Attached files
| file | filename |
|---|---|
| 8-K - 8-K - NEOGENOMICS INC | neo-20210505.htm |

NeoGenomics Investor Presentation May 2021 1 Exhibit 99.1

Forward-Looking Statements 2 This presentation has been prepared by NeoGenomics, Inc. (“we,” ”us,” “our,” “NeoGenomics” or the “Company”) and is made for informational purposes only and does not constitute an offer to sell or a solicitation of an offer to buy securities, nor shall there be any sale of any securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. The information set forth herein does not purport to be complete or to contain all of the information you may desire. Statements contained herein are made as of the date of this presentation unless stated otherwise, and neither this presentation, nor any sale of securities, shall under any circumstances create an implication that the information contained herein is correct as of any time after such date or that information will be updated or revised to reflect information that subsequently becomes available or changes occurring after the date hereof. This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to business, operations, and financial conditions of the Company. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. Although the Company believes the expectations reflected in such forward-looking statements are based upon reasonable assumptions, there can be no assurance that its expectations will be realized. Actual results could differ materially from those projected in the Company’s forward-looking statements due to numerous known and unknown risks and uncertainties. All forward-looking statements speak only as of the date of this presentation and are qualified in their entirety by this cautionary statement. The Company undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. Non-GAAP Adjusted EBITDA “Adjusted EBITDA” is defined by NeoGenomics as net income from continuing operations before: (i) interest expense, (ii) tax expense, (iii) depreciation and amortization expense, (iv) non-cash stock-based compensation expense, and, if applicable in a reporting period, (v) acquisition and integration related expenses, (vi) non-cash impairments of intangible assets, (vii) and other significant non-recurring or non-operating (income) or expenses, including any debt financing costs.
We save lives by improving patient care. By providing uncompromising quality, exceptional service and innovative solutions, we are becoming the world’s leading cancer testing and information company. Quality, integrity, accountability, teamwork, innovation. NeoGenomics We are Focused and Genuine Our Common Purpose Our Values Our Vision 3
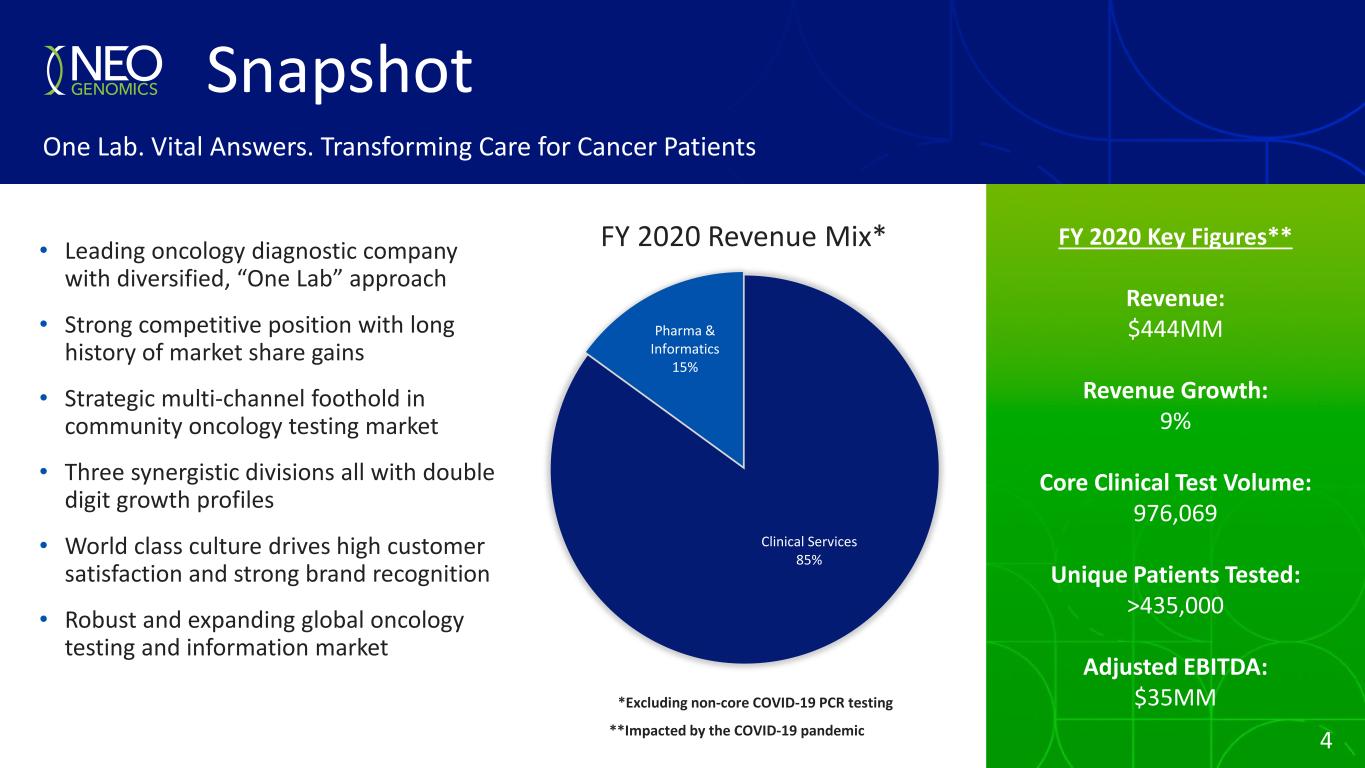
4 FY 2020 Key Figures** Revenue: $444MM Revenue Growth: 9% Core Clinical Test Volume: 976,069 Unique Patients Tested: >435,000 Adjusted EBITDA: $35MM FY 2020 Revenue Mix* Snapshot One Lab. Vital Answers. Transforming Care for Cancer Patients • Leading oncology diagnostic company with diversified, “One Lab” approach • Strong competitive position with long history of market share gains • Strategic multi-channel foothold in community oncology testing market • Three synergistic divisions all with double digit growth profiles • World class culture drives high customer satisfaction and strong brand recognition • Robust and expanding global oncology testing and information market Clinical Services 85% Pharma & Informatics 15% *Excluding non-core COVID-19 PCR testing 4**Impacted by the COVID-19 pandemic
