Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Aldeyra Therapeutics, Inc. | d39940dex992.htm |
| 8-K - 8-K - Aldeyra Therapeutics, Inc. | d39940d8k.htm |

Exhibit 99.1 April 27, 2021 DATA RELEASE Top-Line Results from the Phase 3 INVIGORATE Trial in Allergic Conjunctivitis Nasdaq: ALDX © Aldeyra Therapeutics, Inc. 2021Exhibit 99.1 April 27, 2021 DATA RELEASE Top-Line Results from the Phase 3 INVIGORATE Trial in Allergic Conjunctivitis Nasdaq: ALDX © Aldeyra Therapeutics, Inc. 2021

Disclaimers and Forward-Looking Statements This presentation and various remarks which may be made during this presentation contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Section 21E of the Securities Exchange Act of 1934, as amended, including statements regarding Aldeyra’s possible or assumed future results of operations, expenses and financing needs, business strategies and plans, research and development plans or expectations, political, economic, legal, social and health risks, including the recent COVID-19 outbreak and subsequent public health measures and other responses to it, that may affect Aldeyra’s business or the global economy, the structure, timing and success of Aldeyra’s planned or pending clinical trials, expected milestones, market sizing, pricing and reimbursement, competitive position, regulatory matters, industry environment and potential growth opportunities, among other things. The results of earlier preclinical or clinical trials may not be predictive of future results. As a result of the COVID-19 pandemic, clinical site availability, staffing, and patient recruitment have been negatively affected and the timelines to complete Aldeyra’s clinical trials may be delayed. Forward-looking statements include all statements that are not historical facts and, in some cases, can be identified by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “target,” “design,” “estimate,” “predict,” “potential,” “plan” or similar expressions and the negatives of those terms. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause Aldeyra’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These statements reflect Aldeyra’s current views with respect to future events and are based on assumptions and subject to risks and uncertainties, including the development, clinical and regulatory plans or expectations for Aldeyra’s product candidates and systems-based approaches, later developments with the FDA that may be inconsistent with Aldeyra’s expectations and beliefs, including the risk that the results from earlier clinical trials or portions of clinical trials may not accurately predict results of subsequent trials or the remainder of a clinical trial for the same or different indications, inconsistent expectations regarding FDA acceptance and review of the company’s filings and submitted data sets, and Aldeyra’s continuing review and quality control analysis of clinical data. Important factors that could cause actual results to differ materially from those reflected in Aldeyra's forward-looking statements are described in Aldeyra’s most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q, as well as Aldeyra’s subsequent filings with the Securities and Exchange Commission. All of Aldeyra's development plans and timelines may be subject to adjustment depending on funding, recruitment rate, regulatory review, preclinical and clinical results, and other factors any of which could result in changes to Aldeyra’s development plans and programs or delay the initiation, completion, or reporting of clinical trials. In addition to the risks described above and in Aldeyra's other filings with the SEC, other unknown or unpredictable factors also could affect Aldeyra's results. No forward-looking statements can be guaranteed, and actual results may differ materially from such statements. The information in this presentation is provided only as of April 27, 2021, and Aldeyra undertakes no obligation to update any forward-looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law. 2Disclaimers and Forward-Looking Statements This presentation and various remarks which may be made during this presentation contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Section 21E of the Securities Exchange Act of 1934, as amended, including statements regarding Aldeyra’s possible or assumed future results of operations, expenses and financing needs, business strategies and plans, research and development plans or expectations, political, economic, legal, social