Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Soliton, Inc. | formxinvestorpresentations.htm |
!"#$%&'(#&)*$+,($"&*-.$/0$102$3452647829$:42;082$<96=455401. Rapid Acoustic Pulse Technology (Nasdaq: SOLY) EXHIBIT 99.1

! Forward-Looking Statements All statements contained herein other than statements of historical fact, including statements regarding our future results of operations and financial position, our business strategy and plans, and our objectives for future operations, are forward-looking statements. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” and similar expressions are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy, short-term and long-term business operations and objectives, and financial needs. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under Item 1A. “Risk Factors” in our most recently filed Form 10-K filed with the Securities and Exchange Commission (“SEC”) and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this presentation speak only as of its date. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the future events and trends discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. More detailed information about Soliton is set forth in our filings with the SEC. Security holders are urged to read these documents free of charge on the SEC’s web site at http://www.sec.gov.

" Overview Breakthrough acoustic shockwave device using novel and proprietary Rapid Acoustic Pulse (RAP) technology ! Licensed from MD Anderson Cancer Center ! Induces mechanical disruption to targeted cellular level structures and vacuoles ! No cavitation, heat or collateral tissue damage or pain Platform technology ! Tattoo removal in as little as 1/3 the number office visits compared to standard of care ! Improvement in the appearance of Cellulite (recently cleared by FDA) ! Keloids/other fibrotic disorders – human POC data ! Skin laxity reduction in clinical development Potential $24 billion global market ! Global cellulite reduction market estimated to be ~$4 billion by 2025 ! Global market for tattoo removal is projected to be ~$4 billion by 2023 ! Global keloid scar treatment market estimated to be ~$10 billion by 2025 ! Skin laxity treatment market estimated to be ~$6 billion in 2018 Images provided by Industrial Design firm; actual product may differ somewhat when the product is launched. There is no guarantee of a successful outcome of this revenue strategy.

# Science Advisory Board High profile Key Opinion Leaders Strong podium presence Respected, published researchers

$ Device and Revenue Strategy Sell consoles Hand piece contains replaceable cartridge Replaceable consumable cartridge for one patient visit “razor and blade” recurring revenue model Images provided by Industrial Design firm; actual product may differ somewhat when the product is launched. There is no guarantee of a successful outcome of this revenue strategy.

% ! Electro-hydraulics (arc plasma in saline) utilizes 3000 volts at 3000 amps; creates shockwaves from .25 to 12 MPa @ 100 Hz ! Electronic wave shaping enables very high repetition rate; reduces unwanted frequencies that cause heat and pain ! Custom designed reflector eliminates cavitation, heat, pain; non-focused, slightly diverging waves ! Different cartridges enable different therapies: tattoo removal and cellulite reduction, and if cleared, keloid scars ! 