Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Rubius Therapeutics, Inc. | tm219672d2_8k.htm |
| EX-99.1 - EXHIBIT 99.1 - Rubius Therapeutics, Inc. | tm219672d2_ex99-1.htm |
Exhibit 99.2

REALIZING THE POWER OF RED™ A NEW ERA IN CELLULAR MEDICINEInitial Clinical Results from RTX-240 Phase 1 Clinical TrialMARCH 15, 2021 1

Forward-Looking StatementsThis presentation may contain forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our development plans, our clinical results and other future conditions. All statements other than statements of historical facts contained in this presentation, including statements regarding our future financial or business performance, conditions, plans, prospects, trends or strategies and other financial and business matters; our current and prospective product candidates, planned clinical trials and preclinical activities, including timing related to such trials and expected results, research and development costs, current and prospective collaborations; the estimated size of the market for our product candidates, the timing and success of our development and commercialization of our anticipated product candidates; and the availability of alternative therapies for our target market. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. Although we believe the expectations reflected in such forward- looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party sources to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. Rubius recommends that investors independently evaluate specific investments and strategies. For further information regarding these risks, uncertainties and other factors, you should read the “Risk Factors” section of our Annual Report on Form 10-K filed for the period ended December 31, 2020 and subsequent filings with the SEC. This presentation may contain tradenames, trademarks or servicemarks of other companies. Rubius does not intend the use or display of other parties’ tradenames, trademarks or servicemarks to imply a relationship with, or endorsement or sponsorship of, these other parties.2
Single-Agent Activity with RTX-240 in Advanced Solid Tumors – Two Responses with Favorable Safety ResultsWe Aim to Realize the Power of Immune Agonists by Enabling a Broad Therapeutic WindowThese Initial Clinical Results Increase the Probability of Success of our Oncology Pipeline3
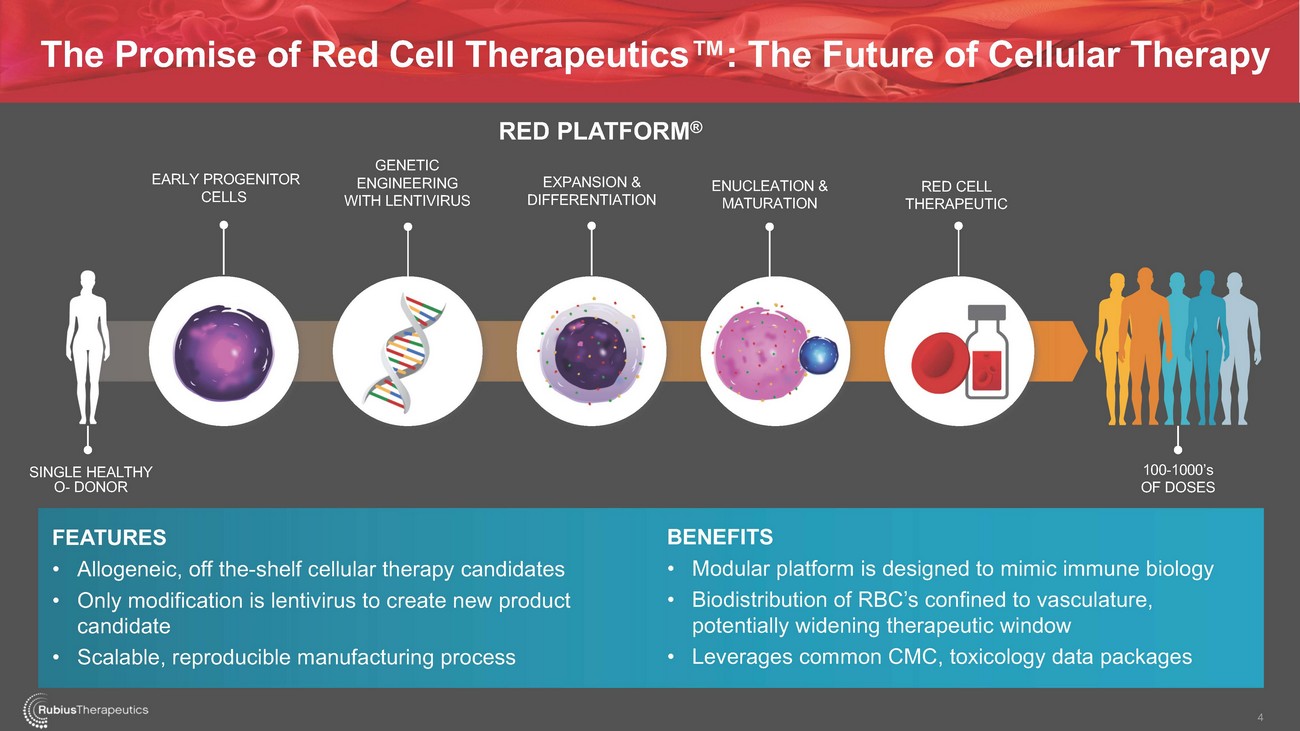
The Promise of Red Cell Therapeutics™: The Future of Cellular TherapyEARLY PROGENITOR CELLSGENETIC ENGINEERING WITH LENTIVIRUSRED PLATFORM®EXPANSION & DIFFERENTIATIONENUCLEATION & MATURATIONRED CELL THERAPEUTICSINGLE HEALTHY O- DONOR100-1000’s OF DOSESFEATURES • Allogeneic, off the-shelf cellular therapy candidates • Only modification is lentivirus to create new product candidate • Scalable, reproducible manufacturing processBENEFITS • Modular platform is designed to mimic immune biology • Biodistribution of RBC’s confined to vasculature, potentially widening therapeutic window • Leverages common CMC, toxicology data packages4
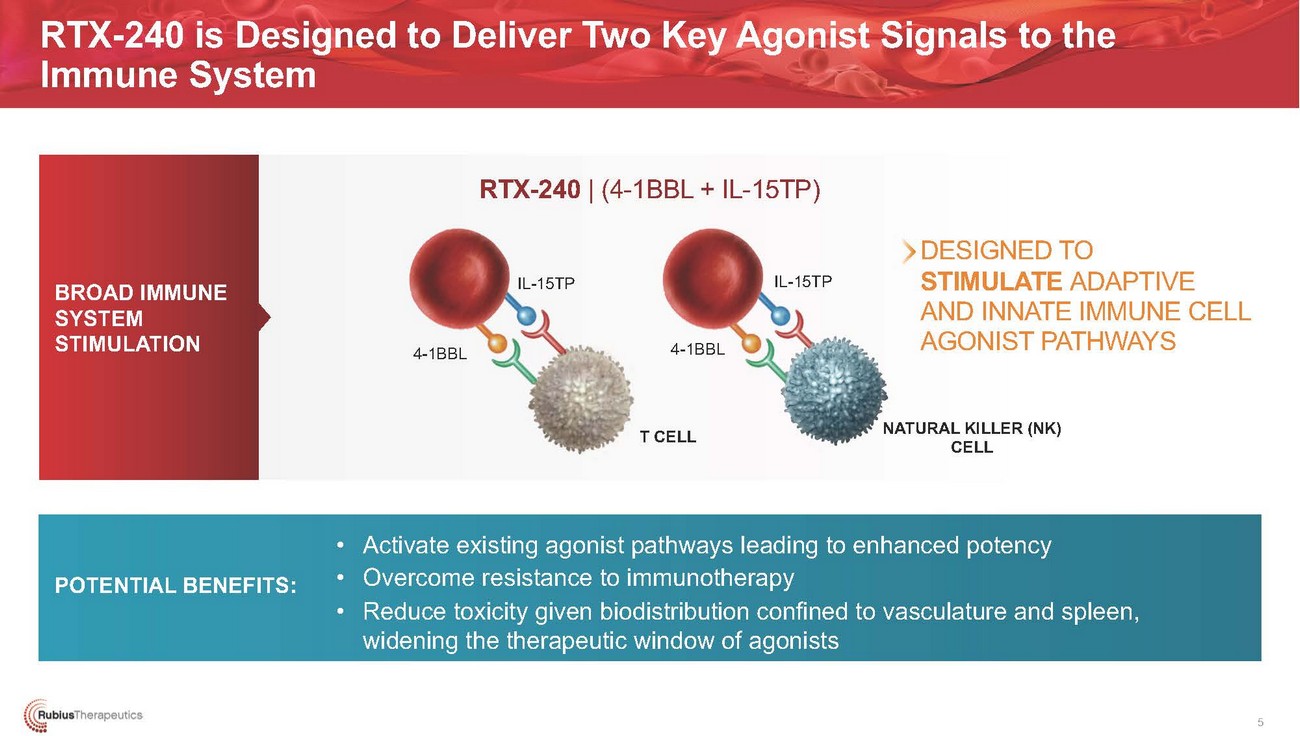
RTX-240 is Designed to Deliver Two Key Agonist Signals to the Immune SystemRTX-240 | (4-1BBL + IL-15TP)BROAD IMMUNE SYSTEM STIMULATIONDESIGNED TO STIMULATE ADAPTIVE AND INNATE IMMUNE CELL AGONIST PATHWAYSNK)POTENTIAL BENEFITS:• Activate existing agonist pathways leading to enhanced potency • Overcome resistance to immunotherapy • Reduce toxicity given biodistribution confined to vasculature and spleen, widening the therapeutic window of agonists5
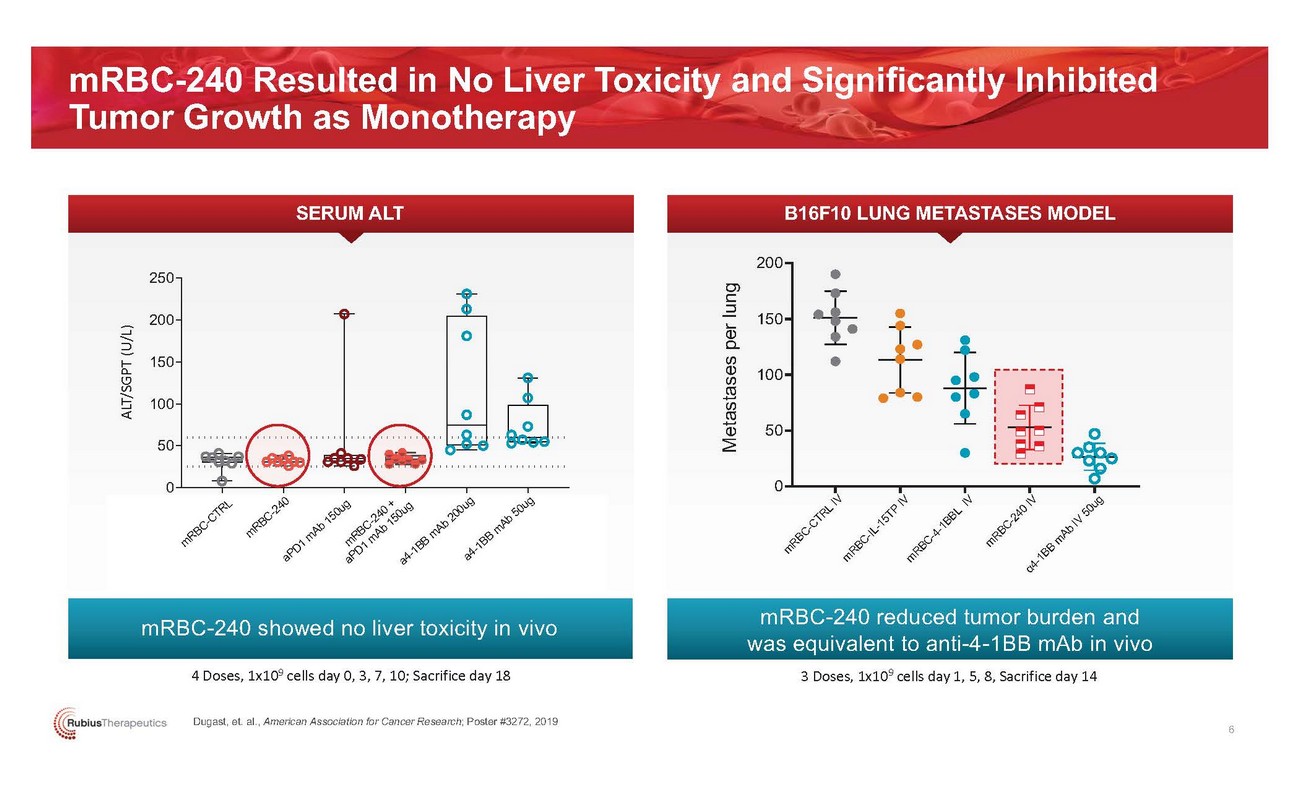
mRBC-240 Resulted in No Liver Toxicity and Significantly Inhibited Tumor Growth as MonotherapySERUM ALT B16F10 LUNG METASTASES MODEL250200200 15015010050100500 0mRBC-240 showed no liver toxicity in vivo4 Doses, 1x109 cells day 0, 3, 7, 10; Sacrifice day 18mRBC-240 reduced tumor burden and was equivalent to anti-4-1BB mAb in vivo 3 Doses, 1x109 cells day 1, 5, 8, Sacrifice day 14Dugast, et. al., American Association for Cancer Research; Poster #3272, 2019 6
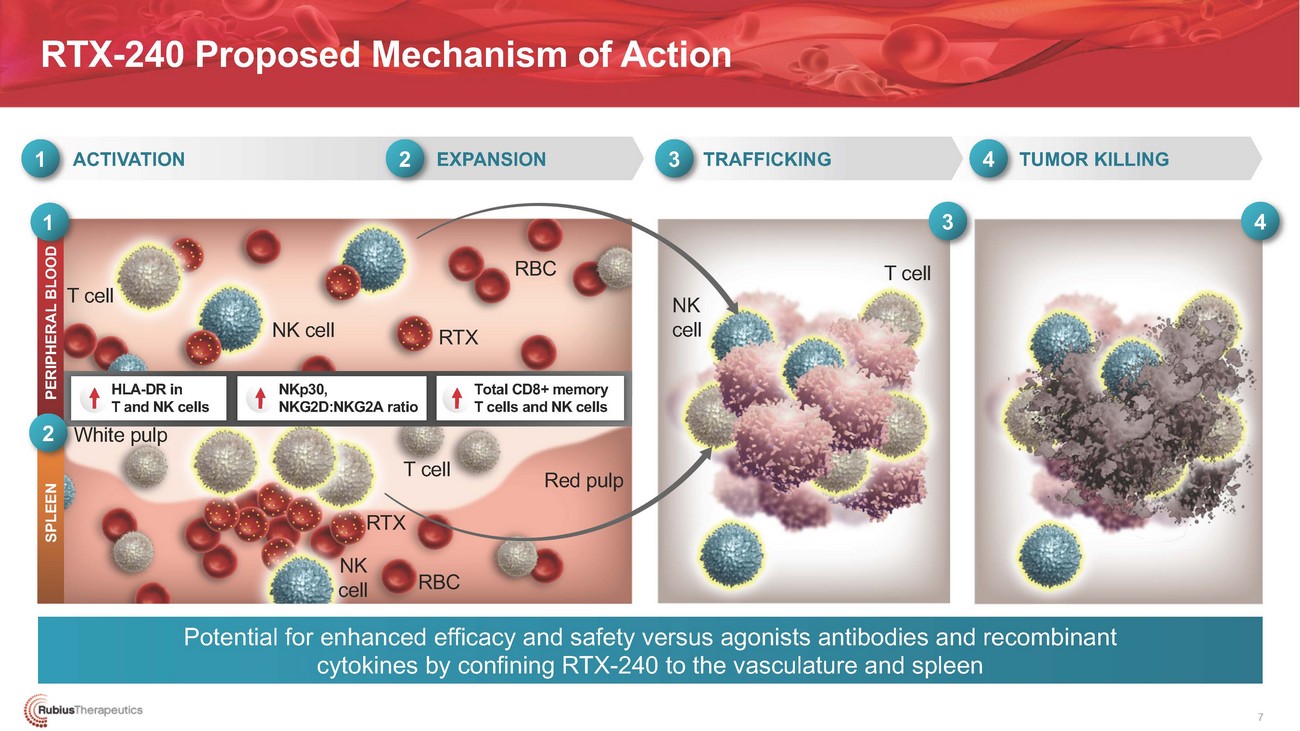
RTX-240 Proposed Mechanism of Action1 ACTIVATION2 EXPANSION3 TRAFFICKING4 TUMOR KILLING1T cellNK cell RTXRBCNK cell3 4T cellHLA-DR in T and NK cells 2 White pulpNKp30, NKG2D:NKG2A ratioTotal CD8+ memory T cells and NK cellsT cell Red pulp RTX NKcellRBCPotential for enhanced efficacy and safety versus agonists antibodies and recombinant cytokines by confining RTX-240 to the vasculature and spleen7

