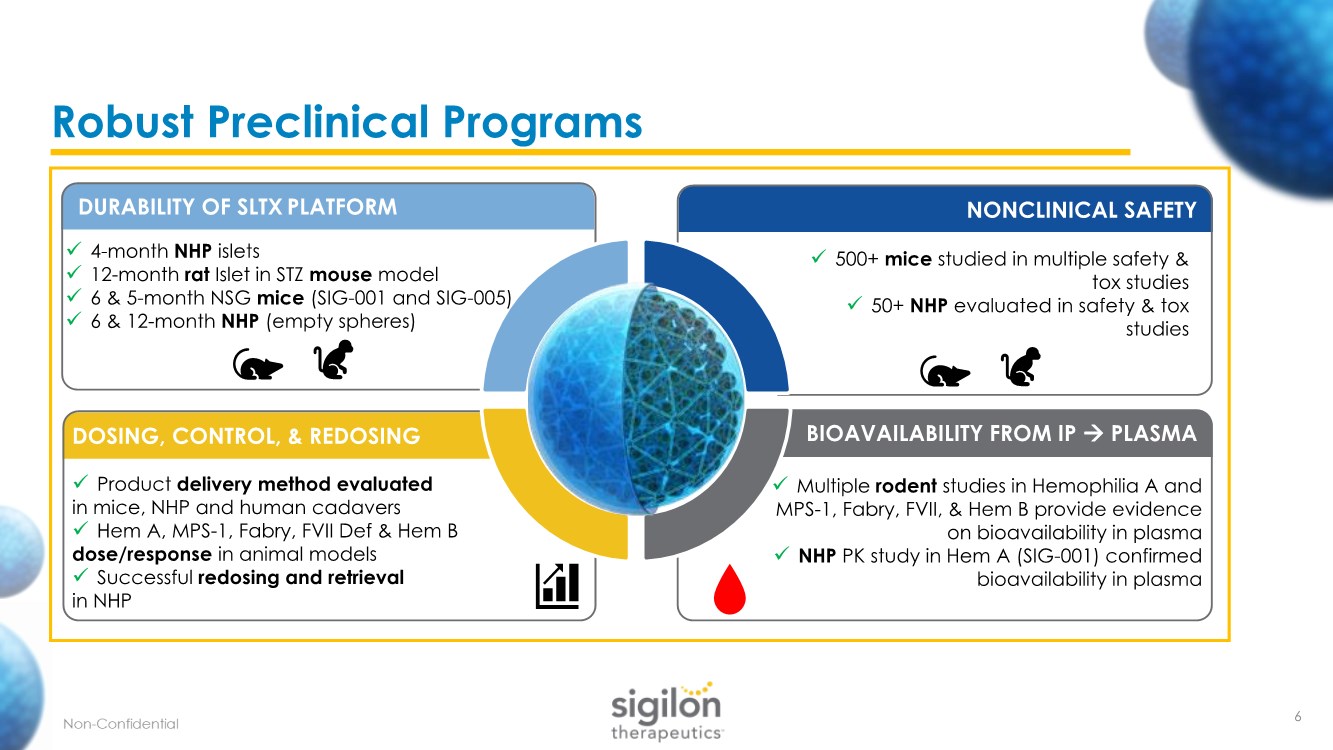
| Robust Preclinical Programs ✓ 4-month NHP islets ✓ 12-month rat Islet in STZ mouse model ✓ 6 & 5-month NSG mice (SIG-001 and SIG-005) ✓ 6 & 12-month NHP (empty spheres) DURABILITY OF SLTX PLATFORM ✓ Product delivery method evaluated in mice, NHP and human cadavers ✓ Hem A, MPS-1, Fabry, FVII Def & Hem B dose/response in animal models ✓ Successful redosing and retrieval in NHP ✓ 500+ mice studied in multiple safety & tox studies ✓ 50+ NHP evaluated in safety & tox studies ✓ Multiple rodent studies in Hemophilia A and MPS-1, Fabry, FVII, & Hem B provide evidence on bioavailability in plasma ✓ NHP PK study in Hem A (SIG-001) confirmed bioavailability in plasma NONCLINICAL SAFETY DOSING, CONTROL, & REDOSING BIOAVAILABILITY FROM IP → PLASMA 6 |
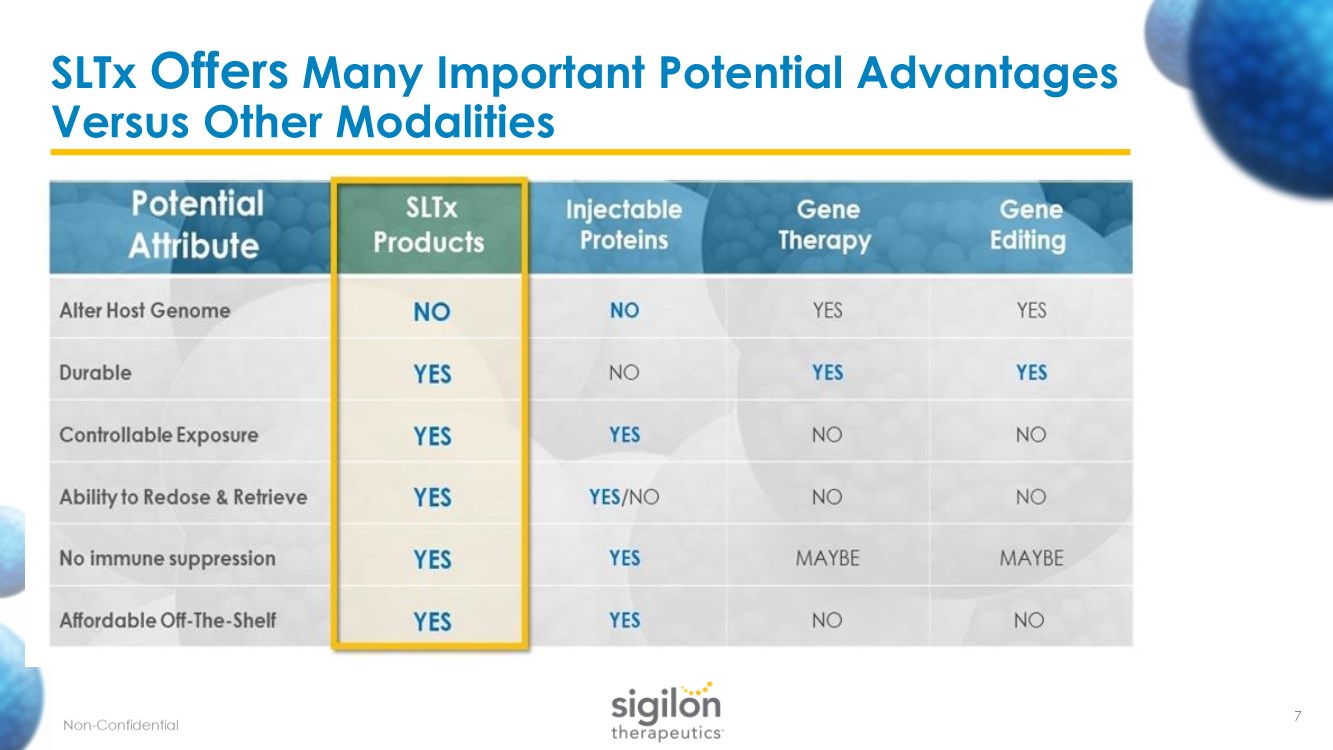
| SLTx Offers Many Important Potential Advantages Versus Other Modalities 7 |
