Attached files
| file | filename |
|---|---|
| EX-99.1 - JOINT PRESS RELEASE OF INTEC PHARMA LTD. AND DECOY BIOSYSTEMS, INC., DATED MARCH - Intec Pharma Ltd. | ea137630ex99-1_intecpharma.htm |
| EX-10.3 - FORM OF LOCK-UP AGREEMENT - Intec Pharma Ltd. | ea137630ex10-3_intecpharma.htm |
| EX-10.2 - FORM OF INTEC SHAREHOLDER SUPPORT AGREEMENT - Intec Pharma Ltd. | ea137630ex10-2_intecpharma.htm |
| EX-10.1 - FORM OF DECOY STOCKHOLDER SUPPORT AGREEMENT - Intec Pharma Ltd. | ea137630ex10-1_intecpharma.htm |
| EX-2.1 - AGREEMENT AND PLAN OF MERGER AND REORGANIZATION, DATED AS OF MARCH 15, 2021, BY - Intec Pharma Ltd. | ea137630ex2-1_intecpharma.htm |
| 8-K - CURRENT REPORT - Intec Pharma Ltd. | ea137630-8k_intecpharma.htm |
Exhibit 99.2

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Project Dillion Investor Presentation

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Forward Looking Statements This presentation contains forward - looking statements with the meaning of the Private Securities Litigation Reform Act. These in clude statements regarding management’s expectations, beliefs and intentions regarding, among other things, our product development efforts, business, financial condition, results of operation s, strategies, plans and prospects. Forward - looking statements can be identified by the use of forward - looking words such as “believe”, “expect”, “intend”, “plan“, “may“, “should“, “could“, “might“, “seek“, “target“, “will”, “project“, “forecast“, “continue” or “anticipate” or their negatives or variations of these words or other comparable words or by the fact that these statements d o n ot relate strictly to historical matters. For example, forward - looking statements are used in this presentation when we discuss Decoy’s future plans and expected timeline of its development pipeli ne. Forward - looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made. Because forward - looking statements relate to matters that have not yet occurred, these statements are inherently subject to risks and uncertainties that could cause our actual results to diffe r m aterially from any future results expressed or implied by the forward - looking statements. In addition, historical results or conclusions from scientific research and clinical studies do not guara nte e that future results would suggest similar conclusions or that historical results referred to herein would be interpreted similarly in light of additional research or otherwise. Many factors could ca use actual activities or results to differ materially from the activities and results anticipated in forward - looking statements, including, but not limited to, the following: risks associated with Intec’s a nd Decoy’s ability to obtain the shareholder approval required to consummate the proposed merger and the timing of the closing of the proposed merger, including the risks that a condition to clo sing would not be satisfied within the expected timeframe or at all or that the closing of the proposed Merger will not occur; risks related to the ability to consummate certain closing conditi ons including the pre - closing financing and the disposition of the Accordion Pill business, the occurrence of any event, change, or other circumstances that could give rise to the termination of the mer ger agreement; the outcome of any legal proceedings that may be instituted against the Company or Decoy following the announcement of the merger agreement and the transactions contemplated the rein; unanticipated difficulties or expenditures relating to the proposed merger, the response of business partners and competitors to the announcement of the proposed merger, and/or potenti al difficulties in employee retention as a result of the announcement and pendency of the proposed merger; the occurrence of any event, change, or other circumstance that could give ris e to the termination of the merger agreement or could otherwise cause the transaction to fail to close; the inability to list the merger shares on Nasdaq or maintain the listing o f I ntec Parent’s shares of common stock on Nasdaq following the proposed merger; the ability to recognize the anticipated benefits of the proposed merger. Risks and uncertainties relating to Decoy t hat may cause actual results to differ materially from those expressed or implied in any forward - looking statement include, but not limited to: Decoy’s plans to develop and potentially commercialize its technology, the timing and cost of Decoy’s planned investigational new drug application and any clinical trials, the completion and receiving favorable results in any clinical trials, Decoy’s abi lity to obtain and maintain regulatory approval of any product candidate, Decoy’s ability to protect and maintain its intellectual property and licensing arrangements, Decoy’s ability to develop, man ufa cture and commercialize its product candidates, the risk of product liability claims, the availability of reimbursement, the influence of extensive and costly government regulation, and Decoy’s es timates regarding future revenue, expenses capital requirements and the need for additional financing following the merger. These risks, as well as other risks and uncertainties associated with th e merger, will be discussed in the proxy statement/prospectus that will be included in the registration statement on Form S - 4 to be filed with the SEC in connection with the merger. Additional risks a nd uncertainties are identified and discussed under the heading “Risk Factors” in Intec’s Annual Report and other period filings with the SEC. All forward - looking statements speak only as of the date of this presentation and are expressly qualified in their entirety by th e cautionary statements included in this presentation. Neither Intec nor Decoy undertake any obligation to update or revise forward - looking statements to reflect events or circumstances that arise after the date made or to reflect the occurrence of unanticipated events, except as required by applicable law. The presentation contains information about investigation - stage drug products under development, which have not yet been approve d by the FDA for commercial distribution in the United States. All representations in this presentation are based upon investigations in certain clinical and other research, but which acco rdi ngly should not be construed as general claims for the safety or efficacy of the products when used by patients. 2