9 patent families filed ! Worldwide exclusive license from MD Anderson ! 60601 Electrical Safety certified; 2 MOPP/MOOP ! IRB “non-significant risk” established Electrode Cartridge Treatment windowReflector design Electrode gap – spark generation and focal point
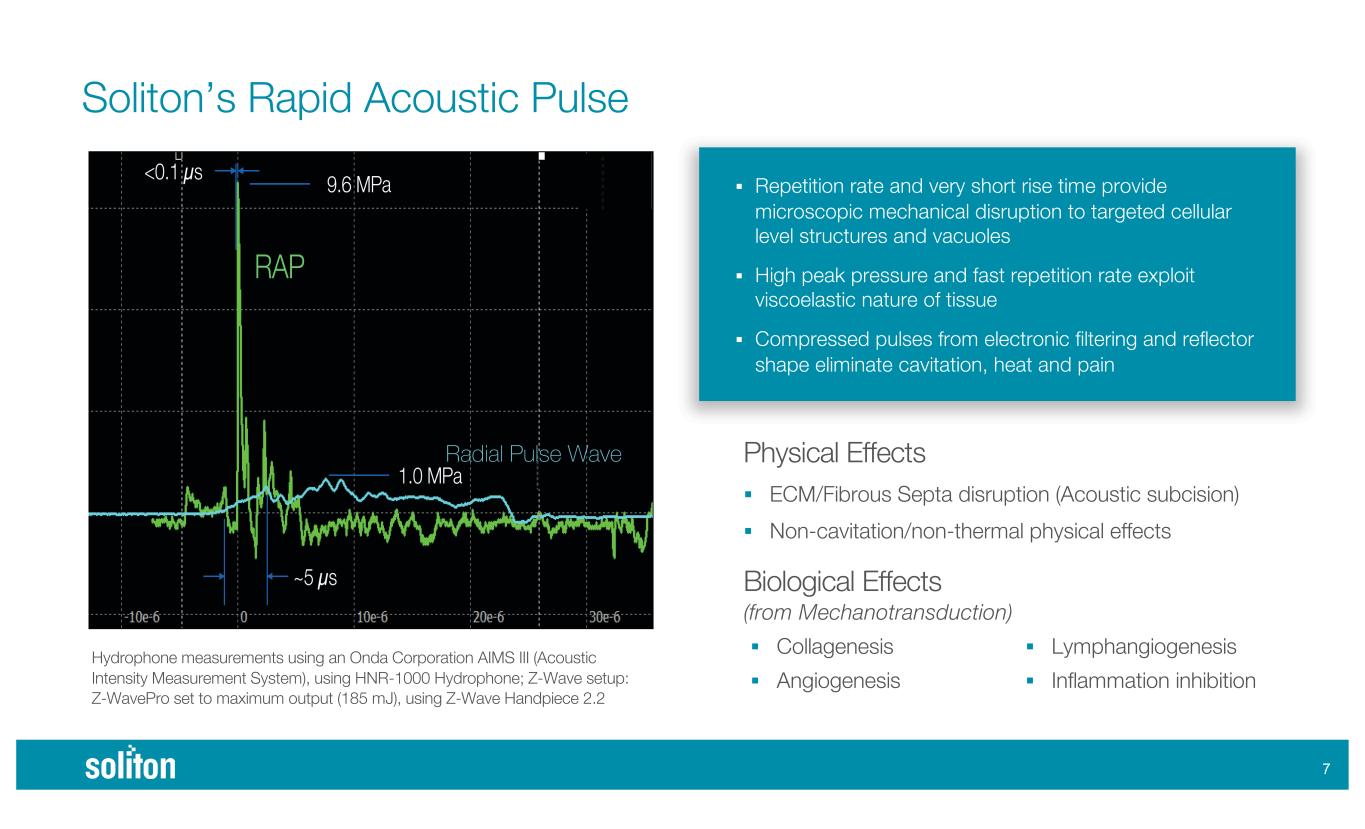
& Hydrophone measurements using an Onda Corporation AIMS III (Acoustic Intensity Measurement System), using HNR-1000 Hydrophone; Z-Wave setup: Z-WavePro set to maximum output (185 mJ), using Z-Wave Handpiece 2.2 Soliton’s Rapid Acoustic Pulse Physical Effects ! ECM/Fibrous Septa disruption (Acoustic subcision) ! Non-cavitation/non-thermal physical effects Biological Effects (from Mechanotransduction) ! Repetition rate and very short rise time provide microscopic mechanical disruption to targeted cellular level structures and vacuoles ! High peak pressure and fast repetition rate exploit viscoelastic nature of tissue ! Compressed pulses from electronic filtering and reflector shape eliminate cavitation, heat and pain ! Collagenesis ! Angiogenesis ! Lymphangiogenesis ! Inflammation inhibition Radial Pulse Wave

' Acoustic ECM Disruption—Breakthrough CONTROL – 20X 1 MIN – 20X 2 MIN – 20X 3 MIN – 20X Septa Disruption—Time Dose Response (Source: Control—Tx#26, Pig202 (68659); Test—Tx#63, Day O, Pig 210a. R1-R3 (68659 vs 91940 vs 91939 vs 91900); Head: Omega set at higher power 2800v, 100HZ, 70ms) (Animal Model)
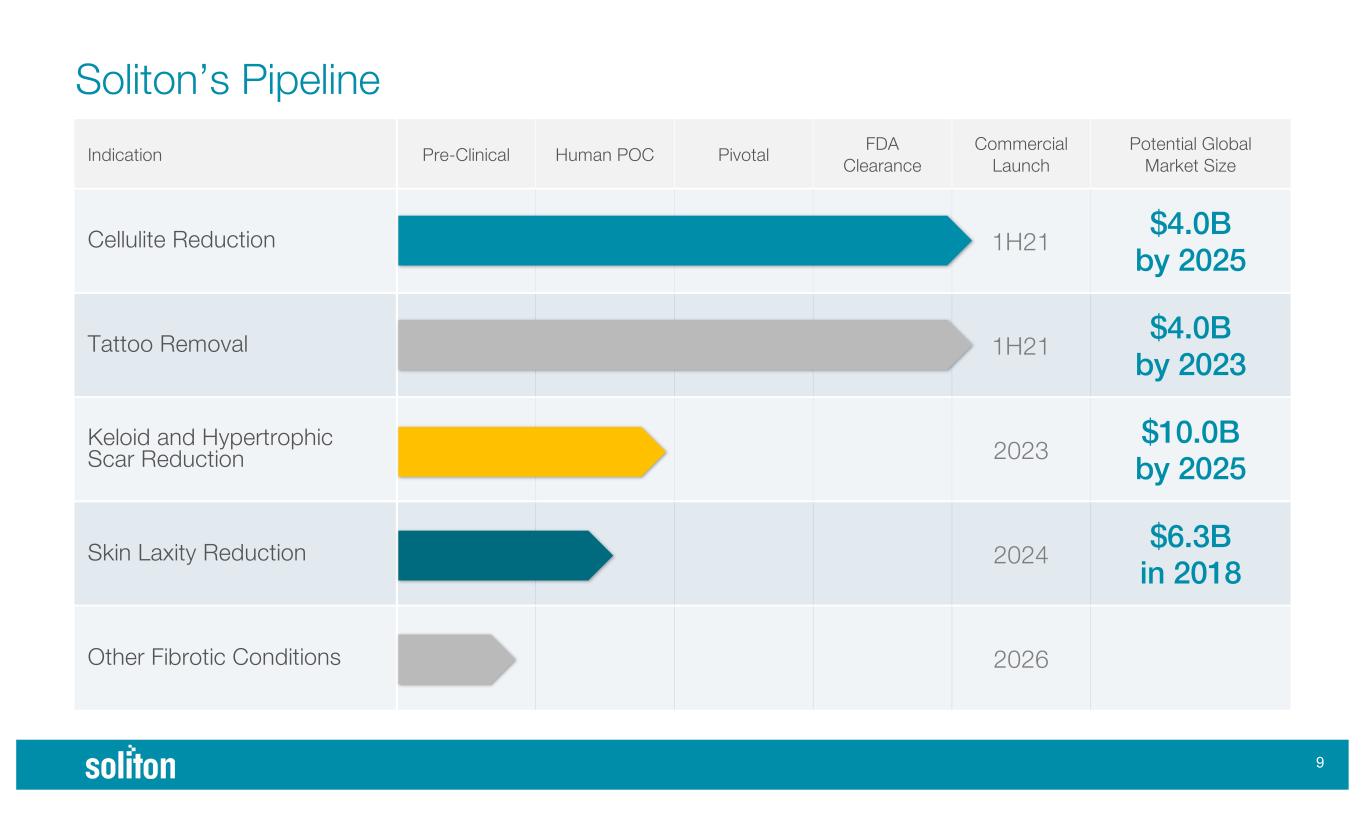
