Attached files
| file | filename |
|---|---|
| EX-32.1 - EX-32.1 - Nuvation Bio Inc. | d124332dex321.htm |
| EX-31.2 - EX-31.2 - Nuvation Bio Inc. | d124332dex312.htm |
| EX-31.1 - EX-31.1 - Nuvation Bio Inc. | d124332dex311.htm |
| EX-4.4 - EX-4.4 - Nuvation Bio Inc. | d124332dex44.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2020
OR
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE TRANSITION PERIOD FROM TO
Commission File Number 001-39351
NUVATION BIO INC.
(Exact name of Registrant as specified in its Charter)
| Delaware | 85-0862255 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
| 1500 Broadway New York, New York |
10036 | |
| (Address of principal executive offices) | (Zip Code) | |
Registrant’s telephone number, including area code: (332) 208-6102
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
| Class A Common Stock, Par Value $0.0001 Per Share Warrants to Purchase Class A Common Stock |
NUVB NUVB.WS |
New York Stock Exchange New York Stock Exchange |
Securities registered pursuant to section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES ☐ NO ☒
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. YES ☐ NO ☒
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES ☒ NO ☐
Indicate by check mark whether the Registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit such files). YES ☒ NO ☐
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company”, and “emerging growth company” in Rule 12b-2 of the Exchange Act:
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ | |||
| Emerging growth company | ☒ | |||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES ☐ NO ☒
The registrant’s common stock was not publicly traded as of the last business day of the registrant’s most recently completed second fiscal quarter.
As of March 9, 2021, the registrant had 216,650,055 shares of Class A common stock and 1,000,000 shares of Class B common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE:
None.
Table of Contents
1
Table of Contents
CAUTIONARY INFORMATION REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K for the year ended December 31, 2020, contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, which are subject to the “safe harbor” created by those sections, concerning our business, operations, and financial performance and condition as well as our plans, objectives, and expectations for business operations and financial performance and condition. Any statements contained herein that are not of historical facts may be deemed to be forward-looking statements. You can identify these statements by words such as “anticipate,” “assume,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “should,” “will,” “would,” and other similar expressions that are predictions of or indicate future events and future trends. These forward-looking statements are based on current expectations, estimates, forecasts, and projections about our business and the industry in which we operate and management’s beliefs and assumptions and are not guarantees of future performance or development and involve known and unknown risks, uncertainties, and other factors that are in some cases beyond our control. As a result, any or all of our forward-looking statements in this Annual Report on Form 10-K may turn out to be inaccurate. Factors that could materially affect our business operations and financial performance and condition include, but are not limited to, those risks and uncertainties described herein under “Item 1A—Risk Factors.” You are urged to consider these factors carefully in evaluating the forward-looking statements and are cautioned not to place undue reliance on the forward-looking statements. The forward-looking statements are based on information available to us as of the filing date of this Annual Report on Form 10-K. Unless required by law, we do not intend to publicly update or revise any forward-looking statements to reflect new information or future events or otherwise. You should, however, review the factors and risks we describe in the reports we will file from time to time with the Securities and Exchange Commission, or the SEC, after the date of this Annual Report on Form 10-K.
SUMMARY RISK FACTORS
Below is a summary of material factors that make an investment in our securities speculative or risky. Importantly, this summary does not address all of the risks and uncertainties that we face. Additional discussion of the risks and uncertainties summarized in this risk factor summary, as well as other risks and uncertainties that we face, can be found under the section titled “Risk Factors” in Part I, Item 1A of this Annual Report on Form 10-K. The below summary is qualified in its entirety by that more complete discussion of such risks and uncertainties. You should consider carefully the risks and uncertainties described under the section titled “Risk Factors” as part of your evaluation of an investment in our securities:
| • | We have a limited operating history and have incurred significant losses since inception and anticipate that we may continue to incur losses for the foreseeable future, and may never achieve or maintain profitability. |
| • | We will need substantial funding to pursue our business objectives. If we are unable to raise capital when needed or on favorable terms, we could be forced to delay, reduce or terminate our product development, other operations or commercialization efforts. Additionally, raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish proprietary rights. |
| • | If we do not obtain regulatory approval for and successfully commercialize our product candidates in one or more indications or we experience significant delays in doing so, we may never generate any revenue or become profitable. |
| • | Our approach to the discovery and development of product candidates based on our Drug-Drug Conjugate platform is unproven and is based on novel technology, and we do not know whether we will be able to develop any products of commercial value, or if competing technological approaches will limit the commercial value of our product candidates or render our platform obsolete. |
| • | Clinical trials are very expensive, time-consuming and difficult to design and implement, and involve uncertain outcomes. Furthermore, results of earlier preclinical studies and clinical trials may not be predictive of results of future preclinical studies or clinical trials. |
| • | We may encounter substantial delays in our preclinical studies or clinical trials or we may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities. |
| • | If any of our product candidates receives marketing approval and we, or others, later discover that the drug is less effective than previously believed or causes undesirable side effects that were not previously identified, its ability to market the drug could be compromised. |
| • | We may become exposed to costly and damaging liability claims, either when testing our product candidates in the clinic or at the commercial stage, and our product liability insurance may not cover all damages from such claims. |
| • | We have never commercialized a product candidate and we may lack the necessary expertise, personnel and resources to successfully commercialize any of our products that receive regulatory approval on our own or together with collaborators. |
| • | We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do. |
| • | Even if we obtain regulatory approval for our product candidates, they will remain subject to ongoing regulatory oversight. |
| • | We rely on third parties to perform the chemistry work associated with our drug discovery and preclinical activities and to conduct our preclinical studies and future clinical trials, and our business could be substantially harmed if these third parties cease performing services or perform in an unsatisfactory manner. |
| • | We do not have our own manufacturing capabilities and will rely on third parties to produce clinical and commercial supplies of NUV-422 and our other current and future product candidates. |
| • | If we are not able to establish collaborations, we may have to alter some of our future development and commercialization plans. If we are not able to establish further collaborations, we may have to alter some of our future development and commercialization plans. |
| • | Our business operations and current and future relationships with investigators, healthcare professionals, consultants, third-party payors and customers will be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws, transparency laws, health information privacy and security laws and other healthcare laws and regulations. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties. |
| • | If we are unable to obtain, maintain, protect and enforce sufficient patent and other intellectual property rights for our product candidates and technology, or if the scope of patent and other intellectual property rights obtained is not sufficiently broad, we may not be able to compete effectively in our market. |
| • | Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed. |
| • | We may become involved in lawsuits to protect or enforce our patents or other intellectual property, which could be expensive, time-consuming and unsuccessful, and issued patents covering our technology and product candidates could be found invalid or unenforceable if challenged. |
| • | Third parties may initiate legal proceedings alleging that we are infringing, misappropriating or otherwise violating their intellectual property rights, the outcome of which would be uncertain and could negatively impact the success of our business. |
| • | Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities. |
| • | Our business, operations and clinical development plans and timelines and supply chain could be adversely affected by the effects of health epidemics, including the ongoing COVID-19 pandemic, on the manufacturing, clinical trial and other business activities performed by us or by third parties with whom we conduct business, including our CMOs, CROs, shippers and others. |
| • | Our future success depends on our ability to retain Dr. Hung and our other key employees, consultants and advisors and to attract, retain and motivate qualified personnel. |
| • | The dual-class structure of our common stock has the effect of concentrating voting power with our Chief Executive Officer, which limits other stockholders’ ability to influence the outcome of important transactions, including a change in control. |
1
Table of Contents
| Item 1. | Business. |
Business Combination of Panacea Acquisition Corp. and Nuvation Bio Inc.
On February 10, 2021, Panacea Acquisition Corp. (“Panacea”) and privately-held Nuvation Bio Inc. (“Legacy Nuvation Bio”) completed a business combination in accordance with the terms of the Agreement and Plan of Merger (the “Business Combination Agreement”), dated as of October 20, 2020, by and among Panacea, Panacea Merger Subsidiary Corp., a wholly owned subsidiary of Panacea (“Merger Sub”) and Legacy Nuvation Bio, pursuant to which Merger Sub merged with and into Legacy Nuvation Bio, with Legacy Nuvation Bio surviving as a wholly owned subsidiary of Panacea. This transaction is referred to as “the Business Combination.” Immediately following the Business Combination, Panacea changed its name to “Nuvation Bio Inc.” In connection with the closing of the Business Combination, our Class A common stock and warrants to purchase shares of our Class A common stock began trading on The New York Stock Exchange under the symbols “NUVB” and “NUVB.WS,” respectively, on February 11, 2021. The disclosure in Items 1 and 1A of this report gives effect to the Business Combination and includes the operations of Legacy Nuvation Bio prior to the Business Combination.
Business Overview
We are a clinical-stage biopharmaceutical company tackling some of the greatest unmet needs in oncology by developing differentiated and novel therapeutic candidates. We were founded by our chief executive officer, David Hung, M.D., who founded Medivation, Inc. and led its successful development of oncology drugs Xtandi® and talazoparib (now marketed as Talzenna®), leading to its $14.3 billion sale to Pfizer Inc. (“Pfizer”) in 2016. We leverage our team’s extensive expertise in medicinal chemistry and drug development to pursue oncology targets validated by strong clinical or preclinical data and discover novel small molecules that improve the activity and overcome the liabilities of currently marketed drugs or best-in-development therapeutic candidates. In addition to our focus on development of small molecules for validated targets, we are also developing novel therapeutic candidates based on our Drug-Drug Conjugate (“DDC”) platform. Utilizing this platform, we are able to conjugate tissue-selective targeted small molecules with anti-tumor agents to create unique therapeutic candidates. We began dosing high-grade glioma patients in a Phase 1/2 clinical trial of our lead product candidate in December 2020 and expect to report top-line data from the Phase 1 portion of this trial in 2022. We plan to initiate multiple other Phase 1 trials through 2022 and to submit up to an additional five IND applications over the next six years across our pipeline of therapeutic product candidates.
Our fully integrated discovery and development team is developing a wholly owned pipeline of targeted oncology product candidates. Our lead product candidate, NUV-422, is a selective inhibitor of key regulators of cell cycle checkpoints cyclin-dependent kinases (“CDK”) 2/4/6. An IND, or a request for authorization from the FDA to administer an investigational product to humans, was accepted, and we initiated a Phase 1/2 clinical trial of NUV-422 for the treatment of high-grade gliomas in December 2020. Our second product candidate is NUV-868, a novel inhibitor of the bromodomain and extraterminal domain (“BET”) family member BRD4, currently in preclinical studies. Our third candidate, NUV-569, is a selective inhibitor of the Wee1 kinase, initially being pursued in combination with DNA-damaging chemotherapy or radiation therapy for the treatment of pancreatic cancer and other solid tumors. We are also characterizing multiple potential lead product candidates from our DDC platform, including NUV-1156, a targeted tumor cell inhibitor that binds to androgen receptor (“AR”)-expressing tissues such as the prostate, and NUV-1176, which binds to estrogen receptor (“ER”)-expressing tissues such as breast.
Our focused approach to address major unmet needs in oncology leverages our group’s significant expertise in discovery, medicinal chemistry, manufacturing, clinical development and commercialization. Through these approaches we have created substantial intellectual property around the composition of matter for our new chemical entities. The foundations of our approach include:
| • | The pursuit of validated targets: We identify and pursue oncology targets validated by strong clinical or preclinical data that provide a high degree of confidence in generating clinically meaningful benefit. We focus on targets where there has been some progress by others in generating clinical candidates or FDA-approved drugs, and we then attempt to design novel therapeutic candidates to overcome the encountered safety liabilities or limitations in efficacy. As an example, in preclinical studies, our lead product candidate, NUV-422, has demonstrated improvements in potency and selectivity of the CDK target class to reduce off-target toxic effects and to overcome specific drug-resistance mechanisms and improve anti-tumor activity. |
| • | Innovative medicinal chemistry expertise. We use our medicinal chemistry capabilities to generate differentiated therapeutic candidates, focused on improving their safety, anti-tumor activity and pharmacologic profiles over other standard of care (“SOC”) therapies. We also use innovative medicinal chemistry approaches to generate novel classes of molecules such as our DDCs. |
2
Table of Contents
| • | A precision oncology approach: Wherever beneficial and practical, we are pursuing a precision approach, initially selecting patients we believe are most likely to respond in indications involving serious or life-threatening conditions in which there is significant unmet medical need. In these indications, we believe that, depending on the results of our preclinical studies, our product candidates could potentially be considered for the FDA’s accelerated approval process based on their potential to demonstrate an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit. |
The following table summarizes our product candidate pipeline:
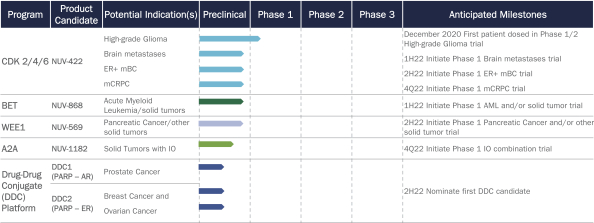
Our lead product candidate, NUV-422, was designed to be a brain-penetrant second-generation CDK4/6 inhibitor with the additional ability to inhibit CDK2, a cell cycle checkpoint found to be altered in many patients with high-grade gliomas including glioblastoma (“GBM”) and also recognized as a mechanism of resistance to currently marketed CDK4/6 inhibitors in breast cancer. NUV-422 also was designed to limit CDK1 inhibition, a potential cause of toxicity in current second-generation inhibitors in development. Currently marketed CDK4/6 inhibitors generated more than $6.0 billion of sales in 2019 and are expected to grow to $14.0 billion of sales in 2025, reflecting the significant patient benefit of this therapeutic class of drugs. We believe that a CDK2/4/6 inhibitor that avoids CDK1 inhibition can bring greater benefit to a broader patient population. We are also advancing NUV-422 to treat patients with breast cancer and potentially other cancer patients with brain metastases, where FDA-approved CDK4/6 inhibitors have shown limited efficacy. Additionally, we are advancing NUV-422 to treat patients with hormone receptor-positive metastatic breast cancer (“ER+ mBC”) where CDK4/6 inhibitors have been successful but CDK2-driven resistance to therapy can develop. Furthermore, we also intend to develop NUV-422 in metastatic castration-resistant prostate cancer (“mCRPC”). The FDA has granted orphan drug designation to NUV-422 for the treatment of patients with malignant gliomas. We began dosing high-grade glioma patients in a Phase 1/2 clinical trial of NUV-422 in December 2020. We plan to expand into a cohort of genetically defined high-grade glioma patients who have a deletion of CDKN2A, a genetic marker of CDK2 activity. CDKN2A deletions are found in nearly 70% of high-grade glioma patients. We anticipate reporting data from the Phase 1 portion of this trial in 2022.
Our second product candidate, NUV-868 is a selective inhibitor of the BET family of epigenetic transcriptional regulators. NUV-868 specifically inhibits the protein BRD4, a key member of the BET family that epigenetically regulates proteins that control tumor growth and differentiation. Notably, BET proteins are believed to be important regulators of the oncogene c-myc. Because c-myc has been implicated as a driver of tumor growth in up to 70% of cancers, BET inhibitors are considered to be a potentially important approach to inhibiting this oncogene, which has been difficult to generate therapeutic drugs against. We have designed NUV-868 to have properties that avoid the therapeutic limiting toxicities of BRD4 inhibitors currently in development focused on optimizing BD2 versus BD1 selectivity. Some first-generation BET inhibitors inhibit BD2 only 1.4-1.5 times better than they inhibit BD1. These first-generation BET inhibitors have been observed to have significant toxicities, especially in the gastrointestinal (“GI”) tract and bone marrow. NUV-868 inhibits the BD2 subdomain of BRD4 almost 1,500 times more potently than it does the BD1 subdomain, which we believe will improve its tolerability. We plan to submit an IND for NUV-868 in the second half of 2021 and initiate Phase 1 clinical trials in patients with acute myeloid leukemia (“AML”) and/or potentially c-myc driven solid tumors in the first half of 2022.
3
Table of Contents
We are also developing several other therapeutic candidates, including NUV-569, a differentiated selective inhibitor of the Wee1 kinase, an important regulator of DNA damage repair. Wee1 is responsible for controlling the cellular checkpoint that can signal to a dividing cell to pause replication while damaged DNA is repaired. Inhibition of this kinase can cause a tumor cell to divide before it has finished repairing its DNA, causing catastrophic DNA damage and programmed cell death. We have designed NUV-569 to avoid off-target effects by improving its kinase selectivity, which we believe could increase its therapeutic window. Because Wee1 inhibitors synergize with DNA-damaging therapies like radiation and certain types of chemotherapy to increase anti-tumor activity. Wee1 inhibitors like NUV-569 may have wide applicability in treating many different types of cancer. We intend to submit an IND for NUV-569 in the first half of 2022 and initiate Phase 1 clinical trials in patients with pancreatic cancer and/or other solid tumors in the second half of 2022.
Our DDC platform is a novel therapeutic approach within the drug-conjugate class of anti-cancer therapies with parallels to Antibody-Drug Conjugates (“ADCs”). ADCs have been effective treatments in oncology, with ten drugs approved by the FDA and an estimated $11.0 billion in worldwide sales expected in 2023. We believe our DDC candidates could expand the therapeutic potential for the drug-conjugate class due to inherently differentiated properties versus ADCs, including a simpler manufacturing process, the potential to cross the cell membrane and recognize intracellular targets, and the potential for oral dosing.
Our DDC platform is designed to selectively deliver potent targeted therapeutics to cancer cells to exert greater toxicity against these target cells than against healthy non-target tissues. We have accomplished this by synthetically fusing a proven anti-cancer small molecule drug to a second small molecule that selectively binds distinct receptors that are preferentially expressed in cancer cells. These tissue-specific receptors create a “sink” that not only may concentrate the targeted drug in cancer cells but may also magnify the effects of the drug in those cells, while preventing similar effects in cells that do not express the targeted receptor. This should allow our DDC candidates to avoid some of the adverse effects commonly seen with many cancer drugs, such as bone marrow suppression and GI toxicity. Because this program at its core fuses the active sites of two or more small molecules to each other to generate a new small molecule with improved activity and targeted specificity, they are called DDCs.
Our first DDC program is focused on targeting an inhibitor of poly ADP ribose polymerase (“PARP”) to androgen receptor, or AR-expressing cancer cells. AR expression is significantly higher in prostate cancer cells than in the GI tract and bone marrow, which are the major sites of current commercial PARP inhibitor toxicities. By fusing a PARP inhibitor to an AR-targeting drug, we believe we may be able to achieve better on-target anti-cancer effects and lower off-target adverse effects than commercially available non-specific and non-targeted agents. We believe such a targeted approach to prostate cancer could potentially be broadly applicable, ranging from the treatment of heavily pretreated metastatic castration resistant prostate cancer to the treatment of newly diagnosed early prostate cancer. The ability of our PARP-AR DDC to kill prostate cancer cells resistant to current therapies suggests that this therapeutic candidate could play a role in advanced stage prostate cancer, particularly in the Xtandi and Zytiga® resistant setting. In the early cancer setting, we believe that PARP-AR DDCs could be a potential pharmacological alternative to surgical prostatectomy and ablative radiation, procedures that often result in comorbidities due to damage to the prostate and surrounding nerve and vascular structures.
Our second DDC program is focused on targeting a PARP inhibitor to ER-expressing cancer cells. We believe we may be able to achieve better on-target anti-cancer effects by delivering a PARP inhibitor to ER-expressing breast and ovarian cancers and avoid adverse effects commonly seen with non-specific, non- targeted PARP inhibitors including bone marrow and gastrointestinal toxicity. Similar to our PARP-AR DDC program, we believe that our PARP-ER DDC could be broadly applicable, ranging from treatment of advanced stage to early stage breast and ovarian cancers. We intend to nominate clinical development candidates in the second half of 2022.
4
Table of Contents
Our adenosine receptor program is focused on targeting the A2a adenosine receptor, an important target in immune-oncology. Accumulation of adenosine in the tumor microenvironment may be a critical factor in limiting the activity of currently available immuno-oncology drugs, including anti-PD(L)1 drugs and anti-cancer chimeric antigen receptor T cells. Thus, targeting the adenosine receptor may overcome this blockade, leading to improved anti-cancer activity in tumors which are resistant to immuno-oncology drugs and adoptive T cell therapies.
We retain worldwide rights to all the products in our pipeline. We have a broad intellectual property portfolio comprised of more than 15 pending U.S. patent applications, over 15 pending PCT patent applications, over 40 pending foreign patent applications and two issued US patents, with expected expirations, excluding any patent term extensions, between 2038 and 2041.
Our Team
Our Chief Executive Officer, David Hung, M.D., has over 15 years of experience as a leader in the biopharmaceutical industry. Dr. Hung founded Medivation in 2003, raised a total of $433.0 million in public offerings over the life of the company and, in 2016, sold Medivation to Pfizer for $14.3 billion, one of the largest biopharma sales ever by a founding Chief Executive Officer. At Medivation, Dr. Hung identified, in-licensed and led bench-to-bedside development of enzalutamide (marketed as Xtandi) for advanced prostate cancer. Xtandi was taken from first in vitro laboratory experiment to FDA approval in seven years, one of the fastest development timelines in pharmaceutical history. Xtandi, approved in 60+ countries, reached blockbuster drug status exceeding $2.0 billion in global annual sales in 2015 and generated $3.7 billion in sales in 2019. Medivation also licensed the PARP inhibitor talazoparib from BioMarin in 2015, and Dr. Hung led its Phase 3 clinical development. In 2018, talazoparib received FDA approval, and it is now marketed as Talzenna for the treatment of breast cancer. Prior to Medivation, Dr. Hung was founder and Chief Executive Officer of Pro Duct Health, a startup company focused on the early detection of breast cancer. Under Dr. Hung’s stewardship, Pro Duct Health raised a total of $22.0 million in venture financing. Three years after its first private financing, Pro Duct Health’s lead product in breast cancer (which Dr. Hung himself invented) received FDA clearance, and Pro Duct Health was then acquired for $168.0 million by Cytyc Corporation.
Our management team has broad expertise and a successful track record of discovering and developing new medicines. Our Chief Medical Officer, Sergey Yurasov, M.D., Ph.D., previously held positions at F. Hoffmann La-Roche (“Roche”), Eli Lilly and Company (“Eli Lilly”), Clovis Oncology Inc. and Immune Design Corp. At Eli Lilly, he was a leading physician for the FDA filing of Cyramza® for patients with non-small cell lung cancer, and, at Clovis Oncology, he led a clinical development group during the FDA filing of Rubraca for patients with ovarian cancer. He was most recently Chief Medical Officer at Immune Design, a publicly traded company acquired by Merck & Co., Inc. (“Merck”) in 2019, where he oversaw clinical and regulatory development of several oncology drug candidates. Our Chief Scientific Officer, Gary Hattersley, Ph.D., was previously at Millennium Pharmaceuticals and Radius Health. At Radius Health, he was a founding scientist and supported the development and subsequent FDA filing of Tymlos® for patients with osteoporosis at high risk of fracture and the development of several oncology drug candidates.
We raised $275.0 million through a Series A preferred stock financing with leading firms that share our vision of delivering new therapies to cancer patients, including, Aisling Capital, Baupost Group, Boxer Capital, Citadel, EcoR1, Fidelity, Omega Funds, Perceptive Advisors, Redmile Group and others.
Strategy
We strive to deliver meaningful benefit to patients with serious unmet medical needs in oncology by developing potentially breakthrough therapies. The core elements of our strategy include:
| • | Rapidly advance the development of our lead product candidate, NUV-422, our selective CDK2/4/6 inhibitor, toward regulatory approval for the treatment of four of the greatest unmet needs in oncology. We have advanced NUV-422 through preclinical studies that have informed a robust clinical development plan to treat (1) CDK2-driven recurrent high-grade gliomas, including GBM. The FDA has granted orphan drug designation to NUV-422 for the treatment of patients with malignant gliomas. Considering NUV-422’s ability to penetrate into the brain, we plan to investigate its anti-tumor activity in (2) breast and potentially other cancer patients with brain metastases, where FDA-approved CDK4/6 inhibitors have shown limited efficacy. We are also advancing NUV-422 to treat patients with (3) ER+ mBC where CDK4/6 inhibitors have been successful but CDK2-driven resistance to therapy can develop. Furthermore, based upon literature that suggests an important role for CDK2/4/6 in driving prostate cancer, we also intend to develop NUV-422 in (4) mCRPC. We began dosing high-grade glioma patients in a Phase 1/2 clinical trial with NUV-422 in December 2020, leveraging a potentially accelerated approval pathway based on its potential to demonstrate an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit in a serious or life-threatening condition for which there are unmet medical needs. We also intend to initiate additional clinical trials in patients with brain metastases, ER+ mBC and mCRPC. |
5
Table of Contents
| • | Advance candidates from our DDC platform to expand our oncology-focused pipeline. We are developing a pipeline of new chemical entities that leverage the tissue-specific targeting capabilities of AR and ER small molecule binders fused to PARP inhibitors. We intend to nominate clinical development candidates in the second half of 2022. |
| • | Advance our deep oncology pipeline of novel therapeutic candidates developed against clinically validated targets. We have designed our candidates with optimized properties including their ability to avoid specific adverse effects of competitive compounds or enhance their anti-tumor potential by targeting additional drivers of tumor resistance. Our early stage pipeline currently includes NUV-868 targeting BET and NUV-569 targeting Wee1. Overall, in addition to the IND already submitted and accepted for NUV-422, we anticipate submitting up to an additional five IND applications to the FDA over the next six years and expect to seek expedited regulatory pathways in multiple programs, such as through Fast Track and Breakthrough Therapy designations. |
| • | Continue to leverage our deep insights in medicinal chemistry to pursue innovative clinical candidates. We have established medicinal chemistry capabilities that have enabled us to rapidly pursue our current pipeline and platform. We intend to leverage these capabilities to pursue both new and validated targets in patients with serious unmet medical needs. |
| • | Evaluate strategic opportunities to accelerate development timelines and maximize value of our product candidate pipeline. We currently own the exclusive worldwide development and commercial rights to each of our product candidates. We intend to evaluate collaborations that could maximize the value of our product candidate pipeline, either through the evaluation of our product candidates in combination with compounds owned by third parties or through geographic collaborations outside of the U.S. that allow us to leverage the existing infrastructure of other companies. |
| • | Build a fully integrated global oncology company. We intend to continue building a fully integrated research, development and commercialization focused company. Our track record of success underscores our proven expertise in discovering, developing and delivering innovative medicines to patients. If our therapeutic candidates are approved, we intend to establish a focused commercial infrastructure and selectively expand our global commercial capabilities. |
Programs
Overview of NUV-422: CDK2/4/6 Inhibitor Program
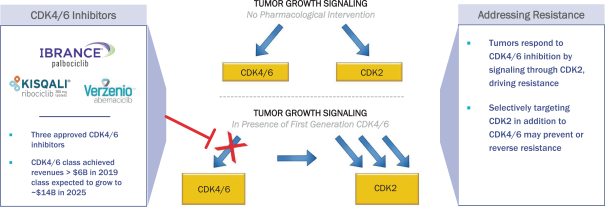
Our lead product candidate, NUV-422, is a potent and selective small molecule inhibitor targeting CDK2, CDK4 and CDK6. These are members of the CDK family of proteins that play a critical role in the regulation of tumor growth. Inhibition of cell-cycle kinases CDK4 and CDK6 results in significant therapeutic effect in patients with advanced hormone ER+ mBC, and these results have led to the approvals of three first-generation CDK inhibitors, palbociclib, ribociclib and abemaciclib. Although these advancements have greatly expanded the treatment options for breast cancer patients, insensitivity to CDK4/6 inhibition has been found in some patients with primary or acquired resistance. As a result, therapeutic resistance and disease progression continue to limit the efficacy and duration of clinical benefit of these therapies. One known mechanism by which some breast cancer patients become resistant to first-generation CDK inhibitors is through CDK2 signaling, which allows cancer cells to bypass CDK4/6 inhibition. Beyond breast cancer, CDK2 activation is known to drive tumorigenesis in multiple solid tumors including brain cancer and prostate cancer, and increased CDK2 activity is associated with lower overall patient survival. NUV-422 selectively inhibits CDK4/6, similar to the approved CDK4/6 inhibitors, but also potently inhibits CDK2. Since its initial discovery in our chemistry program, we have rapidly advanced NUV-422 through preclinical studies that have informed a robust clinical development plan focused on these areas of unmet medical need: (1) high-grade gliomas, including GBM; (2) breast cancer with brain metastases (“BCBM”); (3) ER+ mBC; and (4) mCRPC. We began dosing patients with high-grade gliomas in a Phase 1/2 clinical trial of NUV-422 in December 2020, with additional clinical trials in BCBM, ER+ mBC and mCRPC expected to follow soon thereafter.
