Attached files
| file | filename |
|---|---|
| EX-32.1 - EX-32.1 - Coherus BioSciences, Inc. | chrs-20210331xex32d1.htm |
| EX-31.2 - EX-31.2 - Coherus BioSciences, Inc. | chrs-20210331xex31d2.htm |
| EX-31.1 - EX-31.1 - Coherus BioSciences, Inc. | chrs-20210331xex31d1.htm |
| EX-10.2 - EX-10.2 - Coherus BioSciences, Inc. | chrs-20210331xex10d2.htm |
| 10-Q - 10-Q - Coherus BioSciences, Inc. | chrs-20210331x10q.htm |
Exhibit 10.1
EXECUTION COPY
Confidential
EXCLUSIVE LICENSE AND COMMERCIALIZATION AGREEMENT
BY AND BETWEEN
COHERUS BIOSCIENCES, INC.
AND
SHANGHAI JUNSHI BIOSCIENCES CO., LTD.
DATED AS OF FEBRUARY 1, 2021
TABLE OF CONTENTS
| | | | Page |
Article 1 |
| DEFINITIONS |
| 1 |
Article 2 | | LICENSES AND EXCLUSIVITY | | 17 |
Article 3 | | GOVERNANCE | | 26 |
Article 4 | | DEVELOPMENT | | 30 |
Article 5 | | REGULATORY | | 36 |
Article 6 | | MANUFACTURING | | 40 |
Article 7 | | COMMERCIALIZATION | | 40 |
Article 8 | | FINANCIALS | | 41 |
Article 9 | | INTELLECTUAL PROPERTY | | 47 |
Article 10 | | REPRESENTATIONS, WARRANTIES, AND COVENANTS | | 52 |
Article 11 | | INDEMNIFICATION | | 59 |
Article 12 | | CONFIDENTIALITY | | 61 |
Article 13 | | TERM AND TERMINATION | | 64 |
Article 14 | | EFFECTIVENESS | | 67 |
Article 15 | | DISPUTE RESOLUTION | | 68 |
Article 16 | | MISCELLANEOUS | | 70 |
-i-
EXCLUSIVE LICENSE AND COMMERCIALIZATION AGREEMENT
THIS EXCLUSIVE LICENSE AND COMMERCIALIZATION AGREEMENT (this “Agreement”) is entered into as of 1 February 2021 (the “Execution Date”) by and among Shanghai Junshi Biosciences Co., Ltd., a corporation organized and existing under the laws of the People’s Republic of China, with a registered address at Level 13, Building 2, Nos. 36 and 58, Hai Qu Road, China (Shanghai) Pilot Free Trade Zone, China 201203 (“Junshi”), and Coherus BioSciences, Inc., a Delaware corporation having its principal place of business at 333 Twin Dolphin Drive, Suite 600 Redwood City, CA 94065 (“Coherus”). Junshi and Coherus are referred to herein individually as a “Party” and collectively as the “Parties.”
BACKGROUND
WHEREAS, Junshi and its Affiliates Control certain Patent Rights and Know-How relating to the Licensed Antibody and are conducting research and development with respect to additional products.
WHEREAS, Junshi desires to grant, and Coherus desires to receive, an exclusive license under such Patent Rights and Know-How related to the Licensed Antibody to permit Coherus to Develop, Manufacture, Commercialize, and otherwise Exploit the Licensed Antibody and Licensed Products within the Coherus Territory.
WHEREAS, Junshi desires to grant, and Coherus desires to receive, an exclusive option to receive an exclusive license to Exploit products that are the subject of two other programs Controlled by Junshi.
WHEREAS, Junshi desires to grant, and Coherus desires to receive, a right of first negotiation with respect to the grant of rights to Exploit products that are the subject of two additional programs Controlled by Junshi.
NOW THEREFORE, the Parties agree as follows:
ARTICLE 1
DEFINITIONS
1.1 | “Additional Cure Period” has the meaning set forth in Section 13.3(c) (Disputes Regarding Material Breach). |
1.2 | “Additional Third Party IP” has the meaning set forth in Section 2.5 (Third Party In-Licenses). |
1.3 | “Additional Third Party License” has the meaning set forth in Section 2.5 (Third Party In- Licenses). |
1.4 | “Affiliate” means, with respect to a Party, a person, corporation, partnership, or other entity that controls, is controlled by, controlling or is under common control with such Party, but only for so long as such control will continue. For the purposes of this definition, the word “control” (including, with correlative meaning, the terms “controlled by”, “controlling” or “under the common control with”) means the actual power, either directly or indirectly through one or more intermediaries, to direct or cause the direction of the management and policies of such entity, whether by the ownership of more than fifty percent (50%) of the voting stock of such entity, or by contract or otherwise. |
1.5 | “Agreement” has the meaning set forth in the Preamble. |
1.6 | “Alliance Manager” has the meaning set forth in Section 3.1 (Alliance Manager). |
1.7 | “Antibody” means an antibody, or an antigen binding fragment thereof. |
1.8 | “Antitrust Clearance Date” means the earliest date on which all applicable waiting periods and approvals required under Antitrust Laws in the Clearance Countries with respect to the transactions |
contemplated under this Agreement have expired or have been terminated (in the case of waiting periods) or been received (in the case of approvals), in each case, without the imposition of any conditions.
1.9 | “Antitrust Filing” means filings by Junshi and Coherus with the United States Federal Trade Commission and the United States Department of Justice and any applicable governmental authority in the Clearance Countries, as required under any Antitrust Laws with respect to the transactions contemplated under this Agreement, together with all required documentary attachments thereto. |
1.10 | “Antitrust Laws” means any and all applicable law designed to prohibit, restrict, or regulate actions for the purpose or effect of monopolization or restraint of trade. |
1.11 | “Arising Know-How” has the meaning set forth in Section 9.1(b)(i) (Arising Technology). |
1.12 | “Arising Patent Rights” has the meaning set forth in Section 9.1(b)(i) (Arising Technology). |
1.13 | “Arising Technology” has the meaning set forth in Section 9.1(b)(i) (Arising Technology). |
1.14 | “BLA” means a Biologics License Application submitted to the FDA pursuant to 21 U.S.C. §601.2, for purposes of obtaining Regulatory Approval to introduce a biologic product into interstate commerce in the United States, or any equivalent filing in a country or regulatory jurisdiction other than the United States. |
1.15 | “Business Day” means a day other than a Saturday, Sunday, or a day on which banking institutions in Redwood City, CA or Shanghai, China are required by applicable law to remain closed. |
1.16 | “Calendar Quarter” means a period of three consecutive months ending on the last day of March, June, September, or December, respectively. |
1.17 | “Calendar Year” means a period of 12 consecutive months beginning on January 1 and ending on December 31. |
1.18 | “CD112r” means receptor for cluster of differentiation 112. |
1.19 | “cGMP” means applicable current Good Manufacturing Practices, including, as applicable, (a) the principles detailed in the U.S. Current Good Manufacturing Practices, 21 C.F.R. Parts 4, 210, 211, 600, 610 and 820, (b) European Directive 2003/94/EC and Eudralex 4, (c) the principles detailed in the ICH’s Q7 guidelines, and (d) the applicable laws the Coherus Territory corresponding to (a) through (c) above, each as may be amended and applicable from time to time. |
1.20 | “Chairperson” has the meaning set forth in Section 3.2 (Joint Development Committee). |
1.21 | “Change of Control” means, with respect to a Party, that: (a) any Third Party acquires directly or indirectly the beneficial ownership of any voting security of such Party, or if the percentage ownership of such Third Party in the voting securities of such Party is increased through stock redemption, cancellation, or other recapitalization, and immediately after such acquisition or increase such Third Party is, directly or indirectly, the beneficial owner of voting securities representing more than 50% of the total voting power of all of the then outstanding voting securities of such Party; (b) a merger, consolidation, recapitalization, or reorganization of such Party is consummated that would result in shareholders or equity holders of such Party immediately prior to such transaction, owning more than 50% of the outstanding voting securities of the surviving entity (or its parent entity) immediately following such transaction; or (c) there is a sale or transfer to a Third Party of all or substantially all of such Party’s consolidated assets taken as a whole, through one or more related transactions. |
1.22 | “Claim” has the meaning set forth in Section 11.3 (Indemnification Procedures). |
2
1.23 | “Clearance Countries” means the countries and jurisdictions where antitrust clearance is required under any Antitrust Laws with respect to the transactions contemplated under this Agreement. |
1.24 | “Clinical Trial” means a study in humans to obtain information regarding a product, including information relating to the safety, tolerability, pharmacological activity, pharmacokinetics, dose ranging or efficacy of such product, including a Phase I Clinical Trial, Phase II Clinical Trial, Phase III Clinical Trial, and a Pivotal Trial. |
1.25 | “Closing Disclosure Letter” has the meaning set forth in Section 10.3(a) (Closing Disclosure Letter). |
1.26 | “CMO” means a contract manufacturing organization. |
1.27 | “Coherus” has the meaning set forth in the Preamble. |
1.28 | “Coherus Arising Patent Rights” has the meaning set forth in Section 9.1(b)(i) (Arising Technology). |
1.29 | “Coherus CMO” has the meaning set forth in Section 2.6(a) (Initial Technology Transfer). |
1.30 | “Coherus Indemnitees” has the meaning set forth in Section 11.1 (Indemnification by Junshi). |
1.31 | “Coherus Territory” means the United States and Canada. |
1.32 | “Combination Product” means a Licensed Product that is (a) sold in the form of a combination that contains or comprises a Licensed Antibody together with one or more other therapeutically active pharmaceutical agents (whether coformulated or copackaged or otherwise sold for a single price), (b) sold for a single invoice price together with any (i) delivery device or component therefor, (ii) companion diagnostic related to a Licensed Antibody, or (iii) product, process, service, or therapy (including possibly another Licensed Antibody, such additional therapeutically active pharmaceutical agent and each of (i) – (iii), an “Other Component”); or (c) defined as a “combination product” by the FDA pursuant to 21 C.F.R. §3.2(e) or its foreign equivalent. |
1.33 | “Combination Regimen” means any product or treatment regimen that comprises, or is a combination of (a) a Licensed Product, and (b) any Other Component (whether or not the intellectual property rights for such Other Component are Controlled by a Party), where (a) and (b) are labeled for use together or Regulatory Approval is being sought for use together either simultaneously or in a separate or sequential administration, whether or not sold for a single price. |
1.34 | “Commercialization,” “Commercializing,” or “Commercialize” means any and all activities directed to the marketing, promotion, distribution, offering for sale, sale, having sold, importing, having imported, exporting, having exported or other commercialization of a pharmaceutical or biologic product, but excluding activities directed to Manufacturing, Development, or Medical Affairs. “Commercialize,” “Commercializing,” and “Commercialized” will be construed accordingly. |
1.35 | “Commercially Reasonable Efforts” means, with respect to the efforts to be expended by a Party or its Affiliate with respect to any Development or Commercialization objective, activity, or goal related to a Licensed Product or Option Product (as applicable) under this Agreement, those efforts that a Party would normally use to accomplish such objective, activity, or goal, and specifically means the carrying out of Development and Commercialization activities using efforts that a Party would normally devote to a product at a similar stage in its development or product life and of similar market potential, strategic importance, and profit potential (taking into account payments under this Agreement), based on conditions then prevailing and taking into account efficacy, safety, product labeling, profitability, the competitiveness of alternative products sold by Third Parties in the marketplace, the patent and other proprietary position of the product, the likelihood of regulatory approval given the regulatory structure involved, and all other relevant factors. |
3
Commercially Reasonable Efforts will be determined on a country-by-country and indication-by- indication basis for the applicable Licensed Product and it is anticipated that the level of effort will change over time, reflecting changes in the status of such Licensed Product (as applicable) and the market or country involved.
1.36 | “Competitive Product” means, (a) with respect to the Licensed Antibody and Licensed Products as of the Effective Date, any pharmaceutical or biologic product, other than a Licensed Product, that is an anti-PD-1 monospecific Antibody or anti-PD-L1 monospecific Antibody, excluding, in each case, any other modality molecule including such Antibody (such as a bi-specific or multi- specific Antibody or fused cytokine), (b) with respect to the Licensed Antibody and Licensed Products that are the subject of the Option Program for the Junshi IL-2 Molecule any pharmaceutical or biologic product, other than such Licensed Products that are the subject of the Option Program for the Junshi IL-2 Molecule, that is a molecule derived from IL-2, excluding, in each case, any IL-2 derived molecule fused with an Antibody or any IL-2 derived molecule described in clause (i) and (ii) of the definition of Junshi IL-2 Molecule, and (c) with respect to the Licensed Antibody and Licensed Products that are the subject of the Option Program for the Junshi TIGIT Antibody, any pharmaceutical or biologic product, other than such Licensed Products that are the subject of the Option Program for the Junshi TIGIT Antibody, that is an anti-TIGIT monospecific Antibody, excluding, in each case, any other modality molecule including such Antibody (such as a bi-specific or multi-specific Antibody or fused cytokine). For clarity, any product described in clauses (b) or (c) will not be a “Competitive Product” unless and until Coherus exercises the option with respect to the applicable Option Program. |
1.37 | “Confidential Information” has the meaning set forth in Section 12.1 (Confidentiality; Exceptions). |
1.38 | “Continuing Technology Transfer” has the meaning set forth in Section 2.6(c) (Continuing Technology Transfer). |
1.39 | “Control” or “Controlled” means (a) the possession by a Party (whether by ownership, license, or otherwise other than pursuant to this Agreement) of, (i) with respect to any tangible Know-How, the legal authority or right to physical possession of such tangible Know-How, with the right to provide such tangible Know-How to the other Party on the terms set forth herein, or (ii) with respect to Patent Right, Regulatory Approvals, Regulatory Materials, intangible Know-How, or other intellectual property rights, the legal authority or right to grant a license, sublicense, access, or right to use (as applicable) to the other Party under such Patent Right, Regulatory Approvals, Regulatory Materials, intangible Know-How, or other intellectual property rights on the terms set forth herein, in each case ((i) and (ii)), without breaching or otherwise violating the terms of any arrangement or agreement with a Third Party in existence as of the time such Party or its Affiliates would first be required hereunder to grant the other Party such possession, access, right to use, licenses, or sublicense; and (b) with respect to any product, the possession by a Party of the ability (whether by sole or joint ownership, license or otherwise, other than pursuant to this Agreement) to grant a license or sublicense of Patent Rights that claim such product or proprietary Know-How that is used in connection with the Exploitation of such product. Notwithstanding the foregoing, (A) a Party and its Affiliates will not be deemed to “Control” any Patent Rights, Know-How, or product that, prior to the consummation of a Change of Control of such Party, are owned or in-licensed by a Third Party that becomes an Affiliate of such acquired Party (or that merges or consolidates with such Party) after the Effective Date as a result of such Change of Control, unless after the consummation of the Change of Control, such acquired Party uses any such Patent Rights, Know- How, or product in connection with the Exploitation of a Combination Regimen (if Junshi is the acquired Party); and (B) any Additional Third Party IP that is licensed to a Party will not be deemed to be “Controlled” by such Party unless and until the Parties enter into a written agreement with |
4
terms under which a Party or its Affiliate will grant to the other Party a sublicense thereunder in accordance with Section 2.5 (Third Party In-Licenses).
1.40 | “Cover,” “Covering,” or “Covered” means, when used to refer to the relationship between a particular Patent Right and particular subject matter, that the manufacture, use, sale, offer for sale, or importation of such subject matter would fall within the scope of one or more claims in, or is otherwise claimed by, such Patent Right. |
1.41 | “CPA Firm” has the meaning set forth in Section 8.6 (Books and Records; Audit Rights). |
1.42 | “CTLA-4” means cytotoxic T-lymphocyte antigen 4. |
1.43 | “Date of First Regulatory Approval” has the meaning set forth in Section 7.3 (Commercialization Report). |
1.44 | “Defaulting Party” has the meaning set forth in Section 13.3(c) (Disputes Regarding Material Breach). |
1.45 | “Develop” or “Development” means all internal and external research, development, and regulatory activities related to pharmaceutical or biologic products, including (a) research, non- clinical testing, toxicology, testing and studies, non-clinical and preclinical activities, and Clinical Trials, and (b) preparation, submission, review, and development of data or information for the purpose of submission to a Regulatory Authority to obtain authorization to conduct Clinical Trials and to obtain, support, or maintain Regulatory Approval of a pharmaceutical or biologic product and interacting with Regulatory Authorities following receipt of Regulatory Approval in the applicable country or region for such pharmaceutical or biologic product regarding the foregoing, but excluding activities directed to Manufacturing, Medical Affairs, or Commercialization. Development will include development and regulatory activities for additional forms, formulations, or indications for a pharmaceutical or biologic product after receipt of Regulatory Approval of such product (including label expansion), including Clinical Trials initiated following receipt of Regulatory Approval or any Clinical Trial to be conducted after receipt of Regulatory Approval that was mandated by the applicable Regulatory Authority as a condition of such Regulatory Approval with respect to an approved formulation or indication (such as post-marketing studies, observational studies, implementation and management of registries and analysis thereof, in each case, if required by any Regulatory Authority in any region to support or maintain Regulatory Approval for a pharmaceutical or biologic product in such region). “Develop,” “Developing,” and “Developed” will be construed accordingly. |
1.46 | “Developing Party” has the meaning set forth in Section 4.5 (Independent Development in the Coherus Territory). |
1.47 | “Development Proposal” has the meaning set forth in Section 4.3(a) (Proposals and JDC Review). |
1.48 | “Effective Date” has the meaning set forth in Section 14.1 (Effective Date). |
1.49 | “Execution Date” has the meaning set forth in the Preamble. |
1.50 | “Executive Officer” means (a) in the case of Coherus, the chief executive officer of Coherus, and (b) in the case of Junshi, the chief executive officer of Junshi, neither of who will be a member of the JDC. |
1.51 | “Existing Nondisclosure Agreement” means the Confidentiality Agreement entered into by Coherus and Junshi, effective as of April 1, 2020. |
1.52 | “Exploit” and “Exploitation” means Develop, use, perform Medical Affairs, offer for sale, sell, export, import, Manufacture, have Manufactured, Commercialize, or otherwise exploit. “Exploitation” and “Exploiting” will be construed accordingly. |
5
1.53 | “FDA” means the U.S. Food and Drug Administration or any successor agency thereto. |
1.54 | “Field” means treatment or prevention of diseases and disorders in humans. |
1.55 | “First Commercial Sale” means, with respect to a Licensed Product in a country or region in the Coherus Territory, the first sale to a Third Party of such Licensed Product in such country or region after receipt of Regulatory Approval and, where applicable for the Commercialization of such Licensed Product in such country or region, Pricing and Reimbursement Approval. First Commercial Sale excludes any sale or other distribution of a Licensed Product for promotional or advertising purposes, Clinical Trials, preclinical trials, or other Development purposes, free samples, named patient use, compassionate use, patient assistance, expanded access, or charitable use. |
1.56 | “For the Coherus Territory” means, with respect to any Clinical Trial or other Development of a Licensed Product, that such Development is conducted for purposes of obtaining, maintaining, or supporting Regulatory Approval of such Licensed Antibodies and Licensed Products in the Field in the Coherus Territory, including when Clinical Trials are conducted under an IND in the US or its Canadian counterpart. |
1.57 | “FTE” means the equivalent of the work of one duly qualified employee of either Party full time for one year (consisting of a total of 1,800 hours per year) carrying out Development or Manufacturing activities, or other scientific or technical work under this Agreement. Overtime and work on weekends, holidays, and the like, in each case, will not be counted with any multiplier (e.g., time-and-a-half or double time) toward the number of hours that are used to calculate the FTE contribution. The portion of an FTE billable for one individual during a given accounting period will be determined by dividing the number of hours worked directly by such individual on the work to be conducted under this Agreement during such accounting period and the number of FTE hours applicable for such accounting period based on 1,800 working hours per Calendar Year. |
1.58 | “FTE Rate” means with respect to an employee or contractor of either Party, the amount for an FTE per Calendar Year, which for the Calendar Year ending on December 31, 2021 will be $306,000 per FTE pro-rated for the period beginning on the Effective Date and ending on December 31, 2021, and thereafter will be adjusted annually based on the change in the Chinese Consumer Price Index as published by the National Bureau of Statistics of China (中华人民共和国国家统计局) available at http://data.stats.gov.cn/english/. |
1.59 | “Fully Burdened Manufacturing Costs” means, with respect to any Licensed Antibody or Licensed Product, supplied by or on behalf of the applicable Party to the other Party or its Affiliates hereunder: |
(a) | if and to the extent the Licensed Antibody or Licensed Product is Manufactured by a CMO, the actual Third Party costs of such Manufacturing incurred by the supplying Party, including the costs of raw materials, intermediates and components, reference materials, or standards required for release testing, materials necessary to support stability studies, drug substance and drug product Manufacturing, quality assurance and stability testing, characterization testing, quality control release testing of drug substance and drug product, quality assurance batch record review and release of product, insurance, storage and freight, shipping, tariffs, sales and excise taxes imposed thereon, customs and duty and charges levied by governmental authorities and all costs of packaging and labeling; or |
(b) | if and to the extent the Licensed Antibody or Licensed Product is Manufactured by a Party or its Affiliate, the actual, fully burdened costs that are directly attributable to such Manufacturing, including the cost of raw materials and any costs incurred by such supplying Party for time spent by such Party’s personnel necessary for the other Party to obtain such raw materials, at the applicable FTE Rate, direct labor and benefits, a |
6
proportionate share of indirect Manufacturing costs to the extent the foregoing are allocable to the Manufacture of such Licensed Antibody or Licensed Product (based on actual utilization for such product in a particular Calendar Year as compared to the actual working days during such Calendar Year), and all other reasonable and customary Manufacturing- related costs for such Licensed Antibody or Licensed Product, including management and process improvement costs (collectively, not to exceed 15% of the total of the Fully Burdened Manufacturing Costs for such Licensed Antibody or Licensed Product), factory, plant, or equipment start-up or start-up amortization costs, scale-up expenses, quality assurance and stability testing, characterization testing, quality control release testing of drug substance and drug product, quality assurance batch record review and release of product, insurance, storage and freight, shipping, tariffs, customs and duty and charges levied by governmental authorities (including export fees), and all costs of packaging and labeling. Such fully burdened costs will be calculated in accordance with GAAP (when calculated by a Party or its Affiliate in the USA) or IFRS (when calculated by a Party or its Affiliate outside of the USA), in each case, as consistently applied. Notwithstanding the foregoing, Fully Burdened Manufacturing Cost will be computed on a theoretical basis of no less than 70% capacity, and no overhead, equipment, or facilities costs will be included for unutilized, vacant, or dormant facilities or equipment (and Manufacturing overhead costs related to an underutilized facility or underutilized equipment will be allocated proportionately over the entire Manufacturing production (based on a theoretical full-capacity production schedule) of the facility and applicable equipment, whether or not the entire Manufacturing facility is being utilized).
1.60 | “GCP” means all applicable Good Clinical Practice standards for the design, conduct, performance, monitoring, auditing, recording, analyses and reporting of Clinical Trials, including, as applicable (a) as set forth in the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use Harmonized Tripartite Guideline for Good Clinical Practice E6(R2) and any other guidelines for good clinical practice for trials on medicinal products in the Coherus Territory, (b) the Declaration of Helsinki (2004) as last amended at the 52nd World Medical Association in October 2000 and any further amendments or clarifications thereto, (c) U.S. Code of Federal Regulations Title 21, Parts 50 (Protection of Human Subjects), 56 (Institutional Review Boards), 312 (Investigational New Drug Application) and any other regulations related to good clinical practice for trials on medicinal products in the Coherus Territory, as may be amended from time to time, and (d) the equivalent applicable laws in the region in the Coherus Territory, each as may be amended and applicable from time to time and in each case, that provide for, among other things, assurance that the clinical data and reported results are credible and accurate and protect the rights, integrity, and confidentiality of trial subjects. |
1.61 | “Global Brand Elements” has the meaning set forth in Section 9.6 (Trademarks). |
1.62 | “GLP” means all applicable Good Laboratory Practice standards, including, as set forth in the then- current good laboratory practice standards promulgated or endorsed by the U.S. Food and Drug Administration, as defined in 21 C.F.R. Part 58, and the equivalent applicable laws in the Coherus Territory, each as may be amended and applicable from time to time. |
1.63 | “HKSE” has the meaning set forth in Section 12.2(c) (Disclosure on HKSE and SSE). |
1.64 | “ICH” means the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. |
1.65 | “ICH Guidelines” means guidelines established by ICH regarding quality, safety, efficacy and multidisciplinary topics. |
1.66 | “IL-2” means the interleukin-2 cytokine. |
7
1.67 | “IL-2 Program” means the program of Development of the Junshi IL-2 Molecules. |
1.68 | “IND” means (a) an Investigational New Drug Application as defined in the United States Federal Food, Drug and Cosmetic Act, as amended (the “FD&C Act”) and applicable regulations promulgated thereunder by the FDA, or (b) the equivalent application to the equivalent Regulatory Authority in any other regulatory jurisdiction, the filing of which is necessary to initiate or conduct clinical testing of a pharmaceutical or biological product in humans in such jurisdiction. |
1.69 | “Indemnified Party” has the meaning set forth in Section 11.3 (Indemnification Procedures). |
1.70 | “Indemnifying Party” has the meaning set forth in Section 11.3 (Indemnification Procedures). |
1.71 | “Independent Development Budget” has the meaning set forth in Section 4.5 (Independent Development). |
1.72 | “Independent Development Plan” has the meaning set forth in Section 4.5 (Independent Development). |
1.73 | “Independent Trial” has the meaning set forth in Section 4.5 (Independent Development). |
1.74 | “Initial Technology Transfer” has the meaning set forth in Section 2.6(c) (Initial Technology Transfer). |
1.75 | “JAMS” has the meaning set forth in Section 15.1(b) (Dispute Resolution). |
1.76 | “JAMS Rules” has the meaning set forth in Section 15.1(c) (Dispute Resolution). |
1.77 | “Joint Arising Know-How” has the meaning set forth in Section 9.1(b)(i) (Arising Technology). |
1.78 | “Joint Arising Patent Rights” has the meaning set forth in Section 9.1(b)(i) (Arising Technology). |
1.79 | “Joint Arising Technology” has the meaning set forth in Section 9.1(b)(i) (Arising Technology). |
1.80 | “Joint Development Committee” and “JDC” have the meaning set forth in Section 3.2(a) (Formation; Composition). |
1.81 | “Joint Development Budget” has the meaning set forth in Section 4.4 (Joint Development). |
1.82 | “Joint Development Plan” has the meaning set forth in Section 4.4 (Joint Development). |
1.83 | “Joint Development Proposal” has the meaning set forth in Section 4.3(a) (Proposals and JDC Review). |
1.84 | “JS001” has the meaning set forth in Section 1.99 (Licensed Antibody). |
1.85 | “JS001 Upstream License” means that certain Multi-Product Licence Agreement by and between Junshi and Lonza Sales AG effective as of 4 May 2020, as amended. |
1.86 | “JS001 Upstream License Costs” has the meaning set forth in Section 8.5(a) (JS001 Upstream License). |
1.87 | “JS018-1 Upstream License” means that certain Technology License Contract in respect of Intramolecular Disulfide Bond IL-2 Drug (Contract No. LT20200828), signed on Aug 28, 2020 in Shanghai City. |
1.88 | “JS018-1 Upstream License Costs” has the meaning set forth in Section 8.5(b) (JS018-1 Upstream License). |
1.89 | “Junshi” has the meaning set forth in the Preamble. |
1.90 | “Junshi Arising Patent Rights” has the meaning set forth in Section 9.1(b)(i) (Arising Technology). |
8
1.91 | “Junshi Arising Technology” has the meaning set forth in Section 9.1(b) (Arising Technology). |
1.92 | “Junshi Clinical Trials” means (a) the Ongoing JS001 Clinical Trials, (b) those Independent Trials for which Junshi is the Developing Party, and (c) the Optioned Licensed Product Trials. |
1.93 | “Junshi IL-2 Molecule” means (a) the recombinant cytokine that is known as of the Execution Date as JS018-1, which has the sequence set forth on Schedule 1.93 (JS018-1 Sequence) and (b) any successor molecule that is Controlled by Junshi or any of its Affiliates, is derived from human IL-2, and is specifically engineered to reduce its CD25 binding affinity (compared to wild-type human IL-2), excluding (i) any molecules derived from IL-2 that are specifically engineered to alter the binding or effects of IL-2 on any IL-2 binding partners or effectors other than CD25 or (ii) any such IL-2 derived molecule when it is fused to other cytokines, antibodies, or other functional moiety. |
1.94 | “Junshi Indemnitees” has the meaning set forth in Section 11.2 (Indemnification by Coherus). |
1.95 | “Junshi Territory” means all countries in the world other than the Coherus Territory. |
1.96 | “Junshi TIGIT Antibody” means (a) the monoclonal Antibody that is known as of the Execution Date as JS006, which has the sequence set forth on Schedule 1.96 (JS006 Sequence) and (b) any successor monospecific Antibody that is Controlled by Junshi or any of its Affiliates and is directed to TIGIT, excluding any other modality molecule (such as bi-specific or multi-specific Antibody or fused cytokine) that contains the same CDR region as JS006. |
1.97 | “Know-How” means any data, results, and information of any type whatsoever, in any tangible or intangible form, including trade secrets, practices, techniques, methods, processes, inventions, discoveries, developments, specifications, formulations, formulae, materials or compositions of matter of any type or kind (patentable or otherwise), software, algorithms, marketing reports, clinical and non-clinical study reports, clinical and non-clinical data, regulatory filings and regulatory submission documents and summaries, technology, test data including pharmacological, biological, chemical, biochemical, toxicological, and clinical test data, analytical and quality control data, stability data, studies and procedures and any other know-how, and any physical embodiments of any of the foregoing. |
1.98 | “License Option” has the meaning set forth in Section 2.8(a) (Grant of Options). |
1.99 | “Licensed Antibody” means (a) (i) the monoclonal Antibody known as JS001 and with the International Nonproprietary Name of TORIPALIMAB, which has the sequence set forth on Schedule 1.99 (JS001 Sequence) (“JS001”) and (ii) any successor anti-PD-1 monospecific Antibody that is Controlled by Junshi or its Affiliates, but excluding any other modality molecule (such as bi-specific or multi-specific Antibody or fused cytokine) that contains the same CDR region as JS001 and (b) upon the exercise of a License Option with respect to an Option Program, all Option Molecules that are the subject of such Option Program. |
1.100 | “Licensed Know-How” means any and all Know-How, other than any Joint Arising Know-How, that is owned or Controlled by Junshi or any of its Affiliates as of the Execution Date or during the Term and that is necessary or reasonably useful to Exploit any Licensed Antibody or Licensed Product in the Field. |
1.101 | “Licensed Manufacturing Technology” has the meaning set forth in Section 2.6(b) (Manufacturing Technology Transfer). |
1.102 | “Licensed Patent Rights” means any and all Patent Rights, other than any Joint Arising Patent Rights, that are owned or Controlled by Junshi or any of its Affiliates as of the Execution Date or during the Term and that are necessary or reasonably useful to Exploit any Licensed Antibody or |
9
Licensed Product in the Field. All Licensed Patent Rights existing as of the Execution Date are listed on Schedule 10.2(d) (Existing Patent Rights).