RTX-240 Led to Clinical Benefit in Certain Patients with Pretreated Advanced Solid TumorsPARTIAL RESPONSE CONFIRMEDMETASTATIC ANAL CANCERPARTIAL RESPONSE UNCONFIRMED METASTATIC UVEAL MELANOMASTABLE DISEASE*NON-SMALL CELL LUNG CANCERSTABLE DISEASESOFT TISSUE SARCOMASTABLE DISEASEPANCREATIC CANCERSTABLE DISEASEPROSTATE CANCER• 54% tumor shrinkage of target lesions • Duration of Response >4 months• Treated at 1e8 Q4W • 3L metastatic• Treatment was ongoing at 8 months • Prior anti-PD-L1 (1L) and anti-PD-L1 + ICOS therapy (2L)• 100% tumor shrinkage of target lesion • Resolution of 14/15 hepatic non-target lesions at 16 weeks• Treated at 1e10 Q4W • 2L metastatic• Treatment was ongoing at 4 months • Prior anti-PD-1 + CTLA-4• Disease stabilization at 8 weeks• Treated at 3e10 Q4W • 2L metastatic• Treatment was ongoing at 3 months • Prior anti-PD-L1 + chemo as 1L therapy• Disease stabilization for 4 months• Converted cold tumor to hot with trafficking of NK and T cells and expression of PD-L1• Treated at 1e10 IV/IT • 3L metastatic• ---• Prior lines of therapy with adriamycin + ifosfamide and tazemetostat• Disease stabilization for 3 months• Treated at 1e10 IV • 2L metastatic• ---• Prior therapy with FOLFOXIRI chemotherapy• Disease stabilization for 4 months• Treated at 3e9 Q4W • 4L metastatic• ---• Prior therapy with enzalutamide, abiraterone, docetaxel and tremelimumabInitial clinical data at the time of the cut-off Feb 28, 2021 *Stable disease according to RECIST v.1.1 of at least 12 weeks duration is reported8