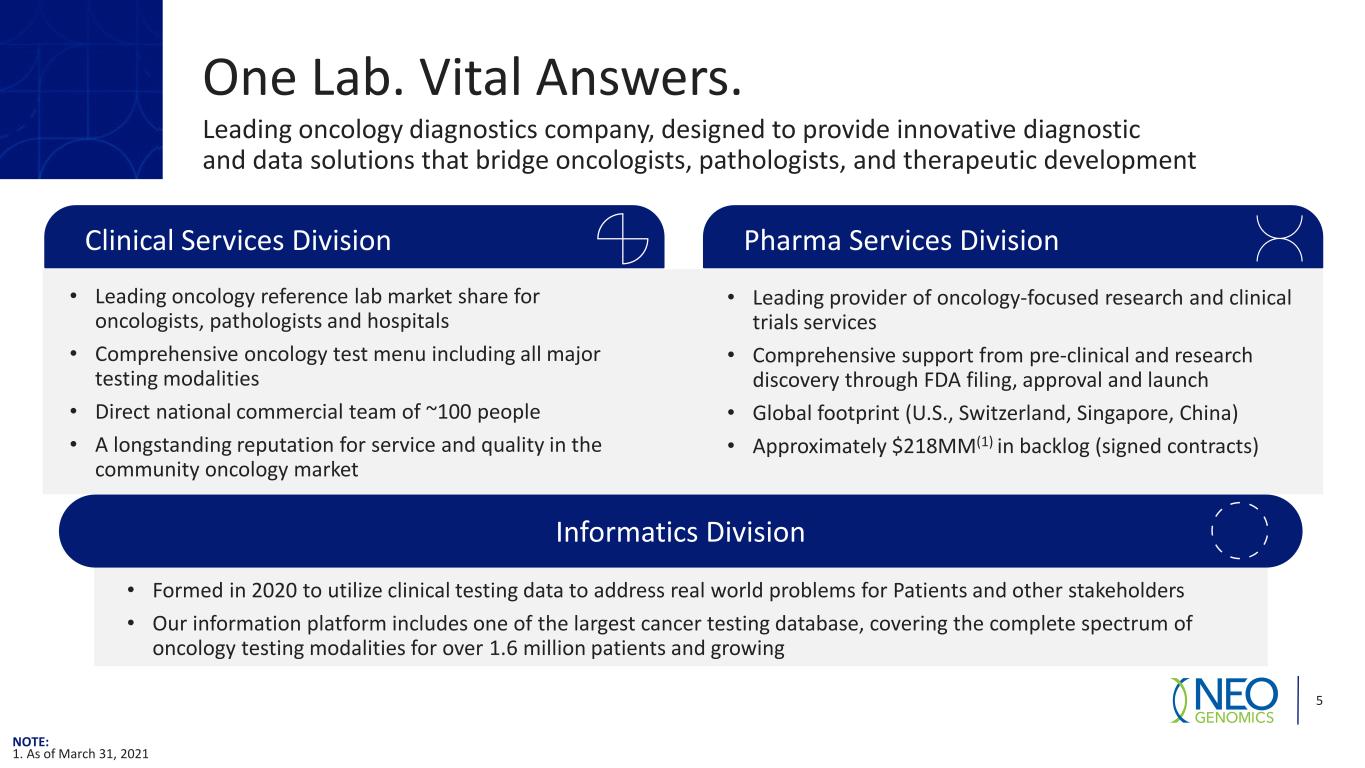
Informatics Division One Lab. Vital Answers. 5 Leading oncology diagnostics company, designed to provide innovative diagnostic and data solutions that bridge oncologists, pathologists, and therapeutic development • Leading oncology reference lab market share for oncologists, pathologists and hospitals • Comprehensive oncology test menu including all major testing modalities • Direct national commercial team of ~100 people • A longstanding reputation for service and quality in the community oncology market • Leading provider of oncology-focused research and clinical trials services • Comprehensive support from pre-clinical and research discovery through FDA filing, approval and launch • Global footprint (U.S., Switzerland, Singapore, China) • Approximately $218MM(1) in backlog (signed contracts) Pharma Services DivisionClinical Services Division • Formed in 2020 to utilize clinical testing data to address real world problems for Patients and other stakeholders • Our information platform includes one of the largest cancer testing database, covering the complete spectrum of oncology testing modalities for over 1.6 million patients and growing NOTE: 1. As of March 31, 2021
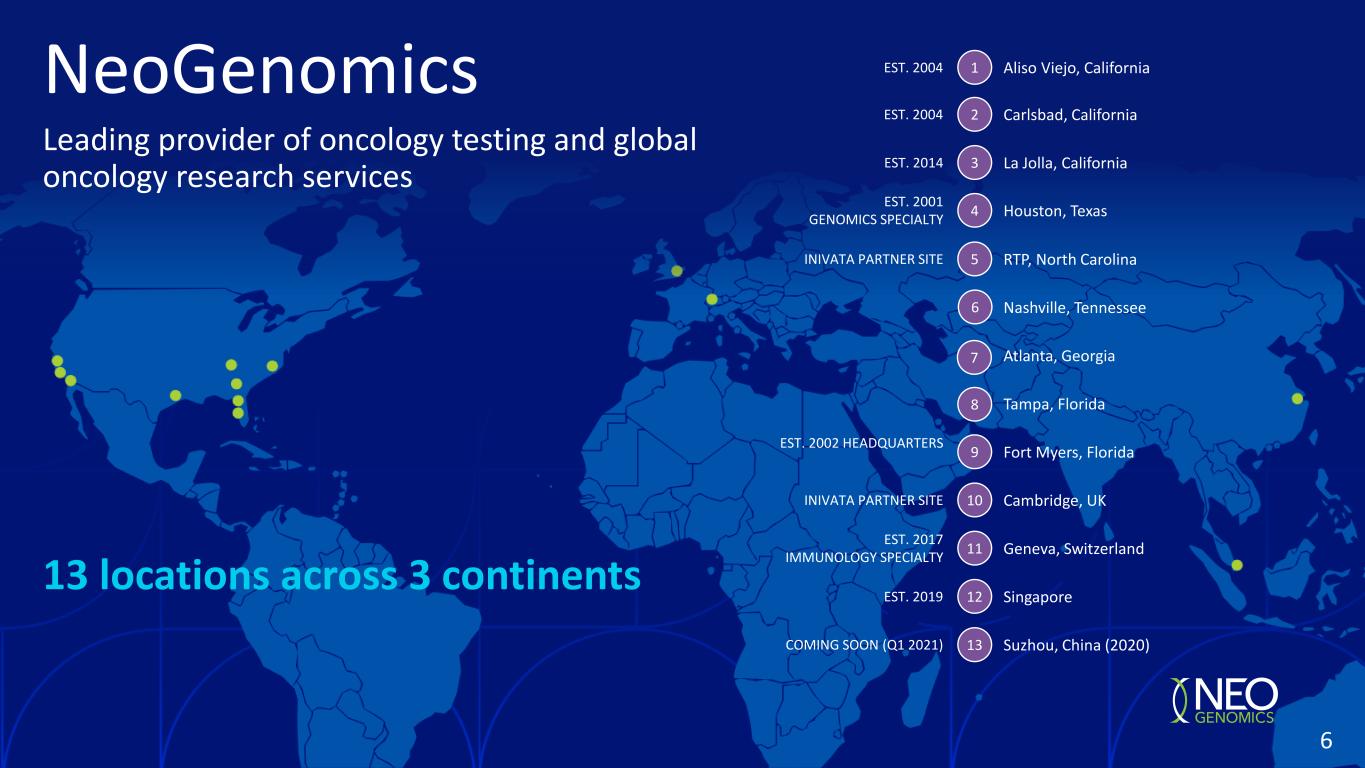
13 locations across 3 continents Leading provider of oncology testing and global oncology research services NeoGenomics Carlsbad, California Houston, Texas La Jolla, California Fort Myers, Florida Tampa, Florida Atlanta, Georgia Nashville, Tennessee RTP, North Carolina Cambridge, UK Geneva, Switzerland Singapore Suzhou, China (2020) EST. 2004 EST. 2014 EST. 2001 GENOMICS SPECIALTY EST. 2002 HEADQUARTERS EST. 2017 IMMUNOLOGY SPECIALTY EST. 2019 COMING SOON (Q1 2021) 2 3 4 5 9 10 11 12 13 INIVATA PARTNER SITE INIVATA PARTNER SITE 6 7 8 Aliso Viejo, CaliforniaEST. 2004 1 6

Oncology Testing Market Tailwinds Estimated 6% to 8% annual market growth with upside potential Demographics − An aging population is resulting in higher cancer incidence − Increased cancer survival rates leading to more follow-on testing Precision Medicine & Drug Development − Proliferation and complexity of therapeutic options driving more testing − Burgeoning oncology drug pipeline underlying current Pharma Services demand and likely to drive demand for future clinical testing − New platforms and tests (NGS, TMB, MSI, liquid biopsy, etc.) creating more test options for diagnosis, prognosis, and therapy selection Upside Potential: Emerging Opportunities − Promising minimal residual disease tests in development such as strategic partner Inivata’s RaDaR assay could create a compelling recurrence monitoring opportunity − We expect to develop a number of innovative value-add data offerings in our growing Informatics division 7
