Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Nemaura Medical Inc. | nmra_8k.htm |
Exhibit 99.1

Corporate Presentation Maxim Group Emerging Growth Virtual Conference Dr Faz Chowdhury, CEO M arch 17 - 18, 2021
| |

Forward - Looking Statements • This presentation includes forward - looking statements that are subject to many risks and uncertainties. These forward - looking statements, such as statements about Nemaura’s short - term and long - term growth strategies, can sometimes be identified by use of terms such as “intend,” “expect,” “plan,” “estimate,” “future,” “strive,” and similar words. These statements involve many risks and uncertainties that may cause actual results to differ from what may be expressed or implied in these statements. • These risks are discussed in Nemaura’s filings with the Securities and Exchange Commission (the “Commission”), including the risks identified under the section captioned “Risk Factors” in Nemaura’s Annual Report on Form 10 - K filed with the Commission o n 29 June 2020, as the same may be updated from time to time. • Nemaura disclaims any obligation to update information contained in these forward - looking statements whether as a result of new information, future events, or otherwise. 2 www.nemauramedical.com
| |

Obesity and Diabetes: TWO of the major drivers of the chronic disease Epidemic The added cost of managing a Type 2 diabetic person is >$6000/year. This can be avoided by preventing or reversing Type 2 Diabetes 3 Pre - Diabetics >80 million Type 2 >25 million
| |

Key Targets 1. Prevent diabetes in >80 million people with pre - diabetes in the USA 1. Reverse Diabetes in >25 million persons in the USA 4
| |

Our Solution A World first daily wear Continuous glucose monitor* Applicable for Pre - diabetes (diabetes prevention), Type 1 (glucose trending) and Type 2 Diabetes (glucose trending and diabetes reversal) Total addressable market exceeding $ 150 Billion 1,2,3 CE approved Class 2b Medical device – adjunct to finger - prick test BEAT diabetes program launched in the USA in December 2020 PMA pending with US FDA *Nemaura’s sensor is non - invasive and can be worn for a few hours each day instead of competing sensors that are worn for between 10 and 14 days consecutively. 1. https ://drug - dev.com/global - type - 2 - diabetes - market - set - to - almost - double - to - 58 - 7 - billion / 2. https://www.prnewswire.com/news - releases/global - digital - diabetes - market - outlook - to - 2026 - a - 16 - billion - industry - opportunity - 300980794. html 3. https://www.absolutemarketsinsights.com/reports/Global - Noninsulin - Therapies - for - Diabetes - Market - 2019 - 2027 - 259 5
| |
Why we are different A World first daily wear Continuous glucose monitor – currently no other sensor technology like it (to best of our knowledge)* Highly competitive pricing allowing broader adoption to address unmet clinical needs Allows us to target the Medical market and consumer market with a device that is fit for both markets Excellent feedback thus far from UK based sugarBEAT® users (via UK licensee) *Nemaura’s sensor is non - invasive and can be worn for a few hours each day instead of competing sensors that are worn for between 10 and 14 days consecutively. 6
| |

Core Technology
| |

| |

| |
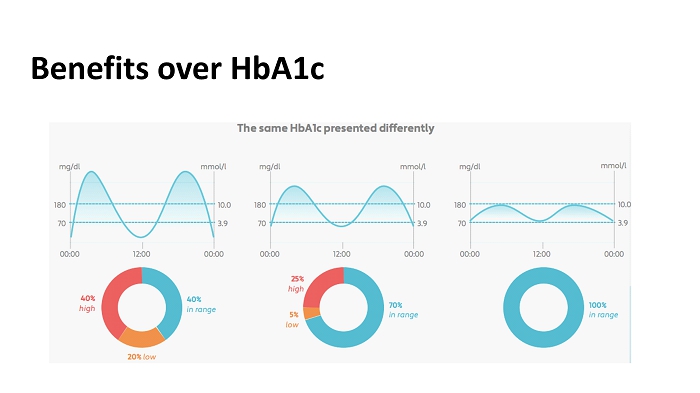
Benefits over HbA1c
| |

11 FULL PROGRAM LAUNCHED DECEMBER 2020 IN USA 3 CORE COMPONENTS 1. Weight loss program originally developed at the Joslin Diabetes Centre – over 12 years of clinical evidence. Sustained long term weight loss achieved without loss of muscle mass 2. proBEAT Ρ glucose profiling – using world’s first daily - wearable glucose sensor, developed in - house 3. Coaching: digital 24/7 using app, and specialist 1 to 1 coaching
| |

Intermittent Glucose Profiling: B enefits 12 7 - point glucose profiles every 4 weeks. Patients received guidance for diet and exercise adjustments based on SMBG. Outcome: Significant reductions in HbA1c, weight, BMI, systolic BP, diastolic BP, and LDL Cholesterol Kempf et. al., Diabetes Technol Ther (2010 )
| |

13 Glucose trend and diet , exercise , and other parameters used to determine individual metabolic health . Combine with education and guidance
| |

14 KEY MILESTONES FOR 2021 1. Adoption of BEATdiabetes program by several large corporations/employers and Insurers in the USA (initially) 2. Launch of consumer device and program addressing Metabolic health and weight loss through glucose control 3. Launch sugarBEAT real time non - invasive continuous glucose monitor in a number of territories outside the USA, and in the USA on PMA approval. 4. Achieve Reimbursement in Germany (submitted to G - BA)
| |

SUMMARY 1. Total addressable market >$150 Billion 2. World’s first daily wearable sensor with unparalleled economics 3. Targeting prevention and/or reversal of Type 2 diabetes with significant long term savings 4. Glucose profiling a few days a month provides a powerful cost - effective tool to facilitate management of Type 2 diabetes. 5. Combining with digital intervention/guidance/coaching, the product can be easily scaled and deployed widely to cater for the approx. 30 million people with Type 2 in the US. 6. Targeting payers such as Corporations and Insurers 7. Outstanding and growing management team 8. Revenue generating from 2021 with first mover and cost advantage 9. Strong balance sheet: over $33m on balance sheet at end of last reported quarter 10. Low quarterly cash burn, <$1.5m/quarter historically 15
| |
