Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Passage BIO, Inc. | tmb-20210505x8k.htm |
| EX-99.1 - EX-99.1 - Passage BIO, Inc. | tmb-20210505xex99d1.htm |
Exhibit 99.2
| Corporate Presentation May 2021 NASDAQ GS: PASG Fulfilling the Promise of Gene Therapies for Central Nervous System Disorders |
| Forward-Looking Statement This presentation includes “forward-looking statements” within the meaning of, and made pursuant to the safe harbor provisions of, the Private Securities Litigation Reform Act of 1995, including, but not limited to: our expectation about timing and execution of anticipated milestones, including our planned initiation of clinical trials and the availability of clinical data from such trials; our expectations about our collaborators’ and partners’ ability to execute key initiatives; estimates regarding our cash forecasts; the expected impact of the COVID-19 pandemic on our operations; and the ability of our lead product candidates to treat their respective target monogenic CNS disorders. These forward-looking statements may be accompanied by such words as “aim,” “anticipate,” “believe,” “could,” “estimate,” “expect,” “forecast,” “goal,” “intend,” “may,” “might,” “plan,” “potential,” “possible,” “will,” “would,” and other words and terms of similar meaning. These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements, including: our ability to develop, obtain regulatory approval for and commercialize PBGM01, PBFT02, PBKR03 and future product candidates; the timing and results of preclinical studies and clinical trials; the risk that positive results in a preclinical study or clinical trial may not be replicated in subsequent trials or success in early stage clinical trials may not be predictive of results in later stage clinical trials; risks associated with clinical trials, including our ability to adequately manage clinical activities, unexpected concerns that may arise from additional data or analysis obtained during clinical trials, regulatory authorities may require additional information or further studies, or may fail to approve or may delay approval of our drug candidates; the occurrence of adverse safety events; failure to protect and enforce our intellectual property, and other proprietary rights; failure to successfully execute or realize the anticipated benefits of our strategic and growth initiatives; risks relating to technology failures or breaches; our dependence on collaborators and other third parties for the development of product candidates and other aspects of our business, which are outside of our full control; risks associated with current and potential delays, work stoppages, or supply chain disruptions caused by the coronavirus pandemic; risks associated with current and potential future healthcare reforms; risks relating to attracting and retaining key personnel; failure to comply with legal and regulatory requirements; risks relating to access to capital and credit markets; and the other risks and uncertainties that are described in the Risk Factors section of our most recent filings with the U.S. Securities and Exchange Commission. These statements are based on our current beliefs and expectations and