1.103 | “Licensed Product” means any product that contains a Licensed Antibody, alone or in combination with one or more therapeutically active pharmaceutical ingredients, in all forms, presentations, compositions, dosages, and formulations. |
1.104 | “Licensed Technology” means Licensed Know-How, Licensed Patent Rights, and Junshi’s and its Affiliates’ interest in the Joint Arising Technology. |
1.105 | “Manufacture” or “Manufacturing” means activities directed to manufacturing, processing, packaging, labeling, filling, finishing, assembly, quality assurance, quality control, testing, and release, shipping, or storage of any pharmaceutical or biologic product (or any components or process steps involving any product or any companion diagnostic), placebo, or comparator agent, as the case may be, including process development, qualification, and validation, scale-up, pre- clinical, clinical, and commercial manufacture and analytic development, product characterization, and stability testing, but excluding activities directed to Development, Commercialization, or Medical Affairs. “Manufacturing” will be construed accordingly. |
1.106 | “Manufacturing Technology Transfer” has the meaning set forth in Section 2.6(b) (Manufacturing Technology Transfer). |
1.107 | “Medical Affairs” means, with respect to a Licensed Product, any and all activities performed by or on behalf of a Party’s or its Affiliates’ medical affairs departments interacting with physicians or other healthcare professionals who utilize or conduct research related to a drug or biological product, including: supporting continuing medical education and other medical programs and communications; development, publication, and dissemination of publications; development and fulfillment of medical information responses; development and execution of disease awareness education including symposia and digital education initiatives; sponsorship and booth exhibition at key congresses; conducting health economic, burden of illness/disease, natural history and real world evidence studies; supporting educational fellowships and research grants, supporting external research efforts such as scientific research agreements and investigator initiated trials (following Regulatory Approval); medical resourcing, training and allocation; medical and scientific platform, content development, publications, and communications; conducting appropriate activities involving opinion leaders, including communications and engagement; conducting medical science liaison activities; advisory boards (to the extent related to medical affairs or clinical guidance) and conducting advisory board meetings or other consultant programs; establishing patient registries and expanded access programs; post-approval investigator initiated trials or scientific research agreements; life cycle management activities and clinical research. |
1.108 | “Net Sales” means, with respect to a Licensed Product, the aggregate gross sales invoiced of such Licensed Product sold by Coherus or any of its Affiliates or Sublicensees (other than Junshi or any distributors, resellers, wholesalers, hospitals, or end users) (each, a “Selling Party”) to a Third Party (including distributors, resellers, wholesalers, hospitals, and end users) (each, a “Buying Party”) in the Coherus Territory, less the following deductions: |
(a) | trade, cash, and quantity discounts, allowances, and credits allowed or paid, in the form of deductions actually allowed with respect to sales of such Licensed Product; |
(b) | retroactive price reductions, allowances, or credits granted upon rejections or returns of Licensed Product, including for recalls or damaged good and billing errors; |
(c) | discounts, chargeback payments, rebates, and reimbursements related to sales of Licensed Products granted to wholesalers and other distributors, pharmacies and other retailers, |
10
managed care organizations, group purchasing organizations, patients (through-co-pay assistance programs), or other buying groups, pharmacy benefit management companies, health maintenance organizations, federal, state, provincial, local, or other governments, and any other providers of health insurance coverage, health care organizations, or other health care institutions (including hospitals), health care administrators, or patient assistance or other similar programs to the extent actually given;
(d) | compulsory payments and cash rebates related to the sales of such Licensed Product paid to a governmental authority (or agent thereof) pursuant to applicable law by reason of any national or local health insurance program or similar program, including required chargebacks and retroactive price reductions, to the extent allowed and taken, including government levied fees as a result of healthcare reform policies (including annual fees due under Section 9008 of the United States Patient Protection and Affordable Care Act of 2010 (Pub. L. No. 111-48)); |
(e) | customary freight, shipping insurance and other transportation expenses to the extent separately itemized and included in the gross amount invoiced and charged to the Buying Party; |
(f) | tariffs, duties, import, export, excise, sales, use, turnover, value-added, and other similar taxes (other than taxes based on income); customs duties; or other government charges, in each case, imposed on the sale of Licensed Product to the extent included in the price and separately itemized on the invoice, including VAT; |
(g) | other similar and customary deductions that are in accordance with GAAP or other accounting standard applicable to such entity as consistently applied and actually given. |
For the avoidance of doubt, if a single item falls into more than one of the categories set forth in clauses (a) to (h) above, then such item may not be deducted more than once. All amounts set forth in clauses (a) to (h) above will only be deducted to the extent permitted under GAAP or other accounting standard applicable to such entity as consistently applied.
Sales and other transfer of a Licensed Product between any Selling Party to another Selling Party will not give rise to Net Sales, but rather Net Sales will be deemed to have arisen upon the subsequent sale of a Licensed Product to a Third Party.
Any Licensed Products used for promotional or advertising purposes, used for Clinical Trials, preclinical trials or other research purposes, free samples, named patient use, compassionate use, patient assistance, expanded access, charitable use or distributed at no charge to patients unable to purchase the same will not be included in Net Sales. Donations, dispositions, or transfers for charitable reasons that are at or below fully-burdened manufacturing costs therefor will also not be included in Net Sales.
Calculations of Net Sales will be consistently applied across all products of a Selling Party. Such amounts will be determined from the books and records of the Selling Party, and will be calculated in accordance with GAAP or other accounting standard applicable to such entity as consistently applied.
In the case of any Combination Product sold in a given country and reporting period, Net Sales for the purpose of determining Royalties and sales milestone events of the Combination Product in such country will be calculated by multiplying actual Net Sales of such Combination Product by the fraction A/(A+B), where A is the invoice price of the Licensed Antibody if sold
11
separately in the same indication in such country, and B is the total invoice price of the Other Components in the Combination Product, if sold separately in the same indication in such country.
If, on a country-by-country basis in a particular reporting period, the Licensed Antibody is sold separately in the same indication in a country, but the Other Components in the Combination Product are not sold separately in the same indication in such country, then Net Sales for the purpose of determining Royalties and sales milestone events of the Combination Product for such country will be calculated by multiplying actual Net Sales of the Combination Product by the fraction A/C, where A is the invoice price of the Licensed Antibody if sold separately in the same indication in such country, and C is the invoice price of the Combination Product in such country.
If, on a country-by-country basis in a particular reporting period, the Licensed Antibody in the Combination Product is not sold separately in the same indication in such country, but the Other Components included in the Combination Product are sold separately in the same indication in such country, then Net Sales for the purpose of determining Royalties and sales milestone events of the Combination Product for such country will be calculated by multiplying actual Net Sales of the Combination Product by the fraction (C-B)/C, where B is the invoice price of the Other Components included in such Combination Product if sold separately in the same indication in such country, and C is the invoice price of the Combination Product in such country.
Notwithstanding the foregoing, in the case as set forth in the preceding paragraphs of any Combination Product that is sold in a given country and reporting period, the Net Sales for each Licensed Antibody for the purpose of determining Royalties and sales milestone events of a Combination Product in such country will never be less than 25% of the total Net Sales for the Combination Product (even if as a result of the applicable calculations using A, B, or C above would lead to an allocation of less than 25% for a Licensed Antibody).
If neither the Licensed Antibody nor the Other Components are sold separately in the same indication in a given country during a particular reporting period, then Net Sales will be calculated based on the fair market value of the Licensed Product as A and the fair market value of each of the Other Components included in such Combination Product as B when A and B would be sold in such indication in such country. In such a case as described in this paragraph, the Net Sales for each Licensed Antibody for the purpose of determining Royalties and sales milestone events of a Combination Product in such country will never be less than 1/(X+Y) of the total Net Sales for the Combination Product, wherein X is the number of active ingredients that are or contain Licensed Antibody(ies) and Y is the number of active ingredients that are Other Component(s) (other than Licensed Antibody(ies).
1.109 | “Non-Defaulting Party” has the meaning set forth in Section 13.3(c) (Disputes Regarding Material Breach). |
1.110 | “Non-Developing Party” has the meaning set forth in Section 4.5 (Independent Development in the Coherus Territory). |
1.111 | “Ongoing JS001 Trial Cap” has the meaning set forth in Section 4.2(b) (Costs to be Reimbursed). |
1.112 | “Ongoing JS001 Trial Costs” has the meaning set forth in Section 4.2(b) (Costs to be Reimbursed). |
1.113 | “Ongoing JS001 Trials” means the Clinical Trials identified on Schedule 1.113 (Ongoing JS001 Trials). |
12
1.114 | “Ongoing JS001 Development Budget” has the meaning set forth in Section 4.2 (Ongoing JS001 Trials). |
1.115 | “Ongoing JS001 Development Plan” has the meaning set forth in Section 4.2 (Ongoing JS001 Trials). |
1.116 | “Option Data Package” means, with respect to each Option Program, the information and materials set forth on Schedule 1.116 (Option Data Package). |
1.117 | “Option Data Package Delivery Date” has the meaning set forth in Section 10.2 (Representations and Warranties by Junshi). |
1.118 | “Option Disclosure Letter” has the meaning set forth in Section 10.2 (Representations and Warranties by Junshi). |
1.119 | “Option Exercise” has the meaning set forth in Section 2.8(g) (Exercise of a License Option). |
1.120 | “Option Exercise Date” has the meaning set forth in Section 2.8(g) (Exercise of a License Option). |
1.121 | “Option Exercise Notice” has the meaning set forth in Section 2.8(g) (Exercise of a License Option). |
1.122 | “Optioned Licensed Products” has the meaning set forth in Section 4.6 (Development of Optioned Licensed Products). |
1.123 | “Optioned Licensed Product Trial” has the meaning set forth in Section 4.6(a) (Trials Ongoing as of Option Exercise). |
1.124 | “Optioned Licensed Product Development Budget” means, for each of the IL-2 Program and the TIGIT Program, the budget of the costs and expenses to be incurred in the performance of activities under the Optioned Licensed Product Development Plan for such program. |
1.125 | “Optioned Licensed Product Development Costs” has the meaning set forth in Section 2.8(h) (Coherus Share of Option Program Development Costs). |
1.126 | “Optioned Licensed Product Development Plan” means, for each of the IL-2 Program and the TIGIT Program, the plan of the Development activities to be conducted with respect to Optioned Licensed Product Trials for the Option Molecules and Optioned Licensed Products that are the subject of such program. Such Optioned Licensed Product Development Plan may include a plan of the Development activities to be conducted both inside and outside the Coherus Territory. |
1.127 | “Option Molecule” means any (a) Junshi TIGIT Antibody or (b) Junshi IL-2 Molecule. |
1.128 | “Option Notice” has the meaning set forth in Section 2.8(b)(i) (Delivery). |
1.129 | “Option Patent Rights” has the meaning set forth in Section 10.2(f). |
1.130 | “Option Product” means any product that contains an Option Molecule, alone or in combination with one or more therapeutically active pharmaceutical ingredients, in all forms, presentations, compositions, dosages, and formulations. |
1.131 | “Option Program” means each of the IL-2 Program and the TIGIT Program. |
1.132 | “Option Term” has the meaning set forth in Section 2.8(g) (Exercise of a License Option). |
13
1.133 | “Other Component” has the meaning set forth in Section 1.32 (Combination Product). |
1.134 | “Panel” has the meaning set forth in Section 4.3(b) (Restrictions on Additional Development). |
1.135 | “Party” and “Parties” have the meaning set forth in the Preamble. |
1.136 | “Patent Proceeding” means any proceeding before a patent office related to the post-grant review of any Patent Right, including any post-grant proceeding brought pursuant to the America Invents Act (such as any inter partes review, post-grant review, opposition, interference, re-examination), or any foreign equivalent thereof. Any appeal, action, or proceeding related to a Patent Right that is brought before any court of competent jurisdiction will be deemed a “Patent Proceeding”; provided that prosecution activities including any ex parte examination and ex parte re-issue examination, appeal of ex parte patent prosecution decisions to a court or a board of a patent office, or supplemental examination before the U.S. Patent Office will not be considered a “Patent Proceeding.” |
1.137 | “Patent Right” means (a) any national, regional, or international patent or patent application, including any provisional patent application, (b) any patent application filed either from such a patent, patent application, or provisional application or from an application claiming priority from any of these, including any divisional, continuation, continuation-in-part, provisional, converted provisional, and continued prosecution application, (c) any patent that has issued or in the future issues from any of the foregoing patent applications ((a) and (b)), including any utility model, petty patent, design patent, and certificate of invention, (d) any extension or restoration by existing or future extension or restoration mechanisms, including any revalidation, reissue, re-examination, and extension (including any supplementary protection certificate and the like) of any of the foregoing patents or patent applications ((a), (b), and (c)), and (e) any similar rights, including so- called pipeline protection, or any importation, revalidation, confirmation or introduction patent, or registration patent or patent of additions to any such foregoing patent application or patent. |
1.138 | “Payee” has the meaning set forth in Section 8.7(b) (Tax Withholding). |
1.139 | “Payor” has the meaning set forth in Section 8.7(b) (Tax Withholding). |
1.140 | “PD-1” means programmed death-1, and refers to an inhibitory checkpoint receptor expressed on T cells, which binds with PD-L1 and PD-L2 to inhibit T cell and cytokine activation. |
1.141 | “PD-1 Program” means the program of Development of JS001 and any other Licensed Antibody described in clause (a) of the definition of Licensed Antibody. |
1.142 | “Person” means any individual, partnership, joint venture, limited liability company, corporation, firm, trust, association, unincorporated organization, Regulatory Authority, or any other entity not specifically listed in this definition. |
1.143 | “Pharmacovigilance Agreement” has the meaning set forth in Section 5.5 (Adverse Event Reporting). |
1.144 | “Phase I Clinical Trial” means a clinical trial in humans that generally provides for the first introduction into humans of a pharmaceutical or biologic product with the primary purpose of determining safety, metabolism, and pharmacokinetic properties and clinical pharmacology of such product, in a manner that meets the requirements of 21 C.F.R. § 312.21(a), as amended (or its successor regulation), or, with respect to any other country or region, the equivalent of such a clinical trial in such other country or region. |
14
1.145 | “Phase II Clinical Trial” means a clinical trial in humans that is intended to explore the feasibility, safety, dose ranging, or efficacy of a pharmaceutical or biologic product that is prospectively designed to generate sufficient data (if successful) to commence a Phase III Clinical Trial for such product, in a manner that meets the requirements of 21 C.F.R. § 312.21(b), as amended (or its successor regulation), or, with respect to any other country or region, the equivalent of such a clinical trial in such other country or region. |
1.146 | “Phase III Clinical Trial” means a clinical trial in humans of a pharmaceutical or biologic product that the FDA permits to be conducted under an open IND and that is performed to gain evidence with statistical significance of the efficacy of such product in a target population, and to obtain expanded evidence of safety for such product that is needed to evaluate the overall benefit-risk relationship of such product, to form the basis for approval of a BLA by a Regulatory Authority and to provide an adequate basis for physician labeling, in a manner that meets the requirements of 21 C.F.R. § 312.21(c), as amended (or its successor regulation), or, with respect to any other country or region, the equivalent of such a clinical trial in such other country or region. Notwithstanding anything to the contrary set forth in this Agreement, treatment of patients as part of an expanded access program, compassionate sales or use program (including named patient program or single patient program), or an indigent program, in each case, will not be included in determining whether or not a clinical trial is a Phase III Clinical Trial or whether a patient has been dosed thereunder. |
1.147 | “Pivotal Trial” means any Clinical Trial of the active substance of a pharmaceutical or biologic product, the results of which, together with prior data and information concerning such product, are intended to be sufficient, without any additional Clinical Trial, to meet the evidentiary standard for demonstrating the safety, purity, and potency of such active substance of such product established by a Regulatory Authority in any particular jurisdiction and is intended to support the acceptance and approval of a BLA by a Regulatory Authority in such jurisdiction. |
1.148 | “Pricing and Reimbursement Approval” means the later of (a) the approval, agreement, determination, or governmental decision establishing a price for a pharmaceutical or biologic product that can be legally charged to consumers, if required in a given jurisdiction or country for the Commercialization of such pharmaceutical or biologic product in such jurisdiction or country; and (b) the approval, agreement, determination, or governmental decision establishing the level of reimbursement for a pharmaceutical or biologic product that will be reimbursed by governmental authorities, if either required or otherwise commercially beneficial in a given jurisdiction or country for the Commercialization of such pharmaceutical or biologic product in such jurisdiction or country. |
1.149 | “Product Marks” has the meaning set forth in Section 9.6 (Trademarks). |
1.150 | “Proprietary Combination Regimen” has the meaning set forth in Section 4.5(a) (Right to Opt- In). |
1.151 | “Quality Agreement” has the meaning set forth in Section 6.1 (Manufacturing by Junshi). |
1.152 | “Regulatory Approval” means all approvals by a governmental authority necessary for the Manufacture, marketing, importation, and sale of a product for one or more indications in a country or regulatory jurisdiction, which may include satisfaction of all applicable regulatory and notification requirements. Regulatory Approvals include approvals by Regulatory Authorities of INDs and BLAs, and all Pricing and Reimbursement Approvals. |
15
1.153 | “Regulatory Authority” means, in a particular country or regulatory jurisdiction, any applicable governmental authority involved in granting Regulatory Approval or, to the extent required in such country or regulatory jurisdiction, Pricing and Reimbursement Approval of a product in such country or regulatory jurisdiction. |
1.154 | “Regulatory Exclusivity” means any exclusive marketing rights or data exclusivity rights conferred by any Regulatory Authority with respect to a Licensed Product other than Patent Rights, including rights conferred in the U.S. under the FDA Modernization Act of 1997 (including pediatric exclusivity), orphan drug exclusivity, or rights similar thereto outside the U.S. |
1.155 | “Regulatory Materials” means regulatory applications, submissions, notifications, registrations, or other filings or documents maintained or made to or with a Regulatory Authority that are necessary or reasonably desirable in order to Develop, Manufacture, market, sell, or otherwise Commercialize a Licensed Product in a particular country or regulatory jurisdiction. Regulatory Materials include INDs and BLAs (as applications, but not the approvals with respect thereto). |
1.156 | “Required Filings” has the meaning set forth in Section 14.2 (Filings). |
1.157 | “ROFN Exercise Notice” has the meaning set forth in Section 2.9 (Right of First Negotiation ). |
1.158 | “ROFN Jurisdiction” has the meaning set forth in Section 2.9 (Right of First Negotiation). |
1.159 | “ROFN Product” has the meaning set forth in Section 2.9 (Right of First Negotiation). |
1.160 | “ROFN Product Activity” has the meaning set forth in Section 2.9 (Right of First Negotiation). |
1.161 | “Royalty” has the meaning set forth in Section 8.4 (Royalties). |
1.162 | “Royalty Report” has the meaning set forth in Section 8.4(b) (Reports; Payment). |
1.163 | “Royalty Term” means, on a country-by-country and Licensed Product-by-Licensed Product basis, the period commencing upon the First Commercial Sale of a Licensed Product in a country, and ending upon the later to occur of (a) the expiration in such country of the last to expire of any Licensed Patent Right Covering the composition of matter, formulation, or approved method of treatment or use of such Licensed Product that would be infringed by the manufacture, use, sale, offer for sale, or import of such Licensed Product in such country; (b) the expiration of all Regulatory Exclusivities for such Licensed Product in such country; or (c) 10 years after the First Commercial Sale in such country of such Licensed Product. |
1.164 | “SEC” has the meaning set forth in Section 12.2(b) (Disclosure to SEC). |
1.165 | “SSE” has the meaning set forth in Section 12.2(c) (Disclosure on HKSE and SSE). |
1.166 | “Sublicensee” means any Third Party granted a sublicense by Coherus under the rights licensed to Coherus pursuant to Article 2 (Licenses and Exclusivity) hereof. |
1.167 | “Supply Agreement” has the meaning set forth in Section 6.1 (Manufacturing by Junshi). |
1.168 | “Supply Price” has the meaning set forth in Section 6.1 (Manufacturing by Junshi). |
1.169 | “Technology Transfer” has the meaning set forth in Section 2.6(c) (Continuing Technology Transfer). |
16
1.170 | “Term” has the meaning set forth in Section 13.1 (Term). |
1.171 | “Third Party” means any entity other than Junshi or Coherus or their respective Affiliates. |
1.172 | “Third Party Agreements” has the meaning set forth in Section 10.2(g) (Third Party Agreements). |
1.173 | “TIGIT” means the T-cell immunoreceptor with immunoglobulin and ITIM domains. |
1.174 | “TIGIT Program” means the program of Development of the Junshi TIGIT Antibodies. |
1.175 | “Transition Plan” has the meaning set forth in Section 5.1 (Regulatory Responsibilities). |
1.176 | “United States” means the United States of America and all of its territories and possessions. |
1.177 | “Upfront Payment” has the meaning set forth in Section 8.1 (Upfront Payment). |
1.178 | “Valid Claim” means a claim of (a) an issued, unexpired, and in-force patent, which claim has not been held invalid or unenforceable by a court or other government agency of competent jurisdiction from which no appeal can be or has been taken and has not been held or admitted to be invalid or unenforceable through re-examination, inter partes review, post grant review or disclaimer, opposition procedure, nullity suit, or otherwise, or (b) a pending patent application that has not been finally abandoned, finally rejected from which no appeal can be or has been taken, or expired; provided, however, that if a claim of a pending patent application will not have issued within seven years after the earliest filing date from which such claim takes priority, then such claim will not constitute a Valid Claim for the purposes of this Agreement unless and until a patent issues with such claim. |
1.179 | “VAT” has the meaning set forth in Section 8.7(c) (VAT in Coherus Territory). |
ARTICLE 2
LICENSES AND EXCLUSIVITY
2.1 | Licenses to Coherus. Subject to the terms and conditions of this Agreement, Junshi, on behalf of itself and its Affiliates, hereby grants to Coherus (a) an exclusive (even as to Junshi and each of its Affiliates), non-transferable (except as permitted in accordance with Section 16.6 (Assignment)) license, with the right to sublicense (solely as permitted in accordance with Section 2.2 (Sublicensing)), under the Licensed Technology, to Exploit the Licensed Antibodies and Licensed Products in the Field in the Coherus Territory; and (b) a non-exclusive, non-transferable (except as permitted in accordance with Section 16.6 (Assignment)) license, with the right to sublicense (solely as permitted in accordance with Section 2.2 (Sublicensing)), under the Licensed Technology, to conduct Clinical Trials using the Licensed Antibodies and Licensed Products in the Field outside of the Coherus Territory solely for purposes of obtaining, maintaining, or supporting Regulatory Approval of such Licensed Antibodies and Licensed Products in the Field in the Coherus Territory. |
2.2 | Sublicensing. |
(a) | Consent; Responsibility. Coherus may grant sublicenses of the rights granted to it under Section 2.1 (Licenses to Coherus) to (i) its Affiliates during only such time as such Person remains an Affiliate of Coherus and (ii) contractors of Coherus and its sublicensed Affiliates for the sole purpose of performing Coherus’ obligations or exercising Coherus’ rights with respect to the Exploitation of Licensed Products in accordance with the terms of this Agreement, in each case, (i) and (ii), without the consent of Junshi so long as such sublicense also satisfies the requirements of Section 2.2(b) (Certain Requirements). |
17
Coherus may grant sublicenses under such rights otherwise only with the advance, written consent of Junshi, such consent not to be unreasonably withheld. Coherus will remain primarily liable to Junshi for the performance of all of Coherus’ obligations under this Agreement and will be liable for any act or failure to act by any such sublicensed Affiliate or Third Party Sublicensee that if committed (or not committed) by such Person would be a breach of any of Coherus’ obligations under this Agreement as though the same were a breach by Coherus.
(b) | Certain Requirements. Without limiting the foregoing, each sublicense to a Third Party must be granted in writing and all sublicenses (to Affiliates and Third Parties) (i) must be consistent with, and are and will be subject to, the terms and conditions of this Agreement, (ii) terminate automatically upon termination of the corresponding licensed rights granted under Section 2.1 (Licenses to Coherus). In addition, in each sublicense, Coherus will include terms that enable Coherus to grant a license or sublicense to Junshi under the Coherus Arising Technology consistent with the terms of Section 2.3 (Licenses to Junshi) under all Coherus Arising Technology. |
(c) | Notice; Copy. Coherus will, within 30 days after granting any sublicense to a Third Party Sublicensee that is not an agent or a consultant, contract manufacturing organization, contract research organization, or other similar type contractor acting for or on behalf of Coherus, notify Junshi of the grant of such sublicense and provide Junshi with a copy of such sublicense; provided that Coherus may redact any portion of such sublicense agreement to the extent not necessary for Junshi to determine compliance with this Agreement. |
2.3 | Licenses to Junshi. Subject to the terms and conditions of this Agreement, Coherus hereby grants to Junshi (a) an irrevocable, non-exclusive, royalty-free license, with the right to sublicense, under the Coherus Arising Technology to Exploit the Licensed Antibodies and Licensed Products in the Field in the Junshi Territory, and (b) an irrevocable, non-exclusive, royalty-free license, with the right to sublicense, under the Coherus Arising Technology to Exploit the Licensed Antibodies and Licensed Products in the Field in the Coherus Territory for the purposes for which Junshi retains rights to Exploit the Licensed Technology under Section 2.4 (No Implied Licenses; Retained Rights). Without limiting the foregoing, each sublicense by Junshi of the foregoing rights to a Third Party must be granted in writing, and all sublicenses must be consistent with, and will be subject to, the terms and conditions of this Agreement, and will terminate automatically upon termination of the corresponding licensed rights granted under this Section 2.3 (Licenses to Junshi). Junshi will, within 30 days after granting any sublicense to a Third Party Sublicensee that is not an agent or a consultant, contract manufacturing organization, contract research organization, or other similar type contractor acting for or on behalf Junshi, notify Coherus of the grant of such sublicense. |
2.4 | No Implied Licenses; Retained Rights. Except as explicitly set forth in this Agreement, neither Party grants to the other Party any license or other rights, express or implied, under any intellectual property rights (whether by implication, estoppel, or otherwise). Notwithstanding the exclusive license granted to Coherus in Section 2.1 (Licenses to Coherus), Junshi retains rights under the Licensed Technology (including its joint interest in any Joint Arising Technology) solely to (and to grant its Affiliates and Third Parties the right to), within the Coherus Territory:(a) conduct the Junshi Clinical Trials, (b) conduct Clinical Trials in the Coherus Territory solely for purposes of Junshi, its Affiliates or its and their respective licensees/sublicensees obtaining, maintaining, or supporting Regulatory Approval of Licensed Antibodies and Licensed Products outside of the Coherus Territory, (c) supply or import Licensed Antibodies and Licensed Products into the Coherus Territory (i) to Coherus or its Affiliates or Sublicensees and (ii) to perform the Junshi Clinical Trials, and (d) Manufacture Licensed Products in the Coherus Territory for use in the Junshi Clinical Trials and for export to, and use and sale outside of, the Coherus Territory. The |
18
Licensed Technology Controlled by Junshi pursuant to any Third Party Agreement is licensed to Coherus under this Agreement subject to sections 4.1, 4.2, 4.3, 4.4, 4.5, 6.1, 8.1 and 10.5 of the JS001 Upstream License, and if Coherus assumes responsibility for making any payments directly to Lonza under Section 8.5(a) (JS001 Upstream License), then also Sections 6.2, 6.4.2 and 6.5 therein, and Sections 5.1(1), 5.1(2), and 5.1(3) of the JS018-1 Upstream License, as such agreements were transmitted to Coherus in emails dated as of January 21, 2021 at 10:05 PM Pacific Time and January 23, 2021 at 7:11 PM Pacific Time, respectively. The Licensed Technology Controlled by Junshi pursuant to any Additional Third Party License is licensed to Coherus under this Agreement subject to the terms agreed by the Parties in accordance with Section 2.5 (Third Party In-Licenses).
2.5 | Third Party In-Licenses. During the Term, if either Party identifies any Patent Right or Know- How owned or controlled by a Third Party that it reasonably believes may be necessary or reasonably useful to Exploit any Licensed Antibody or Licensed Product in the Field in the Coherus Territory (other than a Third Party License Agreement), or absent a license or agreement with such Third Party to such intellectual property, would be infringed by the Exploitation of any Licensed Antibody or Licensed Product in the Field in the Coherus Territory (“Additional Third Party IP”), then it may raise such matter with the JDC. Unless otherwise agreed by the Parties, neither Party will enter into an agreement with a Third Party to obtain a license, covenant not to sue, or other similar rights under any such Patent Right or Know-How (each, an “Additional Third Party License”) for a period of three months after first raising the matter to the JDC unless during such three month period the Parties agree in writing on the cost-sharing terms applicable to any such Additional Third Party License between the Parties and the other terms on which the applicable Party might sublicense rights to the other Party under such Additional Third Party IP. Any such agreement between the Parties should be effective prior to or concurrently with the execution of the Additional Third Party License. If the Parties do not enter into such written agreement within such three month period (or such other period as may be agreed) or the Parties otherwise agree, then the Party desiring to enter into the Additional Third Party License may do so, and such Additional Third Party IP will not be deemed Controlled by the Party that is party to such Additional Third Party License for purposes of this Agreement. Unless the Parties otherwise agree, Coherus may deduct any royalties paid by Coherus to Junshi in consideration for any such sublicensed rights under any Additional Third Party IP in accordance with Section 8.4(c)(iii) (Reduction for Additional Third Party IP). If Coherus is the party to an Additional Third Party License, then Coherus may deduct any royalties paid by Coherus to the Third Party in consideration for any such sublicensed rights in accordance with Section 8.4(c)(iii) (Reduction for Additional Third Party IP). |
2.6 | Technology Transfer. |
(a) | Initial Technology Transfer. As soon as reasonably practicable (i) with respect to JS001 and any Antibody thereof that is the subject of the PD-1 Program, after the Effective Date or (ii) with respect to each Option Molecule for which Coherus exercises a License Option, after exercise of such License Option, and in each case ((i) and (ii)), in accordance with a plan to be agreed between the Parties no later than 60 days after the Effective Date or the Option Exercise Date (as applicable), Junshi will transfer to Coherus electronic copies of appropriate documents, data, regulatory correspondence, clinical and pre-clinical data, or other Know-How included within the Licensed Know-How existing as of the Effective Date or the applicable Option Exercise Date, other than Licensed Manufacturing Technology, which will be transferred to Coherus as contemplated in Section 2.6(b) (Manufacturing Technology Transfer) (the “Initial Technology Transfer”). |
19
(b) | Manufacturing Technology Transfer. In addition to the documents, data, or other Licensed Know-How provided to Coherus pursuant to the Initial Technology Transfer, on a Licensed Antibody-by-Licensed Antibody basis, upon the request of Coherus during the Term (which, for JS001, will be promptly following the Effective Date), Junshi will evaluate jointly with Coherus a CMO designated by Coherus (such CMO designated by Coherus, a “Coherus CMO”). For each Licensed Antibody, Junshi will promptly conduct a transfer to such Coherus CMO (a “Coherus CMO”), of appropriate documents, data (including an appropriate set of base reference Manufacturing process data), other Licensed Know-How, or activities necessary to Manufacture the Licensed Products that include each such Licensed Antibody in accordance with the terms of this Agreement (“Licensed Manufacturing Technology”) and necessary to enable such Coherus CMO to assume the Manufacturing activities of the Licensed Products that include the applicable Licensed Antibody (for each such Licensed Antibody, a “Manufacturing Technology Transfer”); provided that, solely to the extent required under the JS001 Upstream License with respect to JS001, such Coherus CMO is reasonably acceptable to Lonza. The Manufacturing Technology Transfer for each Licensed Antibody (including all Licensed Products that include such Licensed Antibody) will be conducted pursuant to a transfer plan and timeline, and terms and conditions limiting the Coherus CMO’s use and disclosure of Licensed Technology, which terms are set forth on Schedule 2.6(b) (Manufacturing Technology Transfer Additional Terms), (for each Manufacturing Technology Transfer, a “Technology Transfer Agreement”). Thereafter during the Term following completion of the Manufacturing Technology Transfer for a particular Licensed Antibody, Junshi will provide any additional Licensed Manufacturing Technology related to the Manufacture of such Licensed Antibody or Licensed Product that include such Licensed Antibody as part of the Continuing Technology Transfer in accordance with Section 2.6(c) (Continuing Technology Transfer). |
(c) | Continuing Technology Transfer; Coherus Arising Technology. After the completion of the Initial Technology Transfer for each Licensed Antibody, Junshi will transfer to Coherus (through the JDC) or a Coherus CMO (if applicable to the Manufacture of Licensed Products) any additional documents, data (including all data from the Ongoing JS001 Clinical Trials, and data from Independent Trials for which Junshi is the Developing Party after Coherus opts-in thereto pursuant to Section 4.5 (Independent Development)), or other Licensed Know-How, in each case, that is in Junshi’s Control and has not been previously transferred to Coherus or a Coherus CMO (the “Continuing Technology Transfer,” and together with the Initial Technology Transfer and the Manufacturing Technology Transfer, the “Technology Transfer”). The Continuing Technology Transfer will not include any of the foregoing arising from an Independent Development for which Junshi is the Developing Party unless and until Coherus opts-in to share the costs of such activities in accordance with Section 4.5 (Independent Development). After completion of the Manufacturing Technology Transfer for a given Licensed Antibody, Junshi will also transfer to the Coherus CMO any Continuing Technology Transfer for Manufacturing Licensed Products that include such Licensed Antibody in accordance with the terms of the applicable Technology Transfer Agreement (as then existing or as may be amended by the Parties and the Coherus CMO to accommodate the Continuing Technology Transfer related to Manufacture of such Licensed Products). Junshi shall perform such applicable Continuing Technology Transfer reasonably in advance of the last JDC meeting of each Calendar Year and additionally in advance of the second JDC meeting of the Calendar Year for Continuing Technology Transfer not related to Licensed Manufacturing Technology. Coherus will transfer to the JDC all Coherus Arising Technology and data from Independent Trials for which Coherus is the Developing Party after Junshi opts-in thereto |
20
pursuant to Section 4.5 (Independent Development) with the same frequency and about the same time as Junshi is required to undertake the Continuing Technology Transfer pursuant to this Section 2.6(c) (Continuing Technology Transfer; Coherus Arising Technology).
