Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Akero Therapeutics, Inc. | tm219778d2_ex99-1.htm |
| 8-K - FORM 8-K - Akero Therapeutics, Inc. | tm219778d2_8k.htm |
Exhibit 99.2
 |
A Global Disease, A Pioneering Treatment Cohort C Readout: 16-Week Study of EFX in Cirrhotic NASH Patients (F4) March 22, 2021 |
 |
SAFE HARBOR This presentation may contain “forward-looking statements” of Akero Therapeutics, Inc. (“we,” “us,” “our,” “Akero” or the “Company”) within the meaning of the Private Securities Litigation Reform Act of 1995 relating to our business, operations, and financial conditions, including but not limited to current beliefs, expectations and assumptions regarding: the future of our business; future plans and strategies, including our expectations around the therapeutic potential and clinical benefits of Efruxifermin; our development plans for Efruxifermin, including our belief in the unique potential of Efruxifermin as a foundational NASH therapy; our preclinical and clinical results, including our top-line safety/tolerability, laboratory measures and paired biopsy data from our Phase 2a BALANCED study; the potential benefits resulting from the PRIME designation of EFX; plans to report preliminary results for Cohort C of the Phase 2a BALANCED study; the Phase 2b HARMONY study including expected timing to complete enrollment and report preliminary results; statements regarding a potential meeting with the FDA and timing thereof; the availability of a new drug product formulation to support Phase 3 clinical trials; risks related to the competitive landscape; expectations regarding the Company’s use of capital, expenses and other future financial results; and the potential impact of COVID-19 on strategy, our employees, supply chain, future operations and clinical trials. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by law, we assume no obligation to update these forward looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in our most recent annual report on Form 10-K filed with the Securities and Exchange Commission, as well as discussions of potential risks, uncertainties, and other important factors in our other filings with the Securities and Exchange Commission. All information in this presentation is as of the date hereof, and we undertake no duty to update this information unless required by law. Certain information Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. 2 |
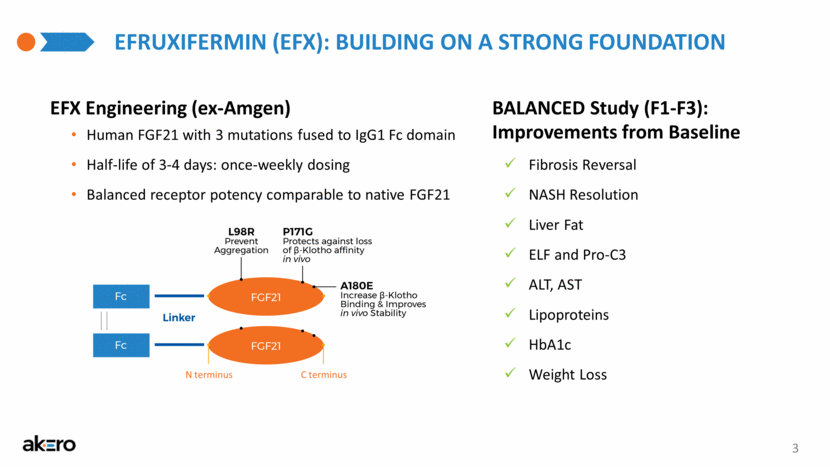 |
EFRUXIFERMIN (EFX): BUILDING ON A STRONG FOUNDATION BALANCED Study (F1-F3): Improvements from Baseline Fibrosis Reversal NASH Resolution Liver Fat ELF and Pro-C3 ALT, AST Lipoproteins HbA1c Weight Loss EFX Engineering (ex-Amgen) Human FGF21 with 3 mutations fused to IgG1 Fc domain Half-life of 3-4 days: once-weekly dosing Balanced receptor potency comparable to native FGF21 3 N terminus C terminus |
 |
EXPANDING EFX TO CIRRHOTIC PATIENT POPULATION (F4) Developing Treatments for Cirrhotic (F4) NASH FDA draft guidance specific for F4 patients Projected ~3.5M US F4 patients in 2030 FDA: The goals of treatment for compensated NASH cirrhosis are to halt or slow progression of fibrosis, prevent clinical decompensation, reduce the need for liver transplantation, and improve survival 4 |
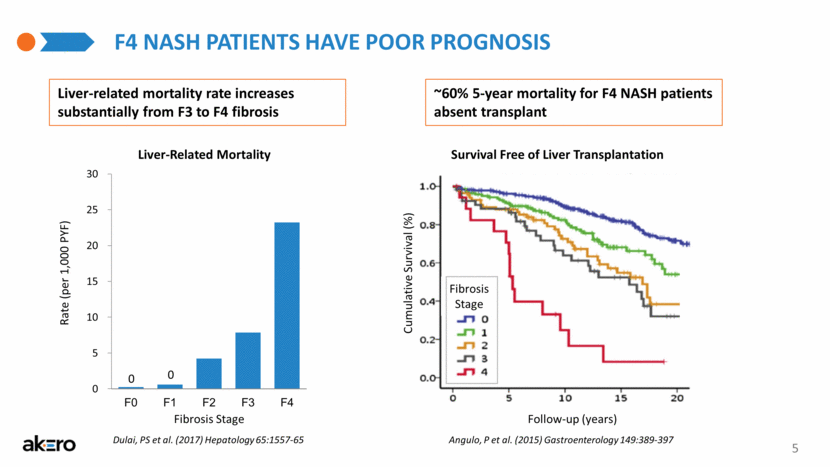 |
5 F4 NASH PATIENTS HAVE POOR PROGNOSIS ~60% 5-year mortality for F4 NASH patients absent transplant Liver-related mortality rate increases substantially from F3 to F4 fibrosis Liver-Related Mortality 0 0 Fibrosis Stage Rate (per 1,000 PYF) Survival Free of Liver Transplantation Follow-up (years) Cumulative Survival (%) Angulo, P et al. (2015) Gastroenterology 149:389-397 Fibrosis Stage Dulai, PS et al. (2017) Hepatology 65:1557-65 0 5 10 15 20 25 30 F0 F1 F2 F3 F4 |