speak only as of the date of this presentation. We do not undertake any obligation to publicly update any forward-looking statements except as required by law. By attending or receiving this presentation you acknowledge that you are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date such statements are made; you will be solely responsible for your own assessment of the market and our market position; and that you will conduct your own analysis and be solely responsible for forming your own view of the potential future performance of Passage Bio. 2 |
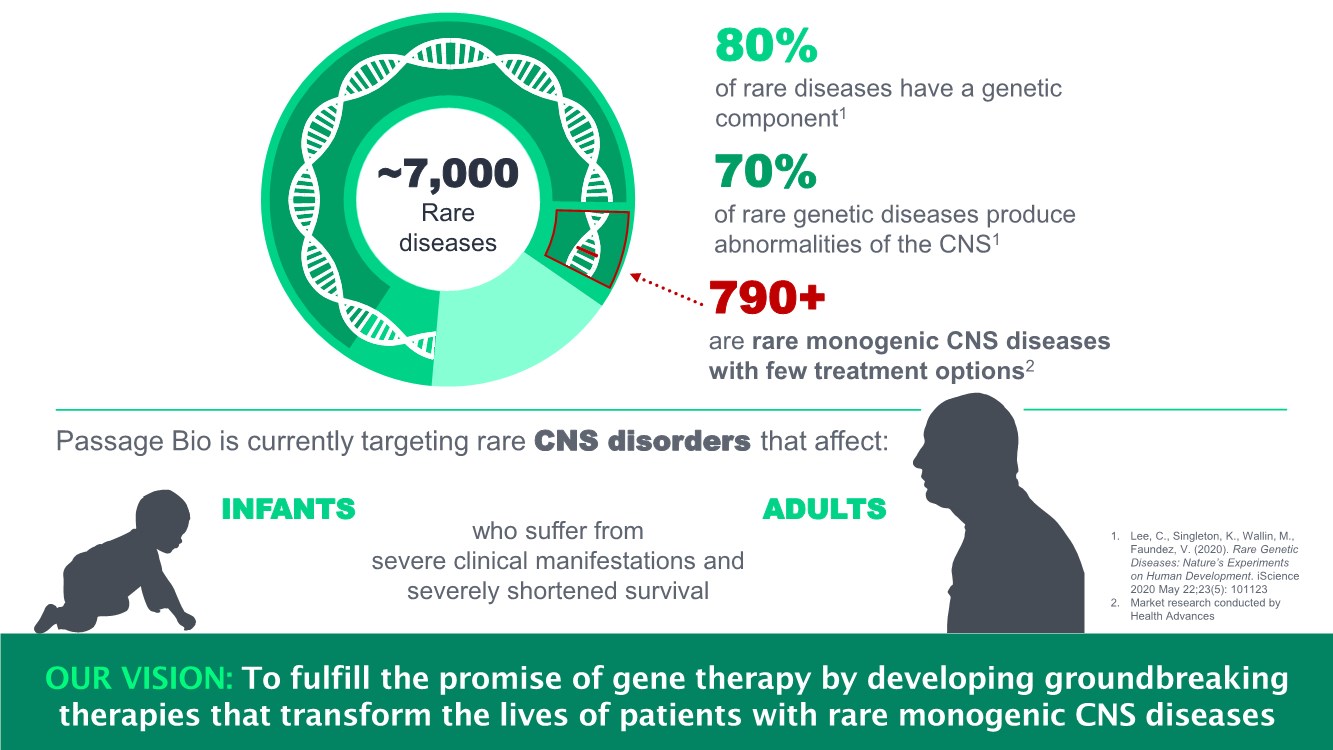
| ~7,000 Rare diseases 70% of rare genetic diseases produce abnormalities of the CNS1 790+ are rare monogenic CNS diseases with few treatment options2 Passage Bio is currently targeting rare CNS disorders that affect: INFANTS ADULTS 1. Lee, C., Singleton, K., Wallin, M., Faundez, V. (2020). Rare Genetic Diseases: Nature’s Experiments on Human Development. iScience 2020 May 22;23(5): 101123 2. Market research conducted by Health Advances 80% of rare diseases have a genetic component1 who suffer from severe clinical manifestations and severely shortened survival 3 OUR VISION: To fulfill the promise of gene therapy by developing groundbreaking therapies that transform the lives of patients with rare monogenic CNS diseases |
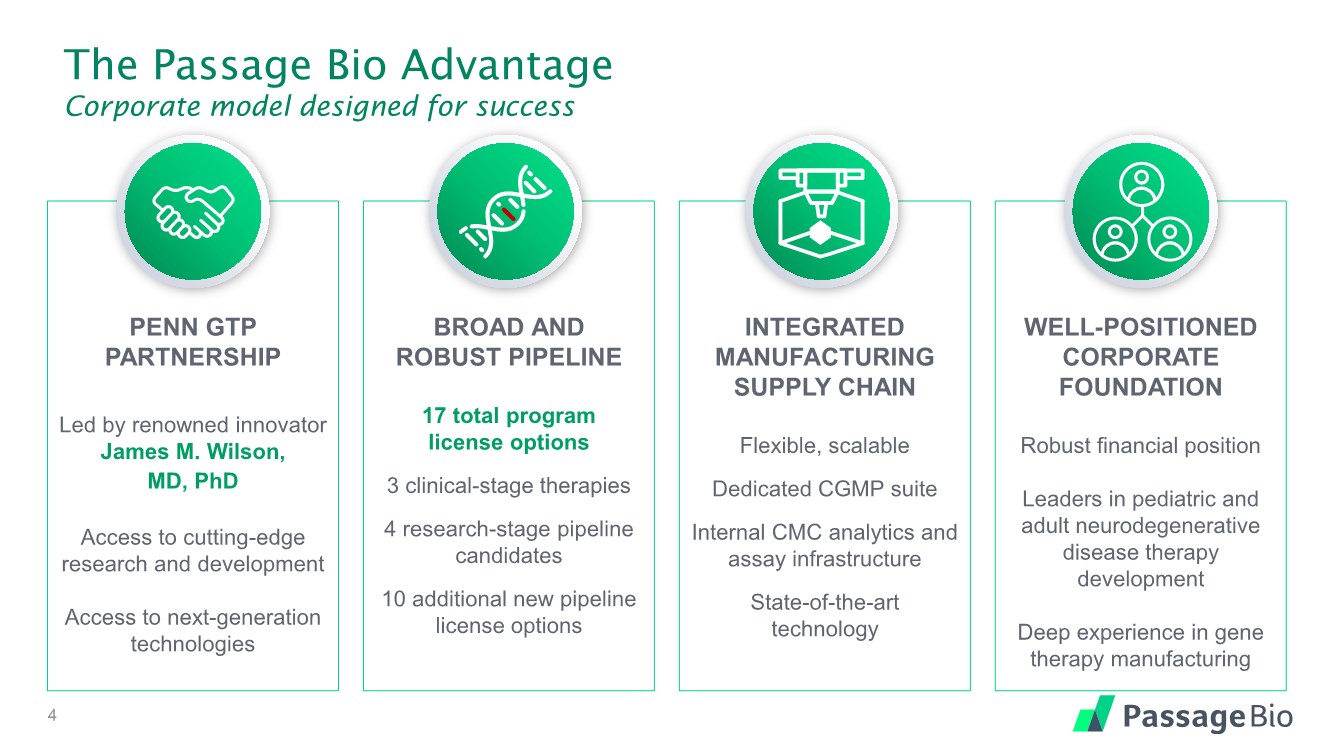
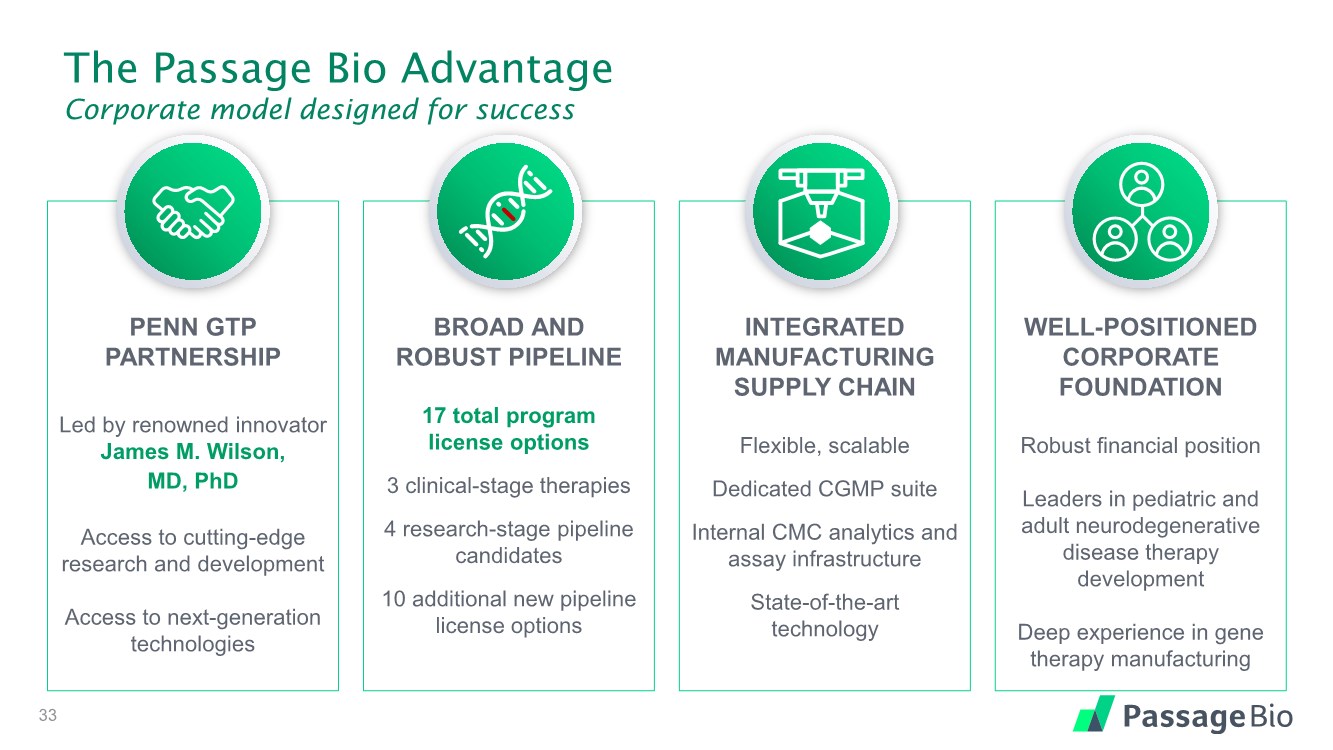
| The Passage Bio Advantage Corporate model designed for success PENN GTP PARTNERSHIP Led by renowned innovator James M. Wilson, MD, PhD Access to cutting-edge research and development Access to next-generation technologies BROAD AND ROBUST PIPELINE 17 total program license options 3 clinical-stage therapies 4 research-stage pipeline candidates 10 additional new pipeline license options INTEGRATED MANUFACTURING SUPPLY CHAIN Flexible, scalable Dedicated CGMP suite Internal CMC analytics and assay infrastructure State-of-the-art technology WELL-POSITIONED CORPORATE FOUNDATION Robust financial position Leaders in pediatric and adult neurodegenerative disease therapy development Deep experience in gene therapy manufacturing 4 |
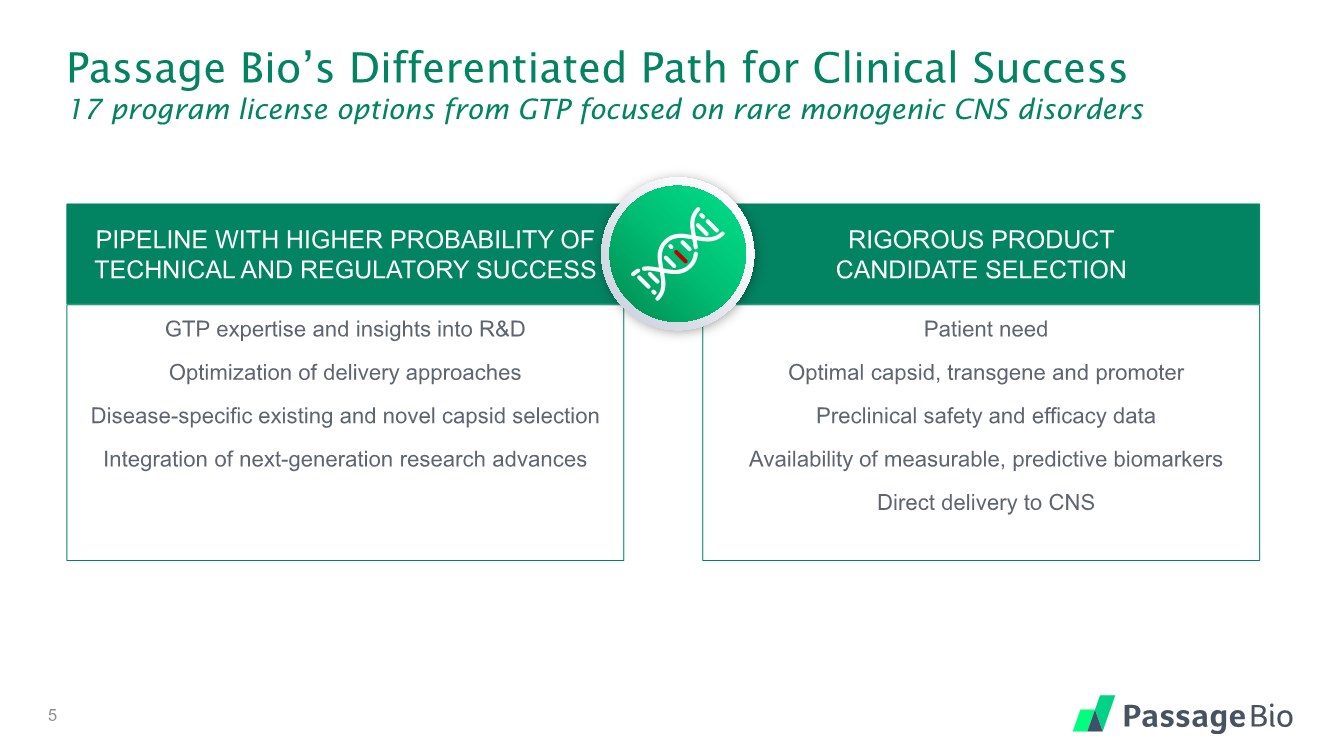
| Passage Bio’s Differentiated Path for Clinical Success 17 program license options from GTP focused on rare monogenic CNS disorders GTP expertise and insights into R&D Optimization of delivery approaches Disease-specific existing and novel capsid selection Integration of next-generation research advances 5 Patient need Optimal capsid, transgene and promoter Preclinical safety and efficacy data Availability of measurable, predictive biomarkers Direct delivery to CNS PIPELINE WITH HIGHER PROBABILITY OF TECHNICAL AND REGULATORY SUCCESS RIGOROUS PRODUCT CANDIDATE SELECTION |
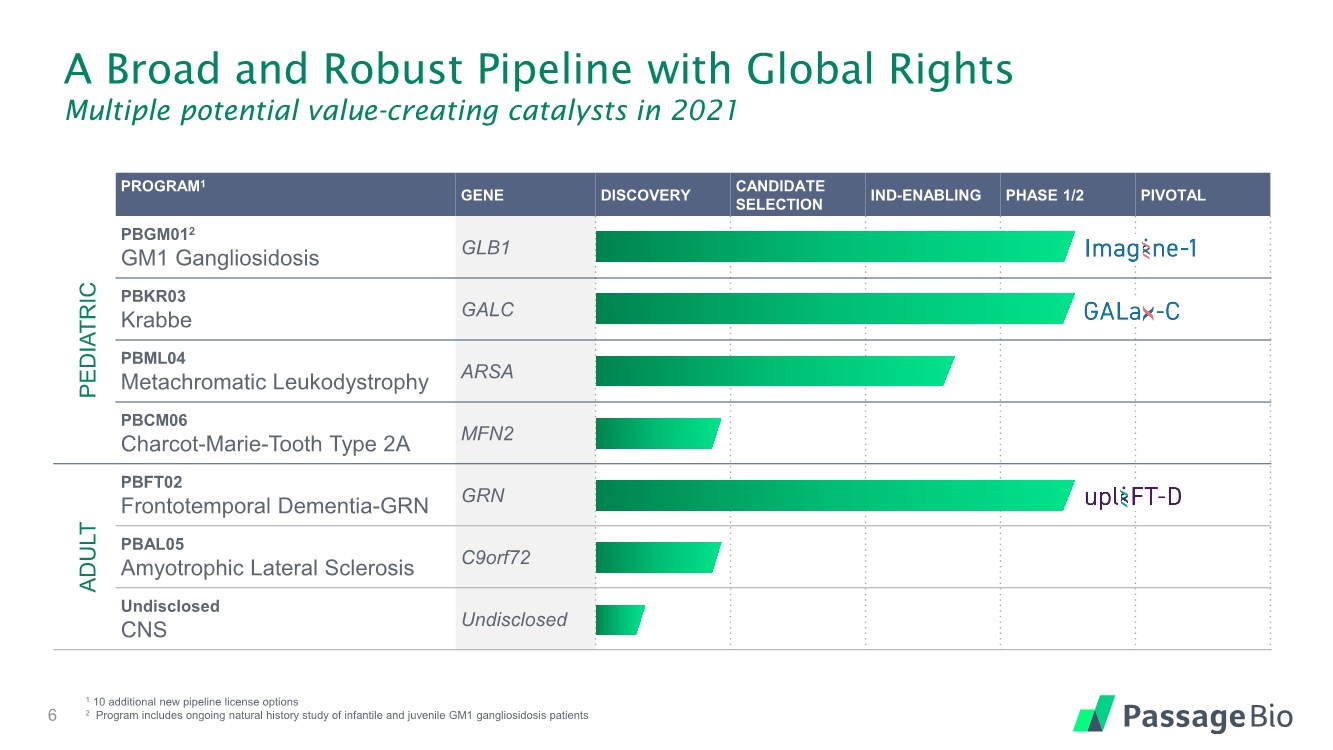
| PROGRAM1 GENE DISCOVERY CANDIDATE SELECTION IND-ENABLING PHASE 1/2 PIVOTAL PEDIATRIC PBGM012 GM1 Gangliosidosis GLB1 PBKR03 Krabbe GALC PBML04 Metachromatic Leukodystrophy ARSA PBCM06 Charcot-Marie-Tooth Type 2A MFN2 ADULT PBFT02 Frontotemporal Dementia-GRN GRN PBAL05 Amyotrophic Lateral Sclerosis C9orf72 Undisclosed CNS Undisclosed A Broad and Robust Pipeline with Global Rights Multiple potential value-creating catalysts in 2021 6 1 10 additional new pipeline license options 2 Program includes ongoing natural history study of infantile and juvenile GM1 gangliosidosis patients |