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Intec Pharma / Decoy Biosystems 2021 Intec Pharma announces merger with Decoy Biosystems 3 • Entry into exciting area of immuno - oncology • Continues pursuit of advancing new technologies • Potential value creating opportunity for shareholders • Leverages senior level expertise Transition into new business expected to be completed in Q3 in conjunction with review of strategic options for the Accordion Pill platform
TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Decoy Investment Thesis • Immunology - based anti - tumor and anti - viral platform with IND filing anticipated 2H ’21 • Pre - clinical data demonstrate Decoy safely primes innate and adaptive immune pathways • Technology active against multiple solid tumor and lymphoma models – As single agent and combo with checkpoint therapy, targeted antibodies or chemotherapy – Sustained durable responses, immunological memory and wide therapeutic index • Platform with activity against chronic hepatitis B virus (HBV) & HIV in animal models • Large opportunities in unmet medical needs in multiple therapeutic areas • Broad, issued patent portfolio 4

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Immune System Well - Equipped to Prevent and Eliminate Tumors Checkpoint inhibitors (CPI) have revolutionized the treatment of oncology (immuno - oncology IO) Successes of Existing CPI • Broad & deep responses in multiple tumor settings with cures of advanced cancer in a small number of indications • Approved for almost ½ of cancer patients (1) • Combined revenue of $23B with 40% growth last year and will become a best - selling drug class (2) Limitations of Existing CPI • Most CPI patients succumb to their disease as tumors develop the ability to evade • The most sensitive indications have already been identified • Targeting of only the T - cell axis is insufficient to cure most patients 5 Adaptive Immune System Innate Immune System Dendritic Cell Tumor Antigen Gamma - Delta T - Cell Macrophage Neutrophil Natural Killer Cell Natural Killer T - Cell CD8+ T - Cell CD4+ T - Cell Checkpoint Inhibitors PD - (L)1, CTLA - 4 Cancer Cells While meaningful, CPI only solve part of the equation (1). JAMA doi:10.1001/jamanetworkopen.2019.2535 (2). PharmaLive 2019 Top 200 Prescription Drugs by Global Sales

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Decoy Technology Activates Innate and Adaptive Pathways • Significant advances will only be possible if we can safely activate both innate & adaptive anti - tumor immune pathways • Decoy therapy safely primes both pathways after short exposure • Potential for single agent activity and synergistic combination with other IO approaches, including CPI, targeted antibodies and chemotherapy 6 Adaptive Immune System Innate Immune System Dendritic Cell Tumor Antigen Gamma - Delta T - Cell Macrophage Neutrophil Natural Killer Cell Natural Killer T - Cell CD8+ T - Cell CD4+ T - Cell Cancer Cells Decoy20

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Harnessing Immune Activators in Bacteria Bacteria contain multiple immune system stimulating “danger signals” – Toll - Like Receptors (TLR) agonists – NOD - Like Receptors (NLR) agonists – Stimulator of Interferon Genes (STING) Bacterial danger signals are potent immune system primers/activators – Key participants in activation of essentially all innate and adaptative immune cell types – Enhance tumor antigen processing/presentation – Also participate in killing of tumor cells via induction of anti - tumor cytokines Coley’s Toxins (1894): killed bacteria – Invented by Dr. William Coley at what is now Memorial Sloan Kettering and cured hundreds of advanced cancer patients – Local or intra - tumoral administration was used to minimize toxicity, but was less effective than i.v. administration – Lack of understanding of mechanisms of immune activation and toxicity hindered optimization – Treatment paradigm changed in the 1950’s with advent of radiation and chemotherapy 7