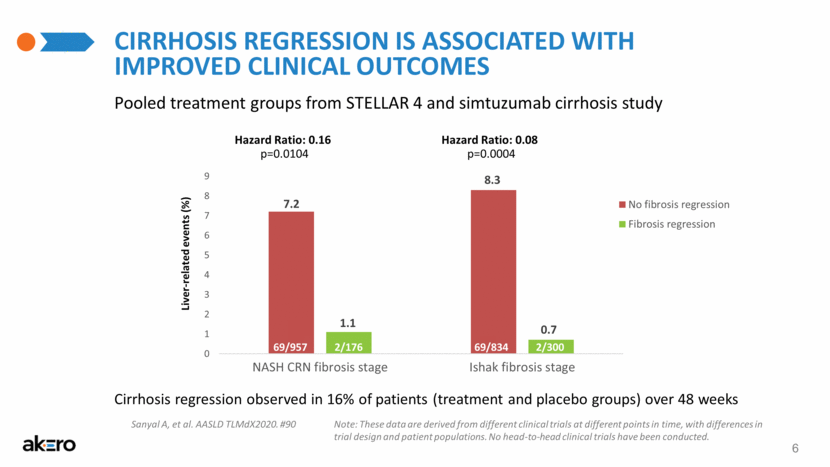 |
6 Cirrhosis Regression is Associated with Improved Clinical Outcomes Pooled treatment groups from STELLAR 4 and simtuzumab cirrhosis study Liver-related events (%) Hazard Ratio: 0.16 p=0.0104 Hazard Ratio: 0.08 p=0.0004 Cirrhosis regression observed in 16% of patients (treatment and placebo groups) over 48 weeks Note: These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. No head-to-head clinical trials have been conducted. Sanyal A, et al. AASLD TLMdX2020. #90 |
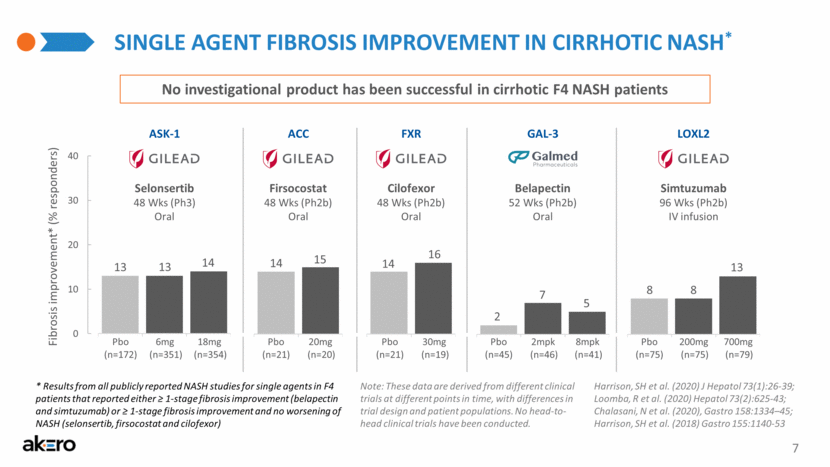 |
SINGLE AGENT FIBROSIS IMPROVEMENT IN CIRRHOTIC NASH* Belapectin 52 Wks (Ph2b) Oral GAL-3 Selonsertib 48 Wks (Ph3) Oral Simtuzumab 96 Wks (Ph2b) IV infusion ASK-1 ACC FXR LOXL2 Firsocostat 48 Wks (Ph2b) Oral Cilofexor 48 Wks (Ph2b) Oral (n=20) (n=21) Pbo 20mg (n=19) (n=21) Pbo 30mg (n=46) (n=45) Pbo 2mpk (n=41) 8mpk (n=75) (n=75) Pbo 200mg (n=79) 700mg (n=351) (n=172) Pbo 6mg (n=354) 18mg 13 14 13 8 13 8 2 5 7 14 16 14 15 * Results from all publicly reported NASH studies for single agents in F4 patients that reported either ≥ 1-stage fibrosis improvement (belapectin and simtuzumab) or ≥ 1-stage fibrosis improvement and no worsening of NASH (selonsertib, firsocostat and cilofexor) 7 No investigational product has been successful in cirrhotic F4 NASH patients Fibrosis improvement* (% responders) Note: These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. No head-to-head clinical trials have been conducted. Harrison, SH et al. (2020) J Hepatol 73(1):26-39; Loomba, R et al. (2020) Hepatol 73(2):625-43; Chalasani, N et al. (2020), Gastro 158:1334–45; Harrison, SH et al. (2018) Gastro 155:1140-53 0 10 20 30 40 |