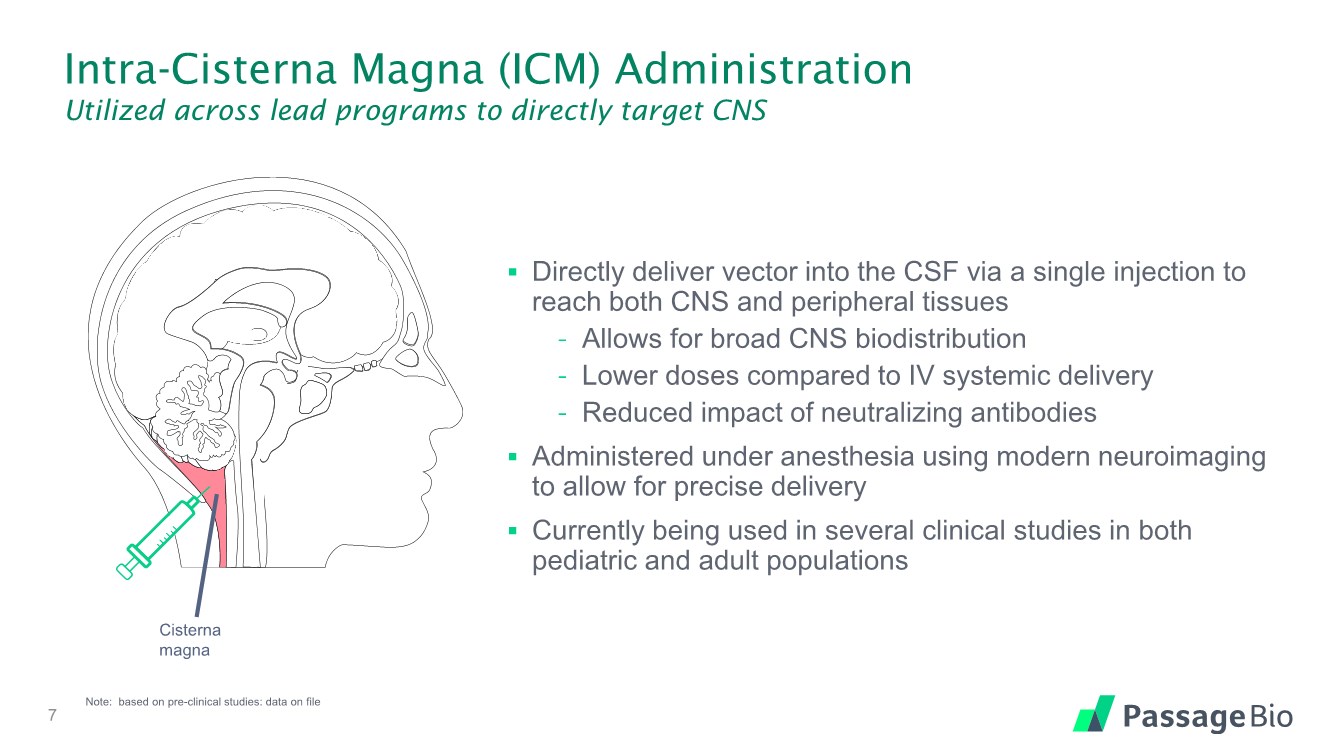
| Intra-Cisterna Magna (ICM) Administration Utilized across lead programs to directly target CNS ▪ Directly deliver vector into the CSF via a single injection to reach both CNS and peripheral tissues - Allows for broad CNS biodistribution - Lower doses compared to IV systemic delivery - Reduced impact of neutralizing antibodies ▪ Administered under anesthesia using modern neuroimaging to allow for precise delivery ▪ Currently being used in several clinical studies in both pediatric and adult populations 7 Note: based on pre-clinical studies: data on file Cisterna magna |
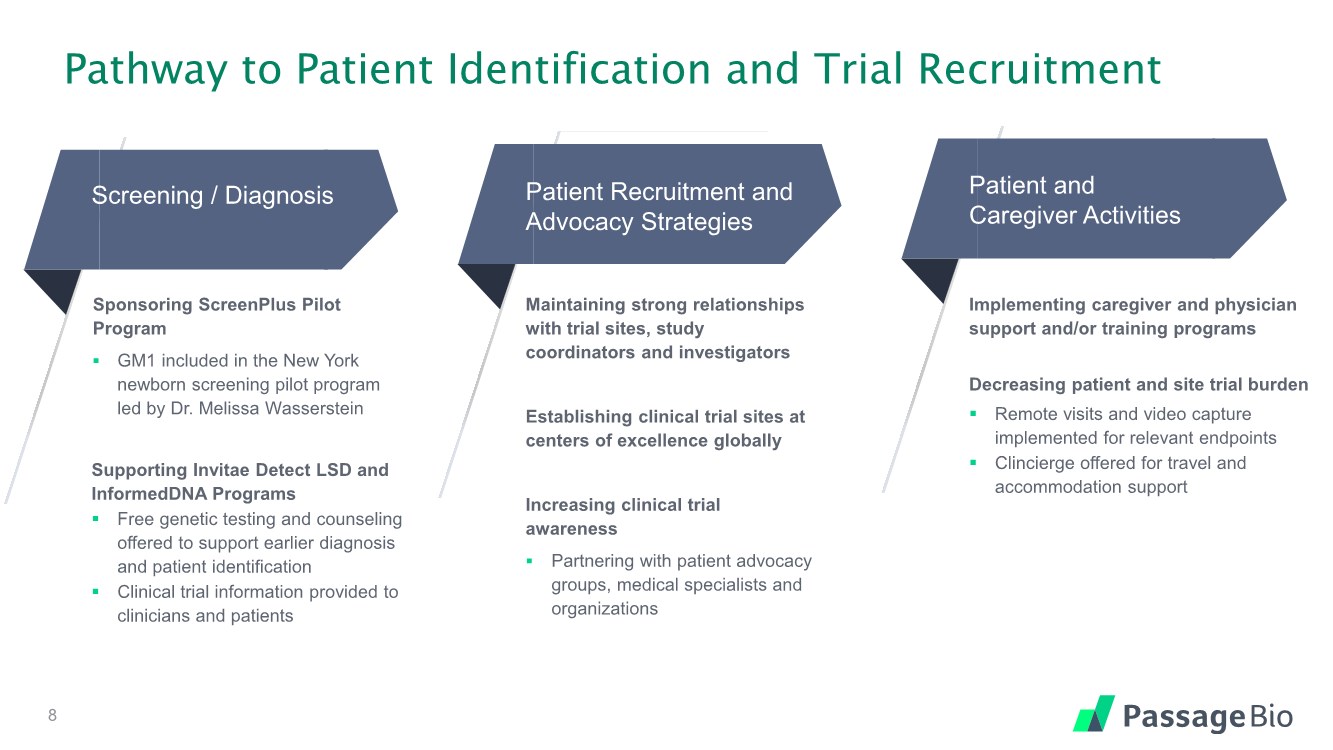
| Pathway to Patient Identification and Trial Recruitment Screening / Diagnosis Patient Recruitment and Advocacy Strategies Patient and Caregiver Activities Sponsoring ScreenPlus Pilot Program ▪ GM1 included in the New York newborn screening pilot program led by Dr. Melissa Wasserstein Supporting Invitae Detect LSD and InformedDNA Programs ▪ Free genetic testing and counseling offered to support earlier diagnosis and patient identification ▪ Clinical trial information provided to clinicians and patients Implementing caregiver and physician support and/or training programs Decreasing patient and site trial burden ▪ Remote visits and video capture implemented for relevant endpoints ▪ Clincierge offered for travel and accommodation support Maintaining strong relationships with trial sites, study coordinators and investigators Establishing clinical trial sites at centers of excellence globally Increasing clinical trial awareness ▪ Partnering with patient advocacy groups, medical specialists and organizations 8 |
| PBGM01 GM1 Gangliosidosis |
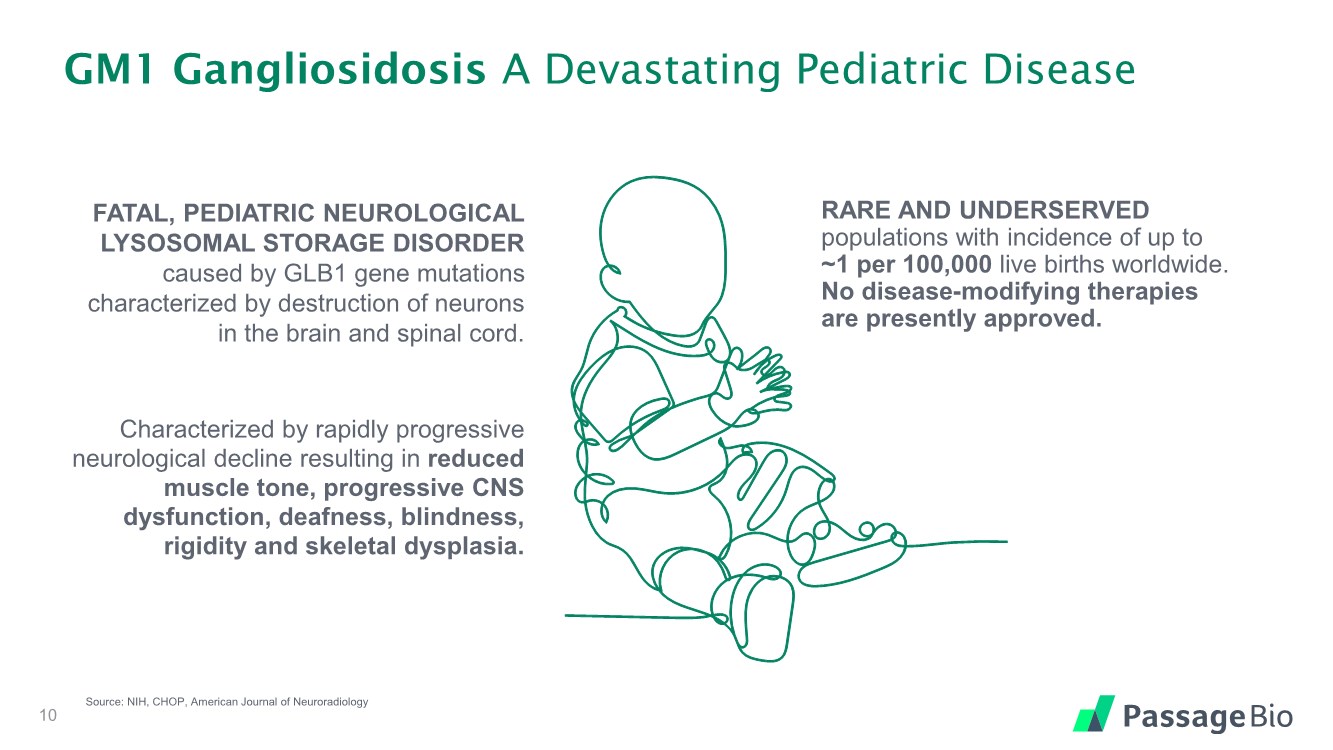
| GM1 Gangliosidosis A Devastating Pediatric Disease 10 Source: NIH, CHOP, American Journal of Neuroradiology FATAL, PEDIATRIC NEUROLOGICAL LYSOSOMAL STORAGE DISORDER caused by GLB1 gene mutations characterized by destruction of neurons in the brain and spinal cord. Characterized by rapidly progressive neurological decline resulting in reduced muscle tone, progressive CNS dysfunction, deafness, blindness, rigidity and skeletal dysplasia. RARE AND UNDERSERVED populations with incidence of up to ~1 per 100,000 live births worldwide. No disease-modifying therapies are presently approved. |
| PBGM01 Potential Transformative Therapy For Rare, Underserved Disorder ▪ Next-generation, proprietary AAVhu68 capsid delivers functional GLB1 gene encoding β-gal to the brain and peripheral tissues ▪ Compelling preclinical data showing meaningful transduction of both central nervous system and critical peripheral organs ▪ Received multiple global regulatory clearances for Phase 1/2 clinical trial ▪ Received Orphan Drug, Rare Pediatric Disease and Fast Track designations by FDA and Orphan designation by EC for treatment in GM1 ▪ First patient dosed in Phase 1/2 trial ▪ Initial safety and 30-day biomarker data from cohort 1 planned for 4Q21 11 |
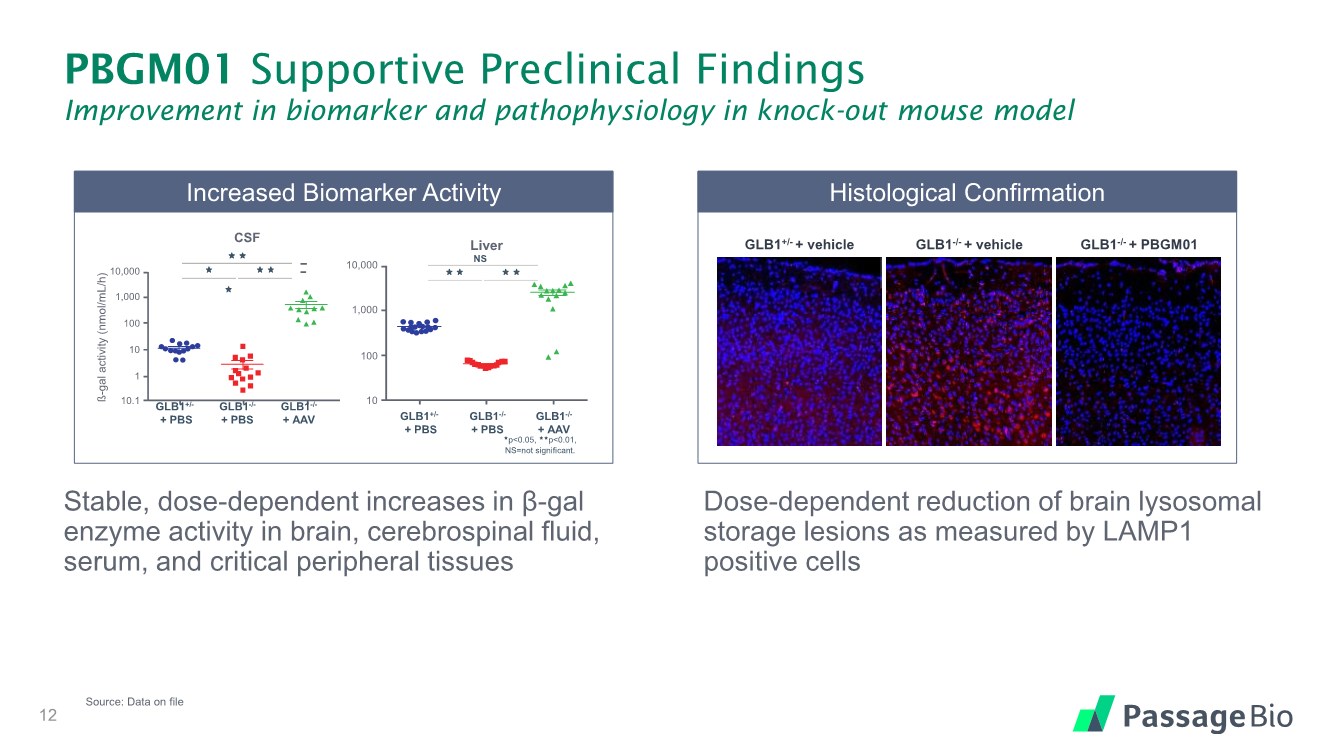