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Decoy’s Innovation Innate and adaptive immunology mechanisms established since the 1990’s suggested a new path – The most potent and broadly acting bacterial danger signal is lipopolysaccharide (LPS) or endotoxin – LPS is the major (75%) component of the outer membrane of Gram - negative bacteria – LPS is important for efficacy, but can also produce significant toxicity when delivered systemically Decoy’s hypothesis – Significantly reducing LPS will lower toxicity, while leaving a small amount will be enough to synergize with other immune activating danger signals – This might make i.v. administration of killed, Gram - negative bacteria both safe and effective inducers of anti - tumor and anti - viral immune responses 8

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Decoy Technology Briefly tricking the body into thinking an infection is present • Start with a single, pure strain of non - pathogenic, Gram - negative bacteria • Reduce LPS - endotoxin level by ~90% • Kill the bacteria and stabilize so they remain intact after i.v. administration • Obtain broad, issued patent coverage Potential for a safe and efficacious i.v. delivered therapy for multiple indications. 9 • Pre - clinical data across multiple tumor and anti - viral models supports success and differentiation from competitor approaches • 100 to 2,500 - fold less toxic in mice (LD 50 ) than live, attenuated bacterial products

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Decoy Pre - Clinical Assessments Decoy bacteria in vivo – Significant anti - tumor and anti - viral activity as a single agent – Synergistic induction of innate and adaptive immune pathways in tumors (cold to hot) – Anti - tumor synergy with anti - PD - 1 CPI, targeted antibodies or chemo, without enhanced toxicity – Durable, complete regressions or remissions • Both