and health risks, including the recent COVID-19 outbreak and subsequent public health measures and other responses to it, that may affect Aldeyra’s business or the global economy, the structure, timing and success of Aldeyra’s planned or pending clinical trials, expected milestones, market sizing, pricing and reimbursement, competitive position, regulatory matters, industry environment and potential growth opportunities, among other things. The results of earlier preclinical or clinical trials may not be predictive of future results. As a result of the COVID-19 pandemic, clinical site availability, staffing, and patient recruitment have been negatively affected and the timelines to complete Aldeyra’s clinical trials may be delayed. Forward-looking statements include all statements that are not historical facts and, in some cases, can be identified by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “target,” “design,” “estimate,” “predict,” “potential,” “plan” or similar expressions and the negatives of those terms. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause Aldeyra’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These statements reflect Aldeyra’s current views with respect to future events and are based on assumptions and subject to risks and uncertainties, including the development, clinical and regulatory plans or expectations for Aldeyra’s product candidates and systems-based approaches, later developments with the FDA that may be inconsistent with Aldeyra’s expectations and beliefs, including the risk that the results from earlier clinical trials or portions of clinical trials may not accurately predict results of subsequent trials or the remainder of a clinical trial for the same or different indications, inconsistent expectations regarding FDA acceptance and review of the company’s filings and submitted data sets, and Aldeyra’s continuing review and quality control analysis of clinical data. Important factors that could cause actual results to differ materially from those reflected in Aldeyra's forward-looking statements are described in Aldeyra’s most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q, as well as Aldeyra’s subsequent filings with the Securities and Exchange Commission. All of Aldeyra's development plans and timelines may be subject to adjustment depending on funding, recruitment rate, regulatory review, preclinical and clinical results, and other factors any of which could result in changes to Aldeyra’s development plans and programs or delay the initiation, completion, or reporting of clinical trials. In addition to the risks described above and in Aldeyra's other filings with the SEC, other unknown or unpredictable factors also could affect Aldeyra's results. No forward-looking statements can be guaranteed, and actual results may differ materially from such statements. The information in this presentation is provided only as of April 27, 2021, and Aldeyra undertakes no obligation to update any forward-looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law. 2

Top-Line Results from Phase 3 INVIGORATE Clinical Trial of Reproxalap in Allergic Conjunctivitis Statistical Significance Results Consistent with Phase 3 Achieved for: ALLEVIATE Allergic Conjunctivitis Clinical Trial and Previous Chamber Results in • Primary Endpoint of Ocular Itching at All Phase 2 Allergic Conjunctivitis Trial and Prespecified Timepoints (p<0.0001) Run-In Cohort of Phase 3 TRANQUILITY • Key Secondary Endpoint of Reduction in Dry Eye Disease Trial Ocular Redness (p<0.0001) Reproxalap Potentially Represents the • Secondary Endpoints of Ocular Tearing First New Allergic Conjunctivitis (p<0.0001) and Total Ocular Severity Score (p<0.0001) Therapeutic Mechanism in Decades 3Top-Line Results from Phase 3 INVIGORATE Clinical Trial of Reproxalap in Allergic Conjunctivitis Statistical Significance Results Consistent with Phase 3 Achieved for: ALLEVIATE Allergic Conjunctivitis Clinical Trial and Previous Chamber Results in • Primary Endpoint of Ocular Itching at All Phase 2 Allergic Conjunctivitis Trial and Prespecified Timepoints (p<0.0001) Run-In Cohort of Phase 3 TRANQUILITY • Key Secondary Endpoint of Reduction in Dry Eye Disease Trial Ocular Redness (p<0.0001) Reproxalap Potentially Represents the • Secondary Endpoints of Ocular Tearing First New Allergic Conjunctivitis (p<0.0001) and Total Ocular Severity Score (p<0.0001) Therapeutic Mechanism in Decades 3