| PBGM01 Supportive Preclinical Findings Improvement in biomarker and pathophysiology in knock-out mouse model Stable, dose-dependent increases in β-gal enzyme activity in brain, cerebrospinal fluid, serum, and critical peripheral tissues Dose-dependent reduction of brain lysosomal storage lesions as measured by LAMP1 positive cells 12 Source: Data on file p<0.05, p<0.01, NS=not significant. GLB1 +/ - + PBS GLB1 - / - + PBS GLB1 - / - + AAV 10,000 10 1 10.1 CSF 100 1,000 ß - gal activity ( nmol /mL/h) GLB1+/- + PBS GLB1-/- + PBS GLB1-/- + AAV GLB1 +/ - + PBS GLB1 - / - + PBS GLB1 - / - + AAV 10,000 1,000 100 10 NS Liver GLB1+/- + PBS GLB1-/- + PBS GLB1-/- + AAV NS Increased Biomarker Activity Histological Confirmation GLB1+/- + vehicle GLB1-/- + vehicle GLB1-/- + PBGM01 |
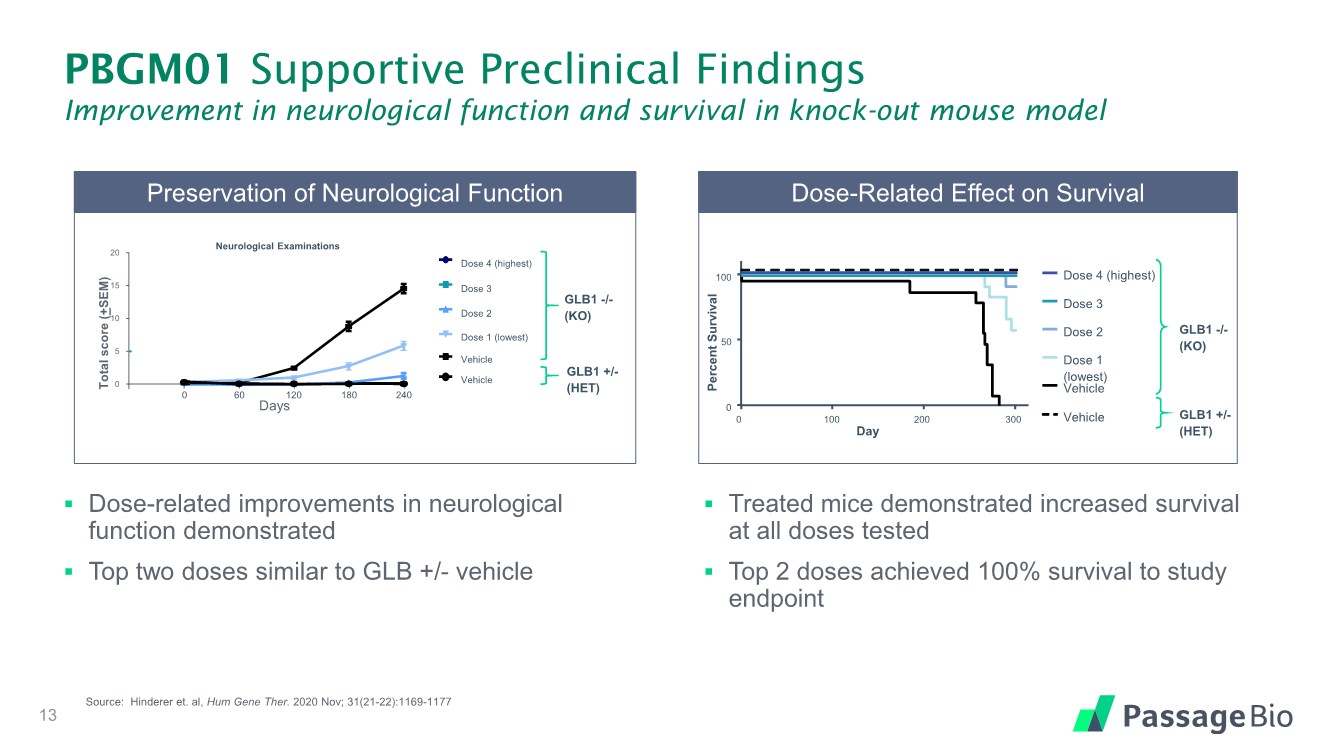
| Dose-Related Effect on Survival GLB1 -/- (KO) GLB1 +/- (HET) Dose 4 (highest) Dose 3 Dose 2 Dose 1 (lowest) Vehicle Vehicle Percent Survival 0 Day 100 200 300 0 50 100 Preservation of Neurological Function 0 5 10 15 20 Total score ( + SEM) Days 240 180 120 60 0 Neurological Examinations GLB1 -/- (KO) GLB1 +/- (HET) Dose 4 (highest) Dose 3 Dose 2 Dose 1 (lowest) Vehicle Vehicle PBGM01 Supportive Preclinical Findings Improvement in neurological function and survival in knock-out mouse model ▪ Dose-related improvements in neurological function demonstrated ▪ Top two doses similar to GLB +/- vehicle ▪ Treated mice demonstrated increased survival at all doses tested ▪ Top 2 doses achieved 100% survival to study endpoint 13 Source: Hinderer et. al, Hum Gene Ther. 2020 Nov; 31(21-22):1169-1177 |
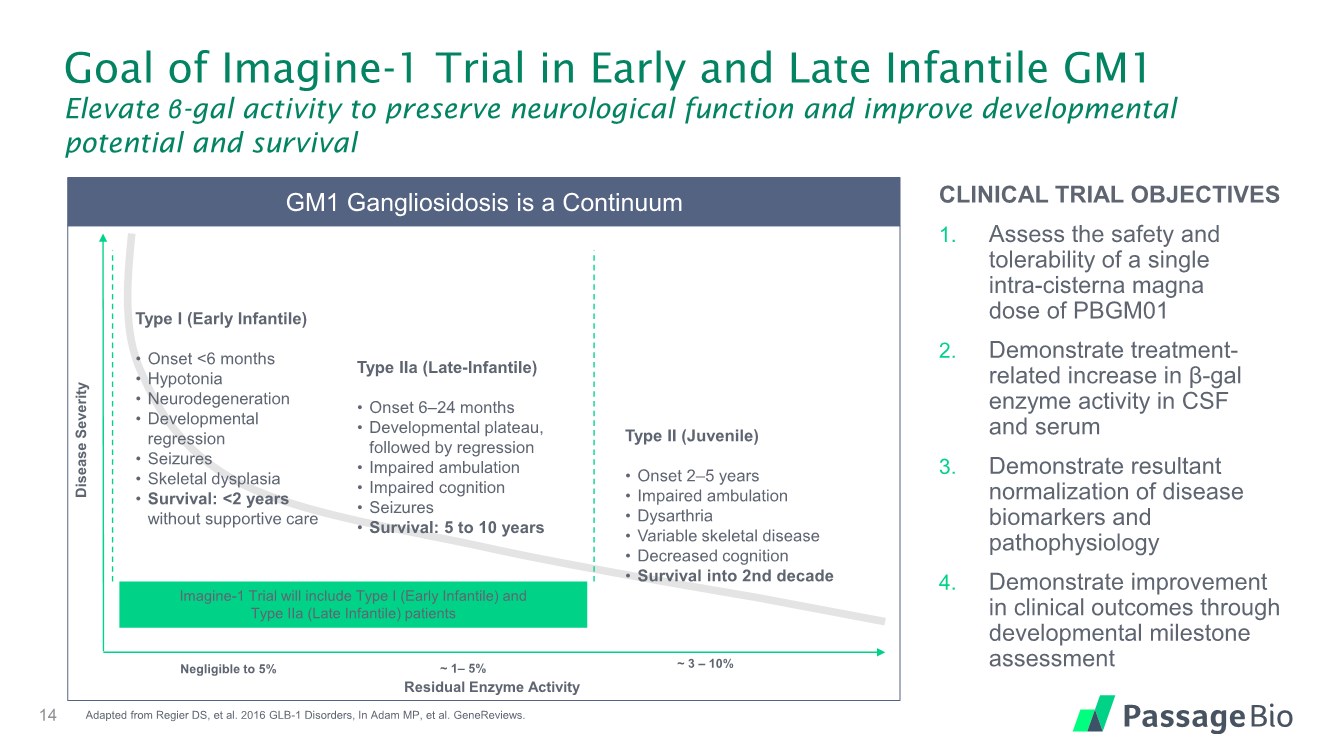
| Goal of Imagine-1 Trial in Early and Late Infantile GM1 Elevate β-gal activity to preserve neurological function and improve developmental potential and survival CLINICAL TRIAL OBJECTIVES 1. Assess the safety and tolerability of a single intra-cisterna magna dose of PBGM01 2. Demonstrate treatment- related increase in β-gal enzyme activity in CSF and serum 3. Demonstrate resultant normalization of disease biomarkers and pathophysiology 4. Demonstrate improvement in clinical outcomes through developmental milestone assessment 14 GM1 Gangliosidosis is a Continuum Disease Severity Residual Enzyme Activity Imagine-1 Trial will include Type I (Early Infantile) and Type IIa (Late Infantile) patients Negligible to 5% ~ 1– 5% ~ 3 – 10% Type I (Early Infantile) • Onset <6 months • Hypotonia • Neurodegeneration • Developmental regression • Seizures • Skeletal dysplasia • Survival: <2 years without supportive care Type IIa (Late-Infantile) • Onset 6–24 months • Developmental plateau, followed by regression • Impaired ambulation • Impaired cognition • Seizures • Survival: 5 to 10 years Type II (Juvenile) • Onset 2–5 years • Impaired ambulation • Dysarthria • Variable skeletal disease • Decreased cognition • Survival into 2nd decade Adapted from Regier DS, et al. 2016 GLB-1 Disorders, In Adam MP, et al. GeneReviews. |
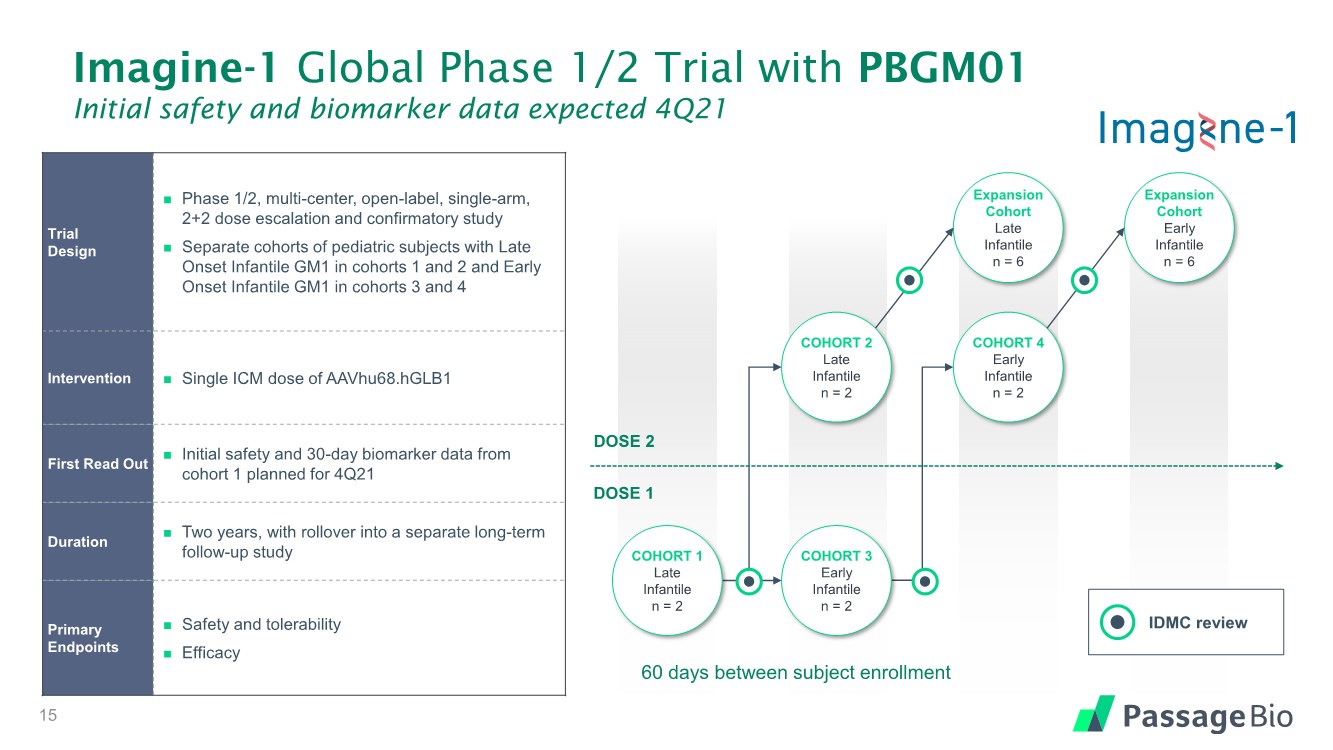