6
Table of Contents
CDK2 as a novel mechanism of resistance and oncogenic driver in multiple cancers
The CDK family of proteins regulates cell cycle progression and transcriptional regulation. Recent advances in treatments using CDK inhibitors have focused on inhibition of CDK4 and CDK6, but preclinical studies and clinical trials suggest CDK2 may play an important role as a driver of tumor cell growth as the underlying mechanism of both primary and acquired resistance to CDK4/6 inhibitors. CDK2 is an essential regulator of the cell division cycle and multiple events within the cell cycle, including centrosome duplication, DNA synthesis and G1-to-S-phase transition. CDK2 can bind both cyclin E and cyclin A, which play roles in the cell cycle. Cyclin D normally binds CDK4/6 and is thus a target of CDK4/6 inhibitors, but in the absence of CDK4/6, cyclin D can activate CDK2, which subsequently drives cell cycle progression.
We believe that CDK2 plays a key role in patients who either do not respond to current therapies or develop primary or secondary resistance to ongoing treatment. We and others have shown that CDK2 function can drive hyperproliferation in multiple cancers, including in gliomas, breast cancer and prostate cancer. The CDK2 expression level is elevated in multiple patient tumor tissues, and increased CDK2 expression correlates with a worse survival outcome (Tadasse, et al 2020, Wang et al 2016). It was also recently shown that nearly 70% of high-grade glioma patients carry a homozygous deletion of CDKN2A, which encodes for p14 and p16, which are tumor suppressors that inhibit CDK4/6 directly and CDK2 through p21 (Reinhardt, et al 2018, Verhaak, et al 2010). These results, some of which are depicted in the diagram and graphs below, suggest that targeting CDK2 in these cancers may lead to a blockade of an important aberrant mechanism of tumor growth and resistance to therapy leading to an improvement of clinical outcomes.
7
Table of Contents
CDKN2A-DELETION DRIVES PRIMARY HIGH-GRADE GLIOMAS
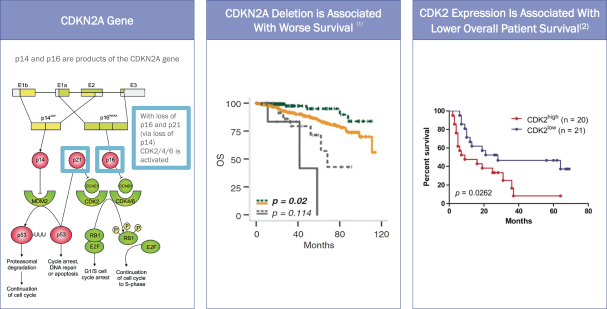
| (1) | Appay et al., 2020 |
| (2) | Wang et al., 2016 |
In addition to primary resistance and de novo tumorigenesis, there are preclinical and clinical data suggesting that CDK2 may be involved in acquired resistance to cancer therapy. Metastatic breast cancer patients enrolled in the PALOMA-3 study who did not benefit from palbociclib therapy demonstrated overexpression of c-myc and cyclin E1 (Turner et al 2018). Since c-myc acts upstream to activate CDK2 and cyclin E1 binds CDK2 to drive the cell cycle, these results suggest CDK2 may be responsible for tumor resistance to palbociclib treatment. Taken together, these preclinical and clinical data demonstrate that CDK2 plays a unique role in promoting tumor growth in multiple types of cancer and that targeting CDK2 in addition to CDK4/6 may help patients overcome the CDK2-mediated resistance to approved therapies, including palbociclib and other CDK4/6 inhibitors.
CDK2 DRIVES RESISTANCE TO CDK4/6 INHIBITORS
8
Table of Contents
Limitations of Other CDK Inhibitors
While CDK4/6 inhibitors demonstrated significant clinical benefit in patients with hormone-receptor-positive breast cancer including an improvement in overall survival, emerging preclinical and clinical evidence suggests that targeting CDK2 in addition to CDK4/6 may provide further benefit to cancer patients whose tumors may be driven by CDK2. These may include breast cancer that does not benefit from treatment with approved CDK4/6 inhibitors and other cancers wherein CDK2 dysregulation may contribute to tumor growth and worse clinical outcomes. It has been reported that of the three FDA approved CDK4/6 inhibitors (ribociclib, palbociclib, abemaciclib), only abemaciclib demonstrated some anti-CDK2 activity, albeit extremely weak activity, in the hundreds of nanomolar half-maximal inhibitory concentration (“IC50”) range (Chen, et al, 2016). The IC50 is a measure of how much of a particular drug or other substance (inhibitor) is required for 50% inhibition of a specific biological or biochemical function, and IC50 values in the hundreds of nanomolar range are considered a sign of relatively weak inhibition. As evidenced by the recently reported divergent outcomes in the breast cancer adjuvant trials of palbociclib (PALLAS and PENELOPE-B studies) and abemaciclib (MonarchE study), patients who received treatment with abemaciclib experienced significant improvement in invasive disease-free survival and distant relapse-free survival (Johnston, et al, 2020), while no such effect was reported for patients in palbociclib trials (Mayer, et al 2020). Moreover, only abemaciclib received approval by the FDA as monotherapy for breast cancer patients, while ribociclib and palbociclib are only approved in combination with hormonal therapy, suggesting a potential benefit of even weak CDK2 inhibition in addition to CDK4/6 inhibition.
We and others have shown that it is critical to target CDK2, CDK4 and CDK6, while sparing CDK1, which is a ubiquitously expressed CDK, the inhibition of which is known to cause severe toxicities in animal models and in patients. Dinaciclib is a potent inhibitor of CDK1 in addition to CDK2 with IC50 for both in the low nanomolar range, indicating strong inhibition. When tested as a once weekly intravenous infusion in a clinical trial (Nemunaitis, et al 2013), despite early signs of anti-tumor activity, 60% of patients experienced grade 3-4 adverse events, including nausea, vomiting, liver enzyme elevation, hyperbilirubinemia and hematological adverse events (neutropenia, anemia). Consequently, clinical development of dinaciclib has been discontinued.
To our knowledge, PF-06873600 is the only other CDK2/4/6 inhibitor in clinical development currently, and while it inhibits CDK2/4/6, it also strongly inhibits CDK1 with an IC50 in the single-digit nanomolar range, which could result in a poor therapeutic index. We believe that sparing CDK1 inhibition is critical to developing a safe and efficacious next-generation CDK inhibitor drug.
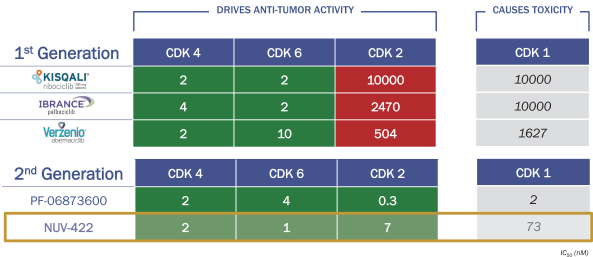
NUV-422 Differentiation
NUV-422 is a next-generation CDK inhibitor discovered in our chemistry program, which potently inhibits CDK2, CDK4 and CDK6, while sparing CDK1 as shown in the table below. NUV-422 is approximately equal to approved drugs ribociclib, palbociclib and abemaciclib in its ability to inhibit CDK4 and CDK6, but it additionally inhibits CDK2, like PF-06873600. But importantly, unlike PF-06873600, NUV-422 does not potently inhibit CDK1, demonstrating a 10 fold lower IC50 for CDK1 than CDK2, CDK4 or CDK6.We believe this positions NUV-422 as a promising next-generation CDK inhibitor with superior CDK2/4/6 vs CDK1 selectivity. In preclinical studies, we have shown that NUV-422 exhibits good drug-like properties, with oral bioavailability, suitable pharmacokinetic and drug metabolism profiles, and a nonclinical safety profile consistent with the class of CDK4/6 inhibitors, as well as a scalable manufacturing process. We have shown that NUV-422 demonstrates strong anti-proliferative activity across multiple human cancer cells.
9
Table of Contents
NUV-422: POTENT INHIBITOR OF CDK2/4/6
IC50 Values: Lower value indicates stronger inhibition
Our Current Opportunities for NUV-422
Overview of Recurrent or Refractory High-Grade Gliomas
Cancer is the second leading cause of mortality in the U.S. and accounts for nearly one in four deaths. Primary tumors of the central nervous system (“CNS”) remain among the most difficult to treat, with a 5-year overall survival of approximately 35%. Gliomas represent 75% of malignant primary brain tumors and GBM accounts for over half of all gliomas. Compared to other areas of oncology, relatively few advances have been made in the treatment of brain cancers. Temozolomide (“TMZ”) is commonly used in front-line settings in combination with radiation, and it was first approved more than fifteen years ago in 2005. Bevacizumab approval soon followed in 2009 for recurrent GBM, but its use remains controversial due to conflicting clinical trial results. Consequently, our initial proposed indication, recurrent or refractory high-grade gliomas, remains a significant unmet medical need with the first de facto option for recurrent GBM patients being clinical trials. Based on the preclinical data we have generated and clinical data others have generated, including patient biopsy, genetic sequencing and survival data, there is a strong biological rationale for targeting CDK2 in gliomas, including GBM. Coupled with preclinical data demonstrating preferential accumulation of NUV-422 in the brain without evidence of CNS toxicity, we believe that NUV-422 has the potential to bring significant clinical benefit to high-grade glioma patients. The FDA has granted orphan drug designation to NUV-422 for the treatment of patients with malignant gliomas.
Clinical Rationale for Targeting CDK2/4/6 in Gliomas
There is strong evidence suggesting CDK inhibition may be a promising therapeutic strategy in gliomas. It was recently reported that CDKN2A deletion occurs in nearly 70% of high-grade gliomas (Reinhardt, et al 2018, Verhaak, et al 2010). Importantly CDKN2A deletion was identified as an independent prognostic factor of poor outcomes including shorter overall survival (Korshunov, et al 2019, Appay, et al 2020). A phase 2 study of abemaciclib in newly diagnosed glioblastoma patients showed that a progression-free survival (PFS) was significantly longer with abemaciclib when compared to the temozolomide containing control arm. However, since abemaciclib demonstrated some anti-CDK2 activity, and there was no evidence of a positive treatment and CDK4 biomarker interaction, CDK2 activity may be an important driver of tumor glioblastoma growth. Since CDKN2A encodes for proteins whose functions are to inhibit CDK2 and CDK4/6, a drug that can inhibit all three of these CDKs may be efficacious in these patients. These results provide strong support for developing NUV-422 for glioma patients.
10
Table of Contents
Preclinical Data
The in vitro anti-proliferative activity of NUV-422 was evaluated in six glioma cell lines, five of which have known CDKN2A deletions. Treatment with NUV-422 resulted in dose-dependent growth inhibition of all six glioma cell lines, with mean absolute IC50 values in the nanomolar range.
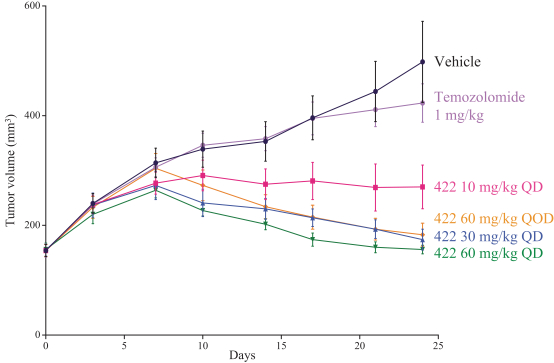
The in vivo antitumor activity of NUV-422 and TMZ was evaluated in a cell line-derived xenograft model, which harbors a CDKN2A deletion, implanted subcutaneously in the flank of immunocompromised mice. NUV-422 was administered orally once daily (“QD”) at 10, 30 and 60 mg/kg and orally once every other day (“QOD”) at 60 mg/kg. NUV-422 treatment at all doses resulted in reduced tumor volume (p < 0.0001) of tumors compared to the vehicle-treated group. In contrast, SOC TMZ had no significant effect on tumor growth compared to the vehicle-treated group. These results are illustrated in the following chart.
NUV-422 INHIBITS TUMOR GROWTH BETTER THAN SOC TMZ IN GLIOBLASTOMA XENOGRAFT MODEL
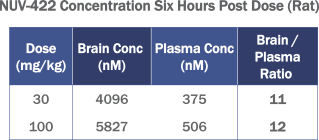
Following a single 30 mg/kg and 100 mg/kg oral dose of NUV-422 in rats, the brain-to-plasma concentration ratios at six hours post-dose ranged from 11 to 12. These data, set forth in the following table, demonstrate high blood-brain barrier (“BBB”) penetration of NUV-422.
HIGH CONCENTRATIONS OF NUV-422 IN THE BRAIN
11
Table of Contents
Clinical Development Plan for NUV-422 in Brain Tumors
We have successfully completed IND-enabling studies of NUV-422, our lead product candidate. The molecule has favorable pharmacological properties with a wide therapeutic index and has demonstrated a consistent nonclinical safety profile supportive of advancement into clinical trials. Most importantly, NUV-422 is unique among CDK inhibitors in that it is much more BBB penetrant and maintains a longer half-life in the brain than in the plasma: approximately 12 times the exposure in the brain compared to the plasma. We believe these characteristics will allow NUV-422 to more potently engage the intended targets in brain tumors compared to other CDK inhibitors that have been tested in brain tumors to date.
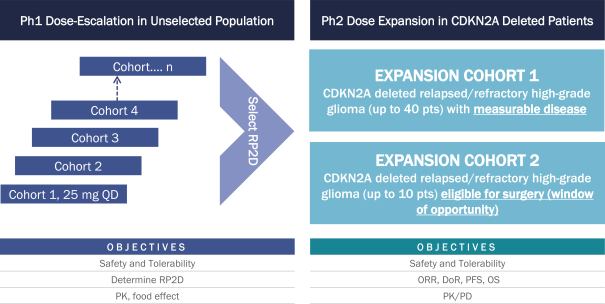
Our IND for NUV-422 was accepted by the FDA, and we began dosing adult patients with recurrent or refractory high-grade gliomas in a Phase 1/2 clinical trial in December 2020. The Phase 1 part of our clinical trial is being conducted as a dose escalation study in an unselected all comer population with recurrent high-grade gliomas, including GBM, with primary objectives to evaluate safety and tolerability, as well as to determine a recommended Phase 2 dose based on the tolerability profile and pharmacokinetic properties of NUV-422. We intend to continue with the Phase 2 dose expansion part of the trial initially focusing on high-grade gliomas, including GBM, with CDKN2A deletion to evaluate overall response rate, duration of response and survival. While we hypothesize that these patients are most likely to experience clinical benefit from NUV-422 dosing, the trial may be amended to include patients irrespective of their CDKN2A status since activity of NUV-422 is not limited to CDK2, and benefit may be observed in other patients. We anticipate reporting data from the Phase 1 portion of this trial in 2022. This trial design is depicted below.
NUV-422-02: SEAMLESS PHASE 1/2 TRIAL DESIGN
Overview of Metastatic Breast Cancer
Breast cancer is the most frequent malignancy in women worldwide, and the second most common cancer worldwide, with an estimated 1.8 million new diagnoses per year. In the U.S., breast cancer has the highest prevalence among all cancers. The Surveillance, Epidemiology, and End Results (“SEER”) Program at National Cancer Institute estimates that in 2020, there will be 276,000 new cases of breast cancer in the U.S. alone, and more than 40,000 deaths. Treatment options for breast cancer depend on many factors, including the stage of cancer. Breast cancer is a heterogeneous disease which is grouped into several clinical subtypes based on the expression of three proteins: ER, progesterone receptor (“PR”) and HER2. Both ER and PR are hormone receptors, and tumors that express either of these receptors are referred to as hormone receptor-positive. The ACS estimates that approximately 75-80% of all breast cancers express estrogen receptor (“ER+”) highlighting the central role of ER signaling in driving a large majority of breast cancer. Although early-stage non-metastatic disease is curable in approximately 70-80% of patients, advanced breast cancer with distant organ metastases is considered incurable with currently available therapies (Harbeck, et al 2019). Advanced breast cancer comprises inoperable locally advanced breast cancer, which has not spread to distant organs, and metastatic (stage IV) breast cancer; common sites of spread are bone, lungs, liver and brain. Currently, it is a treatable but virtually incurable disease, with metastases including to the brain being the cause of death in almost all patients, and a median overall survival of two to three years. Patients with metastatic breast cancer receive treatments that aim to relieve their symptoms and to prolong quality-adjusted life expectancy.
12
Table of Contents
For patients with advanced ER+ breast cancer, endocrine therapy has been the backbone of treatment with a focus on developing a new generation of selective ER modulators (“SERMs”), aromatase inhibitors (“AIs”) and selective ER degraders (“SERDs”) due to emerging resistance to approved drugs. This resistance to endocrine treatment is due to multiple mechanisms, including changes in ER signaling and activation of other molecular pathways, such as CDK, mammalian target of rapamycin (“mTOR”), phosphoinositide 3-kinase (“PI3K”), mitogen-activated protein kinase (“MAPK”) and others (McAndrew & Finn, 2020). Recently, several agents targeting these mechanisms have been approved by the FDA: mTOR inhibitor everolimus (2012), followed by the approval of 3 CDK 4/6 inhibitors (palbociclib [2015], ribociclib [2018] and abemaciclib [2018]), and more recently the PI3-K inhibitor alpelisib for a subgroup of patients with PI3K alterations (2019). For a select group of patients with homologous recombination-deficient (“HR-D”) breast cancer, talazoparib, an oral PARP inhibitor, was approved by the FDA in 2018. All three approved CDK4/6 inhibitors—palbociclib, abemaciclib and ribociclib—are used in metastatic settings, yet nearly half of patients with hormone-receptor positive breast cancer may not respond to first line of treatment with a CDK4/6 inhibitor in combination with hormonal therapy, and many eventually experience progression of cancer.
Clinical Rationale for Targeting CDK2/4/6 in Breast Cancer Patients with Brain Metastases and Other Tumors.
It is estimated that at least 15% and as high as 50% of breast cancer patients will develop brain metastases during the course of their disease (Leone et al 2019). Patients with brain metastasis have a poor prognosis with short overall survival and low quality of life. The prevalence of BCBM is increasing as treatment of primary cancers and imaging techniques improve. In addition, the brain is a “sanctuary site” for breast cancer cells treated with drugs that have poor penetration into CNS. Thus, although a multitude of systemic treatment options exist for extracranial breast metastases, brain metastases continue to pose treatment challenges in clinical practice. For ER+ mBC patients, though recent Phase 3 trials demonstrated a PFS and even an overall survival benefit for CDK4/6 inhibitors in the first or second-line setting, there is limited evidence to inform their CNS-specific activity (Nguyen, et al 2019). Many studies included patients with stable and treated brain metastases or excluded patients with brain metastasis altogether, thus, the potential utility of CDK4/6 inhibitors for the prevention of CNS metastases remains unknown. A study of abemaciclib in BCBM patients demonstrated a slightly over 20% intracranial and extracranial clinical benefit with 5% intracranial response rate (Tolaney et al 2019). While brain exposure was favorable in some of the patients, the overall low response rate in and outside the brain demonstrated that inhibition of just CDK4/6 may not be enough for substantial control of the disease in this patient population. In addition, analyses of breast cancer metastases identified CDKN2A/p16 as a gene potentially associated with development of brain metastases. Patients with a higher p16 score had higher risk of brain metastases and worse overall survival (Furet, et al., 2017). Thus, targeting CDK2 in addition to CDK4/6 may present an important therapeutic strategy in ER+ mBC. In addition, up to 50% of patients with advanced HER2+ breast cancer develop brain metastases, and a combination strategy of CDK2/4/6 inhibition with HER2 targeted therapy may warrant further investigation.
Overall, brain metastases develop in nearly 30% of patients with solid tumors. Cancers of the lung, breast and skin (melanoma) most frequently develop brain metastases and account for 67–80% of patients. Brain metastases from solid extracranial tumors represent an unmet need of increasing relevance as their incidence is rising considerably and is now estimated to be approximately 10 times higher than for primary malignant brain
13
Table of Contents
tumors. Thus, we may opt to study the effect of NUV-422 on brain metastases in patients whose primary tumor location is other than breast (e.g., lung, skin, and/or gastrointestinal tract).
Clinical Rationale for Targeting CDK2/4/6 in ER+ mBC
It was recently reported in the PALOMA-3 trial of ER+ mBC patients that cyclin E1 overexpression is a potential resistance mechanism to palbociclib (Turner, et al 2019). Palbociclib efficacy was approximately halved in patients with high cyclin E1 expression compared to patients with low cyclin E1 expression (median PFS of 7.6 vs 14.1 months, respectively). Since Cyclin E is a known binding partner to CDK2 leading to cell cycle progression, these results reinforce that CDK2 is a key bypass kinase of CDK4/6 inhibition that may be responsible for driving resistance to palbociclib.
Preclinical Data and Development Plan for NUV-422
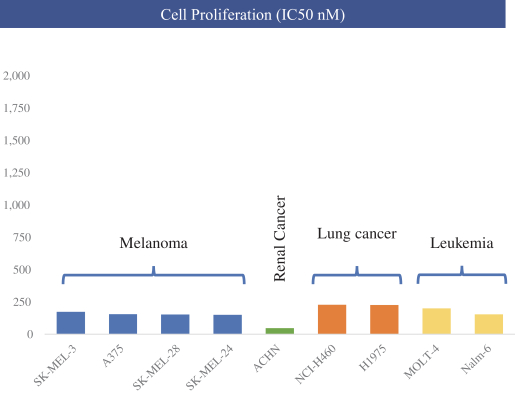
| • | The in vitro anti-proliferative activity of NUV-422 was evaluated in nine cell lines representing four different tumor types that frequently metastasize to the brain. As illustrated in the graph below, treatment with NUV-422 resulted in growth inhibition of all nine cell lines, with mean absolute IC50 values in the less than 200 nanomolar range. |
BEYOND PRIMARY BRAIN TUMORS, ADDITIONAL NUV-422 OPPORTUNITIES IN TUMORS WHICH COMMONLY METASTASIZE TO BRAIN
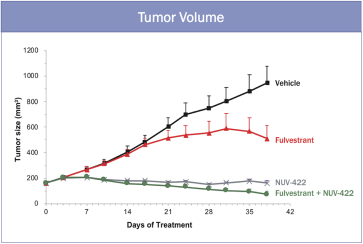
The in vivo antitumor activity of NUV-422 alone and in combination with fulvestrant, an approved anti-estrogen breast cancer drug, was evaluated in a cell line-derived xenograft model, implanted subcutaneously in the flank of immunocompromised mice. NUV-422 was administered orally QD at 30 mg/kg. NUV-422 treatment resulted in reduced tumor volume compared to the vehicle-treated group. While fulvestrant alone had a significant effect on tumor volume, the combination of NUV-422 and fulvestrant results in an even greater decrease in tumor volume. These results are illustrated in the graph below.
14
Table of Contents
NUV-422 IS SUPERIOR TO FULVESTRANT IN XENOGRAFT MODEL OF ER+ METASTATIC BREAST CANCER
We expect to initiate a Phase 1/2 trial of NUV-422 in BCBM patients in first half of 2022 and to initiate a Phase 1/2 trial of NUV-422 in ER+ mBC in second half of 2022.
Clinical Rationale for Targeting CDK2/4/6 in mCRPC.
The role of CDK2 as a crucial factor in development of metastases in patients with prostate cancer has been supported by an extensive analysis of patient gene sequencing data and clinical outcomes (Yin, et al 2018). This analysis identified CDK2 and CDKN2C as one of the most important genes in transcriptional dysregulation in prostate cancer when expression of CDK2 was significantly associated with recurrence of prostate cancer (p = 0.00793). The importance of CDK2 in cancer growth is further supported by knockout experiments suggesting that CDK2 is critical to the cell invasion. While a clinical trial of abemaciclib in prostate cancer is ongoing, a randomized study of palbociclib with abiraterone in mCRPC patients demonstrated that addition of this CDK4/6 inhibitor to AR-targeting therapy did not improve prostate specific antigen endpoints or PFS in this population (Palmbos, et al 2020). Thus, targeting CDK2 in combination with hormonal therapy may be able to address an important unmet medical need in mCRPC patients who progress on current SOC therapy.
Preclinical Data
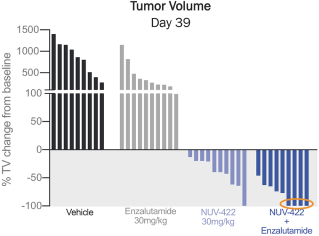
The in vivo antitumor activity of NUV-422 alone and in combination with enzalutamide (Xtandi), an approved prostate cancer drug, was evaluated in a patient-derived xenograft model, implanted subcutaneously in the flank of immunocompromised mice. NUV-422 was administered orally QD at 30 mg/kg. Treatment with NUV-422 alone resulted in reduced tumor volume compared to the vehicle-treated group. As illustrated in the graph below, while enzalutamide alone had very little effect on reducing tumor volume, the combination of NUV-422 and enzalutamide resulted in an enhanced antitumor effect, where all of the treated animals had marked tumor regression.