(d) | Costs of Technology Transfer. Junshi will reasonably cooperate with Coherus to facilitate the Technology Transfer to Coherus or a Coherus CMO (as applicable). In the course of any Technology Transfer, Junshi will provide Coherus or the applicable Coherus CMO with reasonable access by teleconference or in-person at Junshi’s or any of its Affiliates’ facilities to Junshi or any of its Affiliates’ personnel involved in the Development or Manufacture of the Licensed Antibodies and designated by Junshi to provide Coherus or the Coherus CMO with a reasonable level of technical assistance and consultation in connection with all Technology Transfers. Junshi will be responsible for its internal costs for up to a total of 100 hours in connection with all Technology Transfers. Coherus will reimburse Junshi for (i) internal costs (at the FTE Rate) reasonably incurred by or on behalf of Junshi or its Affiliates in connection with Technology Transfer in excess of 100 hours of consultation and assistance related thereto, and (ii) all verifiable external or out-of-pocket costs actually incurred by or on behalf of Junshi or its Affiliates in connection with any Technology Transfer, in each case ((i) and (ii)), within 45 days after receiving Junshi’s invoice therefor. |
2.7 | Exclusivity. |
(a) | Exclusivity Covenant. Subject to Section 2.7(b) (Acquisition by Third Parties) and Section 2.7(c) (Acquisition of Third Parties), during the Term, neither Party will, and will ensure that its Affiliates do not, independently or for or with any Third Party, directly or indirectly, clinically Develop or Commercialize any Competitive Product in the Coherus Territory (or license or otherwise authorize any Third Party to do any of the foregoing) (the “Competitive Activities”) unless agreed in writing by the Parties. |
(b) | Acquisition by Third Parties. If either Party undergoes a Change of Control with a Third Party that is (either directly or through an Affiliate, or in collaboration with another Third Party) performing Competitive Activities with respect to one or more Competitive Products in the Coherus Territory at the closing of the Change of Control transaction, then it will not be in breach of the restrictions set forth in Section 2.7(a) (Exclusivity Covenant) due to such Change of Control with such a Third Party, and such Third Party may continue to perform, or commence the performance of, the applicable Competitive Activities with respect to such Competitive Products after such Change of Control transaction as long as: (i) such Party notifies the other Party of the Change of Control and the nature of the Competitive Activities following the closing of such Change of Control, (ii) no Licensed Technology is used by or on behalf of such Party or its Affiliates in more than a de minimis fashion in connection with any subsequent clinical Development or Commercialization of such Competitive Products, and (iii) such Party and its Affiliates institute commercially reasonable technical and administrative safeguards to ensure the requirements set forth in the foregoing clause (ii) are met, including by creating “firewalls” between the personnel working on such Competitive Products and the personnel working on the Licensed Antibody and Licensed Products or having access to data from activities performed under this Agreement or Confidential Information of the other Party. If (A) Coherus is the Party that undergoes such a Change of Control, (B) the Third Party acquirer or any of its Affiliates is performing Competitive Activities with respect to one or more Competitive Products in the Coherus Territory at the closing of the Change of Control transaction, and (C) (i) any Clinical Trials involving the applicable Licensed Product being conducted or planned to be conducted by Coherus or any of its Affiliates or Sublicensees are discontinued before their clinical endpoint(s) or (ii) on two separate occasions (which more |
21
or may not be consecutive) the Net Sales of any applicable Licensed Product drop from one Calendar Quarter to the next Calendar Quarter, then in either case ((i) or (ii)), Coherus must promptly notify Junshi of such result. If any such discontinuation of such a Clinical Trial or drop in Net Sales is not (1) by written agreement of the Parties, (2) a result of Coherus’ reasonable response to specific guidance from, or action by, a Regulatory Authority in the Coherus Territory with respect to the applicable Licensed Products (such as a clinical hold, or a recall or withdrawal), (3) caused by Junshi’s uncured failure to perform its applicable obligations under and in accordance with this Agreement or the Supply Agreement (such that Coherus is prevented or hindered from performing any such activities that would have advanced the Development or Commercialization of the applicable Licensed Product but for such failure by Junshi), (4) prevented by applicable law, or (5) prevented by a Force Majeure, then, Junshi may notify Coherus that Coherus or such Third Party must (and Coherus or such Third Party will), at Coherus’ election: (I) divest, or cause its relevant Affiliates to divest, whether by sale, assignment, exclusive license or otherwise, its interest in the applicable Competitive Products within 12 months following such notice by Junshi; (II) terminate any further Competitive Activities with respect to such Competitive Products within 12 months following such notice by Junshi; or (III) provide a notice of termination under Section 13.2 (Termination by Coherus) within 15 days after such notice by Junshi.
(c) | Acquisition of Third Parties. If a Party or any of its Affiliates merges or consolidates with, or otherwise acquires a Third Party (whether such transaction occurs by way of a sale of assets, merger, consolidation, or similar transaction) (the “Acquiring Party”) and at such time such Third Party is performing Competitive Activities with respect to one or more Competitive Products or is engaged in activities that would otherwise constitute a breach of Section 2.7(a) (Exclusivity Covenant), then, unless the Parties agree otherwise in writing, such Acquiring Party will not be in breach of Section 2.7(a) (Exclusivity Covenant) if such Party notifies the other Party of the Change of Control and the nature of the Competitive Activities promptly after the closing thereof and thereafter does one of following: (i) divests, or cause its relevant Affiliates to divest, whether by sale, assignment, exclusive license or otherwise, its interest in such Competitive Products within 12 months following such acquisition; (ii) terminates any further Competitive Activities with respect to such Competitive Products within 12 months following such acquisition; (iii) if the Acquiring Party is Coherus, provide within 15 days after such acquisition a notice of termination under Section 13.2 (Termination by Coherus). The Acquiring Party will notify the other Party as to whether it intends to select option (i), (ii), or (iii) above within 15 days following the consummation of such acquisition. The Acquiring Party will keep the other Party reasonably informed of its efforts and progress in effecting such divesture or termination until the Acquiring Party completes the same. If the Acquiring Party selects either option (i) or (ii) above, then until the divestiture or termination is complete, it will ensure that (A) no Licensed Technology is used by or on behalf of the Acquiring Party or its Affiliates in more than a de minimis fashion in connection with any subsequent clinical Development or Commercialization of such Competitive Products, and (B) the Acquiring Party and its Affiliates institutes commercially reasonable technical and administrative safeguards to ensure the requirements set forth in the foregoing clause (A) are met, including by creating “firewalls” between the personnel working on such Competitive Products and the personnel working on the Licensed Antibody and Licensed Products or having access to data from activities performed under this Agreement or Confidential Information of the other Party. |
2.8 | Coherus License Options. |
22
(a) | Grant of Options. Junshi hereby grants Coherus, on an Option Program-by-Option Program basis, the exclusive option during the Option Term for each Option Program to obtain an exclusive license to Exploit the Option Molecule and Option Products that are the subject of each Option Program in the Field in the Coherus Territory (for each Option Program, a “License Option”) upon the same terms in this Agreement as applicable to the Licensed Antibody and Licensed Products existing as of the Effective Date, except as otherwise specified. If Coherus does not exercise the License Option with respect to an Option Program prior to expiration of the applicable Option Term for such Option Program, then Coherus will no longer have any right to exercise the License Option for such Option Program. |
(b) | Option Data Package. |
(i) | Delivery. On an Option Program-by-Option Program basis with respect to a given Option Program, after Junshi has conducted sufficient number of Phase I Clinical Trials to generate safety data and a recommended dosage for conducting Phase II Clinical Trials for a Junshi IL-2 Molecule or Junshi TIGIT Antibody, as applicable, Junshi will deliver to Coherus (collectively, for each Option Program, an “Option Notice”): |
(ii) | the Option Data Package for such Option Program; |
(iii) | a description in reasonable detail of the Development (within and outside of the Coherus Territory) in an Optioned Licensed Product Development Plan, including the Optioned Licensed Product Development Budget for all costs and expenses for such Development to be incurred after the date of the Option Notice; |
(iv) | an Option Disclosure Letter for such Option Program; and |
(v) | a certification of an officer of Junshi as to the accuracy and completeness of the provided information, and a statement that, subject to the disclosures contained in the Option Disclosure Letter for such Option Program, the representations and warranties of Junshi set forth in Section 10.2 (Representations and Warranties of Junshi) being remade as of the Option Data Package Delivery Date are true and correct in all respects with respect to such Option Program as of the Option Data Package Delivery Date. |
(c) | Incomplete Option Data Package. Following receipt of an Option Data Package for an Option Program, Coherus will have the right (subject to the remainder of this Section 2.8(c) (Incomplete Option Data Package)) to promptly notify Junshi if Coherus believes that any such Option Data Package is missing any required information. Junshi will provide Coherus with the missing information identified in such notice within 10 Business Days after the date of Coherus’ request. If, following any such request from Coherus, Junshi does provide any such missing information that is available to Junshi, then the Option Term with respect to such Option Program will be extended only one time to end 20 days after delivery of such missing information. |
(d) | Due Diligence. During the Option Term for a given Option Program, to assist Coherus in conducting thorough due diligence to decide whether to exercise the License Option for such Option Program, at least once every Calendar Quarter Junshi will provide a report of all data and results from all non-clinical and pre-clinical studies and Clinical Trials for Option Products that are the subject of each Option Program, and Junshi will afford to Coherus and its representatives an opportunity to discuss such activities with Junshi |
23
personnel during normal business hours. In addition, during the Option Term following delivery to Coherus of the Option Data Package for an Option Program, as the case may be, upon Coherus’ request, (i) Junshi will use reasonable efforts to afford to Coherus and its representatives reasonable access during normal business hours to Junshi’s and its Affiliates’ personnel, records and data, offices, and laboratories, in each case, as Coherus may reasonably request related to such Option Program to conduct customary and reasonable due diligence of such Option Program and (ii) subject to customary and reasonable due diligence procedures to preserve the confidential nature of any such information, Junshi will promptly provide to Coherus through an electronic data room copies of (A) any documents reasonably requested by Coherus, (B) any patent or regulatory information, and (C) any results of preclinical, clinical and CMC activities relating to such Option Program, in each case ((A) – (C)), then Controlled by Junshi or its Affiliates, to the extent that such information has not been previously provided by or on behalf of Junshi to Coherus and pertains to such Option Program.
(e) | Junshi Restrictions. During the Option Term for a given Option Program, other than with the prior written consent of Coherus, Junshi will not grant to any Third Party any right to Exploit any Option Molecule or Option Products that are the subject of such Option Program in a manner that would conflict with the License Option granted to Coherus hereunder with respect to such Option Program or the rights granted to Coherus if Coherus were to exercise such License Option. |
(f) | Termination of Option. If Coherus does not provide an Option Exercise Notice in respect of a given Option Program prior to the expiration of the Option Term for such Option Program, then Coherus’ right to exercise the License Option for such Option Program will terminate and Junshi will have no further obligations to Coherus with respect to such Option Program and this Agreement will terminate with respect to such Option Program. |
(g) | Exercise of a License Option. Coherus may exercise the License Option for a given Option Program at any time during the period commencing on the Effective Date and ending 60 days following receipt of an Option Notice with respect to such Option Program (the “Option Term” and each such exercise, an “Option Exercise”), by providing Junshi with written notice of its intent with respect thereto (each, an “Option Exercise Notice”) and payment of the amounts due under Section 8.2 (Option Exercise Payment) so long as at such time neither Coherus nor any of its Affiliates is undertaking any activities that would, if the subject Option Molecules or Option Products were Licensed Antibodies or Licensed Product, constitute Competitive Activities at the time of the Option Exercise with respect to the subject Option Molecules and Option Products. If Coherus or any of its Affiliates is undertaking any Competitive Activities at such time, then, in addition to the foregoing conditions, Coherus may only exercise the License Option with the written consent of Junshi. The date on which Coherus exercises a License Option and pays all such amounts for an Option Program is the “Option Exercise Date.” From and after the Option Exercise Date for an Option Program, all Option Molecules and Option Products that are the subject of such Option Program will thereafter become Licensed Antibodies and Licensed Products, as applicable, for purposes of this Agreement upon the same terms in this Agreement as applicable to the other Licensed Antibodies and Licensed Products that existed as of the Effective Date, except as otherwise specified herein, and accordingly Coherus will also have the right to make Development Proposals for such Option Molecules and Optioned Licensed Products in accordance with the provisions of Section 4.3 (Additional Development), conduct joint Development with Junshi under Joint Development Plans approved by the JDC, and conduct Independent Development in connection with such Option Molecules and Optioned Licensed Products. |
24
(h) | Coherus Share of Option Program Development Costs. Coherus will pay its share of costs incurred by Junshi or its Affiliates after the Option Notice according to the applicable Optioned Licensed Product Development Plan and Optioned Licensed Product Development Budget presented to Coherus in the Option Notice and each update to such plan and budget as approved by the JDC (“Optioned Licensed Product Development Costs”) equal to (i) 100% of such costs and expenses attributable to Development activities in the Coherus Territory plus (ii) 25% of such costs and expenses attributable to Development activities outside of the Coherus Territory; provided, however, that Coherus will not be obligated to reimburse Junshi for more than $25,000,000 per Calendar Year (prorated on a daily basis for the partial Calendar Year during which the Option Exercise Date occurs) (the “Option Program Annual Ongoing Development Cost Cap”) for the Optioned Licensed Product Development Costs associated with an Option Program. |
(i) | Invoices and Payments. No later than 30 days after the end of each Calendar Quarter after the Option Exercise Date, Junshi will provide an invoice to Coherus setting forth in reasonable detail Coherus’ share of the Optioned Licensed Product Development Costs incurred by Junshi or its Affiliates during such Calendar Quarter (to the extent less than or equal to the Option Program Annual Ongoing Development Cost Cap for the applicable Calendar Year). Such quarterly invoices will be accompanied by available supporting documentation, receipts, or related information to the extent necessary to verify such share of Optioned Licensed Product Development Costs for a particular Calendar Quarter. Coherus will pay the undisputed share of Optioned Licensed Product Development Costs for each Calendar Quarter (to the extent less than or equal to the Option Program Annual Ongoing Development Cost Cap for the applicable Calendar Year) no later than 45 days after the receipt of such invoice. |
(j) | Plan and Budget Updates. Once every six months following the Option Exercise, Junshi will provide to the JDC to review, discuss, and determine whether to approve, an updated Optioned Licensed Product Development Plan and Optioned Licensed Product Development Budget. |
2.9 | Right of First Negotiation. Junshi hereby grants to Coherus the exclusive right of first negotiation in the event that Junshi or its Affiliates determines to transfer, license, sublicense, assign, grant, or otherwise dispose of rights to any Third Party, other than express or implied licenses/sublicenses granted to an agent or a consultant, contract manufacturing organization, contract research organization, or other similar type contractor acting for or on behalf of Junshi or its Affiliates, to Develop or Commercialize one or more Antibodies Controlled by Junshi or any of its Affiliates directed to CD112r or CTLA-4 (a “ROFN Product”) in one or more countries in the Coherus Territory (a “ROFN Product Activity” and such countries, the “ROFN Jurisdictions”). Promptly upon determining to engage in a ROFN Product Activity, Junshi will notify Coherus in writing of such determination and identify the applicable ROFN Product and ROFN Jurisdictions with respect to which such Development or Commercialization rights would be granted. Coherus will have an exclusive right, exercisable no later than 30 days after receipt of any such written notice from Junshi, to notify Junshi in writing as to whether Coherus desires to negotiate exclusively for the right to Develop or Commercialize such ROFN Product in such ROFN Jurisdiction (for each of CD112r and CTLA-4, each, a “ROFN Exercise Notice”). If Coherus provides a ROFN Exercise Notice to Junshi within such 30 day period indicating its desire to negotiate for such rights to the applicable ROFN Product in the applicable ROFN Jurisdiction, then (a) upon Coherus’ request, Junshi will (i) within 20 Business Days of Coherus’ request, provide Coherus with other information and documentation reasonably requested by Coherus relating to such ROFN Product and ROFN Jurisdiction; and (ii) afford Coherus and its representatives reasonable access during normal business hours to Junshi’s personnel; and (b) Coherus will have the exclusive right for 100 |
25
days from the date of Junshi’s receipt of the ROFN Exercise Notice to enter into an agreement or amendment to this Agreement, as applicable, with respect to the Exploitation by Coherus of such ROFN Product in such ROFN Jurisdiction. If, with respect to a ROFN Product in a ROFN Jurisdiction, either (A) Coherus does not provide the ROFN Exercise Notice to Junshi within such 30 day period, or (B) Coherus and Junshi do not agree on terms under which Coherus would be granted the right to Exploit such ROFN Product in such ROFN Jurisdiction within the 100 day negotiation period after having conducted such negotiations in good faith, then, in each case ((A) and (B)), Junshi will be free to enter into negotiations and an agreement with one or more Third Parties relating to a grant of rights to Develop or Commercialize such ROFN Product (or to Develop or Commercialize any such ROFN Product itself) in such ROFN Jurisdiction.
ARTICLE 3
GOVERNANCE
3.1 | Alliance Manager. Within 30 days of the Effective Date, each Party will appoint an individual (from the Party or from any Affiliate of such Party) who possesses a general understanding of Development and Manufacturing issues regarding pharmaceutical and biologic products to act as the facilitator of the meetings of the JDC and the first point of contact between the Parties with regard to questions relating to this Agreement or the overall business relationship and related matters between the Parties (each, an “Alliance Manager”). Each Party may replace its Alliance Manager at any time upon written notice to the other Party. |
3.2 | Joint Development Committee. |
(a) | Formation; Composition. No later than after 30 days after the Effective Date, the Parties will establish a joint development committee (the “Joint Development Committee” or “JDC”) comprised of three representatives from each Party (or appointed representatives of any Affiliate of such Party) with sufficient seniority within the applicable Party to make decisions arising within the scope of the JDC’s responsibilities. The JDC may change its size from time to time by mutual consent of its members, provided that the JDC will consist at all times of an equal number of representatives of each of Junshi and Coherus. Each Party may replace its JDC representatives at any time upon written notice to the other Party. The JDC may invite non-members to participate in the discussions and meetings of the JDC, provided that such participants will have no voting authority at the JDC. The JDC will be chaired by one of the representatives (“Chairperson”) and will rotate between the Parties every 12 months during the Term. The role of the Chairperson will be to convene and preside at meetings of the JDC. The Chairperson will have no additional powers or rights beyond those held by the other JDC representatives. The Alliance Managers will work with the Chairperson to prepare and circulate agendas and to ensure the preparation of minutes. |
(b) | Specific Responsibilities. The JDC will: |
(i) | facilitate the flow of information between the Parties with respect to the Development and Commercialization of the Licensed Antibodies and Licensed Products; |
(ii) | review, discuss, and determine whether to approve updates to any Ongoing JS001 Development Plan or Ongoing JS001 Development Budget pursuant to Section 4.2(a) (Plans and Budgets); |
(iii) | review and discuss Development Proposals presented by either Party pursuant to Section 4.3(a) (Proposals and JDC Review), and, subject to Section 3.2(b)(iv), Independent Trials, Independent Development Plans, and Independent |
26
Development Budgets presented by either Party pursuant to Section 4.5 (Independent Development);
(iv) | review, discuss, and determine whether to approve each Joint Development Plan and Joint Development Budget, and each update thereto, pursuant to Section 4.4 (Joint Development), which will include any Independent Development Plan or Independent Development Budget that becomes a Joint Development Plan or Joint Development Budget following a Party’s opt-in pursuant to Section 4.5 (Independent Development), and any updates thereto; |
(v) | after the Option Exercise with respect to an Option Program, review, discuss, and determine whether to approve all updates or amendments to any applicable Optioned Licensed Product Trials, Optioned Licensed Product Development Plan, and Optioned Licensed Product Development Budget pursuant to Section 4.6 (Development of Optioned Licensed Products) or Section 2.8 (Coherus License Options); |
(vi) | review and discuss any Development reports provided by either Party pursuant to Section 4.10 (Development Reports); |
(vii) | review, discuss, and determine whether to approve the Transition Plan pursuant to Section 5.1 (Regulatory Responsibilities); |
(viii) | review, discuss, and approve (A) the date by which the Parties will complete all transition activities to enable Coherus to assume regulatory responsibilities for the Licensed Antibodies and Licensed Products in the Coherus Territory (other than those related to the Junshi Clinical Trials), and (B) whether Coherus will assume responsibility for further regulatory activities for the Licensed Antibodies and Licensed Products throughout the Coherus Territory for the Ongoing JS001 Trial following transfer of the applicable Regulatory Approvals and Regulatory Materials for the Ongoing JS001 Trial to Coherus, in each case ((A) – (B)) pursuant to Section 5.2 (Assignment of Regulatory Materials); |
(ix) | review and discuss any Commercialization updates provided by Coherus pursuant to Section 7.3 (Commercialization Report); |
(x) | to the extent not specified in a Joint Development Plan approved by the JDC, review, discuss, and determine which Party will have control and decision-making authority with respect to preparing and submitting regulatory filings and conducting communications with Regulatory Authorities, in each case related to the Licensed Antibodies and Licensed Products that is the subject of the Joint Development Plan approved by the JDC, as applicable; and |
(xi) | perform such other functions as appropriate, to further the purposes of this Agreement, in each case as agreed in writing by the Parties. |
(c) | Meetings. During the Term, the JDC will meet on a quarterly basis, unless otherwise agreed to by the JDC. No later than 10 Business Days prior to any meeting of the JDC, the Alliance Managers will jointly prepare and circulate an agenda for such meeting; provided, however, that either Party may propose additional topics to be included on such agenda, either prior to or in the course of such meeting. Either Party may also call a special meeting of the JDC (by videoconference, teleconference or in person) by providing at least 10 Business Days’ prior written notice to the other Party if such Party reasonably believes that a significant matter must be addressed prior to the next regularly scheduled meeting, in |
27
which event such Party will work with the Chairperson of the JDC to provide the members of the JDC no later than three Business Days prior to the special meeting with an agenda for the meeting and materials reasonably adequate to enable an informed decision on the matters to be considered. The JDC may meet in person, by videoconference or by teleconference. In-person JDC meetings will be held at locations agreed upon by Junshi and by Coherus. Each Party will bear the expense of its respective JDC members’ participation in JDC meetings. Meetings of the JDC will be effective only if at least two JDC members from each Party (which members do not include such Party’s Alliance Manager) are present or participating (including by videoconference or teleconference) in such meeting. The Alliance Managers will be responsible for preparing reasonably detailed written minutes of all JDC meetings that reflect material decisions made and action items identified at such meetings. The Alliance Managers will send draft meeting minutes to each member of the JDC for review and approval within 20 Business Days after each JDC meeting. Such minutes will be deemed approved unless one or more members of the JDC objects to the accuracy of such minutes within five Business Days of receipt. Minutes will be officially endorsed by the JDC at the next JDC meeting, and will be signed by the Chairperson.
(d) | Decision-Making. The representatives from each Party on the JDC will have, collectively, one vote on behalf of that Party, and all decision making will be by consensus. Disputes at the JDC will be handled in accordance with Section 3.3 (Resolution of JDC Disputes). |
3.3 | Resolution of JDC Disputes. |
(a) | Within the JDC. All decisions within the JDC will be made by consensus. If the JDC is unable to reach consensus on any issue for which it is responsible within thirty 30 days after a Party affirmatively states that a decision needs to be made, then either Party may elect, by written notice to the other Party, to submit such issue the Parties’ Executive Officers, in accordance with Section 3.3(b) (Referral to Executive Officers). |
(b) | Referral to Executive Officers. If a Party makes an election under Section 3.3(a) (Resolution of JDC Disputes; Within the JDC) to refer a matter to the Executive Officers, then the Executive Officers will use good faith efforts to resolve promptly such matter, which good faith efforts will include at least one in-person, video or telephonic meeting between such Executive Officers within 15 Business Days after the submission of such matter to them. |
(c) | Final Decision-Making Authority. If the Executive Officers are unable to reach consensus on any such matter within thirty (30) days after its submission to them, then: |
(i) | No Changes; Status Quo. Neither Party will have final decision-making authority over: (A) approval of a Joint Development Plan or any update thereto, (B) changes to the Ongoing JS001 Development Plan or the Ongoing JS001 Development Budget, except with respect to any change to any Ongoing JS001 Development Budget that results in a total budget that is less than 10% higher than the then- current total budget, (C) after an Option Exercise for an Option Program, approval of, or changes to, the corresponding Optioned Licensed Product Development Plan or Optioned Licensed Product Development Budget, except with respect to any change to any Optioned Licensed Product Development Budget that results in a total budget that is less than 10% higher than the then-current total budget, (D) any matter that relates to any Clinical Trial that the Parties are conducting pursuant to a Joint Development Plan, (E) the date by which the Parties will complete all transition activities to enable Coherus to assume regulatory responsibilities for the Licensed Antibodies and Licensed Products in the Coherus Territory (other than |
28
those related to the Junshi Clinical Trials), (F) approval of the Transition Plan or any changes to it after its approval, or (G) whether Coherus will assume responsibility for further regulatory activities for the Licensed Antibodies and Licensed Products throughout the Coherus Territory for the Ongoing JS001 Trial following transfer of the applicable Regulatory Approvals and Regulatory Materials for the Ongoing JS001 Trial to Coherus.
(ii) | Coherus Decisions. Coherus will have final decision-making authority with respect to matters (A) that relate exclusively to the Coherus Territory (other than any Independent Development for which Junshi is the Developing Party), or (B) that relate to Independent Development for the Coherus Territory for which Coherus is the Developing Party, except those matters set forth under Section 3.3(c)(i) (No Changes; Status Quo), Section 3.3(c)(iii) (Junshi Decisions), or Section 3.3(c)(iv) (Limitations on Decision-Making) and provided that Coherus will not exercise its final decision-making authority in a matter that would reasonably be expected to: (1) result in a material quality, safety, toxicity, or side effect concern with respect to the Licensed Antibody or Licensed Product, as such matter, if applicable, may be determined by the Panel in accordance with Section 4.3(b) (Restrictions on Additional Development); or (2) change Coherus’ obligations under this Agreement. |
(iii) | Junshi Decisions. Junshi will have final decision-making authority with respect to (A) matters that relate exclusively to the Junshi Territory (other than Independent Development for which Coherus is the Developing Party), (B) matters that relate to Independent Development for which Junshi is the Developing Party, (C) the Manufacturing of any Licensed Products that include each Licensed Antibody prior to the completion of the Manufacturing Technology Transfer for the applicable Licensed Antibody (except those matters set forth under Section 3.3(c)(i) (No Changes; Status Quo)) or Section 3.3(c)(iv) (Limitations on Decision-Making), or (D) modifications of any then-current Ongoing JS001 Development Budget, Joint Development Budget, or Optioned Licensed Product Development Budget to the extent such modifications result in a total budget that is less than 10% higher than the then-current total budget; in each case, (A) through (D), provided that, Junshi will not exercise its final decision-making authority in a matter that would reasonably be expected to: (1) result in a material quality, safety, toxicity, or side effect concern with respect to the Licensed Antibody or Licensed Product, as such matter, if applicable, may be determined by the Panel in accordance with Section 4.3(b) (Restrictions on Additional Development); or (2) change Junshi’s obligations under this Agreement. |
(iv) | Limitations on Decision-Making. Without the other Party’s prior written consent, neither Party may unilaterally make a decision (in exercise of its final decision-making authority on any such matters) that (A) expands either Party’s contractual rights or reduces either Party’s contractual obligations under this Agreement, (B) results in a material increase in the other Party’s obligations, costs, or expenses or a material limitation to the other Party’s rights under this Agreement, (C) conflicts with this Agreement, or would be reasonably likely to result in a violation of applicable law, the requirement of any Regulatory Authorities or any agreement with any Third Party (including any Additional Third Party License), or result in the infringement or misappropriation of intellectual property rights of any Third Party, or (D) is stated to require the agreement or |
29
consent of the Parties under Section 3.3(c)(i) (No Changes; Status Quo) or that is subject to the determination of the other Party pursuant to Section 3.3(c)(ii) (Coherus Decisions) or Section 3.3(c)(iii) (Junshi Decisions) (as applicable). In addition, no exercise by either Party of such Party’s decision-making authority can amend or waive compliance with any terms of this Agreement.