The Prevalence of Allergic Conjunctivitis is Rising Allergic Rhinitis Allergic Allergic Conjunctivitis Asthma Allergic diseases Allergic conjunctivitis Temperatures Pollen is Allergy seasons are hyperendemic affects more than and CO levels spreading to are getting 2 and prevalence 1 billion people are rising. new areas. longer and more is increasing. worldwide, including severe. 100 million in the U.S. Millions of patients continue to suffer, and new treatments are needed. Slide sources: Ziska LH, Makra L, Harry, SK, et al. Lancet Planetary Health. 2019; Trends in prevalence and treatment of ocular allergy – Curr. Opin. Allergy Clin. Immunol. 2014 Oct; White Book on Allergy (2013 Update) ; Singh K, Axelrod S, Bielory L, J Allergy Clin Immunol. 2010; Pitt AD, Smith AF, Lindsell L, et al. Ophthal. Epidemiol. 2004. 4The Prevalence of Allergic Conjunctivitis is Rising Allergic Rhinitis Allergic Allergic Conjunctivitis Asthma Allergic diseases Allergic conjunctivitis Temperatures Pollen is Allergy seasons are hyperendemic affects more than and CO levels spreading to are getting 2 and prevalence 1 billion people are rising. new areas. longer and more is increasing. worldwide, including severe. 100 million in the U.S. Millions of patients continue to suffer, and new treatments are needed. Slide sources: Ziska LH, Makra L, Harry, SK, et al. Lancet Planetary Health. 2019; Trends in prevalence and treatment of ocular allergy – Curr. Opin. Allergy Clin. Immunol. 2014 Oct; White Book on Allergy (2013 Update) ; Singh K, Axelrod S, Bielory L, J Allergy Clin Immunol. 2010; Pitt AD, Smith AF, Lindsell L, et al. Ophthal. Epidemiol. 2004. 4
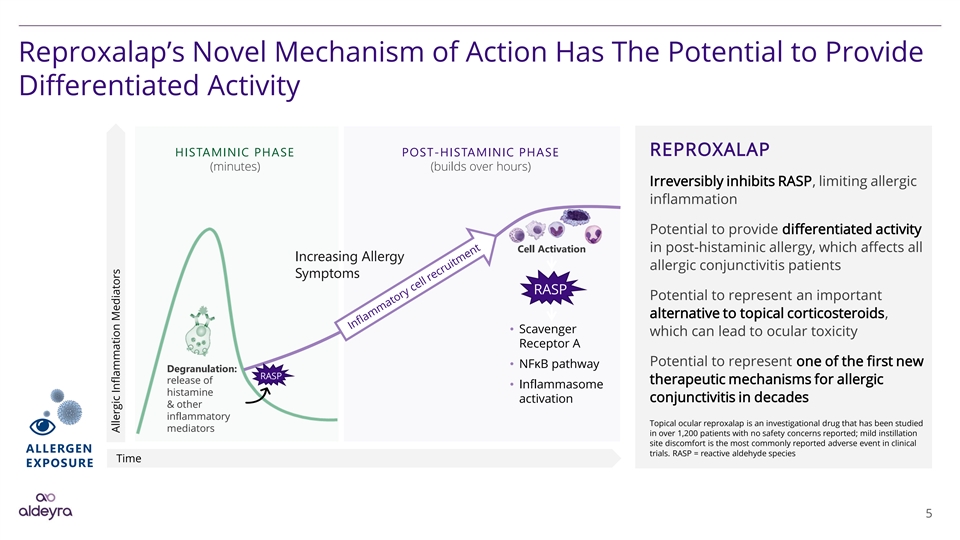
Reproxalap’s Novel Mechanism of Action Has The Potential to Provide Differentiated Activity HISTAMINIC PHASE POST-HISTAMINIC PHASE REPROXALAP (minutes) (builds over hours) Irreversibly inhibits RASP, limiting allergic inflammation Potential to provide differentiated activity Cell Activation in post-histaminic allergy, which affects all Increasing Allergy allergic conjunctivitis patients Symptoms RASP Potential to represent an important alternative to topical corticosteroids, • Scavenger which can lead to ocular toxicity Receptor A Potential to represent one of the first new • NFκB pathway Degranulation: RASP release of therapeutic mechanisms for allergic • Inflammasome histamine conjunctivitis in decades activation & other inflammatory Topical ocular reproxalap is an investigational drug that has been studied mediators in over 1,200 patients with no safety concerns reported; mild instillation site discomfort is the most commonly reported adverse event in clinical ALLERGEN trials. RASP = reactive aldehyde species Time EXPOSURE 5 Allergic Inflammation MediatorsReproxalap’s Novel Mechanism of Action Has The Potential to Provide Differentiated Activity HISTAMINIC PHASE POST-HISTAMINIC PHASE REPROXALAP (minutes) (builds over hours) Irreversibly inhibits RASP, limiting allergic inflammation Potential to provide differentiated activity Cell Activation in post-histaminic allergy, which affects all Increasing Allergy allergic conjunctivitis patients Symptoms RASP Potential to represent an important alternative to topical corticosteroids, • Scavenger which can lead to ocular toxicity Receptor A Potential to represent one of the first new • NFκB pathway Degranulation: RASP release of therapeutic mechanisms for allergic • Inflammasome histamine conjunctivitis in decades activation & other inflammatory Topical ocular reproxalap is an investigational drug that has been studied mediators in over 1,200 patients with no safety concerns reported; mild instillation site discomfort is the most commonly reported adverse event in clinical ALLERGEN trials. RASP = reactive aldehyde species Time EXPOSURE 5 Allergic Inflammation Mediators

The Allergen Chamber: A Demanding Real-World Drug Assessment in Allergic Conjunctivitis To our knowledge, no late-stage investigational allergic conjunctivitis drug has been rigorously tested in an The allergen chamber Subjects are exposed to naturalistic moderate allergen chamber. • enables a controlled, to high levels of ragweed pollen continuously environmental allergen exposure for approximately 3.5 hours. that mimics real-world exposure to • Drug or vehicle is administered prior to airborne allergens. allergen exposure and at 90 minutes, when • allows for detailed assessment of peak symptoms typically occur. prophylaxis and treatment with • Subject-reported ocular itching and tearing