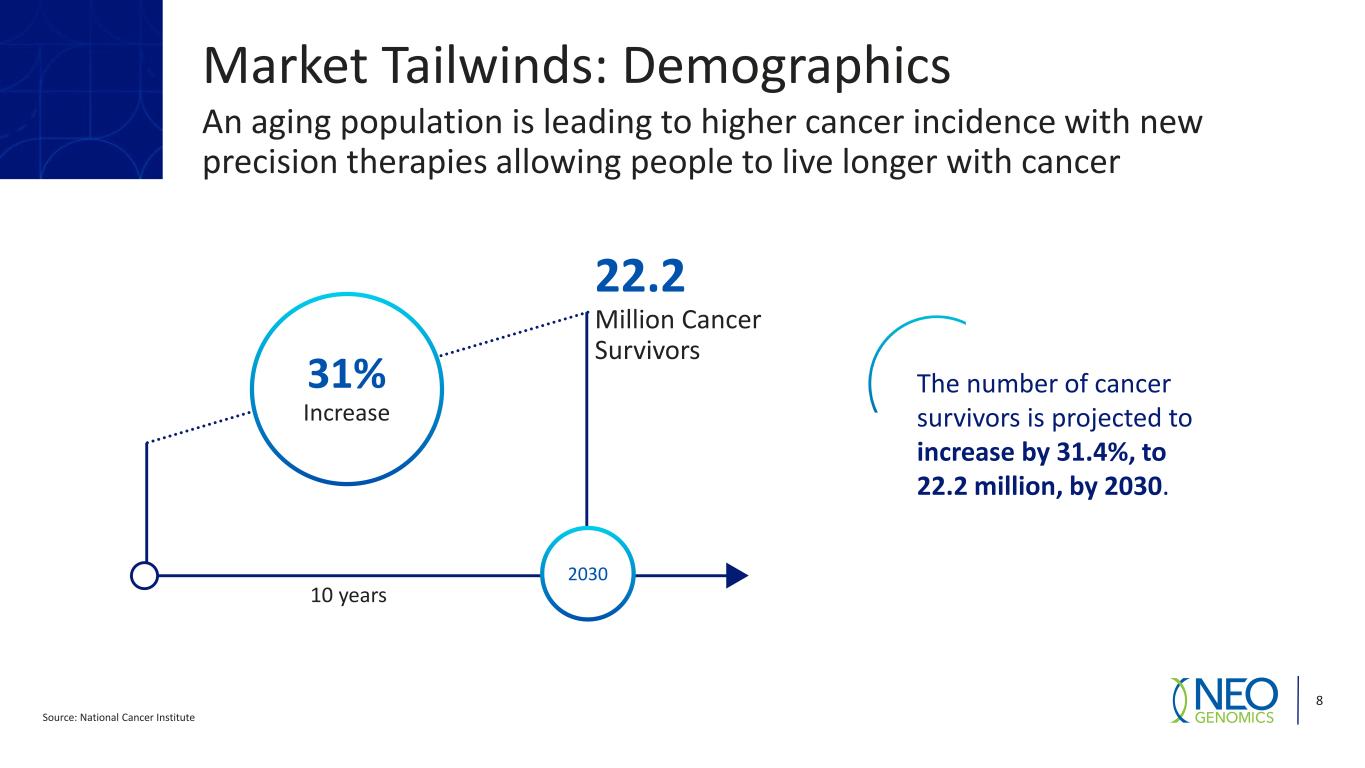
Market Tailwinds: Demographics An aging population is leading to higher cancer incidence with new precision therapies allowing people to live longer with cancer Source: National Cancer Institute 8 2030 22.2 Million Cancer Survivors 10 years 31% Increase The number of cancer survivors is projected to increase by 31.4%, to 22.2 million, by 2030.
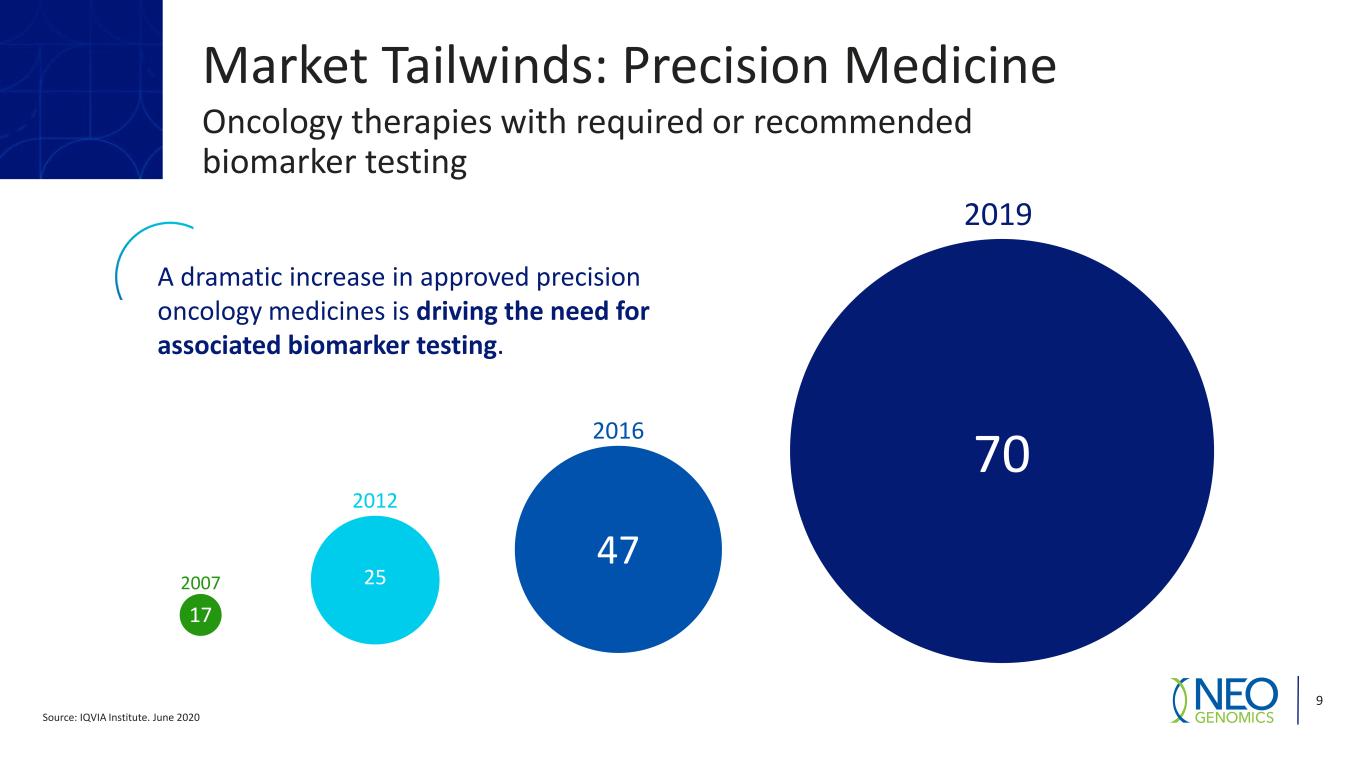
Source: IQVIA Institute. June 2020 9 2007 2012 2016 2019 70 47 25 17 A dramatic increase in approved precision oncology medicines is driving the need for associated biomarker testing. Market Tailwinds: Precision Medicine Oncology therapies with required or recommended biomarker testing
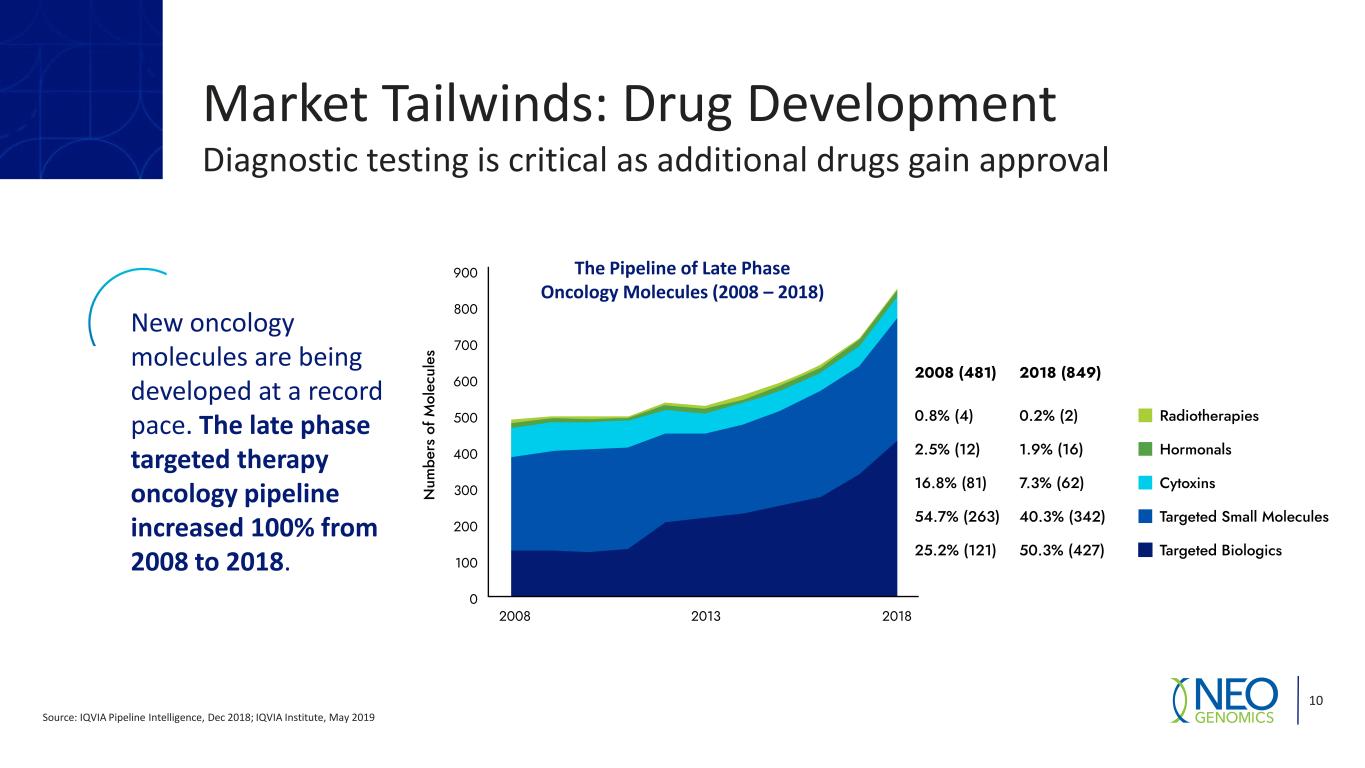
Market Tailwinds: Drug Development Diagnostic testing is critical as additional drugs gain approval Source: IQVIA Pipeline Intelligence, Dec 2018; IQVIA Institute, May 2019 10 New oncology molecules are being developed at a record pace. The late phase targeted therapy oncology pipeline increased 100% from 2008 to 2018. The Pipeline of Late Phase Oncology Molecules (2008 – 2018)
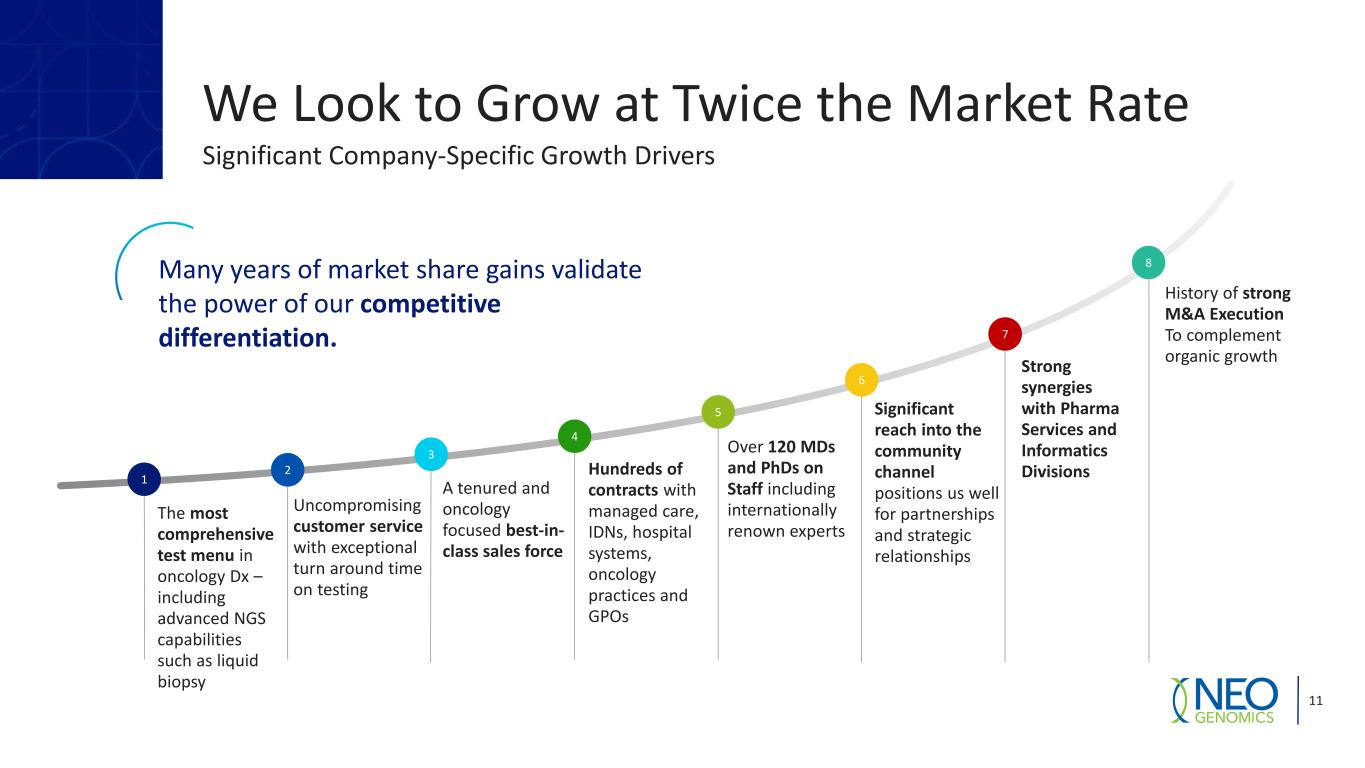
We Look to Grow at Twice the Market Rate 11 Significant Company-Specific Growth Drivers 1 The most comprehensive test menu in oncology Dx – including advanced NGS capabilities such as liquid biopsy 2 Uncompromising customer service with exceptional turn around time on testing 3 4 5 Over 120 MDs and PhDs on Staff including internationally renown experts 6 7 8Many years of market share gains validate the power of our competitive differentiation. Strong synergies with Pharma Services and Informatics Divisions A tenured and oncology focused best-in- class sales force Hundreds of contracts with managed care, IDNs, hospital systems, oncology practices and GPOs History of strong M&A Execution To complement organic growth Significant reach into the community channel positions us well for partnerships and strategic relationships
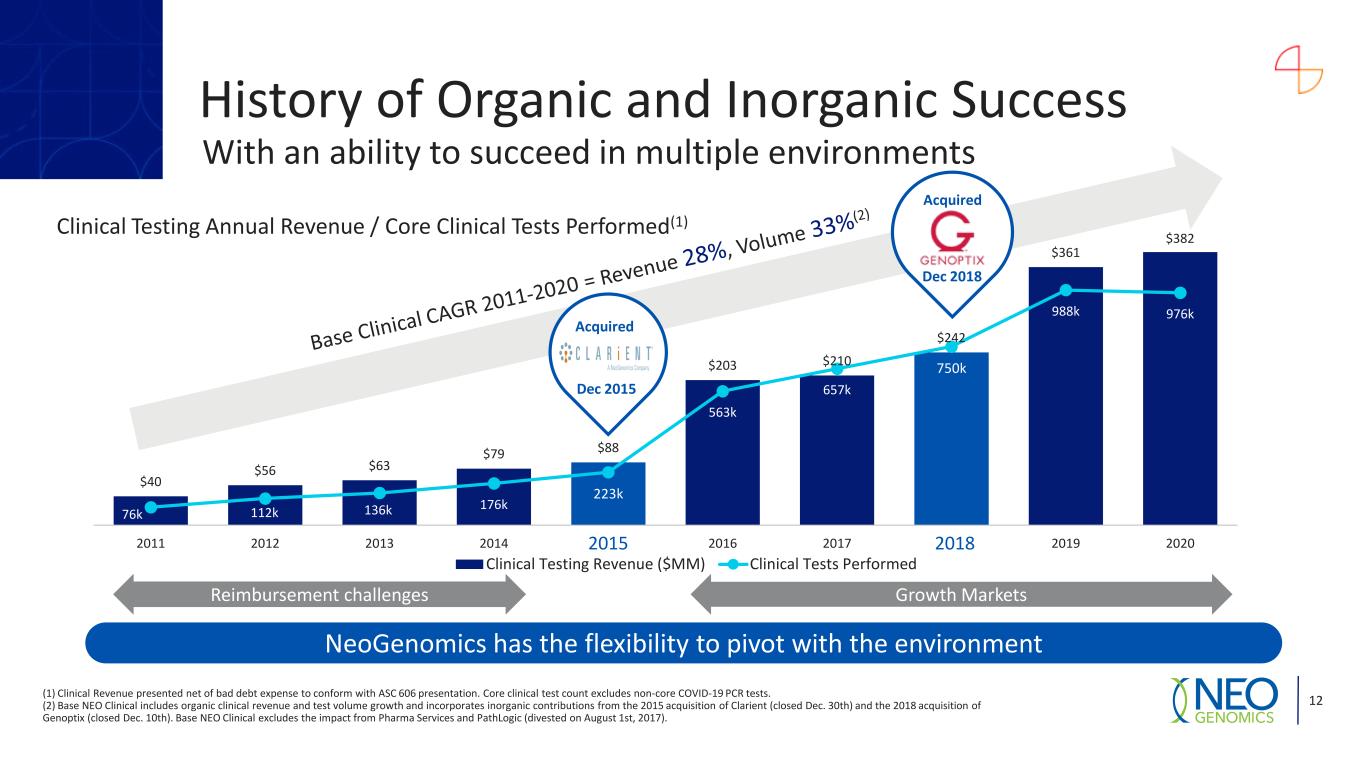
$40 $56 $63 $79 $88 $203 $210 $242 $361 $382 76k 112k 136k 176k 223k 563k 657k 750k 988k 976k 0 200 400 600 800 1,000 1,200 0 50 100 150 200 250 300 350 400 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 Clinical Testing Revenue ($MM) Clinical Tests Performed Clinical Testing Annual Revenue / Core Clinical Tests Performed(1) 82 Acquired Growth MarketsReimbursement challenges NeoGenomics has the flexibility to pivot with the environment History of Organic and Inorganic Success With an ability to succeed in multiple environments 12 Acquired Dec 2015 (1) Clinical Revenue presented net of bad debt expense to conform with ASC 606 presentation. Core clinical test count excludes non-core COVID-19 PCR tests. (2) Base NEO Clinical includes organic clinical revenue and test volume growth and incorporates inorganic contributions from the 2015 acquisition of Clarient (closed Dec. 30th) and the 2018 acquisition of Genoptix (closed Dec. 10th). Base NEO Clinical excludes the impact from Pharma Services and PathLogic (divested on August 1st, 2017). Dec 2018