3.4 | Discontinuation of JDC. The JDC will continue to exist until the Parties agree to disband the JDC. Once the JDC is disbanded, the JDC will have no further obligations under this Agreement and, thereafter, the Alliance Managers will be the points of contact for the exchange of information between the Parties under this Agreement and any references in this Agreement to decisions of the JDC will automatically become references to decisions by and between the Parties in writing, subject to the other terms of this Agreement and consistent with the terms of Section 3.3(c) (Final Decision-Making Authority). |
ARTICLE 4
DEVELOPMENT
4.1 | Development Diligence Obligations. |
(a) | Junshi. Junshi will use Commercially Reasonable Efforts to conduct (i) the Ongoing JS001 Clinical Trials, (ii) a sufficient number of Phase I Clinical Trials to generate safety data and a recommended dosage for conducting Phase II Clinical Trials for a Junshi IL-2 Molecule and for a Junshi TIGIT Antibody, as applicable, and (iii) after the Option Exercise for an Option Program, the corresponding Optioned Licensed Product Trials. |
(b) | Coherus. Subject to Junshi’s satisfaction of its obligations set forth in Section 4.1(a) (Junshi) as applicable, Coherus will use Commercially Reasonable Efforts to Develop and obtain Regulatory Approval (and, where applicable, Pricing and Reimbursement Approval) for (i) a Licensed Product that includes a Licensed Antibody defined in clause (a) of the definition of “Licensed Antibody” in the Field in the United States and Canada and (ii) after the Option Exercise for an Option Program, at least one Licensed Product in the Field in the United States and Canada involving a Junshi IL-2 Molecule or Junshi TIGIT Antibody, as applicable. |
(c) | Joint Development Plans. If a Joint Development Plan is approved by all members of the JDC, then each Party will use Commercially Reasonable Efforts to conduct those Development activities allocated to such Party under and in accordance with the Joint Development Plan and the applicable Joint Development Budget. |
4.2 | Ongoing JS001 Trials. |
(a) | Plans and Budgets. Junshi will be responsible for the completion of, will continue to lead clinical operation of, and will use Commercially Reasonable Efforts to complete the Ongoing JS001 Trials in accordance with the Ongoing JS001 Development Plan (as may be revised from time to time). The development plan for the Ongoing JS001 Trials as of the Execution Date is attached to this Agreement as Schedule 4.2 (Ongoing JS001 Development Plan) (the “Ongoing JS001 Development Plan”), which includes the budget of the costs and expenses for the completion of the Ongoing JS001 Trials(the “Ongoing JS001 Development Budget”). Junshi will provide to Coherus any proposed updates to the Ongoing JS001 Development Plan (including to the Ongoing JS001 Development Budget set forth therein) for Coherus’ review and comment and no changes to the Ongoing JS001 Development Plan will be effective unless and until the JDC determines to approve such changes. |
30
(b) | Costs to be Reimbursed. Coherus will reimburse Junshi, at Junshi’s election, for either: (i) 100% of the documented internal and external costs and expenses (including the cost of allocated FTEs at the FTE Rate for Junshi) in the Coherus Territory or (ii) 25% of the documented internal and external costs and expenses (including the cost of allocated FTEs at the FTE Rate) globally (including within the Coherus Territory and outside the Coherus Territory), in each case ((i) and (ii)), incurred by Junshi or its Affiliates in the performance of the Ongoing JS001 Trials after the Execution Date to the extent in accordance with the then-current Ongoing JS001 Development Plan and Ongoing JS001 Development Budget (the “Ongoing JS001 Trial Costs”) up to a maximum of $25,000,000 per Calendar Year (the “Ongoing JS001 Trial Cap”). |
(c) | Invoices and Payments. No later than 30 days after the end of each Calendar Quarter during which an Ongoing JS001 Trial is conducted, Junshi will provide an invoice to Coherus setting forth in reasonable detail Coherus’ share of the Ongoing JS001 Trial Costs incurred by Junshi or its Affiliates during such Calendar Quarter (to the extent less than or equal to the Ongoing JS001 Trial Cap for the applicable Calendar Year). Such quarterly invoices will be accompanied by available supporting documentation, receipts, or related information to the extent necessary to verify such share of Ongoing JS001 Trial Costs for a particular Calendar Quarter at the rate selected by Junshi under Section 4.2(b) (Costs to be Reimbursed). Coherus will pay the undisputed share of Ongoing JS001 Trial Costs for each Calendar Quarter (to the extent less than or equal to the Ongoing JS001 Trial Cap for the applicable Calendar Year) no later than 45 days after the receipt of such invoice. At the end of each Calendar Year during which an Ongoing JS001 Trial is conducted, Junshi will provide a final invoice for the preceding Calendar Year so that Coherus is invoiced at the greater of the rates under Section 4.2(b) (Costs to be Reimbursed) for such Calendar Year; provided, however, that if such invoice would cause Coherus to exceed the Ongoing JS001 Trial Cap for such Calendar Year, then Junshi will provide to Coherus an invoice for an amount so that Junshi only invoices Coherus a total amount for the subject Calendar Year equal to the Ongoing JS001 Trial Cap for such Calendar Year. |
(d) | Plan and Budget Updates. No less than once per year, or more frequently as may be required, Junshi will provide to the JDC to review, discuss, and determine whether to approve, an updated Ongoing JS001 Development Plan and Ongoing JS001 Development Budget. |
4.3 | Additional Development. |
(a) | Proposals and JDC Review. During the Term, either Party may propose to (i) conduct Clinical Trials using a Licensed Antibody as a monotherapy or (ii) Develop a Combination Regimen that includes a Licensed Antibody, in any such case (i) or (ii), either independently or collaboratively with the other Party and involving Clinical Trials the data from which will be used to obtain, maintain, or support Regulatory Approval for the applicable monotherapy or Combination Regimen solely or partly For the Coherus Territory (but that is not at such time already the subject of the Ongoing JS001 Trials, an Optioned Licensed Product Development Plan, a Joint Development Plan approved by the JDC, or an Independent Development Plan). If a Party desires to undertake such new Development, then such Party will submit a proposal to the JDC setting forth the proposed Development activities and its related timeline (a “Development Proposal”). Any such proposal that proposes costs sharing between the Parties or other collaboration with respect to such Development activities (a “Joint Development Proposal”) should also contain the proposed budget and cost sharing arrangements for the corresponding Development activities. Within 30 days after submission to the JDC, the JDC will review, discuss, and determine whether to approve such Development Proposal. |
31
(b) | Restrictions on Additional Development. Neither Party will conduct any Clinical Trials involving a Licensed Antibody in or For the Coherus Territory (including an Optioned Licensed Product following the Option Exercise for such product) (i) other than as set forth in this Section 4.3 (Additional Development), Section 4.4 (Joint Development), and Section 4.5 (Independent Development) or (ii) if the other Party objects to the conduct of Clinical Trials in or For the Coherus Territory on the basis they are likely to result in a material quality, safety, toxicity, or side effect with respect to the Licensed Antibody or Licensed Product and provides its rationale for such objection in writing to the JDC; provided that, if the Parties disagree as to whether such Clinical Trial is likely to result in a material quality, safety, toxicity, or side effect with respect to such Licensed Antibody or Licensed Product, then either Party may escalate such dispute for resolution to the Executive Officers in accordance with the terms of Section 15.1(a) (Dispute Resolution). If the Executive Officers cannot agree on a resolution within the applicable time period, then neither Party may proceed under Section 15.1(b) (Dispute Resolution), but instead either Party may, within 10 days after expiration of such time period, refer such disagreement to expert arbitration pursuant to the following procedures: |
(A) | The expert arbitration will be overseen by and conducted as a “baseball” form of binding arbitration conducted by a panel of three (3) arbitrators (“Panel”). The Panel will be selected in accordance with the following procedure: Coherus will appoint one (1) arbitrator for the Panel, Junshi will appoint one (1) arbitrator for the Panel, each within fourteen (14) days of the initiation of arbitration, and the two Party-appointed arbitrators will appoint the third arbitrator for the Panel, who will act as the chair, within fourteen (14) days of confirmation of the two Party-appointed arbitrators. The Parties may confer with their respective Party-appointed arbitrators regarding the appointment of the chair. Each arbitrator comprising the Panel will have at least twenty (20) years of experience in the negotiation of biotechnology and pharmaceutical license and collaboration agreements. At the election of any member of the Panel, the Panel may engage one or more independent experts with experience in the subject matter of the dispute to advise the Panel, but final decision-making authority will remain in the Panel. |
(B) | Within twenty-one (21) days after the constitution of the Panel, each Party will submit to both the Panel and the other Party a detailed written proposal of no more than thirty (30) pages setting forth its proposed resolution of the dispute, which proposal should indicate one of the following: (1) that the proposed Clinical Trials can proceed as most recently proposed by the Developing Party to the JDC, (2) that the proposed Clinical Trials cannot proceed, or (3) that the proposed Clinical Trials can proceed but with the modifications as described by such Party in the proposal to the Panel. The Parties will also provide to the Panel a copy of this Agreement, as may be amended at such time. |
(C) | There will be no discovery and there will be no hearing, although the arbitration proceeding will be deemed to have its seat in New York, New York, U.S.A. |
(D) | Within thirty (30) days after the submission of the Parties’ legal briefs, the Panel will select one of the two detailed written proposals (without modification) provided by the Parties that the Panel believes is most |
32
consistent with the intention underlying and agreed principles set forth in this Agreement. The decision of the Panel will be final and unappealable. The detailed written proposal selected by the Panel will automatically be binding on the Parties.
(E) | The Panel must select one of the two detailed written proposals and may not combine elements of both detailed written proposals or take any other action. |
(F) | Each Party will bear its own attorneys’ fees, costs and disbursements arising out of the arbitration, and will pay an equal share of the fees and costs of the Panel. |
4.4Joint Development. If within 30 days after submission of a Joint Development Proposal the JDC determines to further consider such proposal (either as originally proposed or with revisions), then (a)the Parties will develop for approval by the JDC a written plan to govern such Development, including the activities to be conducted by each Party with respect to the Development activities and associated budget and cost sharing arrangement, and (b) the Parties may enter into an amendment of this Agreement or a separate written agreement governing the Parties’ rights and obligations with respect to such Development, if appropriate, to be effective concurrently with the approval of such plan by the JDC. Any such plan approved by all members of the JDC is a “Joint Development Plan” and its associated budget a “Joint Development Budget.” Any Joint Development Proposal not approved by the JDC within 60 days (unless such time is extended by written agreement of the Parties) after it is first presented by a Party to the JDC as a Joint Development Proposal will be deemed not approved by the JDC.
4.5 | Independent Development. If either (a) the JDC does not approve a Joint Development Proposal within the applicable time or (b) the Development Proposal was not presented to the JDC as a Joint Development Proposal, then the proposing Party (the “Developing Party”) will have the right, but not the obligation, to proceed with the Development activities as most recently proposed by such proposing Party to the JDC (“Independent Development”) at its sole cost and expense subject to the following conditions. |
(a) | Right to Opt-In. If a Developing Party desires to proceed with any Independent Development, then it must provide the other Party (the “Non-Developing Party”) with the option to share in the costs of the Clinical Trials associated with such Independent Development on the terms described in this Section 4.5 (Independent Development) below (and be granted rights to the data and results from such Clinical Trials), unless the Independent Development is for a Combination Regimen in which any of the Other Component(s) is/are Controlled by the Developing Party. If the Developing Party desires to proceed with any Independent Development for a Combination Regimen in which any of the Other Component(s) is/are Controlled by the Developing Party (including when any such Other Component(s) is/are still in Development or the subject of one or more Regulatory Approval(s), a “Proprietary Combination Regimen”), then the Developing Party may, but is not required to, provide the Non-Developing Party with such option. |
(b) | Non-Developing Party Opt-In. If a Developing Party undertakes any Independent Development in which the Non-Developing Party has the option to share in the data, results and costs of the Clinical Trials associated with such Independent Development (each such Clinical Trial, an “Independent Trial”) then within 30 days following the JDC’s decision not to approve a Joint Development Proposal or the Developing Party’s presentation to the JDC of such option (if not presented as a possible Joint Development Proposal), the Developing Party will provide to the Non-Developing Party a plan of the Development activities to be conducted with respect to such Independent Development (the |
33
“Independent Development Plan”) and a budget of the costs and expenses to be incurred in the performance of activities under such plan (the “Independent Development Budget”).
(c) | Early Opt-In Cost Sharing. If the Non-Developing Party notifies the Developing Party in writing that it is electing to be granted such rights within 30 days after receipt of the Independent Development Plan and Independent Development Budget, then the applicable Independent Development Plan will become a Joint Development Plan and the applicable Independent Development Budget will become a Joint Development Budget, and: |
(i) | If the Non-Developing Party is Coherus, Coherus will reimburse Junshi each Calendar Quarter for: (A) 100% of the documented internal and external costs and expenses (including the cost of allocated FTEs at the FTE Rate) in the Coherus Territory plus (B) 25% of the documented internal and external costs and expenses (including the cost of allocated FTEs at the FTE Rate) outside of the Coherus Territory, in each case ((A) and (B)), incurred by Junshi or its Affiliates in the performance of the Independent Development Plan to the extent in accordance with the applicable and then-current Independent Development Budget, which reimbursements will be conducted in accordance with the terms of Section 2.8(i) (Invoices and Payments) mutatis mutandis; provided, however, that Coherus will not be obligated to reimburse Junshi for more than $25,000,000 per Calendar Year for the PD-1 Program or $25,000,000 per Calendar Year for an Option Program; or |
(ii) | If the Non-Developing Party is Junshi, Junshi will reimburse Coherus each Calendar Quarter for 35% of the documented internal and external costs and expenses (including the cost of allocated FTEs at the FTE Rate) in the Coherus Territory incurred by Coherus in the performance of the Independent Development Plan to the extent in accordance with the applicable and then current Independent Development Budget; provided, however, that Junshi will not be obligated to reimburse Coherus for more than $25,000,000 per Calendar Year for the PD-1 Program or $25,000,000 per Calendar Year for an Option Program. |
(iii) | No less than once per year, or more frequently as may be required (including to add new Independent Trials), the Developing Party will provide to the JDC to review, discuss, and determine whether to approve, any updates to an existing Independent Development Plan and corresponding Independent Development Budget. |
(d) | Later Opt-In Cost-Sharing Premium. If the Non-Developing Party does not elect to reimburse the Developing Party in accordance with Section 4.5(c) (Early Opt-In Cost Sharing), then the Non-Developing Party will not have any rights with respect to any data or results generated from the Independent Trials or other Development activities of the Independent Development or any related Regulatory Materials, unless and until the Non- Developing Party notifies the Developing Party of its election to be granted such rights and reimburses the Developing Party for 50% more than the Non-Developing Party would be required to pay if the Non-Developing Party had elected to share in the costs and expenses as provided under Section 4.5(c) (Early Opt-In Cost Sharing) for all such costs and expenses incurred by the Developing Party through the date of such election and thereafter. After the Non-Developing Party makes such election and reimburses the Developing Party for its share of such costs and expenses incurred through such time, the Non-Developing Party will be responsible for its applicable costs and expenses incurred after the date of election and the data and results generated from such Independent Trials or other |
34
Independent Development activities will be subject to the Non-Developing Party’s right of use and right of reference under Section 4.8 (Data Exchange and Use) and Section 5.5 (Right of Reference).
4.6 | Development of Optioned Licensed Products. This Section 4.6 (Development of Optioned Licensed Products) only applies to Licensed Products that are the subject of the TIGIT Program or the IL-2 Program following the Option Exercise with respect to the applicable Option Program (“Optioned Licensed Products”). Upon and after the Option Exercise with respect to an Option Program: |
(a) | Trials Ongoing as of Option Exercise. Coherus will have the right to participate in any Clinical Trials conducted by Junshi or its Affiliates with respect to the Optioned Licensed Products that are the subject of the TIGIT Program or the IL-2 Program (as applicable) in the Coherus Territory, according to the applicable Optioned Licensed Product Development Plan and Optioned Licensed Product Development Budget presented to Coherus in the Option Notice and each update to such plan and budget as proposed to JDC pursuant to the terms of this Agreement, whether such Clinical Trials are ongoing as of the Option Exercise Date for such program or thereafter (each, an “Optioned Licensed Product Trial”). |
(b) | Cost Sharing. In addition to the amounts that Coherus must pay to Junshi as part of the Option Exercise as provided in Section 2.8(h) (Coherus Share of Option Program Development Costs), upon and after the Option Exercise, Coherus must co-fund its share of Optioned Licensed Product Development Costs in accordance with Section 2.8(i) (Invoices and Payments), subject to the Option Program Annual Ongoing Development Cost Cap. |
(c) | Rights to Data. Coherus will obtain rights to use and reference the data and results generated from the Development activities conducted in connection with the Optioned Licensed Product Development Plan in accordance with Section 4.8 (Data Exchange and Use) and Section 5.5 (Right of Reference). |
4.7 | Responsibility for Development. Other than with respect to Development related to the Junshi Clinical Trials in accordance with this Article 4 (Development) and Article 3 (Governance), or as otherwise set forth under a Joint Development Plan approved by the JDC, each Party will be solely responsible for Development activities with respect to the Licensed Antibodies and Licensed Products for its respective territory. |
4.8 | Data Exchange and Use. In addition to its adverse event and safety data reporting obligations set forth in Section5.6 (Adverse Event Reporting), each Party will promptly provide the other Party with copies of all data and results and all supporting documentation (e.g., protocols, Investigator’s Brochures, case report forms, analysis plans) Controlled by such Party that are generated by or on behalf of such Party or its Affiliates, Sublicensees, or subcontractors, if applicable, in the Development of any Licensed Product For the Coherus Territory (whether as part of any Clinical Trials inside or outside of the Coherus Territory, involving any monotherapy or Combination Regimens conducted independently or jointly); provided, however, that in the case of Proprietary Combination Regimens, a Party is only required to disclose such data and results about the Licensed Product portion of the Proprietary Combination Regimen. Subject to payment of any amounts agreed in writing by the Parties or specified to be due under Section 4.2 (Ongoing JS001 Trials) through and including Section 4.6 (Development of Optioned Licensed Products), as applicable, each Party will have the right to use and reference such data and results provided by the other Party for the purpose of obtaining, supporting, and maintaining Regulatory Approvals, as applicable, of the Licensed Products (but not any other part of a Proprietary Combination Regimen, if applicable) |
35
in the recipient’s respective territory, without additional consideration. If either Party obtains the right to use or a right of reference to data Controlled by a Third Party with respect to a Combination Regimen, then such Party will attempt to obtain sufficient rights from such Third Party to grant a further right to use or right of reference with respect to such data to the other Party consistent with the foregoing rights to data and results Controlled by a Party.
4.9 | Development Records. Each Party will, and will cause its Affiliates, Sublicensees, and subcontractors to, maintain reasonably complete, current, and accurate records of all Development activities conducted by or on behalf of it and its Affiliates, Sublicensees, and subcontractors, respectively, for any Licensed Product (including any Combination Regimen) For the Coherus Territory and all data and other information resulting from such activities consistent with its usual practices, in validated computer systems that are compliant with 21 C.F.R. §11 and in accordance with applicable law in the Coherus Territory. Each Party will maintain all such records relating to the Development of Licensed Products for a period of five years, or a longer period as may be required by applicable law or regulation, after the end of the Term. Each Party will document all non-clinical and preclinical studies and Clinical Trials in formal written study reports in accordance with GLP, cGMP, and GCP in compliance with ICH Guidelines, as applicable, and in compliance with applicable law. Upon either Party’s reasonable request, not more frequently than once each Calendar Year during which the other Party or its Affiliates, Sublicensees, or subcontractors are performing or having performed Development activities for any Licensed Product (including any Combination Regimen) For the Coherus Territory, the other Party will, and will cause its Affiliates, Sublicensees, and subcontractors to, provide copies of such records (including access to relevant databases) to the requesting Party. |
4.10 | Development Reports. At each JDC meeting for a Calendar Quarter during which either Party is performing, or having performed, Development activities for any Licensed Product (including any Combination Regimen) For the Coherus Territory, such Party will provide a report to the other Party summarizing the Development activities for the Licensed Products performed during the period since the preceding JDC meeting, the Development activities for the Licensed Products in process, and the future Development activities for the Licensed Products that such Party or its Sublicensees or subcontractors expect to initiate, including a summary of the data, timelines, and results of such Development activities. Such reports and any additional information provided by a Party regarding Development activities for the Licensed Products, in each case, will be the Confidential Information of the providing Party and subject to the terms of Article 12 (Confidentiality). |
ARTICLE 5
REGULATORY
5.1 | Regulatory Responsibilities. Subject to the terms and conditions of this Agreement, Junshi and Coherus will be jointly responsible for all regulatory activities with respect to the Ongoing JS001 Clinical Trials in the Coherus Territory, until the transfer and assignment to Coherus, on a Licensed Antibody-by-Licensed Antibody basis, of the applicable Regulatory Approvals and Regulatory Materials in the Coherus Territory related to the Licensed Products that include a given Licensed Antibody pursuant to Section 5.2 (Assignment of Regulatory Materials). After such transfer and assignment, other than with respect to any Junshi Clinical Trials, Coherus will have sole responsibility for, and sole decision-making authority over, all further regulatory activities and associated costs and expenses for the applicable Licensed Antibody and all Licensed Products that include such Licensed Antibody in the Field in the Coherus Territory. Junshi will reasonably cooperate with Coherus in maintaining all Regulatory Approvals for the Licensed Products in the Coherus Territory and all regulatory activities related to the Exploitation of the Licensed Products in the Coherus Territory. Within 60 days after the Effective Date, the Parties will submit to the JDC to review, discuss, and determine whether to approve a plan for transitioning the regulatory |
36
activities with respect to the Ongoing JS001 Clinical Trials from Junshi to Coherus (“Transition Plan”).
5.2 | Assignment of Regulatory Materials. To the extent permissible under applicable law and as specified within the Transition Plan, (a) Junshi will transfer and assign, or will cause the transfer or assignment, to Coherus or its regulatory agent, Junshi’s, or any of Junshi’s Affiliates’, entire rights, title, and interests in and to all Regulatory Approvals and Regulatory Materials in the Coherus Territory with respect to the Licensed Antibodies and Licensed Products (other than those related to any Junshi Clinical Trial) that are owned, Controlled, or possessed by Junshi or any of its Affiliates, and (b) the Parties will complete all other transition activities to enable Coherus to assume the regulatory responsibilities for the Licensed Antibodies and Licensed Products in the Coherus Territory (other than those related to the Junshi Clinical Trials) by such time as agreed by the JDC (i) after the Effective Date with respect to JS001 and (ii) after each Option Exercise Date with respect to all Option Molecules and Option Products that are the subject of the Option Program for which Coherus exercises the License Option. In any event, Junshi will use reasonable efforts to provide any relevant documents to Coherus as soon as practical following the Effective Date or the Option Exercise Date, as applicable. Except as otherwise specified in the Transition Plan, Junshi will continue to hold in its name, throughout the world, all Regulatory Approvals and Regulatory Materials related to the Ongoing JS001 Clinical Trials until the completion thereof, and Junshi will continue to be responsible for all regulatory responsibilities related to the Ongoing JS001 Clinical Trials in the Coherus Territory until completion thereof and receipt of Regulatory Approval in the Coherus Territory for the Licensed Antibody and Licensed Product that is the subject of a trial. Promptly following receipt of any such Regulatory Approval, Junshi will transfer and assign, or will cause the transfer or assignment, to Coherus or its regulatory agent, Junshi’s, or any of Junshi’s Affiliates’, entire rights, title, and interests in and to all such Regulatory Approvals and Regulatory Materials with respect to such Ongoing JS001 Trial in the Coherus Territory that are Controlled by Junshi or any of its Affiliates, and following the completion of such transfer and assignment, unless otherwise agreed by the JDC, Coherus will assume responsibility for all further regulatory activities for the Licensed Antibodies and Licensed Products throughout the Coherus Territory related to such Ongoing JS001 Trial. |
5.3 | Regulatory Filings; Control and Ownership. |
(a) | By Coherus. As between the Parties, other than with respect to the Junshi Clinical Trials or as specified in the Transition Plan or a Joint Development Plan approved by the JDC, Coherus will lead and have sole control over and decision-making authority with respect to preparing and submitting to Regulatory Authorities in the Coherus Territory all regulatory filings related to the Licensed Antibodies and Licensed Products For the Coherus Territory, including all applications for Regulatory Approval for the Licensed Products in the Coherus Territory. As between the Parties, Coherus will own any and all such Regulatory Approvals and Regulatory Materials in the Coherus Territory related to the Licensed Antibodies and Licensed Products (including all Regulatory Approvals and Regulatory Materials in relation to any Ongoing JS001 Trial after such Regulatory Approvals and Regulatory Materials are assigned to Coherus pursuant to Section 5.2 (Assignment of Regulatory Materials)), which after such assignment will be held in the name of Coherus or its regulatory agent. Coherus will use reasonable efforts to: (i) keep Junshi informed of receipt of any Regulatory Approvals in the Coherus Territory with respect to any Licensed Products within one week after approval and (ii) provide copies of any applications for Regulatory Approvals (in its original language) in the Coherus Territory to Junshi within one week after filing for Junshi’s records, in each case to the extent not in violation of any law or contract. If Junshi obtains a BLA for a Combination Regimen in the Coherus Territory, then Coherus will update the label for the corresponding |
37
Licensed Product in the Coherus Territory as reasonably necessary for Coherus to be able to Commercialize the applicable Licensed Product as part of the Combination Regimen in the Coherus Territory, and Junshi will provide all assistance reasonably requested by Coherus related to any such label update. Except as may be required under applicable law, Coherus will not communicate with any Regulatory Authority outside of the Coherus Territory regarding any Regulatory Approval of a Licensed Product outside of the Coherus Territory.
(b) | Junshi Clinical Trials. Subject to Sections 5.1 (Regulatory Responsibilities) and Section 5.2 (Assignment of Regulatory Materials) with respect to the Ongoing JS001 Clinical Trials, as between the Parties, Junshi will lead and have sole control over and decision- making authority with respect to preparing and submitting (i) all regulatory filings related to Junshi Clinical Trials (including Independent Trials for which Junshi is the Developing Party) within or For the Coherus Territory, including all such applications for Regulatory Approval in the Coherus Territory, as well as (ii) all regulatory filings for or within the Junshi Territory. As between the Parties, Junshi will own any and all such Regulatory Approvals in the Coherus Territory and Regulatory Materials submitted to such Regulatory Authorities in the Coherus Territory unless and until they are assigned to Coherus pursuant to Section 5.2 (Assignment of Regulatory Materials)). As between the Parties, Junshi will also own any and all Regulatory Approvals for or within the Junshi Territory and all associated Regulatory Materials. Junshi will use reasonable efforts to: (A) keep Coherus informed of receipt of any such Regulatory Approvals in the Coherus Territory with respect to any Licensed Products within one week after approval and (B) provide copies of any such applications for Regulatory Approvals (in its original language) in the Coherus Territory to Coherus within one week after filing for Coherus’ records, in each case to the extent not in violation of any law or contract. |
(c) | Joint Development Plans. Each Party will have the rights and responsibilities for Regulatory Approvals and Regulatory Materials involving a Joint Development Plan approved by the JDC as specified in such plan or otherwise determined by the JDC. |
5.4 | Interactions with Regulatory Authorities. Until assignment to Coherus of the Regulatory Materials that are the subject of Section 5.2 (Assignment of Regulatory Materials), Junshi will have the joint right with Coherus to conduct communications with Regulatory Authorities in the Coherus Territory related to the Licensed Products that are the subject of those Regulatory Materials, including all meetings, conferences, and discussions (including advisory committee meetings). Unless otherwise agreed by the Parties in writing or determined by the JDC, the Party designated as having control over and decision-making authority with respect to preparing and submitting all regulatory filings related to the Licensed Antibodies and Licensed Products under Section 5.3 (Regulatory Filings Control and Ownership) within or For the Coherus Territory or Junshi Territory (as applicable) also has the sole right to conduct all communications with Regulatory Authorities related to the applicable Licensed Antibody(ies) or Licensed Product(s) for such territory, including all meetings, conferences, and discussions (including advisory committee meetings). The Party who controls such communications will disclose to the JDC within four (4) Business Days of such determination (a) any communications with a Regulatory Authority in the Coherus Territory that such Party reasonably determines would be likely to have a material impact on obtaining Regulatory Approval for a Licensed Product in the Coherus Territory, and (b) any communications with a Regulatory Authority in the Coherus Territory that such Party reasonably determines indicates the Regulatory Authority will require any specific pre-approval or post-approval commitments or requirements for Regulatory Approval or Commercialization in the Coherus Territory. |
38
5.5 | Right of Reference. Each Party will grant, and hereby does grant, to the other Party a right of reference to all Regulatory Approvals and Regulatory Materials pertaining to the Licensed Products in the Field Controlled and submitted by or on behalf of such Party or its Affiliates, subject to payment of any amounts agreed in writing by the Parties or specified to be due under Section 4.2 (Ongoing JS001 Clinical Trials) through and including Section 4.6 (Development of Optioned Licensed Products), as applicable, to obtain such right or reference. Coherus may use such right of reference to such Regulatory Approvals and Regulatory Materials Controlled by Junshi or its Affiliates, if any, solely for the purpose of seeking, obtaining, supporting, and maintaining Regulatory Approval and any Pricing and Reimbursement Approvals of the Licensed Products in the Coherus Territory. Junshi may use such right of reference to such Regulatory Approvals and Regulatory Materials Controlled by Coherus or any of its Affiliates solely for the purpose of seeking, obtaining, supporting, and maintaining Regulatory Approval and any Pricing and Reimbursement Approvals of (a) the Licensed Products in the Junshi Territory and (b) any product Controlled by Junshi that is included in any Combination Regimen in the Coherus Territory. The Party using such right of reference will bear (i) its own costs and expenses and (ii) the reasonable and verifiable costs and expenses incurred by the other Party associated with providing assistance to enable such use of the right of reference pursuant to this Section 5.5 (Right of Reference), including (A) internal costs (calculated at the FTE Rate) reasonably incurred by or on behalf of the other Party or its Affiliates in connection with such activities, and (B) all verifiable external or out- of-pocket costs reasonably incurred by or on behalf of the other Party or its Affiliates in connection with such activities under this Section 5.5, in each case ((A) and (B)), within 45 days after receiving the invoice therefor. Each Party will take such actions as may be reasonably requested by the other Party to give effect to the intent of this Section 5.5 (Right of Reference) and to give the other Party the benefit of the granting Party’s Regulatory Approvals and Regulatory Materials in the other Party’s territory as provided herein, including by executing any letters of authorization or similar correspondence for submitting to an applicable Regulatory Authority to further evidence or give effect to the rights contemplated hereby. |
5.6 | Adverse Event Reporting. No later than 90 days after the Effective Date, and in any event before the conduct of any Clinical Trial by Coherus in the Coherus Territory, Junshi and Coherus will develop and agree in a written agreement on worldwide safety and pharmacovigilance procedures for the Parties with respect to all Licensed Products, such as safety data sharing and exchange, adverse events reporting and prescription events monitoring (the “Pharmacovigilance Agreement”). The Pharmacovigilance Agreement will contain terms no less stringent than those required by ICH or other applicable guidelines in order to allow the Parties to meet the applicable law and other applicable regulatory requirements regarding the management of safety data in the Coherus Territory. The Pharmacovigilance Agreement will provide for (a) reimbursement by Coherus of Junshi’s internal and out-of-pocket costs and expenses associated with reporting adverse events or assisting Coherus with reporting adverse events, in each case, within the Coherus Territory and (b) reimbursement by Junshi of Coherus’ internal and out-of-pocket costs and expenses associated with reporting adverse events or assisting Junshi with reporting adverse events, in each case, outside of the Coherus Territory (in each case (a) and (b), to the extent not already reimbursed as part of any arrangement between the Parties involving Clinical Trials of Licensed Products). |
5.7 | Cost Reimbursement. With respect to the cooperation and assistance of Junshi provided pursuant to this Article 5 (Regulatory) (which will be separate from the cooperation and assistance provided in connection with the Technology Transfers), Coherus will reimburse Junshi for (a) internal costs (at the FTE Rate) reasonably incurred by or on behalf of Junshi or its Affiliates in connection with in relation to such activities in excess of 100 hours, and (b) all verifiable external or out-of-pocket costs actually incurred by or on behalf of Junshi or its Affiliates in connection with such activities, |
39
in each case ((a) and (b)), within 45 days after receiving Junshi’s invoice therefor and to the extent not otherwise reimbursed as part of any arrangement between the Parties involving Clinical Trials of Licensed Products, pursuant to Section 5.5 (Right of Reference), or the terms of the Pharmacovigilance Agreement.