unparalleled standardization. scores, and investigator-assessed ocular redness scores, are obtained approximately every 10 minutes. Slide source: Cliantha Research 6The Allergen Chamber: A Demanding Real-World Drug Assessment in Allergic Conjunctivitis To our knowledge, no late-stage investigational allergic conjunctivitis drug has been rigorously tested in an The allergen chamber Subjects are exposed to naturalistic moderate allergen chamber. • enables a controlled, to high levels of ragweed pollen continuously environmental allergen exposure for approximately 3.5 hours. that mimics real-world exposure to • Drug or vehicle is administered prior to airborne allergens. allergen exposure and at 90 minutes, when • allows for detailed assessment of peak symptoms typically occur. prophylaxis and treatment with • Subject-reported ocular itching and tearing unparalleled standardization. scores, and investigator-assessed ocular redness scores, are obtained approximately every 10 minutes. Slide source: Cliantha Research 6
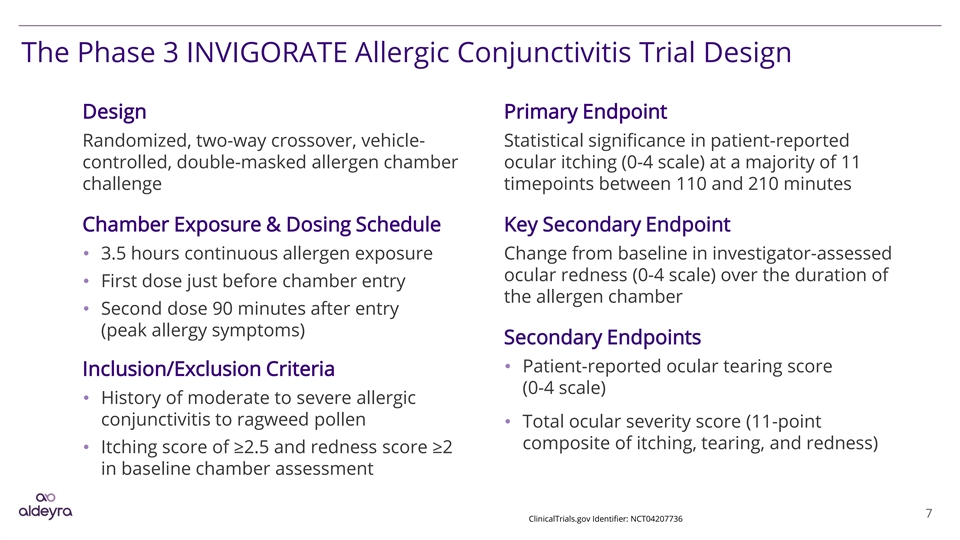
The Phase 3 INVIGORATE Allergic Conjunctivitis Trial Design Design Primary Endpoint Randomized, two-way crossover, vehicle- Statistical significance in patient-reported controlled, double-masked allergen chamber ocular itching (0-4 scale) at a majority of 11 challenge timepoints between 110 and 210 minutes Chamber Exposure & Dosing Schedule Key Secondary Endpoint • 3.5 hours continuous allergen exposure Change from baseline in investigator-assessed ocular redness (0-4 scale) over the duration of • First dose just before chamber entry the allergen chamber • Second dose 90 minutes after entry (peak allergy symptoms) Secondary Endpoints • Patient-reported ocular tearing score Inclusion/Exclusion Criteria (0-4 scale) • History of moderate to severe allergic conjunctivitis to ragweed pollen • Total ocular severity score (11-point composite of itching, tearing, and redness) • Itching score of ≥2.5 and redness score ≥2 in baseline chamber assessment 7 ClinicalTrials.gov Identifier: NCT04207736The Phase 3 INVIGORATE Allergic Conjunctivitis Trial Design Design Primary Endpoint Randomized, two-way crossover, vehicle- Statistical significance in patient-reported controlled, double-masked allergen chamber ocular itching (0-4 scale) at a majority of 11 challenge timepoints between 110 and 210 minutes Chamber Exposure & Dosing Schedule Key Secondary Endpoint • 3.5 hours continuous allergen exposure Change from baseline in investigator-assessed ocular redness (0-4 scale) over the duration of • First dose just before chamber entry the allergen chamber • Second dose 90 minutes after entry (peak allergy symptoms) Secondary Endpoints • Patient-reported ocular tearing score Inclusion/Exclusion Criteria (0-4 scale) • History of moderate to severe allergic conjunctivitis to ragweed pollen • Total ocular severity score (11-point composite of itching, tearing, and redness) • Itching score of ≥2.5 and redness score ≥2 in baseline chamber assessment 7 ClinicalTrials.gov Identifier: NCT04207736
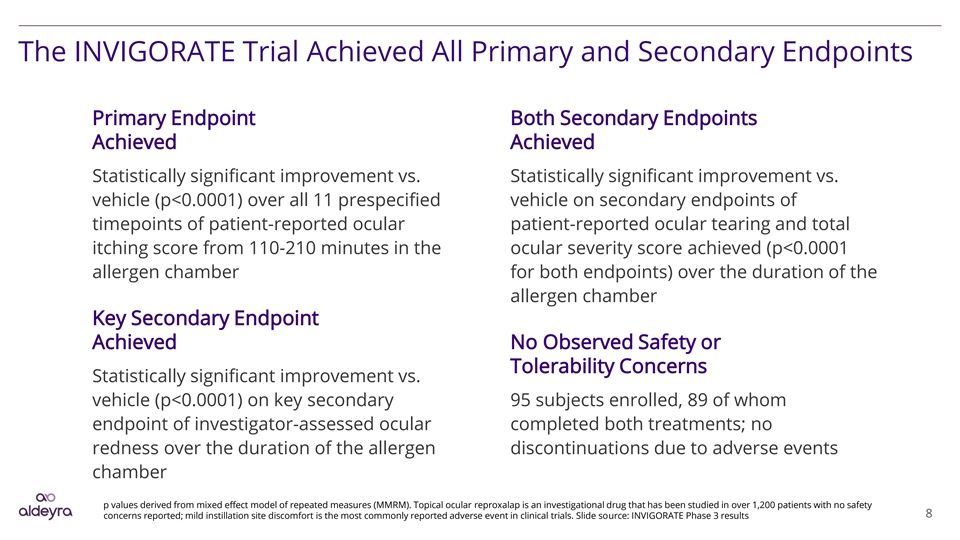
The INVIGORATE Trial Achieved All Primary and Secondary Endpoints Primary Endpoint Both Secondary Endpoints Achieved Achieved Statistically significant improvement vs. Statistically significant improvement vs. vehicle (p<0.0001) over all 11 prespecified vehicle on secondary endpoints of timepoints of patient-reported ocular patient-reported ocular tearing and total itching score from 110-210 minutes in the ocular severity score achieved (p<0.0001 allergen chamber for both endpoints) over the duration of the allergen chamber Key Secondary Endpoint Achieved No Observed Safety or Tolerability Concerns Statistically significant improvement vs. vehicle (p<0.0001) on key secondary 95 subjects enrolled, 89 of whom endpoint of investigator-assessed ocular completed both treatments; no redness over the duration of the allergen discontinuations due to adverse events chamber p values derived from mixed effect model of repeated measures (MMRM). Topical ocular reproxalap is an investigational drug that has been studied in over 1,200 patients with no safety 8 concerns reported; mild instillation site discomfort is the most commonly reported adverse event in clinical trials. Slide source: INVIGORATE Phase 3 resultsThe INVIGORATE Trial Achieved All Primary and Secondary Endpoints Primary Endpoint Both Secondary Endpoints Achieved Achieved Statistically significant improvement vs. Statistically