| Imagine-1 Global Phase 1/2 Trial with PBGM01 Initial safety and biomarker data expected 4Q21 Trial Design ◼ Phase 1/2, multi-center, open-label, single-arm, 2+2 dose escalation and confirmatory study ◼ Separate cohorts of pediatric subjects with Late Onset Infantile GM1 in cohorts 1 and 2 and Early Onset Infantile GM1 in cohorts 3 and 4 Intervention ◼ Single ICM dose of AAVhu68.hGLB1 First Read Out ◼ Initial safety and 30-day biomarker data from cohort 1 planned for 4Q21 Duration ◼ Two years, with rollover into a separate long-term follow-up study Primary Endpoints ◼ Safety and tolerability ◼ Efficacy COHORT 4 Early Infantile n = 2 DOSE 2 DOSE 1 Expansion Cohort Early Infantile n = 6 Expansion Cohort Late Infantile n = 6 COHORT 2 Late Infantile n = 2 COHORT 3 Early Infantile n = 2 COHORT 1 Late Infantile n = 2 IDMC review 15 60 days between subject enrollment |
| PBKR03 Krabbe Disease |
| Krabbe Disease A Devastating Pediatric Disease 17 Source: NIH, CHOP, American Journal of Neuroradiology, Third Party Research FATAL, PEDIATRIC NEUROLOGICAL LYSOSOMAL STORAGE DISORDER caused by GALC gene mutations characterized by demyelination of neurons in the brain and periphery. Disease progression is rapid and highly predictable including loss of acquired milestones, staring episodes, peripheral neuropathy, seizures, blindness and deafness. RARE AND UNDERSERVED populations with incidence of up to ~2.6 per 100,000 live births worldwide. No disease-modifying therapies are presently approved. |
| PBKR03 Potential Transformative Therapy for Rare, Underserved Disorder 18 ▪ Next-generation, proprietary AAVhu68 capsid delivers functional GALC gene encoding galactosylceramidase (GALC) to the brain and peripheral tissues ▪ PBKR03-treated Krabbe dogs had improved central and peripheral myelination, reduced neuroinflammation and increased survival rates with full phenotypic recovery ▪ Received regulatory clearances from FDA, MHRA (UK) and Health Canada for global Phase 1/2 clinical trial ▪ Received Orphan Drug, Rare Pediatric Disease and Fast Track designations by FDA and Orphan designation by EC for treatment of Krabbe disease ▪ Clinical trial initiation expected in 3Q21 |
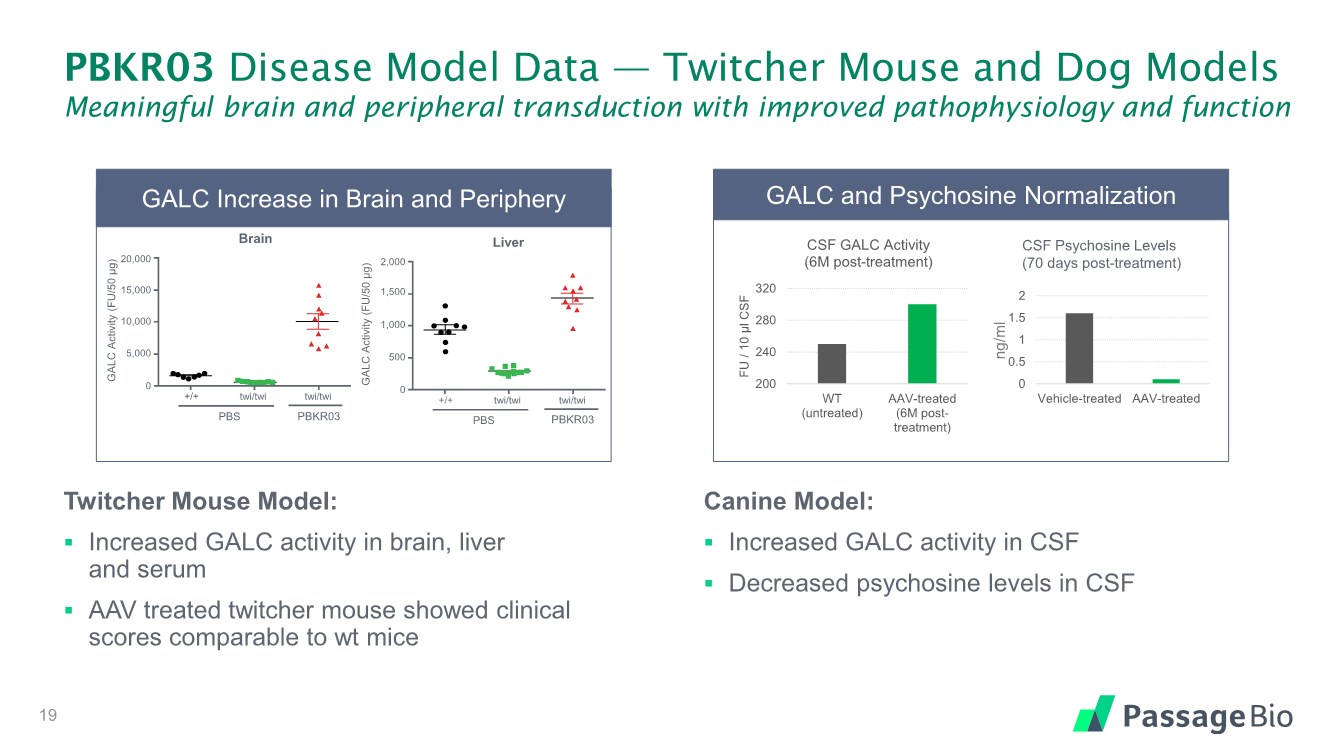
| PBKR03 Disease Model Data — Twitcher Mouse and Dog Models Meaningful brain and peripheral transduction with improved pathophysiology and function Twitcher Mouse Model: ▪ Increased GALC activity in brain, liver and serum ▪ AAV treated twitcher mouse showed clinical scores comparable to wt mice Canine Model: ▪ Increased GALC activity in CSF ▪ Decreased psychosine levels in CSF 19 GALC Increase in Brain and Periphery GALC Activity (FU/50 µg) 20,000 15,000 10,000 0 5,000 +/+ twi/twi twi/twi PBS GTP-206 Brain PBKR03 GALC Activity (FU/50 µg) 2,000 1,500 0 500 +/+ twi/twi twi/twi PBS GTP-206 1,000 Liver PBKR03 GALC and Psychosine Normalization 200 240 280 320 WT (untreated) AAV-treated (6M post- treatment) FU / 10 µl CSF CSF GALC Activity (6M post-treatment) CSF Psychosine Levels (70 days post-treatment) ng/ml 0 0.5 1 1.5 2 Vehicle-treated AAV-treated |
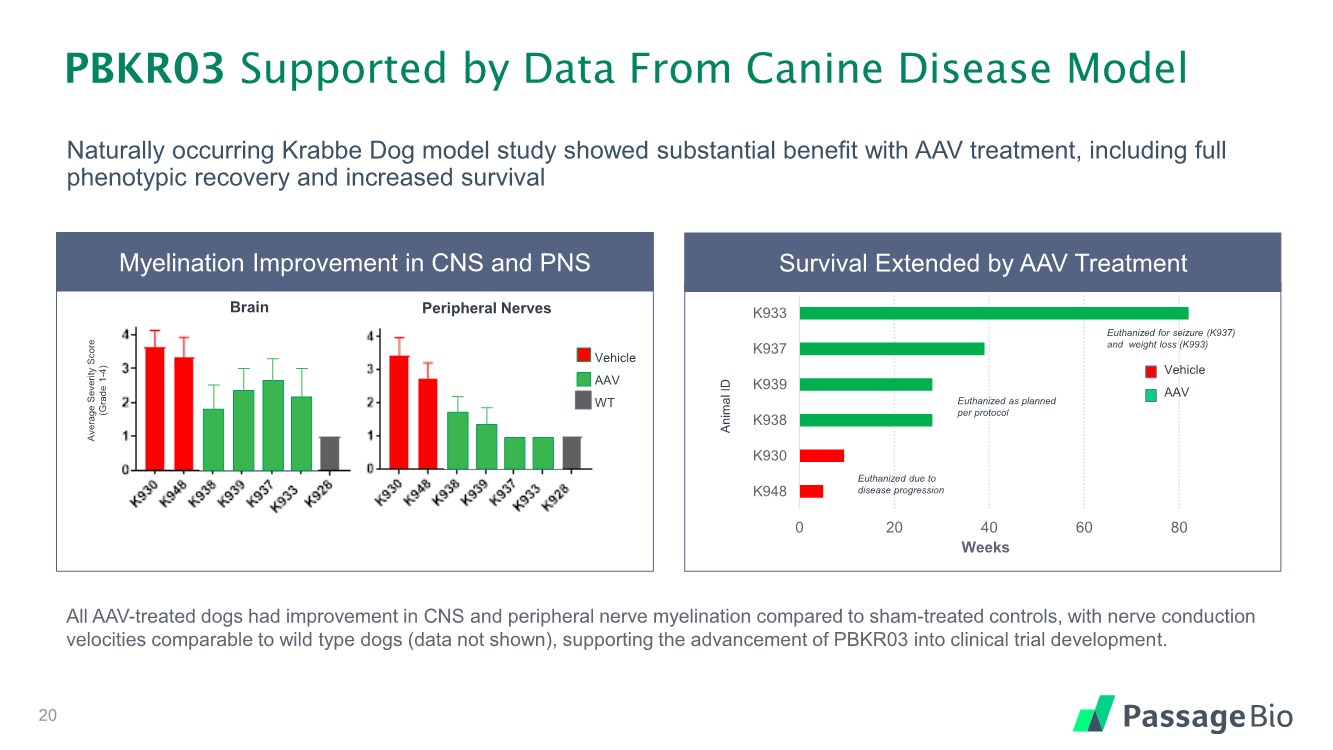