Our Focus Is The Community Setting We bring state-of-the-art oncology testing to the masses 13 NeoGenomics works with >4,400 hospitals, institutions and oncology offices, most in the community setting, to ensure all patients can benefit from high-quality diagnostic tests to support Precision Medicine Community Hospital Community Oncology Office Community Channel 80% to 85% of all cancer patients are treated by community oncologists
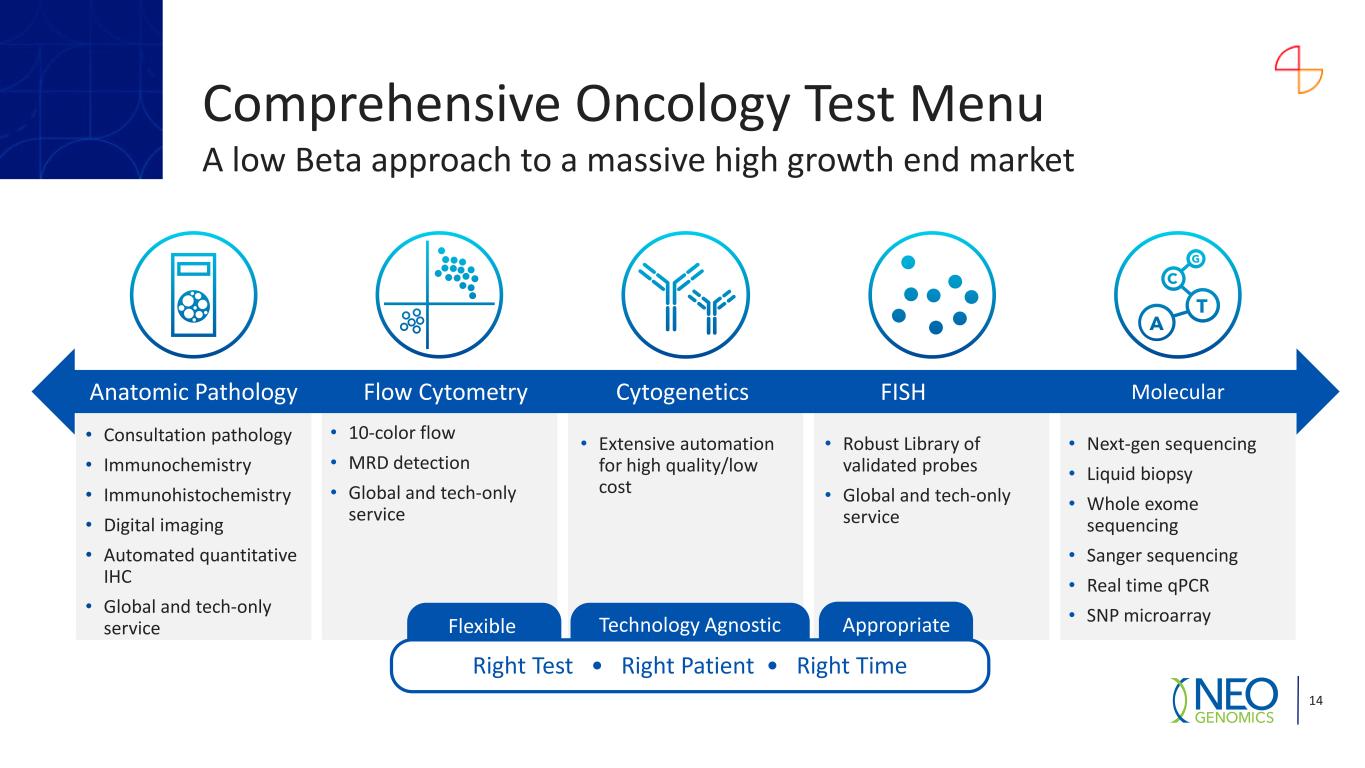
Comprehensive Oncology Test Menu A low Beta approach to a massive high growth end market 14 • Consultation pathology • Immunochemistry • Immunohistochemistry • Digital imaging • Automated quantitative IHC • Global and tech-only service • 10-color flow • MRD detection • Global and tech-only service • Extensive automation for high quality/low cost • Robust Library of validated probes • Global and tech-only service FISH • Next-gen sequencing • Liquid biopsy • Whole exome sequencing • Sanger sequencing • Real time qPCR • SNP microarray MolecularAnatomic Pathology Flow Cytometry Cytogenetics Right Test • Right Patient • Right Time Flexible AppropriateTechnology Agnostic
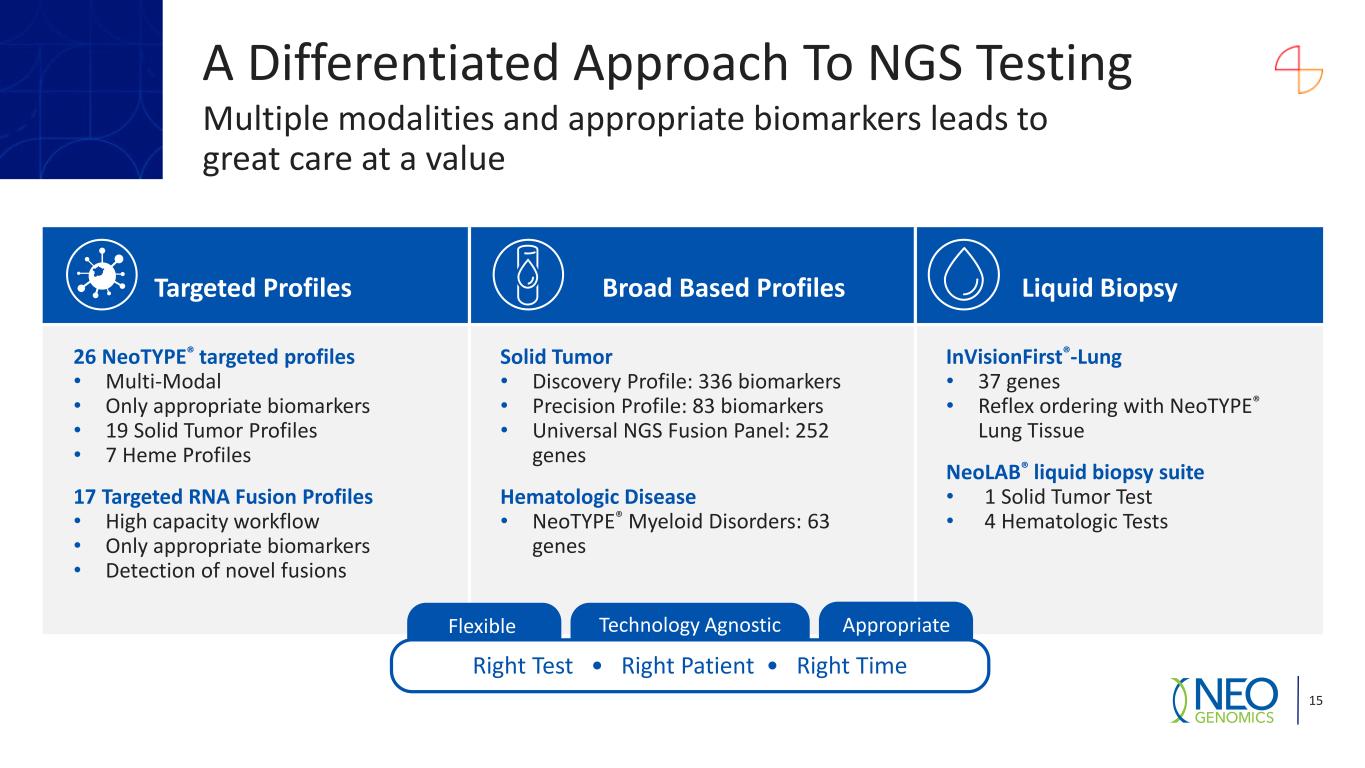
Targeted Profiles Broad Based Profiles Liquid Biopsy 26 NeoTYPE® targeted profiles • Multi-Modal • Only appropriate biomarkers • 19 Solid Tumor Profiles • 7 Heme Profiles 17 Targeted RNA Fusion Profiles • High capacity workflow • Only appropriate biomarkers • Detection of novel fusions Solid Tumor • Discovery Profile: 336 biomarkers • Precision Profile: 83 biomarkers • Universal NGS Fusion Panel: 252 genes Hematologic Disease • NeoTYPE® Myeloid Disorders: 63 genes InVisionFirst®-Lung • 37 genes • Reflex ordering with NeoTYPE® Lung Tissue NeoLAB® liquid biopsy suite • 1 Solid Tumor Test • 4 Hematologic Tests 15 Right Test • Right Patient • Right Time Flexible AppropriateTechnology Agnostic A Differentiated Approach To NGS Testing Multiple modalities and appropriate biomarkers leads to great care at a value

We Are Focused on The Customer NOTES: 1,055 respondents 16 Best-in-class net promoter score Q4 2020 Clinical Client Survey How likely is it that you would recommend this company to a friend or colleague? 0 1 2 3 4 5 6 7 8 9 10 Not at all likely Neutral Extremely likely Detractor Passive Promoter 7% 20% 73% 0 50 100-100 -50 +66 NPS Satisfaction Model Employee Engagement Employee Retention Customer Satisfaction Client Retention >95% Shareholder Satisfaction Achieve Results >Plan % Promoters - % Detractors = NPS (Net Promoter Score)

Clinical Reference Labs with Oncology Divisions Niche Oncology Players High R&D investment and limited test menus 17 Diversified Focus Leading Share in U.S. Clinical Oncology Market Comprehensive, multi-modality “One Lab” position Large and advanced somatic cancer test menu Significant reach into all customer segments National footprint and extensive payer contracts Outstanding client service and partnership models Synergistic Pharma, Clinical and Informatics businesses Pure Play Oncology Diagnostic Lab Comprehensive Test Menu + Sustainable Growth Competing Through Focus, Scale and Scope We enjoy a unique position in the clinical market /