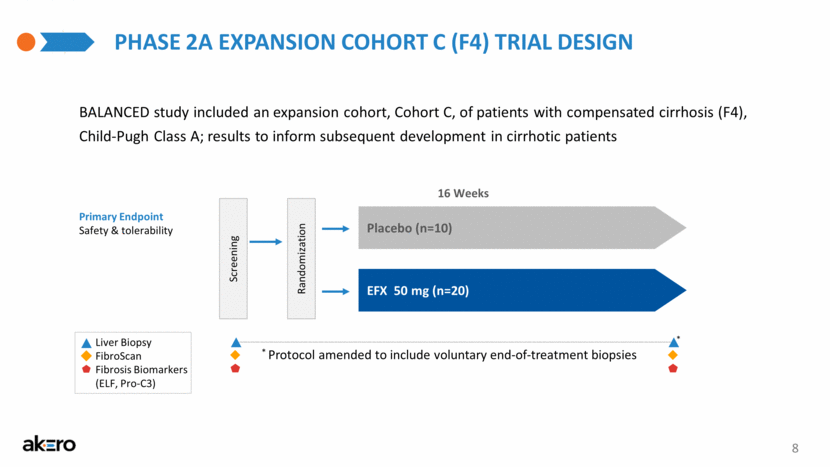 |
* PHASE 2A EXPANSION COHORT C (F4) TRIAL DESIGN 8 Primary Endpoint Safety & tolerability BALANCED study included an expansion cohort, Cohort C, of patients with compensated cirrhosis (F4), Child-Pugh Class A; results to inform subsequent development in cirrhotic patients 16 Weeks Screening Randomization EFX 50 mg (n=20) Placebo (n=10) FibroScan Liver Biopsy Fibrosis Biomarkers (ELF, Pro-C3) * Protocol amended to include voluntary end-of-treatment biopsies |
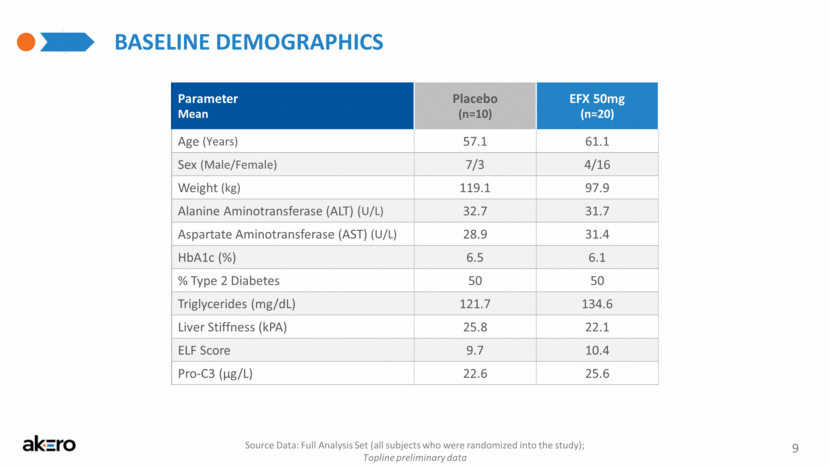 |
Baseline Demographics Parameter Mean Placebo (n=10) EFX 50mg (n=20) Age (Years) 57.1 61.1 Sex (Male/Female) 7/3 4/16 Weight (kg) 119.1 97.9 Alanine Aminotransferase (ALT) (U/L) 32.7 31.7 Aspartate Aminotransferase (AST) (U/L) 28.9 31.4 HbA1c (%) 6.5 6.1 % Type 2 Diabetes 50 50 Triglycerides (mg/dL) 121.7 134.6 Liver Stiffness (kPA) 25.8 22.1 ELF Score 9.7 10.4 Pro-C3 (μg/L) 22.6 25.6 9 Source Data: Full Analysis Set (all subjects who were randomized into the study); Topline preliminary data |
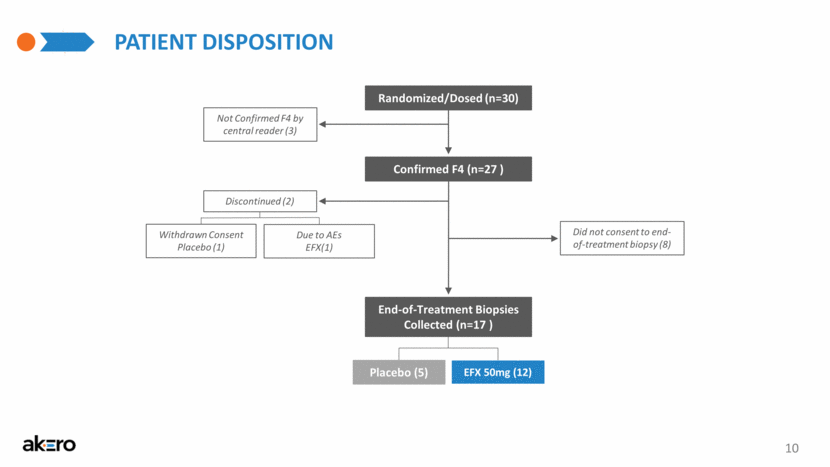 |
10 PATIENT DISPOSITION Randomized/Dosed (n=30) End-of-Treatment Biopsies Collected (n=17 ) Confirmed F4 (n=27 ) Due to AEs EFX(1) Discontinued (2) Withdrawn Consent Placebo (1) EFX 50mg (12) Placebo (5) Not Confirmed F4 by central reader (3) Did not consent to end-of-treatment biopsy (8) |
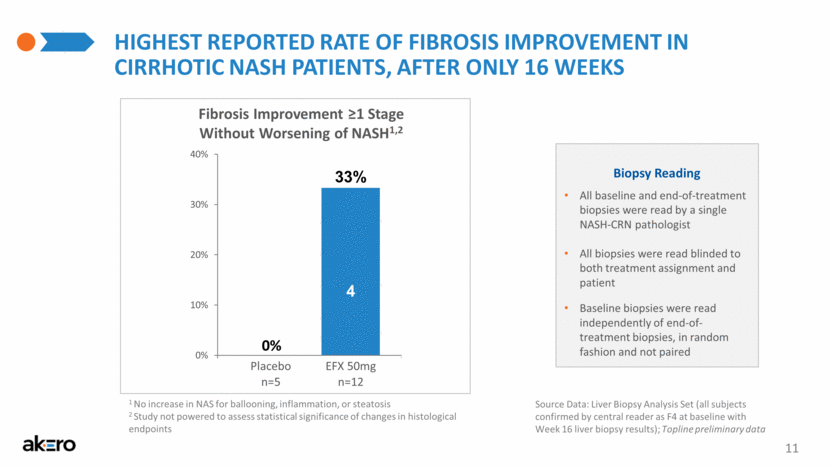 |