| Naturally occurring Krabbe Dog model study showed substantial benefit with AAV treatment, including full phenotypic recovery and increased survival 0 20 40 60 80 K948 K930 K938 K939 K937 K933 Weeks PBKR03 Supported by Data From Canine Disease Model Survival Extended by AAV Treatment Euthanized due to disease progression Euthanized as planned per protocol Euthanized for seizure (K937) and weight loss (K993) Myelination Improvement in CNS and PNS Brain Peripheral Nerves Animal ID Average Severity Score (Grade 1 - 4) Vehicle AAV WT Vehicle AAV All AAV-treated dogs had improvement in CNS and peripheral nerve myelination compared to sham-treated controls, with nerve conduction velocities comparable to wild type dogs (data not shown), supporting the advancement of PBKR03 into clinical trial development. 20 |
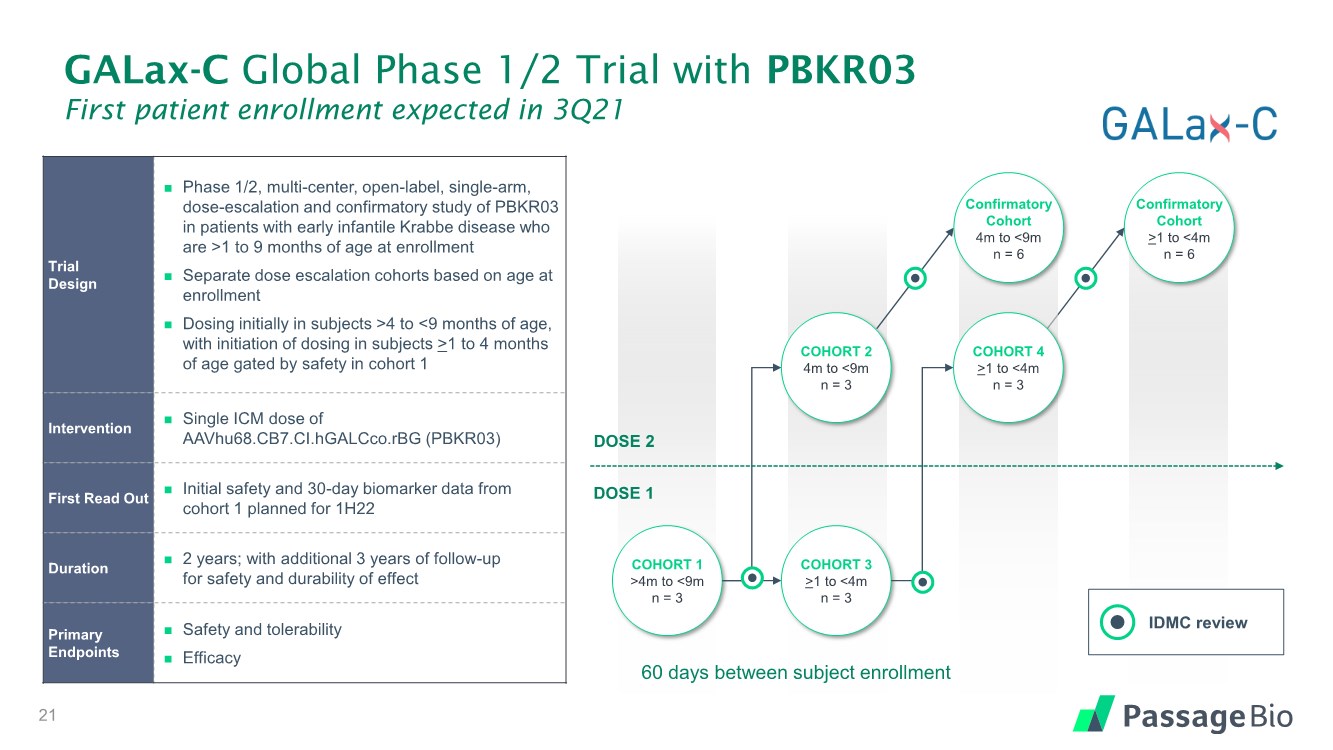
| COHORT 2 4m to <9m n = 3 COHORT 3 >1 to <4m n = 3 COHORT 1 >4m to <9m n = 3 GALax-C Global Phase 1/2 Trial with PBKR03 First patient enrollment expected in 3Q21 Trial Design ◼ Phase 1/2, multi-center, open-label, single-arm, dose-escalation and confirmatory study of PBKR03 in patients with early infantile Krabbe disease who are >1 to 9 months of age at enrollment ◼ Separate dose escalation cohorts based on age at enrollment ◼ Dosing initially in subjects >4 to <9 months of age, with initiation of dosing in subjects >1 to 4 months of age gated by safety in cohort 1 Intervention ◼ Single ICM dose of AAVhu68.CB7.CI.hGALCco.rBG (PBKR03) First Read Out ◼ Initial safety and 30-day biomarker data from cohort 1 planned for 1H22 Duration ◼ 2 years; with additional 3 years of follow-up for safety and durability of effect Primary Endpoints ◼ Safety and tolerability ◼ Efficacy IDMC review Confirmatory Cohort >1 to <4m n = 6 21 Confirmatory Cohort 4m to <9m n = 6 COHORT 4 >1 to <4m n = 3 DOSE 2 DOSE 1 60 days between subject enrollment |
| PBFT02 Frontotemporal Dementia — GRN |
| FTD-GRN A Devastating Adult Disease DEVASTATING FORM OF DEMENTIA caused by a GRN gene mutation resulting in progranulin (PGRN) deficiency. Approximately 5–10% of FTD is caused by a GRN mutation. Disease progression is rapid and degenerative including loss of speech, loss of expression, severe behavioral changes and immobility. RARE AND UNDERSERVED populations with estimated U.S. prevalence of ~3,000 to 6,000 patients. No disease-modifying therapies are presently approved. 23 |
| PBFT02 Potential Transformative Therapy for Rare, Underserved Disorder ▪ Proprietary AAV1 capsid delivers function GRN gene encoding progranulin ▪ PBFT02 demonstrated increases in CSF PGRN concentrations in NHP studies to >50-fold normal human CSF PGRN concentrations ▪ Received regulatory clearances from FDA and Health Canada for global Phase 1/2 clinical trial ▪ Received FDA Orphan Drug and Fast Track designations ▪ Clinical trial initiation expected in 2Q/3Q21 24 |
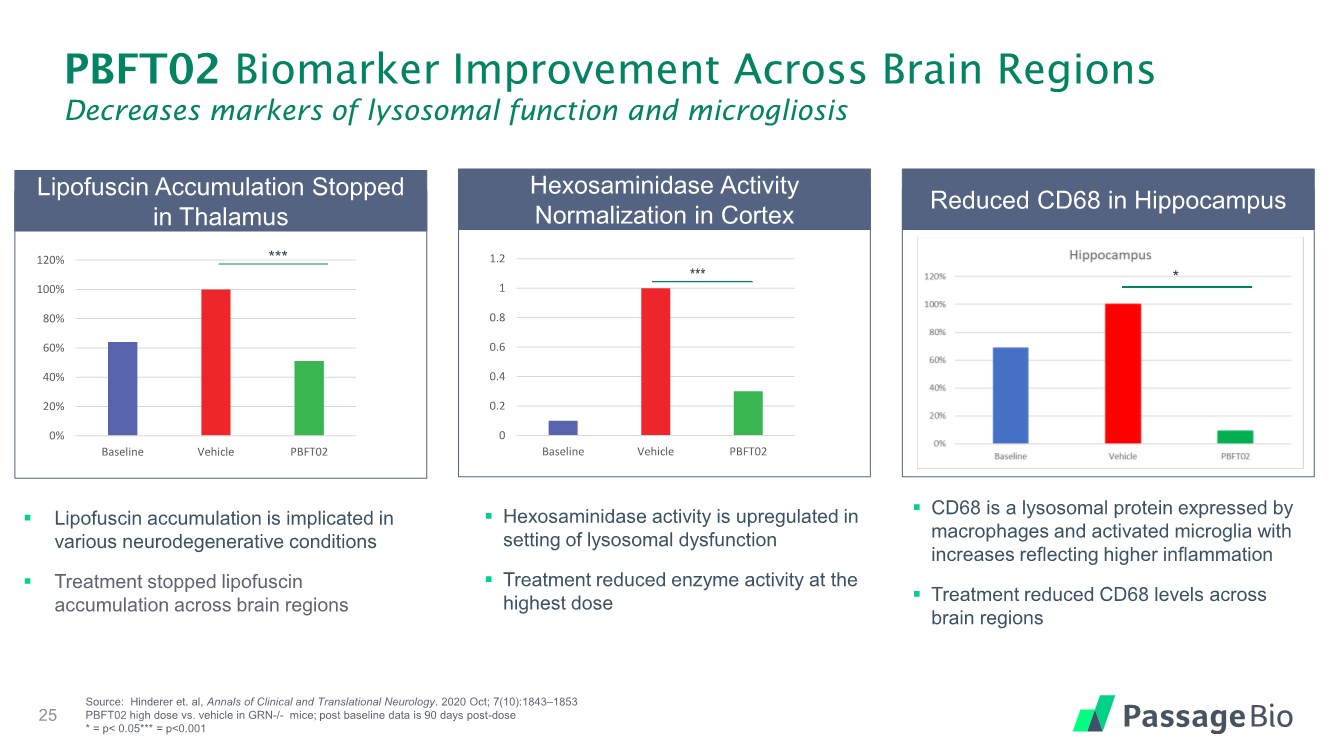
| PBFT02 Biomarker Improvement Across Brain Regions Decreases markers of lysosomal function and microgliosis 25 Source: Hinderer et. al, Annals of Clinical and Translational Neurology. 2020 Oct; 7(10):1843–1853 PBFT02 high dose vs. vehicle in GRN-/- mice; post baseline data is 90 days post-dose * = p< 0.05*** = p<0.001 *** * 0% 20% 40% 60% 80% 100% 120% Baseline Vehicle PBFT02 0 0.2 0.4 0.6 0.8 1 1.2 Baseline Vehicle PBFT02 Lipofuscin Accumulation Stopped in Thalamus Hexosaminidase Activity Normalization in Cortex Reduced CD68 in Hippocampus ▪ Lipofuscin accumulation is implicated in various neurodegenerative conditions ▪ Treatment stopped lipofuscin accumulation across brain regions ▪ Hexosaminidase activity is upregulated in setting of lysosomal dysfunction ▪ Treatment reduced enzyme activity at the highest dose ▪ CD68 is a lysosomal protein expressed by macrophages and activated microglia with increases reflecting higher inflammation ▪ Treatment reduced CD68 levels across brain regions |