CLINICAL TRIAL DESIGN & INITIAL DATA SUMMARY CHRISTINA COUGHLIN, M.D., PH.D., CHIEF MEDICAL OFFICER9

RTX-240 Clinical Development PlanMONOTHERAPY Relapsed/refractory (R/R) or locally advanced solid tumors1e8 (n=2) Q4W IV1e9 (n=3) Q6W IV3e9 (n=3) Q4W IV1e10 (n=4) Q4W iv1e10 (n=3) Q4W iv/it*3e10 Q4W IV (n=1) Open to Enrollment**cFurther explore dose & intervalRTX-240 + PD-1 Relapsed/Refractory Solid Tumors1e10 Q3W IV Open to Enrollment**Phase 1: Dose-Escalation ~3-4 cohorts (leverage monotherapy data)MONOTHERAPY Relapsed/Refractory Acute Myeloid LeukemiaENROLLING: Phase 1 Dose Escalation Phase 2 Expansion* iv/it (intratumoral) dosing is designed with the combination of iv and it in cycle 1 followed by Q4W iv ** Two dose cohorts open in parallel to explore dose (3e10 iv Q4W) and schedule (1e10 iv Q3W)10
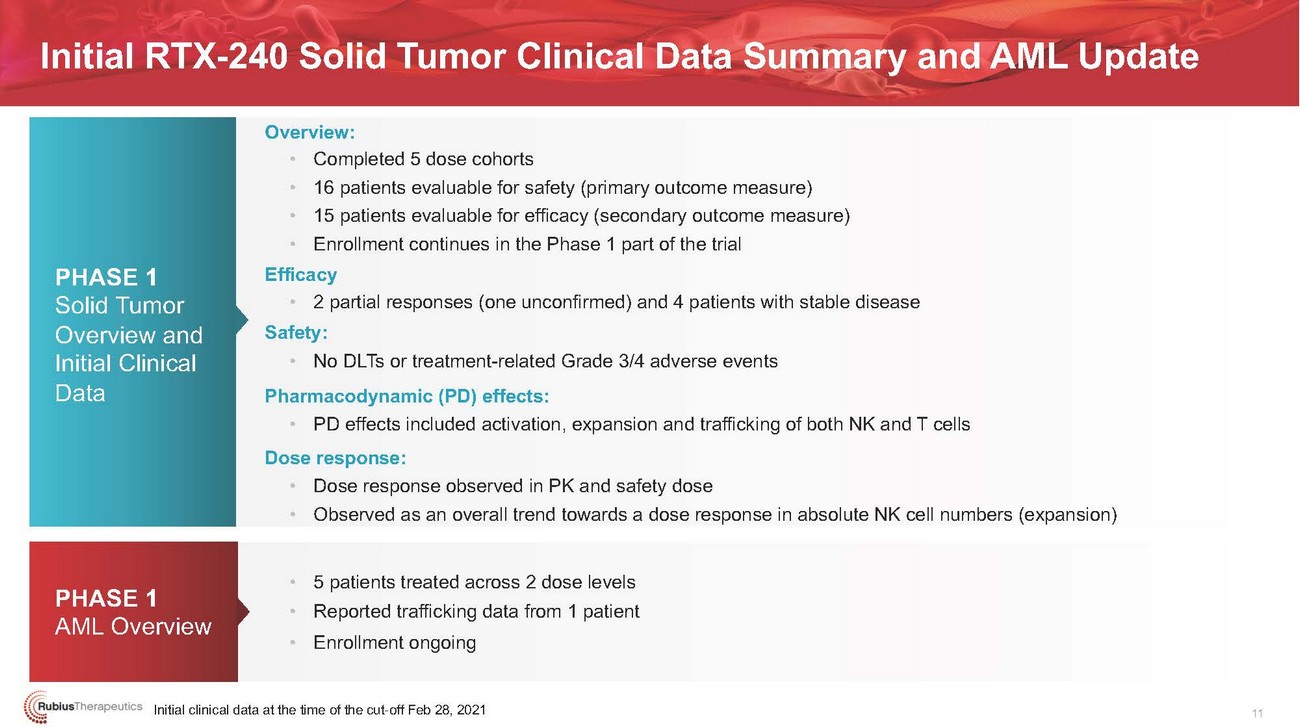
Initial RTX-240 Solid Tumor Clinical Data Summary and AML UpdatePHASE 1 Solid Tumor Overview and Initial Clinical DataPHASE 1 AML OverviewOverview: • • • • Efficacy • Safety: • Pharmacodynamic (PD) effects: • Dose response: • •• 5 patients treated across 2 dose levels • Reported trafficking data from 1 patient • Enrollment ongoingInitial clinical data at the time of the cut-off Feb 28, 202111

......·.·.·.·.·..•.•..• . i·.(·.;(•..R...ubiusTherapeutics •••••••••PATIENT CASE STUDIES12
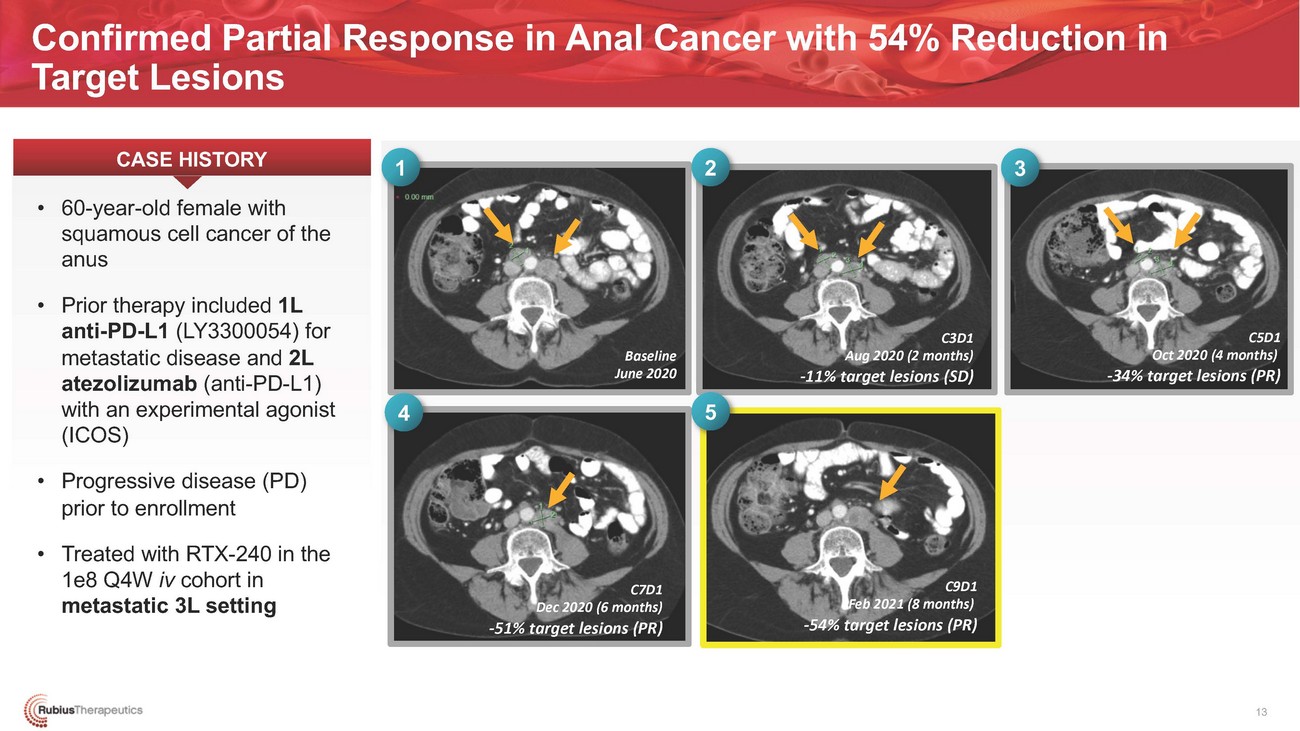
Confirmed Partial Response in Anal Cancer with 54% Reduction in Target LesionsCASE HISTORY1 2 3Baseline June 20204 5C3D1 Aug 2020 (2 months) -11% target lesions (SD)C5D1 Oct 2020 (4 months) -34% target lesions (PR)prior to enrollment • Treated with RTX-240 in the 1e8 Q4W iv cohort in metastatic 3L settingC7D1 Dec 2020 (6 months) -51% target lesions (PR)C9D1 Feb 2021 (8 months) -54% target lesions (PR)13
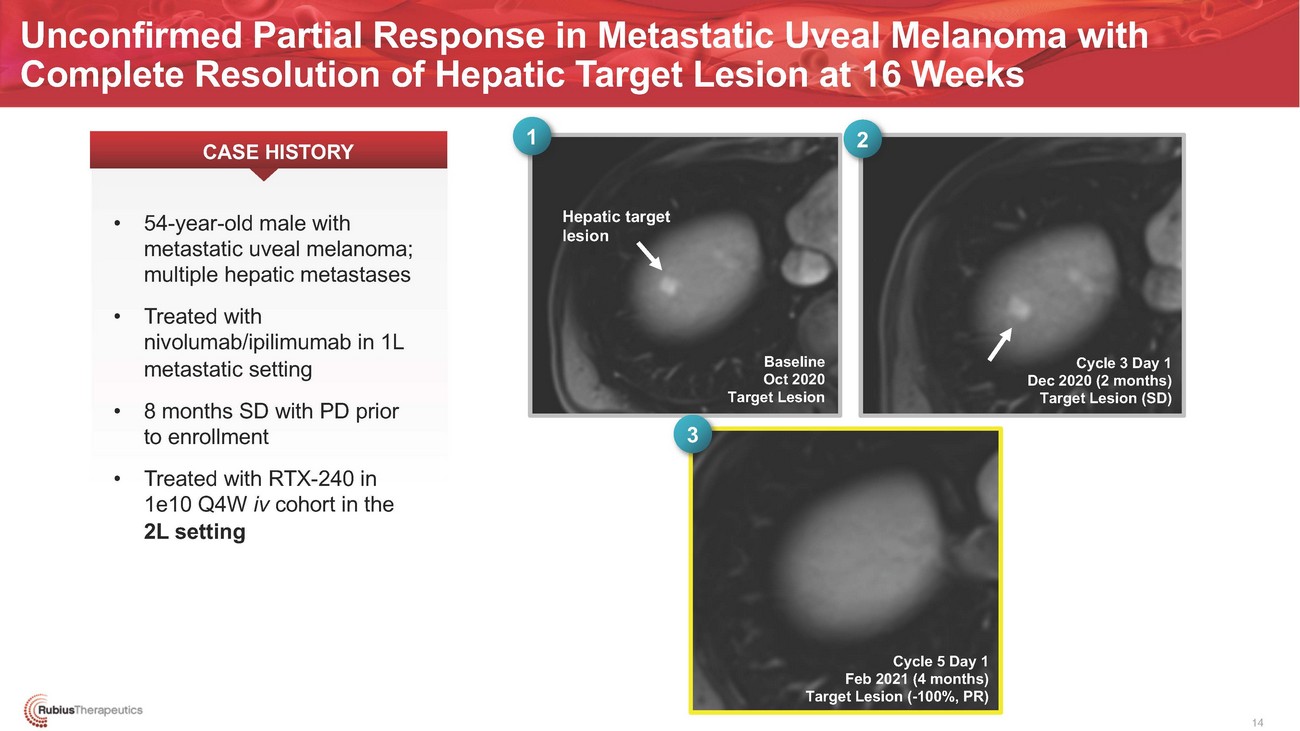
Unconfirmed Partial Response in Metastatic Uveal Melanoma with Complete Resolution of Hepatic Target Lesion at 16 Weeks CASE HISTORY 1 2Hepatic target lesionBaseline Oct 2020 Target Lesion3Cycle 3 Day 1 Dec 2020 (2 months) Target Lesion (SD)1e10 Q4W iv cohort in the 2L settingCycle 5 Day 1 Feb 2021 (4 months) Target Lesion (-100%, PR) 14
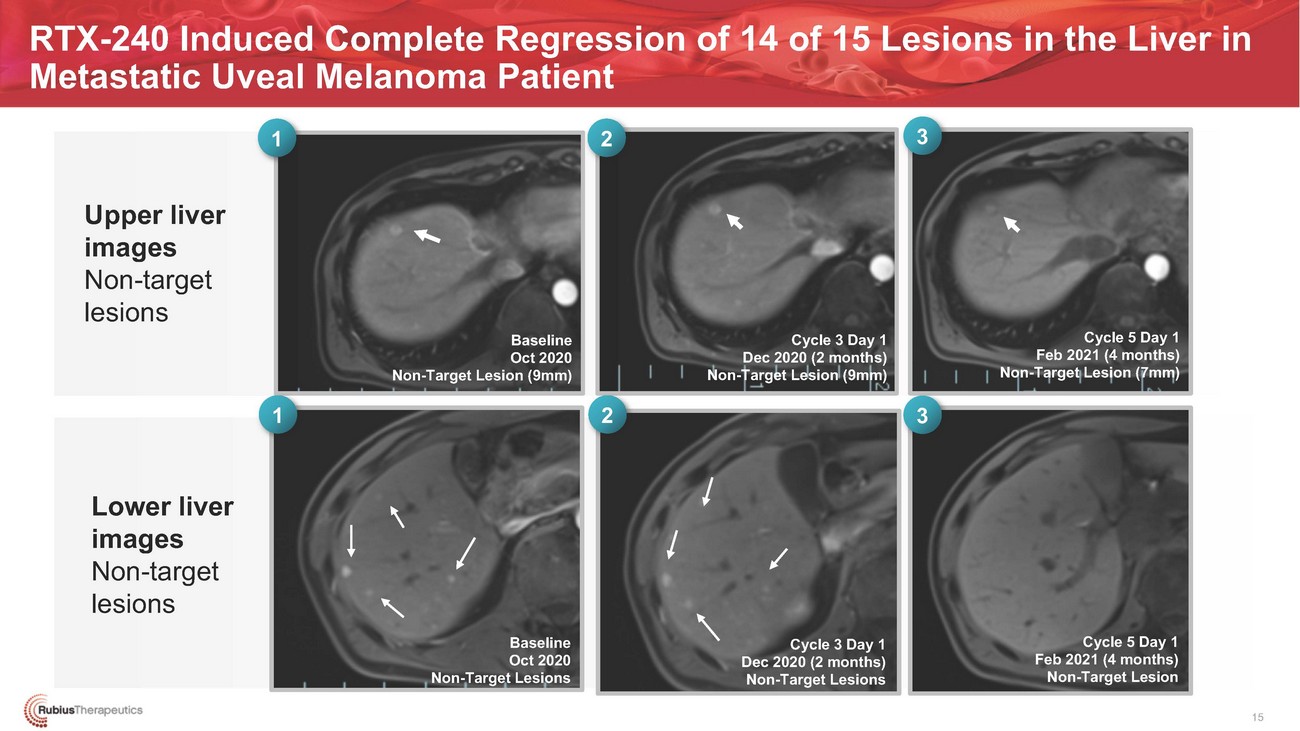
RTX-240 Induced Complete Regression of 14 of 15 Lesions in the Liver in Metastatic Uveal Melanoma Patient1 2 3Baseline Oct 2020 Non-Target Lesion (9mm)1 2Cycle 3 Day 1 Dec 2020 (2 months) Non-Target Lesion (9mm)3Cycle 5 Day 1 Feb 2021 (4 months) Non-Target Lesion (7mm)Lower liver images Non-target lesionsBaseline Oct 2020 Non-Target LesionsCycle 3 Day 1 Dec 2020 (2 months) Non-Target LesionsCycle 5 Day 1 Feb 2021 (4 months) Non-Target Lesion15