( Soliton’s Pipeline Indication Pre-Clinical Human POC Pivotal FDA Clearance Commercial Launch Potential Global Market Size Cellulite Reduction 1H21 $4.0B by 2025 Tattoo Removal 1H21 $4.0B by 2023 Keloid and Hypertrophic Scar Reduction 2023 $10.0B by 2025 Skin Laxity Reduction 2024 $6.3B in 2018 Other Fibrotic Conditions 2026

!"#$%&'(#&)*$+,($"&*-.$/0$102$3452647829$:42;082$<96=455401. Cellulite FDA Cleared January 2021
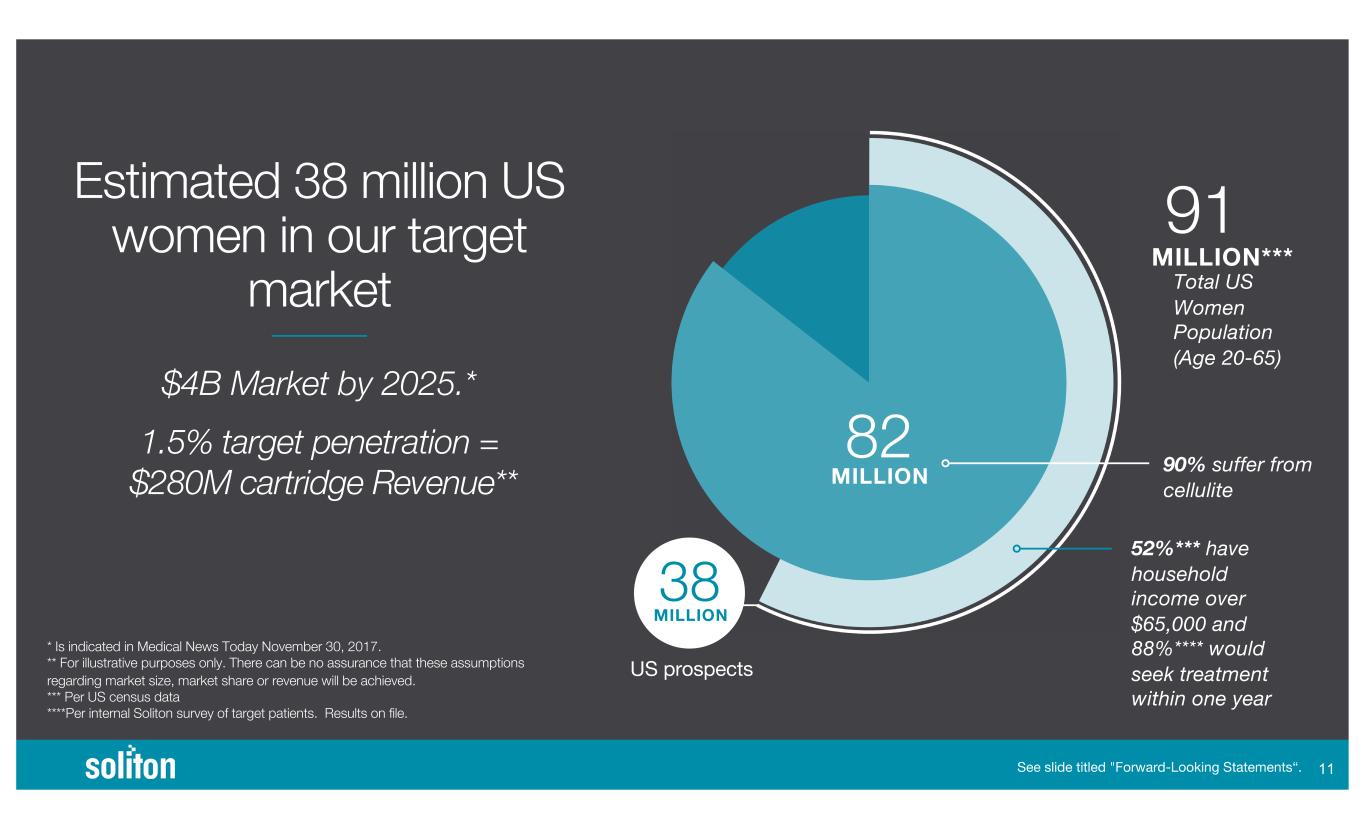
$4B Market by 2025.* Estimated 38 million US women in our target market * Is indicated in Medical News Today November 30, 2017. ** For illustrative purposes only. There can be no assurance that these assumptions regarding market size, market share or revenue will be achieved. *** Per US census data ****Per internal Soliton survey of target patients. Results on file. See slide titled "Forward-Looking Statements“. US prospects MILLION 82 MILLION 38 MILLION*** 91 Total US Women Population (Age 20-65) 90% suffer from cellulite 52%*** have household income over $65,000 and 88%**** would seek treatment within one year )) 1.5% target penetration = $280M cartridge Revenue**

Company Technology Noninvasive Avg Cost # Trmts Long-term Duration Side Effects Patient Satisfaction Syneron Candela RF $1,600 4+ L 60% Cynosure RF & massage 6 L Solta RF $2,475 1 H 67% BTL RF & targeted pressure energy $3,100 4 L 90% Venus Concept RF & pulsed EM fields $2,050 6+ L 62% Soliton Rapid Acoustic Pulse $2,500** 1 *** L 93% Merz Surgical Procedure $4,250 1 H 94% Cynosure Laser used under skin $5,775 1 H 92.5% Endo Injected Drug $3,500 per vial 3 H 62.9% )! *Based on the Company's review of