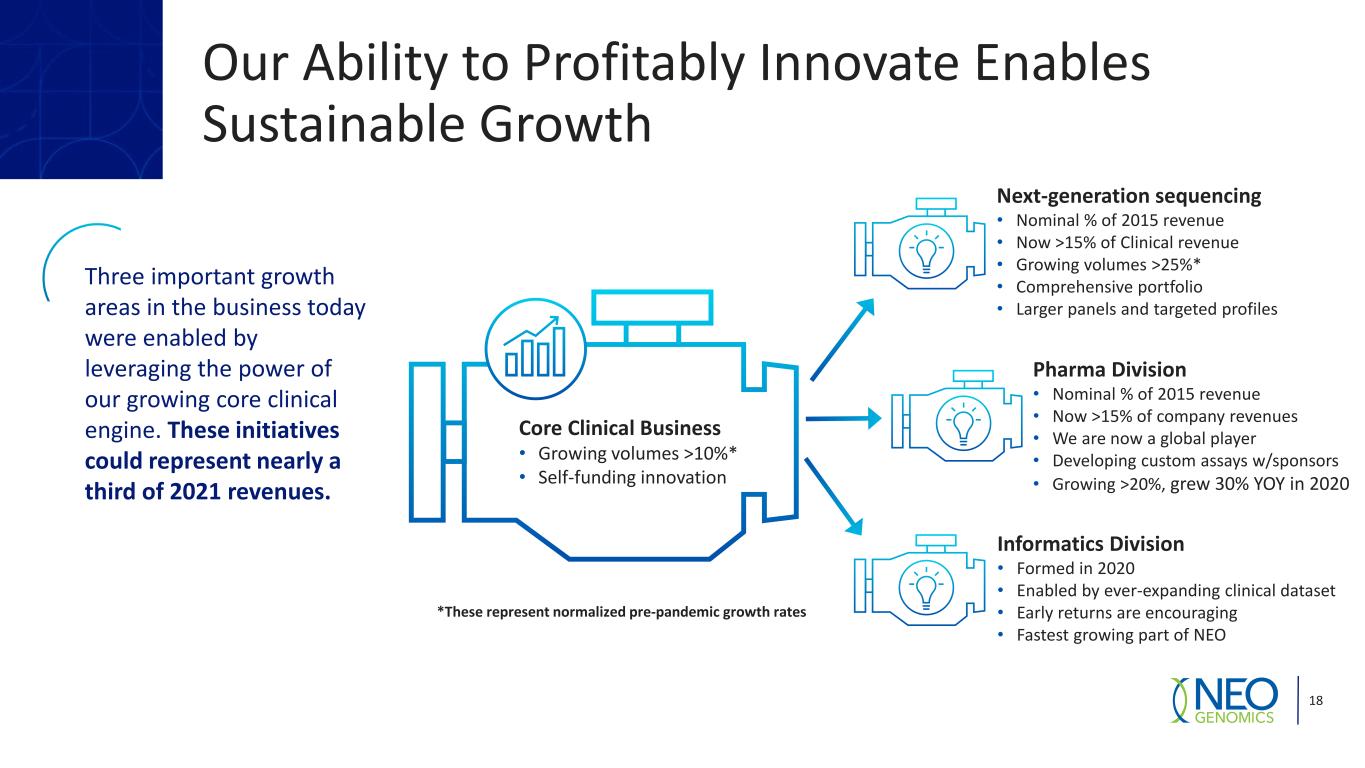
Our Ability to Profitably Innovate Enables Sustainable Growth 18 Core Clinical Business • Growing volumes >10%* • Self-funding innovation Three important growth areas in the business today were enabled by leveraging the power of our growing core clinical engine. These initiatives could represent nearly a third of 2021 revenues. Next-generation sequencing • Nominal % of 2015 revenue • Now >15% of Clinical revenue • Growing volumes >25%* • Comprehensive portfolio • Larger panels and targeted profiles Pharma Division • Nominal % of 2015 revenue • Now >15% of company revenues • We are now a global player • Developing custom assays w/sponsors • Growing >20%, grew 30% YOY in 2020 Informatics Division • Formed in 2020 • Enabled by ever-expanding clinical dataset • Early returns are encouraging • Fastest growing part of NEO *These represent normalized pre-pandemic growth rates
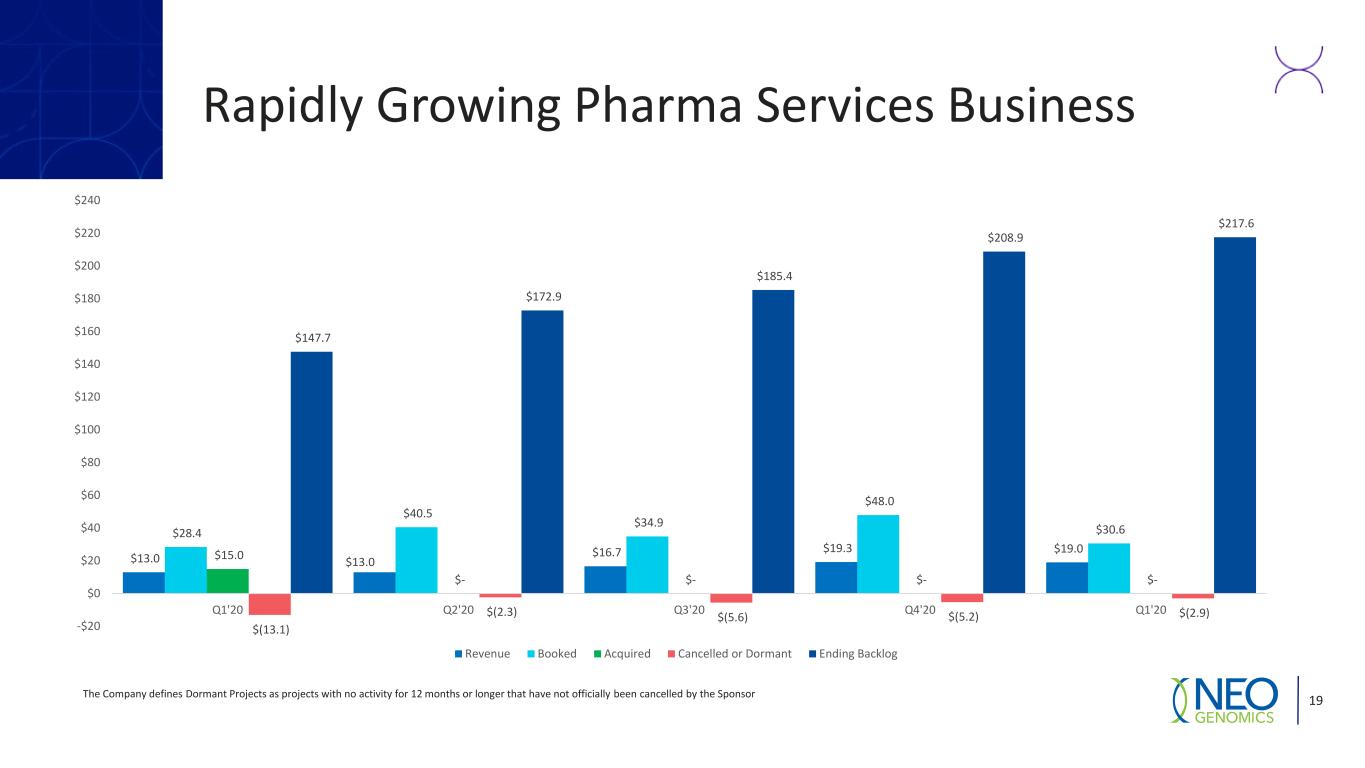
Rapidly Growing Pharma Services Business The Company defines Dormant Projects as projects with no activity for 12 months or longer that have not officially been cancelled by the Sponsor 19 $13.0 $13.0 $16.7 $19.3 $19.0 $28.4 $40.5 $34.9 $48.0 $30.6 $15.0 $- $- $- $- $(13.1) $(2.3) $(5.6) $(5.2) $(2.9) $147.7 $172.9 $185.4 $208.9 $217.6 -$20 $0 $20 $40 $60 $80 $100 $120 $140 $160 $180 $200 $220 $240 Q1'20 Q2'20 Q3'20 Q4'20 Q1'20 Revenue Booked Acquired Cancelled or Dormant Ending Backlog
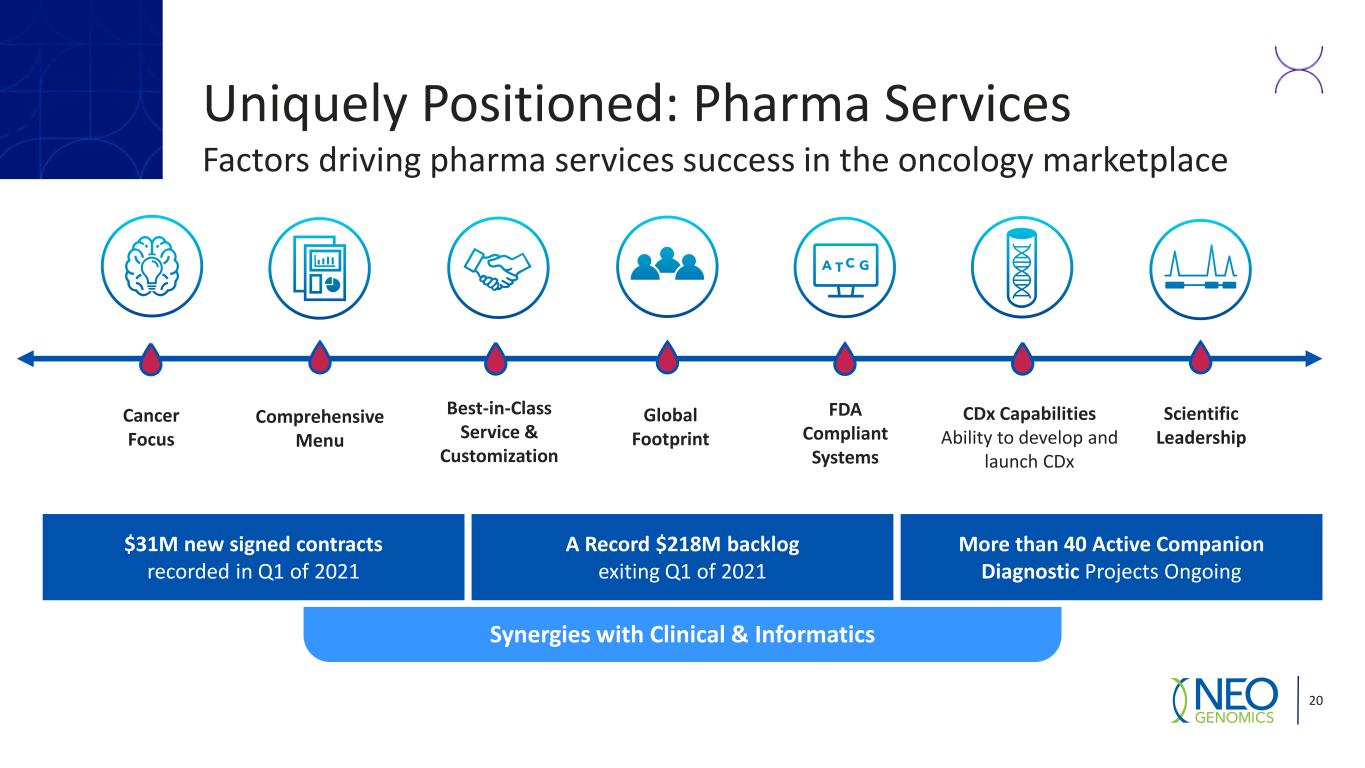
Uniquely Positioned: Pharma Services Factors driving pharma services success in the oncology marketplace 20 $31M new signed contracts recorded in Q1 of 2021 Synergies with Clinical & Informatics More than 40 Active Companion Diagnostic Projects Ongoing A Record $218M backlog exiting Q1 of 2021 Comprehensive Menu Scientific Leadership CDx Capabilities Ability to develop and launch CDx Best-in-Class Service & Customization Cancer Focus Global Footprint FDA Compliant Systems
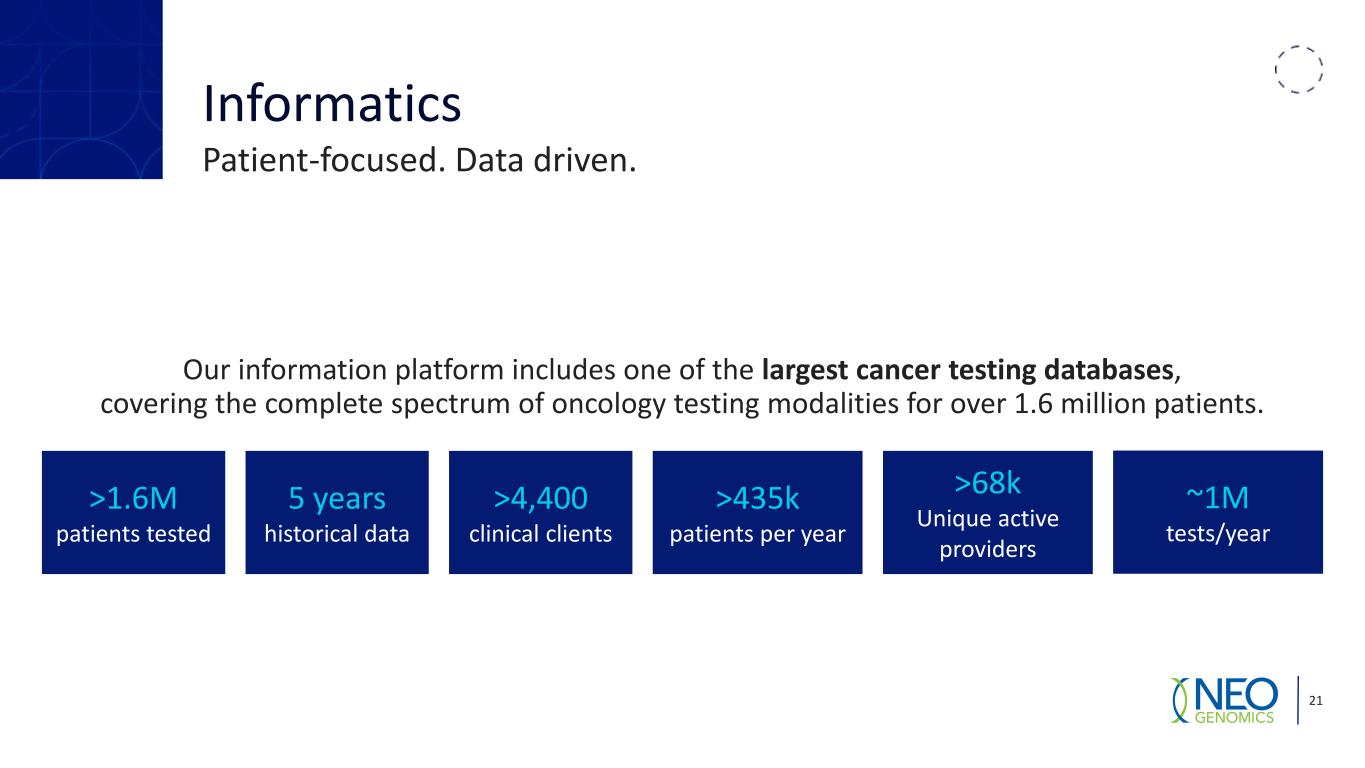
Informatics Patient-focused. Data driven. 21 Our information platform includes one of the largest cancer testing databases, covering the complete spectrum of oncology testing modalities for over 1.6 million patients. >1.6M patients tested 5 years historical data >4,400 clinical clients >435k patients per year >68k Unique active providers ~1M tests/year
Informatics Primary offerings today 22 Diagnostic lab alerts and commercial analytics Clinical trial matching and provider outreach
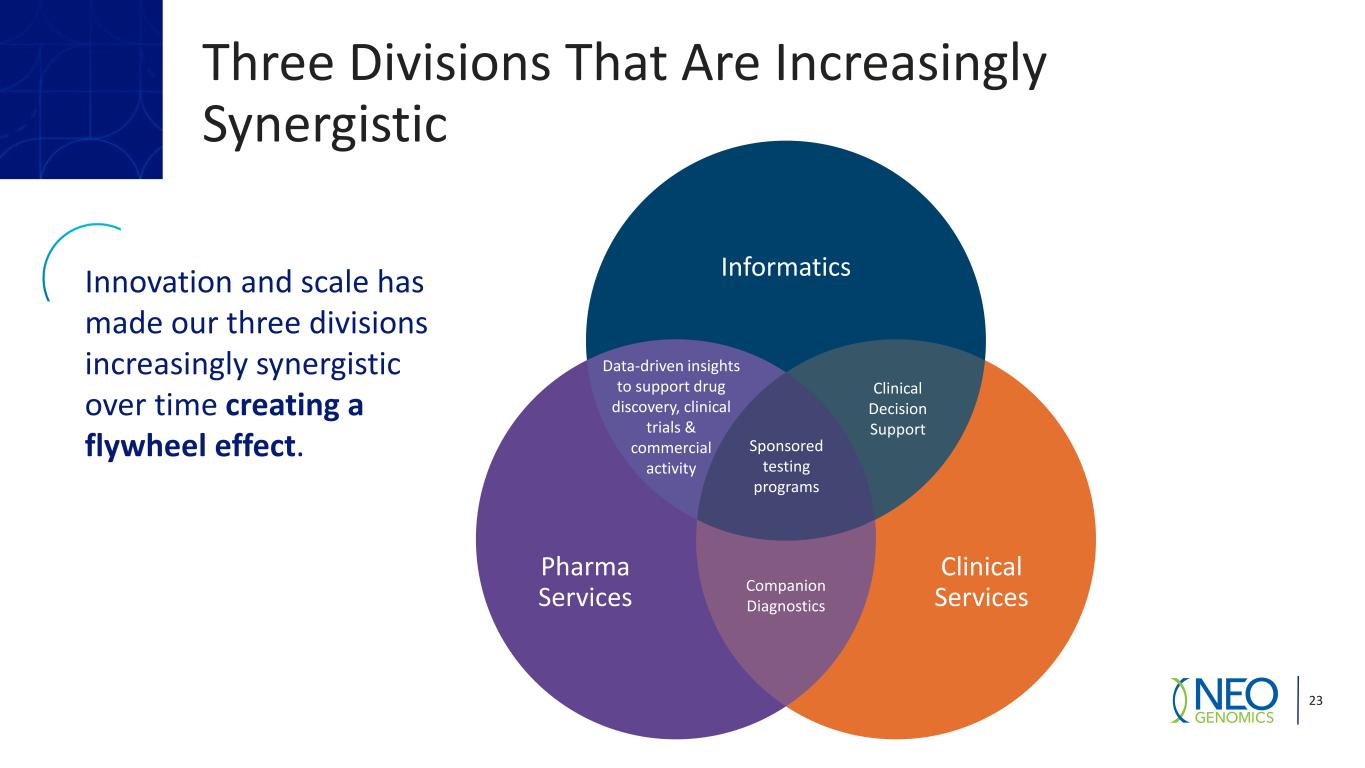
Three Divisions That Are Increasingly Synergistic 23 Innovation and scale has made our three divisions increasingly synergistic over time creating a flywheel effect. Data-driven insights to support drug discovery, clinical trials & commercial activity Clinical Decision Support Sponsored testing programs Clinical Services Pharma Services Informatics Companion Diagnostics