significant improvement vs. vehicle (p<0.0001) over all 11 prespecified vehicle on secondary endpoints of timepoints of patient-reported ocular patient-reported ocular tearing and total itching score from 110-210 minutes in the ocular severity score achieved (p<0.0001 allergen chamber for both endpoints) over the duration of the allergen chamber Key Secondary Endpoint Achieved No Observed Safety or Tolerability Concerns Statistically significant improvement vs. vehicle (p<0.0001) on key secondary 95 subjects enrolled, 89 of whom endpoint of investigator-assessed ocular completed both treatments; no redness over the duration of the allergen discontinuations due to adverse events chamber p values derived from mixed effect model of repeated measures (MMRM). Topical ocular reproxalap is an investigational drug that has been studied in over 1,200 patients with no safety 8 concerns reported; mild instillation site discomfort is the most commonly reported adverse event in clinical trials. Slide source: INVIGORATE Phase 3 results
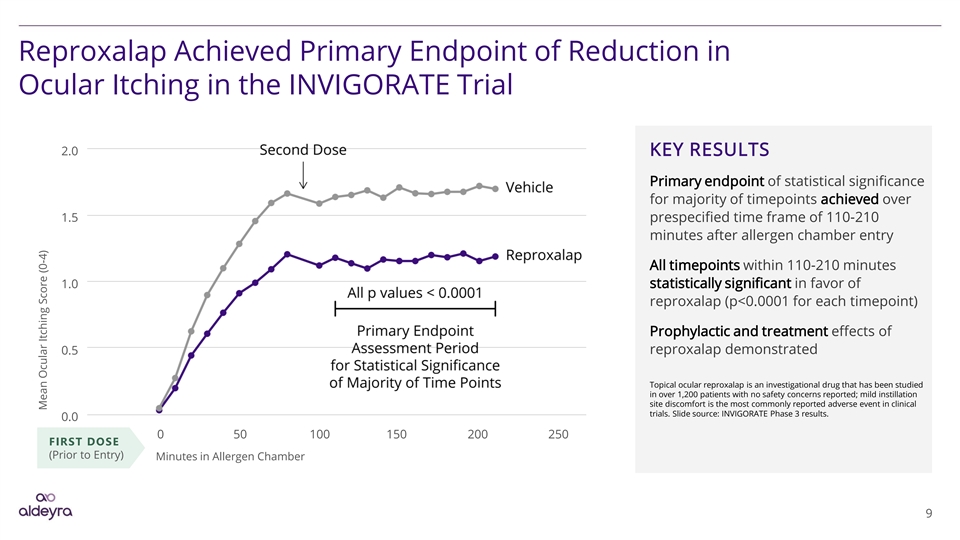
Reproxalap Achieved Primary Endpoint of Reduction in Ocular Itching in the INVIGORATE Trial 2.0 KEY RESULTS Primary endpoint of statistical significance for majority of timepoints achieved over 1.5 prespecified time frame of 110-210 minutes after allergen chamber entry All timepoints within 110-210 minutes 1.0 statistically significant in favor of reproxalap (p<0.0001 for each timepoint) Prophylactic and treatment effects of reproxalap demonstrated 0.5 Topical ocular reproxalap is an investigational drug that has been studied in over 1,200 patients with no safety concerns reported; mild instillation site discomfort is the most commonly reported adverse event in clinical trials. Slide source: INVIGORATE Phase 3 results. 0.0 0 50 100 150 200 250 FIRST DOSE (Prior to Entry) Minutes in Allergen Chamber 9 Mean Ocular Itching Score (0-4)Reproxalap Achieved Primary Endpoint of Reduction in Ocular Itching in the INVIGORATE Trial 2.0 KEY RESULTS Primary endpoint of statistical significance for majority of timepoints achieved over 1.5 prespecified time frame of 110-210 minutes after allergen chamber entry All timepoints within 110-210 minutes 1.0 statistically significant in favor of reproxalap (p<0.0001 for each timepoint) Prophylactic and treatment effects of reproxalap demonstrated 0.5 Topical ocular reproxalap is an investigational drug that has been studied in over 1,200 patients with no safety concerns reported; mild instillation site discomfort is the most commonly reported adverse event in clinical trials. Slide source: INVIGORATE Phase 3 results. 0.0 0 50 100 150 200 250 FIRST DOSE (Prior to Entry) Minutes in Allergen Chamber 9 Mean Ocular Itching Score (0-4)
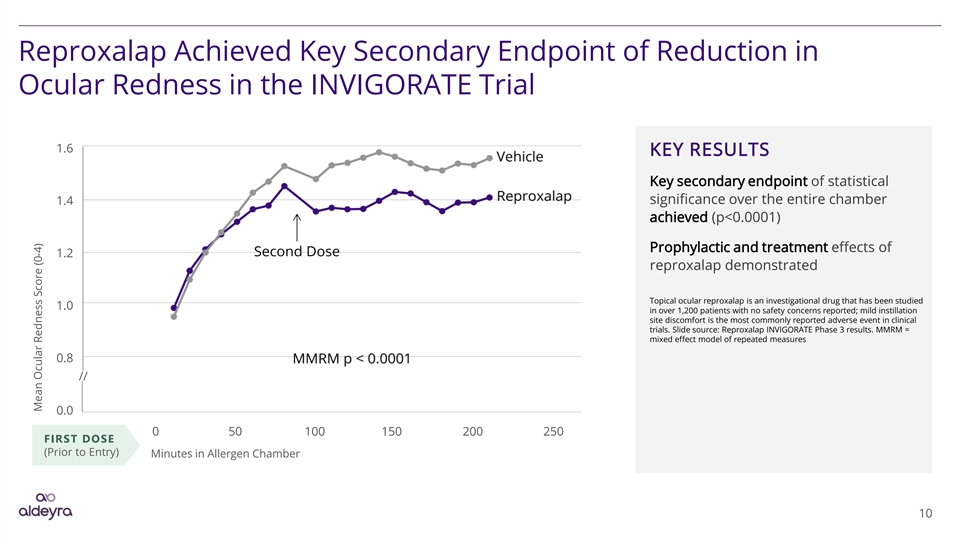
Reproxalap Achieved Key Secondary Endpoint of Reduction in Ocular Redness in the INVIGORATE Trial 1.6 KEY RESULTS Key secondary endpoint of statistical significance over the entire chamber 1.4 achieved (p<0.0001) Prophylactic and treatment effects of 1.2 reproxalap demonstrated Topical ocular reproxalap is an investigational drug that has been studied 1.0 in over 1,200 patients with no safety concerns reported; mild instillation site discomfort is the most commonly reported adverse event in clinical trials. Slide source: Reproxalap INVIGORATE Phase 3 results. MMRM = mixed effect model of repeated measures 0.8 // 0.0 0 50 100 150 200 250 FIRST DOSE (Prior to Entry) Minutes in Allergen Chamber 10 Mean Ocular Redness Score (0-4)Reproxalap Achieved Key Secondary Endpoint of Reduction in Ocular Redness in the INVIGORATE Trial 1.6 KEY RESULTS Key secondary endpoint of statistical significance over the entire chamber 1.4 achieved (p<0.0001) Prophylactic and treatment effects of 1.2 reproxalap demonstrated Topical ocular reproxalap is an investigational drug that has been studied 1.0 in over 1,200 patients with no safety concerns reported; mild instillation site discomfort is the most commonly reported adverse event in clinical trials. Slide source: Reproxalap INVIGORATE Phase 3 results. MMRM = mixed effect model of repeated measures 0.8 // 0.0 0 50 100 150 200 250 FIRST DOSE (Prior to Entry) Minutes in Allergen Chamber 10 Mean Ocular Redness Score (0-4)
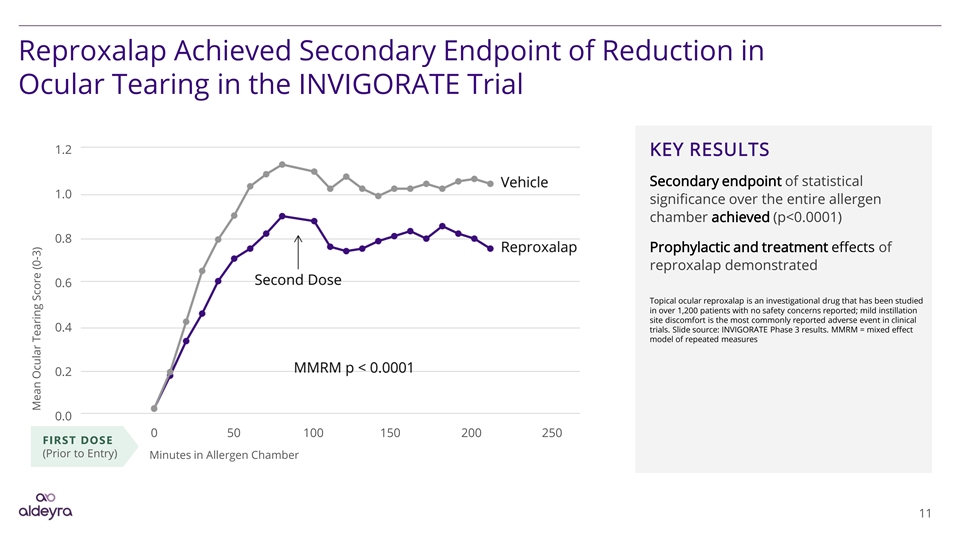