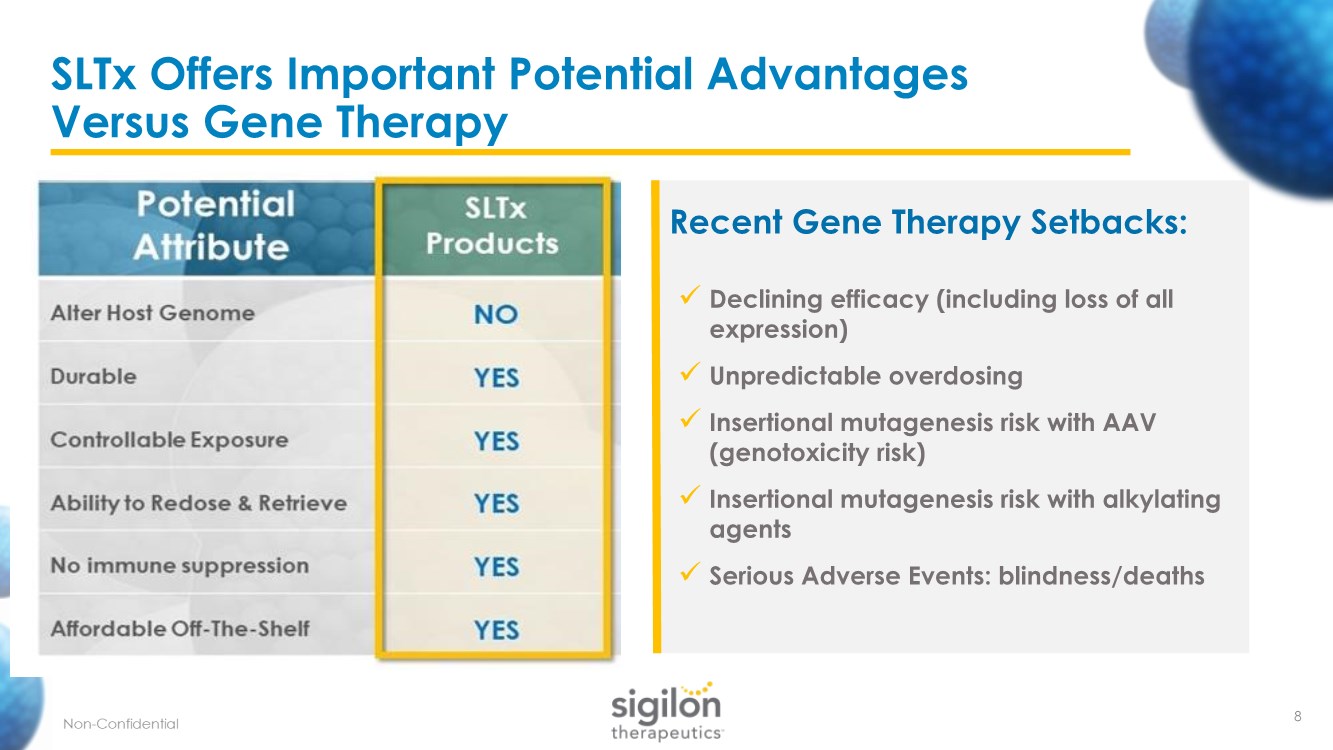
| SLTx Offers Important Potential Advantages Versus Gene Therapy 8 Recent Gene Therapy Setbacks: ✓ Declining efficacy (including loss of all expression) ✓ Unpredictable overdosing ✓ Insertional mutagenesis risk with AAV (genotoxicity risk) ✓ Insertional mutagenesis risk with alkylating agents ✓ Serious Adverse Events: blindness/deaths |
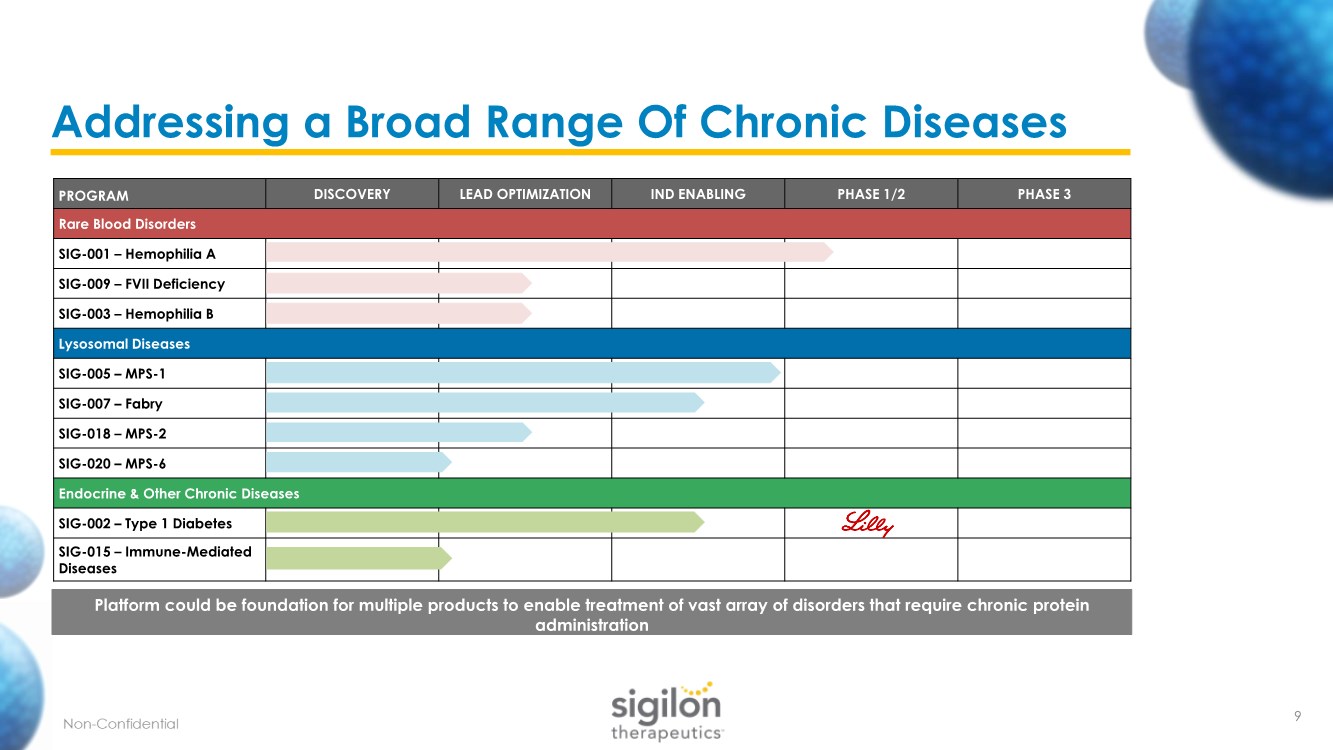
| PROGRAM DISCOVERY LEAD OPTIMIZATION IND ENABLING PHASE 1/2 PHASE 3 Rare Blood Disorders SIG-001 – Hemophilia A SIG-009 – FVII Deficiency SIG-003 – Hemophilia B Lysosomal Diseases SIG-005 – MPS-1 SIG-007 – Fabry SIG-018 – MPS-2 SIG-020 – MPS-6 Endocrine & Other Chronic Diseases SIG-002 – Type 1 Diabetes SIG-015 – Immune-Mediated Diseases Addressing a Broad Range Of Chronic Diseases Platform could be foundation for multiple products to enable treatment of vast array of disorders that require chronic protein administration 9 |