established and metastatic tumors, including colorectal, hepatocellular (liver), pancreatic and non - Hodgkin’s lymphoma – Immunological memory leading to rejection of tumor re - challenge – Efficacy in both mouse and human tumor models – Broad therapeutic (safety) index for induction of tumor regression – Successful pre - clinical toxicology studies indicate potential for safe dosing in humans 10 In vitro and in vivo data demonstrate Decoy safely primes innate and adaptive immune pathways leading to broad and deep anti - tumor and anti - viral responses
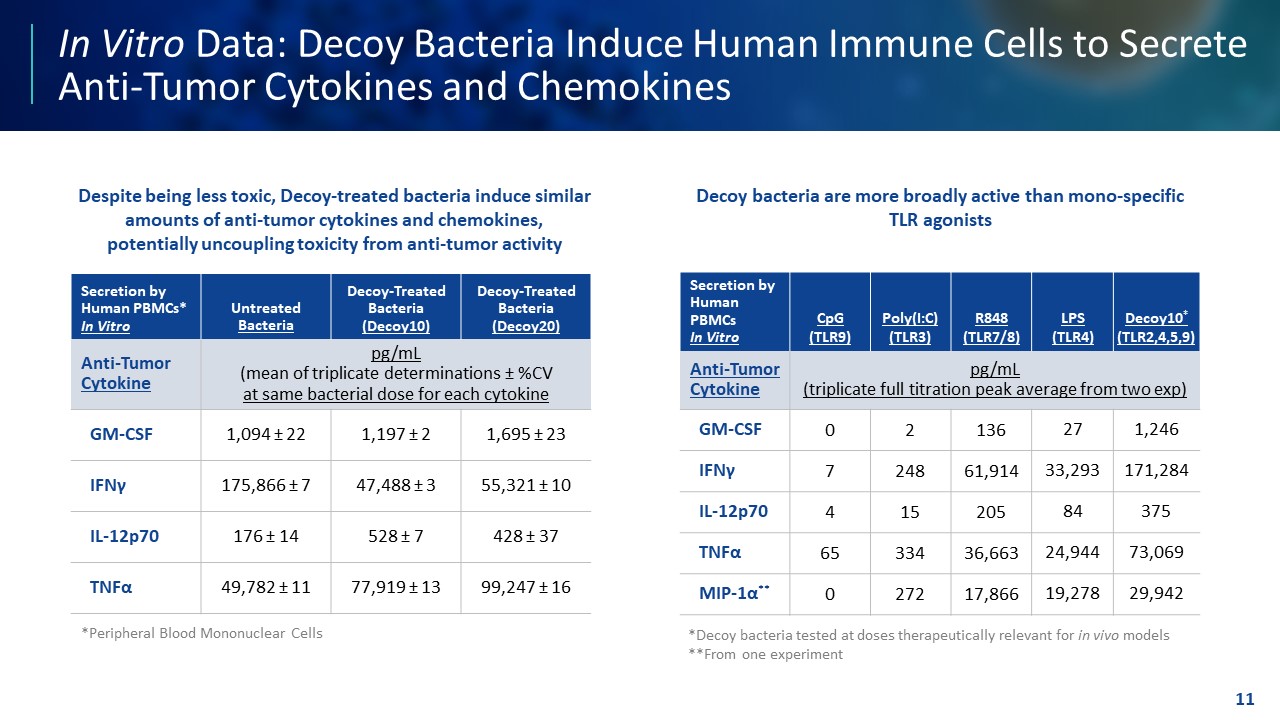
TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. In Vitro Data: Decoy Bacteria Induce Human Immune Cells to Secrete Anti - Tumor Cytokines and Chemokines 11 *Decoy bacteria tested at doses therapeutically relevant for in vivo models **From one experiment Secretion by Human PBMCs* In Vitro Untreated Bacteria Decoy - Treated Bacteria (Decoy10) Decoy - Treated Bacteria (Decoy20) Anti - Tumor Cytokine pg/mL (mean of triplicate determinations ± %CV at same bacterial dose for each cytokine GM - CSF 1,094 ± 22 1,197 ± 2 1,695 ± 23 IFN γ 175,866 ± 7 47,488 ± 3 55,321 ± 10 IL - 12p70 176 ± 14 528 ± 7 428 ± 37 TNF α 49,782 ± 11 77,919 ± 13 99,247 ± 16 Secretion by Human PBMCs In Vitro CpG (TLR9) Poly(I:C) (TLR3) R848 (TLR7/8) LPS (TLR4) Decoy10 * (TLR2,4,5,9) Anti - Tumor Cytokine pg/mL (triplicate full titration peak average from two exp) GM - CSF 0 2 136 27 1,246 IFN γ 7 248 61,914 33,293 171,284 IL - 12p70 4 15 205 84 375 TNF α 65 334 36,663 24,944 73,069 MIP - 1 α ** 0 272 17,866 19,278 29,942 Despite being less toxic, Decoy - treated bacteria induce similar amounts of anti - tumor cytokines and chemokines, potentially uncoupling toxicity from anti - tumor activity Decoy bacteria are more broadly active than mono - specific TLR agonists *Peripheral Blood Mononuclear Cells