Leading Oncology Diagnostics Company 24 Guided By Science And Passion For Patient Care We are a leader in the field of diagnostic testing with a significant share of patient test volume in the US Our extensive patient database allows us to optimize the pairing of patients with clinical trials We act as a collaborative partner to pathologists, oncologists and biopharma to deliver best-in-class services for all By helping the community oncology field, we improve lives Our work is founded in science, driven by data, and upheld to the highest standards We are oncology experts focused on developing foundational and innovative oncology laboratory diagnostic services When you invest in NeoGenomics, you invest in all of oncology


Appendix
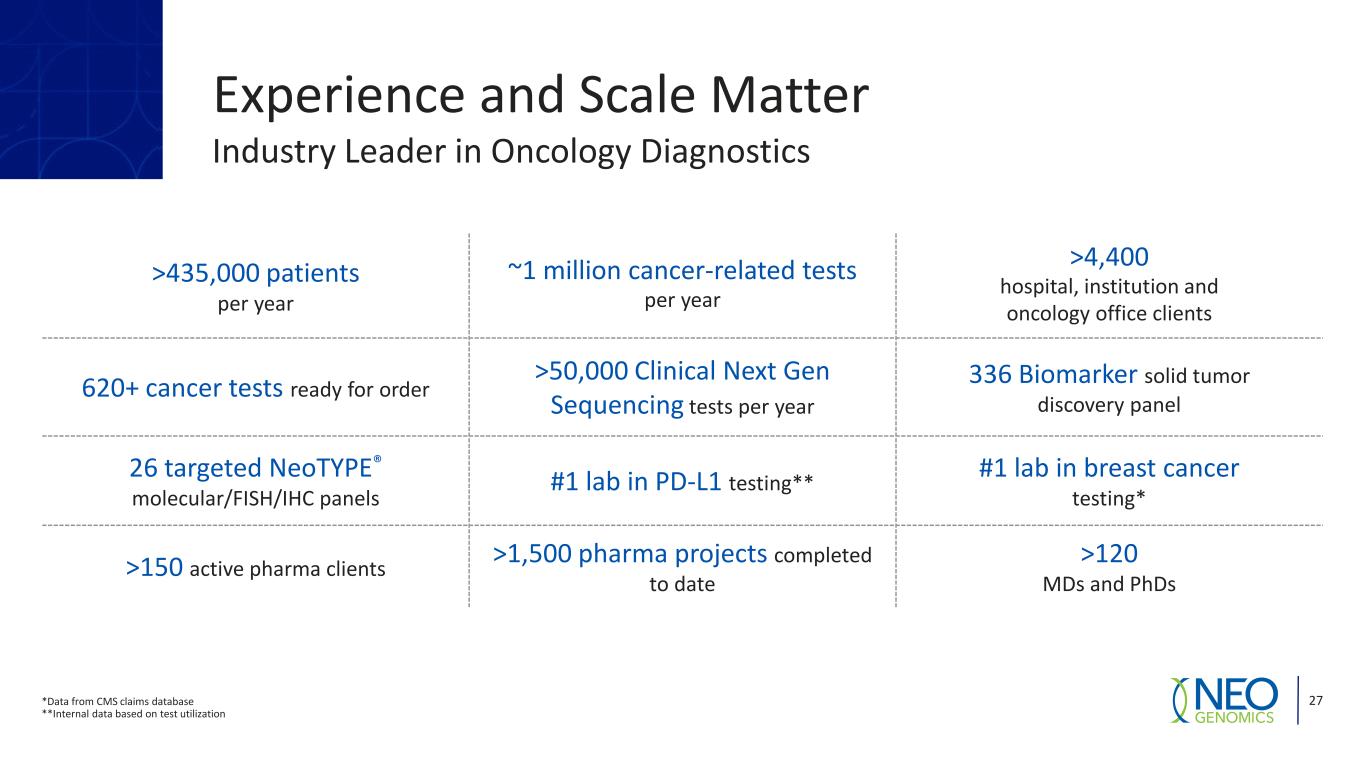
27 Experience and Scale Matter >435,000 patients per year ~1 million cancer-related tests per year >4,400 hospital, institution and oncology office clients 620+ cancer tests ready for order >50,000 Clinical Next Gen Sequencing tests per year 336 Biomarker solid tumor discovery panel 26 targeted NeoTYPE® molecular/FISH/IHC panels #1 lab in PD-L1 testing** #1 lab in breast cancer testing* >150 active pharma clients >1,500 pharma projects completed to date >120 MDs and PhDs *Data from CMS claims database **Internal data based on test utilization Industry Leader in Oncology Diagnostics
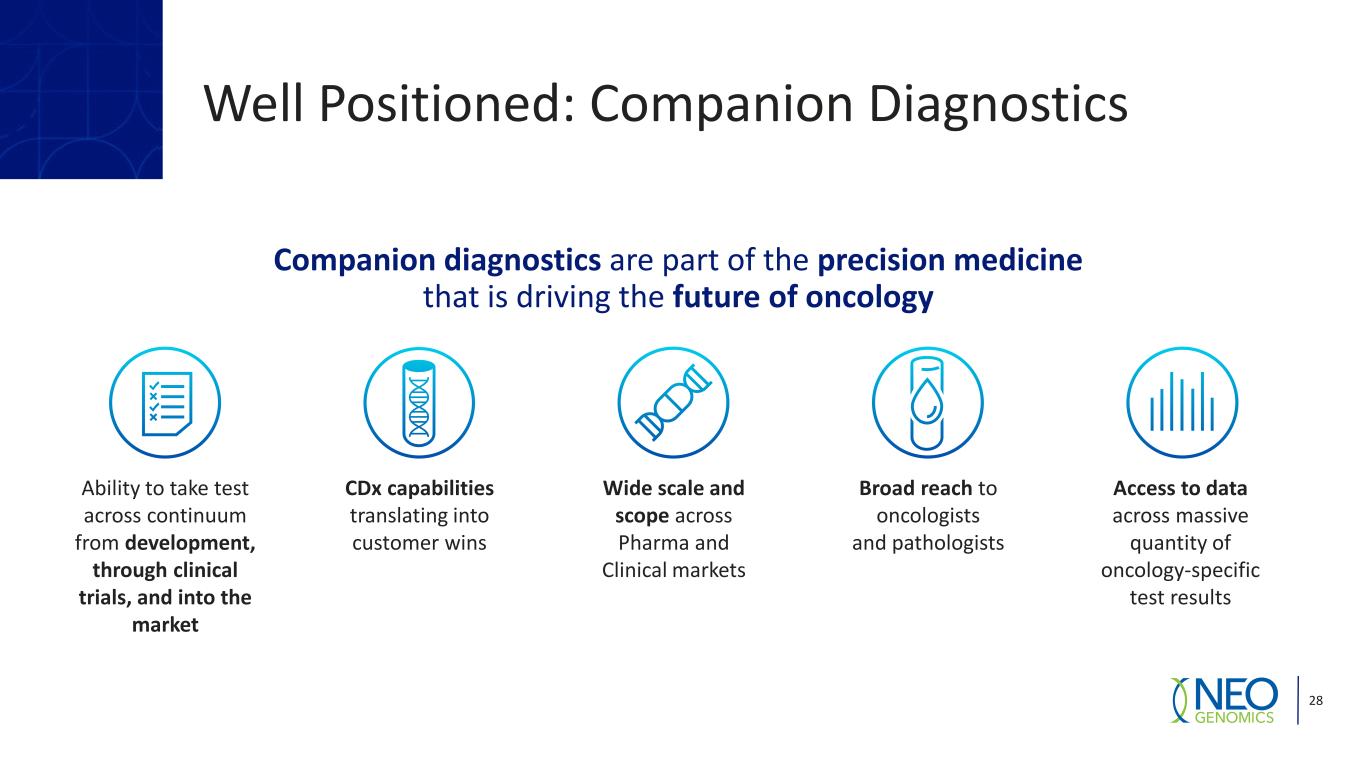
28 Companion diagnostics are part of the precision medicine that is driving the future of oncology Ability to take test across continuum from development, through clinical trials, and into the market CDx capabilities translating into customer wins Wide scale and scope across Pharma and Clinical markets Broad reach to oncologists and pathologists Access to data across massive quantity of oncology-specific test results Well Positioned: Companion Diagnostics

Proving the Point: KEYTRUDA® • Selected by Merck due to IHC expertise • Participated in Early Validation Program for Keytruda • One of only 3 labs to offer PD-L1 testing on Day 1 29 We remain an industry leader in clinical PD-L1 testing

• PIK3CA is a gene that is mutated in many breast cancers • In 2018, when Novartis was in late-stage development for its PIK3CA inhibitor, alpelisib, QIAGEN established a development program to bring to market a molecular test (therascreen® PIK3CA RGQ PCR Kit) as a companion diagnostic to guide the use of alpelisib Proving the Point: PIQRAY® JAN FEB DEC By December 31, 2019: Achievement Novartis revenue was ~$118M and NEO PIK3CA order volume is ~4,000 orders APR MAY May 24, 2019: PIQRAY® (alpelisib) launch and CDx launch Hand-off to clinical: STP ready, all materials ready, clinical sales, marketing and medical activated, medical outreach program, sales contest; Novartis revenue target is $30M and NEO PIK3CA order volume target is 1,000 orders April – June 2019: Launch readiness Pharma Sponsored Testing Program (STP) prepared and ready for day 1 February – June 2019: Clinical launch preparation Sponsored testing program, sales materials and training, website development and digital marketing January – May 2019: Clinical validation NEO pharma services engaged with QIAGEN and Novartis to begin clinical validation process for PIK3CA to ensure PIK3CA testing available on day 1 upon FDA approval 30 In partnership with Novartis and QIAGEN, NeoGenomics was the Day 1 Preferred Laboratory Partner for this critical PIK3CA CDx