15
Table of Contents
DEEP TUMOR REDUCTIONS OBSERVED IN ENZALUTAMIDE-RESISTANT PATIENT-DERIVED XENOGRAFT PROSTATE MODEL
Overview of Our DDC Technology Platform
The foundations of our DDCs are built by employing tissue-targeting small molecules fused to anti-cancer warheads of existing drugs with well-understood mechanisms of action. For example, our current lead PARP-AR DDC, NUV-1156, is composed of the AR binder Xtandi (enzalutamide) fused to the warhead of the PARP inhibitor Lynparza® (olaparib) to address advanced stage prostate cancers with the potential to move into earlier lines typically treated with surgical prostatectomy. Our current lead PARP-ER DDC, NUV-1176, is composed of a PARP inhibitor warhead that is fused to the binding domain of an ER-targeting small molecule to address ER+ breast and ovarian cancer. In preclinical models, NUV-1156 and NUV-1176 potently kill tumor cell lines without killing healthy cells in the bone marrow and the gastrointestinal tract. NUV-1156 and NUV-1176 are currently our lead DDCs from our DDC platform in preclinical development, and we intend to nominate our first clinical development candidate from our DDC platform in the second half of 2022.
Traditional Cancer Therapeutics
Cancer treatment has traditionally included chemotherapy, radiation, surgery or a combination of these approaches. Over the last twenty years, new paradigms of cancer research and treatment have emerged to address the limitations of existing treatments. Monoclonal antibodies, or proteins that bind to antigen targets on tumor cells and inhibit tumor growth, represent one of the most successful approaches. More recently, engineered versions of monoclonal antibody-based therapies have emerged, including ADCs and bispecific antibodies, which collectively aim to exert the tumor-specific power of monoclonal antibodies to drive a larger clinical impact than conventional approaches.
ADCs
ADCs exert their antitumor activity by using monoclonal antibodies to deliver potent cytotoxins directly to tumors. ADCs have three primary components: (1) a monoclonal antibody that recognizes an antigen on the tumor and is responsible for directing the therapy to the tumor; (2) a cytotoxic molecule that causes cell death, typically by interrupting with a critical cell function such as replication; and (3) a linker that attaches the cytotoxin to the antibody. The two main attributes of ADC therapeutics are:
| • | Targeting Only Diseased Tissue. ADCs are designed with a monoclonal antibody that binds to antigen targets that are preferably expressed on the outside of tumor cells and not on healthy tissues. |
16
Table of Contents
| • | Increased Therapeutic Window. The cytotoxin payload of the ADC attached to the targeting monoclonal antibody is directed to specific cancer epitopes on the cell surface, allowing an improved therapeutic index by delivering the cytotoxin to the cancer more than non-target tissues. |
As a result of these two main attributes, ADCs can offer greater antitumor potency while still maintaining an acceptable tolerability profile. Despite these benefits, limitations remain, including:
| • | Intravenous Delivery. ADCs are administered intravenously into the systemic circulation where they home to tumors. While the cytotoxic payload is designed to only cleave when internalized by the targeted tumor cell, challenges with linker chemistry can result in instability and cause the cytotoxic payload to be released within circulation, causing systemic toxicities. |
| • | Inability to Reach Intracellular Targets. Monoclonal antibodies are not capable of penetrating the cell membrane due to their size and are limited to targeting antigens that are present on the surface of a tumor cell. |
| • | Complex Manufacturing. ADCs are complex biologics that require the refinement of several properties in tandem and are expensive to manufacture. They often present significant manufacturing challenges, particularly at a large scale, and generally have a lower gross margin than a small molecule. |
Our Solution—DDCs
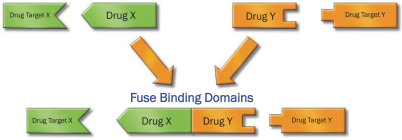
Our DDC platform has generated orally bioavailable small molecules that fuse the binding domains of two different drugs to target two different targets, simultaneously. Our platform leverages our drug discovery and chemistry expertise to find the minimum target binding sites of drug X and drug Y and fuse them together, while maintaining activity. Our DDCs are designed to selectively bind to intracellular targets that are expressed more highly in specific target tissues and to potently deliver anti-cancer warheads to these target tissues. The figure below depicts our DDC approach.
DRUG-DRUG CONJUGATES ARE DESIGNED TO BIND TWO DIFFERENT TARGETS SIMULTANEOUSLY
Key benefits of our DDCs include:
| • | Small molecules that are potentially orally bioavailable; |
| • | Ability to bind to intracellular as well as surface cell membrane targets; and |
| • | Straightforward small molecule manufacturing and attractive gross margins. |
We believe our DDC technology will be broadly applicable and can be replicated across many other existing therapies to transform the SOC across multiple indications for oncology.
NUV-1156: Targeting AR and PARP for Prostate Cancer
NUV-1156, our current lead PARP-AR DDC, is an oral small molecule that is composed of a PARP inhibitor warhead that is fused to the binding domain of an AR-targeting small molecule. In preclinical models, NUV-1156 demonstrated the ability to kill tumor cells associated with high AR-expression, sparing healthy cells in bone marrow and the gastrointestinal tract that do not have high levels of AR expression.
17
Table of Contents
We are exploring the use of NUV-1156 in prostate cancer, initially focused on mCRPC where there is an urgent unmet medical need. The ability of our PARP-AR DDC to kill prostate cancer cells resistant to current therapies suggests that this drug could play a role in advanced stage prostate cancer, particularly in the Xtandi and Zytiga resistant setting.
Additionally, we believe PARP-AR DDCs could play a role in early-stage prostate cancer where the SOC care for newly diagnosed, early-stage patients is radical prostatectomy and radiation therapy which often results in serious side effects, including urinary incontinence, erectile dysfunction and fecal incontinence. We believe a PARP-AR DDC could potentially allow early-stage patients to avoid surgical radical prostatectomy and radiation therapy, which we believe could be a major transformation for the treatment of prostate cancer.
We are currently in preclinical development and intend to nominate our first lead clinical development candidate in the second half of 2022.
NUV-1156 Drug Design and Mechanism of Action
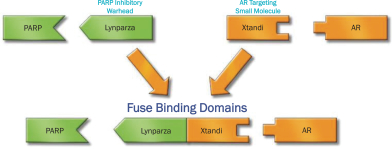
Our PARP-AR DDCs kill cells via an AR-targeted mechanism. NUV-1156 is comprised of the warhead from the PARP inhibitor Lynparza (olaparib) which is fused to an AR-binding domain of Xtandi (enzalutamide). We believe this drug design will potentially allow for a PARP inhibitor to be potently delivered to high AR-expressing tumors, like prostate cancer, while avoiding the off-target toxicities associated with other PARP inhibitors, namely toxicity in the bone marrow and gastrointestinal tract, which are low AR-expressing tissues. The figure below depicts the components of NUV-1156.
NUV-1156 IS A DDC THAT TARGETS AR AND PARP
PARP Inhibitor Overview
Mechanisms of Action
The rapid cell division and attendant required DNA replication seen in cancers causes an increase in single stranded DNA breaks. PARP is the most abundant DNA repair enzyme in the nucleus. Because cancers have an increase in DNA breaks related to their rapid division, their DNA breaks must be repaired by PARP if the cancers are to be able to faithfully replicate their DNA. Furthermore, approximately one-third of tumors have intrinsic DNA repair defects, such as BRCA-mutations and other HR-D. Tumors with HR-D struggle to repair and faithfully replicate DNA. When HR-D is combined with PARP inhibition, DNA repair is so compromised that cancer cells can no longer survive. This is the fundamental reason that all current commercially available PARP inhibitors have superior outcomes in HR-D vs. homologous recombination proficient (“HR-P”) cancers. This mechanism of action of PARP inhibitors has been shown to further enhance the effects of DNA-damaging anti-cancer therapies, such as chemotherapy or radiation.
Existing PARP Inhibitors and Our Opportunity
PARP inhibitors Lynparza (olaparib), Rubraca (rucaparib camsylate), Zejula (niraparib) and Talzenna (talazoparib tosylate) have been approved by the FDA for multiple oncology indications, including ovarian, breast, prostate and pancreatic cancer. Sales of these FDA-approved PARP inhibitors were approximately $1.7 billion in 2019 and are forecasted to be over $7.0 billion in 2025, with Lynparza (olaparib) accounting for $1.2 billion and over $4.0 billion in the 2019 and 2025 totals, respectively.
18
Table of Contents
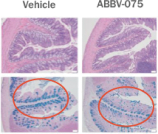
Despite the commercial success of PARP inhibitors, broader adoption is limited by their high rates of GI and bone marrow toxicity which is largely a result of off-target cell killing. Adverse grade 3-4 events from this class of drugs include anemia, thrombocytopenia, neutropenia and alopecia. Other common adverse reactions include nausea, vomiting, diarrhea, fatigue and decreased appetite. We believe a DDC that fuses the warhead of a PARP inhibitor to an AR-binding domain of Xtandi (enzalutamide) will allow us to take advantage of the powerful and proven selectivity of AR therapy in AR-driven tumors by possibly minimizing the toxicities associated with PARP inhibitors in low AR-expressing cells in the gastrointestinal tract and bone marrow and broadening the tumor types (both HR-D and HR-P) in which this approach could be effective.
AR Selectively Expressed in AR-Specific Tissue
The growth and survival of prostate cancer cells depends heavily on the AR. Testosterone fuels prostate cancer cell growth by using the binding of androgens to ARs to trigger abnormal cell growth and tumor progression. In men, AR protein expression is limited primarily to the sex organs, with medium to high AR expression levels seen across the testis, prostate, epididymis and seminal vesicle tissues. In contrast, AR expression is either low or not detected in the bone marrow and the gastrointestinal tract, two organs strongly associated with PARP-inhibitor toxicity.
Existing AR Inhibitors and Our Opportunity
Xtandi (enzalutamide) is an AR inhibitor that acts on different steps in the AR signaling pathway. Xtandi has been shown to potently bind to the AR and effectively compete for this receptor against its native ligand testosterone. Zytiga (abiraterone) is an inhibitor of androgen synthesis and results in decreased AR signaling through ligand depletion. Between 15% and 25% of patients do not respond to either AR signaling pathway inhibitors abiraterone or enzalutamide, and the vast majority of the responsive patients will ultimately become resistant, resulting in limited survival. Zytiga was approved for the treatment of mCRPC in 2011 and generated sales of $2.8 billion in 2019. Xtandi was approved for the treatment of mCRPC in 2012 and generated sales of approximately $3.7 billion in 2019.
Prostate Cancer Overview
Prostate cancer is reported as the second and third leading cause of cancer death for men in the U.S. and in Europe, respectively. SEER cancer statistics estimated that approximately 175,000 men in the U.S. and 450,000 men in the EU5 would be diagnosed with prostate cancer in 2020, potentially resulting in a $15 billion market opportunity given the costs of treatment.
For early stage prostate cancer, the SOC is a radical prostatectomy, the removal of the prostate via surgery, or radiation therapy. While potentially curative, prostatectomy and/or radiation can result in serious side effects, including urinary and fecal incontinence and erectile dysfunction, as a result of damage to surrounding vital structures, blood vessels and nerves. Given the invasive nature of the procedure, prostatectomy surgery also brings the risk of complications with anesthesia, bleeding and infection.
mCRPC is the most advanced form of the disease and there are approximately 35,000 to 45,000 new incidences of mCRPC each year. Men with mCRPC have a poor prognosis and a predicted survival rate of fewer than two years from the initial time of progression.
Current SOC for men with castration-resistant prostate cancer provides that patients should initially receive a combination of androgen deprivation therapy (“ADT”) and either abiraterone, which works by decreasing androgen levels, or enzalutamide, which works by blocking androgen binding to AR. If the disease progresses despite these second-generation hormonal therapies, chemotherapy is considered the next treatment option. Treatment with chemotherapy is generally postponed for as long as possible due to its effect on patient’s quality of life and the potential for severe side effects including neuropathies, nausea, diarrhea, decreased mental capacity and increased risk of infections.
19
Table of Contents
Preclinical Data
In an Xtandi (enzalutamide)-resistant prostate cancer model, NUV-1156 demonstrated the ability to inhibit growth of enzalutamide-resistant prostate cancer cells more than Lynparza (olaparib), Xtandi (enzalutamide) or the combination of olaparib and enzalutamide. Cell proliferation, as measured by IC50, was more than 30,000 nanomolar for enzalutamide, nearly 8,000 nanomolar for olaparib and over 6,000 nanomolar for olaparib + enzalutamide. In contrast, NUV-1156 had an IC50 of 201 nanomolar, demonstrating that forming a DDC of a PARP inhibitor with a targeting agent that targets a receptor highly expressed in prostate cancer leads to orders of magnitude superior therapeutic effects compared to either agent alone, or even a combination of the two agents given in their native state. These results are shown in the table below.
NUV-1156 DDC POTENTLY KILLS PROSTATE CANCER CELLS RESISTANT TO CURRENT SOC
| Drug(s) |
Cell Proliferation IC50 (nM) |
|||
| Xtandi (enzalutamide) |
>30,000 | |||
| Lynparza (olaparib) |
7,844 | |||
| Xtandi (enzalutamide) + Lynparza (olaparib) |
6,152 | |||
| NUV-1156 (PARP x AR DDC) |
201 | |||
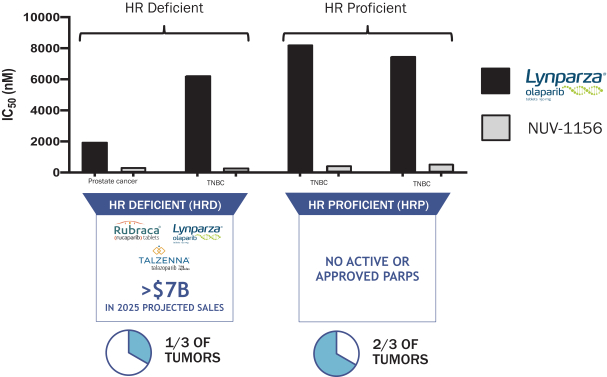
As shown in the figure below, unlike Lynparza (olaparib) which is only approved in HR-D cancers and is not effective in HR-P tumors, NUV-1156 potently kills prostate cancer cells and triple negative breast cancer cells, whether they are HR-D or HR-P. We believe this underscores the superior potency of NUV-1156 as well as the potential for NUV-1156 to be used more broadly than current commercially available PARP inhibitors, which are limited to HR-D driven tumor types.
20
Table of Contents
NUV-1156 IS ACTIVE IN BOTH HR-D AND HR-P CANCER CELL LINES
In an Xtandi (enzalutamide)-resistant prostate cancer cell model, NUV-1156 demonstrated the ability to kill cancer cells while sparing healthy gastrointestinal cells in vitro. In the figure below, the black bars represent prostate cancer cells as measured by a 22RV1 prostate epithelial cell line model and the gray bars represent gastrointestinal epithelial cells, or healthy tissue, as measured by an IEC-6, a standard model for healthy rat gastrointestinal epithelial cells. In this enzalutamide-resistant model, Xtandi (enzalutamide) had no toxicity on such gastrointestinal cells but, had little efficacy on Xtandi-resistant prostate cancer, a suboptimal effect. Lynparza (olaparib) fared even worse, having little efficacy on Xtandi-resistant prostate cancer, but killing gastrointestinal epithelial cells three times more potently than it kills prostate cancer cells. As compared to Lynparza (olaparib) and Xtandi (enzalutamide), NUV-1156 was observed to be significantly more potent and selective for prostate cancer cells than either Lynparza or Xtandi alone, killing Xtandi-resistant prostate cancer with low nanomolar potency while having little toxicity on healthy gastrointestinal epithelial cells. These results are shown in the graph below.
21
Table of Contents
NUV-1156 KILLS ENZALUTAMIDE-RESISTANT PROSTATE CANCER (HIGH AR) CELLS BUT SPARES HEALTHY COLON (LOW AR) CELLS IN VITRO
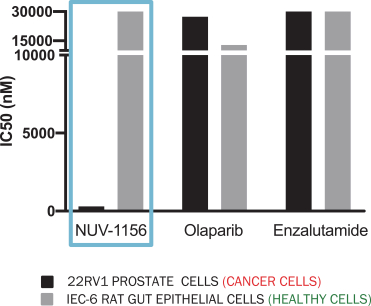
Thus, in preclinical models, NUV-1156 has demonstrated the ability to kill high AR-expressing tissues like prostate cancer while sparing low AR-expressing tissues like healthy gastrointestinal epithelial cells. This level of specificity may potentially allow a prostate-specific DDC to kill prostate cancer cells in the prostate while sparing other low AR-expressing cells like nerve and blood vessel cells, which are directly impacted during prostate ablation procedures like radical prostatectomy and radiation therapy, the current SOC for early stage prostate cancer. While prostatectomy and radiation ablation are potentially curative, these interventions can result in serious side effects, including erectile dysfunction, urinary incontinence and/or fecal incontinence, or other sequelae of invasive surgery, as a result of damage to the tissues surrounding or within the prostate like healthy blood vessels and nerve cells. We believe that NUV-1156 has the potential to become a non-surgical/non-radiation curative alternative for these patients, representing a large potential market opportunity.
Next Steps
We believe NUV-1156 may potentially address significant unmet medical need in mCRPC patients and may also eventually serve as an alternative to patients undergoing a radical prostatectomy or radiation therapy. We are currently in preclinical development and intend to nominate our first lead clinical development candidate in the second half of 2022.
NUV-1176: Targeting ER and PARP for ER+ Breast Cancers
NUV-1176, our lead PARP-ER DDC, is an oral small molecule that is composed of a PARP inhibitor warhead that is fused to the binding domain of an ER targeting small molecule. In preclinical models, NUV-1176 potently kills both HR-D and HR-P ER+ tumor cell lines without killing healthy gastrointestinal epithelial cells. We are exploring the use of NUV-1176 for ER+ breast cancers and ovarian cancer. We are currently in preclinical development and intend to nominate our first lead clinical development candidate in the second half of 2022.
22
Table of Contents
Overview of ER+ Breast cancers
Prevalence and Prognosis
Breast cancer is the second most common cancer worldwide, with an estimated 1.8 million new diagnoses and 500,000 patients receiving treatment per year. In 2020, the ACS estimated there would be approximately 276,000 new cases of female breast cancer and over 40,000 deaths in the U.S. The ACS estimates that approximately 75-80% of all breast cancers are ER+, highlighting the central role of ER signaling in driving a large majority of ER+ mBC. When bound to estrogen, the ER directs the expression of genes that are essential for breast cancer cells’ survival and proliferation.
Although early-stage non-metastatic disease is curable in approximately 70-80% of patients, advanced breast cancer with distant organ metastases is considered incurable with currently available therapies (Harbeck, et al 2019). Advanced breast cancer comprises inoperable locally advanced breast cancer, which has not spread to distant organs, and metastatic (stage IV) breast cancer; common sites of spread are bone, lungs, liver and brain. Currently, it is a treatable but virtually incurable disease, with metastases being the cause of death in almost all patients, and a median overall survival of two to three years. Patients with metastatic breast cancer receive treatments that aim to relieve their symptoms and to prolong quality-adjusted life expectancy.
Treatment of ER+ Breast Cancer
ER+ breast cancer has led the way in drug development given the early appreciation for its dependence on estrogen signaling. The initial SOC for patients with early-stage ER+ breast cancer is at least five years of adjuvant endocrine therapy which commonly uses an ER antagonist (tamoxifen) or an aromatase inhibitor (anastrozole, exemestane or letrozole). For patients with advanced ER+ breast cancer, endocrine therapy has been the backbone of treatment. In 2019, worldwide sales for endocrine and targeted therapies treating ER+ breast cancer patients totaled $9.6 billion. Given the incidence rate and cost of treatment, by 2027 the market size for adjuvant therapy, first line treatments and second line treatments could total $25 billion, $8 billion and $4 billion, respectively.
Due to emerging resistance to endocrine therapy, there has been a focus on developing a new generation of SERMs, AIs and SERDs. Resistance to endocrine treatment is due to multiple mechanisms, including changes in ER signaling and activation of other molecular pathways, such as CDK, mammalian target of mTOR, PI3K, MAPK and others (McAndrew & Finn, 2020). Recently, several agents targeting these mechanisms have been approved by the FDA: mTOR inhibitor everolimus (2012), followed by the approval of three CDK 4/6 inhibitors (palbociclib [2015], ribociclib [2018] and abemaciclib [2018]), and more recently the PI3-K inhibitor alpelisib for a subgroup of patients with PI3K alterations (2019). For a select group of patients with HR-D breast cancer, talazoparib, an oral PARP inhibitor, was approved by the FDA in 2018. Despite the fact that these new therapies in combination with endocrine therapy bring significant clinical benefit to ER+ mBC patients, it has been well established that patients will either not respond to or acquire resistance to treatment over the course of their disease and will eventually require cytotoxic chemotherapy, which is associated with significant side effects. Thus, there remains a significant unmet medical need for ER+ mBC patients who exhausted available therapies and have to opt for the last resort of chemotherapy.
ER Selectively Expressed in ER-Specific Tissue
In women, ER protein expression is limited primarily to the sex organs, with median to high ER expression levels seen across the fallopian tube, breast, vagina, uterine, cervix and endometrium tissues. In contrast, ER expression is either low or not detected in the bone marrow and intestine, organs strongly associated with current commercially available PARP-inhibitor toxicity. Given that ER is more highly expressed in tumors that arise in female sex organ tissues like breast or ovarian cancer than tissues like the bone marrow or gastrointestinal tract, we believe an ER-targeted DDC will have improved anti-tumor activity while avoiding the toxicity profile associated with current commercially available PARP inhibitors.
23
Table of Contents
Preclinical data
We have developed NUV-1176, an ER-targeted DDC that is composed of a PARP inhibitor warhead that is fused to the binding domain of an ER-targeting small molecule. In preclinical models, as shown below, NUV-1176 has demonstrated the ability to potently kill both HR-D and HR-P ER+ tumor cell lines with minimal effects on healthy gastrointestinal cells.
NUV-1176, AN ER-TARGETED DDC, POTENTLY KILLS BOTH HR-D AND HR-P ER+ BREAST CANCER CELLS WITH MINIMAL EFFECTS ON HEALTHY COLON CELLS
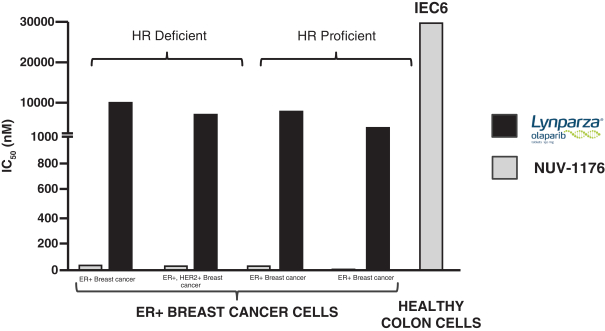
Next Steps
We are currently in preclinical development and intend to nominate our first lead clinical development candidate from our DDC platform in the second half of 2022.
Overview of NUV-868: Bromodomain Inhibitor Program
NUV-868 for AML
NUV-868, our lead candidate from our BET program, is an oral small molecule BET inhibitor that is almost 1,500 times more selective for BD2 over BD1, avoiding the toxicities associated with other non-BD2 selective inhibitors. We are currently in preclinical development and intend to initiate a Phase 1 trial in patients with AML in the first half of 2022.
BET as a Driver of Disease in AML
BET proteins are epigenetic readers that turn on specific genes by binding unique regions of the genome through their ability to read specific chemical tags on chromatin. In some instances, BET proteins turn on genes that are abnormally expressed in a variety of human cancers. BET inhibitors downregulate the expression of key genes that have the potential to cause cancer, or oncogenes, such as c-myc. C-myc is believed to play a role in promoting the growth of up to 70% of all cancers. These observations have resulted in the generation and clinical investigation of BET inhibitors in several cancer subtypes.
24
Table of Contents
BETs are comprised of two sub-domains: BD1, the inhibition of which is known to contribute to toxicity, and BD2, the inhibition of which is known to be important for efficacy. BET inhibitors have historically targeted both BD1 and BD2 less selectively, causing gastrointestinal toxicity and bone marrow suppressive effects like thrombocytopenia.
AML
In the U.S. alone in 2019, there will be an estimated 21,450 new cases of AML diagnosed and 10,920 deaths (Lai, et al2019). With a median age of 68 years and a five-year overall survival of roughly 25%, the prognosis remains poor. While 5-year OS is 40% to 50% for younger (under 50 years) patients with de novo AML, the estimated five-year OS for older patients, those with secondary AML, or relapsed or refractory (“R/R”) disease, is only 5% to 10%. Approximately half of patients over 60 years of age receive intensive induction chemotherapy, with the remainder receiving either non-intensive chemotherapy or supportive care. In the absence of adequate therapies, these R/R patients may be put into clinical trials for new and emerging therapies.
Treatment options for AML have been limited for the past five decades (Lai, et al 2019). The combination of an anthracycline and cytarabine known as “7 + 3” was initially reported in 1973, and induction therapy has remained relatively unchanged since then. While several therapies have been approved by the FDA during the past several years, there remains a significant unmet medical need with patients progressing despite therapy. Many of the new drugs are limited to a subgroup of patients with specific molecular alterations, and, most importantly, all patients progress on current therapies, and available subsequent therapeutic options are often limited to older chemotherapeutics and bone marrow transplantation. The recently approved drugs include fms-related tyrosine kinase 3 (FLT3)-targeting drugs (midostaurin, gilteritinib), isocitrate dehydrogenase (IDH) 1 and 2 inhibitors (enasidenib, ivosidenib), bcl-2inhibitor (venetoclux), smoothened pathway inhibitor (glasdegib) and ADC gemtuzumab ozogamicin.
Our Solution—NUV-868
NUV-868, our lead candidate from our BET program, is an oral small molecule BET inhibitor that is almost 1,500 times more selective for BD2 than BD1, avoiding the toxicities associated with other non-BD2 selective inhibitors. Given BET’s promise as an oncology target, there are several BET inhibitors in development for several cancers. ABBV-774 is a BET inhibitor that is 324 times more potent for BD2 than BD1. Other BET inhibitors that are not as selective for BD2, have been associated with toxicities including gastrointestinal and thrombocytopenia. The selectivity of several BET inhibitors that are currently in development is shown in the table below.
