Attached files
| file | filename |
|---|---|
| 8-K - 8-K - LUMOS PHARMA, INC. | lumo-2021q1erx8k.htm |
| EX-99.1 - PRESS RELEASE - LUMOS PHARMA, INC. | lumo-2021q1x8kxex991.htm |

1 First Quarter 2021 May 5, 2021

2 Forward Looking Statements This presentation contains forward-looking statements of Lumos Pharma, Inc. (the “Company”) that involve substantial risks and uncertainties. All such statements contained in this presentation are forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. The words “forecast,” “projected,” "guidance," "upcoming," "will," “would,” "plan," “intend,” "anticipate," "approximate," "expect," “potential,” “imminent,” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among others, the ability of prior research results to forecast the performance of therapeutic agents in the clinic, anticipated business development activities, anticipated market reception to our treatment regimen for PGHD and other indications, plans related to initiation and execution of clinical trials; plans related to moving additional indications into clinical development; future financial performance, results of operations, cash position and sufficiency of capital resources to fund its operating requirements; and any other statements other than statements of historical fact. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements that the Company makes due to a number of important factors, including the effects of pandemics or other widespread health problems, the outcome of our future interactions with regulatory authorities, our ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, the ability to obtain the necessary patient enrollment for our product candidate in a timely manner, the ability to successfully develop our product candidate, the timing and ability of Lumos to raise additional equity capital as needed and other risks that could cause actual results to differ materially from those matters expressed in or implied by such forward-looking statements as discussed in "Risk Factors" and elsewhere in Lumos Pharma’s Annual Report on Form 10-K for the year ended December 31, 2020 and other reports filed with the SEC. The forward-looking statements in this presentation represent the Company’s views as of the date of this presentation. The Company anticipates that subsequent events and developments will cause their views to change. However, while it may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so. You should, therefore, not rely on these forward-looking statements as representing the Company’s views as of any date subsequent to the date of this presentation. 5.5.2021

3 Agenda • Lisa Miller, Senior Director of Investor Relations • Rick Hawkins, CEO, President & Chairman • John McKew, PhD, COO & CSO • Lori Lawley, SVP of Finance & Incoming CFO

4 First Quarter 2021 Update LUM-201 Data PGHD Trials Solid Cash Position • Final tranche of $26.0M from PRV monetization received in January 2021 • Cash runway through anticipated OraGrowtH210 & OraGrowtH212 trial readouts • Balance sheet flexibility to acquire additional rare disease assets • ENDO Presentation – LUM-201 differentiated from standard GH secretagogues • PK/PD Data – Pulsatile MOA and potential for efficacy in PEM+ PGHD patients • Phase 2b OraGrowtH210 Trial in PGHD – Enrolling with data expected mid-2022 • PK/PD OraGrowtH212 Trial in PGHD – Initiation expected Q2 2021

5 PEMs Enrich Trials for Patients with Functional but Reduced GH Secretion HP-GH – hypothalamic pituitary growth hormone 1 Bright 2021 JES 2 Blum 2021 JES Moderate (PEM+): Included in Clinical Trials Severe (PEM ): Excluded from Clinical Trials Predictive Enrichment Markers (PEMs): Data demonstrate GH response to single LUM-201 dose and baseline IGF-1 have potential to distinguish these populations IGF-1 Pituitary Hypothalamus G H S R 1 a G H S R 1 a GH SST GHRH Pituitary Hypothalamus G H S R 1 a G H S R 1 a GH SST GHRH Non-functional HP-GH axis o Unable to secrete GH o Not expected to respond to LUM-201 o Represents ~40% of PGHD patients2 LUM-201 LUM-201 Functional but reduced HP-GH axis o Able to secrete some, but insufficient, GH o Expected to respond to LUM-2011 o Represents ~60% of PGHD patients2

6 PK/PD Data Show LUM-201 Pulsatile MOA & Potential Efficacy in PGHD Patients PEM Positive PEM Negative Patient A Patient B Patient C Baseline 6months Baseline 6months Baseline 6months IGF-1 (ng/ml) 182 231 53 72 17 15 Q20m 24h GH Mean (ng/ml) 3.4 6.3 1.0 1.3 0.5 0.3 AUC (ng*hr/ml) 75.5 137.3 17.6 25.0 4.9 3.4 Height Velocity (cm/yr) 3.7 7.9 3.5 8.9 1.1 1.8 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 0 6 12 18 24 30 Sampling Time (hr) S er u m G ro w th H o rm o n e (n g /m l) Patient A Baseline 6 months 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 0.0 0.2 0.4 0.6 0.8 1.0 1.2 Sampling Time (hr) S e ru m G ro w th H o rm o n e ( n g /m l) Patient C Baseline 6 months Merck Study 020 patient subset. Cassorla, F. PEM-Positive Patient A PEM-Negative Patient C

7 ENDO 2021 – LUM-201 Differentiated from Standard GH Secretagogues in PGHD Bright, G. ENDO 2021. Comparison of GH Responses to LUM-201 and to 2 standard GH stimulation tests *Note both parameters are displayed on logarithmic scales