Balance Sheet, March 31, 2021 (unaudited, in thousands) 31 March 31, 2021 (Unaudited) December 31, 2020 ASSETS Cash and cash equivalents $ 611,970 $ 228,713 Marketable securities, at fair value 190,710 67,546 Accounts receivable, net 102,922 106,843 Inventories 21,382 29,526 Prepaid assets 11,073 11,547 Other current assets 4,675 4,555 Total current assets 942,732 448,730 Property and equipment (net of accumulated depreciation of $98,746 and $92,895 respectively) 94,315 85,873 Operating lease right-of-use assets 50,904 45,786 Intangible assets, net 118,195 120,653 Goodwill 211,083 211,083 Restricted cash 11,119 21,919 Investment in non-consolidated affiliate 29,555 29,555 Loan receivable from non-consolidated affiliate 10,185 — Prepaid lease asset 21,052 20,229 Other assets 5,273 4,503 Total non-current assets $ 551,681 $ 539,601 TOTAL ASSETS $ 1,494,413 $ 988,331 LIABILITIES AND STOCKHOLDERS’ EQUITY Accounts payable and other current liabilities $ 60,550 $ 65,375 Current portion of equipment financing obligations 2,089 2,841 Current portion of operating lease liabilities 5,111 4,967 Total current liabilities 67,750 73,183 Convertible senior notes, net 530,378 168,120 Operating lease liabilities 46,437 42,296 Deferred income tax liabilities, net 1,744 5,415 Other long-term liabilities 4,390 5,023 Total long-term liabilities 582,949 220,854 TOTAL LIABILITIES $ 650,699 $ 294,037 TOTAL STOCKHOLDERS’ EQUITY $ 843,714 $ 694,294 TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY $ 1,494,413 $ 988,331
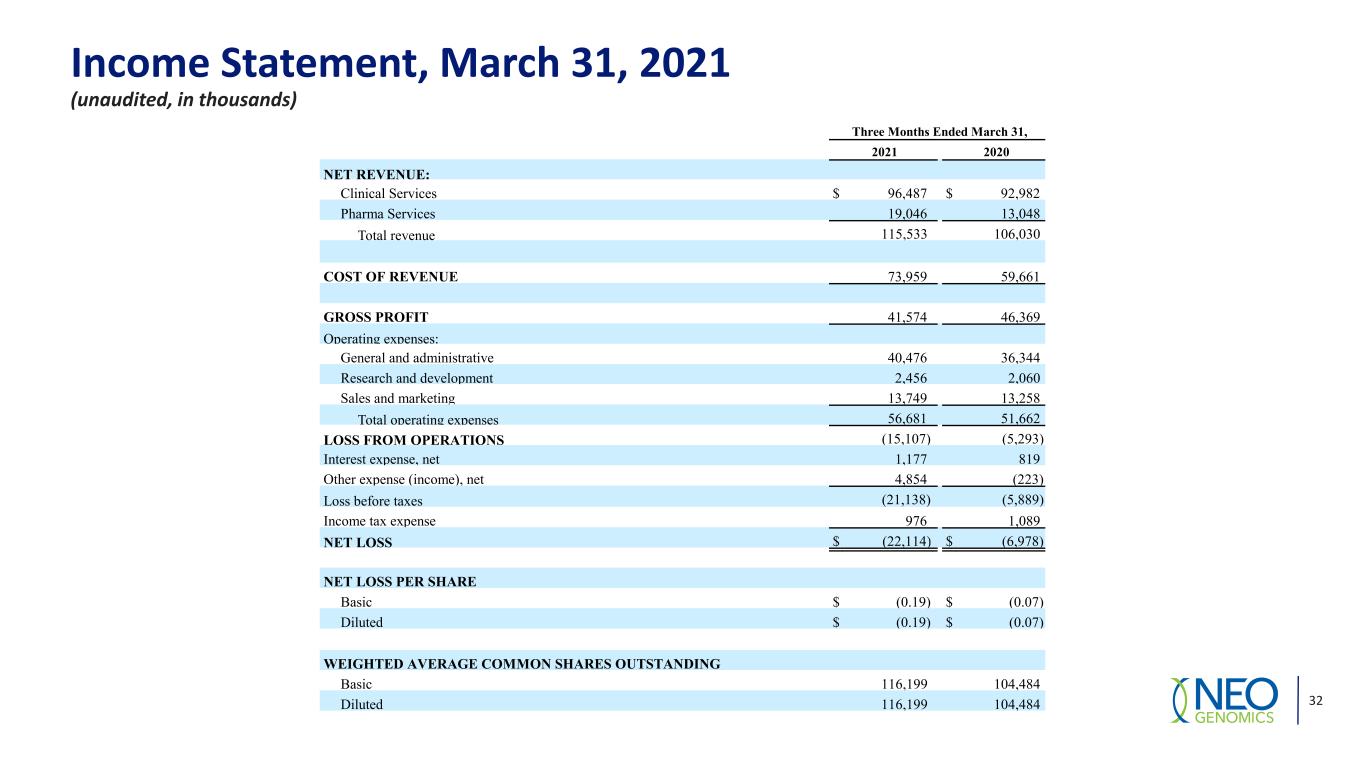
Income Statement, March 31, 2021 (unaudited, in thousands) 32 Three Months Ended March 31, 2021 2020 NET REVENUE: Clinical Services $ 96,487 $ 92,982 Pharma Services 19,046 13,048 Total revenue 115,533 106,030 COST OF REVENUE 73,959 59,661 GROSS PROFIT 41,574 46,369 Operating expenses: General and administrative 40,476 36,344 Research and development 2,456 2,060 Sales and marketing 13,749 13,258 Total operating expenses 56,681 51,662 LOSS FROM OPERATIONS (15,107) (5,293) Interest expense, net 1,177 819 Other expense (income), net 4,854 (223) Loss before taxes (21,138) (5,889) Income tax expense 976 1,089 NET LOSS $ (22,114) $ (6,978) NET LOSS PER SHARE Basic $ (0.19) $ (0.07) Diluted $ (0.19) $ (0.07) WEIGHTED AVERAGE COMMON SHARES OUTSTANDING Basic 116,199 104,484 Diluted 116,199 104,484
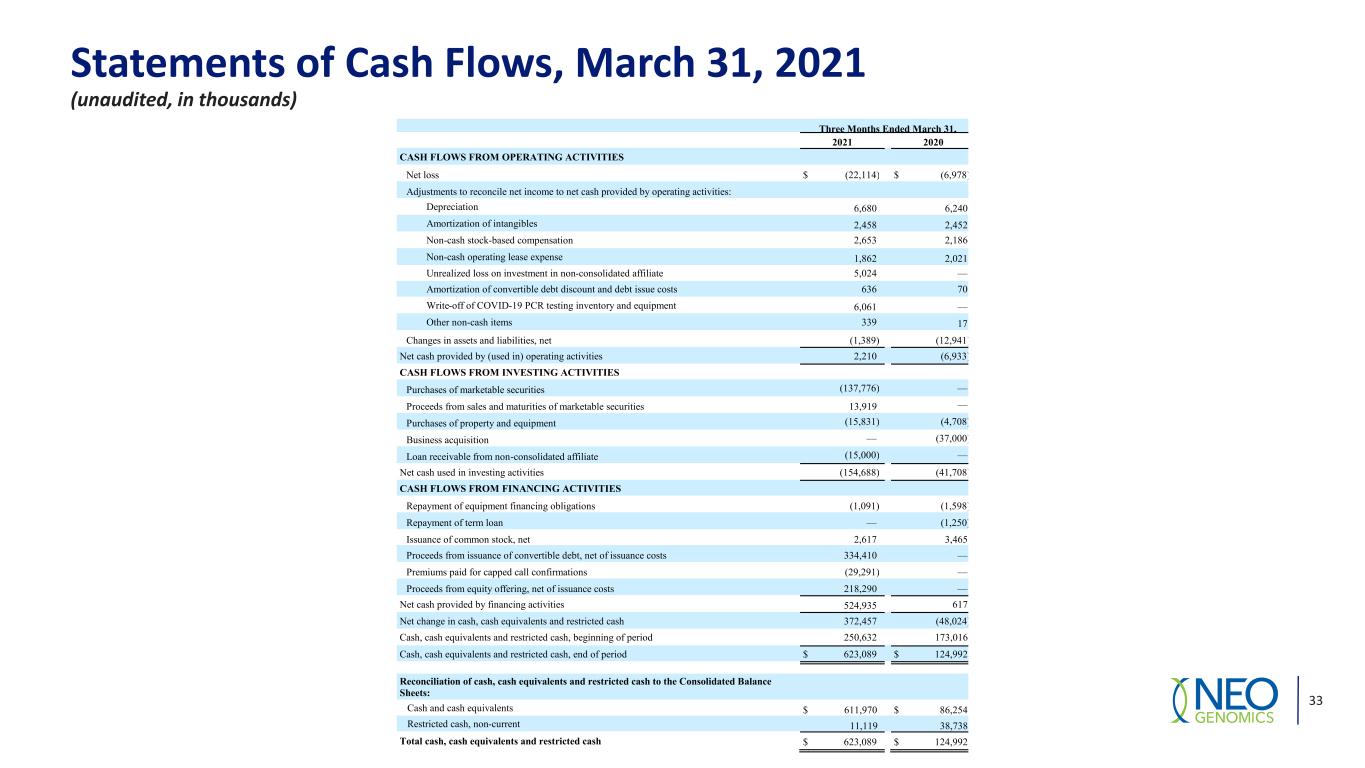
Statements of Cash Flows, March 31, 2021 (unaudited, in thousands) 33 Three Months Ended March 31, 2021 2020 CASH FLOWS FROM OPERATING ACTIVITIES Net loss $ (22,114) $ (6,978) Adjustments to reconcile net income to net cash provided by operating activities: Depreciation 6,680 6,240 Amortization of intangibles 2,458 2,452 Non-cash stock-based compensation 2,653 2,186 Non-cash operating lease expense 1,862 2,021 Unrealized loss on investment in non-consolidated affiliate 5,024 — Amortization of convertible debt discount and debt issue costs 636 70 Write-off of COVID-19 PCR testing inventory and equipment 6,061 — Other non-cash items 339 17 Changes in