......·.·.·.·.·..•.•..• . i·.(·.;(•..R...ubiusTherapeutics •••••••••INITIAL SOLID TUMOR CLINICAL DATA16

Patient Baseline CharacteristicsAge (median, range) Gender (Male/Female) Race/Ethnicity Non-Hispanic/Non-Latino Hispanic/Latino Disease setting1 Colorectal/lower GI cancers, n Melanoma, n Other cancers, n Lines of prior therapy in the metastatic setting, median (range) Prior anti-PD-1/PD-L1 Prior ipilimumab + nivolumab Prior chemotherapy Prior radiationPARAMETER N=16 EVALUABLE 55 (23-80) 8/811 54 5 7 3 (1-10) 11 5 12 71 Lower GI cancers include colorectal cancer (n=2), anal cancer (n=1), pancreatic ductal adenocarcinoma (n=1), gastroesophageal cancer (n=1); melanoma includes cutaneous melanoma (n=2), ocular melanoma (n=2), mucosal melanoma (n=1); and other cancers (all n=1), mesothelioma, ovarian, prostate, soft tissue sarcoma, non-small cell lung, testicular germ cell tumor Safety population n=16 Initial data cut off: February 28, 202117
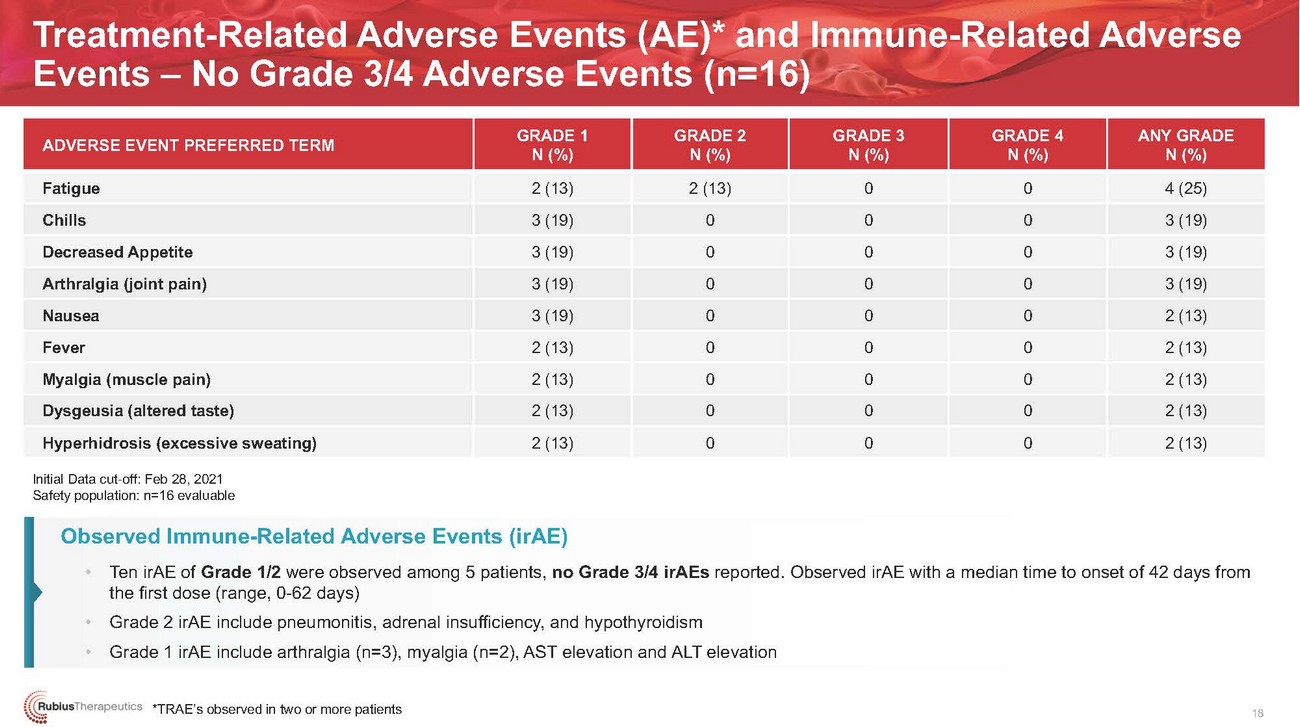
Treatment-Related Adverse Events (AE)* and Immune-Related Adverse Events – No Grade 3/4 Adverse Events (n=16)ADVERSE EVENT PREFERRED TERMFatigue Chills Decreased Appetite Arthralgia (joint pain) Nausea Fever Myalgia (muscle pain) Dysgeusia (altered taste) Hyperhidrosis (excessive sweating)GRADE 1 N (%) 2 (13) 3 (19) 3 (19) 3 (19) 3 (19) 2 (13) 2 (13) 2 (13) 2 (13)GRADE 2 N (%) 2 (13) 0 0 0 0 0 0 0 0GRADE 3 N (%) 0 0 0 0 0 0 0 0 0GRADE 4 N (%) 0 0 0 0 0 0 0 0 0ANY GRADE N (%) 4 (25) 3 (19) 3 (19) 3 (19) 2 (13) 2 (13) 2 (13) 2 (13) 2 (13)Initial Data cut-off: Feb 28, 2021 Safety population: n=16 evaluableObserved Immune-Related Adverse Events (irAE) • Ten irAE of Grade 1/2 were observed among 5 patients, no Grade 3/4 irAEs reported. Observed irAE with a median time to onset of 42 days from the first dose (range, 0-62 days) • Grade 2 irAE include pneumonitis, adrenal insufficiency, and hypothyroidism • Grade 1 irAE include arthralgia (n=3), myalgia (n=2), AST elevation and ALT elevation*TRAE’s observed in two or more patients18