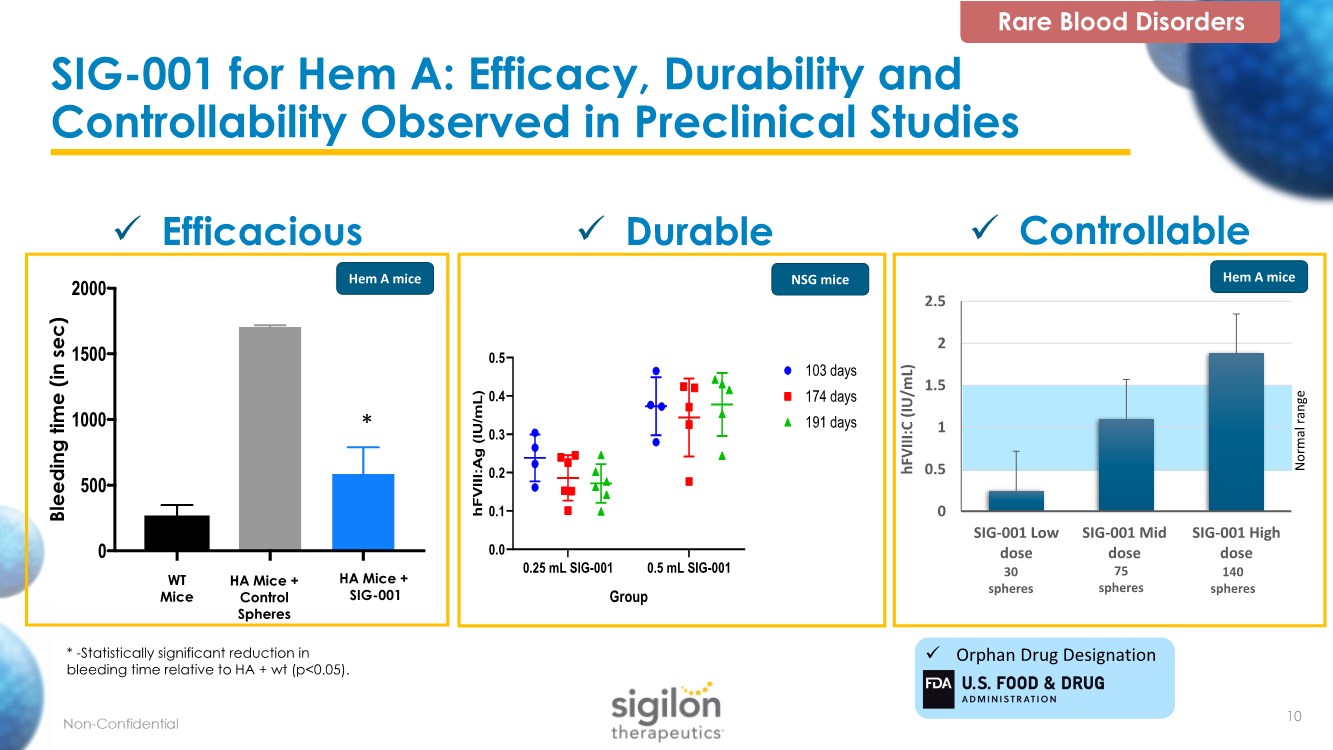
| SIG-001 for Hem A: Efficacy, Durability and Controllability Observed in Preclinical Studies * -Statistically significant reduction in bleeding time relative to HA + wt (p<0.05). Bleeding time (in sec) W T HA + wt cells HA + SIG-001 0 500 1000 1500 2000 WT Mice HA Mice + Control Spheres HA Mice + SIG-001 * 0.25 mL SIG-001 0.5 mL SIG-001 0.0 0.1 0.2 0.3 0.4 0.5 Group h F V I I I : A g ( I U / m L ) 103 days 174 days 191 days ✓ Efficacious ✓ Controllable ✓ Durable Rare Blood Disorders Hem A mice Hem A mice NSG mice 0 0.5 1 1.5 2 2.5 SIG-001 Low dose SIG-001 Mid dose SIG-001 High dose hFVIII:C (IU/mL) Normal range 30 spheres 75 spheres 140 spheres ✓ Orphan Drug Designation 10 |
| SIG-001 Phase 1/2 Dose Escalation Study Represents Significant Milestone For SLTx Platform Rare Blood Disorders • Significant milestones for: • Product platform for our pipeline • GMP Manufacturing • Logistics of Fresh Product • Initial Safety Observations • Measurable plasma FVIII activity • Recent amendments cleared • Next steps: • Continue dose escalation SIG-001 spheres (omentum) Administration catheter 11 8 mm Insufflation & Administration Port 5 mm Camera Port |
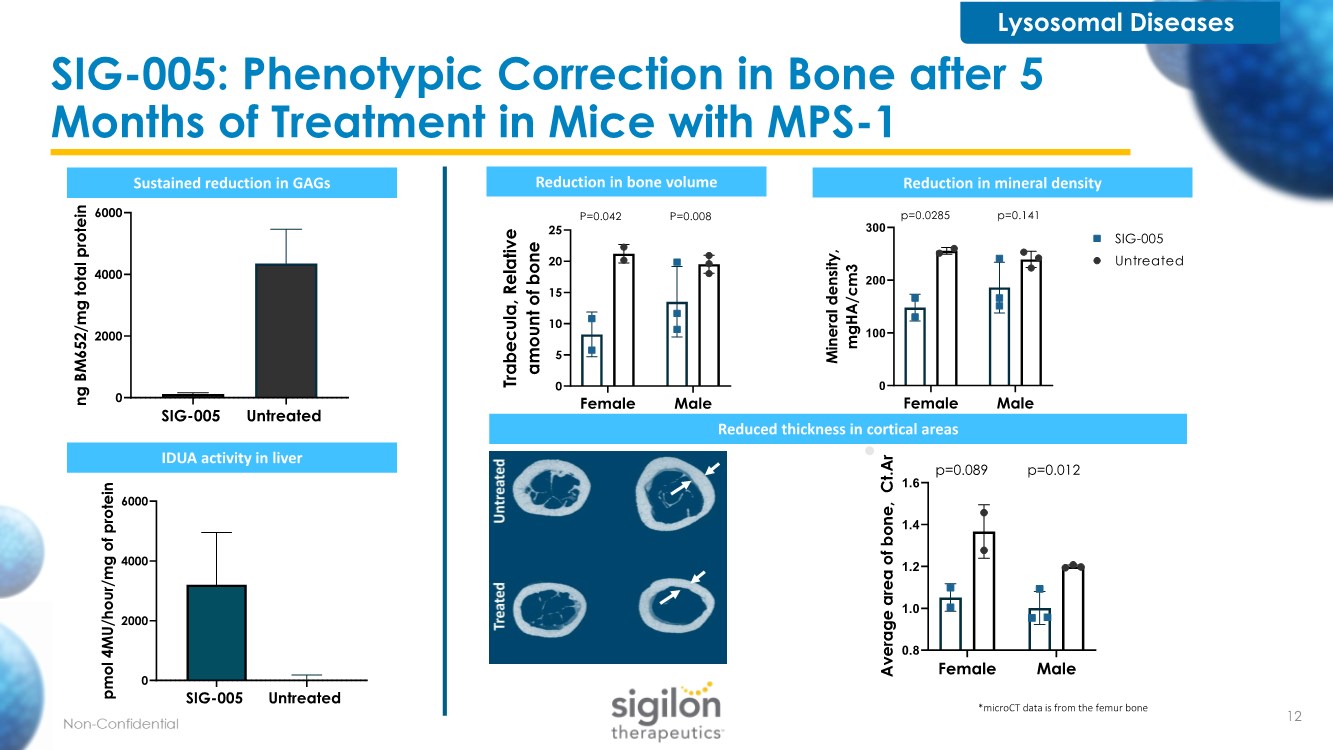
| SIG-005: Phenotypic Correction in Bone after 5 Months of Treatment in Mice with MPS-1 Reduced thickness in cortical areas Sustained reduction in GAGs Female Male 0 5 10 15 20 25 T r a b e c u l a , R e l a t i v e a m o u n t o f b o n e P=0.042 P=0.008 Reduction in bone volume Female Male 0 100 200 300 M i n e r a l d e n s i t y , m g H A / c m 3 Untreated SIG-005 p=0.0285 p=0.141 Reduction in mineral density Female Male 0.8 1.0 1.2 1.4 1.6 A v e r a g e a r e a o f b o n e , C t . A r p=0.089 p=0.012 SIG-005 Untreated 0 2000 4000 6000 n g B M 6 5 2 / m g t o t a l p r o t