publicly available information regarding these technologies and products. **Estimated pricing if FDA clearance is obtained. Not currently FDA-cleared for treatment of cellulite and not commercially available. *** Soliton does not have long-term duration indication through the FDA. Competitive Landscape in Cellulite*
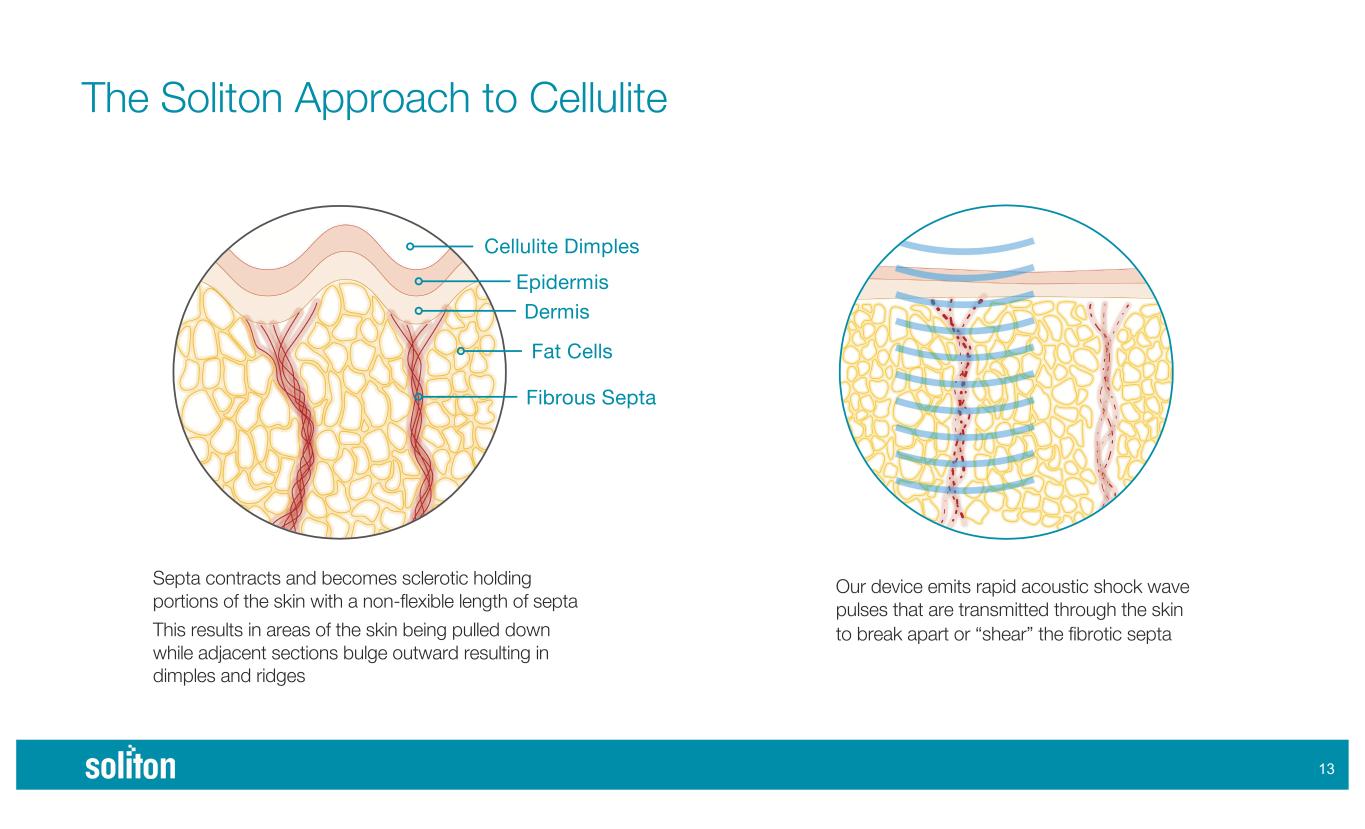
The Soliton Approach to Cellulite )" Acoustic Subcision Septa contracts and becomes sclerotic holding portions of the skin with a non-flexible length of septa This results in areas of the skin being pulled down while adjacent sections bulge outward resulting in dimples and ridges Our device emits rapid acoustic shock wave pulses that are transmitted through the skin to break apart or “shear” the fibrotic septa Cellulite Dimples Epidermis Dermis Fat Cells Fibrous Septa

3.41 2.05 2.40 1.47 1.00 1.50 2.00 2.50 3.00 3.50 4.00 Pivotal Cellulite Trial Results Single Treatment at 12 weeks Acoustic Cellulite Release (n=56) Skin Rejuvenation (n=44) 29.6%** 28.5%*** Pre PrePost Post Hexsel Laxity Score (0-3) CSS Score (0-5) Summary: 12-Week Results Pain Score: 2.4 (on a scale of 0-10) Patient Satisfaction*: 92.9% *Percent of patients that agree or strongly agree their cellulite appeared improved **Cellulite Severity Score (“CSS”) represents the average of the percentage change in CSS for final participants *** Laxity Score (LS) represents the average of the percentage change in LS for final participants Patient n: 56 ** GAIS-Cellulite Imp/Sig Imp: 90.5% (Mean 3 Reviewers) )# Correct Blinded Identification: 96.4%
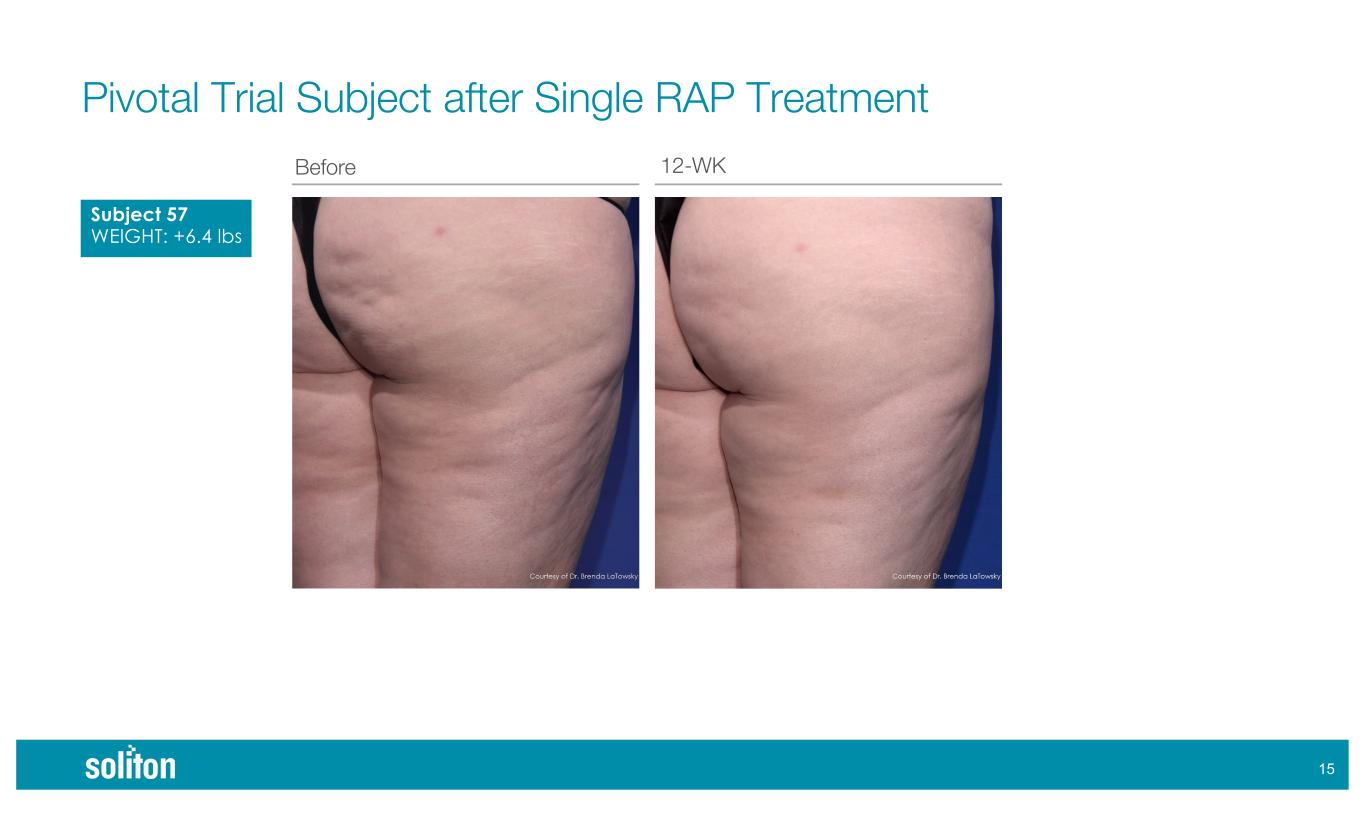