ARTICLE 6
MANUFACTURING
6.1 | Manufacturing by Junshi. Prior to the completion of the Manufacturing Technology Transfer for a Licensed Antibody (and all Licensed Products that include such Licensed Antibody) pursuant to Section 2.6(b) (Manufacturing Technology Transfer), Junshi will, either by itself or through a CMO, Manufacture and supply the Licensed Products to facilitate continuous supply, sale, and registry of Licensed Products to Coherus and its Affiliates and Sublicensees in the Coherus Territory until such time as the applicable Coherus CMO is adequately Manufacturing such Licensed Products for use in the Coherus Territory. During such time, Coherus will purchase its requirements of such Licensed Products from Junshi for use by Coherus and its Affiliates and Sublicensees in the Coherus Territory pursuant to a supply agreement (the “Supply Agreement”) and a quality agreement (the “Quality Agreement”), each to be entered into between the Parties promptly after the Execution Date. The Supply Agreement will specify that Junshi will deliver the Licensed Products to Coherus EXW (Incoterms 2020). Neither Junshi nor its CMO may initiate any changes with respect to the Manufacture of any Licensed Product that could reasonably be expected to affect the quality, performance, or requirements of the FDA in any BLA, in each case, of such Licensed Product as Manufactured by Junshi or its CMO without the prior written consent of Coherus. The terms of the Supply Agreement and Quality Agreement will be consistent with the terms of this Agreement. Junshi will supply the Licensed Products in drug substance or drug product form, as requested by Coherus, in each case, pursuant to this Section 6.1 (Manufacturing by Junshi) at a transfer price equal to the Fully Burdened Manufacturing Costs plus 15% (the “Supply Price”) of Junshi. All Manufacturing activities by or on behalf of Junshi and its Affiliates will at all times be in compliance with cGMP and ICH Guidelines and in compliance with applicable law. |
6.2 | Manufacturing by Coherus. Following the completion of the Manufacturing Technology Transfer for a Licensed Antibody (and all Licensed Products that include such Licensed Antibody), Coherus will have sole control over and decision-making authority with respect to, through the applicable Coherus CMO, the Manufacture of such Licensed Products in the Coherus Territory for clinical and commercial uses in the Coherus Territory. If the Parties have completed a Manufacturing Technology Transfer for the Licensed Antibody then, upon request by Junshi, Coherus will Manufacture and supply to Junshi the applicable Licensed Antibody or Licensed Product at the Supply Price of Coherus. |
ARTICLE 7
COMMERCIALIZATION
7.1 | Commercialization Responsibilities. Coherus will have sole control over and decision-making authority with respect to the Commercialization of all Licensed Products in the Coherus Territory, including the right to determine the price of the Licensed Products sold in the Coherus Territory. |
7.2 | Commercialization Diligence Obligations. Coherus will use Commercially Reasonable Efforts to Commercialize the Licensed Products in each country in the Coherus Territory in which it has obtained Regulatory Approval (and, where applicable, Pricing and Reimbursement Approval) for such Licensed Products, at its sole cost and expense. |
40
7.3 | Commercialization Report. Following the date upon which the first Regulatory Approval for a Licensed Product in the Coherus Territory is granted (the “Date of First Regulatory Approval”), at each meeting of the JDC, Coherus will provide a report summarizing on a Licensed Product-by- Licensed Product and a country-by-country basis the Commercialization activities performed by or on behalf of Coherus and its Affiliates and Sublicensees in the Coherus Territory for the Licensed Products since the prior such report provided by Coherus or, in the case of the first such report, since the Date of First Regulatory Approval. Such reports will be Confidential Information of Coherus and subject to the terms of Article 12 (Confidentiality). Coherus will provide updates to any such report at each meeting of the JDC. |
7.4 | Diversion. Each Party agrees that it will not, and will ensure that its Affiliates and Sublicensees and subcontractors will not, either directly or indirectly, promote, market, distribute, import, sell, or have sold any Licensed Products to any Third Party or to any address or Internet Protocol address or the like in the other Party’s territory, including via the Internet or mail order. Neither Party will engage, nor permit its Affiliates or Sublicensees to engage, in any advertising or promotional activities relating to any Licensed Products for use directed primarily to customers or other buyers or users of the Licensed Products located in any country or jurisdiction in the other Party’s territory, or solicit orders from any prospective purchaser located in any country or jurisdiction in the other Party’s territory. If a Party or its Affiliates or Sublicensees receive any order for any Licensed Products from a prospective purchaser located in a country or jurisdiction in the other Party’s territory, then such Party will immediately refer that order to such other Party and will not accept any such orders. Neither Party will, nor permit its Affiliates or Sublicensees to, deliver or tender (or cause to be delivered or tendered) any Licensed Products to Third Parties for use in the other Party’s territory except in accordance with a Joint Development Plan, to fulfill an obligation under this Agreement or the Supply Agreement to the other Party, or to perform a Junshi Clinical Trial in accordance with this Agreement, or except in connection with a Manufacturing Technology Transfer pursuant to Section 2.6(b) (Manufacturing Technology Transfer). Notwithstanding any provision to the contrary set forth in this Agreement, each Party will have the right to attend conferences and meetings of congresses in the other Party’s territory and to promote and market the Licensed Products to Third Party attendees at such conferences and meetings, subject to this Section 7.4 (Diversion). Each Party will have the right to engage key opinion leaders from outside its territory and to participate in education, advisory, and other activities relating to Licensed Products in the other Party’s territory. |
ARTICLE 8
FINANCIALS
8.1 | Upfront Payment. No later than 20 days after the Effective Date, Coherus will pay a one-time, non-refundable payment in the amount of $150,000,000 (the “Upfront Payment”). |
8.2 | Option Exercise Payment. No later than 10 days after Coherus’ delivery of the Option Exercise Notice for a given License Option for an Option Program, Coherus will pay to Junshi a one-time, non-refundable payment in the amount of $35,000,000. |
8.3 | Regulatory and Sales Milestone Payments. |
(a) | For the PD1 Program. Coherus will make the one-time milestone payments set forth in Table 8.3(a) upon the first achievement by Coherus or its Affiliates or Sublicensees of the corresponding milestone event by the first Licensed Product that contains a Licensed Antibody described in clause (a) of the definition of Licensed Antibody: |
41
Table 8.3(a) – Regulatory and Sales Milestones for the PD1 Program | ||
No. | Milestone Event | Milestone |
1 | Receipt of the first Regulatory Approval from the FDA for a Licensed Product that contains a Licensed Antibody described in clause (a) of the definition of Licensed Antibody in either Urothelial Carcinoma or Esophagus Carcinoma | $25,000,000 |
2 | Receipt of the first Regulatory Approval from the FDA for a Licensed Product that contains a Licensed Antibody described in clause (a) of the definition of Licensed Antibody in Nasopharyngeal Cancer | $25,000,000 |
3 | Receipt of the first Regulatory Approval from the FDA for a Licensed Product that contains a Licensed Antibody described in clause (a) of the definition of Licensed Antibody in Non Small Cell Lung Cancer | $40,000,000 |
4 | Annual Net Sales for Licensed Products that contain a Licensed Antibody described in clause (a) of the definition of Licensed Antibody meet or exceed $250,000,000 | $30,000,000 |
5 | Annual Net Sales of Licensed Products that contain a Licensed Antibody described in clause (a) of the definition of Licensed Antibody meet or exceed $500,000,000 | $50,000,000 |
6 | Annual Net Sales of Licensed Products that contain a Licensed Antibody described in clause (a) of the definition of Licensed Antibody meet or exceed $1,000,000,000 | $90,000,000 |
7 | Annual Net Sales for Licensed Products that contain a Licensed Antibody described in clause (a) of the definition of Licensed Antibody meet or exceed $1,500,000,000 | $120,000,000 |
(b) | For each Option Program. Separately, for each of (i) the Junshi IL-2 Molecules and (ii) the Junshi TIGIT Antibodies for which Coherus exercises the License Option, Coherus will make the one-time milestone payments set forth in Table 8.3(b) upon the first achievement by Coherus or its Affiliates or Sublicensees of the corresponding milestone event by the first Licensed Product that contains the subject Option Molecule: |
Table 8.3(b) – Regulatory and Sales Milestones for each Option Program | ||
No. | Milestone Event | Milestone |
1 | Initiation of the first Pivotal Trial for a Licensed Product that contains an Option Molecule | $20,000,000 |
2 | Receipt of the first Regulatory Approval from the FDA for an Optioned Licensed Product that contains an Option Molecule, which Regulatory Approval is not for Non Small Cell Lung Cancer | $25,000,000 |
3 | Receipt of the first Regulatory Approval from the FDA for an Optioned Licensed Product that contains an Option Molecule in Non Small Cell Lung Cancer | $40,000,000 |
4 | Annual Net Sales of Optioned Licensed Products meet or exceed $250,000,000 | $30,000,000 |
5 | Annual Net Sales of Optioned Licensed Products meet or exceed $500,000,000 | $50,000,000 |
6 | Annual Net Sales of Optioned Licensed Products meet or exceed $1,000,000,000 | $90,000,000 |
42
(c) | Each milestone payment under this Section 8.3 (Regulatory and Sales Milestone Payments) is payable only once for the PD-1 Program, once for the TIGIT Program, and once for the IL-2 Program, upon the first achievement by Coherus or its Affiliate or Sublicensee for a Licensed Product in the Coherus Territory that is the subject of each such program, notwithstanding whether a Licensed Product achieves the milestone event more than once for a given program or whether more than one Licensed Product that is the subject of a given program achieves a milestone event. |
Coherus will provide Junshi with written notice of the achievement of the milestone event in this Section 8.3 (Regulatory and Sales Milestone Payments) no later than 15 Business Days after the achievement of the applicable milestone event by Coherus or any of its Affiliates or Sublicensees. Junshi will invoice Coherus following receipt of such written notice as soon as reasonably practicable and Coherus will pay the associated milestone payment no later than 45 days after the receipt of such invoice. Such payment will be made by wire transfer of immediately available funds into an account designated by Junshi.
8.4 | Royalties. |
(a) | Royalty Rates. During the Royalty Term for a given Licensed Product in a country in the Coherus Territory, on a Licensed Product-by-Licensed Product and country-by-country basis, Coherus will pay Junshi royalties on Net Sales of a given Licensed Product in the Coherus Territory at a royalty rate of (i) 20% with respect to any Licensed Product that is the subject of the PD-1 Program and (ii) 18% with respect to any Licensed Product that is the subject of an Option Program (“Royalties”). |
(b) | Reports; Payment. Coherus will deliver to Junshi a calculation of the Royalties due in a given Calendar Quarter no later than 30 days after the end of each Calendar Quarter, provided that the Royalty calculation for the third month of such Calendar Quarter will be a non-binding good faith estimate (each, a “Royalty Report”). All royalty payments will be payable no later than 45 days after the end of each Calendar Quarter. All payments under this Agreement will be payable, in full, in U.S. dollars, regardless of the country(ies) in which such sales are made. If any Licensed Product is sold in a currency other than United States dollars, the report should list the Net Sales for such Licensed Product in both the original currency and in United States dollars. For purposes of computing the Royalty, the original such currency will be converted into United States dollars at the median of the buying rate and the selling rate of exchange reported by the Wall Street Journal on the last day for the month in which such sales were recorded. |
(c) | Reductions. |
(i) | Royalty Reduction upon Expiration of Valid Claims. If, on a Licensed Product- by-Licensed Product and country-by-country basis, any Royalties are payable pursuant to Section 8.4(a) (Royalty Rates) on Net Sales of a Licensed Product attributable to a country in the Coherus Territory in which there is no Valid Claim within the Licensed Patent Rights Covering the composition of matter, |
43
formulation, or approved method of treatment or use of such Licensed Product that would be infringed by the manufacture, use, sale, offer for sale, or import of such Licensed Product in such country, then the applicable royalty rate applicable to those Net Sales of such Licensed Product for such country will be reduced by 25%.
(ii) | Reduction for Loss of Regulatory Exclusivity. If, on a Licensed Product-by- Licensed Product and country-by-country basis, any Royalties are payable pursuant to Section 8.4(a) (Royalty Rates) on Net Sales of a Licensed Product attributable to a country in the Coherus Territory in which there is both (A) no Valid Claim within the Licensed Patent Rights Covering the composition of matter, formulation, or approved method of treatment or use of such Licensed Product that would be infringed by the manufacture, use, sale, offer for sale, or import of such Licensed Product in such country, and (B) no Regulatory Exclusivity for such Licensed Product in such country, then the applicable royalty rate applicable to those Net Sales of such Licensed Product for such country will be reduced by 50%. |
(iii) | Reduction for Additional Third Party IP. With respect to any Additional Third Party License pursuant to which Coherus is granted rights under any Additional Third Party IP that is necessary to Exploit a Licensed Product in a country or jurisdiction in the Coherus Territory, Coherus will be entitled to deduct from any Royalties payable hereunder with respect to that country or other jurisdiction 50% of royalties paid to such Third Party under such Additional Third Party License to the extent allocable or specific to the Licensed Products; provided that in no event will the Royalties due to Junshi under this Agreement in a Calendar Quarter be reduced by more than 30% of the Royalties that would otherwise be due in such Calendar Quarter for the Licensed Products as a result of the foregoing reductions. However, if Coherus is unable to fully offset against Royalties paid to any Third Party pursuant to an Additional Third Party License in consideration for rights with respect to Additional Third Party IP as permitted under this Section 8.4(c)(iii) (Reduction for Additional Third Party IP) on account of the foregoing 30% floor, then any such permitted deductions may be carried forward to reduce subsequent Calendar Quarter in which Royalties are due (subject to the same floor in such future periods). |
8.5 | Existing License Agreements. |
(a) | JS001 Upstream License. Junshi will be solely responsible for all upfront payments, milestone payments, royalties, or other payments due to the licensor(s) under the JS001 Upstream License (collectively, “JS001 Upstream License Costs”), provided that, to the extent that Coherus does not use Lonza as the Coherus CMO to Manufacture Licensed Antibodies and Licensed Products for Coherus, Coherus will be responsible for all amounts owed by Junshi to Lonza for such Licensed Antibodies and Licensed Products. Coherus will pay to Junshi any amounts due thereunder for a Calendar Quarter within 45 days after the end of the Calendar Quarter in which such payments accrue. Without limiting the foregoing, if (i) Junshi fails to pay any JS001 Upstream License Costs or otherwise fails to maintain the JS001 Upstream License as required by Section 10.3(e) (Control of Upstream Licenses), (ii) Coherus has paid to Junshi all amounts due from Coherus for JS001 Upstream License Costs as a result of Coherus’ selection of the Coherus CMO other than Lonza, and (iii) Coherus pays such JS001 Upstream License Costs on Junshi’s behalf or otherwise incurs any costs in connection with maintaining the JS001 Upstream License, including costs associated with securing and maintaining a replacement agreement if Junshi allows the JS001 Upstream License to terminate or expire in violation of Section 10.3(e) |
44
(Control of Upstream Licenses), then Coherus will be entitled to offset any such costs from any payments due to Junshi hereunder.
(b) | JS018-1 Upstream License. Junshi will be solely responsible for all upfront payments, milestone payments, royalties, or other payments due to the licensor(s) under the JS018-1 Upstream License (collectively, “JS018-1 Upstream License Costs”). Without limiting the foregoing, if (i) Junshi fails to pay any JS018-1 Upstream License Costs or otherwise fails to maintain the JS018-1 Upstream License as required by Section 10.3(e) (Control of Licensed Technology), and (ii) Coherus pays such JS018-1 Upstream License Costs on Junshi’s behalf or otherwise incurs any costs in connection with maintaining the JS018-1 Upstream License, including costs associated with securing and maintaining a replacement agreement if Junshi allows the JS018-1 Upstream License to terminate or expire in violation of Section 10.3(e) (Control of Licensed Technology), then Coherus will be entitled to offset any such costs from any payments due to Junshi hereunder. |
8.6 | Books and Records; Audit Rights. |
(a) | Junshi will have the right to engage, at its own cost and expense, subject to this Section 8.6 (Books and Records; Audit Rights), an independent nationally recognized public accounting firm in the United States chosen by Junshi and reasonably acceptable to Coherus (which accounting firm will not be the external auditor of Junshi, will not have been hired or paid on a contingency basis, and will have experience auditing pharmaceutical companies) (a “CPA Firm”) to conduct an audit of Coherus for the purposes of confirming Coherus’ compliance with the payment provisions of this Agreement. |
(b) | The CPA Firm will be given access to and will be permitted to examine such books and records of Coherus as it will reasonably request, upon 30 days’ prior written notice having been given by Junshi, during regular business hours, for the sole purpose of determining compliance with the payment provisions of this Agreement. Prior to any such examination taking place, the CPA Firm will enter into a confidentiality agreement reasonably acceptable to Coherus with respect to the Know-How to which they are given access and will not contain in its report or otherwise disclose to Junshi or any Third Party any information labeled by Coherus as being confidential customer information regarding pricing or other competitively sensitive proprietary information. |
(c) | Junshi and Coherus will be entitled to receive a full written report of the CPA Firm with respect to its findings and Junshi will provide, without condition or qualification, Coherus with a copy of the report, or other summary of findings, prepared by such CPA Firm promptly following Junshi’s receipt of same. In the event of any dispute between Junshi and Coherus regarding the findings of any such inspection or audit, the Parties will initially attempt in good faith to resolve the dispute amicably between themselves, and if the Parties are unable to resolve such dispute within 30 days after delivery to both Parties of the CPA Firm’s report, each Party will select an internationally recognized independent certified public accounting firm (other than the CPA Firm), and the two firms chosen by the Parties will choose a third internationally recognized independent certified public accounting firm which will resolve the dispute, and such accounting firm’s determination will be binding on both Parties, absent manifest error by such accounting firm. |
(d) | Within 45 days after completion of the CPA Firm’s audit, Coherus will pay to Junshi any deficiency in the payment amount determined by the CPA Firm plus interest pursuant to Section 8.8 (Late Payments). If the report of the CPA Firm shows that Coherus underpaid Junshi by more than 7.5% of the amount due for any Calendar Quarter and such amount |
45
was more than $50,000, then Coherus must pay for the amount charged by the CPA Firm to conduct such audit together with the amount of such deficiency and interest. If the report of the CPA Firm shows that Coherus overpaid, then Coherus will be entitled to off-set such overpayment against any Royalty then owed to Junshi. If no royalty is then owed to Junshi, then Junshi will remit such overpayment to Coherus.
(e) | Junshi’s exercise of its audit rights under this Section 8.6 (Books and Records; Audit Rights) may not (i) be conducted for any Calendar Quarter more than three years after the end of such Calendar Quarter to which such books and records pertain, (ii) be conducted more than once in any 12 month period (unless a previous audit during such 12 month period revealed a material underpayment with respect to such period), or (iii) be repeated for any Calendar Quarter previously audited. |
(f) | Coherus will ensure that it has the right to engage a CPA Firm to conduct an audit of Sublicensees for the purposes of confirming Coherus’ ability to pay amounts due to Coherus under this Agreement based upon activities of the Sublicensees and Sublicensee’s compliance with the terms of this Agreement. Coherus will provide, without condition or qualification, to Junshi with a copy of the report, or other summary of findings (redacted for any materials unrelated to the confirming Coherus’ compliance with the payment provisions of this Agreement), prepared by any such CPA Firm in connection with an audit of a Sublicensee promptly following Coherus’ receipt of same. |
8.7 | Taxes. |
(a) | Taxes on Income. Except as set forth in this Section 8.7 (Taxes), each Party will be solely responsible for and bear all taxes imposed on it under applicable laws in connection with the implementation of this Agreement. |
(b) | Tax Withholding. If applicable laws require a paying Party (“Payor”) to withhold any tax from any payment due to the other Party (“Payee”) under this Agreement (taking into account any legally available reduction or elimination of such tax pursuant to an applicable tax treaty or otherwise), then the Payor will subtract the amount thereof from the payments to the Payee, and pay such amount to the proper taxing authority. The Payor will promptly (as available) submit to the Payee appropriate proof of payment of the withheld taxes as well as the official receipts within a reasonable period of time. The Payor will provide the Payee reasonable assistance in order to allow the Payee to obtain the benefit of any present or future treaty against double taxation or refund or reduction in taxes that may apply to the payments under this Agreement. Without limiting the generality of the foregoing, if the Payee is entitled under any applicable tax treaty to a reduction of rate of, or the elimination of, or recovery of, applicable withholding taxes, it may deliver to the Payor or the appropriate governmental authority the prescribed forms necessary to reduce the applicable rate of withholding or to relieve the Payor of its obligation to withhold taxes. In such case, the Payor will apply the reduced rate of withholding, or not withhold, as the case may be, provided that the Payor is in receipt of evidence, in a form reasonably satisfactory to the Payor (e.g., the Payee’s delivery of all applicable documentation) prior to the time that the applicable payments are due. |
(c) | VAT in Coherus Territory. Notwithstanding Section 8.7(a) (Taxes on Income) and Section 8.7(b) (Tax Withholding), and subject to the following sentence, all sums payable from Coherus to Junshi under this Agreement will be exclusive of sales, use, turnover, value added taxes or similar taxes, including any applicable surcharges on value added |
46
taxes or similar taxes (“VAT”). As between the Parties, Junshi will pay for and bear any VAT applicable to any payment by Coherus to Junshi under this Agreement imposed by any Chinese (central, provincial, or municipal) tax authority. Coherus will pay for and bear any VAT applicable to any payment by Coherus to Junshi under this Agreement imposed by any non-Chinese tax authority.
(d) | Tax Cooperation. Each Party will provide the other with reasonable assistance to enable the recovery of or exemption from, as permitted by applicable law, withholding taxes, VAT, or similar obligations resulting from payments made under this Agreement, such recovery or exemption to be for the benefit of the Party bearing such withholding tax or VAT. |
8.8 | Late Payments. Any payments or portions thereof due hereunder that are not paid on the date such payments are due under this Agreement will bear interest at a rate equal to the lesser of: (a) two percentage points above the prime rate as published by The Wall Street Journal or any successor thereto on the first day of each Calendar Quarter in which such payments are overdue; or (b) the maximum rate permitted by applicable law; in each case, calculated on the number of days such payment is delinquent, compounded monthly. |
8.9 | No Other Compensation. Other than as explicitly set forth (and as applicable) in this Agreement, neither Coherus nor any of its Affiliates will be obligated to pay any additional fees, milestone payments, royalties or other payments of any kind to or on behalf of Junshi or any of its Affiliates under this Agreement. |
8.10 | Other Amounts Payable. With respect to any amounts owed under this Agreement by a Party to the other Party for which no other invoicing and payment procedure is specified in this Agreement, the payee Party will provide an invoice, together with reasonable supporting documentation, to the paying Party for such amounts owed. The paying Party will pay any undisputed amounts no later than 60 days after receipt of the invoice, and will pay any disputed amounts owed by the paying Party no later than 60 days after resolution of the dispute. |
ARTICLE 9
INTELLECTUAL PROPERTY
9.1 | Ownership. |
(a) | Background Technology. As between the Parties, and except with respect to any Arising Technology, which is addressed in Section 9.1(b) (Arising Technology), (i) Junshi will retain all rights, title, and interest in and to any Patent Rights, Know-How, and other intellectual property rights owned or Controlled by Junshi or any of its Affiliates as of the Effective Date or generated or obtained by or on behalf of Junshi or any of its Affiliates during the Term outside of the scope of performance of activities under this Agreement, and (ii) Coherus will retain all rights, title, and interest in and to any Patent Rights, Know- How, and other intellectual property rights owned or Controlled by Coherus or any of its Affiliates as of the Effective Date or generated or obtained by or on behalf of Coherus or any of its Affiliates during the Term outside of the scope of performance of activities under this Agreement. |
(b) | Arising Technology. |
(i) | As between the Parties, ownership will follow inventorship for (A) any and all Know-How developed, created, conceived, or reduced to practice during the Term solely by or on behalf of a Party or any of its Affiliates in a Party’s performance |
47
of activities under this Agreement or Sublicensee’s performance of activities under a sublicense (“Arising Know-How”) and (B) any Patent Right claiming any such Know-How described in clause (A) (the “Arising Patent Rights” and the Arising Know-How and Arising Patent Rights, the “Arising Technology”), with inventorship being determined in accordance with United States patent laws (regardless of where the applicable activities occurred). Arising Know-How invented solely by or on behalf of Junshi or any of its Affiliates, and all Arising Patent Rights claiming any such Arising Know-How (the “Junshi Arising Patent Rights”) will be solely owned by Junshi or any of its Affiliates (“Junshi Arising Technology”). As between the Parties, Arising Technology invented solely by or on behalf of Coherus or any of its Affiliates or Sublicensees, and all Arising Patent Rights claiming any such Arising Know-How (the “Coherus Arising Patent Rights”) will be solely owned by Coherus or any of its Affiliates (“Coherus Arising Technology”). As between the Parties, Arising Technology invented jointly by Junshi or any of its Affiliates and Coherus or any of its Affiliates or Sublicensees, and all Arising Patent Rights claiming any such Arising Know-How (the “Joint Arising Patent Rights”) will be jointly owned by both Parties (“Joint Arising Technology”).
(ii) | Junshi will promptly disclose to Coherus any Junshi Arising Technology or Joint Arising Technology, as applicable, developed, created, conceived, or reduced to practice by or on behalf of Junshi or any of its Affiliates during the Term. Coherus will promptly disclose to Junshi any Coherus Arising Technology or Joint Arising Technology, as applicable, developed, created, conceived, or reduced to practice by or on behalf of Coherus or any of its Affiliates during the Term. |
(iii) | Each Party will have an undivided one-half (1/2) interest in and to the Joint Arising Technology. Each Party will exercise its ownership rights in and to such Joint Arising Technology, including the right to license and sublicense or otherwise to exploit, transfer, or encumber its ownership interest, without an accounting or obligation to, or consent required from, the other Party, but subject to the licenses hereunder and the other relevant terms and conditions of this Agreement. At the reasonable written request of a Party, the other Party will in writing grant such consents and confirm that no such accounting is required to effect the foregoing regarding Joint Arising Technology. Each Party, for itself and on behalf of any of its Affiliates, licensees, and Sublicensees, and employees, subcontractors, consultants, and agents of any of the foregoing, hereby assigns (and to the extent such assignment can only be made in the future hereby agrees to assign), to the other Party a joint and undivided interest in and to all Joint Arising Technology. |
(c) | Notwithstanding any provision to the contrary set forth in this Agreement, neither Party may invoke this Agreement as a “joint research agreement” pursuant to the Cooperative Research and Technology Enhancement Act, 35 U.S.C. § 102(c) without the prior written consent of the other Party. |
9.2 | Prosecution and Maintenance. |
(a) | Coherus Arising Patent Rights. Coherus will have the sole right and discretion to file, prosecute, and maintain all Coherus Arising Patent Rights at its sole cost and expense. |
(b) | Junshi Arising Patent Rights. Subject to Section 9.2(d) (Licensed Patent Rights), Junshi will have the sole right and discretion to file, prosecute, and maintain all Junshi Arising Patent Rights that are not Licensed Patent Rights at its sole cost and expense. |
48
(c) | Joint Arising Patent Rights. Subject to Section 9.3(c) (Patent Proceedings for Joint Arising Patent Rights), Coherus will have the first right, responsibility, and discretion to file, prosecute, and maintain all Joint Arising Patent Rights in the Coherus Territory at its sole cost and expense. Junshi will have the first right, responsibility, and discretion to file, prosecute, and maintain all Joint Arising Patent Rights in the Junshi Territory at its sole cost and expense. The Parties will use good faith efforts to agree on a mutually acceptable strategy and will coordinate with each other for the prosecution and maintenance of all Joint Arising Patent Rights. If the prosecuting Party decides it is no longer interested in the prosecution or maintenance of a particular Patent Right pertaining to the Joint Arising Technology in its territory, then it will promptly provide written notice to the other Party of such decision. The other Party may, upon written notice to the prosecuting Party, assume the prosecution and maintenance of such Patent Right in the applicable territory. |
(d) | Licensed Patent Rights. |
(i) | Junshi Right to Control Prosecution and Maintenance. Subject to Section 9.3(d) (Patent Proceedings for Licensed Patent Rights), from and after the Effective Date, Junshi will have the first right, responsibility, and discretion to file, prosecute, and maintain all Licensed Patent Rights (other than Joint Arising Patent Rights), including all Junshi Arising Patent Rights in (and outside) the Coherus Territory, at Junshi’s cost and expense, as well as, subject to Section 9.7(a) (Patent Right Term Extension), filing for any patent term extensions or similar protections in (and outside) the Coherus Territory. |
(ii) | Abandonment. Junshi will not abandon any issued Licensed Patent Rights in the Coherus Territory without first offering to Coherus the right to perform the activities described in clause (i) above at the discretion and sole cost and expense of Coherus. In the event Coherus assumes control of the prosecution and maintenance in the Coherus Territory of any Licensed Patent Right, then Junshi will (A) provide Coherus with copies of any relevant communications, filings, drafts, and documents, as well as written notice of any pending deadlines or communications applicable thereto, and (B) execute and deliver any legal papers reasonably requested by Coherus to effectuate transfer of control of the filing, prosecution, and maintenance of such Licensed Patent Rights (including papers that transfer any right, title or interest in or to the Licensed Patent Rights to Coherus). |
(iii) | Cooperation. Each Party will reasonably cooperate with the other Party in the filing, prosecution, and maintenance of the Licensed Patent Rights in the Coherus Territory, including with respect to any appeal of any ex parte patent prosecution decision to a court or board of a patent office. Such cooperation includes each Party providing to the other Party copies of substantive correspondence received from any patent office in the Coherus Territory, each Party providing to the other Party with drafts of proposed substantive filings with any such patent office reasonably in advance of the date due, each Party providing prompt feedback to any such proposed filings, and each Party giving reasonable and good faith consideration to any requests of the other Party with respect to such filings provided in a timely manner, provided that Junshi will incorporate any reasonable feedback provided to it by Coherus with respect to such filings. |
9.3 | Patent Proceedings. |
(a) | Patent Proceedings for Coherus Arising Patent Rights. Coherus will have the first right and discretion to institute, defend, and conduct all Patent Proceedings for the Coherus |
49
Arising Patent Rights at its sole cost and expense. If Coherus decides it is no longer interested in controlling a Patent Proceeding pertaining to the Coherus Arising Patent Rights, then it will promptly provide written notice to Junshi of such decision. Junshi may, upon written notice to Coherus, assume control of such Patent Proceeding.
(b) | Patent Proceedings for Junshi Arising Patent Rights. Junshi will have the sole right and discretion to control, at its sole cost and expense, the institution, defense, and conduct of all Patent Proceedings for the Junshi Arising Patent Rights that are not Licensed Patent Rights. |
(c) | Patent Proceedings for Joint Arising Patent Rights. Coherus will have the first right and discretion to control, at its sole cost and expense, the institution, defense, and conduct of all Patent Proceedings for all Joint Arising Patent Rights in the Coherus Territory at its sole cost and expense. Junshi will have the first right and discretion to control, at its sole cost and expense, the institution, defense, and conduct of all Patent Proceedings for all Joint Arising Patent Rights in the Junshi Territory at its sole cost and expense. The Parties will use good faith efforts to agree on a mutually acceptable strategy and will coordinate with each other for the conduct of Patent Proceedings for all Joint Arising Patent Rights. If the controlling Party decides it is no longer interested in controlling a Patent Proceeding pertaining to the Joint Arising Technology in its territory, then it will promptly provide written notice to the other Party of such decision. The other Party may, upon written notice to the controlling Party, assume control of such Patent Proceeding in the applicable territory. Neither Party will voluntarily abandon or admit that any claim of any Joint Arising Patent Right involved in a Patent Proceeding is unpatentable, invalid, or unenforceable without the advance, written consent of the other Party (not to be unreasonably withheld). |
(d) | Patent Proceedings for Licensed Patent Rights. From and after the Effective Date, Coherus will have the first right and discretion to control, at its sole cost and expense, the institution, defense, and conduct of all Patent Proceedings for the Licensed Patent Rights within the Coherus Territory. If Coherus decides it is no longer interested in controlling a Patent Proceeding pertaining to Licensed Patent Rights, then it will promptly provide written notice to Junshi of such decision. Junshi may, upon written notice to Coherus, assume control of such Patent Proceeding. Junshi will have the sole right, responsibility and discretion to control, at its sole cost and expense, the institution, defense, and conduct of all Patent Proceedings for the Licensed Patent Rights within the Junshi Territory. Coherus will not voluntarily abandon or admit that any claim of any Licensed Patent Rights involved in a Patent Proceeding is unpatentable, invalid or unenforceable without the advance, written consent of Junshi (not to be unreasonably withheld). |
(e) | Cooperation. Each Party will reasonably cooperate with the other Party in the institution, defense, and conduct of any Patent Proceeding within the Coherus Territory. Such cooperation includes each Party providing to the other Party copies of substantive correspondence related to such Patent Proceeding, each Party providing to the other Party with drafts of proposed substantive filings for such Patent Proceeding reasonably in advance of the date due, each Party providing prompt feedback to any such proposed filings, and each Party giving reasonable and good faith consideration to any requests of the other Party with respect to such filings provided in a timely manner. |
9.4 | Defense and Settlement of Third Party Claims. From and after the Effective Date, if a Third Party asserts that a Patent Right or other right owned by it is infringed by the Exploitation of any Licensed Antibody or Licensed Product in the Field in the Coherus Territory, then Coherus will have the sole right to defend against any such assertions at Coherus’ sole cost or elect to settle such |
50
claims (except as set forth below). Junshi or any of its Affiliates will assist Coherus and cooperate in any such litigation at Junshi’s request. Junshi may join any defense pursuant to this Section 9.4 (Defense and Settlement of Third Party Claims), with its own counsel, at its sole cost and expense. Coherus or any of its Affiliates may settle or consent to the entry of any judgment in any enforcement action hereunder without Junshi’s prior consent; provided, however, that any such settlement or consent judgment will not, without the prior written consent of Junshi (such consent not to be unreasonably withheld, conditioned or delayed), impose any liability or obligation on Junshi or any of its Affiliates. Each Party will give the other Party prompt written notice of any allegation by any Third Party that a Patent Right or other right owned by it is infringed by the Exploitation of any Licensed Antibody or Licensed Product in the Coherus Territory.