e i n SIG-005 Untreated 0 2000 4000 6000 p m o l 4 M U / h o u r / m g o f p r o t e i n IDUA activity in liver *microCT data is from the femur bone Lysosomal Diseases 12 |
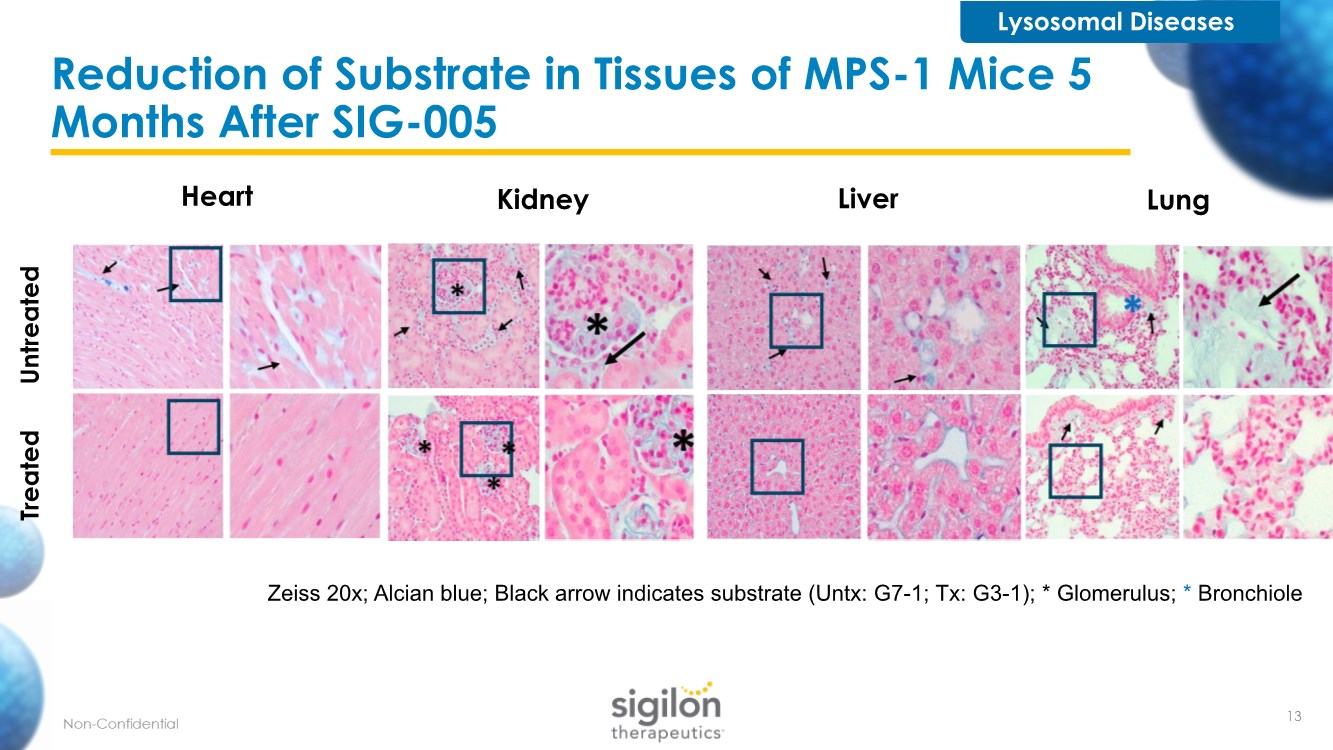
| Reduction of Substrate in Tissues of MPS-1 Mice 5 Months After SIG-005 Heart Kidney Liver Lung Treated Untreated Zeiss 20x; Alcian blue; Black arrow indicates substrate (Untx: G7-1; Tx: G3-1); * Glomerulus; * Bronchiole Lysosomal Diseases 13 |
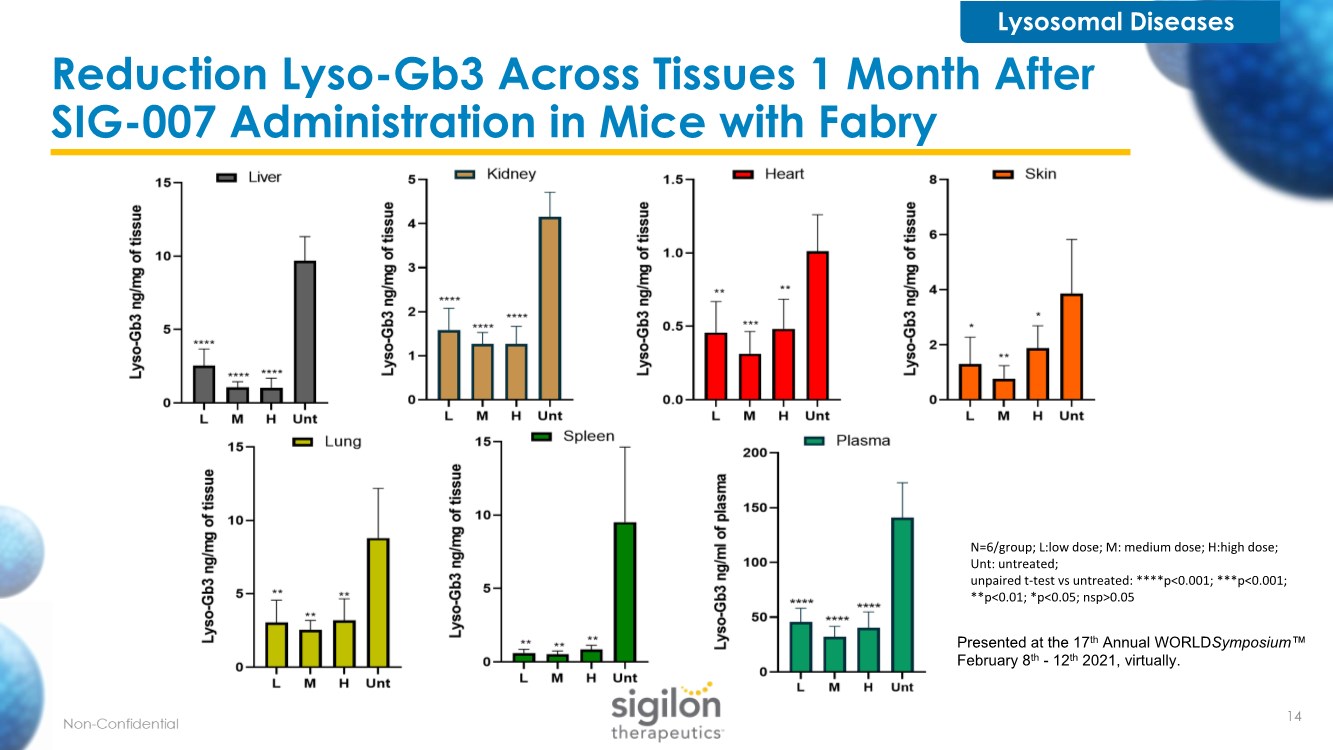
| Reduction Lyso-Gb3 Across Tissues 1 Month After SIG-007 Administration in Mice with Fabry Lysosomal Diseases Presented at the 17th Annual WORLDSymposium™ February 8th - 12th 2021, virtually. N=6/group; L:low dose; M: medium dose; H:high dose; Unt: untreated; unpaired t-test vs untreated: ****p<0.001; ***p<0.001; **p<0.01; *p<0.05; nsp>0.05 14 |