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Single Agent Decoy Extends Survival of Mice with Metastatic Mouse Pancreatic Carcinoma 12 0 10 20 30 40 50 60 70 80 90 0 20 40 60 80 100 Days Post Tumor Cell Inoculation (Treatment Started on Day 5 With 7 Mice/Group) P e r c e n t S u r v i v a l No Treatment Gemcitabine 50 mg/kg Decoy20 5x10^7 Decoy20 2x10^8 Log - Rank Test Decoy20 vs. No Treatment Both Doses P ≤ 0.0008 Pancreatic tumor cells were injected into the spleen on Day 0 All untreated mice develop large tumors in spleen, pancreas and liver Decoy20 2x10^8 Decoy20 5x10^7 Gemcitabine 2x/week x 7 No Treatment Decoy i.v. 2x per week x 3 Decoy 2x10^8 Decoy 5x10^7 No Treatment Decoy 5x10^7 Gem Decoy 2x10^8 Median Survival 27 Days 35 Days P<0.01 41 Days P<0.01 75 Days P<0.01 Percent Survival Days Post Tumor Cell Inoculation (Treatment started on Day 5 with 7 mice/group)

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Single Agent Decoy Inhibits Metastasis and Extends Survival of Mice with Orthotopic Mouse Colorectal Carcinoma 13 0 7 14 21 28 35 42 49 56 63 70 77 84 91 98 105112119 0 10 20 30 40 50 60 70 80 90 100 Days After Tumor Fragment Implant (Treatment Started on Day 5 With 7 Mice/Group) P e r c e n t S u r v i v a l Decoy10 Vehicle Decoy10 2x10^8 (2x per week x 4) Tumor fragments were sewn onto the cecum wall on Day 0 (7 mice/group) Log - rank P = 0.0004 Dose and regimen not optimized Also, combination - based eradications with s.c. tumors Metastasis • 11 total in 5 sacrificed mice • 0 in mouse sacrificed on Day 118 Decoy 2x10^8 (i.v. 2x per week x 3) Metastasis • >41 total in 6 sacrificed mice Decoy Vehicle

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Decoy Synergizes with Anti - PD - 1 Checkpoint Therapy to Safely Regress Established Mouse Hepatocellular Carcinoma (HCC) 14 Decoy + Anti - PD - 1 6/6 CR (5/6 durable at Day 91) (6 mice per group) Anti - PD - 1 1/6 CR Decoy 2/6 CR 0 500 1,000 1,500 2,000 2,500 3,000 3,500 7 14 21 28 35 42 49 56 63 70 Mean Tumor Volume (mm 3 “ SEM) Days After Tumor Cell Implant Indomethacin Indomethacin + 2x10^8 Decoy10 1x/week x 6 Indomethacin + 10 mg/kg anti-PD-1 2x/week x 2 Indomethacin + Decoy10 + Anti-PD-1 Control Decoy Anti - PD - 1 10 mg/kg i.p. 2x per week x 2 Decoy + anti - PD - 1 Control Start treatment with ~200 mm 3 subcutaneous (s.c.) tumors Six mice per group 9.8 6.1 3.7 2.2 5.2 0.8 2x10^8 i.v. 1x per week x 6 * Twice per week Decoy produced 3 - 4/6 full regressions Days After Tumor Cell Implant Mean Tumor Volume (mm 3 ± SEM) * Max % transient weight loss each week for combo treatment No increase in tox with combos All animals also received a non - steroidal anti - inflammatory drug (NSAID)

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Synergistic Full Regression of Murine HCC by Decoy + Anti - PD - 1 Exhibits a Very Wide Therapeutic Index (≥33 - fold) All Decoy - treated groups received the same standard regimen of mouse anti - PD - 1 15 0 200 400 600 800 1,000 7 14 21 28 35 42 49 56 63 70 77 84 91 Tumor Volume (mm 3 ) Days After Tumor Cell Implant 3x10 7 Decoy 0 200 400 600 800 1,000 7 14 21 28 35 42 49 56 63 70 77 84 91 Tumor Volume (mm 3 ) Days After Tumor Cell Implant 1x10 8 Decoy 0 200 400 600 800 1,000 7 14 21 28 35 42 49 56 63 70 77 84 91 Tumor Volume (mm 3 ) Days After Tumor Cell Implant 3x10 8 Decoy 0 200 400 600 800 1,000 7 14 21 28 35 42 49 56 63 70 77 84 91 Tumor Volume (mm 3 ) Days After Tumor Cell Implant 1x10 9 Decoy 0 1,000 2,000 3,000 4,000 7 14 21 28 35 42 49 56 63 70 77 84 91 Tumor Volume (mm 3 ) Days After Tumor Cell Implant No Treatment (6 mice per group) * - 0.15% * - 4.10% * - 4.40% * - 8.12% *Maximum transient body weight loss relative to start of treatment All tumors were still at 0 volume at 143 days 5 tumors were still at 0 volume at 143 days All Decoy - treated groups also received the same standard regimen of mouse anti - PD - 1 Haven’t reached toxic dose: No deaths and no requirement to stop dosing due to weight loss Start treatment at ~200 mm 3 All animals also received a non - steroidal anti - inflammatory drug (NSAID)

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Mice Cured by Decoy + Anti - PD - 1 and Re - Challenged with Fresh HCC Tumor Cells Reject the Tumors (Immunological Memory) Eleven Cured Mice were Re - Challenged with Fresh HCC Tumor Cells on Day 91 on the Opposite Flank from the First Challenge Six Naïve Mice were Challenged with the Same Tumor Cells as the Cured Mice on the Same Day 16 0 100 200 300 400 91 98 105 112 119 126 133 140 147 Tumor Volume (mm 3 ) Days After 1 st Tumor Cell Implant * *All 1 st challenge tumor sites remained tumor - free Cured Mice* 0 1,000 2,000 3,000 4,000 0 7 14 21 28 35 42 49 56 Tumor Volume (mm 3 ) Days After Tumor Cell Implant Naïve Mice