HIGHEST REPORTED RATE OF Fibrosis Improvement IN CIRRHOTIC NASH PATIENTS, AFTER ONLY 16 WEEKS 11 Source Data: Liver Biopsy Analysis Set (all subjects confirmed by central reader as F4 at baseline with Week 16 liver biopsy results); Topline preliminary data 1 No increase in NAS for ballooning, inflammation, or steatosis 2 Study not powered to assess statistical significance of changes in histological endpoints Fibrosis Improvement ≥1 Stage Without Worsening of NASH1,2 Placebo n=5 0% EFX 50mg n=12 33% 4 Biopsy Reading All baseline and end-of-treatment biopsies were read by a single NASH-CRN pathologist All biopsies were read blinded to both treatment assignment and patient Baseline biopsies were read independently of end-of-treatment biopsies, in random fashion and not paired 0% 10% 20% 30% 40% Overall |
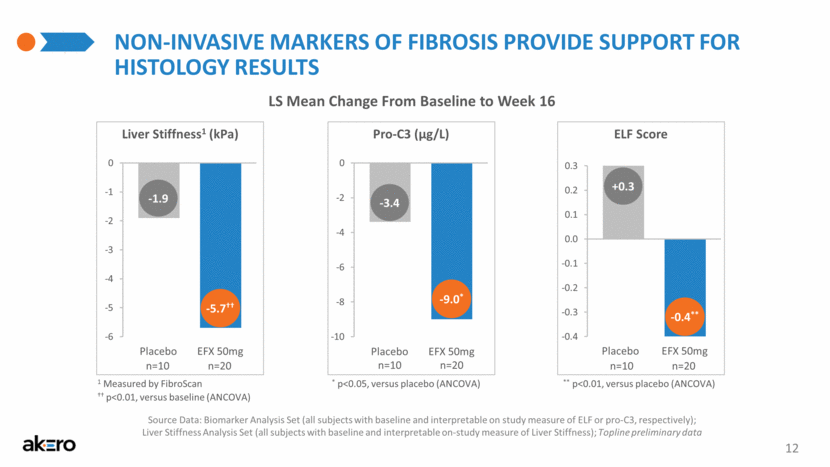 |
NON-INVASIVE MARKERS OF FIBROSIS PROVIDE SUPPORT FOR HISTOLOGY RESULTS Source Data: Biomarker Analysis Set (all subjects with baseline and interpretable on study measure of ELF or pro-C3, respectively); Liver Stiffness Analysis Set (all subjects with baseline and interpretable on-study measure of Liver Stiffness); Topline preliminary data LS Mean Change From Baseline to Week 16 ELF Score ** p<0.01, versus placebo (ANCOVA) -0.4** +0.3 -9.0* -3.4 Pro-C3 (µg/L) * p<0.05, versus placebo (ANCOVA) -5.7†† -1.9 Liver Stiffness1 (kPa) †† p<0.01, versus baseline (ANCOVA) 1 Measured by FibroScan EFX 50mg Placebo EFX 50mg Placebo EFX 50mg Placebo 12 n=20 n=10 n=20 n=10 n=20 n=10 -0.4 -0.3 -0.2 -0.1 0.0 0.1 0.2 0.3 CFB -10 -8 -6 -4 -2 0 CFB -6 -5 -4 -3 -2 -1 0 CFB |
 |
ADDITIONAL PATIENTS RESOLUTION ACHIEVED NASH achieving fibrosis improvement 1 NAS score of 0 or 1 for lobular inflammation and a score of 0 for ballooning 2 Study not powered to assess statistical significance of histological endpoints Source Data: Liver Biopsy Analysis Set; Topline preliminary data 13 7 out of 12 (58%) EFX patients achieved either fibrosis improvement or NASH resolution, compared to 0 of 5 (0%) placebo patients No overlap between patients and achieving NASH resolution NASH Resolution1,2 30% 20% 10% 0%Placebo EFX 50mg n=5 n=12 25% 0% 3 |