Reproxalap Achieved Secondary Endpoint of Reduction in Ocular Tearing in the INVIGORATE Trial 1.2 KEY RESULTS Secondary endpoint of statistical 1.0 significance over the entire allergen chamber achieved (p<0.0001) 0.8 Prophylactic and treatment effects of reproxalap demonstrated 0.6 Topical ocular reproxalap is an investigational drug that has been studied in over 1,200 patients with no safety concerns reported; mild instillation site discomfort is the most commonly reported adverse event in clinical 0.4 trials. Slide source: INVIGORATE Phase 3 results. MMRM = mixed effect model of repeated measures 0.2 0.0 0 50 100 150 200 250 FIRST DOSE (Prior to Entry) Minutes in Allergen Chamber 11 Mean Ocular Tearing Score (0-3)Reproxalap Achieved Secondary Endpoint of Reduction in Ocular Tearing in the INVIGORATE Trial 1.2 KEY RESULTS Secondary endpoint of statistical 1.0 significance over the entire allergen chamber achieved (p<0.0001) 0.8 Prophylactic and treatment effects of reproxalap demonstrated 0.6 Topical ocular reproxalap is an investigational drug that has been studied in over 1,200 patients with no safety concerns reported; mild instillation site discomfort is the most commonly reported adverse event in clinical 0.4 trials. Slide source: INVIGORATE Phase 3 results. MMRM = mixed effect model of repeated measures 0.2 0.0 0 50 100 150 200 250 FIRST DOSE (Prior to Entry) Minutes in Allergen Chamber 11 Mean Ocular Tearing Score (0-3)
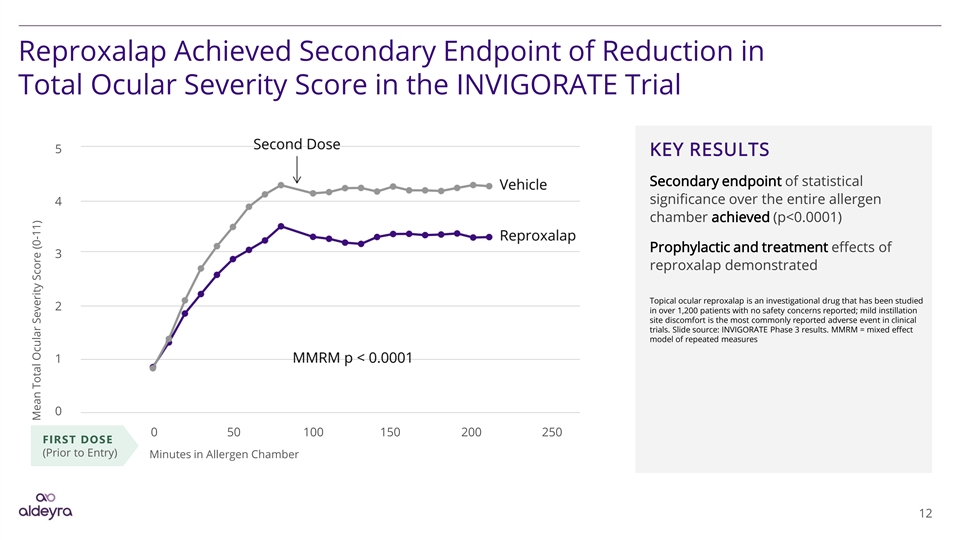
Reproxalap Achieved Secondary Endpoint of Reduction in Total Ocular Severity Score in the INVIGORATE Trial 5 KEY RESULTS Secondary endpoint of statistical significance over the entire allergen 4 chamber achieved (p<0.0001) Prophylactic and treatment effects of 3 reproxalap demonstrated Topical ocular reproxalap is an investigational drug that has been studied 2 in over 1,200 patients with no safety concerns reported; mild instillation site discomfort is the most commonly reported adverse event in clinical trials. Slide source: INVIGORATE Phase 3 results. MMRM = mixed effect model of repeated measures 1 0 0 50 100 150 200 250 FIRST DOSE (Prior to Entry) Minutes in Allergen Chamber 12 Mean Total Ocular Severity Score (0-11)Reproxalap Achieved Secondary Endpoint of Reduction in Total Ocular Severity Score in the INVIGORATE Trial 5 KEY RESULTS Secondary endpoint of statistical significance over the entire allergen 4 chamber achieved (p<0.0001) Prophylactic and treatment effects of 3 reproxalap demonstrated Topical ocular reproxalap is an investigational drug that has been studied 2 in over 1,200 patients with no safety concerns reported; mild instillation site discomfort is the most commonly reported adverse event in clinical trials. Slide source: INVIGORATE Phase 3 results. MMRM = mixed effect model of repeated measures 1 0 0 50 100 150 200 250 FIRST DOSE (Prior to Entry) Minutes in Allergen Chamber 12 Mean Total Ocular Severity Score (0-11)
Reproxalap Was Generally Well Tolerated and No Safety Concerns Were Observed in the INVIGORATE Trial TOPICAL OCULAR NO observed safety or NO observed clinically REPROXALAP tolerability concerns significant findings on administered to safety assessments, more than NO discontinuations due to including: adverse events • visual acuity 1,200 patients • intraocular pressure Consistent with other topically ACROSS administered drugs, most • slit lamp biomicroscopy common treatment-emergent 14 clinical trials • dilated fundoscopy events related to transient Topical ocular reproxalap is an investigational drug that has been instillation site discomfort studied in over 1,200 patients with no safety concerns reported; mild instillation site discomfort is the most commonly reported adverse event in clinical trials. Source: INVIGORATE Phase 3 results. MMRM = mixed effect model of repeated measures 13Reproxalap Was Generally Well Tolerated and No Safety Concerns Were Observed in the INVIGORATE Trial TOPICAL OCULAR NO observed safety or NO observed clinically REPROXALAP tolerability concerns significant findings on administered to safety assessments, more than NO discontinuations due to including: adverse events • visual acuity 1,200 patients • intraocular pressure Consistent with other topically ACROSS administered drugs, most • slit lamp biomicroscopy common treatment-emergent 14 clinical trials • dilated fundoscopy events related to transient Topical ocular reproxalap is an investigational drug that has been instillation site discomfort studied in over 1,200 patients with no safety concerns reported; mild instillation site discomfort is the most commonly reported adverse event in clinical trials. Source: INVIGORATE Phase 3 results. MMRM = mixed effect model of repeated measures 13