NUV-868 IS A MORE SELECTIVE BD2 INHIBITOR
IC50 values of NUV-868 and other BET inhibitors in development
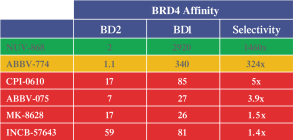
In two AML xenograft models, including a Kasumi-1 and an MV-4-11 model, NUV-868 demonstrated anti-tumor activity as compared to vehicle across three doses (5 mg/kg, 10 mg/kg and 20 mg/kg) out to 21 days, as shown in the graphs below. Notably, near complete tumor regression was observed in the 10-20 mg/kg NUV-868 group.
25
Table of Contents
NUV-868 IS HIGHLY POTENT IN KILLING AML CELLS IN IN VIVO XENOGRAFT MODELS
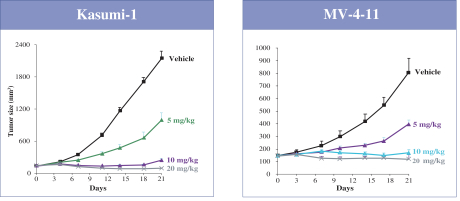
NUV-868’s BD2 selectivity avoids gut toxicity observed with other dual BD1 / BD2 BET inhibitors. In tissue samples from a rat small intestine treated with vehicle and ABBV-075 and NUV-868, treatment with ABBV-075 led to a marked reduction in healthy goblet cells, which are central in protecting the mucous membrane in the GI tract. By comparison, a notably higher dose (30mg/kg) of NUV-868 showed no apparent evidence of goblet cell loss. These results are shown in the images below. We believe this data supports the potential for NUV-868 to limit the gastrointestinal toxicities that are associated with other BET inhibitors.
HIGH SELECTIVITY FOR BD2 OVER BD1 REDUCES THE GUT TOXICITY OBSERVED WITH OTHER BET INHIBITORS
| ABBV-075 (Dual BD1 / BD2)
Faivre et al 2020 |
NUV-868 (BD2 Selective) | |
|
|
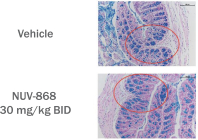
| |
The other main toxicity associated with BET inhibitors is thrombocytopenia. While most non-selective BET inhibitors lower platelet levels and cause thrombocytopenia, NUV-868 has demonstrated higher platelet levels as a function of reversing platelet suppression associated with untreated tumor burden and a lack of bone marrow-suppressive side effects. In the table below, platelet counts are measured in a MV 4-11 AML xenograft hematology panel 24 hours post the final dose of NUV-868 on day 21 across three dose levels. As compared to treatment with vehicle, platelet counts rose for the NUV-868 cohorts across the low (5 mg/kg), medium (10 mg/kg) and high (20 mg/kg) doses.
26
Table of Contents
NUV-868 REVERSES PLATELET SUPPRESSION IN AML
MV 4-11 AML Xenograft Hematology Panel
(24-hours post final dose on Day 21)
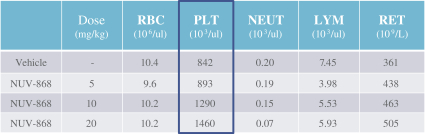
Next Steps
We are currently in preclinical development and intend to initiate a Phase 1 trial of NUV-868 in patients with AML and/or solid tumors in the first half of 2022.
Overview of NUV-569: Wee1 Program
NUV-569, is a differentiated selective inhibitor of Wee1 kinase, an important regulator of DNA damage repair, which we are initially developing for the treatment of pancreatic cancer and solid tumors. Wee1 is responsible for controlling the cellular checkpoint that can signal to a dividing cell to pause while damaged DNA is repaired. Inhibition of this kinase can cause catastrophic DNA damage to a tumor cell triggering programmed cell death. We have designed NUV-569 to avoid off-target effects that could increase the therapeutic window for this class of therapeutic candidates and have wide applicability to treating many different types of cancer. We intend to submit an IND for NUV-569 in the first half of 2022 and initiate Phase 1 trials in patients with pancreatic cancer and/or other solid tumors in the second half of 2022.
Background on Wee1 and DNA Damage Repair
DNA damage occurs frequently throughout the cell cycle, and even more frequently in rapidly dividing cancer cells, as a result of the challenge of endogenous and exogenous DNA insults and stressors. In response to DNA damage, cells have evolved a network of complex, coordinated DNA damage response (“DDR”). The DDR involves a network of DNA repair pathways and DNA damage checkpoints that are linked through various signaling mechanisms responsible for sensing and responding to specific types of DNA damage that affect DNA repair, cell cycle regulation replication stress responses and apoptosis. Defects in the DDR result in genomic instability and ultimately promote the cloning of cancer cells.
Wee1 is one of many kinases involved in regulation of signaling within the cell cycle and DNA damage identification and repair within the DDR. Specifically, Wee1 is a tyrosine kinase that allows cells with DNA damage to repair and survive by activating the G2/M cell cycle checkpoint through inhibition of the phosphorylation of CDK1/2, thus suspending the process of cell division in healthy cells. In cancer cells, tumors activate their Wee1 checkpoint in order to arrest the process of cell division, thus allowing them to repair their damaged DNA and replicate, resulting in tumor growth. Inhibition of cellular regulation and repair mechanisms within the cell cycle and DDR, such as Wee1, may potentially play a crucial role in the induction of apoptosis, improve the efficacy of DNA-damaging cancer therapies to which cancer cells have already developed multiple mechanisms of resistance, and may improve the efficacy of DNA-damaging radiation treatment. Specifically, Wee1 inhibitors force tumor cells to replicate prior to DNA repair, leading to incorrect DNA replication and ultimately tumor cell death.
27
Table of Contents
Wee1 Inhibitors in Clinical Development and Limitations
We are aware of only several Wee1 inhibitors currently in clinical development, including AZD1775. AZD1775 is currently being evaluated in Phase 1 and 2 clinical trials in ovarian cancer and a variety of other solid tumors, both as monotherapy and in combination with other cancer therapies. In these trials, multiple patients with advanced or metastatic tumors for whom no standard therapy was available achieved partial responses when dosed with AZD1775 in combination with chemotherapy agents. For example, in a Phase 2 clinical trial in 24 patients (21 of whom were evaluable for efficacy) with relapsed ovarian cancer, the combination of AZD1775 and carboplatin demonstrated an overall response rate of 43% and one patient exhibited a complete response lasting over 42 months.
In addition, AZD1775 demonstrated encouraging overall survival in a recent Phase 1 clinical trial in patients with locally advanced pancreatic cancer as compared to historical trials in this patient population. AZD1775 in combination with SOC gemcitabine, and radiation resulted in a median overall survival of 21.7 months. More recently, AZD1775 demonstrated an overall response rate of 30% in a Phase 2 clinical trial in patients with recurrent uterine serous carcinoma, an aggressive subtype of endometrial carcinoma characterized by TP53 mutations.
Although AZD1775 has shown encouraging clinical efficacy data in patients with uterine serous carcinoma and ovarian and pancreatic cancers, we believe it has the following limitations with regards to its safety profile.
| • | Potent inhibition of polo-like kinase 1 (“PLK1”). PLK1 is a cell kinase that phosphorylates Wee1 as the cell approaches the G2/M cell cycle checkpoint thus promoting and enabling the cell replication process. PLK1 may be responsible for gastrointestinal and bone marrow toxicity. AZD1775 is a highly potent inhibitor of PLK1, having demonstrated an IC50 of 15 nanomolar in biochemical studies, and thus may contribute to bone marrow and GI toxicity. |
| • | Liver enzyme inhibition. AZD1775 inhibits liver enzyme CYP3A4, which is responsible for elimination of drug and drug metabolites from the body. |
| • | Tolerability. In a recent Phase 1 clinical trial in patients with locally advanced pancreatic cancer, AZD1775 in combination with gemcitabine, an FDA-approved chemotherapy, and radiation, eight patients (24%) experienced a dose-limiting toxicity, most commonly anorexia, nausea, or fatigue, thus preventing continuous dosing of AZD1775. |
Our Solution—NUV-569
NUV-569, our lead candidate from our Wee1 program, is an oral small molecule Wee1 inhibitor that we have designed in order to avoid off-target effects that we believe could increase the therapeutic window for this class of therapeutic candidates. We believe that NUV-569 could have wide applicability to treating many different types of cancer. Our Wee1 inhibitor is highly potent against Wee1 and is designed to have low inhibition of PLK1, thus potentially reducing the toxicity that has been seen in other Wee1 inhibitors, such as AZD1775. Specifically, in our preclinical studies NUV-569 demonstrated single digit nanomolar inhibition of Wee1, 45-fold lower PLK1 inhibition than AZD1775 and 9-fold lower potency in inhibiting proliferation of rat gut epithelial cells (IEC6), than AZD1775, which we believe suggests NUV-569 may have better GI tolerability than AZD1775. The following table demonstrates the favorable potency and selectivity profile of NUV-569 compared to AZD1775.
28
Table of Contents
NUV-569 IS POTENT AGAINST WEE1 BUT CAUSES LESS INHIBITION OF PLK1 AND RAT GUT EPITHELIAL CELLS THAN AZD1775 IN PRECLINICAL STUDIES
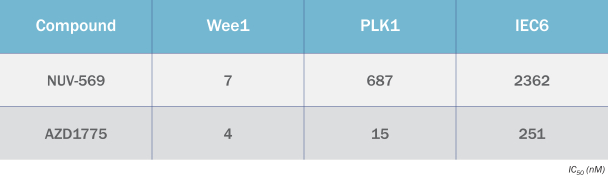
Preclinical Results
In an in vitro preclinical study of pancreatic cancer in combination with gemcitabine and radiation therapy, we observed NUV-569s anti-tumor activity and potency in inducing apoptosis in pancreatic cancer cells, as shown in the graph below.
NUV-569 INCREASES IN VITRO KILLING OF PANCREATIC CANCER CELLS BY CHEMO AND/OR RADIATION
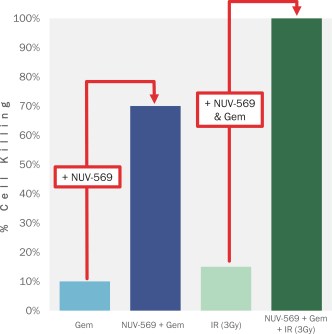
Next Steps
We are currently in preclinical development and intend to initiate Phase 1 trial of NUV-569 in patients with pancreatic cancer and/or other solid tumors in the second half of 2022. In addition to pancreatic cancer, we are also evaluating NUV-569 in breast, ovarian and endometrial cancer.
29
Table of Contents
A2a Adenosine Receptor Program
Our adenosine receptor program is focused on targeting the A2a adenosine receptor. The A2a adenosine receptor plays multiple critical roles in human physiology and pathophysiology including anti-cancer immunity, which makes it an important drug target. Accumulation of adenosine in the tumor microenvironment may be a critical factor in limiting the activity of currently available immune-oncology drugs, including anti-PD1/PD-L1 drugs and anti-cancer chimeric T cells. Thus, targeting the adenosine receptor may overcome this blockade, leading to improved anti-cancer activity in tumors which are resistant to immune-oncology drugs and T cell therapies. We are conducting preclinical studies in order to support the initiation of a Phase 1 trial in the fourth quarter of 2022.
In our preclinical studies, our adenosine receptor inhibitor, NUV-1182, has high affinity for the A2a adenosine receptor demonstrating single digit nanomolar binding affinity. NUV-1182 was also designed to have reduced affinity for the adenosine A1 receptor which may potentially improve tolerability. Our preclinical studies demonstrate that NUV-1182 has a desirable pharmacokinetic profile, with high exposure following a single oral dose in mice. Furthermore, in a melanoma cell line derived xenograft model, daily oral dosing with NUV-1182 enhanced the tumor suppressive activity when combined with either an anti-PD1 antibody or an anti-PD-L1 antibody.
Intellectual Property
Our commercial success depends in large part on our ability to obtain and maintain patent protection in the U.S. and other countries for our investigational products, to operate without infringing valid and enforceable patents and proprietary rights of others, and to prevent others from infringing on our proprietary or intellectual property rights. We seek to protect our proprietary position by filing, in the U.S. and certain other countries, patent applications intended to cover the composition of matter of our investigational products, their methods of use and related discoveries, technologies, inventions and improvements that may be commercially important to our business. We may also rely on trade secrets and know-how to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection. We also intend to take advantage of regulatory protection afforded through data exclusivity, market exclusivity and patent term extensions where available.
We have investigational products in a number of therapeutic targets. As of December 31, 2020, our company-owned patent portfolio consists of two issued U.S. patents, over 15 pending U.S. patent applications, over 15 pending PCT patent applications, and over 40 pending foreign patent applications, in jurisdictions such as Australia, Brazil, Canada, Europe, China, Japan, India, Israel, New Zealand, Mexico, Singapore, South Africa, Republic of Korea, Hong Kong and Taiwan, directed to compositions of matter, methods of synthesis and methods of use related to our investigational products. Of these, four patent families are directed to investigational products in our CDK Program, three patent families are directed to investigational products in our DDC Program, five patent families are directed to investigational products in our BET Program, eight patent families are directed to investigational products in our Wee1 Program and eight patent families are directed to investigational products in our Adenosine Program. The term of any patents that issue from our company-owned U.S. and foreign patent applications will vary in accordance with the laws of each jurisdiction, but is typically 20 years from the earliest non-provisional filing date. Any patents that may issue in the future from our company-owned pending patent applications are projected to expire between 2038 and 2041, unless extended or otherwise adjusted.
The patent positions for biotechnology and pharmaceutical companies like us are generally uncertain and can involve complex legal, scientific and factual issues. Changes in either the patent laws or their interpretation in the U.S. and other countries may diminish our ability to protect our investigational products and enforce the patent rights that we own, and could affect the value of such intellectual property and the business. With respect to our company-owned intellectual property, we cannot guarantee that the patent applications we are currently pursuing or may file in the future will issue as patents in any particular jurisdiction or whether the claims of any issued patents will provide sufficient proprietary protection from competitors. Our competitors may independently develop similar investigational products or technologies that are outside the scope of the rights granted under any company-owned patents that may issue. We cannot be sure that any patents granted to us will be commercially useful in protecting our products or their methods of use or manufacture. Moreover, even issued patents do not guarantee us the right to commercialize our products. For example, third parties may have blocking patents that could be used to prevent us from commercializing or manufacturing our investigational products.
30
Table of Contents
Because of the extensive time required for development, testing and regulatory review of an investigational product, it is possible that, before a product can be commercialized, any patent protection for such product may expire or remain in force for only a short period following commercialization, thereby reducing the commercial advantage the patent provides. In the U.S., the term of a patent covering an FDA-approved product may, in certain cases, be eligible for a patent term extension under the Hatch-Waxman Act as compensation for the loss of patent term during the FDA regulatory review process. The period of extension may be up to five years, but cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval. Only one patent among those eligible for an extension may be extended and the amount of available extension to any PTE-eligible patent depends on a variety of factors, including the date on which the patent issues and certain dates related to the regulatory review period. Possible extensions may be available in Europe and in certain other jurisdictions to extend the term of a patent that covers an approved product. While we intend to seek patent term extensions in any jurisdictions where they are available to us, there is no guarantee that the applicable authorities, including the FDA or the USPTO, will agree with our assessment of whether such extensions should be granted, and even if granted, the length of such extensions.
We cannot be sure that any patents will issue from any pending or future company-owned patent applications. Even if patents do issue, we cannot be sure that the claims of these patents will be held valid or enforceable by a court of law or governmental agency, will provide us with any significant protection against competitive products, or will afford us a commercial advantage over competitive products. For example:
| • | we might not have been the first to file patent applications for the inventions covered by our pending patent applications and any patents that issue therefrom; |
| • | others may independently develop similar or alternative technologies without infringing our intellectual property rights; |
| • | some or all of our pending patent applications may not result in issued patents or the claims that issue may be narrow in scope and not provide us with a competitive advantage; |
| • | any patents that issue from any of our pending patent applications may be challenged by a third party and invalidated; |
| • | any patents that issue from our pending patent applications may be subject to post-grant proceedings, oppositions or other administrative or court proceedings that may result in a reduction in their scope or their loss altogether; |
| • | we may not develop proprietary technologies or investigational products that are patentable; and |
| • | the patents of others may prevent us from discovering, developing or commercializing our investigational products. |
The defense and prosecution of intellectual property infringement suits, post-grant proceedings, oppositions and related legal and administrative proceedings are costly, time-consuming to pursue and divert resources. The outcome of these types of proceedings is uncertain and could significantly harm our business.
The development of our investigational products and the commercialization of any resulting drugs may be impacted by patents of other companies or by companies engaged in the development of competitive programs or those with significantly greater resources. This could result in the expenditure of significant legal fees and management resources.
31
Table of Contents
We also rely on trade secrets to protect our technology and product candidates, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are often difficult to protect, especially outside of the U.S. While we believe that we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors, partners and other advisors may unintentionally or willfully disclose our trade secrets to others, including competitors. Enforcing a claim that a third party illegally disclosed, obtained or is using our trade secrets would be expensive and time-consuming, and the outcome would be unpredictable. Even if we are able to maintain our trade secrets as confidential, our competitors may independently develop information that is equivalent or similar to our trade secrets.
Collaboration Agreement with SPARCBIO LLC
On January 21, 2019, we entered into a Collaboration Agreement with SPARCBIO LLC under which SPARCBIO LLC conducts drug discovery and development activities for our programs. We pay SPARCBIO LLC’s costs to conduct these activities, at least $5,000,000 per year, and we own all inventions and results. The Collaboration Agreement has a five-year term and may be terminated by either party for the other party’s uncured material breach. If SPARCBIO LLC terminates the Collaboration Agreement for our uncured material breach within the first three years, we remain obligated to pay the annual fee for the first three years. We may terminate the Collaboration Agreement without cause after the first three years.
Manufacturing and Supply
We do not own or operate, and currently have no plans to establish, any manufacturing facilities. We rely, and expect to continue to rely, on third parties for the manufacture of our investigational products for preclinical and clinical testing, as well as for commercial manufacture if any of our investigational products obtain marketing approval. We also rely, and expect to continue to rely, on third parties to package, label, store and distribute our investigational products, as well as for our commercial products if marketing approval is obtained. We believe that this strategy allows us to maintain a more efficient infrastructure by eliminating the need for us to invest in our own manufacturing facilities, equipment and personnel while also enabling us to focus our expertise and resources on the development of our investigational products.
To date, we have obtained APIs and drug product for our investigational products from single-source third-party CMOs. We are in the process of developing our supply chain for each of our investigational products and intend to put in place framework agreements under which CMOs will generally provide us with necessary quantities of API and drug product on a project-by-project basis based on our development needs, and which agreements will provide us with intellectual property rights necessary to conduct the business. We seek to use a different CMO for each investigational product and will consider further diversification of drug product and supply organizations as circumstances warrant. Overall, as we advance our investigational products through development, we will start by seeking multiple sources for raw materials and address other potential points in concern over time.
Commercialization
We intend to retain significant development and commercial rights to our investigational products and, if marketing approval is obtained, to commercialize our investigational products on our own, or potentially with a partner, in the U.S. and other regions. We intend to build the necessary infrastructure and sales, marketing and commercial product distribution capabilities for the U.S., and potentially other regions, following further advancement of our investigational products. Clinical data, the size of the addressable patient population and the size of the commercial infrastructure and manufacturing needs and economics related to the foregoing may all influence or alter our commercialization plans.
32
Table of Contents
Competition
The pharmaceutical and biotechnology industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. While we believe that our technology, development experience and scientific knowledge provide us with competitive advantages, we face potential competition from many different sources, including large pharmaceutical and biotechnology companies, academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for the research, development, manufacturing and commercialization of cancer therapies. Any investigational products that we successfully develop and commercialize will compete with new therapies that may become available in the future.
We compete in the segments of the pharmaceutical, biotechnology and other related markets that develop small molecules and drug conjugates as treatments for cancer patients. There are many other companies that have commercialized and/or are developing such treatments for cancer including large pharmaceutical and biotechnology companies, such as AstraZeneca plc, Bristol-Myers Squibb Company (“BMS”), Eli Lilly, Merck, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc. in partnership with Sanofi Genzyme (“Sanofi”) and Roche.
For our CDK2/4/6 inhibitor, we are aware of several clinical-stage CDK inhibitors being developed as monotherapy or in combination with other drugs, including, but not limited to, product candidates being developed by Adastra Pharmaceuticals Inc., Merck, Pfizer, Tiziana Life Sciences plc, Gan & Lee, Cyclacel, Onconova and G1 Therapeutics. In addition, CDK 4/6 inhibitors from Pfizer, Novartis Pharmaceuticals Corporation (Novartis) and Eli Lilly are commercially available for patients with breast cancer and are also in clinical trials for other types of cancer. G1 Therapeutics recently received approval with its CDK4/6 inhibitor to decrease the incidence of chemotherapy-induced myelosuppression extensive-stage small cell lung cancer.
For our BET inhibitor, we are aware of several clinical-stage BET inhibitors being developed for patients with hematological malignancies and solid tumors, including, but not limited to, product candidates from AbbVie, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Constellation Pharmaceuticals, Forma Therapeutics, GlaxoSmithKline, Incyte Corporation, Merck/Oncoethix, Orion Pharma, Plexxikon (part of Daiichi Sankyo), Resverlogix, Roche and Zenith Epigenetics (Newsoara Biopharma). To our knowledge, there is currently no commercially available BET inhibitor and the most advanced BET inhibitor is in a Phase 3 clinical trial. Some BET inhibitors are also developed for non-oncology indications.
For our Wee1 inhibitor, we are aware of several clinical-stage Wee1 inhibitors being developed for patients with hematological malignancies and solid tumors, including product candidates from AstraZeneca, Debiopharm (Almac Discovery) and Zentalis. To our knowledge, there is currently no commercially available Wee1 inhibitor and the most advanced Wee1 inhibitor is in Phase 2 development.
For our adenosine receptor antagonist, we are aware of several other clinical-stage adenosine antagonists being developed, including, but not limited to, product candidates from Arcus Biosciences, Inc./Gilead Sciences, Inc., AstraZeneca, Corvus Pharmaceuticals Inc, CStone Pharmaceuticals, Incyte Corporation, iTeos Therapeutics Inc., Palobiofarma/Novartis and Ryvu Therapeutics SA. To our knowledge, there is currently no adenosine receptor antagonist approved for the treatment of cancer and the most advanced adenosine receptor antagonist is in Phase 2 development.
Our DDC programs targeting hormone receptors in cancer cells apply to types of cancer that may depend on hormone receptors for their growth, such as ER+ mBC, prostate cancer and ovarian cancer. All of these tumors have commercially available therapies including therapies from AstraZeneca, Bayer, Clovis Oncology, Dendreon, Eli Lilly, GSK, Janssen Pharmaceutical Companies, Novartis, Pfizer, Roche and Sanofi. In addition, many new drug candidates are being developed as monotherapy or in combination with other drugs for these tumors, and the most advanced of these development programs are in Phase 3 and may lead to near-term regulatory approval and subsequent commercialization. These development programs include those of the companies named above as well as numerous others. Some of these drugs and drug candidates target hormone receptor pathways directly, while many others may affect cancer cell growth through different mechanisms of action.
Many of the companies against which we are competing or against which we may compete in the future have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved drugs than we do. Mergers and acquisitions in the pharmaceutical, biotechnology and diagnostic industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and enrolling subjects for our clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
33
Table of Contents
We could see a reduction or elimination of our commercial opportunity if our competitors develop and commercialize products that are safer or more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we or our collaborators may develop. Our competitors also may obtain FDA or foreign regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we or our collaborators are able to enter the market. The key competitive factors affecting the success of all of our investigational products, if approved, are likely to be their degree of efficacy, tolerability profile, convenience and price, the effectiveness of companion diagnostics (if required), the level of biosimilar or generic competition and the availability of reimbursement from government and other third-party payors.
Government Regulation
Government authorities in the U.S. at the federal, state and local level and in other countries regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of drug and biological products. Generally, before a new drug can be marketed, considerable data demonstrating its quality, safety and efficacy must be obtained, organized into a format specific for each regulatory authority, submitted for review and approved by the regulatory authority.
U.S. Drug Development
In the U.S., the FDA regulates drugs under the Food, Drug, and Cosmetic Act (“FDCA”). Drugs also are subject to other federal, state and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or post-market may subject an applicant to administrative or judicial sanctions. These sanctions could include, among other actions, the FDA’s refusal to approve pending applications, withdrawal of an approval, a clinical hold, untitled or warning letters, product recalls or market withdrawals, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement and civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on us.
Our product candidates are considered small molecule drugs and must be approved by the FDA through the new drug application (“NDA”), process before they may be legally marketed in the U.S. The process generally involves the following:
| • | completion of extensive preclinical studies in accordance with applicable regulations, including studies conducted in accordance with GLP; |
| • | submission to the FDA of an IND, which must become effective before human clinical trials may begin; |
| • | approval by an independent IRB or ethics committee at each clinical trial site before each trial may be initiated; |
34
Table of Contents
| • | performance of adequate and well controlled human clinical trials in accordance with applicable IND regulations, GCP requirements and other clinical trial-related protocols and regulations to establish substantial evidence of the safety and efficacy of the investigational product for each proposed indication; |
| • | submission to the FDA of an NDA after completion of all pivotal trials; |
| • | determination by the FDA within 60 days of its receipt of an NDA to accept the filing for substantive review; |
| • | satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities where the drug will be produced to assess compliance with cGMP requirements to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; |
| • | potential FDA audit of the preclinical study and/or clinical trial sites that generated the data in support of the NDA filing; |
| • | FDA review and approval of the NDA, including consideration of the views of any FDA advisory committee, prior to any commercial marketing or sale of the drug in the U.S.; and |
| • | compliance with any post-approval requirements, including the potential requirement to implement a REMS and the potential requirement to conduct post-approval studies. |
The data required to support an NDA are generated in two distinct developmental stages: preclinical and clinical. The preclinical and clinical testing and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for any current and future product candidates will be granted on a timely basis, or at all.
Preclinical Studies and IND
The preclinical developmental stage generally involves laboratory evaluations of drug chemistry, formulation and stability, as well as studies to evaluate toxicity in animals, which support subsequent clinical testing. The sponsor must submit the results of the preclinical studies, together with manufacturing information, analytical data, any available clinical data or literature and a proposed clinical protocol, to the FDA as part of the IND. An IND is a request for authorization from the FDA to administer an investigational product to humans and must become effective before human clinical trials may begin.
Preclinical studies include laboratory evaluation of product chemistry and formulation, as well as in vitro and animal studies to assess the potential for adverse events and in some cases to establish a rationale for therapeutic use. The conduct of preclinical studies is subject to federal regulations and requirements, including GLP regulations for safety/toxicology studies. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and plans for clinical studies, among other things, to the FDA as part of an IND. Some long-term preclinical testing, such as animal tests of reproductive adverse events and carcinogenicity, may continue after the IND is submitted. An IND automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to one or more proposed clinical trials and places the trial on clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. As a result, submission of an IND may not result in the FDA allowing clinical trials to commence.