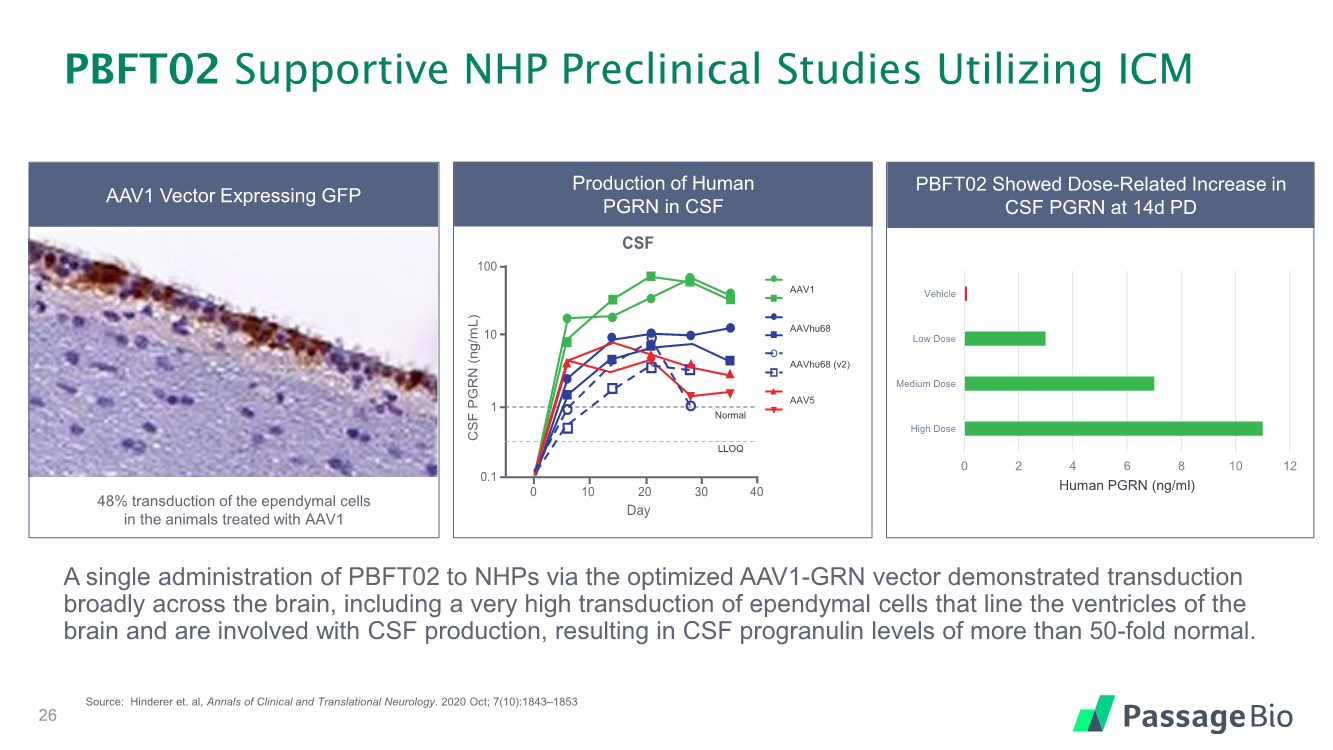
| PBFT02 Supportive NHP Preclinical Studies Utilizing ICM A single administration of PBFT02 to NHPs via the optimized AAV1-GRN vector demonstrated transduction broadly across the brain, including a very high transduction of ependymal cells that line the ventricles of the brain and are involved with CSF production, resulting in CSF progranulin levels of more than 50-fold normal. 26 Source: Hinderer et. al, Annals of Clinical and Translational Neurology. 2020 Oct; 7(10):1843–1853 PBFT02 Showed Dose-Related Increase in CSF PGRN at 14d PD 0 2 4 6 8 10 12 High Dose Medium Dose Low Dose Vehicle Human PGRN (ng/ml) Production of Human PGRN in CSF CSF PGRN (ng/mL) 0 10 20 30 40 CSF 0.1 1 10 100 LLOQ Normal Day RA2981 RA2982 RA3027 RA3153 RA3151 RA3170 RA3155 RA3160 AAVhu68 AAVhu68 (v2) AAV1 AAV5 RA2981 RA2982 RA3027 RA3153 RA3151 RA3170 R A3155 RA3160 A A V hu 68 A A V hu 68 (v 2) AAV1 AAV5 RA2981 RA2982 RA3027 RA3153 RA3151 RA3170 R A3155 RA3160 A A V hu 68 A A V hu 68 (v 2) AAV1 AAV5 AAVhu68 AAVhu68 (v2) AAV5 AAV1 Normal LLOQ AAVhu68 AAV1 Ependyma 1–2% transduction 48% transduction AAV1 Vector Expressing GFP 48% transduction of the ependymal cells in the animals treated with AAV1 |
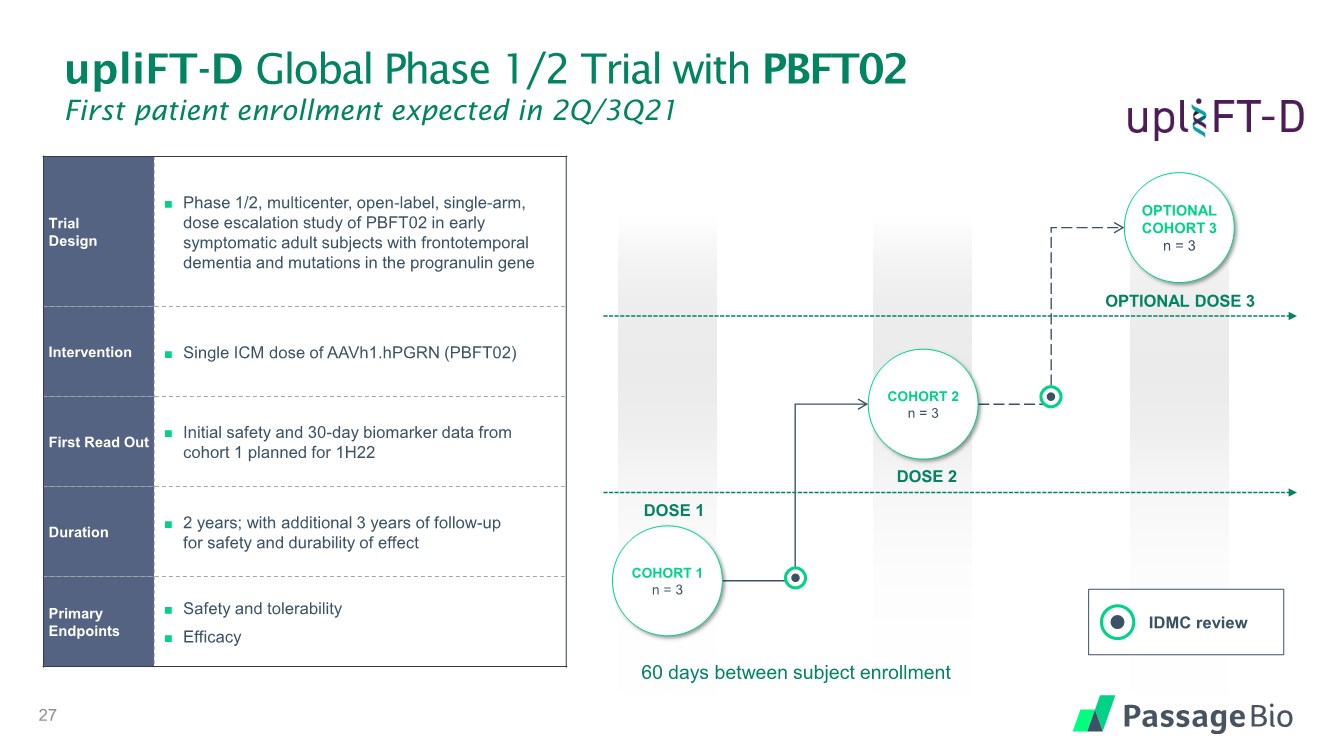
| upliFT-D Global Phase 1/2 Trial with PBFT02 First patient enrollment expected in 2Q/3Q21 Trial Design ◼ Phase 1/2, multicenter, open-label, single-arm, dose escalation study of PBFT02 in early symptomatic adult subjects with frontotemporal dementia and mutations in the progranulin gene Intervention ◼ Single ICM dose of AAVh1.hPGRN (PBFT02) First Read Out ◼ Initial safety and 30-day biomarker data from cohort 1 planned for 1H22 Duration ◼ 2 years; with additional 3 years of follow-up for safety and durability of effect Primary Endpoints ◼ Safety and tolerability ◼ Efficacy OPTIONAL DOSE 3 DOSE 2 DOSE 1 27 60 days between subject enrollment COHORT 1 n = 3 COHORT 2 n = 3 OPTIONAL COHORT 3 n = 3 IDMC review |
| State-of-the-Art Manufacturing |
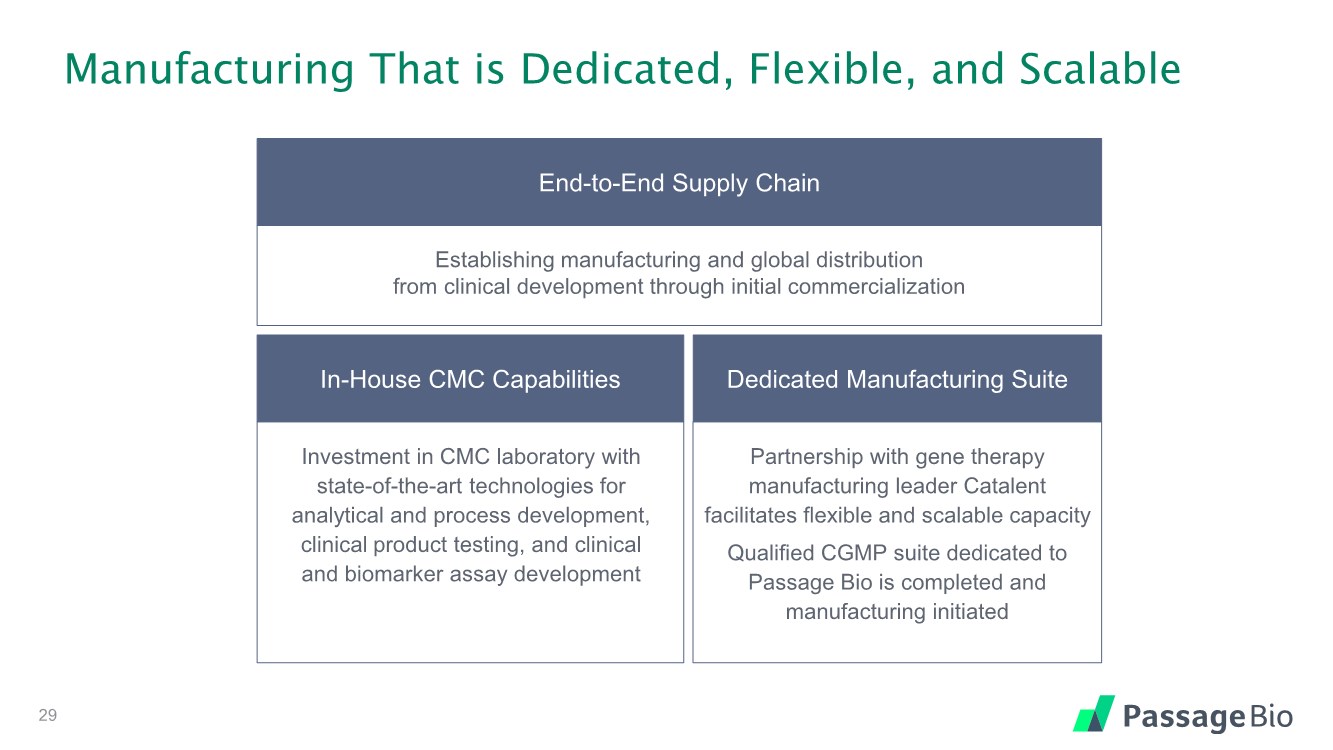
| Manufacturing That is Dedicated, Flexible, and Scalable In-House CMC Capabilities Investment in CMC laboratory with state-of-the-art technologies for analytical and process development, clinical product testing, and clinical and biomarker assay development End-to-End Supply Chain Establishing manufacturing and global distribution from clinical development through initial commercialization Dedicated Manufacturing Suite Partnership with gene therapy manufacturing leader Catalent facilitates flexible and scalable capacity Qualified CGMP suite dedicated to Passage Bio is completed and manufacturing initiated 29 |
| Corporate Foundation 30 SOURCE: www.brandywinerealty.com |