Best Response Observed in Patients with Solid TumorsCLINICAL EFFICACY RESULTS• The best response is recorded from the start of the treatment until disease progression with 14 patients evaluable for best response• Patients are considered evaluable for response with measurable disease at baseline (per RECIST v1.1) and at least one follow-up scan at least 1 dose of RTX-240• Scans are performed at baseline and every 8 weeks* * * * * * * ** * *cPR (ongoing)+20% (PD)-30% (PR)Objective response evaluable population: n=14 Initial data cut-off: Feb 28, 2021uPR (ongoing)*Patients receiving prior PD-1/PD-L1 therapy are indicated with an asterisk(*)
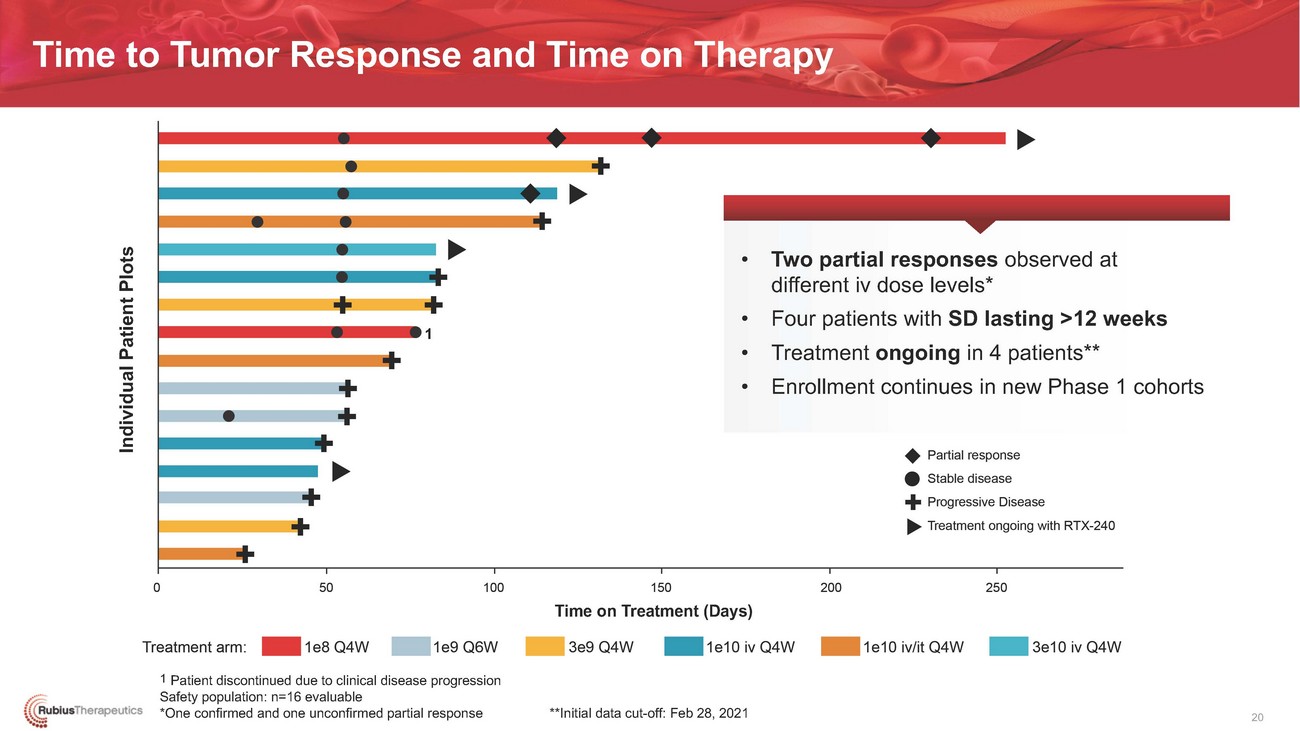
Time to Tumor Response and Time on Therapy• Two partial responses observed at different iv dose levels* • Four patients with SD lasting >12 weeks 1 • Treatment ongoing in 4 patients** • Enrollment continues in new Phase 1 cohortsTime on Treatment (Days)1 Patient discontinued due to clinical disease progression Safety population: n=16 evaluable *One confirmed and one unconfirmed partial response **Initial data cut-off: Feb 28, 2021 20

....•.·•·•·•·······. ·•·· i{(Rub ius The rap eutics •••••••••••••••INITIAL PHARMACODYNAMIC DATA21
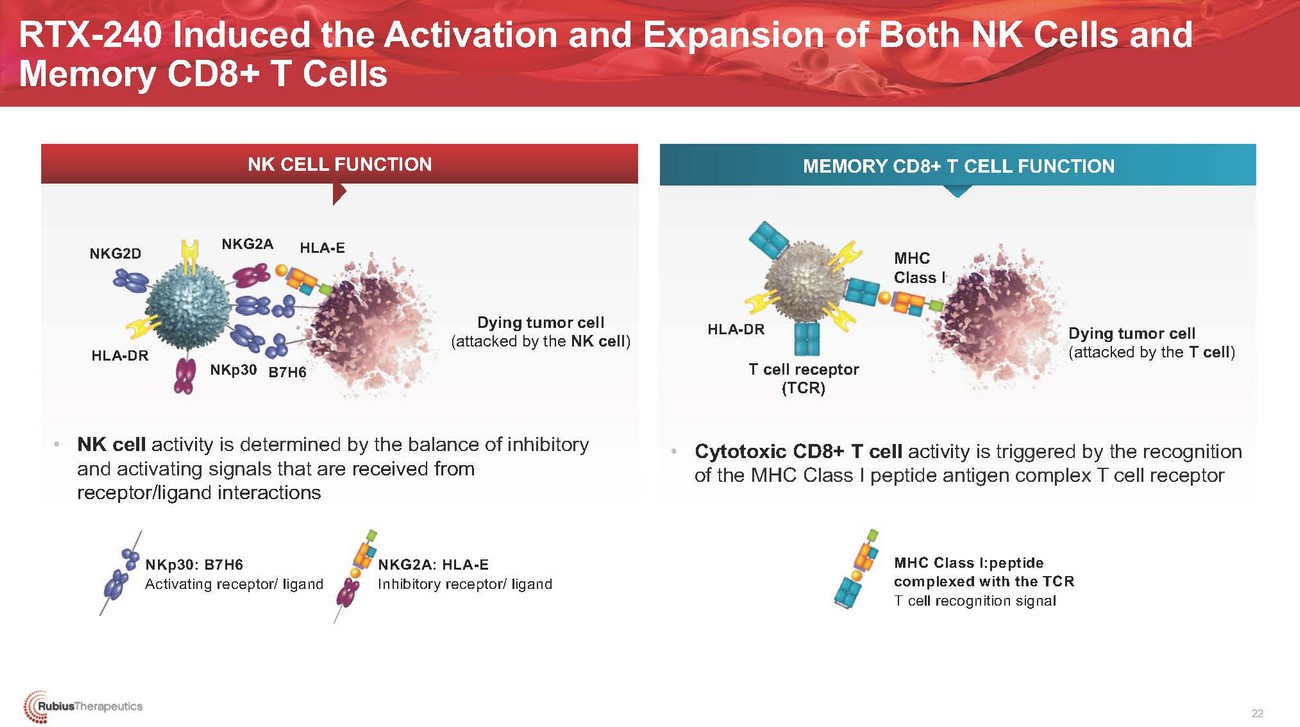
RTX-240 Induced the Activation and Expansion of Both NK Cells and Memory CD8+ T CellsNK CELL FUNCTIONMEMORY CD8+ T CELL FUNCTIONNKG2DHLA-DRNKG2AHLA-EDying tumor cell (attacked by the NK cell)HLA-DRMHC Class IDying tumor cell (attacked by the T cell)NKp30 B7H6T cell receptor (TCR)• NK cell activity is determined by the balance of inhibitory and activating signals that are received from receptor/ligand interactions• Cytotoxic CD8+ T cell activity is triggered by the recognition of the MHC Class I peptide antigen complex T cell receptorNKp30: B7H6 Activating receptor/ ligandNKG2A: HLA-E Inhibitory receptor/ ligandMHC Class I:peptide complexed with the TCR T cell recognition signal22
RTX-240 Stimulated Adaptive and Innate Immunity, Supporting Proof of Mechanism• Demonstrated activation and/or expansion of NK or memory CD8+ T cells or both in all patients with solid tumors (n=16) ▪ Resulted in partial responses in 2 patients• Induced two key NK cell subsets associated with cytotoxicity and cytokine production ▪ CD16+CD56dim (mature NK, killer phenotype) and CD56bright (immature NK, cytokine secretion) ▪ Increased percentage of both NK cell subsets• Induced a specific signature of NK cell receptor expression, including ▪ Expansion of NKp30+ NK cells (activation) and increased ratio of the receptors NKG2D (activation) / NKG2A (inhibitory) ▪ Signature may allow selection of specific tumor types with predicted sensitivity to RTX-24023