| Sigilon and Lilly Collaborating to Develop Potential Functional Cure for Type 1 Diabetes • Sigilon is responsible for execution of the program through IND • Eli Lilly, a global leader in diabetes, will develop and commercialize program worldwide if approved • Financial Terms: • $75 million initial commitment • $415 million in milestones & tiered (from single- to-low double digit) sales-based royalties Type 1 Diabetes 15 |
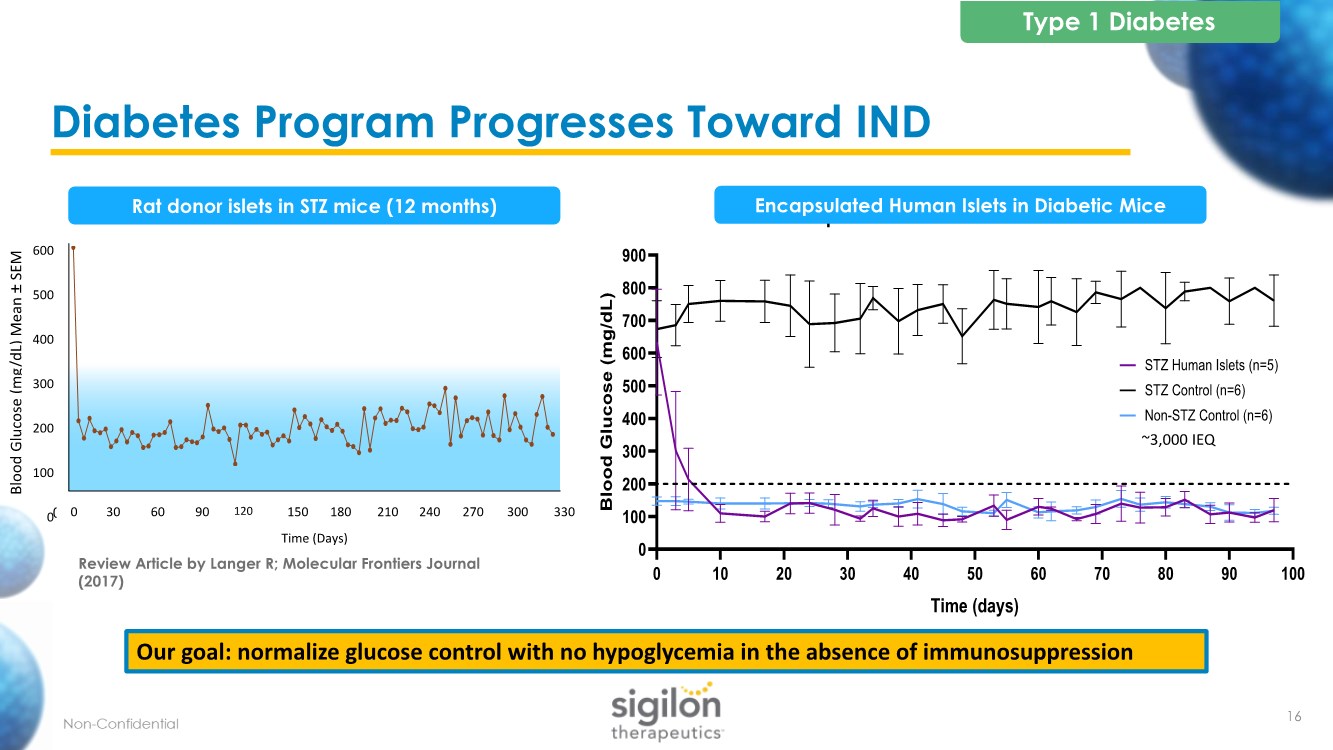
| Diabetes Program Progresses Toward IND Blood Glucose (mg/dL) Mean ± SEM 600 500 400 300 200 100 0 Time (Days) 600 500 400 300 200 100 0 330 300 270 240 210 180 150 120 90 60 30 0 Review Article by Langer R; Molecular Frontiers Journal (2017) Rat donor islets in STZ mice (12 months) Type 1 Diabetes 16 0 10 20 30 40 50 60 70 80 90 100 0 100 200 300 400 500 600 700 800 900 Encapsulated Human Islets in Diabetic Mice Time (days) B l o o d G l u c o s e ( m g / d L ) STZ Human Islets (n=5) STZ Control (n=6) Non-STZ Control (n=6) ~3,000 IEQ 0 10 20 30 40 50 60 70 80 90 100 0 100 200 300 400 500 600 700 800 900 Encapsulated Human Islets in Diabetic Mice Time (days) B l o o d G l u c o s e ( m g / d L ) STZ Human Islets (n=5) STZ Control (n=6) Non-STZ Control (n=6) Encapsulated Human Islets in Diabetic Mice Our goal: normalize glucose control with no hypoglycemia in the absence of immunosuppression |
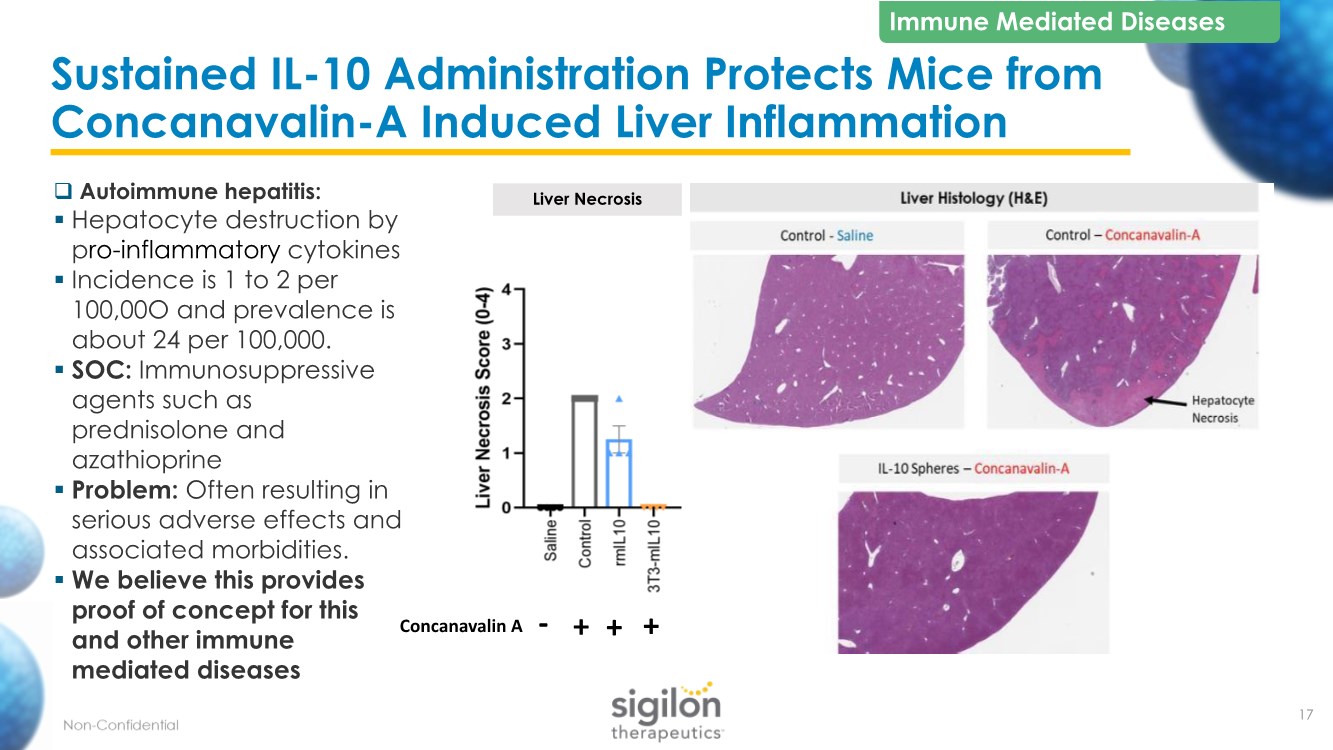
| Sustained IL-10 Administration Protects Mice from Concanavalin-A Induced Liver Inflammation 17 Liver Necrosis + + - + Concanavalin A Immune Mediated Diseases ❑ Autoimmune hepatitis: ▪ Hepatocyte destruction by pro-inflammatory cytokines ▪ Incidence is 1 to 2 per 100,00O and prevalence is about 24 per 100,000. ▪ SOC: Immunosuppressive agents such as prednisolone and azathioprine ▪ Problem: Often resulting in serious adverse effects and associated morbidities. ▪ We believe this provides proof of concept for this and other immune mediated diseases |