Clinical Trials
The clinical-stage of development involves the administration of the investigational product to healthy volunteers or patients under the supervision of qualified investigators, generally physicians not employed by or under the trial sponsor’s control, in accordance with GCP requirements, which include the requirement that all research subjects provide their informed consent for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria and the parameters to be used to monitor subject safety and assess efficacy. Each protocol, and any subsequent amendments to the protocol, must be submitted to the FDA as part of the IND. Furthermore, each clinical trial must be reviewed and approved by an IRB for each institution at which the clinical trial will be conducted to ensure that the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB must also approve the informed consent form that must be provided to each clinical trial subject or his or her legal representative and must monitor the clinical trial until completed. There also are requirements governing the reporting of ongoing clinical trials and completed clinical trial results to public registries.
35
Table of Contents
A sponsor who wishes to conduct a clinical trial outside of the U.S. may, but need not, obtain FDA authorization to conduct the clinical trial under an IND. If a foreign clinical trial is not conducted under an IND, the sponsor may submit data from the clinical trial to the FDA in support of an NDA. The FDA will generally accept a well designed and well conducted foreign clinical trial not conducted under an IND if the clinical trial is conducted in compliance with GCP and. the FDA is able to validate the data through an onsite inspection, if deemed necessary. An NDA based solely on foreign clinical data meeting U.S. criteria for marketing approval may be approved if (1) the foreign data are applicable to the U.S. population and U.S. medical practice, (2) the studies have been performed by clinical investigators of recognized competence and (3) the FDA is able to validate the data through an onsite inspection or other appropriate means, if deemed necessary
Clinical trials in the U.S. generally are conducted in three sequential phases, known as Phase 1, Phase 2 and Phase 3, and may overlap.
| • | Phase 1 clinical trials generally involve a small number of healthy volunteers or disease-affected patients who are initially exposed to a single dose and then multiple doses of the product candidate. The primary purpose of these clinical trials is to assess the metabolism, pharmacologic action, tolerability and safety of the drug. |
| • | Phase 2 clinical trials involve studies in disease-affected patients to determine the dose and dosing schedule required to produce the desired benefits. At the same time, safety and further pharmacokinetic and pharmacodynamic information is collected, possible adverse effects and safety risks are identified, and a preliminary evaluation of efficacy is conducted. |
| • | Phase 3 clinical trials generally involve a large number of patients at multiple sites and are designed to provide the data necessary to demonstrate the effectiveness of the product for its intended use, its safety in use and to establish the overall benefit/risk relationship of the product and provide an adequate basis for product approval. These trials may include comparisons with placebo and/or other comparator treatments. The duration of treatment is often extended to mimic the actual use of a product during marketing. |
Post-approval trials, sometimes referred to as Phase 4 clinical trials, are conducted after initial marketing approval. These trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication. In certain instances, the FDA may mandate the performance of Phase 4 clinical trials as a condition of approval of an NDA.
Progress reports detailing the results of the clinical trials, among other information, must be submitted at least annually to the FDA. Sponsor is also responsible for submitting written IND safety reports, including reports of serious and unexpected suspected adverse events, findings from other studies suggesting a significant risk to humans exposed to the drug, findings from animal or in vitro testing that suggest a significant risk for human subjects, and any clinically significant increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure.
36
Table of Contents
Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, if at all. The FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients. Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group provides authorization for whether a trial may move forward at designated check-points based on access to certain data from the trial.
Concurrent with clinical trials, companies usually complete additional animal safety studies and also must develop additional information about the chemistry and physical characteristics of the drug as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process, as performed by the manufacturing facility, must be capable of consistently producing quality batches of our product candidates. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that our product candidates do not undergo unacceptable deterioration over their labeled shelf life.
We may be required to develop and implement additional clinical trial policies and procedures designed to help protect subjects from the COVID-19 virus. For example, in March 2020, the FDA issued a guidance, which the FDA subsequently updated, on conducting clinical trials during the pandemic, which describes a number of considerations for sponsors of clinical trials impacted by the pandemic, including the requirement to include in the clinical trial report contingency measures implemented to manage the clinical trial, and any disruption of the clinical trial as a result of the COVID-19 pandemic; a list of all subjects affected by the COVID-19-pandemic related study disruption by unique subject identifier and by investigational site and a description of how the individual’s participation was altered; and analyses and corresponding discussions that address the impact of implemented contingency measures (e.g., participant discontinuation from investigational product and/or study, alternative procedures used to collect critical safety and/or efficacy data) on the safety and efficacy results reported for the clinical trial. In June 2020, FDA also issued a guidance on good manufacturing practice considerations for responding to COVID-19 infection in employees in drug products manufacturing, including recommendations for manufacturing controls to prevent contamination of drugs.
NDA Review Process
Following completion of the clinical trials, data is analyzed to assess whether the investigational product is safe and effective for the proposed indicated use or uses. The results of preclinical studies and clinical trials are then submitted to the FDA as part of an NDA, along with proposed labeling, chemistry and manufacturing information to ensure product quality and other relevant data. In short, the NDA is a request for approval to market the drug in the U.S. for one or more specified indications and must contain proof of safety and efficacy for a drug.
The application must include both negative and ambiguous results of preclinical studies and clinical trials, as well as positive findings. Data may come from company-sponsored clinical trials intended to test the safety and efficacy of a product’s use or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and efficacy of the investigational product to the satisfaction of FDA. FDA approval of an NDA must be obtained before a drug may be legally marketed in the U.S.
Under the Prescription Drug User Fee Act (“PDUFA”), as amended, each NDA must be accompanied by a user fee. FDA adjusts the PDUFA user fees on an annual basis. PDUFA also imposes an annual program fee for each marketed human drug. Fee waivers or reductions are available in certain circumstances, including a waiver of the application fee for the first application filed by a small business. Additionally, no user fees are assessed on NDAs for products designated as orphan drugs, unless the product also includes a non-orphan indication.
37
Table of Contents
The FDA reviews all submitted NDAs before it accepts them for filing and may request additional information rather than accepting the NDA for filing. The FDA must make a decision on accepting an NDA for filing within 60 days of receipt. Once the submission is accepted for filing, the FDA begins an in-depth review of the NDA. Under the goals and policies agreed to by the FDA under PDUFA, the FDA has 10 months, from the filing date, in which to complete its initial review of a new molecular-entity NDA and respond to the applicant, and six months from the filing date of a new molecular-entity NDA designated for priority review. The FDA does not always meet its PDUFA goal dates for standard and priority NDAs, and the review process is often extended by FDA requests for additional information or clarification.
Before approving an NDA, the FDA will conduct a pre-approval inspection of the manufacturing facilities for the new product to determine whether they comply with cGMP requirements. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. The FDA also may audit data from clinical trials to ensure compliance with GCP requirements. Additionally, the FDA may refer applications for novel drug products or drug products which present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions, if any. The FDA is not bound by recommendations of an advisory committee, but it considers such recommendations when making decisions on approval. The FDA likely will reanalyze the clinical trial data, which could result in extensive discussions between the FDA and the applicant during the review process. After the FDA evaluates an NDA, it will issue an approval letter or a Complete Response Letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. A Complete Response Letter indicates that the review cycle of the application is complete, and the application will not be approved in its present form. A Complete Response Letter usually describes all of the specific deficiencies in the NDA identified by the FDA. The Complete Response Letter may require additional clinical data, additional pivotal Phase 3 clinical trial(s) and/or other significant and time-consuming requirements related to clinical trials, preclinical studies and/or manufacturing. If a Complete Response Letter is issued, the applicant may either resubmit the NDA, addressing all of the deficiencies identified in the letter, or withdraw the application. Even if such data and information are submitted, the FDA may decide that the NDA does not satisfy the criteria for approval. Data obtained from clinical trials are not always conclusive and the FDA may interpret data differently than we interpret the same data.
Orphan Drugs
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biological product intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the U.S., or more than 200,000 individuals in the U.S. and for which there is no reasonable expectation that the cost of developing and making the product available in the U.S. for this type of disease or condition will be recovered from sales of the product.
Orphan drug designation must be requested before submitting an NDA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan drug exclusivity, which means that the FDA may not approve any other applications to market the same drug for the same indication for seven years from the date of such approval, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity by means of greater effectiveness, greater safety or providing a major contribution to patient care or in instances of drug supply issues. However, competitors may receive approval of either a different product for the same indication or the same product for a different indication but that could be used off-label in the orphan indication. Orphan drug exclusivity also could block the approval of one of our product candidates for seven years if a competitor obtains approval before we do for the same product, as defined by the FDA, for the same indication we are seeking approval, or if a product candidate is determined to be contained within the scope of the competitor’s product for the same indication. If one of our product candidates designated as an orphan drug receives marketing approval for an indication broader than that which is designated, it may not be entitled to orphan drug exclusivity. Orphan drug status in the European Union has similar, but not identical, requirements and benefits.
38
Table of Contents
Expedited Development and Review Programs
The FDA has a fast track program that is intended to expedite or facilitate the process for reviewing new drugs that meet certain criteria. Specifically, new drugs are eligible for fast track designation if they are intended to treat a serious or life-threatening condition and preclinical or clinical data demonstrate the potential to address unmet medical needs for the condition. Fast track designation applies to both the product and the specific indication for which it is being studied. The sponsor can request the FDA to designate the product for fast track status any time before receiving NDA approval, but ideally no later than the pre-NDA meeting with the FDA.
Any product submitted to the FDA for marketing, including under a fast track program, may be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. Any product is eligible for priority review if it treats a serious or life-threatening condition and, if approved, would provide a significant improvement in safety and effectiveness compared to available therapies.
A product may also be eligible for accelerated approval, if it treats a serious or life-threatening condition and generally provides a meaningful advantage over available therapies. In addition, it must demonstrate an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality (“IMM”), which is reasonably likely to predict an effect on IMM or other clinical benefit. As a condition of approval, the FDA may require that a sponsor of a drug receiving accelerated approval perform adequate and well-controlled post-marketing clinical trials. FDA may withdraw drug approval or require changes to the labeled indication of the drug if confirmatory post-market trials fail to verify clinical benefit or do not demonstrate sufficient clinical benefit to justify the risks associated with the drug. If the FDA concludes that a drug shown to be effective can be safely used only if distribution or use is restricted, it may require such post-marketing restrictions as it deems necessary to assure safe use of the product.
Additionally, a drug may be eligible for designation as a breakthrough therapy if the product is intended, alone or in combination with one or more other drugs or biologics, to treat a serious or life-threatening condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over currently approved therapies on one or more clinically significant endpoints. The benefits of breakthrough therapy designation include the same benefits as fast track designation, plus intensive guidance from the FDA to ensure an efficient drug development program. Fast track designation, priority review, accelerated approval and breakthrough therapy designation do not change the standards for approval, but may expedite the development or approval process. Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened.
Post-approval Requirements
Following approval of a new product, the manufacturer and the approved product are subject to continuing regulation by the FDA, including, among other things, monitoring and record-keeping requirements, requirements to report adverse events and comply with promotion and advertising requirements, which include restrictions on promoting drugs for unapproved uses or patient populations, known as “off-label promotion,” and limitations on industry-sponsored scientific and educational activities. Although physicians may prescribe legally available drugs for off-label uses, manufacturers may not market or promote such uses. Prescription drug promotional materials must be submitted to the FDA in conjunction with their first use. Further, if there are any modifications to the drug, including changes in indications, labeling or manufacturing processes or facilities, the applicant may be required to submit and obtain FDA approval of a new NDA or NDA supplement, which may require the development of additional data or preclinical studies and clinical trials.
39
Table of Contents
The FDA may also place other conditions on approvals including the requirement for REMS, to assure the safe use of the product. A REMS could include medication guides, physician communication plans or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. Any of these limitations on approval or marketing could restrict the commercial promotion, distribution, prescription or dispensing of products. Product approvals may be withdrawn for non-compliance with regulatory standards or if problems occur following initial marketing.
The FDA may withdraw approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical studies to assess new safety risks or imposition of distribution restrictions or other restrictions under a REMS program. Other potential consequences include, among other things:
| • | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market, or product recalls; |
| • | fines, warning letters, or holds on post-approval clinical studies; |
| • | refusal of the FDA to approve pending applications or supplements to approved applications; |
| • | suspension or revocation of product approvals; |
| • | product seizure or detention; |
| • | refusal to permit the import or export of products; and |
| • | injunctions or the imposition of civil or criminal penalties. |
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
Other U.S. Regulatory Matters
Pharmaceutical manufacturers are subject to various healthcare laws, regulation, and enforcement by the federal government and by authorities in the states and foreign jurisdictions in which they conduct their business. Our conduct, including those of our employees, as well as our business operations and relationships with third parties, including current and future arrangements with healthcare providers, third-party payors, customers, and others may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations, which may constrain the business or financial arrangements and relationships through which we research, as well as, sell, market, and distribute any products for which we obtain marketing approval. The applicable federal, state and foreign healthcare laws and regulations that may affect our ability to operate include, but are not limited to:
| • | The federal Anti-Kickback Statute, which makes it illegal for any person or entity, including a prescription drug manufacturer (or a party acting on its behalf), to knowingly and willfully solicit, receive, offer or pay any remuneration that is intended to induce or reward referrals, including the purchase, recommendation, order or prescription of a particular drug, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. Moreover, the Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act. |
40
Table of Contents
| • | The federal false claims, including the civil False Claims Act that can be enforced by private citizens through civil whistleblower or qui tam actions, and civil monetary penalties law prohibit individuals or entities from, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government. |
| • | HIPAA prohibits, among other things, executing or attempting to execute a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters. |
| • | HIPAA, as amended by HITECH, and their implementing regulations also impose obligations on covered entities such as health insurance plans, healthcare clearinghouses, and certain healthcare providers and their respective business associates and their covered subcontractors, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information. |
| • | The federal Physician Payments Sunshine Act requires applicable manufacturers of covered drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to annually report to CMS information regarding certain payments and other transfers of value to physicians, as defined by such law, and teaching hospitals as well as information regarding ownership and investment interests held by physicians and their immediate family members; additionally, the Substance Use-Disorder Prevention that Promoted Opioid Recovery and Treatment for Patients and Communities Act, under the provision titled “Fighting the Opioid Epidemic with Sunshine,” in part, extends the reporting and transparency requirements for physicians under the Physician Payments Sunshine Act to physician assistants, nurse practitioners, and other mid-level practitioners, with reporting requirements going into effect in 2022 for payments made, or ownership and investment interests held, in 2021. |
| • | Analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, state laws that require biotechnology companies to comply with the biotechnology industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government; state and local laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures and require the registration of their sales representatives, state laws that require biotechnology companies to report information on the pricing of certain drug products, and state and foreign laws that govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Pricing and rebate programs must also comply with the Medicaid rebate requirements of the U.S. Omnibus Budget Reconciliation Act of 1990 and more recent requirements in the Affordable Care Act. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. Manufacturing, sales, promotion and other activities also are potentially subject to federal and state consumer protection and unfair competition laws. In addition, the distribution of pharmaceutical products is subject to additional requirements and regulations, including extensive record-keeping, licensing, storage and security requirements intended to prevent the unauthorized sale of pharmaceutical products. Products must meet applicable child-resistant packaging requirements under the U.S. Poison Prevention Packaging Act as well as other applicable consumer safety requirements.
41
Table of Contents
The failure to comply with any of these laws or regulatory requirements subjects firms to possible legal or regulatory action. Depending on the circumstances, failure to meet applicable regulatory requirements can result in significant civil, criminal and administrative penalties, including damages, fines, disgorgement, imprisonment, exclusion from participation in government funded healthcare programs, such as Medicare and Medicaid, integrity oversight and reporting obligations, contractual damages, reputational harm, diminished profits and future earnings, injunctions, requests for recall, seizure of products, total or partial suspension of production, denial or withdrawal of product approvals or refusal to allow a firm to enter into supply contracts, including government contracts.
U.S. Patent-Term Restoration and Marketing Exclusivity
Depending upon the timing, duration and specifics of FDA approval of any future product candidates, some of our U.S. patents, if issued, may be eligible for limited patent term extension under the Hatch-Waxman Act. The Hatch-Waxman Act permits restoration of the patent term of up to five years as compensation for patent term lost during product development and FDA regulatory review process. Patent-term restoration, however, cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent-term restoration period is generally one-half the time between the effective date of an IND or the issue date of the patent, whichever is later, and the submission date of an NDA plus the time between the submission date of an NDA or the issue date of the patent, whichever is later, and the approval of that application, except that the review period is reduced by any time during which the applicant failed to exercise due diligence. Only one patent applicable to an approved drug is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent. The USPTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we may apply for restoration of patent term for our currently owned or licensed patents to add patent life beyond its current expiration date, depending on the expected length of the clinical trials and other factors involved in the filing of the relevant NDA.
Market exclusivity provisions under the FDCA also can delay the submission or the approval of certain applications. The FDCA provides a five-year period of non-patent marketing exclusivity within the U.S. to the first applicant to gain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an abbreviated new drug application (“ANDA”), or a 505(b)(2) NDA submitted by another company for a generic version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FDCA also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, new indications, dosages or strengths of an existing drug. This three-year exclusivity covers only the conditions of use associated with the new clinical investigations and does not prohibit the FDA from approving ANDAs for drugs containing the original active agent. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness or generate such data themselves.
European Union Drug Development
Similar to the United States, the various phases of preclinical and clinical research in the European Union are subject to significant regulatory controls. Although the EU Clinical Trials Directive 2001/20/EC has sought to harmonize the EU clinical trials regulatory framework, setting out common rules for the control and authorization of clinical trials in the EU, the EU Member States have transposed and applied the provisions of the Directive differently. This has led to significant variations in the member state regimes. Under the current regime, before a clinical trial can be initiated, it must be approved in each of the EU countries where the trial is to be conducted by two distinct bodies: the National Competent Authority (“NCA”), and one or more Ethics Committees (“ECs”). Under the current regime all suspected unexpected serious adverse reactions to the investigated drug that occur during the clinical trial have to be reported to the NCA and ECs of the Member State where they occurred.
42
Table of Contents
The EU clinical trials legislation currently is undergoing a transition process mainly aimed at harmonizing and streamlining clinical-trial authorization, simplifying adverse-event reporting procedures, improving the supervision of clinical trials and increasing their transparency. Recently enacted Clinical Trials Regulation EU No 536/2014 ensures that the rules for conducting clinical trials in the EU will be identical. In the meantime, Clinical Trials Directive 2001/20/EC continues to govern all clinical trials performed in the EU.
European Union Drug Review and Approval
In the European Economic Area (“EEA”), which comprises the 28 Member States of the European Union and three European Free Trade Association States (Norway, Iceland and Liechtenstein), medicinal products can only be commercialized after obtaining a Marketing Authorization (“MA”). There are two types of MAs.
| • | The Community MA is issued by the European Commission through the Centralized Procedure, based on the opinion of the Committee for Medicinal Products for Human Use, of the EMA, and is valid throughout the entire territory of the EEA. The Centralized Procedure is mandatory for certain types of products, such as biotechnology medicinal products, orphan medicinal products, advanced-therapy medicines such as gene-therapy, somatic cell-therapy or tissue-engineered medicines and medicinal products containing a new active substance indicated for the treatment of HIV, AIDS, cancer, neurodegenerative disorders, diabetes, auto-immune and other immune dysfunctions and viral diseases. The Centralized Procedure is optional for products containing a new active substance not yet authorized in the EEA, or for products that constitute a significant therapeutic, scientific or technical innovation or which are in the interest of public health in the EU. |
| • | National MAs, which are issued by the competent authorities of the Member States of the EEA and only cover their respective territory, are available for products not falling within the mandatory scope of the Centralized Procedure. Where a product has already been authorized for marketing in a Member State of the EEA, this National MA can be recognized in another Member States through the Mutual Recognition Procedure. If the product has not received a National MA in any Member State at the time of application, it can be approved simultaneously in various Member States through the Decentralized Procedure. Under the Decentralized Procedure an identical dossier is submitted to the competent authorities of each of the Member States in which the MA is sought, one of which is selected by the applicant as the Reference Member State (“RMS”). The competent authority of the RMS prepares a draft assessment report, a draft summary of the product characteristics (“SOPC”), and a draft of the labeling and package leaflet, which are sent to the other Member States (referred to as the Member States Concerned) for their approval. If the Member States Concerned raise no objections, based on a potential serious risk to public health, to the assessment, SOPC, labeling or packaging proposed by the RMS, the product is subsequently granted a national MA in all the Member States (i.e., in the RMS and the Member States Concerned). |
Under the above-described procedures, before granting the MA, EMA or the competent authorities of the Member States of the EEA make an assessment of the risk-benefit balance of the product on the basis of scientific criteria concerning its quality, safety and efficacy. Similar to the U.S. patent term-restoration, Supplementary Protection Certificates (“SPCs”) serve as an extension to a patent right in Europe for up to five years. SPCs apply to specific pharmaceutical products to offset the loss of patent protection due to the lengthy testing and clinical trials these products require prior to obtaining regulatory marketing approval.
Coverage and Reimbursement
Sales of our products, if approved, will depend, in part, on the extent to which our products will be covered by third-party payors, such as government health programs, commercial insurance and managed healthcare organizations. There is significant uncertainty related to third-party payor coverage and reimbursement of newly approved products. In the U.S., for example, principal decisions about reimbursement for new products are typically made by CMS. CMS decides whether and to what extent a new product will be covered and reimbursed under Medicare, and private third-party payors often follow CMS’s decisions regarding coverage and reimbursement to a substantial degree. However, no uniform policy of coverage and reimbursement for drug products exists. Accordingly, decisions regarding the extent of coverage and amount of reimbursement to be provided for any of our products will be made on a payor-by-payor basis.
43
Table of Contents
Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. Further, such payors are increasingly challenging the price, examining the medical necessity and reviewing the cost effectiveness of medical product candidates. There may be especially significant delays in obtaining coverage and reimbursement for newly approved drugs. Third-party payors may limit coverage to specific product candidates on an approved list, known as a formulary, which might not include all FDA-approved drugs for a particular indication. We may need to conduct expensive pharmacoeconomic studies to demonstrate the medical necessity and cost effectiveness of our products. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be obtained. Additionally, coverage policies and third party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (“MMA”), established the Medicare Part D program to provide a voluntary prescription drug benefit to Medicare beneficiaries. Under Part D, Medicare beneficiaries may enroll in prescription drug plans offered by private entities that provide coverage of outpatient prescription drugs. Unlike Medicare Part A and B, Part D coverage is not standardized. While all Medicare drug plans must give at least a standard level of coverage set by Medicare, Part D prescription drug plan sponsors are not required to pay for all covered Part D drugs, and each drug plan can develop its own drug formulary that identifies which drugs it will cover and at what tier or level. However, Part D prescription drug formularies must include drugs within each therapeutic category and class of covered Part D drugs, though not necessarily all the drugs in each category or class. Any formulary used by a Part D prescription drug plan must be developed and reviewed by a pharmacy and therapeutic committee. Government payment for some of the costs of prescription drugs may increase demand for products for which we receive marketing approval. However, any negotiated prices for our products covered by a Part D prescription drug plan likely will be lower than the prices we might otherwise obtain. Moreover, while the MMA applies only to drug benefits for Medicare beneficiaries, private third-party payors often follow Medicare coverage policy and payment limitations in setting their own payment rates.
In addition, in case a drug product needs companion diagnostics, then companion diagnostic tests require coverage and reimbursement separate and apart from the coverage and reimbursement for their companion pharmaceutical or biological products. Similar challenges to obtaining coverage and reimbursement, applicable to pharmaceutical or biological products, will apply to companion diagnostics.
In addition, in most foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing and reimbursement vary widely from country to country. For example, the European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, products launched in the European Union do not follow price structures of the U.S. and generally prices tend to be significantly lower.
44
Table of Contents
Healthcare Reform
The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost containment programs to limit the growth of government-paid healthcare costs, including price-controls, restrictions on reimbursement and requirements for substitution of generic products for branded prescription drugs. For example, the Affordable Care Act substantially changed the way healthcare is financed by both the government and private insurers, and continues to significantly impact the U.S. pharmaceutical industry. The Affordable Care Act contains provisions that may reduce the profitability of drug products through increased rebates for drugs reimbursed by Medicaid programs, extension of Medicaid rebates to Medicaid managed care plans, mandatory discounts for certain Medicare Part D beneficiaries and annual fees based on pharmaceutical companies’ share of sales to federal healthcare programs. The Medicaid Drug Rebate Program requires pharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the HHS Secretary as a condition for states to receive federal matching funds for the manufacturer’s outpatient drugs furnished to Medicaid patients. The Affordable Care Act made several changes to the Medicaid Drug Rebate Program, including increasing pharmaceutical manufacturers’ rebate liability by raising the minimum basic Medicaid rebate on most branded prescription drugs from 15.1% of average manufacturer price (“AMP”), to 23.1% of AMP and adding a new rebate calculation for “line extensions” (i.e., new formulations, such as extended release formulations) of solid oral dosage forms of branded products, as well as potentially impacting their rebate liability by modifying the statutory definition of AMP. The Affordable Care Act also expanded the universe of Medicaid utilization subject to drug rebates by requiring pharmaceutical manufacturers to pay rebates on Medicaid managed care utilization and by enlarging the population potentially eligible for Medicaid drug benefits. Additionally, for a drug product to receive federal reimbursement under the Medicaid or Medicare Part B programs or to be sold directly to U.S. government agencies, the manufacturer must extend discounts to entities eligible to participate in the 340B drug pricing program. The required 340B discount on a given product is calculated based on the AMP and Medicaid rebate amounts reported by the manufacturer.