)$ Before 12-WK Pivotal Trial Subject after Single RAP Treatment Subject 57 WEIGHT: +6.4 lbs
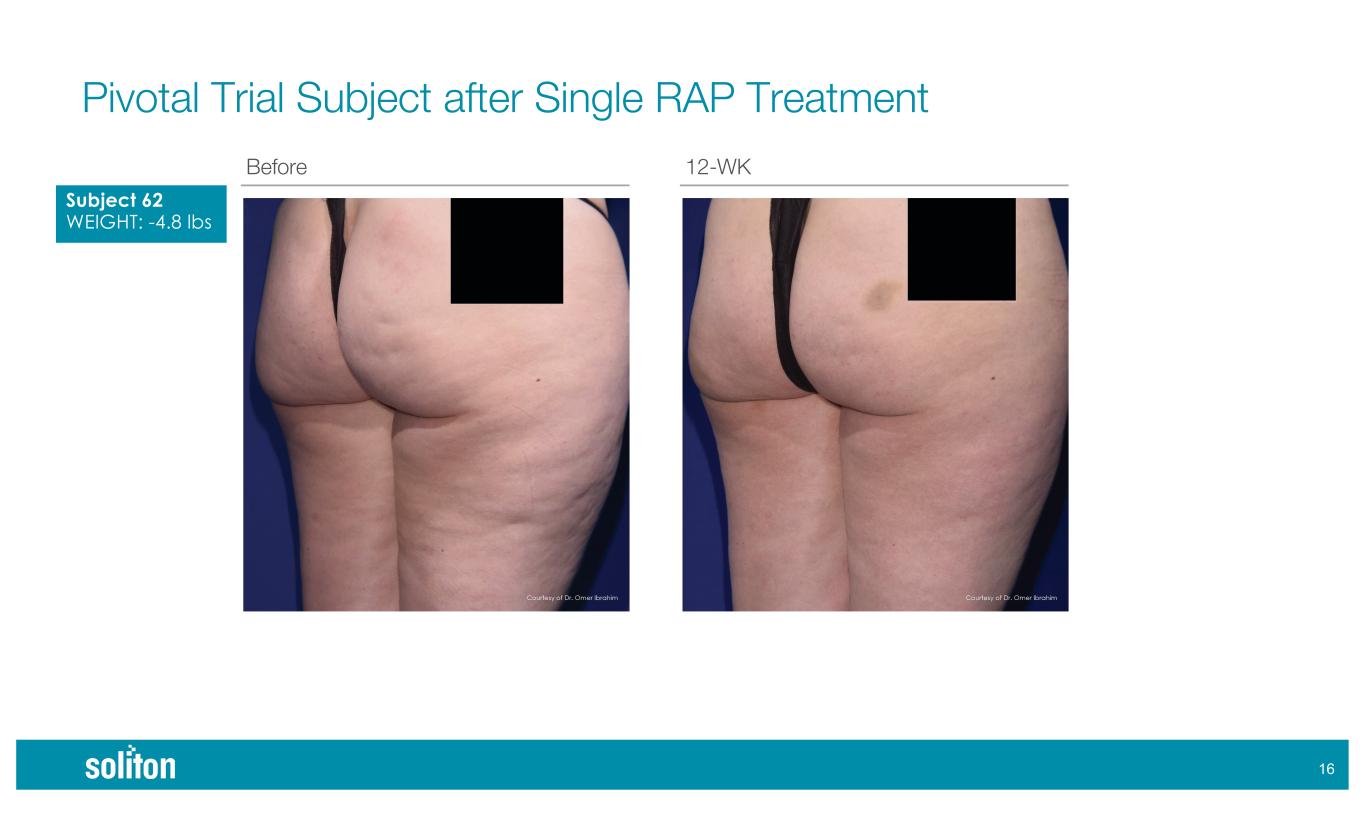
)% Before 12-WK Pivotal Trial Subject after Single RAP Treatment Subject 62 WEIGHT: -4.8 lbs
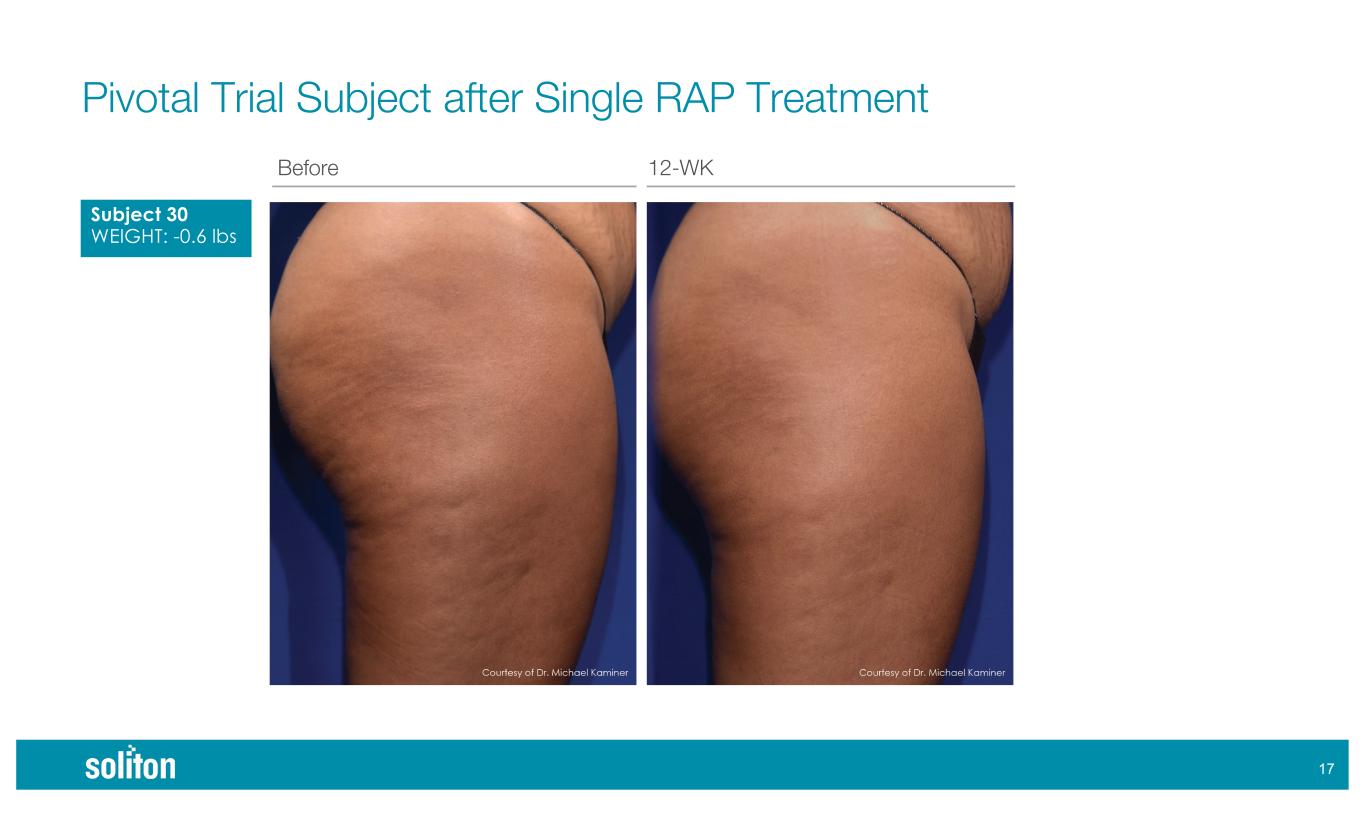
)& Before 12-WK Pivotal Trial Subject after Single RAP Treatment Subject 30 WEIGHT: -0.6 lbs

)' Before 12-WK Pivotal Trial Subject after Single RAP Treatment Subject 18 WEIGHT: -5.2 lbs Courtesy of Dr. Brenda LaTowskyCourtesy of Dr. Brenda LaTowsky

Pivotal Trial Subject after Single RAP Treatment Before 12-WK )( Subject 63 WEIGHT: +0.6 lbs
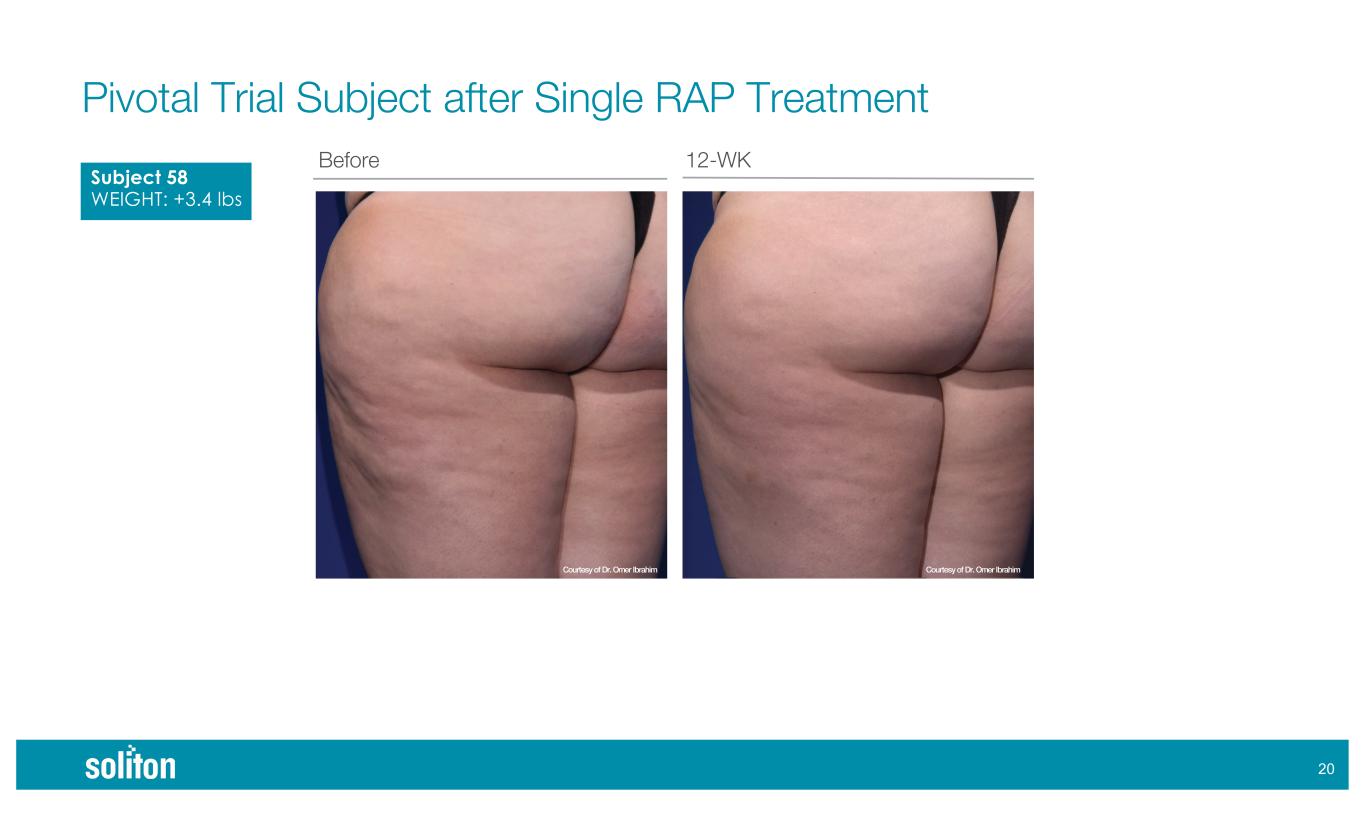
!* Before 12-WK Pivotal Trial Subject after Single RAP Treatment Subject 58 WEIGHT: +3.4 lbs Courtesy of Dr. Omer IbrahimCourtesy of Dr. Omer Ibrahim

Tattoo Removal We received clearance from the FDA for the tattoo removal !) FDA Cleared in 2019
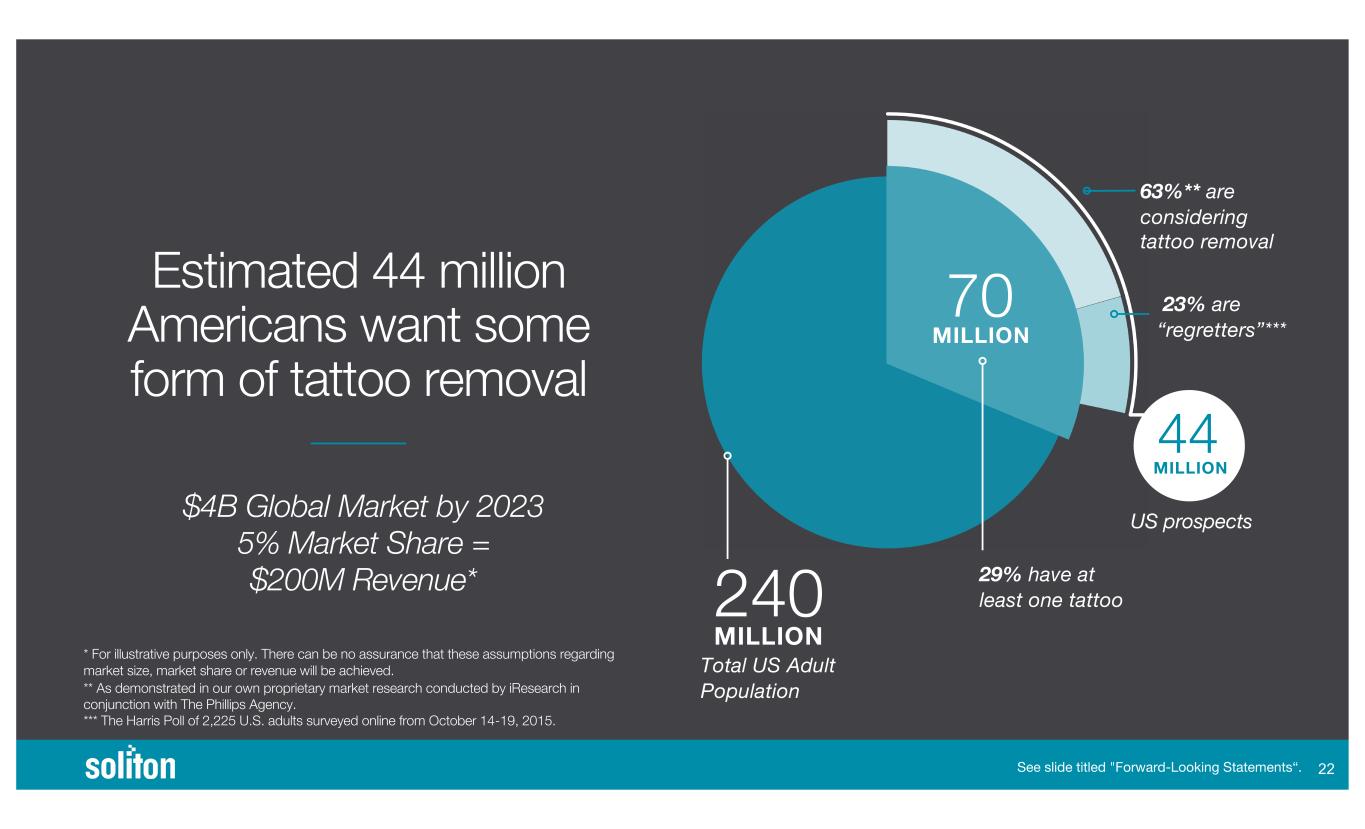
$4B Global Market by 2023 5% Market Share = $200M Revenue* Estimated 44 million Americans want some form of tattoo removal * For illustrative purposes only. There can be no assurance that these assumptions regarding market size, market share or revenue will be achieved. ** As demonstrated in our own proprietary market research conducted by iResearch in conjunction with The Phillips Agency. *** The Harris Poll of 2,225 U.S. adults surveyed online from October 14-19, 2015. 23% are “regretters”*** US prospects MILLION 240 MILLION 70 MILLION 44 Total US Adult Population 29% have at least one tattoo 63%** are considering tattoo removal See slide titled "Forward-Looking Statements“. !!