RTX-240 Induced Activation and Expansion of NK and T Cell Populations (n=16)NK CELL ACTIVATION AND EXPANSION MEMORY CD8 T CELL ACTIVATION AND EXPANSIONLow dose = 1e8-3e9 cells/dose (n=8); High dose = 1e10-3e10 cells/dose (n=8)RTX-240 activated and expanded NK and T cells across RTX-240 dose levelsInitial data cut-off: Feb 28, 2021 24
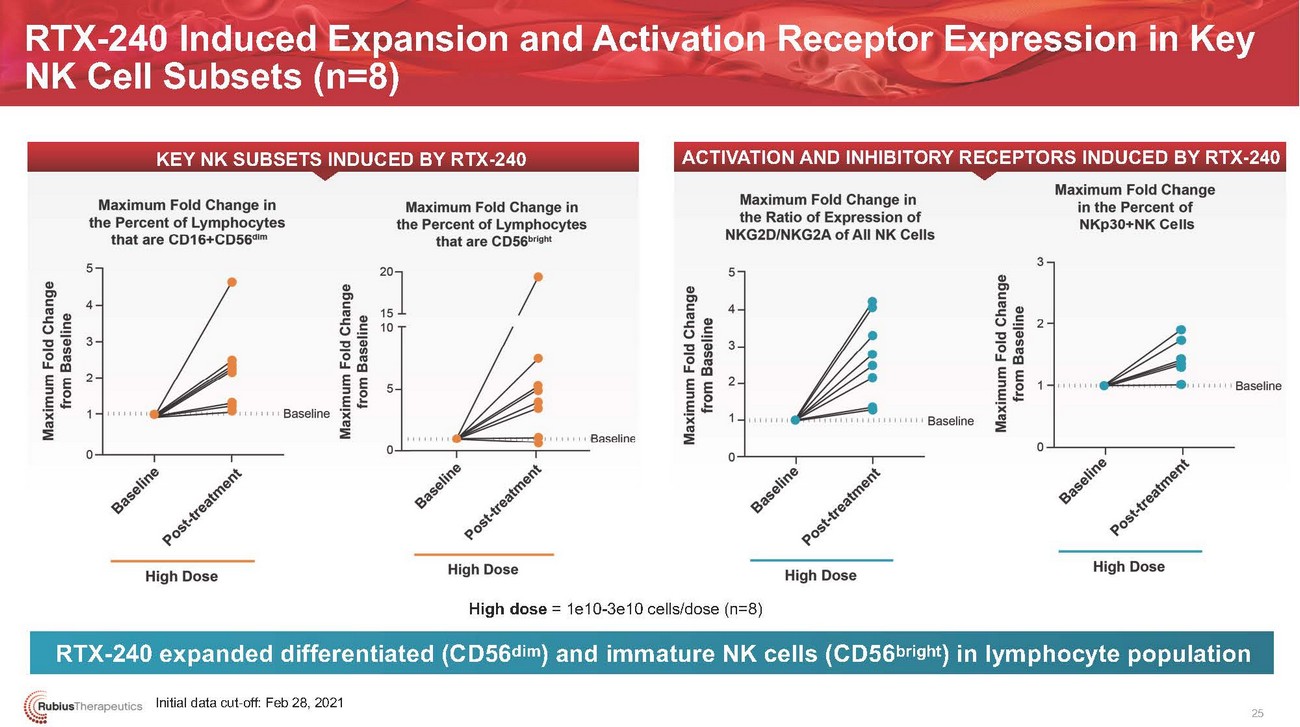
RTX-240 Induced Expansion and Activation Receptor Expression in Key NK Cell Subsets (n=8)KEY NK SUBSETS INDUCED BY RTX-240ACTIVATION AND INHIBITORY RECEPTORS INDUCED BY RTX-240High dose = 1e10-3e10 cells/dose (n=8)RTX-240 expanded differentiated (CD56dim) and immature NK cells (CD56bright) in lymphocyte populationInitial data cut-off: Feb 28, 2021 25