There remain judicial and Congressional challenges to certain aspects of the Affordable Care Act, as well as efforts by the administration to repeal or replace certain aspects of the Affordable Care Act. Since January 2017, there have been several executive orders and other directives designed to delay the implementation of certain provisions of the Affordable Care Act or otherwise circumvent some of the requirements for health insurance mandated by the Affordable Care Act. Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the Affordable Care Act. While Congress has not passed comprehensive repeal legislation, several bills affecting the implementation of certain taxes under the Affordable Care Act have passed. In 2017, the Tax Act repealed, effective January 1, 2019, the tax-based shared responsibility payment imposed by the Affordable Care Act on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” In addition, the 2020 federal spending package permanently eliminated, effective January 1, 2020, the Affordable Care Act’s mandated “Cadillac” tax on high-cost employer-sponsored health coverage and medical device tax and, effective January 1, 2021, also eliminates the health insurer tax. The Bipartisan Budget Act of 2018, among other things, amended the Affordable Care Act, effective January 1, 2019, to close the coverage gap in most Medicare Part D drug plans. In December 2018, CMS published a new final rule permitting further collections and payments to and from certain ACA-qualified health plans and health insurance issuers under the Affordable Care Act risk adjustment program in response to the outcome of federal district court litigation regarding the method CMS uses to determine this risk adjustment. In April 2020, the U.S. Supreme Court reversed a federal circuit decision that previously upheld Congress’ denial of $12.0 billion in “risk corridor” funding. In December 2018, a Texas U.S. District Court Judge ruled that the Affordable Care Act is unconstitutional in its entirety because the “individual mandate” was repealed by Congress as part of the Tax Act. Additionally, in December 2019, the U.S Court of Appeals for the Fifth Circuit upheld the District Court ruling that the individual mandate was unconstitutional and remanded the case back to the District Court to determine whether the remaining provisions of the Affordable Care Act are invalid as well. In March 2020, the U.S. Supreme Court granted the petitions for writs of certiorari to review this case. It is unclear how such litigation and other efforts to repeal and replace the Affordable Care Act will impact the Affordable Care Act and our business. We will continue to evaluate the effect that the Affordable Care Act and its possible repeal and replacement has on our business. Complying with any new legislation, resulting in a material adverse effect on our business.
45
Table of Contents
Other legislative changes have been proposed and adopted in the U.S. since the Affordable Care Act was enacted. These changes included aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, effective April 1, 2013, which, due to subsequent legislative amendments, will stay in effect through 2030 unless additional congressional action is taken. The CARES Act, which was signed into law in March 2020, and designed to provide financial support and resources to individuals and businesses affected by COVID-19 pandemic, suspended the 2% Medicare sequester from May 1, 2020, through December 31, 2020, and extended the sequester by one year, through 2030, in order to offset the added expense of the 2020 suspension. The American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on customers for our drugs, if approved, and accordingly, our financial operations.
Additionally, there has been heightened governmental scrutiny recently over the manner in which drug manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs and reform government program reimbursement methodologies for drug products. For example, at the federal level, the administration’s budget proposals for fiscal year 2021 includes a $135.0 billion allowance to support legislative proposals seeking to reduce drug prices, increase competition, lower out-of-pocket drug costs for patients, and increase patient access to lower-cost generic and biosimilar drugs. On March 10, 2020, the administration sent “principles” for drug pricing to Congress, calling for legislation that would, among other things, cap Medicare Part D beneficiary out-of-pocket pharmacy expenses, provide an option to cap Medicare Part D beneficiary monthly out-of-pocket expenses, and place limits on pharmaceutical price increases. Additionally, the administration previously released a “Blueprint” to lower drug prices and reduce out of pocket costs of drugs that contained proposals to increase manufacturer competition, increase the negotiating power of certain federal healthcare programs, incentivize manufacturers to lower the list price of their products and reduce the out of pocket costs of drug products paid by consumers. Although a number of these and other measures may require additional authorization to become effective, Congress and the administration have each indicated that it will continue to seek new legislative and/or administrative measures to control drug costs. For example, on July 24, 2020, the administration announced four executive orders to lower drug prices, including allowing importation of certain drugs, changing how drug rebates are negotiated by middlemen, like pharmacy benefit managers, and directing such rebates to be passed to patients as point-of-sale discounts, and requiring Medicare to pay certain Part B drugs at the lowest price available in economically comparable countries (the details of which were released on September 13, 2020 and also expanded the policy to cover certain Part D drugs). The president has delayed the effective date of the international drug pricing order, pending discussion with major drug companies. How these executive orders will be implemented and their impact on the industry remain uncertain. Additionally, the FDA recently released a final rule, effective November 30, 2020, implementing a portion of the importation executive order providing guidance for states to build and submit importation plans for drugs from Canada. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. It is possible that additional governmental action is taken in response to the ongoing COVID-19 pandemic, which may impact our business. We are unable to predict the future course of federal or state healthcare legislation in the U.S. directed at broadening the availability of healthcare and containing or lowering the cost of healthcare. These and any further changes in the law or regulatory framework that reduce our revenue or increase our costs could also have a material and adverse effect on our business, financial condition and results of operations.
46
Table of Contents
Facilities
Our principal executive office is located in New York, New York, where we lease approximately 7,900 square feet of office space under a lease that terminates in 2027. We also occupy approximately 8,200 square feet of office space in San Francisco, California, under a lease that terminates in 2022. We believe that these existing facilities will be adequate for our current needs and that suitable additional or alternative space will be available in the future on commercially reasonable terms, if required.
Human Capital
As of February 28, 2021, we had 36 employees, all of whom were full-time and 15 of whom were engaged in research and development activities. Of these employees, 20 are located in New York, New York, 14 are located in San Francisco, California and two are located in Massachusetts. None of our employees are represented by labor unions or covered by collective bargaining agreements. We consider our relationship with our employees to be good.
We recognize that attracting, motivating and retaining talent at all levels is vital to our continued success. Our employees are a significant asset and we aim to create an environment that is equitable, inclusive and representative in which our employees can grow and advance their careers, with the overall goal of developing, expanding and retaining our workforce to support our current pipeline and future business goals. By focusing on employee retention and engagement, we also improve our ability to support our clinical-stage platform, business and operations, and also protect the long-term interests of our securityholders. Our success also depends on our ability to attract, engage and retain a diverse group of employees. Our efforts to recruit and retain a diverse and passionate workforce include providing competitive compensation and benefits packages and ensuring we listen to our employees.
We value agility, passion and teamwork, and are building a diverse environment where our employees can thrive and one that inspires exceptional contributions and professional and personal development in order to achieve our mission to significantly change the practice of oncology. Our human capital resources objectives include, as applicable, identifying, recruiting, retaining, incentivizing and integrating our existing and new employees, advisors and consultants. The principal purposes of our equity and cash incentive plans are to attract, retain and reward personnel through the granting of stock-based and cash-based compensation awards, in order to increase stockholder value and the success of our company by motivating such individuals to perform to the best of their abilities and achieve our objectives. We are committed to providing a competitive and comprehensive benefits package to our employees. Our benefits package provides a balance of protection along with the flexibility to meet the individual health and wellness needs of our employees.
We plan to continue to develop our efforts related to attracting, retaining and motivating our workforce as we grow and develop and hire more employees.
Legal Proceedings
From time to time, we may become involved in legal proceedings or be subject to claims arising in the ordinary course of our business. We are not currently a party to any material legal proceedings. Regardless of outcome, such proceedings or claims can have an adverse impact on us because of defense and settlement costs, diversion of resources and other factors, and there can be no assurances that favorable outcomes will be obtained.
Available Information
We were incorporated in Delaware in April 2020 as a blank check company under the name Panacea Acquisition Corp. On February 10, 2021, Nuvation Bio and Panacea consummated the transactions contemplated under the Business Combination Agreement, following the approval at a special meeting of our stockholders. In connection with the closing of the Business Combination, we changed our name to Nuvation Bio Inc.
We file electronically with the U.S. Securities and Exchange Commission, or SEC, our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended. We make available on our website at www.nuvationbio.com, free of charge, copies of these reports as soon as reasonably practicable after filing these reports with, or furnishing them to, the SEC.
| Item 1A. | Risk Factors. |
Our business and investing in our securities involve significant risks, some of which are described below. Before you make a decision to buy our securities, in addition to the risks and uncertainties discussed in the section titled “Cautionary Information Regarding Forward-Looking Statements,” you should carefully consider the risks and uncertainties described below together with all of the other information contained in this Annual Report on Form 10-K, including our financial statements and related notes and in the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” The occurrence of any of the events or developments described in the following risk factors and the risks described elsewhere in this report could harm our business, financial condition, results of operations, cash flows, the trading price of our common stock and our growth prospects. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business operations. This report on Form 10-K also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of factors that are described in the following risk factors and the risks described elsewhere in this report.
47
Table of Contents
Risks Related to Our Financial Position and Need for Additional Capital
We have a limited operating history and have incurred significant losses since inception and anticipate that we may continue to incur losses for the foreseeable future, and may never achieve or maintain profitability.
Biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. We are an oncology company with a limited operating history upon which you can evaluate our business and prospects. All of our development programs are in preclinical development or in the drug discovery stage. We commenced operations in 2018, and to date, we have focused primarily on organizing and staffing our company, business planning, raising capital, identifying product candidates, establishing our intellectual property portfolio and conducting research, preclinical studies and clinical trials. Our approach to the discovery and development of product candidates is unproven, and we do not know whether we will be able to develop any product candidates that succeed in clinical development or products of commercial value. As an organization, we have not yet completed any clinical trials, obtained regulatory approvals, manufactured a commercial-scale product (or arranged for a third party to do so on our behalf), or conducted sales and marketing activities necessary for successful product commercialization. Consequently, any predictions made about our future success or viability may not be as accurate as they could be if we had a history of successfully developing and commercializing biopharmaceutical products.
Since inception, we have not generated any product revenue and have incurred significant operating losses. Our net losses were $33.6 million and $41.7 million in 2019 and 2020, respectively. As of December 31, 2020, we had an accumulated deficit of $76.0 million. We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future. Since inception, we have devoted substantially all of our efforts to research and preclinical and clinical development of our product candidates, as well as to building our management team and infrastructure. It could be at least several years, if ever, before we have a commercialized drug. The net losses we incur may fluctuate significantly from quarter to quarter and year to year. We anticipate that our expenses will increase substantially if, and as, we:
| • | continue to advance our research and preclinical and clinical development of our product candidates; |
| • | expand and initiate further clinical trials for our product candidates; |
| • | seek to identify additional product candidates; |
| • | seek marketing approvals for our product candidates that successfully complete clinical trials, if any; |
| • | establish a sales, marketing and distribution infrastructure to commercialize any products for which we may obtain marketing approval; |
| • | maintain, expand, protect and enforce our intellectual property portfolio and obtain licenses to third-party intellectual property; |
| • | attract, hire and retain additional administrative, clinical, regulatory and scientific personnel; |
48
Table of Contents
| • | enter into third-party relationships for clinical trials, manufacturing and supply; and |
| • | incur additional legal, accounting and other expenses in operating our business, including the additional costs associated with operating as a public company. |
In addition, because of the numerous risks and uncertainties associated with pharmaceutical products and development, we are unable to accurately predict the timing or amount of increased expenses and when, or if, we will be able to achieve profitability. Our expenses could increase and profitability could be further delayed if we decide to or are required by the U.S. Food and Drug Administration (“FDA”) or other regulatory authorities such as the European Medicines Agency (“EMA”), or the U.K. Medicines & Healthcare Products Regulatory Agency (the “MHRA”), to perform studies or trials in addition to those currently expected, or if there are any delays in the development or completion of any current or future preclinical studies or clinical trials of our current and future product candidates. Even if we complete the development and regulatory processes described above, we anticipate incurring significant costs associated with launching and commercializing our current and future product candidates.
Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would decrease our value and could impair our ability to raise capital, maintain our research and development efforts, expand our business or continue our operations. A decline in value also could cause you to lose all or part of your investment.
We will need substantial funding to pursue our business objectives. If we are unable to raise capital when needed or on favorable terms, we could be forced to delay, reduce or terminate our product development, other operations or commercialization efforts.
Identifying and developing potential product candidates and conducting preclinical studies and clinical trials is a time-consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain regulatory approval and begin selling any approved products. We expect our expenses to increase in connection with our ongoing activities, particularly as we conduct our ongoing and planned preclinical studies and clinical trials, initiate additional clinical trials for our product candidates and seek regulatory approval for our current product candidates and any future product candidates we may develop. Our expenses could increase beyond our current expectations if the FDA requires us to perform clinical trials and other studies in addition to those that we currently anticipate. In addition, if we obtain marketing approval for any of our product candidates, we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing and distribution. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we will be forced to delay, reduce or terminate our research and development programs or future commercialization efforts.
As of December 31, 2020, we had $215.8 million in cash and investments, and an accumulated deficit of $76.0 million. Based upon our current operating plan, we believe that our existing cash, cash equivalents and short-term investments, including the net proceeds from the PIPE investment, will be sufficient to fund our operations for at least the 12 months following the closing of the merger. This estimate is based on assumptions that may prove to be wrong, and we could use our available capital resources sooner than we expect. Changes may occur beyond our control that would cause the us to consume our available capital before that time, including changes in and progress of our development activities and changes in regulation. Our future capital requirements will depend on many factors, including:
| • | the scope, rate of progress, results and costs of drug discovery, preclinical development, laboratory testing and clinical trials for our product candidates; |
| • | the number and development requirements of product candidates that we may pursue, and other indications for our current product candidates that we may pursue; |
| • | the costs, timing and outcome of regulatory review of our product candidates; |
49
Table of Contents
| • | the scope and costs of manufacturing development and commercial manufacturing activities; |
| • | the cost associated with commercializing any approved product candidates; |
| • | the cost and timing of developing our ability to establish sales and marketing capabilities, if any; |
| • | the costs of preparing, filing and prosecuting patent applications, maintaining, enforcing and protecting our intellectual property rights, defending intellectual property-related claims and obtaining licenses to third-party intellectual property; |
| • | our ability to establish and maintain collaborations on favorable terms, if at all; and |
| • | the extent to which we acquire or in-license other product candidates and technologies and associated intellectual property. |
We may require additional capital to complete our planned clinical development programs for our lead product candidate NUV-422 and our other product candidates to obtain regulatory approval. Any additional capital raising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our current and future product candidates, if approved.
In addition, we cannot guarantee that future financing will be available on a timely basis, in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may adversely affect the holdings or the rights of our stockholders and our issuance of additional securities, whether equity or debt, or the market perception that such issuances are likely to occur, could cause the market price of our common stock to decline. If we are unable to obtain funding on a timely basis on acceptable terms, we may be required to delay, reduce or terminate one or more of our research and development programs or the commercialization of any product candidates that may be approved. This could harm our business and could potentially cause us to cease operations.
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish proprietary rights.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, collaborations, strategic alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
If we raise additional funds through collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, reduce or terminate our product development or future commercialization efforts or grant rights to third parties to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
We might not be able to utilize a significant portion of our net operating loss carryforwards.
As of December 31, 2020, we had federal and state net operating loss, (“NOL”), carryforwards of $19.4 million and $12.9 million, respectively. Under the Tax Cuts and Jobs Act (the “Tax Act”), as modified by the Coronavirus Aid, Relief and Economic Security Act (the “CARES Act”), federal NOLs incurred in taxable years beginning after December 31, 2017 and in future taxable years may be carried forward indefinitely, but the deductibility of such federal net operating losses in taxable years beginning after December 31, 2020 is limited to 80% of taxable income. It is uncertain if and to what extent various states will conform to the Tax Act or the CARES Act.
50
Table of Contents
Separately, under Section 382 of the Internal Revenue Code of 1986, as amended, or the Internal Revenue Code, and corresponding provisions of state law, if a corporation undergoes an “ownership change,” which is generally defined as a greater than 50% change, by value, in its equity ownership over a three-year period, the corporation’s ability to use its pre-change NOL carryforwards and other pre-change tax attributes, such as research tax credits, to offset its post-change income or taxes may be limited. The completion of this merger, together with private placements and other transactions that have occurred since our inception, may trigger such an ownership change pursuant to Section 382. We have not completed a Section 382 analysis, and therefore, there can be no assurances that the NOLs are not already limited.
We may experience ownership changes as a result of subsequent shifts in our stock ownership, some of which may be outside of our control. As a result, if we earn net taxable income, our ability to use our prechange NOL carryforwards to offset U.S. federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us. In addition, at the state level, there may be periods during which the use of NOL carryforwards is suspended or otherwise limited such as recent California legislation limiting the usability of NOLs for tax years beginning in 2020 and before 2023, which could accelerate or permanently increase state taxes owed. If an ownership change occurs and our ability to use our net operating loss carryforwards is materially limited, we would harm our future operating results by effectively increasing our future tax obligations.
Risks Related to the Development of our Product Candidates
If we do not obtain regulatory approval for and successfully commercialize our product candidates in one or more indications or we experience significant delays in doing so, we may never generate any revenue or become profitable.
We do not have any products that have received regulatory approval and may never be able to develop marketable product candidates. We are very early in our development efforts. We have invested substantially all of our efforts in developing and identifying potential product candidates and conducting preclinical studies. We expect that a substantial portion of our efforts and expenses over the next several years will be devoted to the development of NUV-422 in our current and planned clinical trials in patients for the treatment of high-grade gliomas, as well as the development of our other product candidates. As a result, our business currently depends heavily on the successful development, regulatory approval and, if approved, commercialization of NUV-422. We cannot be certain that NUV-422 or any other product candidate will receive regulatory approval or will be successfully commercialized even if we receive regulatory approval. The research, testing, manufacturing, safety, efficacy, labeling, approval, sale, marketing and distribution of product candidates is, and will remain, subject to comprehensive regulation by the FDA and similar foreign regulatory authorities. Before obtaining regulatory approvals for the commercial sale of any product candidate, we must demonstrate through preclinical studies and clinical trials that the product candidate is safe and effective for use in each target indication. Drug development is a long, expensive and uncertain process, and delay or failure can occur at any stage of any of our preclinical studies or clinical trials. Failure to obtain regulatory approval for our product candidates will prevent us from commercializing and marketing our product candidates. The success of our product candidates will depend on several additional factors, including:
| • | successful completion of preclinical studies; |
| • | successful initiation of clinical trials; |
| • | successful patient enrollment in, and completion, of clinical trials that demonstrate their safety and efficacy; |
| • | receiving marketing approvals from applicable regulatory authorities; |
| • | obtaining, maintaining, protecting and enforcing patent, trade secret and other intellectual property rights and regulatory exclusivity for our product candidates; |
| • | completing any post-marketing studies required by applicable regulatory authorities; |
51
Table of Contents
| • | making and maintaining arrangements with third-party manufacturers, or establishing manufacturing capabilities, for both clinical and commercial supplies of our product candidates; |
| • | establishing sales, marketing and distribution capabilities and successfully launching commercial sales of our products, if and when approved, whether alone or in collaboration with others; |
| • | the prevalence and severity of adverse events experienced with our product candidates; |
| • | acceptance of our product candidates by patients, the medical community and third-party payors; |
| • | a continued acceptable safety profile following approval; |
| • | obtaining and maintaining healthcare coverage and adequate reimbursement for our product candidates; |
| • | competing effectively with other cancer therapies, including with respect to the sales and marketing of our product candidates, if approved; and |
| • | obtaining licenses to any third-party intellectual property we deem necessary or desirable. |
Many of these factors are beyond our control, including the time needed to adequately complete preclinical studies, clinical testing and the regulatory submission process, our ability to obtain and protect intellectual property rights and changes in the competitive landscape. It is possible that none of our product candidates will ever obtain regulatory approval, even if we expend substantial time and resources seeking such approval. If we do not achieve one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully complete clinical trials, obtain regulatory approval or, if approved, commercialize our product candidates, which would materially harm our business, financial condition, results of operations and prospects.
In addition, the clinical trial requirements of the FDA, the EMA, the MHRA and other regulatory agencies and the criteria these regulators may use to determine the safety and efficacy of a product candidate vary substantially according to the type, complexity, novelty and intended use and market of the potential products. The regulatory approval process for novel product candidates can be more expensive and take longer than for other, better known or extensively studied pharmaceutical or other product candidates.
Our approach to the discovery and development of product candidates based on our DDC platform is unproven, and we do not know whether we will be able to develop any products of commercial value, or if competing technological approaches will limit the commercial value of our product candidates or render our platform obsolete.
The success of our business depends in part upon our ability to identify, develop and commercialize products based on our proprietary Drug-Drug Conjugate (“DDC”) platform, which leverages a novel and unproven therapeutic approach within the drug-conjugate class of anti-cancer therapies. While we have had favorable preclinical study results based on our technology, we have not yet succeeded and may not succeed in demonstrating safety and efficacy for any product candidates in clinical trials or in obtaining marketing approval thereafter. Our product candidates arising from our DDC platform are in pre-clinical development and we have not yet completed any clinical trials for any such product candidate. Our research methodology and novel approach to oncology using our DDC platform may be unsuccessful in identifying additional product candidates, and any product candidates based on our technology may be shown to have harmful side effects or may have other characteristics that may necessitate additional clinical testing, or make the product candidates unmarketable or unlikely to receive marketing approval. In addition, adverse developments with respect to one of our DDC platform-based programs may have a significant adverse impact on the actual or perceived likelihood of success and value of similar programs.
In addition, the biotechnology and biopharmaceutical industries are characterized by rapidly advancing technologies. Our future success will depend in part on our ability to maintain a competitive position with our DDC platform. If we fail to stay at the forefront of technological change in utilizing our DDC platform to create and develop product candidates, we may be unable to compete effectively. Our competitors may render our DDC platform obsolete, or limit the commercial value of our product candidates, by advances in existing technological approaches or the development of new or different approaches, potentially eliminating the advantages in our drug discovery process that we believe we derive from our research approach and proprietary technologies. By contrast, adverse developments with respect to other companies that attempt to use a similar approach to our approach may adversely impact the actual or perceived value of our DDC platform and potential of our DDC platform-based product candidates. If any of these events occur, we may be forced to abandon our development efforts for a program or programs, which would harm our business.
52
Table of Contents
Our DDC platform-based product candidates are based on a novel technology, which makes it difficult to predict the time and cost of product candidate development.
We have concentrated our product research and development efforts on our novel DDC platform, and our future success depends in part on the successful development product candidates arising from our DDC platform. There can be no assurance that any development problems we may experience in the future related to our DDC platform will not cause significant delays or unanticipated costs, or that such development problems can be efficiently solved. We may also experience delays in developing a sustainable, reproducible and scalable manufacturing process or transferring that process to commercial partners, which may prevent us from completing our clinical trials or commercializing our product candidates on a timely or profitable basis, if at all.
We may in the future develop product candidates in combination with other therapies and that may expose us to additional risks.
We may develop future product candidates for use in combination with one or more currently approved cancer therapies. Even if any product candidate we develop was to receive marketing approval or be commercialized for use in combination with other existing therapies, we would continue to be subject to the risks that the FDA or similar foreign regulatory authorities could revoke approval of the therapy used in combination with our product candidate or that safety, efficacy, manufacturing or supply issues could arise with these existing therapies. Combination therapies are commonly used for the treatment of cancer, and we would be subject to similar risks if we develop any of our product candidates for use in combination with other drugs or for indications other than cancer. This could result in our own products being removed from the market or being less successful commercially.
We may also evaluate our product candidates in combination with one or more other cancer therapies that have not yet been approved for marketing by the FDA or similar foreign regulatory authorities. We will not be able to market and sell our product candidates we develop in combination with any such unapproved cancer therapies that do not ultimately obtain marketing approval.
If the FDA or similar foreign regulatory authorities do not approve these other drugs or revoke their approval of, or if safety, efficacy, manufacturing or supply issues arise with, the drugs we choose to evaluate in combination with our product candidates we may be unable to obtain approval of or market our product candidates.
Clinical trials are very expensive, time-consuming and difficult to design and implement, and involve uncertain outcomes. Furthermore, results of earlier preclinical studies and clinical trials may not be predictive of results of future preclinical studies or clinical trials.
The risk of failure for our product candidates is high. It is impossible to predict when or if any of our product candidates will prove effective or safe or effective in humans or will receive regulatory approval. To obtain the requisite regulatory approvals to market and sell any of our product candidates, we must demonstrate through extensive preclinical studies and clinical trials that our product candidates are safe and effective in
53
Table of Contents
humans for use in each target indication. Preclinical investigation and clinical testing is expensive and can take many years to complete, and the outcome is inherently uncertain. Failure can occur at any time during the preclinical investigation or clinical trial process.
In addition, the results of preclinical studies and earlier clinical trials may not be predictive of the results of later-stage preclinical studies or clinical trials. The results generated to date in preclinical studies for our product candidates do not ensure that later preclinical studies or clinical trials will demonstrate similar results. We have not generated any clinical data for any of our product candidates. Product candidates in later stages of clinical trials, although it has none at this stage as of yet, may fail to show the desired safety and efficacy traits despite having progressed through preclinical and earlier stage clinical trials. In later-stage clinical trials, we will likely be subject to more rigorous statistical analyses than in completed earlier stage clinical trials. A number of companies in the pharmaceutical industry have suffered significant setbacks in later-stage clinical trials due to adverse safety profiles or lack of efficacy, notwithstanding promising results in earlier trials, and we cannot be certain that we will not face similar setbacks. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their products.
In some instances, there can be significant variability in safety or efficacy results between different clinical trials of the same product candidate due to numerous factors, including changes in clinical trial procedures set forth in protocols, differences in the size and type of the patient populations, adherence to the dosing regimen and other clinical trial protocols, and the rate of dropout among clinical trial participants. If we fail to produce positive results in our planned preclinical studies or clinical trials of any of our product candidates, the development timeline and regulatory approval and commercialization prospects for our product candidates, and, correspondingly, our business and financial prospects, would be materially and adversely affected.
We may encounter substantial delays in our preclinical studies or clinical trials or we may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities.
Before obtaining marketing approval from regulatory authorities for the sale of our product candidates, we must conduct extensive clinical trials to demonstrate the safety and efficacy of the product candidate for its intended indications. Preclinical studies and clinical trials are expensive, time-consuming and uncertain as to outcome. We cannot guarantee that any preclinical studies or clinical trials will be conducted as planned or completed on schedule, if at all. A failure of one or more preclinical studies or clinical trials can occur at any stage of testing. Events that may prevent successful or timely completion of preclinical or clinical development include:
| • | delays in conducting experiments or preclinical studies or unsatisfactory results from such experiments or studies; |
| • | delays in reaching a consensus with regulatory authorities on trial design; |
| • | delays in reaching agreement or failing to agree on acceptable terms with prospective CROs and clinical trial sites; |
| • | delays in opening sites and recruiting suitable patients to participate in our clinical trials; |
| • | delays in enrollment due to travel or quarantine policies, or other factors, related to COVID-19, other pandemics or other events outside our control; |
| • | imposition of a clinical hold by regulatory authorities as a result of a serious adverse event, concerns with a class of product candidates or after an inspection of our clinical trial operations or trial sites; |
| • | delays in having patients complete participation in a trial or return for post-treatment follow-up; |
54
Table of Contents
| • | occurrence of serious adverse events associated with the product candidate that are viewed to outweigh its potential benefits; or |
| • | changes in regulatory requirements and guidance that require amending or submitting new clinical protocols. |
For instance, the ongoing COVID-19 pandemic and the measures taken by the governmental authorities could disrupt the supply chain and the manufacture or shipment of drug substances and finished drug products for our product candidates for use in our research and clinical trials, delay, limit or prevent our employees and CROs from continuing research and development activities, impede the ability of patients to enroll or continue in clinical trials, or impede testing, monitoring, data collection and analysis or other related activities, any of which could delay our clinical trials and increase our development costs, and have a material adverse effect on our business, financial condition and results of operations.