9.5 | Enforcement. |
(a) | Enforcement and Cooperation. If in the Coherus Territory, (i) Junshi or Coherus becomes aware of any actual or suspected infringement of any Licensed Patent Right or Joint Arising Patent Right, or (ii) any such Licensed Patent Right or Joint Arising Patent Right is challenged in any action or proceeding (other than any Patent Proceeding, which is addressed in Section 9.3 (Patent Proceedings)), then such Party will notify the other Party promptly, and following such notification, the Parties will confer. Coherus will have the first right, but will not be obligated, to defend any such action or proceeding in the Coherus Territory or bring an infringement action with respect to such infringement in the Coherus Territory at its own expense, in its own name, and entirely under its own direction and control, or settle any such action or proceeding by sublicense (including, at Coherus’ sole discretion, granting a sublicense, covenant not to sue or other right with respect to an Antibody or product in the Field in the Coherus Territory in accordance with Section 2.2 (Sublicensing)). If Coherus fails to defend such action, abate such infringement, or file an action to abate such infringement in the Coherus Territory within 180 days after a written request from the Junshi to do so, or if Coherus discontinues the prosecution of any such action after filing without abating such infringement, then Junshi will have the second right, but will not be obligated, to defend any such action or proceeding in the Coherus Territory or bring an infringement action with respect to such infringement in the Coherus Territory at its own expense, in its own name and under its own direction and control. Regardless of which Party exercises its right under this Section 9.5(a) (Enforcement and Cooperation), the other Party and its Affiliates will reasonably assist such enforcing Party in any action or proceeding being defended or prosecuted if so requested, and will agree to be named in or join such action or proceeding if requested by such enforcing Party and necessary for standing under the applicable rules of procedure. If the other Party elects to be represented by legal counsel, then the enforcing Party will bear all of such Party’s related and reasonable legal costs and expenses if the other Party is required to be named in or joined in such action or proceeding or is joined in such action or proceeding at the enforcing Party’s request. |
(b) | Damages. In the event that either Party exercises the rights conferred in this Section 9.5 (Enforcement) and recovers any damages, payments, or other sums in such action or proceeding or in settlement thereof, then such damages or other sums recovered will first be applied to all out-of-pocket costs and expenses incurred by such enforcing Party in connection therewith (including attorney’s fees). If such recovery is insufficient to cover all such costs and expenses of both Parties, then the enforcing Party’s costs will be paid in full first before any of the other Party’s costs. If after such reimbursement any funds will remain from such damages or other sums recovered and Coherus is the enforcing Party, then Junshi will be entitled to 40% of such funds and Coherus may retain the remainder. If after such reimbursement any funds will remain from such damages or other sums |
51
recovered and Junshi is the enforcing Party, then Junshi will be entitled to retain 50% of such funds and Coherus may retain the remainder
9.6 | Trademarks. The Parties may develop and adopt certain distinctive colors, logos, images, symbols, and trademarks to be used in connection with the Commercialization of the Licensed Products on a global basis (such branding elements, collectively, the “Global Brand Elements”). Each Party will have the right to brand the Licensed Products in its respective territory using trademarks, logos, and trade names that it determines appropriate, which may vary by region or within a region, and that are consistent with the Global Brand Elements (the “Product Marks”). Each Party will solely own all rights, title, and interest in and to any Product Marks adopted for use with the Licensed Products in its respective territory, and will be responsible for the registration, filing, maintenance, and enforcement thereof at its own cost and expense. |
9.7 | Patent Right Extensions; Regulatory Exclusivity. |
(a) | Patent Right Term Extension. If elections with respect to obtaining patent term extension or supplemental protection certificates or their equivalents in any country in the Coherus Territory with respect to any Licensed Product becomes available, upon Regulatory Approval or otherwise, then, following discussions with Coherus and consideration in good faith of Coherus’ comments thereon, Junshi will have the sole right to file for patent term extension or supplemental protection certificates or their equivalents and to determine which issued patent to extend. Coherus and its Affiliates and Sublicensees will reasonably cooperate with Junshi so as to enable Junshi to exercise its rights under this Section 9.7(a) (Patent Right Term Extension). Such cooperation includes promptly executing all documents, requiring inventors to be available to discuss and review any filings, and requiring inventors, subcontractors, employees, consultants, and agents of Coherus or any of its Affiliates or Sublicensees to execute all documents, as reasonable and appropriate so as to enable Junshi to exercise its rights under this Section 9.7(a) (Patent Right Term Extension). |
(b) | Regulatory Exclusivity. With respect to regulatory exclusivity periods (such as orphan drug exclusivity and any available pediatric extensions), Coherus will have the sole right to seek and maintain all such regulatory exclusivity periods that may be available for the Licensed Products in the Field in the Coherus Territory. |
ARTICLE 10
REPRESENTATIONS, WARRANTIES, AND COVENANTS
10.1 | Mutual Representations, Warranties, and Covenants. Each Party hereby represents and warrants to the other Party as of the Execution Date and the Effective Date, and covenants, as applicable, as a material inducement for such other Party’s entry into this Agreement, as follows: |
(a) | Corporate Existence and Power. It is a company or corporation duly organized, validly existing, and in good standing under the laws of the jurisdiction in which it is incorporated, and has full corporate power and authority and the legal right to own and operate its property and assets and to carry on its business as it is now being conducted and as contemplated in this Agreement, including the right to grant the licenses granted by it hereunder. |
(b) | Authority and Binding Agreement. (i) It has the corporate power and authority and the legal right to enter into this Agreement and perform its obligations hereunder; (ii) it has taken all necessary corporate action on its part required to authorize the execution and delivery of this Agreement and the performance of its obligations hereunder; and (iii) this Agreement has been duly executed and delivered on behalf of such Party, and constitutes |
52
a legal, valid, and binding obligation of such Party that is enforceable against it in accordance with its terms.
(c) | No Conflict. It is not a party to and will not enter into any agreement that would prevent it from granting the rights or exclusivity granted or intended to be granted to the other Party under this Agreement or performing its obligations under this Agreement. |
(d) | Consents. All consents, approvals, and authorizations from all governmental authorities or other Third Parties required to be obtained by such Party in connection with this Agreement have been obtained, other than, with respect to Junshi, from Lonza Sales AG pursuant to the JS001 Upstream License to grant the licenses to Coherus under Section 2.1 (Licenses to Coherus). |
(e) | Bankruptcy; Insolvency. It and its Affiliates are not subject to any action or petition, pending or otherwise, for bankruptcy or insolvency in any state, country, or other jurisdiction, and it is not aware of any facts or circumstances that could result in such Party or any of its Affiliates becoming or being declared insolvent, bankrupt, or otherwise incapable of meeting its obligations under this Agreement as they become due in the ordinary course of business. |
(f) | No Debarment. Neither it nor any of its employees nor to its knowledge, any of the agents performing hereunder, has ever been, is currently, or is the subject of a proceeding that could lead to it or such employees or agents becoming, as applicable, a Debarred Entity or Debarred Individual, an Excluded Entity, or Excluded Individual or a Convicted Entity or Convicted Individual. For purposes of this Agreement, the following definitions will apply: |
(i) | A “Debarred Individual” is an individual who has been debarred by the FDA pursuant to 21 U.S.C. §335a (a) or (b) from providing services in any capacity to a person that has an approved or pending drug or biological product application. |
(ii) | A “Debarred Entity” is a corporation, partnership or association that has been debarred by the FDA pursuant to 21 U.S.C. §335a (a) or (b) from submitting or assisting in the submission of any abbreviated drug application, or a subsidiary or Affiliate of a Debarred Entity. |
(iii) | An “Excluded Individual” or “Excluded Entity” is (A) an individual or entity, as applicable, who has been excluded, debarred, suspended or is otherwise ineligible to participate in federal health care programs such as Medicare or Medicaid by the Office of the Inspector General (OIG/HHS) of the U.S. Department of Health and Human Services, or (B) is an individual or entity, as applicable, who has been excluded, debarred, suspended or is otherwise ineligible to participate in federal procurement and non-procurement programs, including those produced by the U.S. General Services Administration (GSA). |
(iv) | A “Convicted Individual” or “Convicted Entity” is an individual or entity, as applicable, who has been convicted of a criminal offense that falls within the ambit of 21 U.S.C. §335a (a) or 42 U.S.C. §1320a - 7(a), but has not yet been excluded, debarred, suspended, or otherwise declared ineligible. |
10.2 | Representations and Warranties by Junshi. Junshi further represents and warrants to Coherus (a) with respect to the content below applicable to the PD-1 Program or the Licensed Antibodies, Licensed Products, Licensed Patent Rights, and Licensed Technology (as each such term is defined on the Execution Date), as of the Execution Date and, if not specified as applicable “as of the Execution Date”, then again on the Effective Date), and (b) with respect to the content below |
53
applicable to an Option Program or its corresponding Option Molecule, Option Product, or Option Patent Rights, on an Option Program-by-Option Program basis, as of the Execution Date and, if not specified as applicable “as of the Execution Date”, then again on the date of delivery to Coherus of the Option Data Package for such Option Program in accordance with Section 2.8(b) (Option Data Package) (the “Option Data Package Delivery Date”), except as set forth in the disclosure letter delivered to Coherus on the Option Data Package Delivery Date (the “Option Disclosure Letter”), as follows:
(a) | No Conflicts. Neither Junshi nor any of its Affiliates has entered into any agreement (other than agreements with subcontractors) granting any right, interest or claim in or to, any Licensed Technology to any Third Party that would conflict with the licenses and other rights granted to Coherus under this Agreement. The Licensed Technology constitutes all intellectual property rights Controlled by Junshi and any of its Affiliates that are necessary or reasonably useful for the Exploitation of the Licensed Antibodies or Licensed Products in the Field in the Coherus Territory. All Licensed Patent Rights are solely owned by Junshi or any of its Affiliates free and clear of any liens, charges, security interests, encumbrances, licenses claims or covenants that would conflict with or limit the scope of any of the rights or licenses granted to Coherus hereunder or would give rise to any Third Party claims for payment against Coherus or any of its Affiliates. The Licensed Patent Rights that have issued are subsisting, and, to the knowledge of Junshi, enforceable and valid. |
(b) | No Notice of Infringement, Misappropriation or Invalidity. As of the Execution Date and the Option Package Delivery Date, as applicable: (i) neither Junshi nor any of its Affiliates have received or is aware of any written notice from any Third Party asserting or alleging that any Exploitation of any Licensed Technology, any Licensed Antibody, any Licensed Products, any Know-How or Patent Rights Controlled by Junshi or its Affiliates related to (with respect to such Know-How) or Covering (with respect to such Patent Rights) an Option Molecule, or Option Molecule, in each case, has infringed or misappropriated, or would infringe or misappropriate, the intellectual property rights of any Third Party, and (ii) no claim is pending, and Junshi and any of its Affiliates and, to Junshi’s knowledge, any Third Party collaborator, has not received from a Third Party notice of a claim or threatened claim to the effect that any granted Patent Right rights within the Licensed Technology licensed to Coherus under this Agreement or any Patent Rights Controlled by Junshi or its Affiliates that Cover an Option Molecule, in each case, is invalid or unenforceable. Additionally, as of the Execution Date, to Junshi’s knowledge, there is no unauthorized use, infringement or misappropriation by any Third Party of any Licensed Technology or any Know-How or Patent Rights Controlled by Junshi or its Affiliates related to (with respect to such Know-How) or Covering (with respect to such Patent Rights) an Option Molecule. Junshi has provided to Coherus a translated complete copy of all freedom to operate analyses and reports it has conducted as of the Execution Date with respect to the Licensed Technology, Licensed Antibody, Option Molecule, and Option Product. Except as Junshi has disclosed to Coherus pursuant to a letter from Junshi to Coherus dated 30 January 2021, as of the Execution Date, to Junshi’s knowledge, the Exploitation of the Licensed Products does not infringe, misappropriate, or otherwise violate the intellectual property rights of any Third Party. |
(c) | No Misappropriation. No employee, consultant, agent or independent contractor of Junshi, any of its Affiliates, or Third Party, has, to Junshi’s knowledge as of the Execution Date, (i) misappropriated any Licensed Know-How or (ii) misappropriated any Know-How related to any Option Molecule. |
54
(d) | Licensed Technology. All Patent Rights Controlled by Junshi or its Affiliates, and that are necessary or reasonably useful for the Exploitation of the Licensed Antibodies or Licensed Products in the Field are listed on Schedule 10.2(d) (Existing Patent Rights) as of the Execution Date. As of the Execution Date, the Effective Date, and the Option Package Delivery Date, all Patent Rights listed on Schedule 10.2(d) (Existing Patent Rights) (as such schedule is updated on the Effective Date and the Option Data Package Delivery Date) have been and are being prosecuted in the respective patent offices in the Coherus Territory in accordance with applicable law, have been and are being filed and appropriately maintained, and all applicable fees have been paid on or before the due date for payment, and, to Junshi’s knowledge, are not invalid or unenforceable, in whole or in part. Junshi does not own or hold rights to any Patent Rights that would otherwise fall within the definition of Licensed Patent Rights but for the fact that it does not Control such Patent Rights. |
(e) | Option Programs. the Development, Commercialization, or other Exploitation of the Option Molecules and Option Products as contemplated herein will not conflict with any other license or agreement to which Junshi or any of its Affiliates is a party. In addition, Junshi Controls Know-How related to, and the Option Patent Rights that cover: (A) the recombinant cytokine that is known as of the Execution Date as JS018-1, which has the sequence set forth on Schedule 1.91 (Junshi IL-2 Molecule (JS018-1) Sequence); and (B) the monoclonal Antibody that is known as of the Execution Date as JS006, which has the sequence set forth on Schedule 1.94 (Junshi TIGIT Antibody (JS006) Sequence). |
(f) | Option Technology. (i) Section 10.2(f) (Option Patent Rights) sets forth a complete and accurate list of all Patent Rights that are Controlled by Junshi or any of its Affiliates and that are necessary or reasonably useful to Develop, Manufacture, Commercialize, or otherwise Exploit any Option Molecule or Option Product in the Field in the Coherus Territory (the “Option Patent Rights”), (ii) Junshi does not own or hold rights to any Patent Rights that would otherwise fall within the foregoing clause (i) but for the fact that it does not Control such Patent Rights; and (iii) except as otherwise noted on Section 10.2(f) (Option Patent Rights), Junshi solely owns all rights, title, and interests in and to all Option Patent Rights. |
(g) | Third Party Agreements. Neither Junshi nor any of its Affiliates have entered into any agreement with any Third Party pursuant to which Junshi Controls or grants any intellectual property rights with respect to the Licensed Technology, Option Patent Rights, or any Licensed Antibody or Option Molecule for the Field in the Coherus Territory other than those agreements that are set forth in Schedule 10.2(g) (Third Party Agreements) (the “Third Party Agreements”). Each Third Party Agreement is valid and binding. |
(h) | Licensed Antibody; Option Molecules. Junshi has disclosed to Coherus all Antibodies that Junshi or any of its Affiliates owns or in-licenses that or the subject of the PD-1 Program (as of the Execution Date) or each Option Program, as applicable. |
(i) | Junshi Assignment. Junshi or any of its Affiliates have secured from all employees, consultants, and contractors of Junshi or any of its Affiliates who have contributed to the Development, creation, conception or invention of any of the Licensed Patent Rights or Option Patent Rights a written agreement assigning to Junshi or any of its Affiliates all rights to such Developments, creations, conceptions or inventions, or Licensed Patent Rights or Option Patent Rights, and neither Junshi nor any of its Affiliates has received any written communication challenging Junshi’s ownership or right to the Licensed Patent Rights or Option Patent Rights. |
55
(j) | All Material Information Furnished. |
(i) | As of the Execution Date, Junshi has furnished or made available to Coherus or its agents or representatives (A) all information requested by Coherus on or before January 27, 2021, (B) all material safety and efficacy data, and (C) all material regulatory filings and other material correspondence with Regulatory Authorities within or For the Coherus Territory, in each case ((A) through (C)) concerning the Licensed Antibodies, the Licensed Products, and the Licensed Technology, and as of each such date all such information and data, regulatory filings and other correspondence with Regulatory Authorities is accurate, complete, and true in all material respects. |
(ii) | As of the Option Data Package Delivery Date, Junshi has furnished or made available to Coherus or its agents or representatives (A) all information requested by Coherus, (B) all material safety and efficacy data, and (C) all material regulatory filings and other material correspondence with Regulatory Authorities within or For the Coherus Territory, in each case ((A) through (C)), concerning the Option Molecules and the Option Products, and as of each such date all such information and data, regulatory filings and other correspondence with Regulatory Authorities is accurate, complete, and true in all material respects. |
(k) | Conduct of Research and Development. Junshi and its Affiliates have conducted all Development of Licensed Antibody and Licensed Products for the Coherus Territory in accordance with all applicable law. |
(l) | Upstream Licenses. Junshi is in compliance with all material terms of the JS001 Upstream License and the JS018-1 Upstream License. The provisions listed in Section 2.4 (No Implied Licenses) are the only terms of the JS001 Upstream License and the JS018-1 Upstream License necessary for Junshi’s grant of the sublicenses thereunder to Coherus upon the terms and conditions of this Agreement. |
(m) | Regulatory Materials. Junshi maintains Control over all Regulatory Approvals and Regulatory Materials pertaining to the Licensed Products and Option Products in the Field in the Coherus Territory. |
10.3 | Junshi Covenants. |
(a) | Closing Disclosure Letter. As of the Effective Date, Junshi will provide to Coherus a disclosure letter containing any additional information that is responsive to any of the representations and warranties set forth in Section 10.2 (Representations and Warranties) as if each such representation was made as of the Effective Date (the “Closing Disclosure Letter”) an updated schedule reflecting the Patent Rights described in the first sentence of Section 10.2(d); provided that any representation that is to be made as of the Effective Date must be true and correct notwithstanding any such additional disclosures made in the Closing Disclosure Letter. To the extent not provided before the Execution Date, then Junshi will provide to Coherus the information requested by Coherus from Junshi on January 28, 2021 on or before the Effective Date. |
(b) | No Conflicting Grants. Junshi will not, and will cause its Affiliates not to, enter into any agreement with any Affiliate or Third Party that conflicts with or contradicts the terms and conditions set forth in this Agreement, including any agreement that would limit the grant of licenses or rights hereunder to the Licensed Technology. |
56
(c) | Compliance with Applicable Law. Junshi will comply with the applicable law (including applicable anti-corruption laws), including as applicable GLP, GCP, and cGMP and any applicable anti-corruption or anti-bribery laws or regulations of any governmental authority with jurisdiction over the activities performed by or on behalf of Junshi or its Affiliates in furtherance of such obligations, in each case, in the course of performing its obligations or exercising its rights pursuant to this Agreement. Neither Junshi nor its Affiliates will engage any agent to perform activities under this Agreement that has ever been, is currently, or is the subject of a proceeding that could lead to it or such employees or agents becoming, as applicable, a Debarred Entity or Debarred Individual, an Excluded Entity or Excluded Individual, or a Convicted Entity or Convicted Individual. |
(d) | Control of Licensed Technology. During the period between the Execution Date and the Effective Date, Junshi and its Affiliates will (i) maintain (A) exclusive ownership and Control of all Licensed Technology, Know-How related to any Option Molecule, and Option Patent Right, in each case, owned by Junshi or its Affiliates as of the Execution Date and (B) Control of all Licensed Technology, Know-How related to any Option Molecule, and Option Patent Right, in each case, in-licensed by Junshi or its Affiliates as of the Execution Date. In addition, during the period between the Execution Date and the first to occur of the Option Exercise Date or expiration of the Option Term Junshi and its Affiliates will maintain (A) exclusive ownership and Control of all Know-How related to any Option Molecule and Option Patent Rights, in each case, owned by Junshi or its Affiliates at any time during such period and (B) Control of all Know-How related to any Option Molecule and Option Patent Rights, in each case, in-licensed by Junshi or its Affiliates at any time during such period, and (ii) not assign, transfer, encumber, or otherwise grant any Third Party any rights with respect to the Licensed Technology, Know- How related to any Option Molecule, or Option Patent Right, in each case, in any manner that would conflict with, limit the scope of, or adversely affect the rights granted to Coherus under this Agreement. |
(e) | Control of Upstream Licenses. Junshi will not, without Coherus’ prior written consent, (i) waive, amend, cancel, or terminate any material provision of, or fail to maintain, any JS001 Upstream License or JS018-1 Upstream License in any manner that would adversely affect the rights granted to Coherus hereunder or that would impose additional or greater obligations on Coherus, or (ii) take or fail to take any action that would give any counterparty to any JS001 Upstream License or JS018-1 Upstream License the right to terminate any rights under JS001 Upstream License or JS018-1 Upstream License applicable to the Field within or For the Coherus Territory. The foregoing obligation with respect to the JS018-1 Upstream License terminates if Coherus does not exercise its option with respect to the Junshi IL-2 Molecule within the applicable Option Term. |
(f) | Assignment of Inventions. Junshi will, and will ensure that its Affiliates, licensees, and contractors, obtain written agreements from any and all Persons involved in or performing any activities under this Agreement by or on behalf of Junshi that assign such Persons’ rights, title, and interests in and to any Licensed Technology to Junshi prior to any such Person performing such activities. |
(g) | Debarment. In the performance of activities under this Agreement, Junshi will not employ or use any Person that: (i) has ever been Debarred or is subject to debarment or convicted of a crime for which a Person could be a Debarred Entity or Debarred Individual; or (ii) has ever been under indictment for a crime for which a Person could be so Debarred. Junshi will inform Coherus in writing immediately if it or any Person that is performing activities |
57
under this Agreement is Debarred or is subject to debarment or is the subject of a conviction described in Section 306 of the FD&C Act, or if any action, suit, claim, investigation, or legal or administrative proceeding is pending or threatened, relating to the debarment or conviction of Junshi or any Person used in any capacity by Junshi or any of its Affiliates with respect to this Agreement or the performance of its other obligations or exercise of its rights under this Agreement.
(h) | CFIUS. Notwithstanding any provision to the contrary in this Agreement or any other agreement between the Parties, Junshi will neither be permitted to nor will seek (i) control rights (as defined in 31 C.F.R. § 800.208) in respect of Coherus or any Coherus subsidiary; (i) membership or observer rights on the board or equivalent governing body of any Coherus subsidiary; (ii) access to any material nonpublic technical information in the possession of Coherus or any Coherus subsidiary; or (iii) involvement, other than through voting of shares, in substantive decision making of Coherus or any Coherus subsidiary regarding: (A) the use, development, acquisition, safekeeping, or release of sensitive personal data of U.S. citizens maintained or collected by Coherus or any Coherus subsidiary; (B) the use, development, acquisition, or release of critical technologies; or (C) the management, operation, manufacture, or supply of covered critical infrastructure (in each case of (ii)-(iii), within the meaning of 31 C.F.R. § 800.211(b)). Junshi hereby waives any such rights to which it may be entitled under the this Agreement or any other agreement between the Parties or otherwise, including any statutory rights to such information as a stockholder of Coherus. |
(i) | Lonza Consents. Junshi will, within 30 days of the Effective Date, use reasonable efforts to obtain from Lonza any consent, approval or authorization necessary or required from Lonza under the JS001 Upstream License to grant the licenses to Coherus under Section |
2.1 (Licenses to Coherus).
10.4 | Coherus Covenants and CFIUS Representation. |
(a) | No Conflicting Grants. Coherus will not, and will cause its Affiliates not to, enter into any agreement with any Affiliate or Third Party that conflicts with or contradicts the terms and conditions set forth in this Agreement, including any agreement that would limit the grant of licenses or rights hereunder to the Arising Technology. |
(b) | Compliance with Applicable Law. Coherus will comply with the applicable law (including applicable anti-corruption laws), including as applicable GLP, GCP, and cGMP and any applicable anti-corruption or anti-bribery laws or regulations of any governmental authority with jurisdiction over the activities performed by or on behalf of Coherus or its Affiliates in furtherance of such obligations, in each case, in the course of performing its obligations or exercising its rights pursuant to this Agreement. Neither Coherus nor its Affiliates will engage any agent to perform activities under this Agreement that has ever been, is currently, or is the subject of a proceeding that could lead to it or such employees or agents becoming, as applicable, a Debarred Entity or Debarred Individual, an Excluded Entity or Excluded Individual, or a Convicted Entity or Convicted Individual. |
(c) | Assignment of Inventions. Coherus will, and will ensure that its Affiliates, Sublicensees, and contractors, obtain written agreements from any and all Persons involved in or performing any activities under this Agreement by or on behalf of Coherus that assign such Persons’ rights, title, and interests in and to any Arising Technology to Coherus prior to any such Person performing such activities on behalf of Coherus. |
58
(d) | Debarment. In the performance of activities under this Agreement, Coherus will not employ or use any Person that: (i) has ever been Debarred or is subject to debarment or convicted of a crime for which a Person could be a Debarred Entity or Debarred Individual; or (ii) has ever been under indictment for a crime for which a Person could be so Debarred. Coherus will inform Junshi in writing immediately if it or any Person that is performing activities under this Agreement is Debarred or is subject to debarment or is the subject of a conviction described in Section 306 of the FD&C Act, or if any action, suit, claim, investigation, or legal or administrative proceeding is pending or threatened, relating to the debarment or conviction of Coherus or any Person or entity used in any capacity by Coherus or any of its Affiliates with respect to this Agreement or the performance of its other obligations or exercise of its rights under this Agreement. |
(e) | CFIUS. Coherus represents and warrant to Junshi as of the Execution Date and as of the Effective Date that Coherus is not a “TID business” as defined in 31 C.F.R. § 800.248. |
10.5 | NO OTHER REPRESENTATIONS OR WARRANTIES. EXCEPT AS EXPRESSLY STATED IN THIS Article 10 (REPRESENTATIONS, WARRANTIES AND COVENANTS), NO REPRESENTATIONS OR WARRANTIES WHATSOEVER, WHETHER EXPRESS OR IMPLIED, INCLUDING WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, NON-INFRINGEMENT, OR NON-MISAPPROPRIATION OF THIRD PARTY INTELLECTUAL PROPERTY RIGHTS, IS MADE OR GIVEN BY OR ON BEHALF OF A PARTY. EXCEPT AS EXPRESSLY STATED IN THIS AGREEMENT, ALL REPRESENTATIONS AND WARRANTIES, WHETHER ARISING BY OPERATION OF LAW OR OTHERWISE, ARE HEREBY EXPRESSLY EXCLUDED. |
ARTICLE 11
INDEMNIFICATION
11.1 | Indemnification by Junshi. Subject to the remainder of this Article 11 (Indemnification), Junshi will defend, indemnify, and hold Coherus, its Affiliates, and its and their respective officers, directors, employees, and agents (the “Coherus Indemnitees”) harmless from and against any and all liabilities, losses, costs, damages, fees, expenses, or other amounts payable to a Third Party claimant, as well as any reasonable attorneys’ fees and costs of litigation incurred by such Coherus Indemnitees, all to the extent resulting from claims, suits, proceedings, or causes of action brought by or on behalf of such Third Party against such Coherus Indemnitees that arise from or relate to: (a) the Exploitation of any Licensed Antibody, Licensed Product, Option Molecule, or Option Product by or on behalf of Junshi or any of its Affiliates or licensees (other than Coherus and its Sublicensees); (b) a breach of any of Junshi’s representations, warranties, or obligations under this Agreement; (c) the willful misconduct or grossly negligent acts of Junshi or any of its Affiliates; or (d) violation of applicable law by any Junshi Indemnitee; excluding, in each case ((a), (b), (c), and (d)), any damages or other amounts for which Coherus has an obligation to indemnify any Junshi Indemnitee pursuant to Section 11.2 (Indemnification by Coherus). |
11.2 | Indemnification by Coherus. Subject to the remainder of this Article 11 (Indemnification), Coherus will defend, indemnify, and hold Junshi, its Affiliates, and each of their respective officers, directors, employees, and agents (the “Junshi Indemnitees”) harmless from and against any and all liabilities, losses, costs, damages, fees, expenses, or other amounts payable to a Third Party claimant, as well as any reasonable attorneys’ fees and costs of litigation incurred by such Junshi Indemnitees, all to the extent resulting from any claims, suits, proceedings, or causes of action brought by such Third Party against such Junshi Indemnitees that arise from or relate to: (a) the Exploitation of Licensed Antibody or Licensed Products by or on behalf of Coherus or any of its |
59
Affiliates or Sublicensees; (b) a breach of any of Coherus’ representations, warranties, or obligations under this Agreement; (c) the willful misconduct or grossly negligent acts of Coherus or any of its Affiliates; or (d) violation of applicable law by any Coherus Indemnitee; excluding, in each case ((a), (b), (c), and (d)), any damages or other amounts for which Junshi has an obligation to indemnify any Coherus Indemnitee pursuant to Section 11.1 (Indemnification by Junshi).
11.3 | Indemnification Procedures. The Party claiming indemnity under this Article 11 (Indemnification) (the “Indemnified Party”) will give written notice to the Party from whom indemnity is being sought (the “Indemnifying Party”) promptly after learning of the claim, suit, proceeding or cause of action for which indemnity is being sought (“Claim”). The Indemnifying Party’s obligation to defend, indemnify, and hold harmless pursuant to Section 11.1 (Indemnification by Junshi) or Section 11.2 (Indemnification by Coherus), as applicable, will be reduced to the extent the Indemnified Party’s delay in providing notification pursuant to the previous sentence results in actual prejudice to the Indemnifying Party; provided, however, that the failure by an Indemnified Party to give such notice or otherwise meet its obligations under this Section 11.3 (Indemnification Procedures) will not relieve the Indemnifying Party of its indemnification obligation under this Agreement. At its option, the Indemnifying Party may assume the defense and have exclusive control, at its own expense, of any Claim for which indemnity is being sought by giving written notice to the Indemnified Party within 30 days after receipt of the notice of the Claim. The assumption of defense of the Claim will not be construed as an acknowledgment that the Indemnifying Party is liable to indemnify any Indemnified Party in respect of the Claim, nor will it constitute waiver by the Indemnifying Party of any defenses it may assert against the Indemnified Party’s claim for indemnification. The Indemnified Party will provide the Indemnifying Party with reasonable assistance, at the Indemnifying Party’s expense, in connection with the defense. The Indemnified Party may participate in and monitor such defense with counsel of its own choosing at its sole expense; provided, however, the Indemnifying Party will have the right to assume and conduct the defense of the Claim with counsel of its choice. The Indemnifying Party will not settle any Claim without the prior written consent of the Indemnified Party, not to be unreasonably withheld, unless the settlement involves only the payment of money. The Indemnified Party will not settle any such Claim without the prior written consent of the Indemnifying Party, which consent will not be unreasonably withheld. If the Indemnifying Party does not assume and conduct the defense of the Claim as provided above, (a) the Indemnified Party may defend against, and consent to the entry of any judgment or enter into any settlement with respect to the Claim in any manner the Indemnified Party may deem reasonably appropriate (and the Indemnified Party need not consult with, or obtain any consent from, the Indemnifying Party in connection therewith), and (b) the Indemnified Party reserves any right it may have under this Article 11 (Indemnification) to obtain indemnification from the Indemnified Party. |
11.4 | Limitation of Liability. IN NO EVENT WILL EITHER PARTY BE LIABLE TO THE OTHER FOR ANY SPECIAL, CONSEQUENTIAL, INCIDENTAL, PUNITIVE, EXEMPLARY, OR INDIRECT DAMAGES OF ANY KIND ARISING FROM OR RELATING TO ANY BREACH OF THIS AGREEMENT OR ANY CLAIMS ARISING HEREUNDER, HOWEVER CAUSED AND ON ANY THEORY OF LIABILITY (WHETHER IN CONTRACT, TORT (INCLUDING NEGLIGENCE), STRICT LIABILITY OR OTHERWISE), REGARDLESS OF ANY NOTICE OF THE POSSIBILITY OF SUCH DAMAGES. NOTWITHSTANDING THE FOREGOING, NOTHING IN THIS SECTION 11.4 (LIMITATION OF LIABILITY) IS INTENDED TO OR WILL LIMIT OR RESTRICT (A) THE INDEMNIFICATION RIGHTS OR OBLIGATIONS OF ANY PARTY UNDER SECTION 11.1 (INDEMNIFICATION BY LICENSOR) OR SECTION 11.2 (INDEMNIFICATION BY COHERUS), (B) DAMAGES AVAILABLE IN THE CASE OF A PARTY’S FRAUD, GROSS NEGLIGENCE, OR INTENTIONAL MISCONDUCT, OR (C) DAMAGES AVAILABLE TO A PARTY FOR A BREACH BY THE OTHER PARTY OF THE CONFIDENTIALITY OBLIGATIONS UNDER Article 12 (CONFIDENTIALITY), |
60
MISAPPROPRIATION OR INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OWNED OR CONTROLLED BY SUCH PARTY, OR THE OTHER PARTY’S BREACH OF ITS OBLIGATIONS UNDER SECTION 2.7 (EXCLUSIVITY).