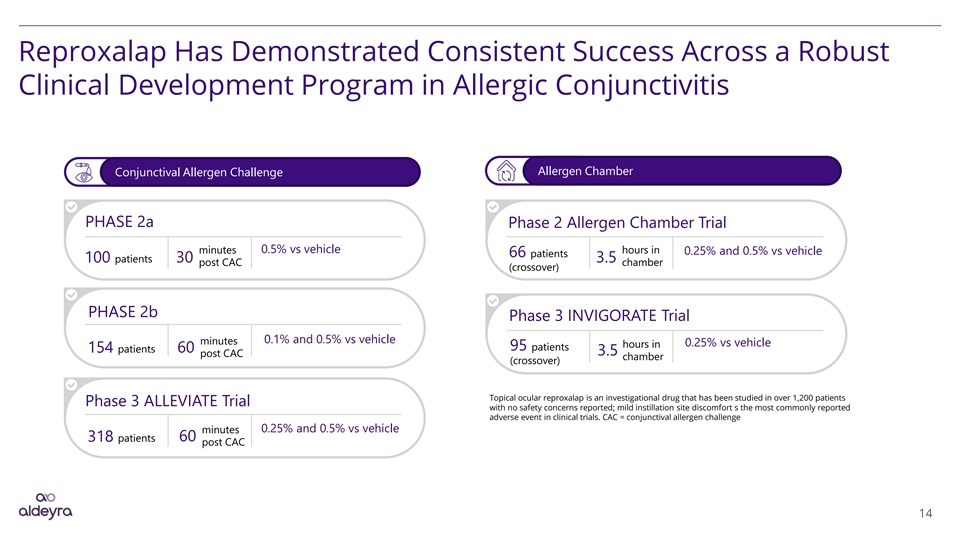
Reproxalap Has Demonstrated Consistent Success Across a Robust Clinical Development Program in Allergic Conjunctivitis Allergen Chamber Conjunctival Allergen Challenge PHASE 2a Phase 2 Allergen Chamber Trial 0.5% vs vehicle minutes hours in 0.25% and 0.5% vs vehicle 66 patients 100 patients 30 3.5 post CAC chamber (crossover) PHASE 2b Phase 3 INVIGORATE Trial 0.1% and 0.5% vs vehicle minutes 0.25% vs vehicle hours in 95 patients 154 patients 60 3.5 post CAC chamber (crossover) Topical ocular reproxalap is an investigational drug that has been studied in over 1,200 patients Phase 3 ALLEVIATE Trial with no safety concerns reported; mild instillation site discomfort s the most commonly reported adverse event in clinical trials. CAC = conjunctival allergen challenge 0.25% and 0.5% vs vehicle minutes 60 318 patients post CAC 14Reproxalap Has Demonstrated Consistent Success Across a Robust Clinical Development Program in Allergic Conjunctivitis Allergen Chamber Conjunctival Allergen Challenge PHASE 2a Phase 2 Allergen Chamber Trial 0.5% vs vehicle minutes hours in 0.25% and 0.5% vs vehicle 66 patients 100 patients 30 3.5 post CAC chamber (crossover) PHASE 2b Phase 3 INVIGORATE Trial 0.1% and 0.5% vs vehicle minutes 0.25% vs vehicle hours in 95 patients 154 patients 60 3.5 post CAC chamber (crossover) Topical ocular reproxalap is an investigational drug that has been studied in over 1,200 patients Phase 3 ALLEVIATE Trial with no safety concerns reported; mild instillation site discomfort s the most commonly reported adverse event in clinical trials. CAC = conjunctival allergen challenge 0.25% and 0.5% vs vehicle minutes 60 318 patients post CAC 14
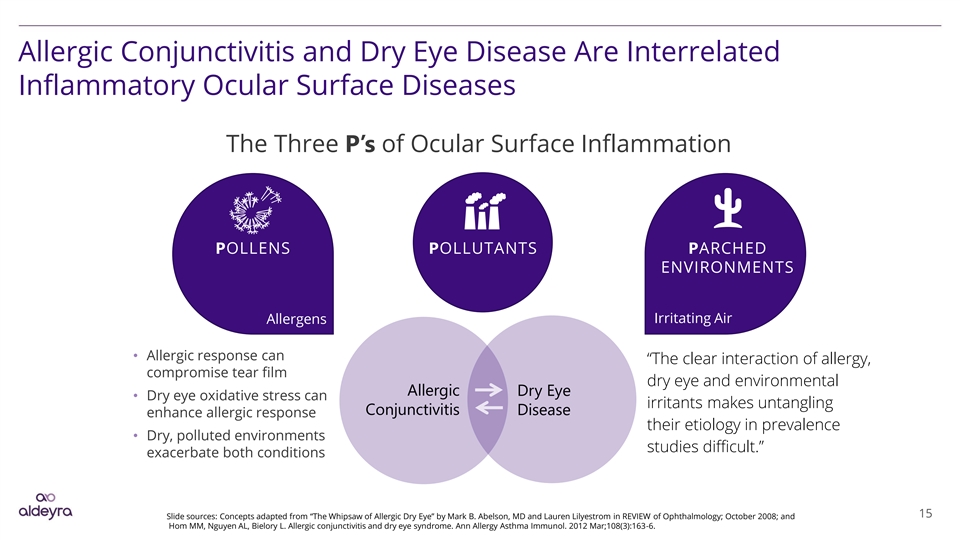
Allergic Conjunctivitis and Dry Eye Disease Are Interrelated Inflammatory Ocular Surface Diseases The Three P’s of Ocular Surface Inflammation POLLENS POLLUTANTS PARCHED ENVIRONMENTS Irritating Air Allergens • Allergic response can “The clear interaction of allergy, compromise tear film dry eye and environmental Allergic Dry Eye • Dry eye oxidative stress can irritants makes untangling Conjunctivitis Disease enhance allergic response their etiology in prevalence • Dry, polluted environments studies difficult.” exacerbate both conditions 15 Slide sources: Concepts adapted from “The Whipsaw of Allergic Dry Eye” by Mark B. Abelson, MD and Lauren Lilyestrom in REVIEW of Ophthalmology; October 2008; and Hom MM, Nguyen AL, Bielory L. Allergic conjunctivitis and dry eye syndrome. Ann Allergy Asthma Immunol. 2012 Mar;108(3):163-6.Allergic Conjunctivitis and Dry Eye Disease Are Interrelated Inflammatory Ocular Surface Diseases The Three P’s of Ocular Surface Inflammation POLLENS POLLUTANTS PARCHED ENVIRONMENTS Irritating Air Allergens • Allergic response can “The clear interaction of allergy, compromise tear film dry eye and environmental Allergic Dry Eye • Dry eye oxidative stress can irritants makes untangling Conjunctivitis Disease enhance allergic response their etiology in prevalence • Dry, polluted environments studies difficult.” exacerbate both conditions 15 Slide sources: Concepts adapted from “The Whipsaw of Allergic Dry Eye” by Mark B. Abelson, MD and Lauren Lilyestrom in REVIEW of Ophthalmology; October 2008; and Hom MM, Nguyen AL, Bielory L. Allergic conjunctivitis and dry eye syndrome. Ann Allergy Asthma Immunol. 2012 Mar;108(3):163-6.
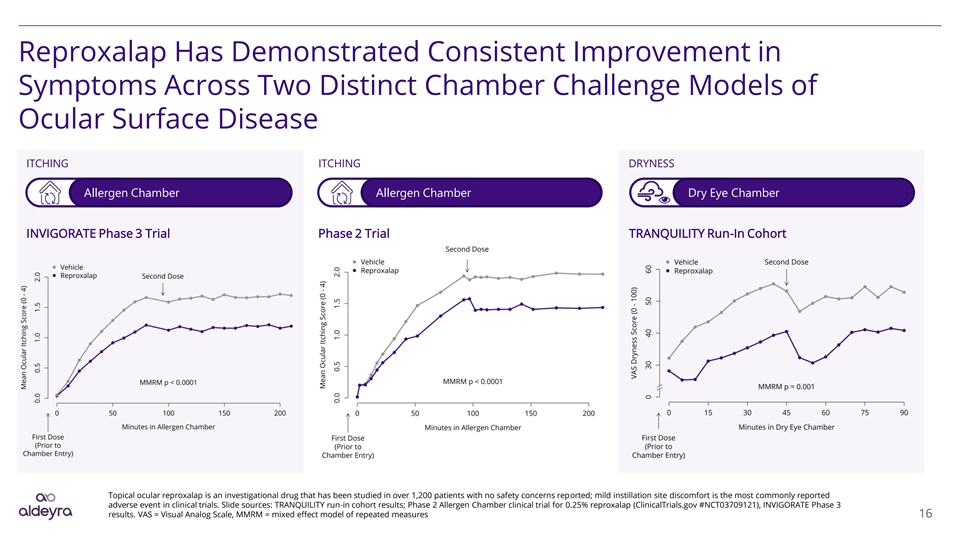
Reproxalap Has Demonstrated Consistent Improvement in Symptoms Across Two Distinct Chamber Challenge Models of Ocular Surface Disease ITCHING ITCHING DRYNESS Allergen Chamber Allergen Chamber Dry Eye Chamber INVIGORATE Phase 3 Trial Phase 2 Trial TRANQUILITY Run-In Cohort Topical ocular reproxalap is an investigational drug that has been studied in over 1,200 patients with no safety concerns reported; mild instillation site discomfort is the most commonly reported adverse event in clinical trials. Slide sources: TRANQUILITY run-in cohort results; Phase 2 Allergen Chamber clinical trial for 0.25% reproxalap (ClinicalTrials.gov #NCT03709121), INVIGORATE Phase 3 results. VAS = Visual Analog Scale, MMRM = mixed effect model of repeated measures 16Reproxalap Has Demonstrated Consistent Improvement in Symptoms Across Two Distinct Chamber Challenge Models of Ocular Surface Disease ITCHING ITCHING DRYNESS Allergen Chamber Allergen Chamber Dry Eye Chamber INVIGORATE Phase 3 Trial Phase 2 Trial TRANQUILITY Run-In Cohort Topical ocular reproxalap is an investigational drug that has been studied in over 1,200 patients with no safety concerns reported; mild instillation site discomfort is the most commonly reported adverse event in clinical trials. Slide sources: TRANQUILITY run-in cohort results; Phase 2 Allergen Chamber clinical trial for 0.25% reproxalap (ClinicalTrials.gov #NCT03709121), INVIGORATE Phase 3 results. VAS = Visual Analog Scale, MMRM = mixed effect model of repeated measures 16