Any inability to timely and successfully complete preclinical and clinical development could result in additional costs to us or impair our ability to achieve regulatory and commercialization milestones. In addition, if we maks manufacturing or formulation changes to our product candidates, we may need to conduct additional testing to bridge our modified product candidate to earlier versions. Clinical trial delays could also shorten any periods during which we may have the exclusive right to commercialize our product candidates, if approved, or allow our competitors to bring comparable drugs to market before we do, which could impair our ability to successfully commercialize our product candidates and may harm our business, financial condition, results of operations and prospects.
Additionally, if the results of our clinical trials are inconclusive or if there are safety concerns or serious adverse events associated with our product candidates, we may:
| • | be delayed in obtaining marketing approval, if at all; |
| • | obtain approval for indications or patient populations that are not as broad as intended or desired; |
| • | obtain approval with labeling that includes significant use or distribution restrictions or safety warnings; |
| • | be subject to additional post-marketing testing requirements; |
| • | be required to perform additional clinical trials to support approval or be subject to additional post-marketing testing requirements; |
| • | have regulatory authorities withdraw, or suspend, their approval of the drug or impose restrictions on its distribution in the form of a modified risk evaluation and mitigation strategy, or REMS; |
| • | be subject to the addition of labeling statements, such as warnings or contraindications; |
| • | be sued; or |
| • | experience damage to our reputation. |
Our drug development costs will also increase if we experience delays in testing or obtaining marketing approvals. We do not know whether any of our preclinical studies or clinical trials will begin as planned, need to be restructured or be completed on schedule, if at all.
Further, we, the FDA or an institutional review board (“IRB”) may suspend our clinical trials at any time if it appears that we or our collaborators are failing to conduct a trial in accordance with regulatory requirements, including the FDA’s current Good Clinical Practice, (“GCP”), regulations, that we are exposing participants to unacceptable health risks or if the FDA finds deficiencies in our Investigational New Drug (“IND”) Applications, or INDs, or the conduct of these trials. Therefore, we cannot predict with any certainty the schedule for commencement and completion of future clinical trials. If we experience delays in the commencement or completion of our clinical trials, or if we terminate a clinical trial prior to completion, the commercial prospects of our product candidates could be negatively impacted, and our ability to generate revenues from our product candidates may be delayed or eliminated entirely.
55
Table of Contents
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we focus on research programs that we identify for specific indications. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications, even those that it has begun investigating and that may have shown promise, that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial therapies or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, it may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate.
If any of our product candidates receives marketing approval and we, or others, later discover that the drug is less effective than previously believed or causes undesirable side effects that were not previously identified, its ability to market the drug could be compromised.
Clinical trials of our product candidates are conducted in carefully defined subsets of patients who have agreed to enter into clinical trials. Consequently, it is possible that our clinical trials may indicate an apparent positive effect of a product candidate that is greater than the actual positive effect, if any, or alternatively fail to identify undesirable side effects. If one or more of our product candidates receives regulatory approval, and we, or others, later discover that they are less effective than previously believed, or cause undesirable side effects, a number of potentially significant negative consequences could result, including:
| • | withdrawal or limitation by regulatory authorities of approvals of such product; |
| • | seizure of the product by regulatory authorities; |
| • | recall of the product; |
| • | restrictions on the marketing of the product or the manufacturing process for any component thereof; |
| • | requirement by regulatory authorities of additional warnings on the label, such as a “black box” warning or contraindication; |
| • | requirements that we implement a REMS or create a medication guide outlining the risks of such side effects for distribution to patients; |
| • | commitment to expensive additional safety studies prior to approval or post-marketing studies required by regulatory authorities of such product; |
| • | adverse impact on the product’s competitiveness; |
| • | initiation of regulatory investigations and government enforcement actions; |
| • | initiation of legal action against us to hold us liable for harm caused to patients; and |
| • | harm to our reputation and resulting harm to physician or patient acceptance of our products. |
Any of these events could prevent us from achieving or maintaining market acceptance of the particular product candidate, if approved, and could harm our business, financial condition, results of operations and prospects.
56
Table of Contents
If we encounter difficulties enrolling patients in our clinical trials, our clinical development activities could be delayed or otherwise adversely affected.
We may experience difficulties in patient enrollment in our clinical trials for a variety of reasons, including challenges resulting from the ongoing COVID-19 pandemic. The timely completion of clinical trials in accordance with their protocols depends, among other things, on our ability to enroll a sufficient number of patients who remain in the study until its conclusion. The enrollment of patients depends on many factors, including:
| • | the patient eligibility criteria defined in the protocol; |
| • | the size and health of the patient population required for analysis of the trial’s primary endpoints; |
| • | the proximity of patients to study sites; |
| • | the design of the trial; |
| • | our ability to recruit clinical trial investigators with the appropriate competencies and experience; |
| • | clinicians’ and patients’ perceptions as to the potential advantages of the product candidate being studied in relation to other available therapies, including any new drugs that may be approved for the indications we are investigating; |
| • | our ability to obtain and maintain patient consents; and |
| • | the risk that patients enrolled in clinical trials will drop out of the trials before completion. |
In addition, our clinical trials will compete with other clinical trials for product candidates that are in the same therapeutic areas as our product candidates, and this competition will reduce the number and types of patients available to us, because some patients who might have opted to enroll in our trials may instead opt to enroll in a trial being conducted by one of our competitors. Since the number of qualified clinical investigators is limited, we expect to conduct some of our clinical trials at the same clinical trial sites that some of our competitors use, which will reduce the number of patients who are available for our clinical trials at such clinical trial site. Moreover, because our product candidates represent a departure from more commonly used methods for cancer treatment, potential patients and their doctors may be inclined to use conventional therapies rather than enroll patients in any future clinical trial.
Delays in patient enrollment may result in increased costs or may affect the timing or outcome of our current or planned clinical trials, which could prevent completion of these trials and adversely affect our ability to advance the development of our product candidates.
We may become exposed to costly and damaging liability claims, either when testing our product candidates in the clinic or at the commercial stage, and our product liability insurance may not cover all damages from such claims.
We are exposed to potential product liability and professional indemnity risks that are inherent in the research, development, manufacturing, marketing and use of pharmaceutical products. We currently have no products that have been approved for commercial sale. However, the current and future use of product candidates by us in clinical trials, and the sale of any approved products in the future, may expose us to liability claims. These claims might be made by patients who use the product, healthcare providers, pharmaceutical companies or others selling such products. Any claims against us, regardless of their merit, could be difficult and costly to defend or settle, and could compromise the market acceptance of our product candidates or any prospects for commercialization of our product candidates, if approved. For more information regarding the risks associated with intellectual property-related litigation, see “Risk Factors—Risks Related to Our Intellectual Property.”
Although the clinical trial process is designed to identify and assess potential side effects, it is always possible that a drug, even after regulatory approval, may exhibit unforeseen side effects. If any of our product candidates were to cause adverse side effects during clinical trials or after approval of the product candidate, we may be exposed to substantial liabilities. Physicians and patients may not comply with any warnings that identify known potential adverse effects and patients who should not use our product candidates.
57
Table of Contents
Although we maintain product liability insurance coverage, such insurance may not be adequate to cover all liabilities that we may incur. We may need to increase our insurance coverage each time we commence a clinical trial and if we successfully commercialize any product candidate. As the expense of insurance coverage is increasing, we may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise. If a successful product liability claim or series of claims is brought against us for uninsured liabilities or in excess of insured liabilities, our assets may not be sufficient to cover such claims and our business operations could be impaired.
Risks Related to Commercialization of Our Product Candidates
We have never commercialized a product candidate and we may lack the necessary expertise, personnel and resources to successfully commercialize any of our products that receive regulatory approval on our own or together with collaborators.
We have never commercialized a product candidate. Our operations to date have been limited to organizing and staffing our company, business planning, raising capital, and undertaking preclinical studies of our product candidates. We currently have no sales force, marketing, manufacturing or distribution capabilities. To achieve commercial success of our product candidates, if any are approved, we will have to develop our own sales, marketing and manufacturing capabilities or outsource these activities to a third party.
Factors that may affect our ability to commercialize our product candidates on our own include recruiting and retaining adequate numbers of effective sales and marketing personnel, persuading adequate numbers of physicians to prescribe our product candidates and other unforeseen costs associated with creating an independent sales and marketing organization. Developing a sales and marketing organization requires significant investment, is time-consuming and could delay the launch of our product candidates. We may not be able to build an effective sales and marketing organization in the U.S., the European Union or other key global markets. If we are unable to build our own distribution and marketing capabilities or to find suitable partners for the commercialization of our product candidates, we may have difficulties generating revenue from them.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of new drug products is highly competitive. We face competition with respect to our current product candidates, and will face competition with respect to any product candidates that we may seek to develop or commercialize in the future, from major pharmaceutical, specialty pharmaceutical and biotechnology companies among others. We compete in the segments of the pharmaceutical, biotechnology and other related markets that develop immunotherapies for the treatment of cancer. There are other companies working to develop immunotherapies for the treatment of cancer including divisions of large pharmaceutical and biotechnology companies of various sizes. Some of these competitive products and therapies are based on scientific approaches that are the same as or similar to our approach, and others are based on entirely different approaches. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
We are developing our initial product candidates for the treatment of cancer, and currently none of these therapies are approved. There are already a variety of available drug therapies marketed for cancer and some of the currently approved drug therapies are branded and subject to patent protection, and others are available on a generic basis. Many of these approved drugs are well established therapies and are widely accepted by physicians, patients and third-party payors. Insurers and other third-party payors may also encourage the use of generic products. We expect that if our product candidates are approved, they will be priced at a significant premium over competitive generic products. This may make it difficult for us to achieve our business strategy of replacing existing therapies with our product candidates.
58
Table of Contents
Our competitors may succeed in developing, acquiring or licensing, on an exclusive basis, products that are more effective or less costly than any product candidate that we may develop. In addition, most of these companies have substantially greater sales, marketing and other experience and reserves than we do. Competition may further increase as a result of advances in the commercial applicability of technologies for drug discovery and development and greater availability of capital for investment in cancer therapies.
Established pharmaceutical companies may invest heavily to accelerate discovery and development of novel compounds or to in-license novel compounds that could make our product candidates less competitive. In addition, any new product that competes with an approved product must demonstrate compelling advantages in efficacy, convenience, tolerability and safety in order to overcome price competition and to be commercially successful. Accordingly, our competitors may succeed in obtaining patent protection, discovering, developing, receiving FDA approval for or commercializing drugs before we do, which would have an adverse impact on our business and results of operations.
The availability of our competitors’ products could limit the demand and the price we are able to charge for any product candidate we commercialize, if any. The inability to compete with existing or subsequently introduced drugs would harm our business, financial condition, results of operations and prospects.
Even if any of our product candidates receive marketing approval, they may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success.
If NUV-422 and our other current and future product candidates receive marketing approval, whether as a single agent or in combination with other therapies, they may nonetheless fail to gain sufficient market acceptance by physicians, patients, third-party payors and others in the medical community. For example, current approved immunotherapies, and other cancer treatments like chemotherapy and radiation therapy, are well established in the medical community, and doctors may continue to rely on these therapies. If any of our product candidates do not achieve an adequate level of acceptance, we may not generate significant product revenues and we may never become profitable. The degree of market acceptance of our product candidates, if approved for commercial sale, will depend on a number of factors, including:
| • | efficacy and potential advantages compared to alternative treatments; |
| • | the ability to offer our products, if approved, for sale at competitive prices; |
| • | convenience and ease of administration compared to alternative treatments; |
| • | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
| • | the strength of marketing and distribution support; |
| • | sufficient third-party coverage or reimbursement, including of combination therapies; |
| • | the willingness of patients to pay all, or a portion of, out-of-pocket costs associated with our products in the absence of sufficient third-party coverage and adequate reimbursement; |
| • | adoption of a companion diagnostic or complementary diagnostic; and |
| • | the prevalence and severity of any side effects. |
59
Table of Contents
The successful commercialization of certain of our product candidates will depend in part on the extent to which governmental authorities and health insurers establish adequate coverage, reimbursement levels and pricing policies. Failure to obtain or maintain adequate coverage and reimbursement for our product candidates, if approved, could limit our ability to market those products and decrease our ability to generate revenue.
The availability and adequacy of coverage and reimbursement by governmental healthcare programs such as Medicare and Medicaid, private health insurers and other third-party payors are essential for most patients to be able to afford products such as our product candidates, if approved. Our ability to achieve acceptable levels of coverage and reimbursement for products by governmental authorities, private health insurers and other organizations will have an effect on our ability to successfully commercialize our product candidates and, if desired, attract collaboration partners to invest in the development of our product candidates. Coverage under certain government programs, such as Medicare, Medicaid, the 340B drug pricing program and TRICARE, may not be available for certain of our product candidates. Assuming we obtain coverage for a given product by a third-party payor, the resulting reimbursement payment rates may not be adequate or may require co-payments that patients find unacceptably high. We cannot be sure that coverage and reimbursement in the U.S., the European Union or elsewhere will be available for any product that we may develop, and any reimbursement that may become available may be decreased or eliminated in the future.
Third-party payors increasingly are challenging prices charged for pharmaceutical products and services, and many third-party payors may refuse to provide coverage and reimbursement for particular drugs when an equivalent generic drug, biosimilar or a less expensive therapy is available. It is possible that a third-party payor may consider our product candidates and other therapies as substitutable and only offer to reimburse patients for the less expensive product. Even if we show improved efficacy or improved convenience of administration with our product candidates, pricing of existing drugs may limit the amount we will be able to charge for our product candidates. These payors may deny or revoke the reimbursement status of a given product or establish prices for new or existing marketed products at levels that are too low to enable us to realize an appropriate return on our investment in product development. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize our product candidates, and may not be able to obtain a satisfactory financial return on products that we may develop.
There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products. In the U.S., third-party payors, including private and governmental payors, such as the Medicare and Medicaid programs, play an important role in determining the extent to which new drugs and biologics will be covered. The Medicare and Medicaid programs increasingly are used as models for how private payors and other governmental payors develop their coverage and reimbursement policies for drugs and biologics. Some third-party payors may require pre-approval of coverage for new or innovative devices or drug therapies before they will reimburse healthcare providers who use such therapies. It is difficult to predict at this time what third-party payors will decide with respect to the coverage and reimbursement for our product candidates.
Obtaining and maintaining reimbursement status is time-consuming and costly. No uniform policy for coverage and reimbursement for products exists among third-party payors in the U.S. Therefore, coverage and reimbursement for products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance. Furthermore, rules and regulations regarding reimbursement change frequently, in some cases at short notice, and we believe that changes in these rules and regulations are likely.
Moreover, increasing efforts by governmental and third-party payors in the U.S. and abroad to cap or reduce healthcare costs may cause such organizations to limit both coverage and the level of reimbursement for newly approved products and, as a result, they may not cover or provide adequate payment for our product candidates.
60
Table of Contents
We expect to experience pricing pressures in connection with the sale of any of our product candidates due to the trend toward managed healthcare, the increasing influence of health maintenance organizations, and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. The continuing efforts of the government, insurance companies, managed care organizations and other payors of health care services to contain or reduce costs of health care may adversely affect:
| • | the demand for any products for which we may obtain regulatory approval; |
| • | our ability to set a price that we believe is fair for our products; |
| • | our ability to obtain coverage and reimbursement approval for a product; |
| • | our ability to generate revenues and achieve or maintain profitability; and |
| • | the level of taxes that we are required to pay. |
Even if we receive marketing approval for any of our product candidates, we may not achieve market acceptance, which would limit the revenue that we can generate from sales of any of our product candidates if approved.
Even if the FDA approves the marketing of any product candidates that we develop, physicians, patients, third-party payors or the medical community may not accept or use them. Efforts to educate the medical community and third-party payors on the benefits of our product candidates may require significant resources and may not be successful. Market acceptance of NUV-422 and our other product candidates, if any are approved, will depend on a number of factors, including, among others:
| • | the ability of NUV-422 and our other product candidates to treat cancer, as compared with other available drugs, treatments or therapies; |
| • | the prevalence and severity of any adverse side effects associated with NUV-422 and our other product candidates; |
| • | limitations or warnings contained in the labeling approved for NUV-422 or our other product candidates by the FDA; |
| • | availability of alternative treatments; |
| • | the size of the target patient population, and the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
| • | the strength of marketing and distribution support and timing of market introduction of competitive products; |
| • | publicity for our product candidates and competing products and treatments; |
| • | pricing and cost effectiveness; |
| • | the effectiveness of our sales and marketing strategies; |
| • | our ability to increase awareness of our product candidates through marketing efforts; |
| • | our ability to obtain sufficient third-party coverage and adequate reimbursement; and |
| • | the likelihood that the FDA may impose additional requirements that limit the promotion, advertising, distribution or sales of our product candidates. |
If any one of our product candidates is approved but does not achieve an adequate level of acceptance by patients, physicians and third-party payors, we may not generate sufficient revenue to become or remain profitable and our business may be harmed.
61
Table of Contents
Even if we obtain regulatory approval for our product candidates, they will remain subject to ongoing regulatory oversight.
Even if we obtain regulatory approval for any of our product candidates, they will be subject to extensive and ongoing regulatory requirements for manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion, sampling and record-keeping. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with current good manufacturing practices, (“cGMP”), regulations and GCPs, for any clinical trials that we conduct post-approval, all of which may result in significant expense and limit our ability to commercialize such products. In addition, any regulatory approvals that we receive for our product candidates may also be subject to limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor the safety and efficacy of the product candidate. The FDA may also require a REMS as a condition of approval of our product candidates, which could include requirements for a medication guide, physician communication plans or additional elements to ensure safe use, such as restricted distribution methods, patient registries and other risk minimization tools.
The FDA’s and other regulatory authorities’ policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the U.S. or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability. Moreover, if there are changes in the application of legislation or regulatory policies, or if problems are discovered with a product or our manufacture of a product, or if we or one of our distributors, licensees or co-marketers fails to comply with regulatory requirements, the regulators could take various actions. These include:
| • | issuing warning or untitled letters; |
| • | seeking an injunction or imposing civil or criminal penalties or monetary fines; |
| • | suspension or imposition of restrictions on operations, including product manufacturing; |
| • | seizure or detention of products, refusal to permit the import or export of products or request that we initiate a product recall; |
| • | suspension or withdrawal of our marketing authorizations; |
| • | suspension of any ongoing clinical trials; |
| • | refusal to approve pending applications or supplements to applications submitted by us; or |
| • | requiring us to conduct additional clinical trials, change our product labeling or submit additional applications for marketing authorization. |
If any of these events occurs, our ability to sell such product may be impaired, and we may incur substantial additional expense to comply with regulatory requirements, which could harm our business, financial condition, results of operations and prospects.
62
Table of Contents
If any of our product candidates are approved for marketing and commercialization and we are unable to establish sales and marketing capabilities or enter into agreements with third parties to sell and market our product candidates, we will be unable to successfully commercialize our product candidates if and when they are approved.
We have no sales, marketing or distribution capabilities or experience. To achieve commercial success for any approved product for which we retain sales and marketing responsibilities, we must either develop a sales and marketing organization, which would be expensive and time consuming, or outsource these functions to other third parties. In the future, we may choose to build a focused sales and marketing infrastructure to sell, or participate in sales activities with our collaborators for, some of our product candidates if and when they are approved.
There are risks involved with both establishing our own sales and marketing capabilities and entering into arrangements with third parties to perform these services. For example, recruiting and training a sales force is expensive and time consuming and could delay any product launch. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
Factors that may inhibit our efforts to commercialize our product candidates on our own include:
| • | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
| • | the inability of sales personnel to obtain access to physicians or persuade adequate numbers of physicians to prescribe any future product candidates; |
| • | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
| • | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
If we enter into arrangements with third parties to perform sales, marketing and distribution services, our product revenues or the profitability of these product revenues to us are likely to be lower than if we were to market and sell any products that we develop ourselves. In addition, we may not be successful in entering into arrangements with third parties to sell and market our product candidates or may be unable to do so on terms that are favorable to us. In entering into third-party marketing or distribution arrangements, any revenue we receive will depend upon the efforts of the third parties and we cannot assure you that such third parties will establish adequate sales and distribution capabilities or devote the necessary resources and attention to sell and market our product candidates effectively. If we do not establish sales and marketing capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates.
Risks Related to Our Dependence on Third Parties
We rely on third parties to perform the chemistry work associated with our drug discovery and preclinical activities and to conduct our preclinical studies and future clinical trials, and our business could be substantially harmed if these third parties cease performing services or perform in an unsatisfactory manner.
We do not have any laboratory facilities and has relied to date on a single third party, SPARCBIO LLC, which itself subcontracts certain of our activities to Integral BioSciences private Limited (“IBS”), to perform substantially all of the medicinal chemistry work associated with our drug discovery activities. Scientists employed by IBS and SPARCBIO LLC are named inventors on many of the patent applications covering our product candidates and have assigned their inventions to us as called for by a five-year Collaboration Agreement entered into between our company and SPARCBIO LLC in January 2019 and predecessor agreements entered into in April 2018 and February 2017. We have only limited control over IBS’s and SPARCBIO LLC’s performance of the activities SPARCBIO LLC is required to perform under the Collaboration Agreement.
We also do not currently have the ability to independently conduct preclinical studies or clinical trials without outside assistance. We have relied on CROs to conduct all of our preclinical studies to date and intends to conduct our future clinical trials by leveraging expertise and assistance from CROs as appropriate. We plan to rely upon medical institutions, clinical investigators, contract laboratories and other third parties, such as CROs, to conduct or assist us in conducting GCP-compliant clinical trials on our product candidates properly and on time, and may not currently have all of the necessary contractual relationships in place to do so. Once we have established contractual relationships with such third-party CROs, we will have only limited control over their actual performance of these activities.
63
Table of Contents
We and our CROs and other vendors are required to comply with cGMP, GCP, and good laboratory practice (“GLP”), which are regulations and guidelines enforced by the FDA, the Competent Authorities of the Member States of the European Union and any comparable foreign regulatory authorities for all of our product candidates in preclinical and clinical development. Regulatory authorities enforce these regulations through periodic inspections of trial sponsors, principal investigators, clinical trial sites and other contractors. Although we will rely on CROs to conduct any current or planned GLP-compliant preclinical studies and GCP-compliant clinical trials and has limited influence over their actual performance, we remain responsible for ensuring that each of our preclinical studies and clinical trials is conducted in accordance with our investigational plan and protocol and applicable laws and regulations, and our reliance on the CROs does not relieve us of our regulatory responsibilities. If we or any of our CROs or vendors fail to comply with applicable regulations, the data generated in our preclinical studies and clinical trials may be deemed unreliable and the FDA, EMA, MHRA or any comparable foreign regulatory agency may require us to perform additional preclinical studies and clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory agency, such regulatory agency will determine that all of our clinical trials comply with GCP regulations. In addition, our clinical trials must be conducted with products produced under cGMP requirements. Our failure to comply with these requirements may require us to repeat clinical trials, which would delay the regulatory approval process.
While we or our subcontractors have or will have agreements governing their activities, IBS, SPARCBIO LLC and our other CROs are contractors, and we will not be able to control whether or not they devote sufficient time and resources to our future chemistry work and preclinical and clinical programs. These CROs may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials or other chemistry or drug discovery or development activities. We face the risk of potential unauthorized disclosure, infringement, misappropriation or other violation of our intellectual property by CROs, which may reduce our trade secret protection and allow our potential competitors, and other third parties, to access and exploit our proprietary technology. CROs also may use our proprietary information and intellectual property in such a way as to invite litigation or other intellectual property-related proceedings that could jeopardize or invalidate our proprietary information and intellectual property. If SPARCBIO LLC or other CROs do not successfully carry out their contractual duties or obligations, fail to meet expected deadlines or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for any other reason, our clinical trials or other drug discovery or development activities may be extended, delayed or terminated, the clinical data generated in our clinical trials may be deemed unreliable, and we may not be able to obtain regulatory approval for, or successfully commercialize any product candidate that we develop. As a result, our financial results and the commercial prospects for any product candidate that we develop would be harmed, our costs could increase, and our ability to generate revenue could be delayed.
If our relationships with SPARCBIO LLC or other CROs were to terminate, we might not be able to enter into arrangements with alternative CROs or do so on commercially reasonable terms. Switching or adding additional CROs involves substantial cost and requires management time and focus, and could delay the discovery, development and commercialization of our product candidates. In addition, there is a natural transition period when a new CRO commences work. As a result, delays occur, which can negatively impact our ability to meet our desired clinical development timelines. Though we intend to carefully manage our relationships with our CROs, there can be no assurance that we will not encounter challenges or delays in the future or that these delays or challenges will not have a negative impact on our business and financial condition.
64
Table of Contents
We do not have our own manufacturing capabilities and will rely on third parties to produce clinical and commercial supplies of NUV-422 and our other current and future product candidates.
We have limited experience in drug formulation and manufacturing and do not own or operate, and we do not expect to own or operate, facilities for drug manufacturing, storage, distribution or testing. To date, we have obtained active pharmaceutical ingredients (“APIs”) and drug product for our investigational products from single-source third-party CMOs. We are in the process of developing our supply chain for each of our investigational products and intend to put in place framework agreements under which CMOs will generally provide us with necessary quantities of API and drug product on a project-by-project basis based on our development needs. We seek to use a different CMO for each investigational product and will consider further diversification of drug product and supply organizations as circumstances warrant.
Third-party CMOs may be unable or unwilling to supply us with sufficient clinical and commercial grade quantities of our clinical materials due to production shortages or other supply interruptions resulting from the ongoing COVID-19 pandemic or otherwise, because they are purchased by one of our competitors or another company that decides not to continue supplying us with these materials, or for other reasons. If one or more of these events occur and we are unable to timely establish an alternate supply from one or more third-party CMOs, we could experience delays in our development efforts as we locate and qualify new manufacturers. Under such circumstances, we may be required to receive drug substance for use on a purchase order basis, and as such, there can be no assurance that we actually receive sufficient quantities. See also the risk factor titled “—Our business, operations and clinical development plans and timelines and supply chain could be adversely affected by the effects of health epidemics, including the ongoing COVID-19 pandemic, on the manufacturing, clinical trial and other business activities performed by us or by third parties with whom we conduct business, including our CMOs, CROs, shippers and others.”