11.5 | Insurance. During the Term, each Party will obtain and maintain, at its individual sole expense, liability insurance in an amount adequate to cover its obligations under this Agreement during the Term. Each Party is required to obtain and maintain clinical trial insurance only for those trials they are sponsoring. Each Party will also maintain any mandatory insurance, including workers compensation coverage, in accordance with all applicable laws and regulations. Each Party will furnish to the other Party, on request, certificates of insurance evidencing the insurance, including notice of cancellation to be provided in accordance with the terms of the insurance policies. Such insurance will not be construed to create a limit of the insured Party’s liability with respect to its indemnification obligations under this Article 11 (Indemnification). |
ARTICLE 12
CONFIDENTIALITY
12.1 | Confidentiality; Exceptions. Except to the extent expressly authorized by this Agreement or otherwise agreed in writing, during the Term and for 10 years thereafter, the Parties agree that the receiving Party will keep confidential and will not publish or otherwise disclose or use for any purpose other than as provided for in this Agreement any information and materials furnished to such Party or any of its Affiliates by or on behalf of the other Party or any of its Affiliates or generated pursuant to this Agreement (collectively, “Confidential Information”). For any Confidential Information that constitutes trade secrets of either Party, the foregoing non-disclosure obligations will continue for as long as such Confidential Information remains trade secrets. |
(a) | Specific Examples of Confidential Information. Confidential Information of a Party or any of its Affiliates will include all information and materials disclosed by such Party or any of its Affiliates or their respective designees that (a) is marked as “Confidential,” “Proprietary,” or with similar designation at the time of disclosure or (b) by its nature can reasonably be expected to be considered Confidential Information by the recipient. Know- How disclosed orally will not be required to be identified as such to be considered Confidential Information. The terms of this Agreement and all Licensed Know-How will be deemed to be the Confidential Information of both Parties. All reports delivered by one Party to the other Party hereunder will be the Confidential Information of the providing Party. |
(b) | Exceptions. Notwithstanding the foregoing, Confidential Information will not include any information to the extent that it can be established by written documentation by the receiving Party that such information (a) was already known to the receiving Party, other than under an obligation of confidentiality (except to the extent such obligation has expired or an exception is applicable under the relevant agreement pursuant to which such obligation was established), at the time of disclosure, (b) was generally available to the public or otherwise part of the public domain at the time of its disclosure to the receiving Party, (c) became generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or omission of the receiving Party in breach of this Agreement, (d) was independently developed by the receiving Party as demonstrated by written documentation prepared contemporaneously with such independent development, or (e) was disclosed to the receiving Party, other than under an obligation of confidentiality (except to the extent such obligation has expired or an exception is applicable under the relevant agreement pursuant to which such obligation was established), by a Third Party who had no obligation to the disclosing Party not to disclose such information to others. |
61
12.2 | Authorized Disclosure. |
(a) | Permitted Disclosure. Except as expressly provided otherwise in this Agreement, each Party may use and disclose Confidential Information of the other Party solely as follows: (i) under appropriate confidentiality provisions substantially equivalent to those in this Agreement (but of shorter duration, if customary): (A) in connection with the performance of its obligations or as necessary or useful in the exercise of its rights under this Agreement, including the right to grant licenses or sublicenses as permitted hereunder, (B) to the extent such disclosure is reasonably necessary or useful in conducting Clinical Trials under this Agreement, or (C) to actual or potential (sub)licensees, acquirers or assignees, collaborators, investment bankers, investors or lenders (including in connection with any royalty factoring transaction), or; (ii) to the extent such disclosure is to a governmental authority as reasonably necessary in filing or prosecuting Patent Right, copyright, and trademark applications in accordance with this Agreement, prosecuting or defending litigation related to this Agreement, complying with applicable governmental regulations with respect to performance under this Agreement (including any disclosure to any securities exchange), obtaining Regulatory Approval or fulfilling post-approval regulatory obligations for the Licensed Antibody or Licensed Products, or otherwise required by applicable law; provided, however, that if a Party is required by applicable law or the rules or requests of any securities exchange or automated quotation system to make any such disclosure of the other Party’s Confidential Information then it will, except where impracticable for necessary disclosures (for example, in the event of medical emergency), endeavors to give reasonable advance notice to the other Party of such disclosure requirement and, in each of the foregoing, will use its reasonable efforts to secure confidential treatment of such Confidential Information required to be disclosed and will limit the disclosure of that Confidential Information required to be disclosed; (iii) to advisors (including lawyers and accountants) on a need to know basis, in each case under appropriate confidentiality provisions or professional standards of confidentiality substantially equivalent to those of this Agreement, or (iv) to the extent agreed to by the Parties. |
(b) | Disclosure to SEC. Each Party acknowledges and agrees that the other Party may submit this Agreement to the U.S. Securities and Exchange Commission (the “SEC”) and if a Party does submit this Agreement to the SEC, then such Party agrees to consult with the other Party with respect to the preparation and submission of, a confidential treatment request for this Agreement. If a Party is required by applicable law to make a disclosure of the terms of this Agreement in a filing with or other submission to the SEC, and (i) such Party has provided copies of the disclosure to the other Party as far in advance of such filing or other disclosure as is reasonably practicable under the circumstances, (ii) such Party has promptly notified the other Party in writing of such requirement and any respective timing constraints, and (iii) such Party has given the other Party a reasonable time under the circumstances from the date of notice by such Party of the required disclosure to comment upon, request confidential treatment or approve such disclosure, then such Party will have the right to make such public disclosure at the time and in the manner reasonably determined by its counsel to be required by applicable law. Notwithstanding any provision to the contrary herein, it is hereby understood and agreed that if a Party seeking to make a disclosure to the U.S. Securities and Exchange Commission as set forth in this Section 12.2 (Authorized Disclosure), and the other Party provides comments within the respective time periods or constraints specified herein or within the respective notice, the Party seeking to make such disclosure or its counsel, as the case may be, will in good faith (A) consider incorporating such comments and (B) use |
62
reasonable efforts to incorporate such comments, limit disclosure, or obtain confidential treatment to the extent reasonably requested by the other Party to the extent permitted by applicable law.
(c) | Disclosure on HKSE and SSE. Each Party acknowledges and agrees that the other Party may submit this Agreement to the Hong Kong Stock Exchange (“HKSE”) and the Shanghai Stock Exchange (“SSE”) and if a Party does submit this Agreement to HKSE or SSE, then such Party agrees to consult with the other Party with respect to the preparation and submission of, a confidential treatment request for this Agreement. If a Party is required by applicable law, rules, or requests to make a disclosure or filing of this Agreement or the terms hereof, (i) such Party will, to the extent practicable, provide copies of the disclosure to the other Party as far in advance of such filing or other disclosure, and |
(ii) such Party will promptly notify the other Party in writing of such requirement and any respective timing constraints. Notwithstanding any provision to the contrary set forth in this Agreement, it is hereby understood and agreed that if a Party seeking to make a disclosure as set forth in this Section 12.2 (Authorized Disclosure), and the other Party provides comments within the respective time periods or constraints specified herein or within the respective notice, then the Party seeking to make such disclosure or its counsel, as the case may be, will in good faith (A) consider incorporating such comments and (B) use reasonable efforts to incorporate such comments, limit disclosure, or obtain confidential treatment to the extent reasonably requested by the other Party to the extent permitted by applicable law.
(d) | Press Release. Other than public disclosure permitted by Section 12.2(a) (Permitted Disclosures), Section 12.2(b) (Disclosure to SEC), Section 12.2(c) (Disclosure on HKSE and SSE) and disclosures required by applicable law and rules of applicable stock exchanges, the Parties agree that the portions of any other news release or other public announcement relating to this Agreement or the performance hereunder that would disclose information that is not already in the public domain, must be reviewed and discussed by both Parties. After a disclosure or other public announcement has been reviewed and approved by both Parties under this Section 12.2 (Authorized Disclosure), either Party may make subsequent public disclosures reiterating such information without having to obtain the other Party’s prior consent and approval, so long as the information in such disclosure or other public announcement remains true, correct, and the most current information with respect to the subject matters set forth therein. |
12.3 | Prior Agreement. This Agreement supersedes the Existing Nondisclosure Agreement. All confidential information exchanged between the Parties under the Existing Nondisclosure Agreement will be deemed Confidential Information of the disclosing Party and will be subject to the terms of this Agreement. |
12.4 | Residual Knowledge. Notwithstanding any provision to the contrary set forth in this Agreement, use or disclosure by an authorized representative of a receiving Party of Confidential Information that is knowledge, technique, experience, or Know-How retained in the unaided memory of such authorized representative of the receiving Party that had authorized access to such Confidential Information (“Residual Knowledge”) will not violate the confidentiality, non-use and non- disclosure obligations set forth in this Article, provided that such authorized representative did not intentionally memorize such Confidential Information for use outside of this Agreement. Any use made by the receiving Party of any such Residual Knowledge is on an “as is, where is” basis, with all faults and all representations and warranties disclaimed and at its sole risk. |
12.5 | Publications. |
63
(a) | Coherus Publication Rights. As between the Parties, Coherus will: (i) control and be responsible for the publication strategy and logistics for all publications with respect to the Licensed Antibodies and Licensed Products within the Coherus Territory, subject to any rights of investigators that conduct any of the Clinical Trials for any of the Licensed Antibodies or Licensed Products in the Coherus Territory, (ii) have the exclusive right to issue publications and make presentations with respect to the Licensed Antibodies and Licensed Products within or For the Coherus Territory authored solely by representatives of Coherus, and (iii) subject to any rights of investigators that conduct any of the Clinical Trials for any of the Licensed Antibodies or Licensed Products in the Coherus Territory, have the sole and exclusive right to author and publish data generated and observations arising from Clinical Trials conducted within or For the Coherus Territory involving monotherapy treatments of Licensed Antibodies and Licensed Products and Development associated with Independent Trials for which Coherus is the Developing Party, in each case, without the prior consent of Junshi, provided that no such publication may include the Confidential Information of Junshi; except that the Parties will jointly author and publish such data and observations arising from the Ongoing JS001 Clinical Trials or activities under the Optioned Licensed Product Development Plan as well as for Clinical Trials conducted jointly by the Parties, subject to Coherus’ publication strategy and logistical control within or For the Coherus Territory. |
(b) | Junshi Publication Rights. As between the Parties, Junshi will have the exclusive right to issue publications and make presentations with respect to the Licensed Antibodies and Licensed Products authored solely by representatives of Junshi and, before the Option Exercise, the Option Molecules. As between the Parties, and subject to any rights of investigators that conduct any of the Clinical Trials for any of the Licensed Antibodies or Licensed Products, Junshi will have the sole and exclusive right to author publish data generated and observations arising from Development of the Option Molecules Clinical Trials before the Option Exercise, Development conducted outside the Coherus Territory involving Licensed Antibodies and Licensed Products, and Development associated with Independent Trials within the Coherus Territory for which Junshi is the Developing Party, in each case, without the prior consent of Coherus, provided that no such publication may include the Confidential Information of Coherus. |
ARTICLE 13
TERM AND TERMINATION
13.1 | Term. This Agreement will commence on the Effective Date and, unless earlier terminated pursuant to this Article 13 (Term and Termination), will expire on a Licensed Product-by-Licensed Product and country-by-country basis at the end of the applicable Royalty Term (the “Term”). Following the end of the Term for a Licensed Product in a country by expiration of the applicable Royalty Term (but not earlier termination for any other reason) and payment of all amounts due with respect thereto, including milestones that first become due after a Royalty Term, the license granted to Coherus under Section 2.1 (Licenses to Coherus) will become perpetual, irrevocable, fully paid-up, and royalty-free. |
13.2 | Termination by Coherus. Coherus will have the right for any or no reason to terminate this Agreement in its entirety or on a Licensed Product-by-Licensed Product basis (a) prior to the first Regulatory Approval for a Licensed Product in the Field in the Coherus Territory, upon six months’ prior written notice to Junshi; and (b) upon or after the first Regulatory Approval for a Licensed Product in the Field in the Coherus Territory, upon 12 months’ prior written notice to Junshi. |
13.3 | Termination for Cause. |
64
(a) | By Coherus. In the event of a material breach of this Agreement by Junshi, which material breach remains uncured for 90 days measured from the date of written notice of such material breach by Coherus that identifies the material breach and the actions or conduct that Coherus considers would be an acceptable cure of such material breach, Coherus may terminate this Agreement in whole or with respect to one or more Licensed Antibodies (and all Licensed Products that include such Licensed Antibody) at any time during the Term of this Agreement by written notice of termination to Junshi, provided that such 90- day cure period will be extended for a period of up to an additional 90 days so long as Junshi continues to use good faith efforts to cure such breach during such extension. |
(b) | By Junshi. In the event of a material breach of this Agreement by Coherus with respect to any of the Licensed Antibodies or Licensed Products, which material breach remains uncured for 90 days measured from the date of written notice of such material breach by Junshi that identifies the material breach and the actions or conduct that it considers would be an acceptable cure of such material breach, Junshi may terminate this Agreement by notice (with immediate effect) solely with respect to the Licensed Antibody and corresponding Licensed Products that contributed to the breach and for which the Term has not yet expired, provided that such 90-day cure period will be extended for a period of up to an additional 90 days so long as Coherus continues to use good faith efforts to cure such breach during such extension. |
(c) | Disputes Regarding Material Breach. In case the Party (the “Defaulting Party”) alleged by the other Party (the “Non-Defaulting Party”) to have committed a material breach under Section 13.3(a( (By Coherus) or Section 13.3(b) (By Junshi) disputes occurrence of such material breach, then the issue of whether the Non-Defaulting Party may properly terminate this Agreement on expiration of the applicable cure period will be resolved in accordance with Article 15 (Dispute Resolution). If as a result of such dispute resolution process, it is determined that the Defaulting Party committed a material breach of this Agreement and the Defaulting Party does not cure such material breach within 90 days after the date of such determination, (the “Additional Cure Period”), then such termination will be effective as of the expiration of the Additional Cure Period. If the Parties dispute whether such material breach was so cured, then such dispute will also be determined in accordance with Article 15 (Dispute Resolution). This Agreement will remain in full force and effect during the pendency of any such dispute resolution proceeding and the cure periods set forth in Section 13.3(a) (By Coherus) or Section 13.3(b) (By Junshi), as applicable, and any Additional Cure Period, in each case, will be tolled during any such dispute resolution proceeding, such proceeding will not suspend any obligations of either Party hereunder, and each Party will use reasonable efforts to mitigate any damage. If as a result of such dispute resolution proceeding it is determined that the Defaulting Party did not commit such material breach (or such material breach was cured in accordance with this Section 13.3 (Termination for Cause)), then no termination will be effective, and this Agreement will continue in full force and effect. |
(d) | Competitive Change of Control of or by Coherus. Without limiting Junshi’s rights under Section 13.3(b) (By Junshi), if Coherus does not comply with its obligations under the last sentence of Section 2.7(b) (Acquisition by Third Parties) with respect to a subset of Competitive Products described by clause (a), (b), or (c) in such definition, then Junshi may terminate this Agreement with respect to the Licensed Antibody and Licensed Products corresponding to such subset immediately by notice. Any such breach will be deemed a material breach but no cure period will apply. |
13.4 | Effects of Termination. Upon the termination of this Agreement (in addition to any other rights and obligations under this Article 13 (Term and Termination)): |
65
(a) | Licenses. As of the effective date of termination of this Agreement with respect to a Licensed Product, all licenses and all other rights granted by Junshi to Coherus under Section 2.1 (Licenses to Coherus) with respect to such Licensed Product will terminate. If this Agreement is terminated by Coherus under Section 13.3(a) (By Coherus), then as of the effective date of such termination, the licenses granted by Coherus to Junshi under Section 2.3 (Licenses to Junshi) will terminate. If this Agreement is terminated for any other reason, then as of the effective date of termination with respect to the terminated Licensed Products, subject to the terms and conditions of this Agreement, Coherus hereby grants to Junshi an irrevocable, non-exclusive, royalty-free, worldwide license, with the right to sublicense in accordance with the terms and restrictions set forth in Section 2.3 (Licenses to Junshi), under the Coherus Arising Technology existing as of the effective date of such termination to Exploit the terminated Licensed Products in the Field in the form such products exist as of the effective date of such termination. |
(b) | Regulatory Materials. Coherus will and hereby does, and will cause its Affiliates and Sublicensees to, no later than 30 days after the effective date of termination of this Agreement, assign and transfer to Junshi or its designee all of Coherus’ rights, title, and interests in and to all Regulatory Approvals and Regulatory Materials for the terminated Licensed Products. |
(c) | Ongoing Clinical Trials. |
(i) | Transfer to Junshi. If, as of the effective date of termination of this Agreement with respect to a Licensed Product, Coherus or its Affiliates are conducting any Clinical Trials for such Licensed Product, then, at Junshi’s election on a Clinical Trial-by-Clinical Trial basis, Coherus will either (A) reasonably cooperate, and ensure that its Affiliates reasonably cooperate, with Junshi to transfer the conduct of such Clinical Trial to Junshi or its designees, or (B) subject to Coherus’ approval, continue to conduct such Clinical Trial, at Junshi’s cost. Junshi will assume any and all liability for the conduct of such transferred Clinical Trial for such Licensed Products after the effective date of such transfer (except to the extent arising prior to the transfer date or from any willful misconduct or negligent act or omission by Coherus, its Affiliates or their respective employees, agents, and contractors). |
(ii) | Wind-Down. If Junshi does not elect to assume control of any such Clinical Trials for any Licensed Product, then Coherus will, in accordance with accepted pharmaceutical industry norms and ethical practices, wind-down the conduct of any such Clinical Trial in an orderly manner. Coherus will be responsible for any costs and expenses associated with such wind-down (unless this Agreement is terminated by Coherus pursuant to Section 13.3 (Termination for Cause), in which case Junshi will bear all such costs and expenses). |
(d) | Return of Confidential Information. Each Party will promptly return to the other Party (or as directed by such other Party destroy and certify to such other Party in writing as to such destruction) all of such other Party’s Confidential Information provided by or on behalf of such other Party hereunder that is in the possession or control of such Party (or any of its Affiliates, Sublicensees or subcontractors), except that (a) such Party will have the right to retain one copy of intangible Confidential Information of such other Party for legal purposes and (b) Junshi will not be obligated to return or destroy any of Coherus’ Confidential Information necessary or reasonably useful for Junshi to exercise the rights granted to it upon termination of this Agreement, provided that all such Confidential Information will continue to be subject to all confidentiality obligations under this |
66
Agreement (with Coherus as the disclosing Party with respect to such Confidential Information). Notwithstanding any provision to the contrary set forth in this Agreement, the receiving Party of any Confidential Information will not be required to destroy electronic files containing such Confidential Information that are made in the ordinary course of its business information back-up procedures pursuant to its electronic record retention and destruction practices that apply to its own general electronic files and information.
(e) | Other Remedies. Termination or expiration of this Agreement for any reason will not release either Party from any liability or obligation that already has accrued prior to such expiration or termination, nor affect the survival of any provision hereof to the extent it is expressly stated to survive such termination. Termination or expiration of this Agreement for any reason will not constitute a waiver or release of, or otherwise be deemed to prejudice or adversely affect, any rights, remedies or claims, whether for damages or otherwise, that a Party may have hereunder or that may arise out of or in connection with such termination or expiration. |
13.5 | Survival. Termination or expiration of this Agreement will not affect rights or obligations of the Parties under this Agreement that have accrued prior to the date of termination or expiration of this Agreement. Notwithstanding any provision to the contrary, the following provisions will survive and apply after expiration or termination of this Agreement in its entirety: Article 1 (Definitions), Section 2.3 (Licenses to Junshi), Section 4.9 (Development Records), Section 8.4 (Royalties) (but only with respect to Net Sales made during the Term), Section 8.6 (Books and Records; Audit Rights) (but only with respect to payment obligations accruing during the Term and only for a period of three years after expiration or termination), Section 8.8 (Late Payments) (but only with respect to payment obligations accruing during the Term), Section 9.1 (Ownership), Section 10.5 (No Other Representations or Warranties), Article 11 (Indemnification), Article 12 (Confidentiality), Section 13.1 (Term), Section 13.4 (Effects of Termination), this Section 13.5 (Survival), Article 15 (Dispute Resolution), and Article 16 (Miscellaneous). In addition, the other applicable provisions of Article 8 (Financials) will survive such expiration or termination of this Agreement in their entirety to the extent required to make final reimbursements, reconciliations or other payments incurred or accrued prior to the date of termination or expiration. For any surviving provisions requiring action or decision by the JDC or an Executive Officer, each Party will appoint representatives to act as its JDC members or Executive Officer, as applicable. All provisions not surviving in accordance with the foregoing will terminate upon expiration or termination of this Agreement and be of no further force and effect. |
ARTICLE 14
EFFECTIVENESS
14.1 | Effective Date. Except for the Parties’ obligations under Article 12 (Confidentiality) and this Article 14 (Effectiveness), which will be effective as of the Execution Date, this Agreement will not become effective until the first Business Day after the Antitrust Clearance Date (the “Effective Date”); provided that the Effective Date will not occur (a) if either Party exercises its termination right under Section 14.3 (Outside Date) prior to the Antitrust Clearance Date, or (b) if and for so long as there is in force any applicable law (i) enjoining or prohibiting the consummation of the transactions contemplated by this Agreement or (ii) imposing any conditions in connection with such effectiveness, and no action, proceeding, or investigation brought by a governmental authority is pending that would reasonably be expected to lead to any of the foregoing that would be material in the context of the transactions contemplated by this Agreement. |
14.2 | Filings. Each Party will unless otherwise agreed by the Parties, within 10 Business Days following the Execution Date, file those Antitrust Filings required under the applicable Antitrust Laws (the |
67
“Required Filings”). The Parties will reasonably cooperate with one another to the extent necessary in the preparation and execution of all such documents that are required to be filed pursuant to the Required Filings. Each Party will be responsible for its own costs and expenses associated with any such Required Filing, including premerger filing fees incurred by each Party associated with any such Required Filing. With respect to the Required Filings, the Parties will use reasonable efforts to seek early termination of the applicable waiting period and each use reasonable efforts to ensure that any applicable waiting period under the applicable Antitrust Law expires or is terminated as soon as practicable and to obtain any necessary approvals or consents under such applicable Antitrust Law, at the earliest possible date after the date of filing. To the extent permitted under applicable law and by the applicable governmental authorities, the Parties will (a) provide each other reasonable advance written notice of any meetings or telephone conferences with a governmental authority under the HSR Act relating to the transactions contemplated by this Agreement, and (b) permit each other to attend and participate in those meetings and telephone conferences. Each Party will (i) provide the other with reasonable opportunity to review and comment on any written submissions, and will consider comments in good faith, and (ii) keep the other Party apprised of the status of any communications with, and any inquiries or requests for information from, any governmental authority under the HSR Act, regardless of whether such other Party declines to participate in any meetings or telephone conferences; provided that neither Party will be obligated to disclose any commercially sensitive or privileged information, and to the extent the Parties agree to share information of this nature, such exchange and review will be limited to the Parties’ outside counsel only. Notwithstanding any provision to the contrary set forth in this Agreement, nothing in this Agreement (including this Section 14.2 (Filings)) will require either Party or any of its Affiliates to (a) disclose to the other Party or any of its Affiliates any information that is subject to obligations of confidentiality or non use owed to Third Parties (nor will either Party be required to conduct joint meetings with any governmental authority in which such information might be shared with the other Party), (b) commit to any consent decree or similar undertaking, or any divestiture, license (in whole or in part), or any arrangement to hold separate (or any similar arrangement) with respect to any of its products or assets, or (c) litigate.
14.3 | Outside Date. This Agreement will terminate at the election of either Party, immediately upon written notice to the other Party, (a) if any governmental authority in any Clearance Country seeks a permanent injunction under applicable Antitrust Laws against the Parties to enjoin the transactions contemplated by this Agreement; or (b) in the event that the Antitrust Clearance Date will not have occurred on or prior to 180 days after the submission of the Required Filing, and the Parties have not agreed in writing to extend the Antitrust Clearance Date. In the event of such termination, this Agreement will be of no further force and effect. |
ARTICLE 15
DISPUTE RESOLUTION
15.1 | Dispute Resolution. |
(a) | In the event of any dispute between the Parties under this Agreement, the Parties will first attempt in good faith to resolve such dispute by negotiation and consultation between themselves. In the event that such dispute is not resolved on an informal basis within 15 Business Days, either Party may refer the matter to the Executive Officers of the Parties for attempted resolution, whereupon the Executive Officers will confer and attempt in good faith to resolve such dispute by negotiation and consultation for a 30 day period following such referral. |
(b) | Subject to the Section 4.3(b) (Restrictions on Additional Development), if the Executive Officers do not resolve such dispute within such thirty (30) day period, either Party may at |
68
any time thereafter proceed to binding arbitration in accordance with this Section 15.1 (Dispute Resolution). A Party may submit such dispute to arbitration with JAMS New York (“JAMS”), and notify the other Party, in writing, of such dispute. Within thirty (30) days after receipt of such notice, the Parties will each designate in writing an arbitrator to resolve the dispute. Both of the designated arbitrators will elect a third arbitrator; provided, however, that if the designated arbitrators cannot agree on a third arbitrator within 20 days after both arbitrators have been designated, the third arbitrator will be selected by JAMS. The arbitrator will be a lawyer with biotechnology and/or pharmaceutical industry legal experience, and will not be an Affiliate, employee, consultant, officer, director or stockholder of any Party.
(c) | Within 30 days after the designation of the arbitrator, the arbitrator and the Parties will meet, at which time the Parties will be required to set forth in writing all disputed issues and a proposed ruling on the merits of each such issue. The Parties will have the right to be represented by counsel. Except as provided herein, the arbitration will be governed by the Comprehensive Arbitration Rules and Procedures (the “JAMS Rules”). The arbitration proceedings will be conducted in English. |
(d) | The arbitrator will use his or her best efforts to rule on each disputed issue within 30 days after the completion of any hearings associated with the arbitration. The determination of the arbitrator as to the resolution of any dispute will be binding and conclusive upon all Parties. The arbitrator will issue a written award that contains a reasoned opinion setting forth the findings of fact and conclusions upon which the award is based, including the calculation of any damages awarded. |
(e) | The (i) attorneys’ fees of the Parties in any arbitration, (ii) fees of the arbitrator and (iii) costs and expenses of the arbitration will be borne by the Parties as determined by the arbitrator. |
(f) | Any arbitration pursuant to this Section 15.1 (Dispute Resolution) will be conducted in New York, NY. |
(g)The Parties intend that each award by an arbitrator in an arbitration pursuant to this Section 15.1(Dispute Resolution) will be rendered in accordance with the United Nations Convention on the Recognition and Enforcement of Arbitral Awards and will be enforceable in accordance therewith.
(h) | The arbitrator will take appropriate actions to prevent, remediate, and/or sanction abusive conduct or other actions that threaten to undermine the fair, speedy and cost-effective resolution of the matter. |
(i) | In addition, during the pendency of any dispute under this Agreement initiated before the end of any applicable cure period under Section 13.3 (Termination for Cause), (i) this Agreement will remain in full force and effect, (ii) the provisions of this Agreement relating to termination for material breach will not be effective, (iii) the time periods for cure under 13.3 (Termination for Cause) as to any termination notice given prior to the initiation of the proceeding will be tolled, and (iv) neither Party will issue a notice of termination pursuant to this Agreement based on the subject matter of the proceeding (and no effect will be given to previously issued termination notices), until the arbitrator has confirmed the existence of the facts claimed by a Non-Defaulting Party to be the basis for the asserted material breach. |
69
15.2 | Injunctive Relief. Nothing in this Article 15 (Dispute Resolution) will preclude either Party from seeking equitable relief or interim or provisional relief from a court of competent jurisdiction, including a temporary restraining order, preliminary injunction or other interim equitable relief, concerning a dispute either prior to or during any proceeding if necessary to protect the interests of such Party or to preserve the status quo pending the proceeding. Therefore, in addition to its rights and remedies otherwise available at law, including the recovery of damages for breach of this Agreement, upon an adequate showing of material breach, and without further proof of irreparable harm other than this acknowledgement, such Non-Defaulting Party will be entitled to seek (a) immediate equitable relief, specifically including both interim and permanent restraining orders and injunctions, and (b) such other and further equitable relief as the court may deem proper under the circumstances. For clarity, nothing in this Section 15.2 (Injunctive Relief; Remedy for Breach of Exclusivity) will otherwise limit a Defaulting Party’s opportunity to cure a material breach as permitted in accordance with 13.3 (Termination for Cause). |
ARTICLE 16
MISCELLANEOUS
16.1 | Entire Agreement; Amendment. This Agreement, including the Schedules hereto, set forth the complete, final and exclusive agreement and all the covenants, promises, agreements, warranties, representations, conditions and understandings between the Parties hereto with respect to the subject matter hereof and supersedes all prior agreements and understandings between the Parties existing as of the Execution Date with respect to the subject matter hereof. In the event of any inconsistency between any plan hereunder and this Agreement, the terms of this Agreement will prevail. There are no covenants, promises, agreements, warranties, representations, conditions or understandings, either oral or written, between the Parties other than as are set forth herein and therein. No subsequent alteration, amendment, change or addition to this Agreement will be binding upon the Parties unless reduced to writing and signed by an authorized officer of each Party. |
16.2 | Force Majeure. Neither Party will be held liable to the other Party nor be deemed to have breached this Agreement for failure or delay performing any obligation under this Agreement to the extent that such failure or delay is caused by or results from acts of God, embargoes, war, acts of war (whether war be declared or not), terrorism, insurrections, riots, civil commotions, strikes, lockouts, or other labor disturbances (other than strikes, lockouts, or labor disturbances involving a Party’s own employees), government actions, fire, earthquakes, floods, epidemics, pandemics, or quarantines (“Force Majeure”) and for so long as such failure or delay continues to be caused by or result from such Force Majeure event. The Parties agree the effects of the COVID-19 pandemic that is ongoing as of the Execution Date may be invoked as a Force Majeure for the purposes of this Agreement even though the pandemic is ongoing to the extent those effects are not reasonably foreseeable by the Parties as of the Execution Date. The affected Party will notify the other Party in writing of any Force Majeure circumstances that may affect its performance under this Agreement as soon as reasonably practical, will provide a good faith estimate of the period for which its failure or delay in performance under the Agreement is expected to continue based on currently available information, and will undertake reasonable efforts necessary to mitigate and overcome such Force Majeure circumstances and resume normal performance of its obligations hereunder as soon a reasonably practicable under the circumstances. If the Force Majeure circumstance continues, then the affected Party will update such notice to the other Party on a weekly basis, or more frequently if requested by the other Party, to provide updated summaries of its mitigation efforts and its estimates of when normal performance under the Agreement will be able to resume. |
16.3 | Notices. Any notice required or permitted to be given under this Agreement will be in writing, will specifically refer to this Agreement, and will be addressed to the appropriate Party at the address |
70
specified below or such other address as may be specified by such Party in writing in accordance with this Section 16.3 (Notices), and will be deemed to have been given for all purposes (a) when received, if hand-delivered or sent by a reputable international expedited delivery service, or (b) five Business Days after mailing, if mailed by first class certified or registered mail, postage prepaid, return receipt requested. This Section 16.3 (Notices) is not intended to govern the day-to- day business communications necessary between the Parties in performing their obligations under the terms of this Agreement.
If to Junshi: | |
| SHANGHAI JUNSHI BIOSCIENCES CO., LTD. Level 13, Building 2, Nos. 36 and 58, Hai Qu Road, Shanghai, China 201203 Attention: CEO And to: SHANGHAI JUNSHI BIOSCIENCES CO., LTD. Level 13, Building 2, Nos. 36 and 58, Hai Qu Road, Shanghai, China 201203 Attention: Board Secretary, Securities Department |
With a copy to (which will not constitute notice): | Jones Day 4655 Executive Drive, Suite 1500 San Diego, CA 92130 Attention: Thomas A. Briggs |
If to Coherus: | Coherus BioSciences, Inc. |
| 333 Twin Dolphin Drive, Suite 600 Redwood City, CA 94065 |
| Attention: CEO |
With a copy to (which will not constitute notice): | Ropes & Gray 800 Boylston Street Boston MA 02199 Attention: David M. McIntosh and Hannah H. Freeman |
71
16.4 | No Strict Construction; Headings. This Agreement has been prepared jointly and will not be strictly construed against either Party. Ambiguities, if any, in this Agreement will not be construed against any Party, irrespective of which Party may be deemed to have authored the ambiguous provision. The headings of each Article and Section in this Agreement have been inserted for convenience of reference only and are not intended to limit or expand on the meaning of the language contained in the particular Article or Section. |
16.5 | Interpretation. Except where the context expressly requires otherwise, (a) the use of any gender herein will be deemed to encompass references to either or both genders, and the use of the singular will be deemed to include the plural (and vice versa), (b) the words “include”, “includes” and “including” will be deemed to be followed by the phrase “without limitation,” (c) the word “will” will be construed to have the same meaning and effect as the word “shall,” (d) any definition of or reference to any agreement, instrument or other document herein will be construed as referring to such agreement, instrument or other document as from time to time amended, supplemented or otherwise modified (subject to any restrictions on such amendments, supplements or modifications set forth herein), (e) any reference herein to any person or entity will be construed to include the person’s or entity’s successors and assigns, (f) the words “herein,” “hereof,” and “hereunder”, and words of similar import, will be construed to refer to this Agreement in its entirety and not to any particular provision hereof, (g) all references herein to Sections or Schedules will be construed to refer to Sections or Schedules of this Agreement, and references to this Agreement include all Schedules hereto, (h) the word “notice” means notice in writing (whether or not specifically stated) and will include notices, consents, approvals and other written communications contemplated under this Agreement, (i) provisions that require that a Party, the Parties or any committee hereunder “agree,” “consent,” or “approve” or the like will require that such agreement, consent or approval be specific and in writing, whether by written agreement, letter, approved minutes or otherwise (including e-mail, but excluding instant messaging), (j) references to any specific law, rule or regulation, or article, section or other division thereof, will be deemed to include the then- current amendments thereto or any replacement or successor law, rule or regulation thereof, (k) the term “or” will be interpreted in the inclusive sense commonly associated with the term “and/or,” and (l) references to any Sections include Sections and subsections that are part of the related Section (e.g., a section numbered “Section 2.2” would be part of “Section 2”, and references to “Section 2.2” would also refer to material contained in the subsection described as “Section 2.2(a)”) Each Party has had the opportunity to consult with counsel in connection with the review, drafting and negotiation of this Agreement. Accordingly, the rule of construction that any ambiguity in this Agreement will be construed against the drafting Party will not apply. |
16.6 | Assignment. Neither this Agreement nor any interest hereunder is assignable by either Party without the prior written consent of the other Party, except as follows: (a) either Party may, subject to the terms of this Agreement, assign its rights and obligations under this Agreement in whole to its successor-in-interest in connection with the sale of all or substantially all of its assets to which this Agreement relates, whether in a merger, acquisition, or similar transaction or series of related transactions, provided that such sale is not primarily for the benefit of its creditors, and (b) either Party may assign its rights and obligations under this Agreement to any of its Affiliates, provided that such Party will remain liable for all of its rights and obligations under this Agreement. A Party will promptly notify the other Party of any assignment or transfer under the provisions of this Section 16.6 (Assignment). This Agreement will be binding upon the successors and permitted assigns of the Parties and the name of a Party appearing herein will be deemed to include the names |
72
of such Party’s successors and permitted assigns to the extent necessary to carry out the intent of this Agreement. Any assignment or attempted assignment by either Party in violation of the terms of this Section 16.6 (Assignment) will be null, void and of no legal effect. If an assignment of this Agreement, in whole or in part, by either Party causes a higher withholding or direct tax rate than would be applicable absent such assignment, then such additional withholding or taxes will be borne by the assigning Party, including taxes resulting therefrom.