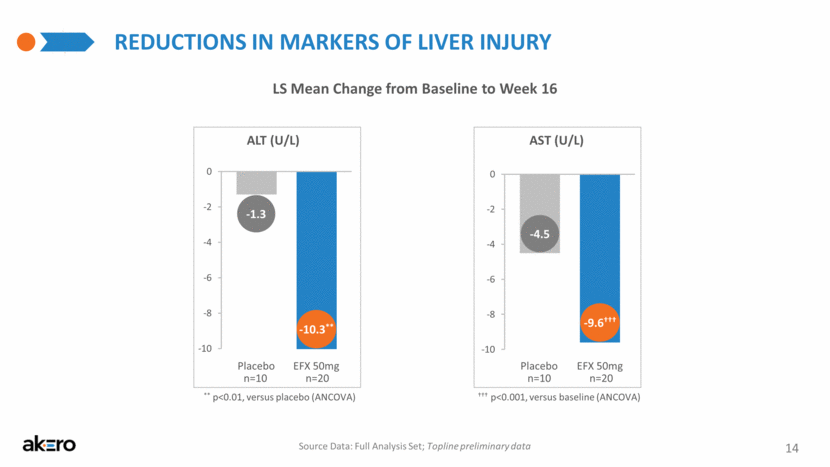 |
REDUCTIONS IN MARKERS OF LIVER INJURY LS Mean Change from Baseline to Week 16 ** p<0.01, versus placebo (ANCOVA) Placebo EFX 50mg ALT (U/L) Placebo EFX 50mg AST (U/L) ††† p<0.001, versus baseline (ANCOVA) Source Data: Full Analysis Set; Topline preliminary data -10.3** -1.3 -9.6††† -4.5 14 n=20 n=10 n=20 n=10 -10 -8 -6 -4 -2 0 CFB -10 -8 -6 -4 -2 0 CFB |
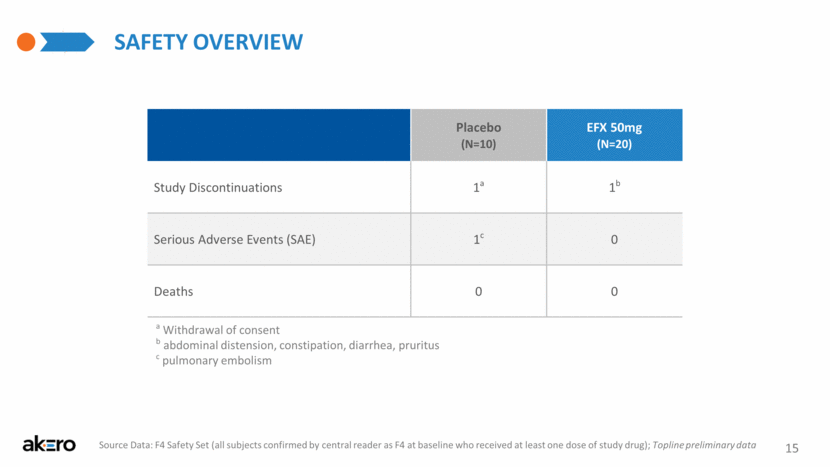 |
Safety overview 15 Placebo (N=10) EFX 50mg (N=20) Study Discontinuations 1a 1b Serious Adverse Events (SAE) 1c 0 Deaths 0 0 a Withdrawal of consent b abdominal distension, constipation, diarrhea, pruritus c pulmonary embolism Source Data: F4 Safety Set (all subjects confirmed by central reader as F4 at baseline who received at least one dose of study drug); Topline preliminary data |
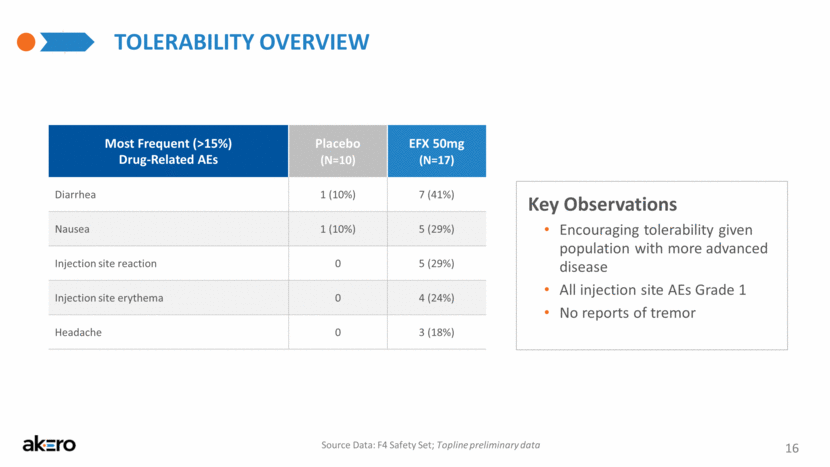 |
Key Observations Encouraging tolerability given population with more advanced disease All injection site AEs Grade 1 No reports of tremor TOLERABILITY OVERVIEW Most Frequent (>15%) Drug-Related AEs Placebo (N=10) EFX 50mg (N=17) Diarrhea 1 (10%) 7 (41%) Nausea 1 (10%) 5 (29%) Injection site reaction 0 5 (29%) Injection site erythema 0 4 (24%) Headache 0 3 (18%) 16 Source Data: F4 Safety Set; Topline preliminary data |
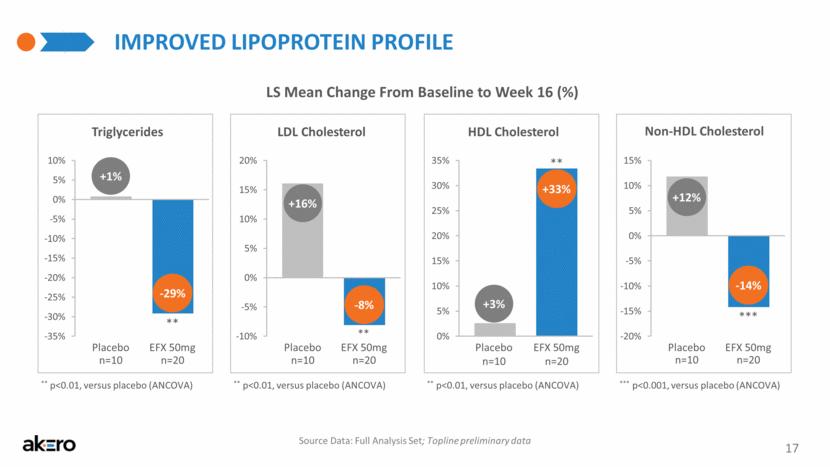 |
LDL Cholesterol Placebo EFX 50mg -8% +16% ** IMPROVED LIPOPROTEIN PROFILE 17 LS Mean Change From Baseline to Week 16 (%) ** p<0.01, versus placebo (ANCOVA) Source Data: Full Analysis Set; Topline preliminary data Triglycerides Placebo EFX 50mg -29% +1% ** HDL Cholesterol Placebo