assets and liabilities, net (1,389) (12,941) Net cash provided by (used in) operating activities 2,210 (6,933) CASH FLOWS FROM INVESTING ACTIVITIES Purchases of marketable securities (137,776) — Proceeds from sales and maturities of marketable securities 13,919 — Purchases of property and equipment (15,831) (4,708) Business acquisition — (37,000) Loan receivable from non-consolidated affiliate (15,000) — Net cash used in investing activities (154,688) (41,708) CASH FLOWS FROM FINANCING ACTIVITIES Repayment of equipment financing obligations (1,091) (1,598) Repayment of term loan — (1,250) Issuance of common stock, net 2,617 3,465 Proceeds from issuance of convertible debt, net of issuance costs 334,410 — Premiums paid for capped call confirmations (29,291) — Proceeds from equity offering, net of issuance costs 218,290 — Net cash provided by financing activities 524,935 617 Net change in cash, cash equivalents and restricted cash 372,457 (48,024) Cash, cash equivalents and restricted cash, beginning of period 250,632 173,016 Cash, cash equivalents and restricted cash, end of period $ 623,089 $ 124,992 Reconciliation of cash, cash equivalents and restricted cash to the Consolidated Balance Sheets: Cash and cash equivalents $ 611,970 $ 86,254 Restricted cash, non-current 11,119 38,738 Total cash, cash equivalents and restricted cash $ 623,089 $ 124,992
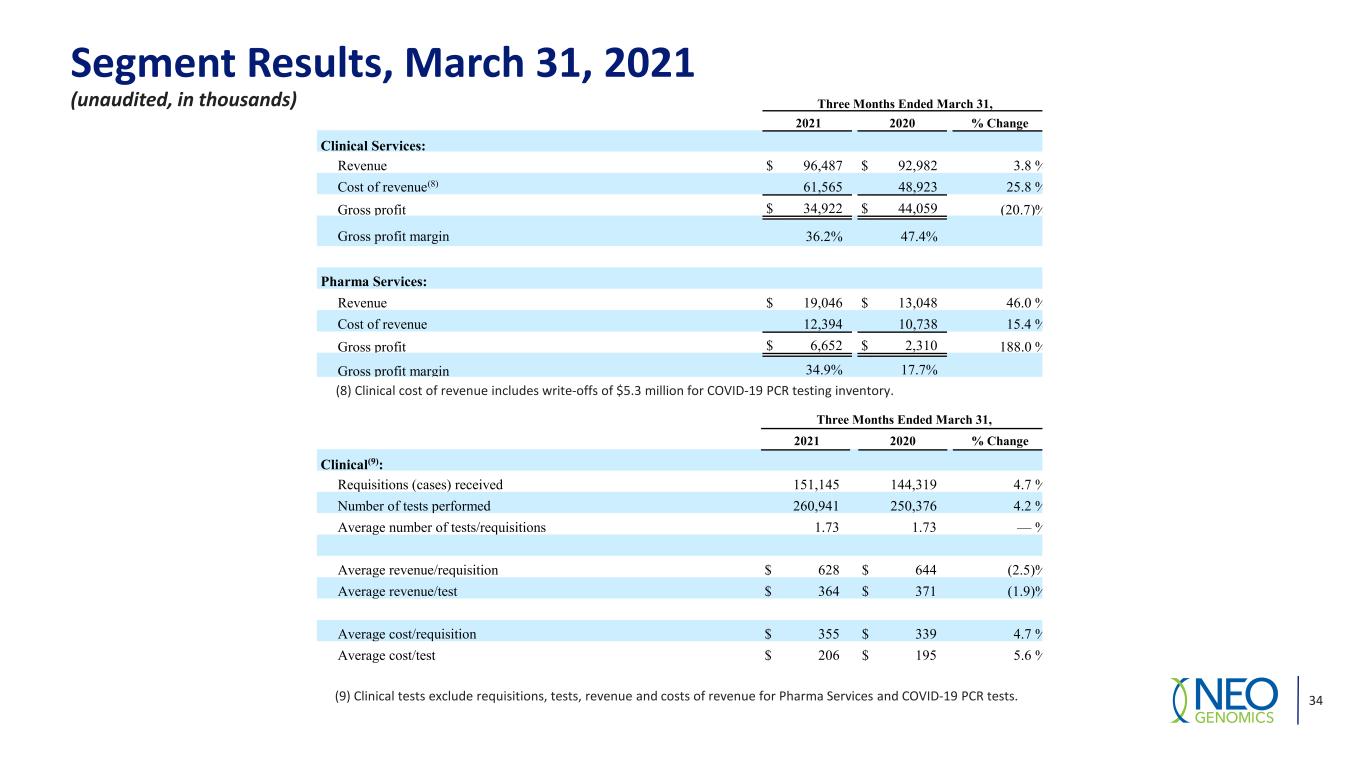
Segment Results, March 31, 2021 (unaudited, in thousands) (9) Clinical tests exclude requisitions, tests, revenue and costs of revenue for Pharma Services and COVID-19 PCR tests. 34 Three Months Ended March 31, 2021 2020 % Change Clinical Services: Revenue $ 96,487 $ 92,982 3.8 % Cost of revenue(8) 61,565 48,923 25.8 % Gross profit $ 34,922 $ 44,059 (20.7) % Gross profit margin 36.2% 47.4% Pharma Services: Revenue $ 19,046 $ 13,048 46.0 % Cost of revenue 12,394 10,738 15.4 % Gross profit $ 6,652 $ 2,310 188.0 % Gross profit margin 34.9% 17.7% (8) Clinical cost of revenue includes write-offs of $5.3 million for COVID-19 PCR testing inventory. Three Months Ended March 31, 2021 2020 % Change Clinical(9): Requisitions (cases) received 151,145 144,319 4.7 % Number of tests performed 260,941 250,376 4.2 % Average number of tests/requisitions 1.73 1.73 — % Average revenue/requisition $ 628 $ 644 (2.5) % Average revenue/test $ 364 $ 371 (1.9) % Average cost/requisition $ 355 $ 339 4.7 % Average cost/test $ 206 $ 195 5.6 %
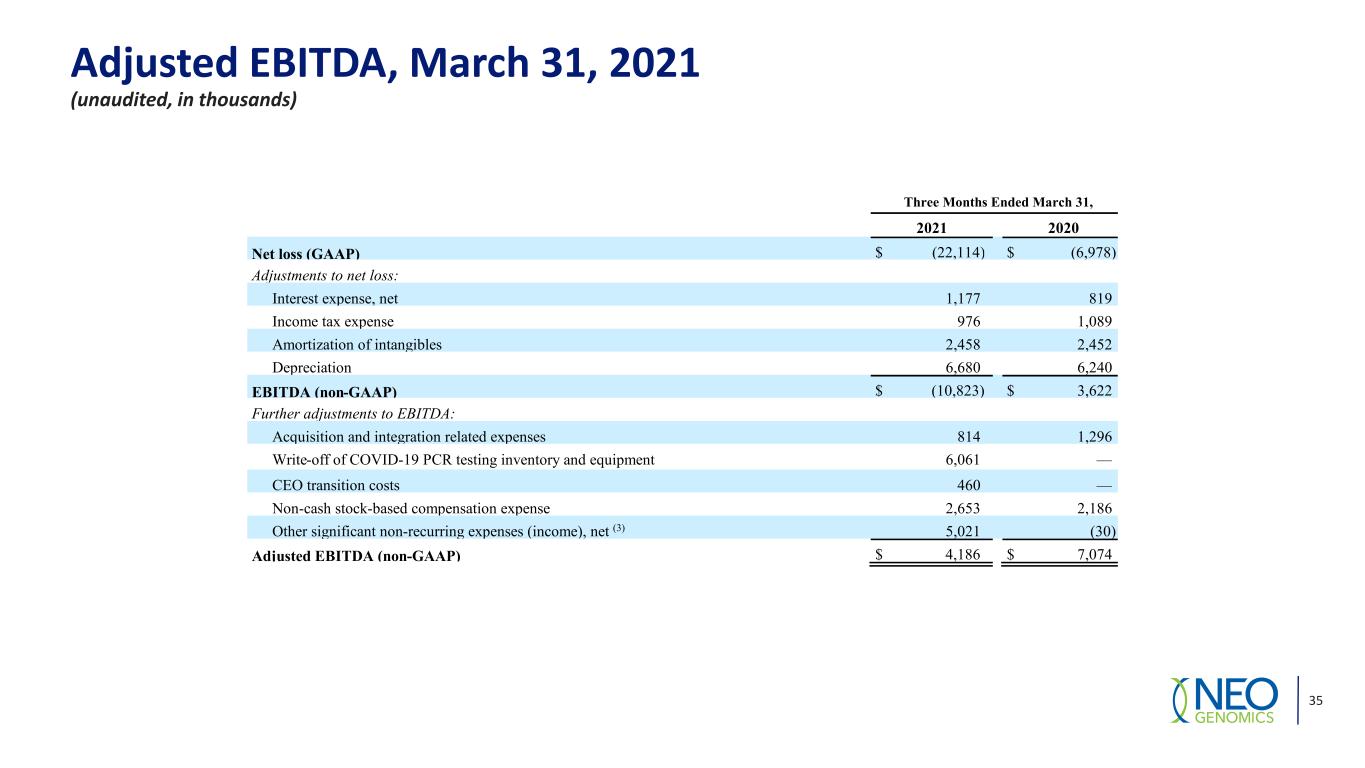
Adjusted EBITDA, March 31, 2021 (unaudited, in thousands) 35 Three Months Ended March 31, 2021 2020 Net loss (GAAP) $ (22,114) $ (6,978) Adjustments to net loss: Interest expense, net 1,177 819 Income tax expense 976 1,089 Amortization of intangibles 2,458 2,452 Depreciation 6,680 6,240 EBITDA (non-GAAP) $ (10,823) $ 3,622 Further adjustments to EBITDA: Acquisition and integration related expenses 814 1,296 Write-off of COVID-19 PCR testing inventory and equipment 6,061 — CEO transition costs 460 — Non-cash stock-based compensation expense 2,653 2,186 Other significant non-recurring expenses (income), net (3) 5,021 (30) Adjusted EBITDA (non-GAAP) $ 4,186 $ 7,074