TUMOR INFILTRATION DATA: SOLID TUMORS AND AML26

Tumor Trafficking from Periphery into the Tumor Microenvironment• Trafficking of both T and NK cells was observed in 3/5 patients (1.6 to 10-fold increases) ▪ Shown in patients with metastatic mesothelioma (n=1), metastatic soft tissue sarcoma (n=1) and refractory AML (n=1) ▪ Tumor infiltration was not observed in patients with ovarian cancer (n=1) and heavily pre-treated melanoma (n=1)• Increased expression of PD-L1 and/or increased ratio of M1/M2 macrophages observed in 3/4 patients with solid tumors ▪ Suggests improved immune-permissive TME, which may enhance innate and adaptive tumor-associated immune cell responses• Trafficking of activated NK and T cells into the bone marrow was associated with increases in the cellularity of the marrow in the AML patient ▪ Increased activated effector cells (granzyme B+ NK and T cells) were observed at the first two doses of RTX-24027
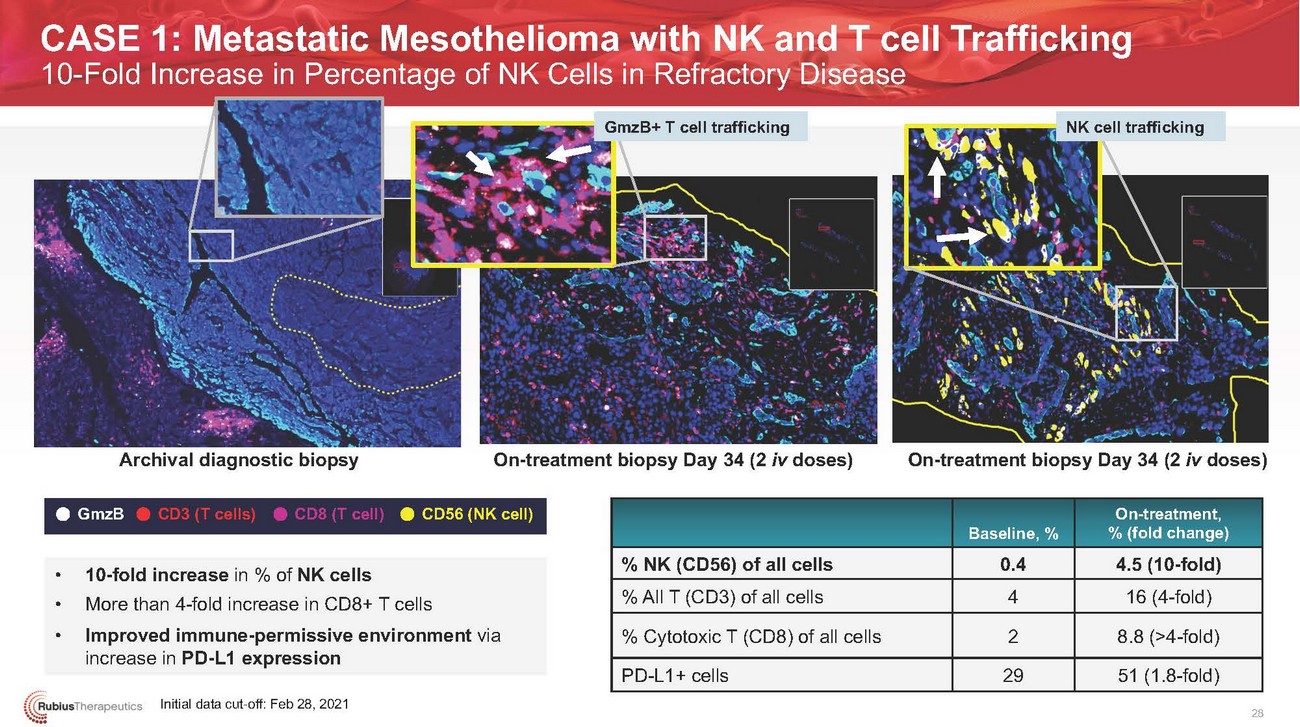
CASE 1: Metastatic Mesothelioma with NK and T cell Trafficking 10-Fold Increase in Percentage of NK Cells in Refractory DiseaseGmzB+ T cell trafficking NK cell traffickingArchival diagnostic biopsy On-treatment biopsy Day 34 (2 iv doses) On-treatment biopsy Day 34 (2 iv doses)GmzBCD3 (T cells) CD8 (T cell)CD56 (NK cell)Baseline, %On-treatment, % (fold change)• 10-fold increase in % of NK cells • More than 4-fold increase in CD8+ T cells • Improved immune-permissive environment via increase in PD-L1 expressionInitial data cut-off: Feb 28, 2021% NK (CD56) of all cells % All T (CD3) of all cells % Cytotoxic T (CD8) of all cells PD-L1+ cells0.4 4 2 294.5 (10-fold) 16 (4-fold) 8.8 (>4-fold) 51 (1.8-fold) 28
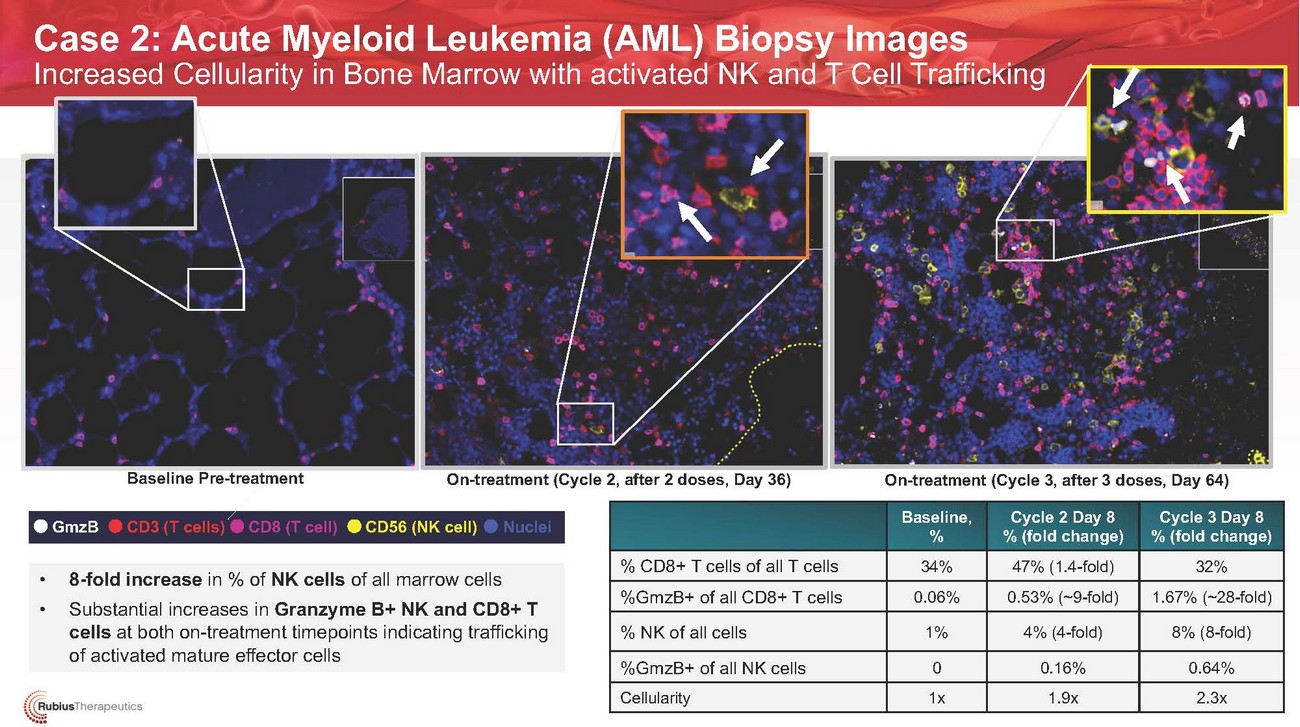
Case 2: Acute Myeloid Leukemia (AML) Biopsy Images Increased Cellularity in Bone Marrow with activated NK and T Cell TraffickingBaseline Pre-treatmentOn-treatment Cycle 2 Day 8 (2 doses, Day 36) On-treatment (Cycle 2, after 2 doses, Day 36)On-treatment (Cycle 3, after 3 doses, Day 64)GmzBCD3 (T cells) CD8 (T cell)CD56 (NK cell) NucleiBaseline, %Cycle 2 Day 8 % (fold change)Cycle 3 Day 8 % (fold change)• 8-fold increase in % of NK cells of all marrow cells • Substantial increases in Granzyme B+ NK and CD8+ T cells at both on-treatment timepoints indicating trafficking of activated mature effector cells% CD8+ T cells of all T cells %GmzB+ of all CD8+ T cells % NK of all cells %GmzB+ of all NK cells Cellularity34% 0.06%1%0 1x47% (1.4-fold) 0.53% (~9-fold)4% (4-fold)0.16% 1.9x32% 1.67% (~28-fold)8% (8-fold)0.64% 2.3x
28
......·.·.·.·.·..•.•..• . i·.(·.;(•..R...ubiusTherapeutics •••••••••RTX-240 CLINICAL DEVELOPMENT PLAN30
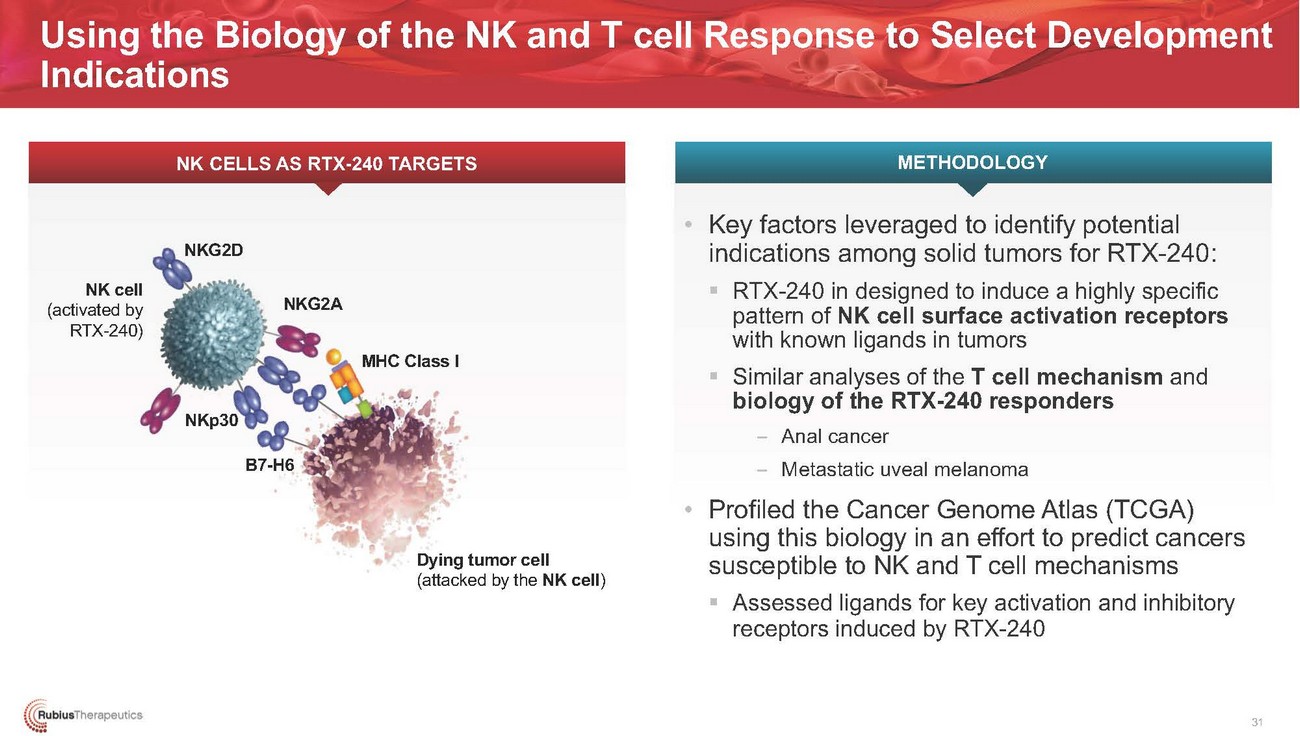
Using the Biology of the NK and T cell Response to Select Development IndicationsNK CELLS AS RTX-240 TARGETS METHODOLOGYNK cell (activated by RTX-240)NKG2DNKp30NKG2AB7-H6MHC Class IDying tumor cell (attacked by the NK cell)• Key factors leveraged to identify potential indications among solid tumors for RTX-240: ▪ RTX-240 in designed to induce a highly specific pattern of NK cell surface activation receptors with known ligands in tumors ▪ Similar analyses of the T cell mechanism and biology of the RTX-240 responders – Anal cancer – Metastatic uveal melanoma • Profiled the Cancer Genome Atlas (TCGA) using this biology in an effort to predict cancers susceptible to NK and T cell mechanisms ▪ Assessed ligands for key activation and inhibitory receptors induced by RTX-24031
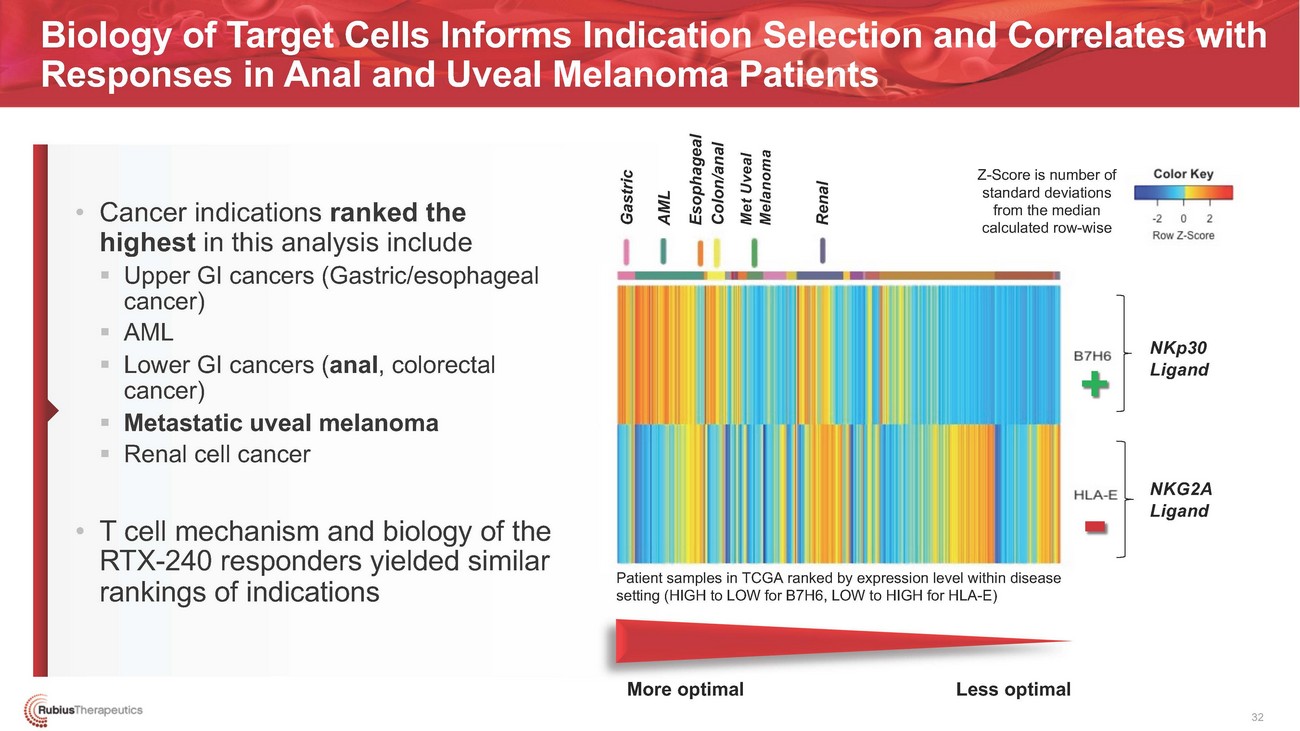
Biology of Target Cells Informs Indication Selection and Correlates with Responses in Anal and Uveal Melanoma Patients• Cancer indications ranked the highest in this analysis include ▪ Upper GI cancers (Gastric/esophageal cancer) ▪ AML ▪ Lower GI cancers (anal, colorectal cancer) ▪ Metastatic uveal melanoma ▪ Renal cell cancer• T cell mechanism and biology of the RTX-240 responders yielded similar rankings of indicationsZ-Score is number of standard deviations from the median calculated row-wise+- Patient samples in TCGA ranked by expression level within disease setting (HIGH to LOW for B7H6, LOW to HIGH for HLA-E)NKp30 LigandNKG2A LigandMore optimal Less optimal 32