EFX 50mg +33% +3% ** Non-HDL Cholesterol Placebo EFX 50mg -14% +12% *** ** p<0.01, versus placebo (ANCOVA) ** p<0.01, versus placebo (ANCOVA) *** p<0.001, versus placebo (ANCOVA) n=20 n=10 n=20 n=10 n=20 n=10 n=20 n=10 -10% -5% 0% 5% 10% 15% 20% 1 -35% -30% -25% -20% -15% -10% -5% 0% 5% 10% 1 0% 5% 10% 15% 20% 25% 30% 35% 1 -20% -15% -10% -5% 0% 5% 10% 15% 1 |
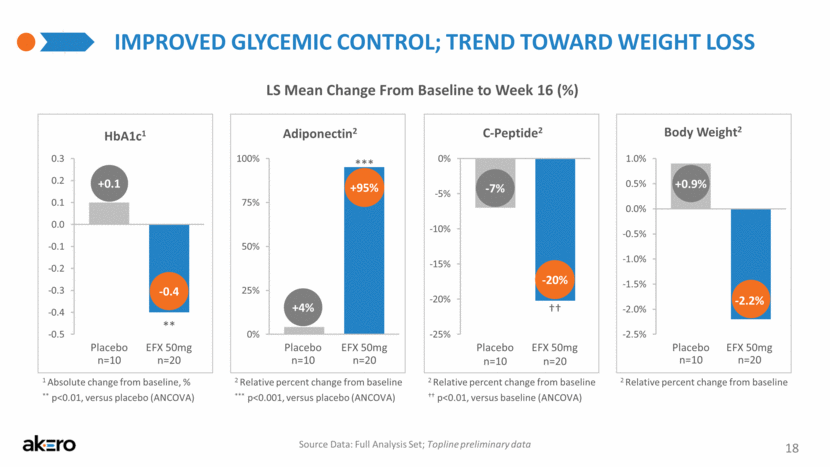 |
-0.4 +0.1 IMPROVED GLYCEMIC CONTROL; TREND TOWARD WEIGHT LOSS 18 Adiponectin2 C-Peptide2 LS Mean Change From Baseline to Week 16 (%) ** p<0.01, versus placebo (ANCOVA) Source Data: Full Analysis Set; Topline preliminary data 1 Absolute change from baseline, % 2 Relative percent change from baseline +95% +4% -20% -7% ** *** +0.9% -2.2% Body Weight2 2 Relative percent change from baseline 2 Relative percent change from baseline *** p<0.001, versus placebo (ANCOVA) †† †† p<0.01, versus baseline (ANCOVA) Placebo EFX 50mg Placebo EFX 50mg Placebo EFX 50mg Placebo EFX 50mg n=20 n=10 n=20 n=10 n=20 n=10 n=20 n=10 HbA1c1 0% 25% 50% 75% 100% 1 -25% -20% -15% -10% -5% 0% 1 -0.5 -0.4 -0.3 -0.2 -0.1 0.0 0.1 0.2 0.3 CFB -2.5% -2.0% -1.5% -1.0% -0.5% 0.0% 0.5% 1.0% 1 |
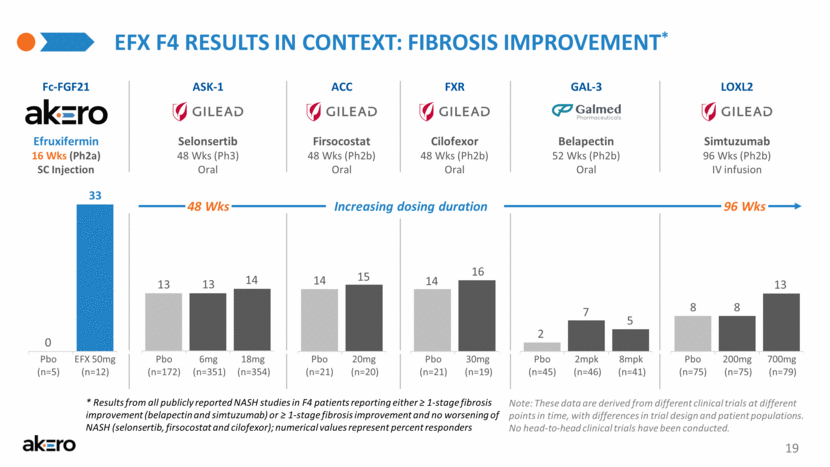 |
Belapectin 52 Wks (Ph2b) Oral GAL-3 EFX F4 RESULTS IN CONTEXT: FIBROSIS IMPROVEMENT* Selonsertib 48 Wks (Ph3) Oral Simtuzumab 96 Wks (Ph2b) IV infusion ASK-1 ACC FXR LOXL2 Firsocostat 48 Wks (Ph2b) Oral Cilofexor 48 Wks (Ph2b) Oral (n=20) (n=21) Pbo 20mg Efruxifermin 16 Wks (Ph2a) SC Injection Fc-FGF21 (n=19) (n=21) Pbo 30mg (n=12) (n=5) Pbo EFX 50mg (n=46) (n=45) Pbo 2mpk (n=41) 8mpk (n=75) (n=75) Pbo 200mg (n=79) 700mg (n=351) (n=172) Pbo 6mg (n=354) 18mg 13 14 13 8 13 8 2 5 7 14 16 14 15 0 33 19 Increasing dosing duration 96 Wks 48 Wks Note: These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. No head-to-head clinical trials have been conducted. * Results from all publicly reported NASH studies in F4 patients reporting either ≥ 1-stage fibrosis improvement (belapectin and simtuzumab) or ≥ 1-stage fibrosis improvement and no worsening of NASH (selonsertib, firsocostat and cilofexor); numerical values represent percent responders |
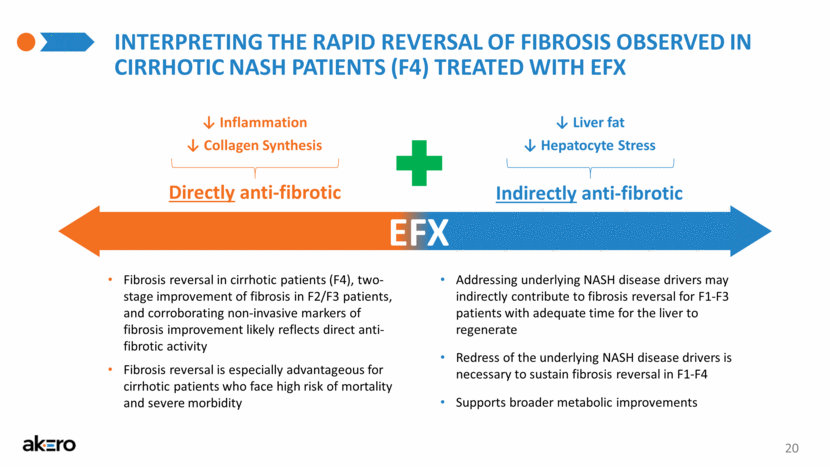 |
20 INTERPRETING THE RAPID REVERSAL OF FIBROSIS OBSERVED IN CIRRHOTIC NASH PATIENTS (F4) TREATED WITH EFX Indirectly anti-fibrotic Directly anti-fibrotic ↓ Hepatocyte Stress ↓ Inflammation ↓ Liver fat ↓ Collagen Synthesis EFX Addressing underlying NASH disease drivers may indirectly contribute to fibrosis reversal for F1-F3 patients with adequate time for the liver to regenerate Redress of the underlying NASH disease drivers is necessary to sustain fibrosis reversal in F1-F4 Supports broader metabolic improvements Fibrosis reversal in cirrhotic patients (F4), two-stage improvement of fibrosis in F2/F3 patients, and corroborating non-invasive markers of fibrosis improvement likely reflects direct anti-fibrotic activity Fibrosis reversal is especially advantageous for cirrhotic patients who face high risk of mortality and severe morbidity |
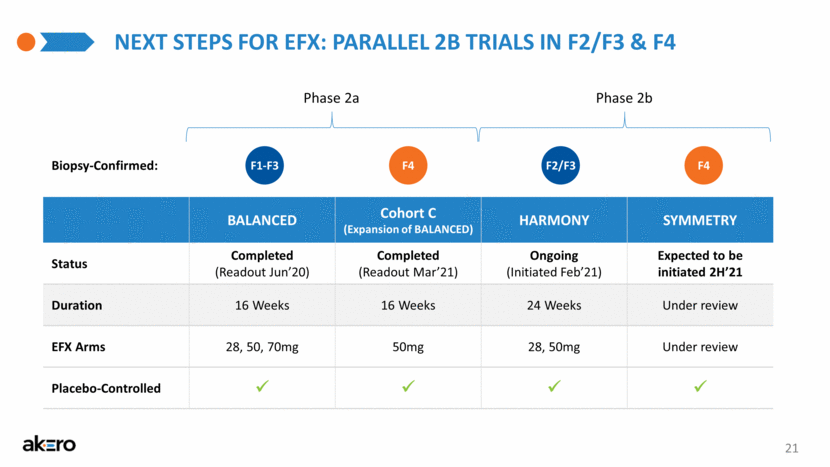 |
NEXT STEPS FOR EFX: PARALLEL 2B TRIALS IN F2/F3 & F4 BALANCED Cohort C (Expansion of BALANCED) HARMONY SYMMETRY Status Completed (Readout Jun’20) Completed (Readout Mar’21) Ongoing (Initiated Feb’21) Expected to be initiated 2H’21 Duration 16 Weeks 16 Weeks 24 Weeks Under review EFX Arms 28, 50, 70mg 50mg 28, 50mg Under review Placebo-Controlled F1-F3 F4 F2/F3 F4 Phase 2a Phase 2b 21 Biopsy-Confirmed: |
 |
A Global Disease, A Pioneering Treatment NASDAQ: AKRO |