16.7 | Performance by Affiliates. Each Party may perform any obligations and exercise any right hereunder through any of its Affiliates, provided that such Party will remain primarily responsible for the acts of such Affiliates to other Party hereunder. Each Party hereby guarantees the performance by any of its Affiliates of such Party’s obligations under this Agreement, and will cause its Affiliates to comply with the provisions of this Agreement in connection with such performance. Any breach by a Party’s Affiliate of any of such Party’s obligations under this Agreement will be deemed a breach by such Party, and the other Party may proceed directly against such Party without any obligation to first proceed against such Party’s Affiliate. If the performance of any obligations, in whole or in part, by an Affiliate of a Party causes a higher withholding or direct tax rate than would be applicable if such Party had performed such obligation, then such additional withholding or taxes will be borne by such Party, including taxes resulting therefrom. |
16.8 | Further Actions. Each Party agrees to execute, acknowledge, and deliver such further instruments, and to do all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of this Agreement. |
16.9 | Severability. If any one or more of the provisions of this Agreement is held to be invalid or unenforceable by an arbitrator or by any court of competent jurisdiction from which no appeal can be or is taken, then the provision will be considered severed from this Agreement and will not serve to invalidate any remaining provisions hereof. The Parties will make a good faith effort to replace any invalid or unenforceable provision with a valid and enforceable one such that the objectives contemplated by the Parties when entering into this Agreement may be realized. |
16.10 | No Waiver. Any delay in enforcing a Party’s rights under this Agreement or any waiver as to a particular default or other matter will not constitute a waiver of such Party’s rights to the future enforcement of its rights under this Agreement, except with respect to an express written and signed waiver relating to a particular matter for a particular period of time. |
16.11 | Independent Contractors. Each Party will act solely as an independent contractor, and nothing in this Agreement will be construed to give either Party the power or authority to act for, bind, or commit the other Party in any way. Nothing herein will be construed to create the relationship of partners, principal and agent, or joint-venture partners between the Parties. |
16.12 | Counterparts. This Agreement may be executed in one or more counterparts, each of which will be deemed an original, but all of which together will constitute one and the same instrument. |
16.13 | Choice of Law. This Agreement will be governed by, and enforced and construed in accordance with, the laws of the State of New York, without regard to its conflicts of law provisions. |
[Signature Page Follows]
73
IN WITNESS WHEREOF, the Parties have executed this Agreement by their duly authorized representatives as of the Execution Date.
SHANGHAI JUNSHI BIOSCIENCES CO., LTD. | COHERUS BIOSCIENCES, INC. | |||
| | | | |
| | | | |
By: | /s/ Ning Li | | By: | |
Name: | Ning Li | | Name: | |
Title: | CEO | | Title: | |
IN WITNESS WHEREOF, the Parties have executed this Agreement by their duly authorized representatives as of the Execution Date.
SHANGHAI JUNSHI BIOSCIENCES CO., LTD. | COHERUS BIOSCIENCES, INC. | |||
| | | | |
| | | | |
By: | | | By: | /s/ Dennis M. Lanfear |
Name: | | | Name: | Dennis M. Lanfear |
Title: | | | Title: | Chairman & Chief Executive |
SCHEDULE 1.93
Junshi IL-2 Molecule (JS018-1) Sequence
As of the Execution Date, Junshi has not selected the clinical candidate for JS018-1. Junshi expects to select such candidate within about 6 months after the Execution Date.
SCHEDULE 1.96
Junshi TIGIT Antibody (JS006) Sequence
Heavy chain:
QVQLVQSGAEVKKPGASVKVSCKTSGYAFTEYTMHWVRQAPGKGLEWMGGINPNTGGTTYNQ
KFQGRVTLTVDKSSSTAYMELSSLRSEDTVVYYCAKLLRLMYYFDYWGQGTLVTVSSASTKGP
SVFPLAPCSRSTSESTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVP
SSSLGTKTYTCNVDHKPSNTKVDKRVESKYGPPCPPCPAPEFLGGPSVFLFPPKPKDTLMISRTPE
VTCVVVDVSQEDPEVQFNWYVDGVEVHNAKTKPREEQFNSTYRVVSVLTVLHQDWLNGKEYK
CKVSNKGLPSSIEKTISKAKGQPREPQVYTLPPSQEEMTKNQVSLTCLVKGFYPSDIAVEWESNG
QPENNYKTTPPVLDSDGSFFLYSRLTVDKSRWQEGNVFSCSVMHEALHNHYTQKSLSLSLGK
Light chain:
DIQMTQSPSSLSASVGDRVTITCQASQDVRTAVAWYQQKPGKAPKLLIYSASYRYTGVP
SRFSGSGSGTDFTFTISSLQPEDIATYYCHQHYITPWTFGGGTKVEIKRTVAAPSVFIFPPS
DEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTL
TLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGEC
SCHEDULE 1.99
JS001 Sequence
Heavy chain
QGQLVQSGAE VKKPGASVKV SCKASGYTFT DYEMHWVRQA PIHGLEWIGV 50
IESETGGTAY NQKFKGRVTI TADKSTSTAY MELSSLRSED TAVYYCAREG 100
ITTVATTYYW YFDVWGQGTT VTVSSASTKG PSVFPLAPCS RSTSESTAAL 150
GCLVKDYFPE PVTVSWNSGA LTSGVHTFPA VLQSSGLYSL SSVVTVPSSS 200
LGTKTYTCNV DHKPSNTKVD KRVESKYGPP CPPCPAPEFL GGPSVFLFPP 250
KPKDTLMISR TPEVTCVVVD VSQEDPEVQF NWYVDGVEVH NAKTKPREEQ 300
FNSTYRVVSV LTVLHQDWLN GKEYKCKVSN KGLPSSIEKT ISKAKGQPRE 350
PQVYTLPPSQ EEMTKNQVSL TCLVKGFYPS DIAVEWESNG QPENNYKTTP 400
PVLDSDGSFF LYSRLTVDKS RWQEGNVFSC SVMHEALHNH YTQKSLSLSL 450
GK 452
Light chain
DVVMTQSPLS LPVTLGQPAS ISCRSSQSIV HSNGNTYLEW YLQKPGQSPQ50
LLIYKVSNRF SGVPDRFSGS GSGTDFTLKI SRVEAEDVGV YYCFQGSHVP100
LTFGQGTKLE IKRTVAAPSV FIFPPSDEQL KSGTASVVCL LNNFYPREAK150
VQWKVDNALQ SGNSQESVTE QDSKDSTYSL SSTLTLSKAD YEKHKVYACE 200
VTHQGLSSPV TKSFNRGEC 219
SCHEDULE 1.113
Ongoing JS001 Clinical Trials
Twenty-four Ongoing and Five Planned Toripalimab Clinical Trials
NO. | Brief Title | ClinicalTrials.gov | Trial |
CT10 | A Phase I open label study to evaluate Toripalimab concurrent with Radiotherapy in Patients with Advanced Triple-Negative Breast Cancer | NCT03151447 | I |
CT13 | A Phase Ib, Open label, Dose-escalation Clinical Study Investigating the Tolerability and Pharmacokinetics of Toripalimab Combined with Axitinib in Patients with Advanced Renal Carcinoma and Melanoma | NCT03086174 | I |
TAB001-01 | A Multi-Center, Open Label Phase I study to Evaluate the Safety, Tolerability and Pharmacokinetics of Toripalimab in Subjects with Advanced Malignancies | NCT03474640 | I |
CT5 | A Multi-Center, Open Label Phase Ib/II Clinical Study to Evaluate Toripalimab in Patients with Advanced Gastric Adenocarcinoma, Esophageal Squamous Cell Carcinoma, Nasopharyngeal Carcinoma and Head and Neck Squamous Cell Carcinoma | NCT02915432 | Ib/II |
CT20 | An Open-Label Study Evaluating the Efficacy and Safety of Toripalimab versus Placebo as Neoadjuvant Therapy in Resectable Hepatocellular Carcinoma or Intrahepatic Cholangiocarcinoma | NCT03867370 | Ib |
CT8 | A Randomized, Controlled, Multi-Center Phase II Study of Toripalimab versus High-Dose Interferon in Patients With Completely surgical resected Mucosal Melanoma | NCT03178123 | II |
CT12 | A Single-arm, Open-label, Multi-center Phase II Study to evaluate the Safety and Efficacy of Toripalimab for Patients with Locally Advanced or Metastatic Bladder Urothelial Carcinoma after Standard Treatment Failed | NCT03113266 | II |
CT18 | A Study of Toripalimab in combination with Pemetrexed and Carboplatin in Patients With EGFR-mutation Positive and T790M Negative NSCLC After Progression on EGFR-TKI Treatment | NCT03513666 | II |
CT33 | A Single-arm, Open-label, Multi-center Phase II Study to Evaluate the Efficacy and Safety of Toripalimab in the Patients with Recurrent or Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma Who Have Failed At least Two Prior Lines of Therapy and Are Positive for Specific Biomarkers | NCT04603040 | II |
CT34 | A Single-arm, Open-label, Multi-center Phase II Clinical Study to Evaluate the Safety and Efficacy of Toripalimab in Combination with Bevacizumab as the First- line Therapy for Advanced Hepatocellular Carcinoma | NCT04605796 | II |
CT16 | A Phase II/III, Randomized, Double-Blind, Placebo- Controlled Study evaluating the Efficacy and Safety of Toripalimab versus Placebo as Adjuvant Therapy in Patients with Local Advanced Hepatocellular Carcinoma after Curative Hepatic Surgery. | NCT03859128 | III |
CT15 | A Randomized, Placebo-Controlled, Multicenter, Double-Blind, Phase III Study Comparing Toripalimab versus Placebo in Combination with Chemotherapy as 1st line treatment of Recurrent or Metastatic Nasopharyngeal Cancer | NCT03581786 | III |
CT17 | A Randomized, Multi-center, Phase III Study to Compare Toripalimab Versus Dacarbazine as First Line Therapy for Unresectable or Metastatic Melanoma | NCT03430297 | III |
CT19 | A Randomized, Controlled, Multi-center, Phase III Study to Evaluate the Efficacy and Safety of Toripalimab Versus Placebo Combined with Chemotherapy in Patients with Treatment-naive Advanced NSCLC | NCT03856411 | III |
CT21 | A Randomized, Controlled, Multi-center, Phase III Study to Compare Toripalimab Versus Placebo in combination with Paclitaxel and Cisplatin as First Line Therapy in Patients with Advanced or Metastatic Esophageal Squamous Cell Cancer | NCT03829969 | III |
CT25 | A Randomized, Controlled, Multi-center, Phase III Study to Evaluate Toripalimab Versus Placebo in combination with Pemetrexed and Carboplatin in Patients With EGFR-mutated NSCLC After Progression on EGFR-TKI Treatment | NCT03924050 | III |
CT26 | A Randomized, Controlled, Multi-center, Phase III Study to Evaluate Toripalimab Versus Placebo in Combination with Nab- Paclitaxel In Patients With Metastatic or Recurrent Triple-Negative Breast Cancer | NCT04085276 | III |
CT27 | A Randomized, Controlled, Multi-center, Phase III Study to Evaluate toripalimab + Lenvatinib vs. Lenvatinib alone as a 1st line treatment for patients with advanced HCC | NCT04523493 | III |
CT28 | A Randomized, Controlled, Multi-center, Phase III Study to Evaluate Toripalimab Versus Placebo in Combination with Platinum and Etoposide in Patients with Extensive-Stage Small Cell Lung Cancer | NCT04012606 | III |
CT29 | A randomized, double-blind, placebo- controlled, multicenter, phase III clinical study of Toripalimab Versus Placebo combined with platinum-based doublet chemotherapy as Neoadjuvant/adjuvant Therapy in Patients with resectable stage IIIA NSCLC | NCT04158440 | III |
CT35 | A randomized, double-blind, placebo- controlled, multicenter, phase III Study to Evaluate Toripalimab Versus Placebo in Combination with Bevacizumab as the First-line Therapy for Advanced Hepatocellular Carcinoma | NCT04723004 | III |
CT36 | A randomized, multicenter, phase III Study to Evaluate the Efficacy and Safety of Toripalimab in Combination with Axitinib Versus Sunitinib Monotherapy as first line treatment in Advanced Renal Cell Carcinoma | NCT04394975 | III |
CT38 | A Multi-center, Randomized, Phase III Study to Evaluate Toripalimab Versus Placebo in Combination with Standard Chemotherapy as a First-line Treatment for Locally Advanced or Metastatic Urothelial Carcinoma | NCT04568304 | III |
CT43 | A Multi-center, Randomized, Open-label Phase III Study to Evaluate Toripalimab + Axitinib versus Pembrolizumab as First-line Therapy in Patients with Unresectable, Locally Advanced or Metastatic Mucosal Melanoma | IND Approved | III |
CT37 | A Phase II Study to Evaluate Toripalimab plus Cetuximab in patients with head and neck squamous cell carcinoma (HNSCC) | IND Stage | II |
CT41 | A Phase II Study to Evaluate Toripalimab+ Anlotinib with or without chemo as the first line therapy for advanced or metastatic colorectal cancer | IND Stage | II |
CT39 | A Multi-center, Randomized Phase III Study to Evaluate Toripalimab in combination with Lenvatinib and GEMOX versus GEMOX to as first-line treatment for advanced or unresectable intrahepatic cholangiocarcinoma | IND Stage | III |
CT42 | A Multi-center, Randomized Phase III Study to Evaluate Toripalimab versus Placebo Plus Chemotherapy as Neoadjuvant/adjuvant Therapy in Patients with Resectable Esophageal Squamous Cell Carcinoma | IND Stage | III |
CT44 | A Multi-center, Randomized Phase III Study to Evaluate Toripalimab versus Placebo as Neoadjuvant/adjuvant Therapy in Patients with Resectable Gastric Cancer | IND Stage | III |
SCHEDULE 1.116
Option Data Package
1. | A detailed description of all Option Molecules and Option Products that are the subject of such Option Program. |
2. | Copies of all (a) communications from any patent authority and (b) applications, filings, and responses made to any patent authority and instructions relating thereto, in each case ((a) and (b)) regarding or otherwise relating to any Licensed Patent Rights with respect to such Option Program. |
3. | All documents (including briefing documents), meeting minutes, correspondence, and information provided (a) by or on behalf of Junshi or its Affiliates to any Regulatory Authority, or (b) by or on behalf of any Regulatory Authority to Junshi or its Affiliates, in each case ((a) and (b)) in connection with (i) any Option Product that is the subject of such Option Program or (ii) any product that is referenced in any regulatory material with respect to such Option Product (a “Referenced Product”). |
4. | A summary of all relevant safety concerns with respect to the applicable Option Products, if an IND were to have been open at the time of observation. |
5. | All available (a) draft or final molecular characterization, preclinical and non-clinical efficacy/safety study reports, including non-GLP and GLP toxicology, drug metabolism, pharmacokinetics, and pharmacodynamics study data and reports, from pre-IND and IND-enabling studies for the applicable Option Products and Referenced Products, and (b) data tables and completed data sets from any other non-clinical or preclinical experiments that evaluate the mechanism of action, dose range, pharmacokinetics, pharmacodynamics, or biochemical/functional efficacy of such Option Products or Referenced Products. |
6. | The complete data set from the applicable Phase I Clinical Trial. |
7. | A finalized statistical analysis plan and the dataset therefor. |
8. | All available (a) CMC records, including all analytical method development and validation reports, certificates of analysis, and accompanying release test reports, and all related starting materials (e.g., cell lines, plasmids, and primers), (b) manufacturing process development, characterization, and validation reports, (c) drug substance and drug product stability reports, and (d) batch records and manufacturing cost records, in each case ((a)-(d)) for the applicable Option Products. |
9. | Update as to the status of the Manufacturing of the applicable Option Products, including where such Option Products are Manufactured, and current quantities available. |
SCHEDULE 2.6(b)
Manufacturing Technology Transfer Additional Terms
1. | Under the oversite of the JDC there will be a Technology Transfer Plan outlining the technology transfer key timelines, roles and responsibilities of JDC and subordinate teams. This is deemed a project management document. |
2. | In coordination with Junshi current technology transfer procedures the following framework is used for technology transfers to internal and external parties. This consists of a 7 Element framework. Junshi will provide latest bilingual (Chinese/English) working drafts of these documents for any given transfer which can then be used as a starting point for the Coherus CMO implementation of the technology transfer for a given molecule. The Manufacturing partner will generate local English Language equivalents (or functional equivalents) to these items. |
| Stage of Transfer | |
Element | Engineering Runs | PPQ Runs |
Process Transfer Protocol | TTP – Technology Transfer Protocol | PQMP - Process Qualification Master Plan |
Analytical Methodology | AMTP – Analytical Method Transfer Plan | |
Control of Process | PCS - Process Control Strategy | |
Measure Outcome | C&S Plan – Comparability and Stability Plan | |
Process Fit | Material Balance | |
Risk Management | QRA – Quality Risk Assessment (for Process) | |
Reporting | ESR – Engineering Summary Report | VSR – Validation Summary Report |
3. | For each transfer to a given manufacturing site or specific scale at a given site, the Element documents listed (or their functional equivalent) will be subject to Junshi’s review and approval. These include for Engineering Runs: TTP, AMTP, PCS, C&S Plan, QRA, ESR. These include for the PPQ Runs: PQMP, PCS, C&S Plan, QRA, VSR. While it is understood different formats for these documents may be currently used by Lonza or other Coherus CMOs, the intention is to follow the above framework in spirit insomuch as Junshi is provided technology oversight and approval to the Element documents (or their functional equivalent) in the reaching of a validated process “state”. |
4. | Coherus CMO data as it relates to manufacturing including and not limited to all CPPs and CQAs for any Engineering and Validation Runs will be shared in a timely manner with Junshi in order to assure consistent and transparent process lifecycle management knowledge is maintained inside Junshi. All release testing data for all Engineering and PPQ batches will be shared with Junshi in a timely manner. |
5. | Process changes that affect regulatory filing established ranges or change previously developed QbD based PCS ranges must be reviewed and approved by Junshi in advance. |
6. | Annual reports content filed with the Regulatory Authorities at Coherus CMO that pertain to manufacturing data for Junshi processes will be shared with Junshi when submitted to the relevant authorities. |
SCHEDULE 4.2
Ongoing JS001 Development Plan
Investigational product: | Toripalimab Injection (JS001) |
English name of investigational product: | Toripalimab Injection |
Company: | Shanghai Junshi Biosciences Co., Ltd. Floor 13, Building 2, No. 36 and 58 Haiqu Road, Pudong New District, Shanghai, China 201203 |
Version date: | Jan 30, 2021, Version 2.0 |
1.Clinical development of Toripalimab Injection
1.1 | Summary |
Toripalimab, also known as JS001 or TAB001, is a humanized IgG4κ monoclonal antibody specific for human programmed death-1 (PD-1). It binds to PD-1 with high affinity and selectively blocks the binding of PD-1 to its ligands PD-L1 and PD-L2, which leads to activation of T-lymphocytes and enhanced proliferation of T-lymphocytes and secretion of cytokines, especially IFN-γ. For nonclinical pharmacology results of toripalimab including pharmacology, PK, and toxicology data please refer to the toripalimab Investigator’s Brochure.
On December 27, 2018, toripalimab received marketing approval in China for the treatment of patients with advanced or metastatic melanoma who had failed 1st line therapy. The approved dose is toripalimab 3 mg/kg Q2W.
As of 16 December 2019, 26 Phase I-III clinical trials of toripalimab have been conducted in China and US. The summary of trials was shown in Figure 1. The safety profile observed with toripalimab is
consistent with other PD-1 inhibitors. Please see the Investigator’s Brochure for further information.
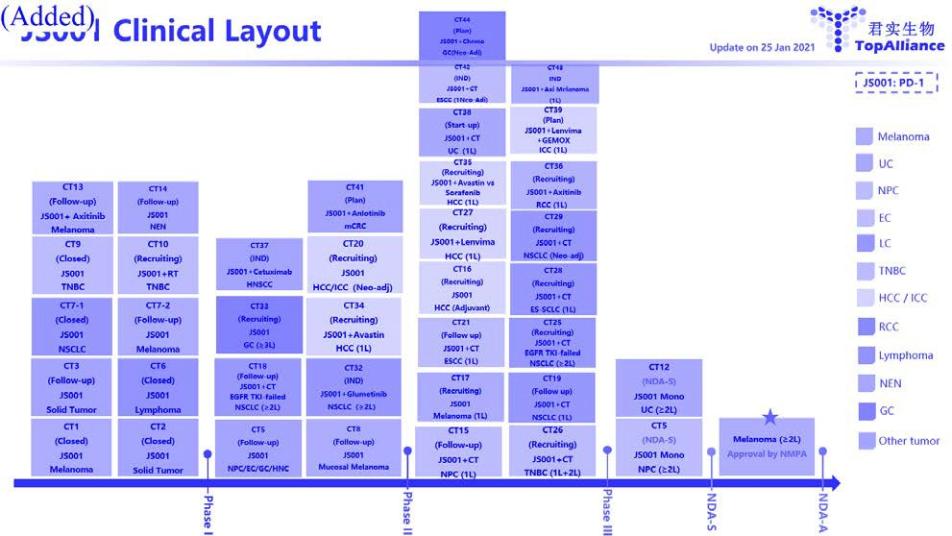
1.2 | Overall of Clinical studies of Toripalimab |
The clinical studies conducted with toripalimab have evaluated the efficacy and safety of toripalimab alone or toripalimab in combination with other drugs. As of Jan 30, 2021, there have been a total of 9 completed clinical studies and 24 ongoing clinical studies. It is estimated that 1546 subjects were exposed to Toripalimab, of which 1189 subjects were exposed to Toripalimab monotherapy and 358 subjects were exposed to Toripalimab combination therapy. It is estimated that 2128 subjects have been randomized to double blinded studies. In addition, approximately 1225 patients were enrolled in collaborative clinical studies sponsored by partners or investigator sponsored studies. For a complete list of completed, on- going and planned study list of company sponsored studies, please see Table 1.
Table 1:Overview of ongoing and completed clinical studies with JS001 by Jan 30, 2021
Study number | Study phase | Study population | Number of enrolled subjects Planned /actual | Study Status |
Advanced solid tumors | | | ||
CT1 | I | Advanced solid tumors | 36 /36 | Completed |
CT2 | I | Advanced solid tumors | 12/25 | Completed |
CT3 | I | Advanced solid tumors | 36/33 | Completed |
Lymphoma | | | ||
CT6 | I | Recurrent/refractory malignant lymphoma | 12/13 | Completed |
Neuroendocrine tumor | | | ||
CT14 | I | Advanced neuroendocrine tumor | 40/40 | Completed |
Study number | Study phase | Study population | Number of enrolled subjects Planned /actual | Study Status |
| | after failure of standard of care | | |
Multiple types of tumor, multiple cohorts | | | ||
CT5 | Ib/II | Advanced gastric adenocarcinoma, esophageal squamous cell carcinoma, nasopharyngeal carcinoma, and squamous cell carcinoma of head and neck | Enrollment closed Monotherapy for nasopharyngeal carcinoma (180/190 subjects) Combined therapy for nasopharyngeal carcinoma (12/12 subjects) Monotherapy for esophageal squamous cell carcinoma (58/60 subjects) Combined therapy for esophageal squamous cell carcinoma (12/12 subjects) Monotherapy for gastric cancer (58/58 subjects) Combined therapy for gastric cancer (36/33 subjects) Monotherapy for squamous cell carcinoma of head and neck (30/34 subjects) Combined therapy for squamous cell carcinoma of head and neck (12/3 subjects) | Follow up |
NPC arm under NDAs | ||||
Triple negative breast cancer | | |||
CT9 | I | Advanced triple Negative Breast Cancer | 24/20 subjects | Completed |
CT10 | I | Advanced triple Negative Breast Cancer (combined with radiotherapy) | 6/36 subjects | Enrollment ongoing |
CT26 | III | As 1st/2nd-line therapy for triple Negative Breast Cancer. | 528/284 | Enrollment ongoing |
Melasma | | | ||
CT4 | II | Locally advanced or metastatic melanoma | 120/128 subjects | Completed |
Study number | Study phase | Study population | Number of enrolled subjects Planned /actual | Study Status |
| | after failure of standard of care | | |
CT7-2 | I | To examine the pharmacokinetic similarity before and after process change in patients with advanced melanoma | 24/26 subjects | Completed |
CT8 | II | As adjuvant therapy for completely resected mucosal melanoma | Enrollment closed 145 subjects (73 subjects in JS001 group, 72 subjects in control group) | Follow up |
CT13 | I | Advanced melanoma (combined with Axitinib) | 24/33 subjects | Data clean |
CT17 | III | As 1st-line therapy for unresectable or metastatic melanoma | 230/168 | Enrollment ongoing |
CT43 | III | As First-line Therapy in Patients with Unresectable, Locally Advanced or Metastatic Mucosal Melanoma | 220 | IND approved |
Urothelial carcinoma | | | ||
CT12 | II | Locally advanced or metastatic urothelial carcinoma after failure of standard of care | 150/151 | Follow up |
Nasopharyngeal carcinoma | | | ||
CT15 | III | As 1st-line therapy for nasopharyngeal carcinoma | 280/289 | Follow up |
Esophageal cancer | | | ||
CT21 | III | Systematic chemotherapy-naïve advanced or metastatic esophageal squamous cell carcinoma | 500/514 | Enrollment completed and follow up Follow up |
HCC | | | ||
CT16 | III | Post-operative adjuvant therapy for liver cancer | 402/278 | Enrollment ongoing |
Study number | Study phase | Study population | Number of enrolled subjects Planned /actual | Study Status |
CT20 | Ib | As neoadjuvant therapy for hepatocellular carcinoma | 40/20 | Enrollment ongoing |
CT27 | III | As 1st-line therapy for advanced hepatocellular carcinoma(+Lev) | 519/45 | Enrollment ongoing |
CT34 | II | As 1st-line therapy for advanced hepatocellular carcinoma(+Bev) | 50/50 | Follow up |
CT35 | III | As 1st-line therapy for advanced hepatocellular carcinoma(+Bev) | 280/1 | Enrollment ongoing |
NSCLC | | | ||
CT7-1 | I | To examine the pharmacokinetic similarity before and after process change in patients with advanced NSCLC | 30/41 | Completed |
CT18 | II | EGFR mutation positive advanced NSCLC after failure of EGFR-TKI therapy | 40 /40 | Follow up |
CT19 | III | As 1st-line therapy for NSCLC (squamous cell carcinoma + adenocarcinoma) | 450/465 | Enrollment completed and follow up |
CT25 | III | As 1st-line therapy for NSCLC after failure of EGFR-TKI therapy | 350/262 | Enrollment ongoing |
CT29 | III | Perioperative systematic therapy for stage IIIA NSCLC | 406/167 | Enrollment ongoing |
SCLC | | | ||
CT28 | III | As 1st-line therapy for SCLC | 420/310 | Enrollment ongoing |
GC | ||||
CT33 | II | Failed at least two prior lines of GC | 100/1 | Enrollment ongoing |
Study number | Study phase | Study population | Number of enrolled subjects Planned /actual | Study Status |
| | | | |
RCC | ||||
CT36 | III | As 1st-line therapy for RCC | 380/54 | Enrollment ongoing |
UC | | |||
CT38 | III | As 1st line therapy for Pd-L1+ UC | 364/0 | Enrollment ongoing |
Clinical trial conducted in US | | | ||
TAB00 1-01 | I | Advanced solid tumors | Enrollment ongoing | Enrollment ongoing |
1.3 | Proposed study of JS001 |
Two Phase II clinical trial for HNSCC and mCRC and three Phase III registration studies in ICC, EC and GC will be initiated in Q2 & Q3 in 2021 .
Study number | Study phase | Study population | Number of enrolled subjects Actual/planned | Study Status |
CT37 | II | head and neck squamous cell carcinoma (HNSCC) | 40 | IND stage |
CT41 | II | JS001 plus Anlotinib with or without chemo as first line in mCRC | 208 | IND stage |
CT39 | III | Intrahepatic cholangiocarcinoma ICC (1st line) JS001+TKI/GEMOX | 285 | IND stage |
CT42 | III | EC (neoadjuvant and adjuvant) | 500 | IND stage |
CT44 | III | GC (neoadjuvant and adjuvant) | 1000 | IND stage |
Note: The budget calculation is based on the ongoing and planned trials (listed in the above tables)
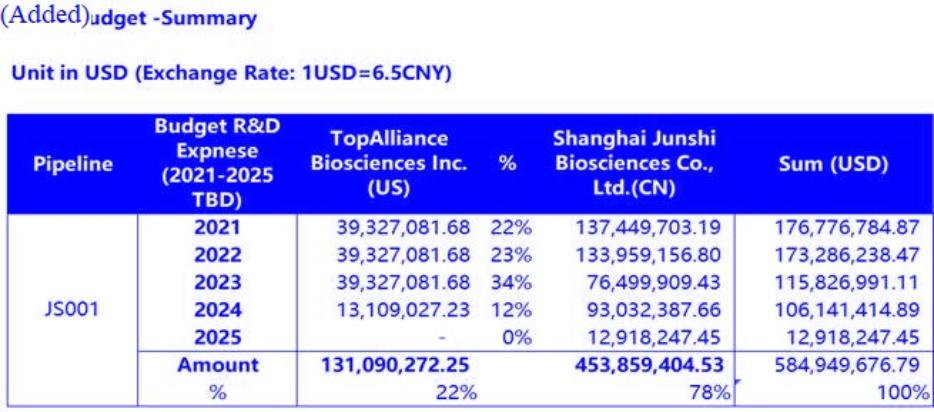
Schedule 10.2(d) Existing Patent Rights
US Patent No. 10,066,013
US Patent No. 10,815,302
US Patent Application No. 17022719
PCT/CN2020/085046
CN2020100908290
CN202010090965X
CN2020100822088
CN2020108796448
CN2020108839123
Schedule 10.2(f)
Option Patent Rights
IL-2 Program: Certain Patent Rights are not owned by Junshi but are licensed to Junshi pursuant to the JS018-1 Upstream License.
JS006: PCT application No. PCT/CN2020/101883
JS018-1: PCT Application No. PCT/CN2020/070748 (licensed from Beijing Zhidao)
Schedule 10.2(g) Third Party Agreements
JS001 Upstream License
JS018-1 Upstream License