Key Takeaways from Initial Data• RTX-240 demonstrated a favorable, emerging safety results across dose levels• Single-agent activity with partial responses per RECIST v1.1 in two patients• Pharmacodynamic effects demonstrated the activation and expansion of the key NK and T cell types• Tumor cell infiltration of activated NK and T cells into the tumor microenvironment in solid tumors and AMLEnrollment Continues and Dose and Schedule Optimization Ongoing33

......·.·.·.·.·..•.•..• . i·.(·.;(•..R...ubiusTherapeutics •••••••••PIPELINE34

Clinical-Stage Company Developing a Broad Wholly Owned PipelinePRODUCT CATEGORYPROGRAMRTX-240PRECLINICALR/R Solid TumorsIND ENABLINGPHASE 1Phase 2 initiation 1Q’22*PHASE 2RTX-240R/R Acute Myeloid LeukemiaInitial clinical results by year-end*CANCERAUTOIMMUNE DISEASESRTX-240RTX-321 aAPC (HPV 16+)RTX-224RTX-aAPCRTX-T1DRTX-240 + PD-1 R/R Solid TumorsR/R HPV-16+ Solid TumorsR/R Solid TumorsCancerType 1 DiabetesPhase 1 trial initiation 2H’21*Initial clinical results by 1Q’22*File IND by year-end*Present additional RTX-240 Phase 1 clinical results for solid tumors and integrated clinical program for RTX-240 by year-end, including expansion cohorts*Anticipated milestones35

......·.·.·.·.·..•.•..• . i·.(·.;(•..R...ubiusTherapeutics •••••••••Q&A AND APPENDIX36

Supporting Rubius Preclinical Publications• RTX-240 ▪ 2020 Society for Immunotherapy of Cancer (SITC) Annual Meeting• RTX-321 ▪ 2020 Federation of Clinical Immunology Societies (FOCIS) Virtual Annual Meeting; ▪ 2020 American Association for Cancer Research (AACR) Tumor Immunology Conference; and ▪ 2020 American Society of Gene & Cell Therapy 23rd Annual Meeting• RTX-224 ▪ 2019 American Association of Cancer Research Annual Meeting37
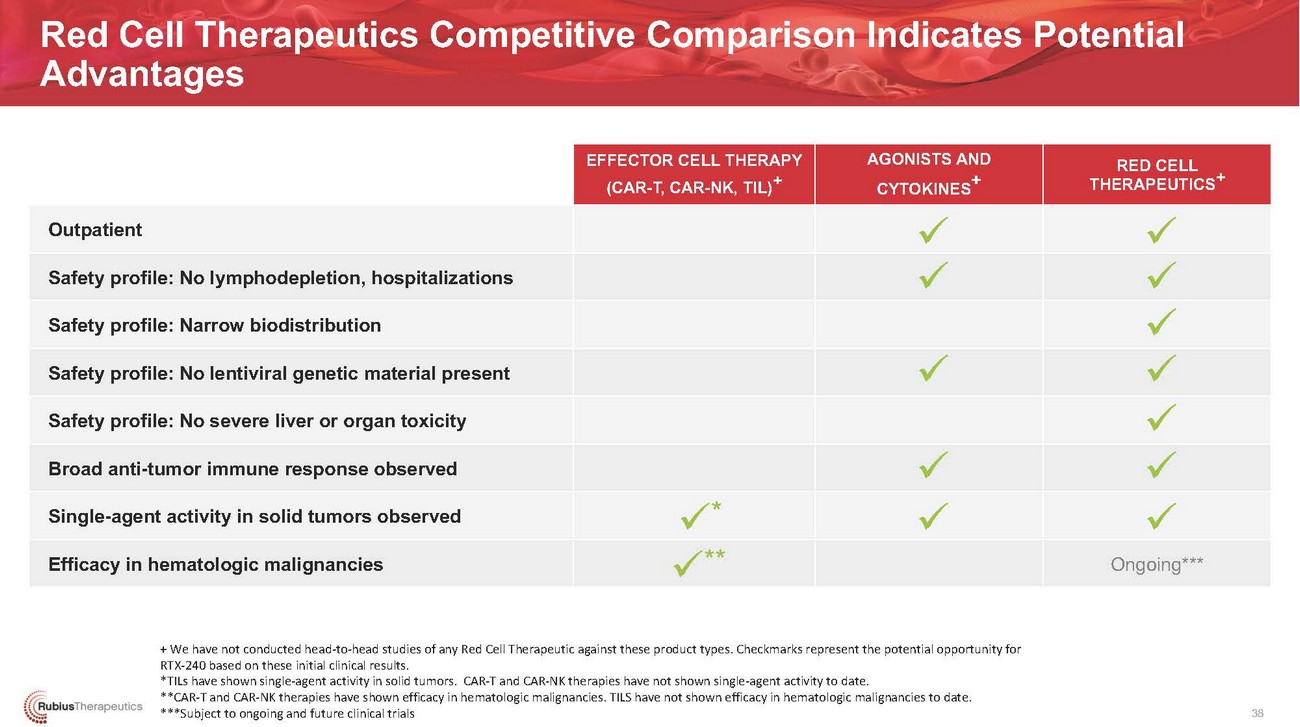
Red Cell Therapeutics Competitive Comparison Indicates Potential AdvantagesOutpatientSafety profile: No lymphodepletion, hospitalizations Safety profile: Narrow biodistribution Safety profile: No lentiviral genetic material present Safety profile: No severe liver or organ toxicity Broad anti-tumor immune response observed Single-agent activity in solid tumors observed Efficacy in hematologic malignanciesEFFECTOR CELL THERAPY (CAR-T, CAR-NK, TIL)+✓* ✓**AGONISTS AND CYTOKINES+ ✓ ✓✓✓ ✓RED CELL THERAPEUTICS✓ ✓ ✓ ✓ ✓ ✓ ✓ Ongoing***+ We have not conducted head-to-head studies of any Red Cell Therapeutic against these product types. Checkmarks represent the potential opportunity for RTX-240 based on these initial clinical results. *TILs have shown single-agent activity in solid tumors. CAR-T and CAR-NK therapies have not shown single-agent activity to date. **CAR-T and CAR-NK therapies have shown efficacy in hematologic malignancies. TILS have not shown efficacy in hematologic malignancies to date. ***Subject to ongoing and future clinical trials38
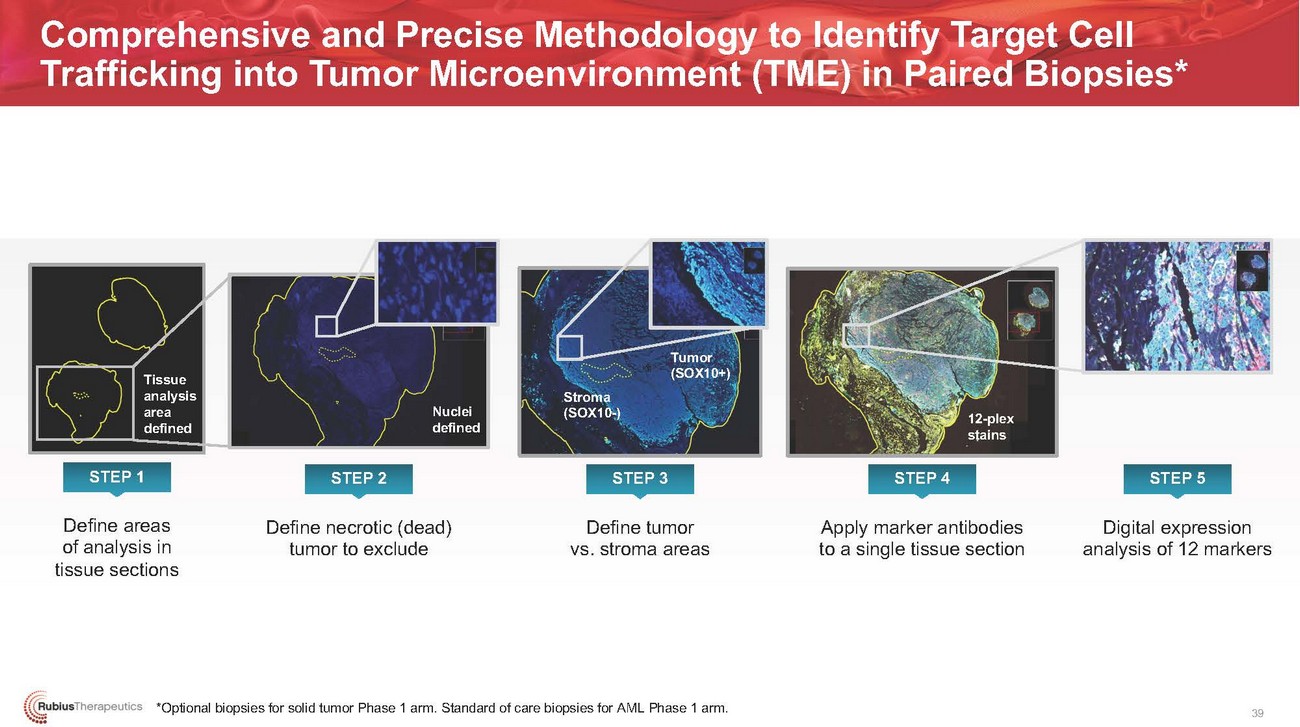
Comprehensive and Precise Methodology to Identify Target Cell Trafficking into Tumor Microenvironment (TME) in Paired Biopsies*Tissue analysis area definedNuclei definedStroma (SOX10-)Tumor (SOX10+)12-plex stainsSTEP 1 STEP 2 STEP 3 STEP 4 STEP 5Define areas of analysis in tissue sectionsDefine necrotic (dead) tumor to excludeDefine tumor vs. stroma areasApply marker antibodies to a single tissue sectionDigital expression analysis of 12 markers*Optional biopsies for solid tumor Phase 1 arm. Standard of care biopsies for AML Phase 1 arm.39