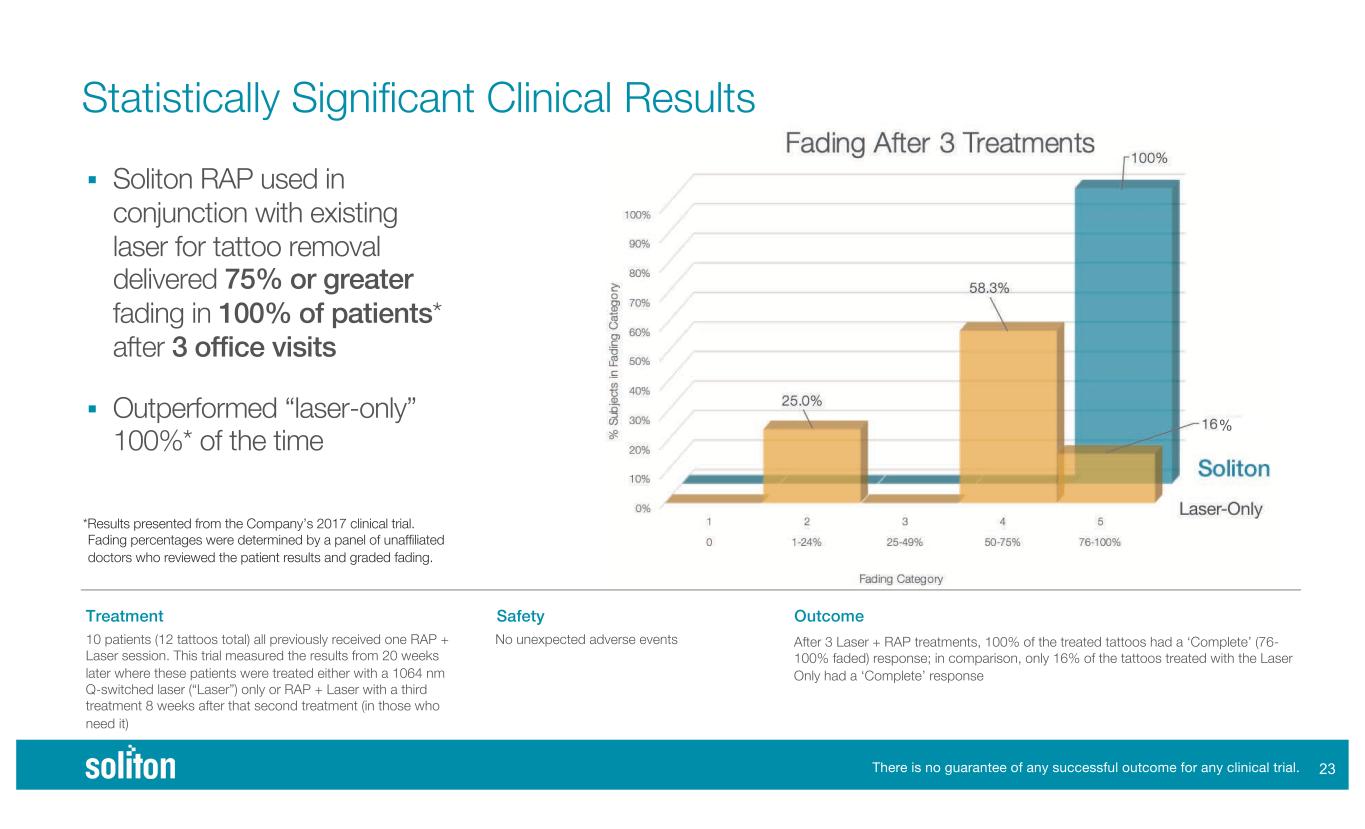
!" Statistically Significant Clinical Results *Results presented from the Company’s 2017 clinical trial. Fading percentages were determined by a panel of unaffiliated doctors who reviewed the patient results and graded fading. ! Soliton RAP used in conjunction with existing laser for tattoo removal delivered 75% or greater fading in 100% of patients* after 3 office visits ! Outperformed “laser-only” 100%* of the time There is no guarantee of any successful outcome for any clinical trial. Treatment 10 patients (12 tattoos total) all previously received one RAP + Laser session. This trial measured the results from 20 weeks later where these patients were treated either with a 1064 nm Q-switched laser (“Laser”) only or RAP + Laser with a third treatment 8 weeks after that second treatment (in those who need it) Safety No unexpected adverse events Outcome After 3 Laser + RAP treatments, 100% of the treated tattoos had a ‘Complete’ (76- 100% faded) response; in comparison, only 16% of the tattoos treated with the Laser Only had a ‘Complete’ response +

!# Complete removal of treated ink after 3 treatment sessions* Before After 3 Treatments Individual results will vary. For illustrative purposes only. *At each session, treated area was treated with multiple laser passes, with each laser pass followed by a treatment with the RAP device.