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Decoy Produces Similar Results in Multiple Mouse Models 17 0 1,000 2,000 3,000 4,000 5,000 13 20 27 34 41 48 55 62 69 76 Decoy Control LDC/no regressions Decoy + LDC 6/6 full regressions 0 500 1,000 1,500 2,000 13 20 27 34 41 48 55 62 69 76 83 90 97 104 111 118 125 0 1,000 2,000 3,000 4,000 5,000 6,000 7,000 0 7 14 21 28 35 42 49 Tumor Volume (mm 3 ) Days After 2 nd Tumor Cell Implant 0 1,000 2,000 3,000 4,000 5,000 6,000 7,000 0 7 14 21 28 35 42 49 Tumor Volume (mm 3 ) Days After Tumor Cell Implant Small 33 - 145 mm 3 tumors appeared on 6/8 mice between days 7 to 18 All tumors rejected Experiment done twice Days After Tumor Cell Implant Days After 1 st Tumor Cell Implant Mean Tumor Volume (mm3m ± ) Tumor Volume (mm 3 ) Full regressions also seen with combination of Decoy + LDC + Rituximab in a human NHL xenograft model Treat with Decoy + LDC for 2 weeks starting day 13 (8/8 mice cured) Rechallenged with tumor cells on opposite flank on day 77 (above left) Tumor Cell Re - Challenged on Opposite Flank on 8 Cured Mice on day 77 Tumor Cell - Match Challenge on 5 Naive Mice on day 77 Decoy Bacteria Synergize with Low - Dose Chemotherapy (LDC) to Safely Induce Full Regression of s.c. Mouse Non - Hodgkin’s - Lymphoma (NHL) Mice Cured by Decoy + LDC and Re - Challenged with Fresh NHL Tumor Cells Reject the Tumors (Immunological Memory)

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Target Indications Include 6 of the World’s 12 Deadliest Cancers 18 Unmet Need - % 5 Year Survival * 20% 30% 40% 50% 60% 70% 80% 90% 10% Competitive Environment Ongoing Phase 3 Studies ** 20 200 100 Patients do worse Patients do better Pancreas 115K incidence *** Hepatocellular 90K incidence *** Sources: *US NCI SEER Explorer Database; **ClinicalTrials.gov database; ***US, EU5, JPN incidence – BiomedTRacker, US SEER database Cervical 50K incidence *** CRC 500K incidence *** Bladder/Urinary Tract 185K incidence *** NHL 150K incidence *** Checkpoint Inhibitor 2019 Revenue $23B ~40% Growth from ‘18 12 Deadliest Cancers World - Wide ( Decoy Targets ) % of Yearly Deaths % of Yearly Cases 1 Lung 18.4 11.6 2 Colorectal 9.0 10.0 3 Stomach 8.2 5.7 4 Liver 8.2 4.7 5 Breast 6.6 11.6 6 Esophagus 5.3 3.2 7 Pancreas 4.5 2.5 8 Prostate 3.8 7.1 9 Cervical 3.3 3.2 10 Leukemia 3.2 2.4 11 N - H Lymphoma 2.6 2.8 12 Bladder 2.1 3.0 Decoy Indications % of Total 29.7% 26.2% Source: CA CANCER J CLIN 2018;68:394 - 424

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Decoy Technology Platform Potential utility as anti - viral therapy Other Infections 19 HBV Oncology Cancer immunotherapy principles (opportunities and challenges) also apply to chronic viral infections Long - standing scientific rationale for use of TLR agonists to treat HBV and HIV; but no commercial success to date Decoy bacteria produce significant single agent activity against chronic HBV and HIV infections in standard pre - clinical models

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. 2021 2022 2023 2024 2025 Q1/2 Q3/4 Q1/2 Q3/4 Q1/2 Q3/4 Q1/2 Q3/4 Q1/2 Q3/4 Dose Escalation Expansion (~2 tumors) Ph1b Combination Checkpoint / Targeted Abs / Chemo Decoy Clinical Development Plan 20 Preparations Underway for IND Filing (expected 2H 2021) • 28 - day Rabbit toxicology study / In - life completed • Decoy20 GMP batch released / Additional characterization underway • ≥ 6 - month stability studies with Decoy20 GMP batch in progress • Preliminary IND preparation underway • Preliminary CRO planning underway Key Milestones 1) Determine MTD / Recommended Ph2 dose / Possible efficacy 2) Single agent efficacy 3) Combination efficacy (Solid tumor all comers + lymphoma) IND Filing