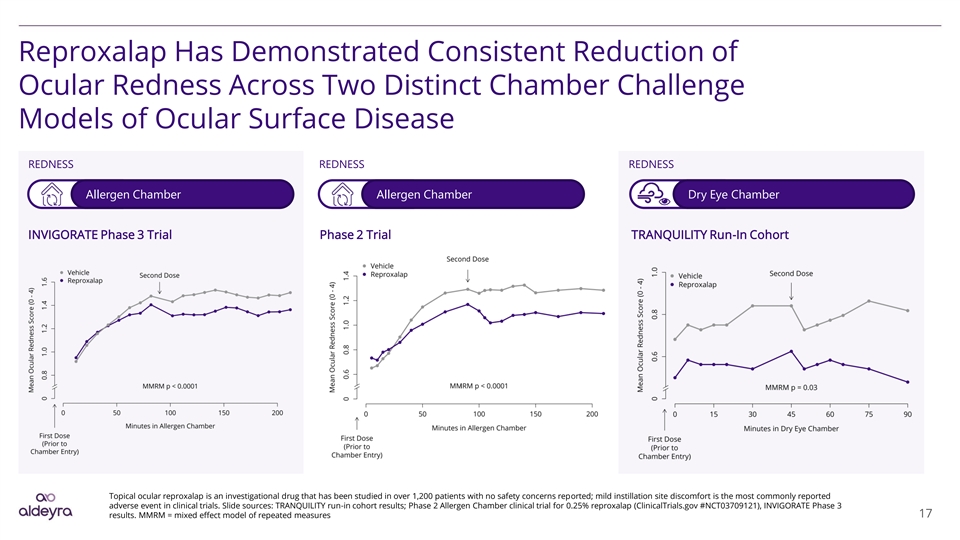
Reproxalap Has Demonstrated Consistent Reduction of Ocular Redness Across Two Distinct Chamber Challenge Models of Ocular Surface Disease REDNESS REDNESS REDNESS Allergen Chamber Allergen Chamber Dry Eye Chamber INVIGORATE Phase 3 Trial Phase 2 Trial TRANQUILITY Run-In Cohort Topical ocular reproxalap is an investigational drug that has been studied in over 1,200 patients with no safety concerns reported; mild instillation site discomfort is the most commonly reported adverse event in clinical trials. Slide sources: TRANQUILITY run-in cohort results; Phase 2 Allergen Chamber clinical trial for 0.25% reproxalap (ClinicalTrials.gov #NCT03709121), INVIGORATE Phase 3 results. MMRM = mixed effect model of repeated measures 17Reproxalap Has Demonstrated Consistent Reduction of Ocular Redness Across Two Distinct Chamber Challenge Models of Ocular Surface Disease REDNESS REDNESS REDNESS Allergen Chamber Allergen Chamber Dry Eye Chamber INVIGORATE Phase 3 Trial Phase 2 Trial TRANQUILITY Run-In Cohort Topical ocular reproxalap is an investigational drug that has been studied in over 1,200 patients with no safety concerns reported; mild instillation site discomfort is the most commonly reported adverse event in clinical trials. Slide sources: TRANQUILITY run-in cohort results; Phase 2 Allergen Chamber clinical trial for 0.25% reproxalap (ClinicalTrials.gov #NCT03709121), INVIGORATE Phase 3 results. MMRM = mixed effect model of repeated measures 17
* Upcoming Expected Reproxalap Development Milestones Reproxalap Reproxalap Reproxalap dry eye disease allergic conjunctivitis dry eye disease Phase 3 TRANQUILITY Phase 3 INVIGORATE Phase 3 TRANQUILITY main cohort initiation top-line results and TRANQUILITY-2 top- H1 2021 H1 2021 line results H2 2021 Aldeyra plans to meet with the U.S. FDA in the second half of 2021 to discuss the INVIGORATE results and the potential submission of a New Drug Application. *The timing of clinical trial initiation and completion depends, in part, on restrictions related to COVID-19, the availability of clinical research facilities and staffing, the ability to recruit patients, and regulatory feedback. 18* Upcoming Expected Reproxalap Development Milestones Reproxalap Reproxalap Reproxalap dry eye disease allergic conjunctivitis dry eye disease Phase 3 TRANQUILITY Phase 3 INVIGORATE Phase 3 TRANQUILITY main cohort initiation top-line results and TRANQUILITY-2 top- H1 2021 H1 2021 line results H2 2021 Aldeyra plans to meet with the U.S. FDA in the second half of 2021 to discuss the INVIGORATE results and the potential submission of a New Drug Application. *The timing of clinical trial initiation and completion depends, in part, on restrictions related to COVID-19, the availability of clinical research facilities and staffing, the ability to recruit patients, and regulatory feedback. 18
A New Paradigm for the Treatment of Anterior Ocular Inflammation: * A Potential Single Approach for Dry Eye Disease and Allergic Conjunctivitis Dry Eye Disease Allergic Conjunctivitis REPROXALAP 0.25% REPROXALAP 0.25% Rapid symptom and redness Clinically significant and durable improvement within minutes symptom response across two models of ocular allergy Broad and durable symptom Potentially the first new allergic control conjunctivitis therapeutic mechanism in decades * Contingent on funding, regulatory review, clinical results, and other factors 19A New Paradigm for the Treatment of Anterior Ocular Inflammation: * A Potential Single Approach for Dry Eye Disease and Allergic Conjunctivitis Dry Eye Disease Allergic Conjunctivitis REPROXALAP 0.25% REPROXALAP 0.25% Rapid symptom and redness Clinically significant and durable improvement within minutes symptom response across two models of ocular allergy Broad and durable symptom Potentially the first new allergic control conjunctivitis therapeutic mechanism in decades * Contingent on funding, regulatory review, clinical results, and other factors 19