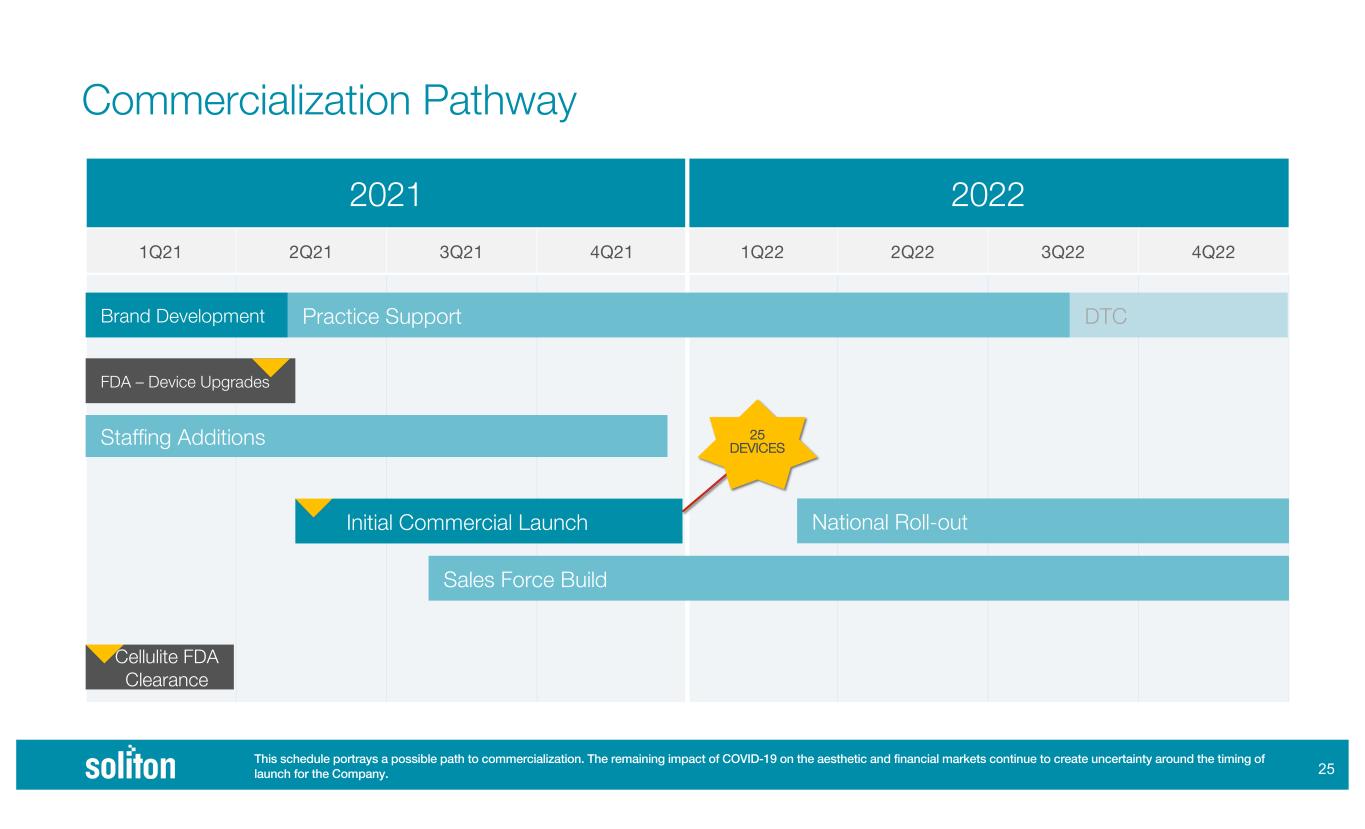
!$ 2021 2022 1Q21 2Q21 3Q21 4Q21 1Q22 2Q22 3Q22 4Q22 Brand Development FDA – Device Upgrades Staffing Additions Initial Commercial Launch Sales Force Build Cellulite FDA Clearance This schedule portrays a possible path to commercialization. The remaining impact of COVID-19 on the aesthetic and financial markets continue to create uncertainty around the timing of launch for the Company. Commercialization Pathway Practice Support National Roll-out 25 DEVICES DTC
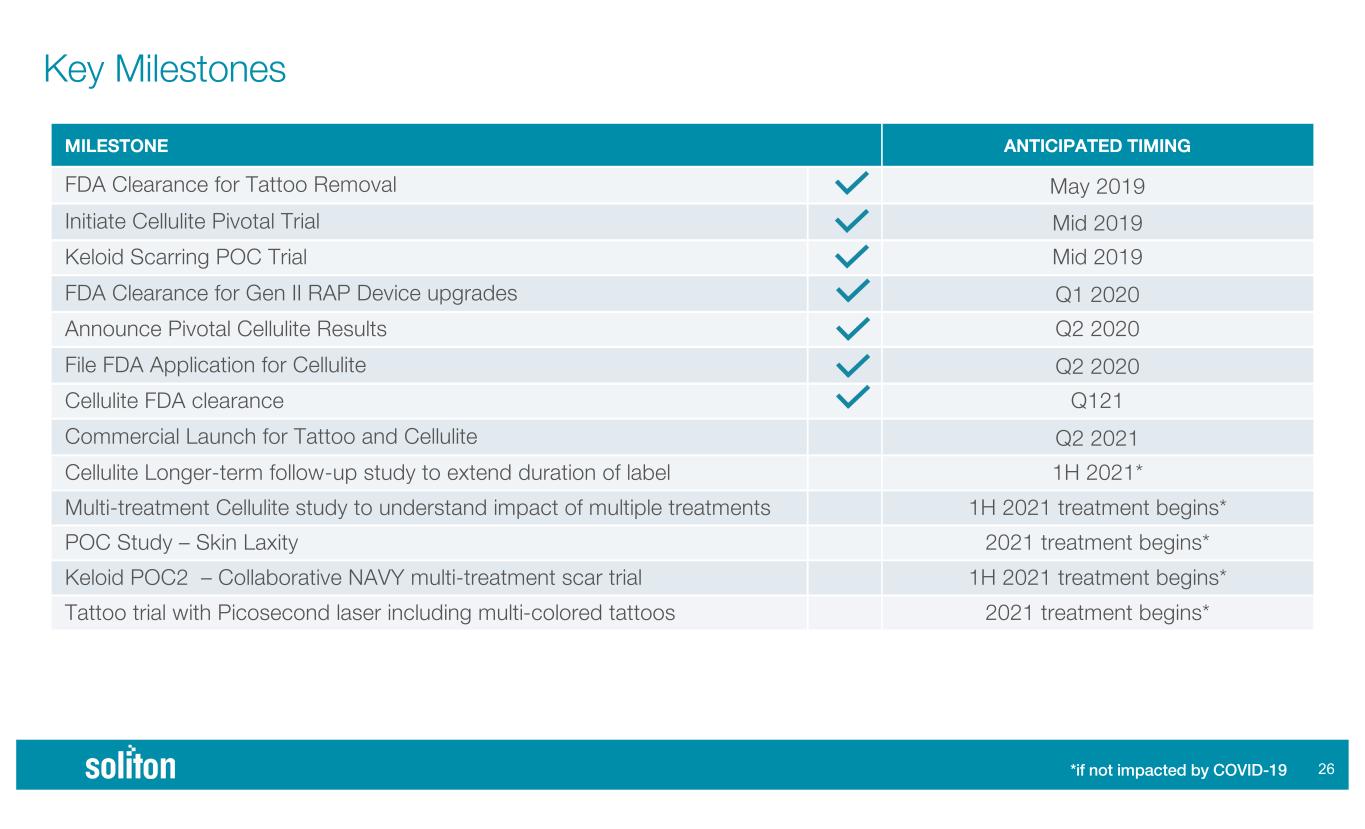
MILESTONE ANTICIPATED TIMING FDA Clearance for Tattoo Removal May 2019 Initiate Cellulite Pivotal Trial Mid 2019 Keloid Scarring POC Trial Mid 2019 FDA Clearance for Gen II RAP Device upgrades Q1 2020 Announce Pivotal Cellulite Results Q2 2020 File FDA Application for Cellulite Q2 2020 Cellulite FDA clearance Q121 Commercial Launch for Tattoo and Cellulite Q2 2021 Cellulite Longer-term follow-up study to extend duration of label 1H 2021* Multi-treatment Cellulite study to understand impact of multiple treatments 1H 2021 treatment begins* POC Study – Skin Laxity 2021 treatment begins* Keloid POC2 – Collaborative NAVY multi-treatment scar trial 1H 2021 treatment begins* Tattoo trial with Picosecond laser including multi-colored tattoos 2021 treatment begins* !% Key Milestones *if not impacted by COVID-19
!"#$%&'(#&)*$+,($"&*-.$/0$102$3452647829$:42;082$<96=455401. Rapid Acoustic Pulse Technology (Nasdaq: SOLY) Thank you