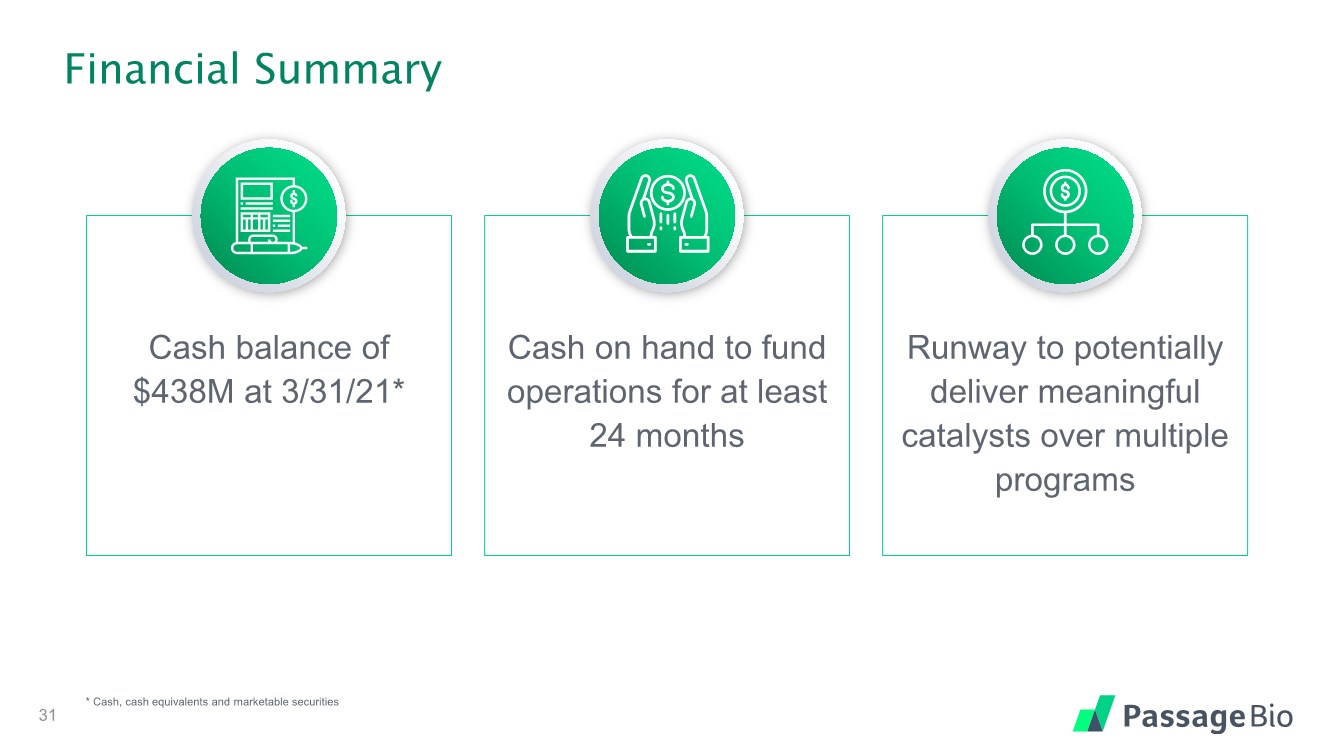
| Financial Summary 31 * Cash, cash equivalents and marketable securities Cash balance of $438M at 3/31/21* Cash on hand to fund operations for at least 24 months Runway to potentially deliver meaningful catalysts over multiple programs |
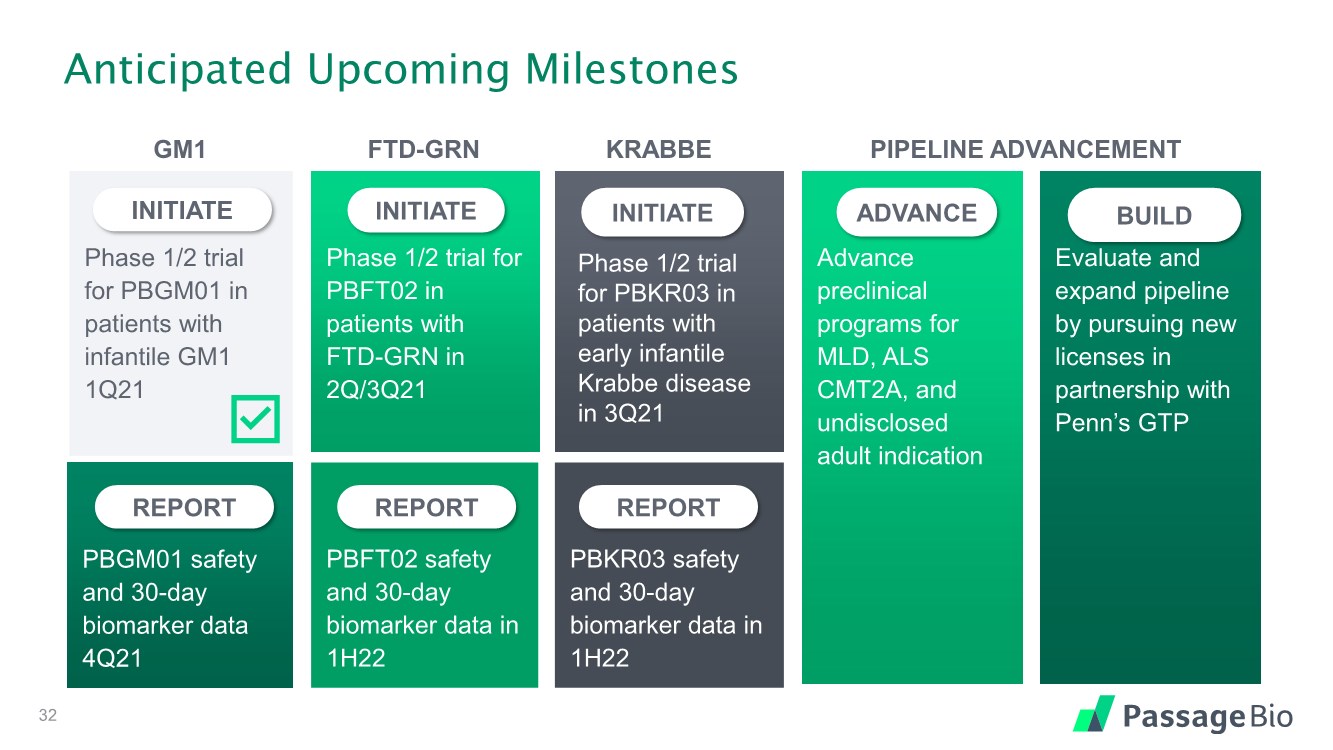
| Anticipated Upcoming Milestones Phase 1/2 trial for PBGM01 in patients with infantile GM1 1Q21 INITIATE Phase 1/2 trial for PBFT02 in patients with FTD-GRN in 2Q/3Q21 INITIATE Advance preclinical programs for MLD, ALS CMT2A, and undisclosed adult indication ADVANCE Evaluate and expand pipeline by pursuing new licenses in partnership with Penn’s GTP BUILD INITIATE PBGM01 safety and 30-day biomarker data 4Q21 REPORT PBFT02 safety and 30-day biomarker data in 1H22 REPORT PBKR03 safety and 30-day biomarker data in 1H22 REPORT GM1 FTD-GRN KRABBE PIPELINE ADVANCEMENT Phase 1/2 trial for PBKR03 in patients with early infantile Krabbe disease in 3Q21 32 |
| The Passage Bio Advantage Corporate model designed for success PENN GTP PARTNERSHIP Led by renowned innovator James M. Wilson, MD, PhD Access to cutting-edge research and development Access to next-generation technologies BROAD AND ROBUST PIPELINE 17 total program license options 3 clinical-stage therapies 4 research-stage pipeline candidates 10 additional new pipeline license options INTEGRATED MANUFACTURING SUPPLY CHAIN Flexible, scalable Dedicated CGMP suite Internal CMC analytics and assay infrastructure State-of-the-art technology WELL-POSITIONED CORPORATE FOUNDATION Robust financial position Leaders in pediatric and adult neurodegenerative disease therapy development Deep experience in gene therapy manufacturing 33 |
| www.passagebio.com NASDAQ GS: PASG THANK YOU |
| Demonstrated Leadership Deep experience in rare disease, CNS disorders and gene therapy LEADERSHIP TEAM BOARD OF DIRECTORS Tachi Yamada, M.D. (Chair) Frazier Athena Countouriotis, M.D. Turning Point Bruce Goldsmith, Ph.D. Passage Bio Maxine Gowen, Ph.D. Tamuro Bio Patrick Heron Frazier Saqib Islam, J.D. SpringWorks Sandip Kapadia Intercept Liam Ratcliffe, M.D., Ph.D. Access Industries Tom Woiwode, Ph.D. Versant Rich Morris Chief Financial Officer Chip Cale General Counsel Alex Fotopoulos Chief Technology Officer Bruce Goldsmith, Ph.D. Chief Executive Officer Jill M. Quigley Chief Operating Officer Gary Romano, M.D., Ph.D. Chief Medical Officer Eliseo Salinas, M.D. Chief R&D Officer 35 |
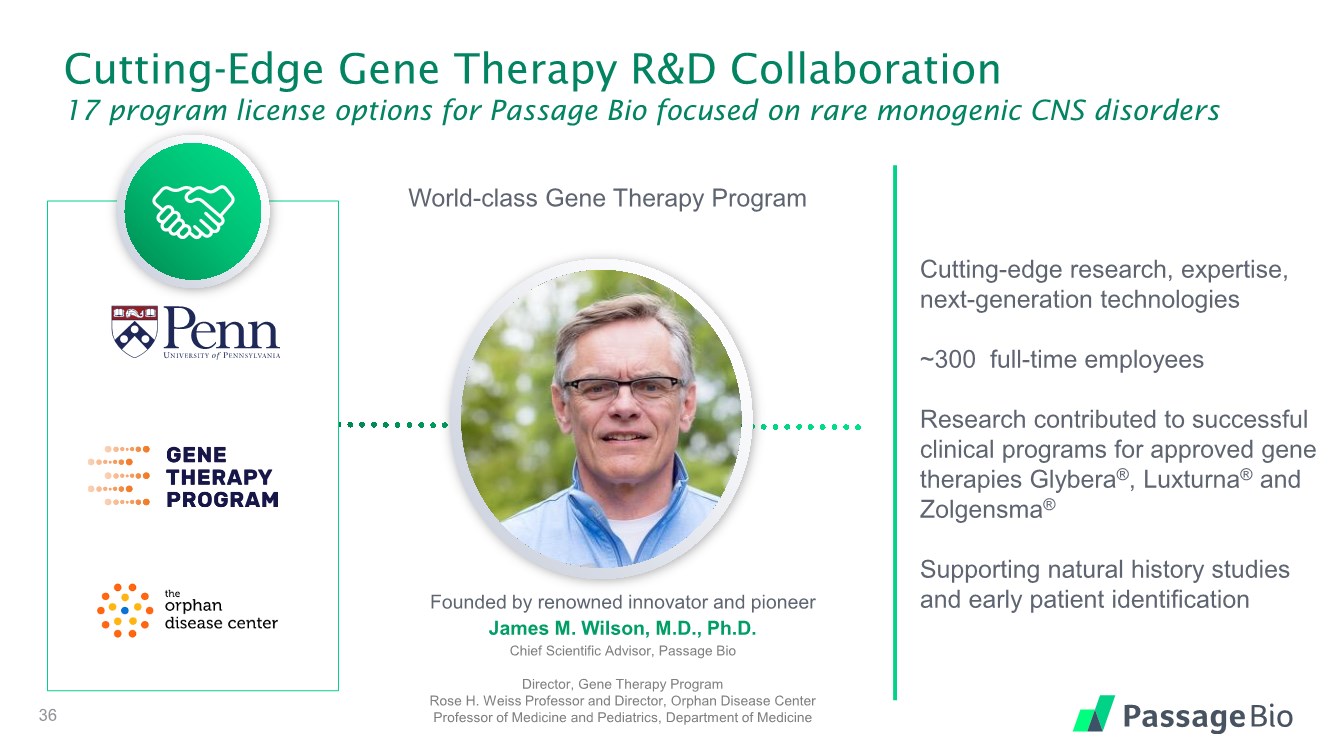
| Cutting-Edge Gene Therapy R&D Collaboration 17 program license options for Passage Bio focused on rare monogenic CNS disorders Founded by renowned innovator and pioneer James M. Wilson, M.D., Ph.D. Chief Scientific Advisor, Passage Bio Director, Gene Therapy Program Rose H. Weiss Professor and Director, Orphan Disease Center Professor of Medicine and Pediatrics, Department of Medicine Cutting-edge research, expertise, next-generation technologies ~300 full-time employees Research contributed to successful clinical programs for approved gene therapies Glybera®, Luxturna® and Zolgensma® Supporting natural history studies and early patient identification World-class Gene Therapy Program 36 |