8 OraGrowtH210 Trial: Phase 2b Trial Evaluating LUM-201 in PGHD N=20 Daily rhGH injection N=20 LUM-201: 3.2 mg/kg/day N=20 LUM-201: 1.6 mg/kg/day N=20 LUM-201: 0.8 mg/kg/day • n = 80 • PEM(+) PGHD patients • Inclusion: stim GH ≥ 5ng/ml and baseline IGF-1 > 30ng/ml • rhGH treatment naïve • 40-50 trial sites US & International • Trial opened Q4 2020 Goals: • Prospectively confirm utility of PEM strategy • Confirm reproducibility of PEM classification • Determine optimal dose for Phase 3 Primary Endpoint: • Annualized Height Velocity (AHV) Anticipate Phase 2b OraGrowtH210 Trial data readout mid-2022 Total Study Duration – 6 months Objectives TreatmentRandomizationScreening R

9 • n = 24 • Open-label study • PGHD patients • rhGH-treatment naïve • 6-month dosing • Single, specialized clinical site • Q10 minute sampling for 12 hours OraGrowtH212 Trial: Pharmacokinetic / Pharmacodynamic Trial in PGHD Goals: • Confirm prior adult PK/PD data • Support future regulatory filings & commercialization Primary Endpoints: • Assess LUM-201 effect on endogenous GH pulsatility • Evaluate PK in children Anticipated initiation in Q2 2021 To run concurrent with Phase 2b OraGrowtH210 Trial Total Study Duration – 6 months Objectives 12 - LUM-201: 3.2 mg/kg/day 12 - LUM-201: 1.6 mg/kg/day R TreatmentRandomizationScreening
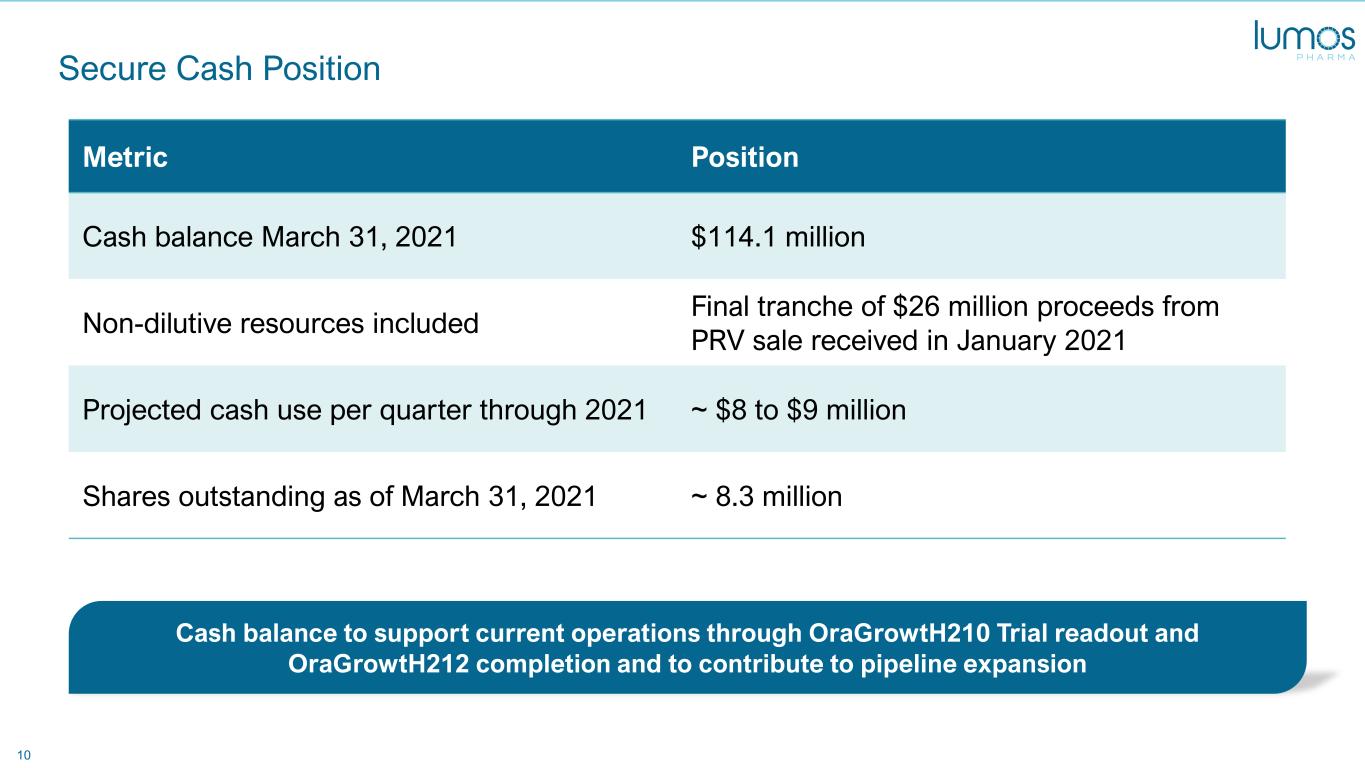
10 Secure Cash Position Metric Position Cash balance March 31, 2021 $114.1 million Non-dilutive resources included Final tranche of $26 million proceeds from PRV sale received in January 2021 Projected cash use per quarter through 2021 ~ $8 to $9 million Shares outstanding as of March 31, 2021 ~ 8.3 million Cash balance to support current operations through OraGrowtH210 Trial readout and OraGrowtH212 completion and to contribute to pipeline expansion

11 Therapeutics For Rare Diseases