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Experienced Board of Directors 21 Roger J. Pomerantz, M.D. Chairman elect Michael J. Newman, Ph.D. Founder, CSO, Director Jeffrey Meckler CEO, Director Anthony J. Maddaluna Director W. Brad Hayes Director Brian O’Callaghan Director Hila Karah Director Hoonmo Lee Director Leadership experience in new modalities and early development

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Favorable Risk/Opportunity Profile 22 Attribute Milestones Intellectual Property • 31 issued or granted patents, including composition of matter (plus applications) In Vivo Activity • Single agent activity and combination - based tumor eradications in multiple models (solid tumors and lymphoma), including large established & metastatic tumors Therapeutic Index • 10 to 33 - fold range for combination - based tumor regressions Immunological Memory • Induction of both innate and adaptive immunological memory Exogenous Antigen Requirement • Not required for Decoy therapy (e.g. tumor agnostic) Platform Potential • Also active against chronic HBV and HIV infection in vivo Toxicity • Toxicology studies demonstrate potential for safe dosing in humans IND - enabling GLP Tox in - life completed CMC • Successful GMP batch released / Additional characterization in progress Clinical Validation • Coley’s Toxins produced durable responses in hundreds of advanced cancer patients • LPS - endotoxin administered safely i.v. to >1,000 healthy human volunteers

TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Additional Information and Where You Can Find It This communication relates to the proposed domestication merger of Intec and the merger of Intec and Decoy. In connection wit h t he proposed domestication merger and merger, Intec will file a Registration Statement on Form S - 4 with the Securities and Exchange Commission (“SEC”), which will include a document that serve s as a proxy statement and prospectus of Intec, and Intec plans to file other documents regarding the proposed merger with the SEC. INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THE PROXY S TAT EMENT/PROSPECTUS AND OTHER RELEVANT DOCUMENTS FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY, WHEN THEY BECOME AVAILABLE, BECAUSE THEY WILL CONTAIN IMPORTANT IN FORMATION THAT SHAREHOLDERS SHOULD CONSIDER BEFORE MAKING ANY DECISION REGARDING THE PROPOSED DOMESTICATION MERGER AND MERGER AND RELATED MATTERS. A definitive pro xy statement/prospectus will be sent to the Company’s shareholders. Investors and security holders will be able to obtain these documents (when available) free of ch arg e from the SEC’s website at www.sec.gov. The documents filed by Intec with the SEC may also be obtained free of charge from the Company by requesting them by mail at Intec Pharma Ltd., 12 H art om Street, Har Hotzvim, Jerusalem 9777512, Israel. Participants in the Solicitation Intec, and its respective directors and executive officers and other members of management and employees and certain of their re spective significant shareholders may be deemed to be participants in the solicitation of proxies from Intec’s shareholders in respect of the proposed merger. Decoy may also be deemed a partic ipa nt in such solicitation. Information about the Company’s directors and executive officers is available in Intec’s proxy statement, filed June 8, 2020 for the 2020 Annual Meeting of Shareholder s, the Company’s Annual Report on Form 10 - K for the fiscal year ended December 31, 2019, which was filed with the SEC on March 13, 2020 and the Company’s Current Report on Form 8 - K filed on July 17, 2020. Information regarding the persons who may, under the rules of the SEC, be deemed participants in the proxy solicitation and a description of their direct and indirect interests, by security holding or otherwise, will be contained in the proxy statement/prospectus and other relevant materials to be filed with the SEC regarding the proposed Merger when they become ava ila ble. Investors should read the proxy statement/prospectus carefully when it becomes available before making any voting or investment decisions. You may obtain free copies of these doc ume nts from the SEC and Intec as indicated above. No Offer or Solicitation This communication is not intended and does not constitute an offer to sell or the solicitation of an offer to buy any securi tie s, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such ju ris diction. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act, as amended. 23
TEMPLATE NOT FINAL. DO NOT CIRCULATE FOR USE. Thank you. 24