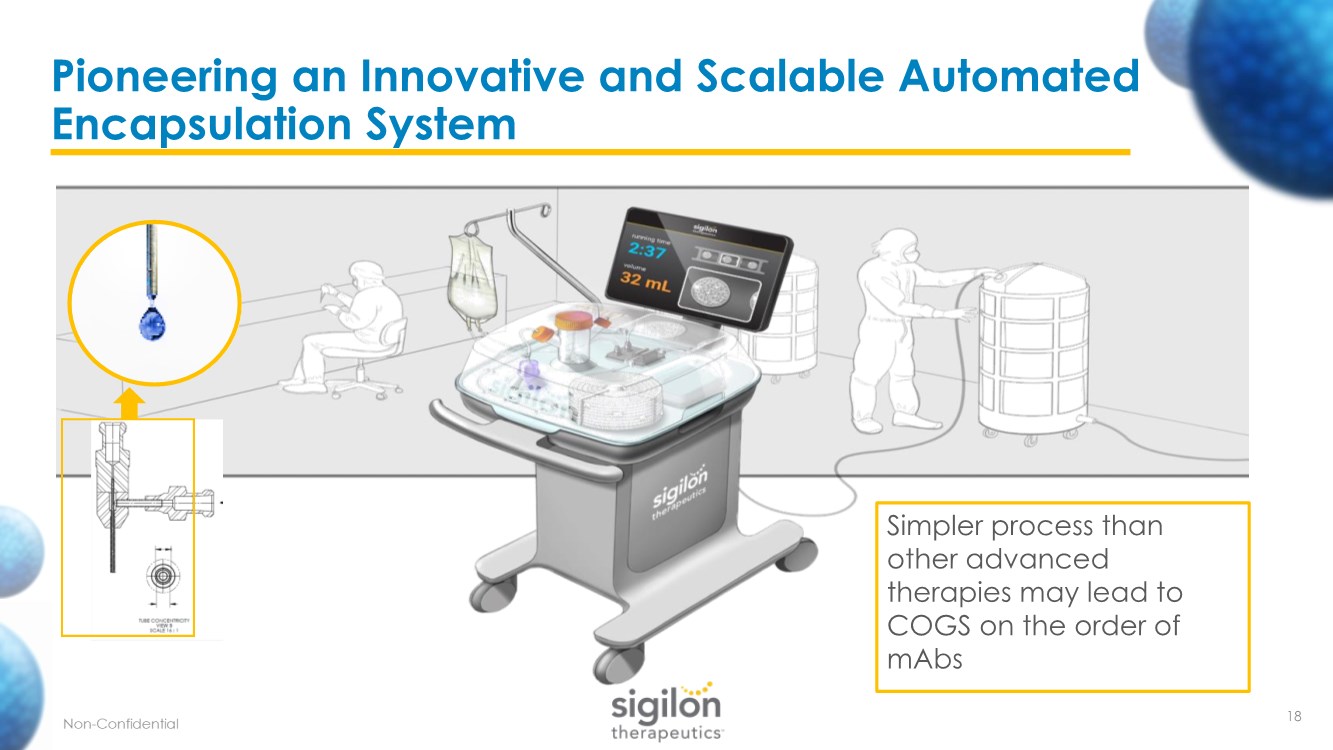
| Pioneering an Innovative and Scalable Automated Encapsulation System Simpler process than other advanced therapies may lead to COGS on the order of mAbs 18 |
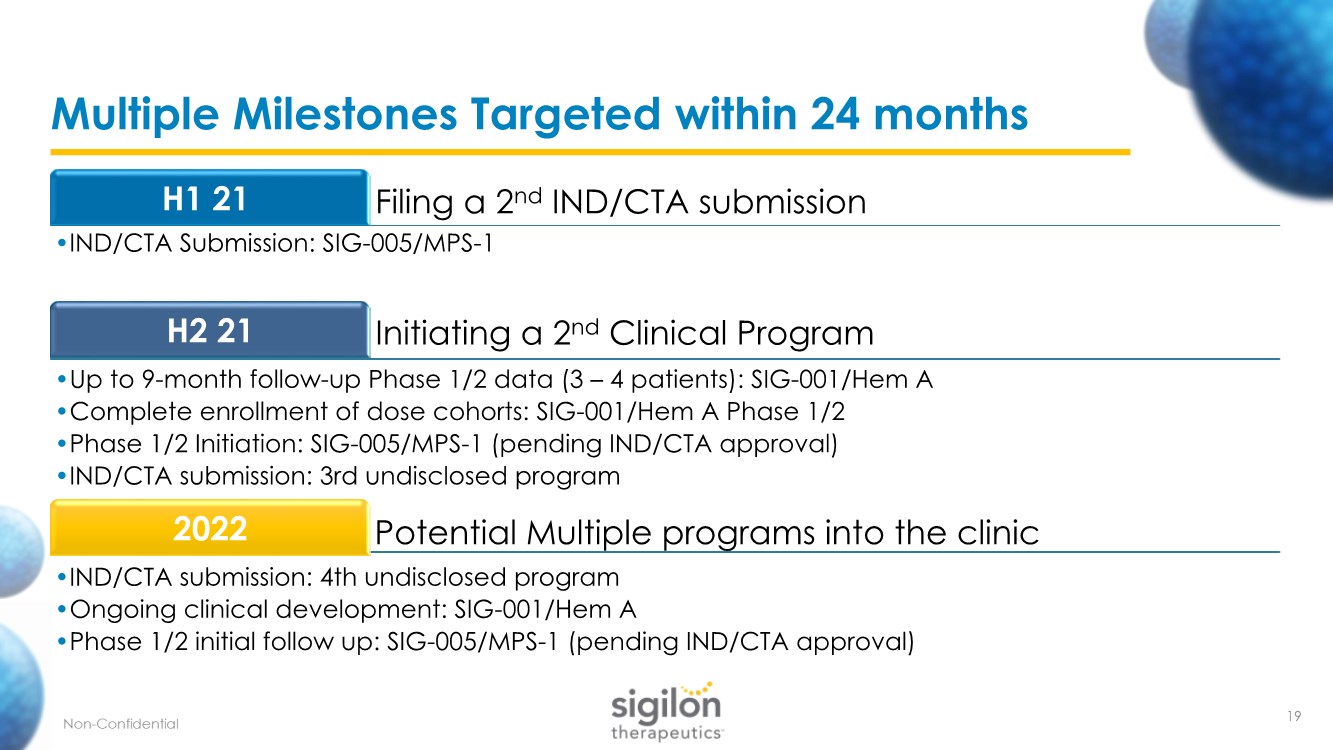
| Multiple Milestones Targeted within 24 months Filing a 2nd IND/CTA submission H1 21 •IND/CTA Submission: SIG-005/MPS-1 Initiating a 2nd Clinical Program H2 21 •Up to 9-month follow-up Phase 1/2 data (3 – 4 patients): SIG-001/Hem A •Complete enrollment of dose cohorts: SIG-001/Hem A Phase 1/2 •Phase 1/2 Initiation: SIG-005/MPS-1 (pending IND/CTA approval) •IND/CTA submission: 3rd undisclosed program Potential Multiple programs into the clinic 2022 •IND/CTA submission: 4th undisclosed program •Ongoing clinical development: SIG-001/Hem A •Phase 1/2 initial follow up: SIG-005/MPS-1 (pending IND/CTA approval) 19 |
| Thanks |