Further, our reliance on third-party manufacturers exposes us to risks beyond our control, including the risk of:
| • | inability to meet our product specifications and quality requirements consistently; |
| • | delay or inability to procure or expand sufficient manufacturing capacity; |
| • | manufacturing and quality issues, including related to scale-up of manufacturing; |
| • | costs and validation of new equipment and facilities required for additional scale-up; |
| • | failure of the manufacturer to comply with cGMP and similar foreign standards; |
| • | inability to negotiate manufacturing agreements with third parties on commercially reasonable terms; |
| • | termination or nonrenewal of manufacturing agreements with third parties in a manner or at a time that is costly or damaging to us; |
| • | reliance on a limited number of sources, and in some cases, single sources for components, such that if we are unable to secure a sufficient supply of these drug components, we will be unable to manufacture and sell NUV-422 or other product candidates in a timely fashion, in sufficient quantities or under acceptable terms; |
| • | lack of qualified backup suppliers for those components that are currently purchased from a sole or single source supplier; |
| • | operations of our third-party manufacturers or suppliers could be disrupted by conditions unrelated to our business or operations, including the bankruptcy of the manufacturer or supplier or the issuance of a FDA Form 483 notice or warning letter; |
| • | carrier disruptions or increased costs that are beyond our control; and |
| • | failure to deliver our products under specified storage conditions and in a timely manner. |
65
Table of Contents
Some of these events could be the basis for FDA action, including injunction, recall, seizure or total or partial suspension of production. In addition, our third-party manufacturers and suppliers are subject to FDA inspection from time to time. Failure by our third-party manufacturers and suppliers to pass such inspections and otherwise satisfactorily complete the FDA approval regimen with respect to our product candidate may result in regulatory actions such as the issuance of FDA Form 483 notices of observations, warning letters or injunctions or the loss of operating licenses. In addition, our third-party manufacturers and suppliers are subject to numerous environmental, health and safety laws and regulations, including those governing the handling, use, storage, treatment and disposal of waste products, and failure to comply with such laws and regulations could result in significant costs associated with civil or criminal fines and penalties for such third parties. Based on the severity of the regulatory action, our clinical or commercial supply of drug and packaging and other services could be interrupted or limited, which could harm our business.
In addition, our CMOs are or may be engaged with other companies to supply and manufacture materials or products for such companies, which also exposes our suppliers and manufacturers to regulatory risks for the production of such materials and products. As a result, failure to meet the regulatory requirements for the production of those materials and products may also affect the regulatory clearance of a contract supplier’s or manufacturer’s facility. If the FDA or a comparable foreign regulatory agency does not approve these facilities for the supply or manufacture of our product candidates, or if it withdraws its approval in the future, we may need to find alternative supply or manufacturing facilities, which would negatively impact our ability to develop, obtain regulatory approval of or market our product candidates, if approved.
Any of these events could lead to clinical trial delays, failure to obtain regulatory approval or impact our ability to successfully commercialize any potential future product candidates. If we are not able to establish collaborations, we may have to alter some of our future development and commercialization plans.
As we prepare for later-stage clinical trials and potential commercialization, we will need to take steps to increase the scale of production of our product candidates, which may include transferring production to new third-party suppliers or manufacturers. In order to conduct larger or late-stage scale clinical trials for our product candidates and supply sufficient commercial quantities of the resulting drug product and our components, if that product candidate is approved for sale, our CMOs and suppliers will need to produce our product candidates in larger quantities, more cost effectively and, in certain cases, at higher yields than they currently achieve. These third-party contractors may not be able to successfully increase the manufacturing capacity for any such product candidates in a timely or cost-effective manner or at all. Significant scale up of manufacturing may require additional processes, technologies and validation studies, which are costly, may not be successful and which the FDA and foreign regulatory authorities must review and approve. In addition, quality issues may arise during those scale-up activities because of the inherent properties of a product candidate itself or of a product candidate in combination with other components added during the manufacturing and packaging process, or during shipping and storage of the APIs or the finished product. If our third-party CMOs are unable to successfully scale up the manufacture of any of our product candidates in sufficient quality and quantity and at commercially reasonable prices, and we are unable to find one or more replacement suppliers or manufacturers capable of production at a substantially equivalent cost in substantially equivalent volumes and quality, and we are unable to successfully transfer the processes on a timely basis, the development of that product candidate and regulatory approval or commercial launch for any resulting products may be delayed, or there may be a shortage in supply, either of which could significantly harm our business, financial condition, results of operations and prospects.
If we are not able to establish further collaborations, we may have to alter some of our future development and commercialization plans.
Our product development programs and the potential commercialization of our product candidates will require substantial additional capital to fund expenses. We may enter into collaboration agreements with pharmaceutical and biotechnology companies for the future development and potential commercialization of our product candidates. If it does enter into one or more such collaborations, we will likely have limited control over the amount and timing of resources that our collaborators dedicate to the development or commercialization of any product candidates we may seek to develop with them. We cannot predict the success of any collaboration that we may enter into.
66
Table of Contents
We face significant competition in seeking appropriate collaborators, and a number of more established companies may also be pursuing strategies to license or acquire third-party intellectual property rights that we may consider attractive. These established companies may have a competitive advantage over us due to their size, financial resources and greater clinical development and commercialization experience and capabilities. In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. Whether we reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of clinical trials, the likelihood of approval by the FDA, EMA, MHRA or similar foreign regulatory authorities, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge, and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with us for our product candidate. We may also be restricted under future license agreements from entering into agreements on certain terms with potential collaborators. Collaborations are complex and time-consuming to negotiate and document. In addition, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators.
We may not be able to negotiate collaboration agreements on a timely basis, on acceptable terms, or at all. Even if we are able to obtain a license to intellectual property of interest, we may not be able to secure exclusive rights, in which case others could use the same rights and compete with us. Our collaboration partners, if any, may not prioritize our product candidates or otherwise not effectively pursue the development of our product candidates which may delay, reduce or terminate the development of such product candidate, reduce or delay its development program or delay its potential commercialization. Further if we are unable to successfully obtain rights to required third-party intellectual property rights or maintain and protect the existing intellectual property rights we have, we may have to delay, reduce or terminate the development of our product candidates, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of any sales or marketing activities or increase our expenditures and undertake development or commercialization activities at our own expense. Doing so will likely harm our ability to execute our business plans. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms or at all. If we do not have sufficient funds, we may not be able to further develop our product candidates or bring them to market and generate product revenue.
Risks Related to Regulatory Compliance
Enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and affect the prices we may charge for such product candidates.
The U.S. and many foreign jurisdictions have enacted or proposed legislative and regulatory changes affecting the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product for which we obtain marketing approval.
The Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively, the “Affordable Care Act”), includes measures that have significantly changed the way healthcare is financed by both governmental and private insurers. There remain judicial, executive and congressional challenges to certain aspects of the Affordable Care Act. Since 2017, there have been Executive Orders and other directives designed to delay the implementation of certain provisions of the Affordable Care Act or otherwise circumvent some of the requirements for health insurance mandated by the Affordable Care Act. In addition, while Congress has not passed comprehensive repeal legislation, it has enacted laws that modify certain provisions of the Affordable Care Act such as removing penalties, effective January 1, 2019, for not complying with the Affordable Care Act’s individual mandate to carry health insurance. Additionally, the 2020 federal spending package permanently eliminated, effective January 1, 2020, the Affordable Care Act-mandated “Cadillac” tax on high-cost employer-sponsored health coverage and medical device tax and, effective January 1, 2021, also eliminates the health insurer tax. In 2018, a U.S. District Court ruled that the Affordable Care Act is unconstitutional in its entirety because the “individual mandate” was effectively repealed by Congress as part of the Tax Act. Additionally, in 2019, the U.S. Court of Appeals for the 5th Circuit upheld the District Court ruling that the individual mandate was unconstitutional and remanded the case back to the District Court to determine whether the remaining provisions of the Affordable Care Act are invalid as well. The U.S. Supreme Court is scheduled to hear oral argument on the case on November 10, 2020 and is expected to render a decision during its 2020-2021 term. It is unclear how such litigation and other efforts to repeal and replace the Affordable Care Act will impact the Affordable Care Act and our business. We continue to evaluate the effect that the Affordable Care Act and its possible repeal and replacement has on our business.
67
Table of Contents
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. For example, the Budget Control Act of 2011, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee on Deficit Reduction did not achieve a targeted deficit reduction, which triggered the legislation’s automatic reduction to several government programs. This includes aggregate reductions to Medicare payments to providers of, on average, 2% per fiscal year through 2030 unless Congress takes additional action. The CARES Act, which was signed into law in March 2020, and designed to provide financial support and resources to individuals and businesses affected by COVID-19 pandemic, suspended the 2% Medicare sequester from May 1, 2020, through December 31, 2020. Recently, there has been increasing legislative and enforcement interest in the U.S. with respect to specialty drug pricing practices. Specifically, there have been several recent U.S. congressional inquiries and legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs and reform government program reimbursement methodologies for drugs. For example, at the federal level, the administration’s budget proposals for fiscal year 2021 includes a $135.0 billion allowance to support legislative proposals seeking to reduce drug prices, increase competition, lower out-of-pocket drug costs for patients, and increase patient access to lower-cost generic and biosimilar drugs. On March 10, 2020, the administration sent “principles” for drug pricing to Congress, calling for legislation that would, among other things, cap Medicare Part D beneficiary out-of-pocket pharmacy expenses, provide an option to cap Medicare Part D beneficiary monthly out-of-pocket expenses, and place limits on pharmaceutical price increases. Additionally, the administration previously released a “Blueprint” to lower drug prices and reduce out of pocket costs of drugs that contained proposals to increase manufacturer competition, increase the negotiating power of certain federal healthcare programs, incentivize manufacturers to lower the list price of their products and reduce the out of pocket costs of drug products paid by consumers. Although a number of these and other measures may require additional authorization to become effective, Congress and the administration have each indicated that it will continue to seek new legislative and/or administrative measures to control drug costs. For example, on July 24, 2020, the administration announced four executive orders to lower drug prices, including allowing importation of certain drugs, changing how drug rebates are negotiated by middlemen, like pharmacy benefit managers, and directing such rebates to be passed to patients as point-of-sale discounts, and requiring Medicare to pay certain Part B drugs at the lowest price available in economically comparable countries (the details of which were released on September 13, 2020 and also expanded the policy to cover certain Part D drugs). The president has delayed the effective date of the international drug pricing order, pending discussion with major drug companies. How these executive orders will be implemented and their impact on the industry remain uncertain. Additionally, the FDA recently released a final rule, effective November 30, 2020, implementing a portion of the importation executive order providing guidance for states to build and submit importation plans for drugs from Canada. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. It is possible that additional governmental action is taken in response to the COVID-19 pandemic, which may impact our business. We are unable to predict the future course of federal or state healthcare legislation in the U.S. directed at broadening the availability of healthcare and containing or lowering the cost of healthcare. These and any further changes in the law or regulatory framework that reduce our revenue or increase our costs could also have a material and adverse effect on our business, financial condition and results of operations.
68
Table of Contents
We expect that the healthcare reform measures that have been adopted and may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved product and could seriously harm our future revenues. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our products.
Our business operations and current and future relationships with investigators, healthcare professionals, consultants, third-party payors and customers will be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws, transparency laws, health information privacy and security laws and other healthcare laws and regulations. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
Although we do not currently have any products on the market, our current and future operations may be, directly or indirectly through our prescribers, customers and third-party payors, subject to various U.S. federal and state healthcare laws and regulations, including, without limitation, the U.S. federal Anti-Kickback Statute, the U.S. federal civil and criminal false claims laws and the Physician Payments Sunshine Act and regulations. Healthcare providers, physicians and others play a primary role in the recommendation and prescription of any products for which we obtain marketing approval. These laws may impact, among other things, our current business operations, including our clinical research activities, and proposed sales, marketing and education programs and constrain the business of financial arrangements and relationships with healthcare providers, physicians and other parties through which we may market, sell and distribute our products for which we obtain marketing approval. In addition, we may be subject to patient data privacy and security regulation by both the U.S. federal government and the states in which we conduct our business. Finally, we may be subject to additional healthcare, statutory and regulatory requirements and enforcement by foreign regulatory authorities in jurisdictions in which we conduct our business. The laws that may affect our ability to operate include:
| • | the U.S. federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, offering, receiving or paying any remuneration (including any kickback, bribe or certain rebates), directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, lease, order or recommendation of, any good, facility, item or service, for which payment may be made, in whole or in part, under U.S. federal and state healthcare programs such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
| • | the U.S. federal false claims, including the False Claims Act, which can be enforced through whistleblower actions, and civil monetary penalties laws, which, among other things, impose criminal and civil penalties against individuals or entities for knowingly presenting, or causing to be presented, to the U.S. federal government, claims for payment or approval that are false or fraudulent, knowingly making, using or causing to be made or used, a false record or statement material to a false or fraudulent claim, or from knowingly making a false statement to avoid, decrease or conceal an obligation to pay money to the U.S. federal government. In addition, the government may assert that a claim including items and services resulting from a violation of the U.S. federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act; |
69
Table of Contents
| • | the U.S. federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement, in connection with the delivery of, or payment for, healthcare benefits, items or services; similar to the U.S. federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
| • | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and its implementing regulations, and as amended again by the Modifications to the HIPAA Privacy, Security, Enforcement and Breach Notification Rules Under HITECH and the Genetic Information Nondiscrimination Act; Other Modifications to the HIPAA Rules, commonly referred to as the Final HIPAA Omnibus Rule, published in January 2013, which imposes certain obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information without appropriate authorization by covered entities subject to the Final HIPAA Omnibus Rule, i.e. health plans, healthcare clearinghouses and certain healthcare providers, as well as their business associates that perform certain services for or on their behalf involving the use or disclosure of individually identifiable health information; |
| • | the U.S. Federal Food, Drug and Cosmetic Act, which prohibits, among other things, the adulteration or misbranding of drugs, biologics and medical devices; |
| • | the U.S. federal legislation commonly referred to as Physician Payments Sunshine Act, enacted as part of the Affordable Care Act, and its implementing regulations, which requires certain manufacturers of drugs, devices, biologics and medical supplies that are reimbursable under Medicare, Medicaid or the Children’s Health Insurance Program to report annually to the CMS information related to certain payments and other transfers of value to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as ownership and investment interests held by the physicians described above and their immediate family members; |
| • | analogous state laws and regulations, including: state anti-kickback and false claims laws, which may apply to our business practices, including, but not limited to, research, distribution, sales and marketing arrangements and claims involving healthcare items or services reimbursed by any third-party payor, including private insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the U.S. federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws and regulations that require drug manufacturers to file reports relating to pricing and marketing information, which requires tracking gifts and other remuneration and items of value provided to healthcare professionals and entities; state and local laws requiring the registration of pharmaceutical sales representatives; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts; and |
| • | European and other foreign law equivalents of each of the laws, including reporting requirements detailing interactions with and payments to healthcare providers. |
Ensuring that our internal operations and future business arrangements with third parties comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations,
70
Table of Contents
agency guidance or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of the laws described above or any other governmental laws and regulations that may apply to us, we may be subject to significant penalties, including civil, criminal and administrative penalties, damages, fines, exclusion from U.S. government funded healthcare programs, such as Medicare and Medicaid, or similar programs in other countries or jurisdictions, disgorgement, imprisonment, contractual damages, reputational harm, diminished profits, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws and the delay, reduction, termination or restructuring of our operations. Further, defending against any such actions can be costly and time-consuming, and may require significant financial and personnel resources. Therefore, even if we are successful in defending against any such actions that may be brought against us, our business may be impaired. If any of the physicians or other providers or entities with whom we expect to do business is found to not be in compliance with applicable laws, they may be subject to significant criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs and imprisonment. If any of the above occur, it could adversely affect our ability to operate our business and our results of operations.
Failure to comply with current or future federal, state and foreign laws and regulations and industry standards relating to privacy and data protection laws could lead to government enforcement actions (which could include civil or criminal penalties), private litigation, and/or adverse publicity and could negatively affect our operating results and business.
We and our collaborators and third-party providers may be subject to federal, state and foreign data privacy and security laws and regulations. In the U.S., numerous federal and state laws and regulations, including federal health information privacy laws, state data breach notification laws, state health information privacy laws and federal and state consumer protection laws, such as Section 5 of the Federal Trade Commission Act, that govern the collection, use, disclosure and protection of health-related and other personal information could apply to our operations or the operations of our collaborators and third-party providers.
In many jurisdictions, enforcement actions and consequences for noncompliance are rising. In the U.S., these include enforcement actions in response to rules and regulations promulgated under the authority of federal agencies and state attorneys general and legislatures and consumer protection agencies. In addition, privacy advocates and industry groups have regularly proposed, and may propose in the future, self-regulatory standards that may legally or contractually apply to us. If we fail to follow these security standards, even if no customer information is compromised, we may incur significant fines or experience a significant increase in costs. Many state legislatures have adopted legislation that regulates how businesses operate online, including measures relating to privacy, data security and data breaches. Laws in all 50 states require businesses to provide notice to customers whose personally identifiable information has been disclosed as a result of a data breach. The laws are not consistent, and compliance in the event of a widespread data breach is costly. States are also constantly amending existing laws, requiring attention to frequently changing regulatory requirements. Furthermore, California recently enacted the California Consumer Privacy Act (the “CCPA”), which became effective in January 2020. The CCPA gives California residents expanded rights to access and delete their personal information, opt out of certain personal information sharing and receive detailed information about how their personal information is used. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. At this time, we do not collect personal data on residents of California but should we begin to do so, the CCPA will impose new and burdensome privacy compliance obligations on our business and will raise new risks for potential fines and class actions.
Foreign data protection laws, including EU General Data Protection Regulation (the “GDPR”), may also apply to health-related and other personal information obtained outside of the U.S. The GDPR, which came into effect in 2018, introduced new data protection requirements in the European Union, as well as potential fines for noncompliant companies of up to the greater of €20.0 million or 4% of annual global revenue. The regulation imposes numerous new requirements for the collection, use and disclosure of personal information, including
71
Table of Contents
more stringent requirements relating to consent and the information that must be shared with data subjects about how their personal information is used, the obligation to notify regulators and affected individuals of personal data breaches, extensive new internal privacy governance obligations and obligations to honor expanded rights of individuals in relation to their personal information (e.g., the right to access, correct and delete their data). Among other requirements, the GDPR regulates transfers of personal data subject to the GDPR to third countries that have not been found to provide adequate protection to such personal data, including the U.S., and the efficacy and longevity of current transfer mechanisms between the EU and the U.S. remains uncertain. For example, in 2016, the EU and U.S. agreed to a transfer framework for data transferred from the EU to the U.S., called the Privacy Shield, but the Privacy Shield was invalidated in July 2020 by the Court of Justice of the European Union. At this time, we do not believe we are subject to the GDPR, but should this change, the GDPR will increase our responsibility and potential liability in relation to personal data that we process, and we may be required to put in place additional mechanisms to ensure compliance with the new EU data protection rules.
Compliance with U.S. and foreign data protection laws and regulations could require us to take on more onerous obligations in our contracts, restrict our ability to collect, use and disclose data, or in some cases, impact our ability to operate in certain jurisdictions. Failure by us or our collaborators and third-party providers to comply with U.S. and foreign data protection laws and regulations could result in government enforcement actions (which could include civil or criminal penalties), private litigation and/or adverse publicity and could negatively affect our operating results and business. Moreover, clinical trial subjects about whom we or our potential collaborators obtain information, as well as the providers who share this information with us, may contractually limit our ability to use and disclose the information. Claims that we have violated individuals’ privacy rights, failed to comply with data protection laws or breached our contractual obligations, even if we are not found liable, could be expensive and time consuming to defend, could result in adverse publicity and could have a material adverse effect on our business, financial condition, results of operations and prospects.
Risks Related to Our Intellectual Property
If we are unable to obtain, maintain, protect and enforce sufficient patent and other intellectual property rights for our product candidates and technology, or if the scope of patent and other intellectual property rights obtained is not sufficiently broad, we may not be able to compete effectively in our market.
Our success depends in significant part on our ability and the ability of any licensors and collaborators to obtain, maintain, protect and enforce patents and other intellectual property rights with respect to our product candidates and technology and to operate our business without infringing, misappropriating or otherwise violating the intellectual property rights of others. As of February 28, 2021, we have only two issued patents, although we also have more than 15 pending U.S. patent applications, over 15 pending Patent Cooperation Treaty (“PCT”) patent applications and over 40 pending foreign patent applications.
The patent prosecution process is uncertain, expensive and time-consuming. We and our current or future licensors, licensees or collaborators may not be able to prepare, file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we or our licensors will fail to identify patentable aspects of our research and development output in time to obtain patent protection or fail to file patent applications covering inventions made in the course of development and commercialization activities before a competitor or another third party files a patent application covering, or publishes information disclosing, a similar, independently-developed invention. Such competitor’s or other third party’s patent application may pose obstacles to our ability to obtain patent protection or limit the scope of the patent protection we may obtain. Although we enter into non-disclosure and confidentiality agreements with parties who have access to confidential or patentable aspects of our research and development output, such as our employees, collaborators, CROs, contract manufacturers, consultants, advisors and other third parties, any of these parties may breach the agreements and disclose such output before a patent application is filed, thereby jeopardizing our ability to seek patent protection in certain jurisdictions. In addition, publications of discoveries in the scientific literature often lag behind actual discoveries, and patent applications in the U.S. and other jurisdictions are typically not
72
Table of Contents
published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we or our licensors were the first to conceive the inventions claimed in our owned or licensed patents or pending patent applications, or were the first to file for patent protection of such inventions.
The patent position of biotechnology and pharmaceutical companies generally is uncertain, involves complex legal and factual questions and is the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our and our current or future licensors’ patent rights are uncertain. Our and our licensors’ pending and future patent applications may not mature into patents or result in issued patents that protect our technology or product candidates, in whole or in part, or which effectively exclude others from commercializing competitive technologies and product candidates. The patent examination process may require us or our licensors to narrow the scope of the claims of our pending and future patent applications, and therefore, even if such patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors or other third parties from competing with us or otherwise provide us with any competitive advantage. Our and our licensors’ patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent issues from such applications, and then only to the extent the issued claims cover such technology. Any patents that we hold or in-license may be challenged, narrowed, circumvented or invalidated by third parties. Consequently, we do not know whether any of our product candidates will be protectable or remain protected by valid and enforceable patents. Our competitors or other third parties may be able to circumvent our patents by developing similar or alternative technologies or products in a non-infringing manner. Any of the foregoing could harm our competitive position, business, financial condition, results of operations and prospects.
The patent protection we obtain for our product candidates and technology may be challenged or not sufficient enough to provide us with any competitive advantage.
Even if our owned patent applications issue as patents, the issuance of any such patents is not conclusive as to their inventorship, scope, validity or enforceability, and such patents may be challenged, invalidated, narrowed or held to be unenforceable, including in the courts or patent offices in the U.S. and abroad, or circumvented. We may be subject to a third-party preissuance submission of prior art to the U.S. Patent and Trademark Office the (“USPTO”), a federal court or equivalent foreign bodies, or become involved in opposition, derivation, revocation, re-examination, post-grant and inter partes review or interference proceedings, or other similar proceedings, challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. Moreover, we, or one of our licensors, may have to participate in interference or derivation proceedings declared by the USPTO to determine priority or ownership of invention or in post-grant challenge proceedings, such as oppositions in a foreign patent office, that challenge priority of invention or other features of patentability. Such proceedings and any other patent challenges may result in loss of patent rights, loss of exclusivity, loss of priority or in patent claims being narrowed, invalidated or held unenforceable, which could limit our ability to stop others from using or commercializing similar or identical technology and products or limit the duration of the patent protection of our technology and product candidates. Such proceedings also may result in substantial cost and require significant time from our scientists and management, even if the eventual outcome is favorable to us. Moreover, there could be public announcements of the results of hearings, motions or other developments related to any of the foregoing proceedings. If securities analysts or investors perceive those results to be negative, it could cause the price of shares of our common stock to decline. Any of the foregoing could harm our business, financial condition, results of operations and prospects.
Moreover, some of our owned or in-licensed patents and patent applications are, and may in the future be, co-owned with third parties. If we are unable to obtain an exclusive license to any such co-owners’ interest in such patents or patent applications, such co-owners may be able to license their rights to other third parties, including our competitors, who could market competing products and technology. In addition, we may need the cooperation of any such co-owners in order to enforce such patents against third parties, and such cooperation may not be provided to us.
73
Table of Contents
Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
Because we rely on third parties to discover, develop and manufacture our product candidates, we must, at times, share certain of its trade secrets with them. We seek to protect our proprietary technology in part by entering into agreements containing confidentiality provisions, including if applicable, confidentiality agreements, material transfer agreements, collaborative research agreements, consulting agreements or other similar agreements with our collaborators, advisors, employees and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose our confidential information, such as trade secrets. Despite these agreements with third parties, sharing trade secrets and other confidential information increases the risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others or are disclosed or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, a competitor’s discovery of our trade secrets or other unauthorized use or disclosure could impair our competitive position and may harm our business.
In addition, these agreements typically restrict the ability of our advisors, employees, third-party contractors and consultants to publish data potentially relating to our trade secrets, although our agreements may contain certain limited publication rights. Despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of our agreements with third parties, independent development or publication of information by any of our third-party collaborators. A competitor’s discovery of our trade secrets could impair our competitive position and have an adverse impact on our business.
We may become involved in lawsuits to protect or enforce our patents or other intellectual property, which could be expensive, time-consuming and unsuccessful, and issued patents covering our technology and product candidates could be found invalid or unenforceable if challenged.
Competitors and other third parties may infringe, misappropriate or otherwise violate our issued patents or other intellectual property or the patents or other intellectual property of our licensors. In addition, our patents or the patents of our licensors may become involved in inventorship or priority disputes. Our pending patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent issues from such applications. To counter infringement or other unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their patents or that our patents are invalid or unenforceable. In a patent infringement proceeding, a court may decide that a patent of ours is invalid or unenforceable, in whole or in part, construe the patent’s claims narrowly or refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology. An adverse result in any litigation proceeding could put one or more of our owned or licensed patents at risk of being invalidated, held unenforceable, interpreted narrowly or interpreted in a manner that would not prevent competitors from entering the market. Further, we may find it impractical or undesirable to enforce our intellectual property against some third parties.
In patent litigation in the U.S., defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, non-enablement or insufficient written description. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO or made a misleading statement during prosecution. Third parties may also raise similar claims before the USPTO or an equivalent foreign body, even outside the context of litigation. Potential proceedings include re-examination, post-grant review, inter partes review, interference proceedings, derivation proceedings and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result in the revocation of, cancellation of, or amendment to our patents in such a way that they no longer cover our technology or any product candidates that we may develop. The
74
Table of Contents
outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection on the applicable product candidates or technology covered by the patent rendered invalid or unenforceable. Such a loss of patent protection could materially harm our business, financial condition, results of operations and prospects.
Interference or derivation proceedings provoked by third parties or brought by us or declared by the USPTO may be necessary to determine the ownership or priority of inventions with respect to our patents or patent applications. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Such licenses may not be available on commercially reasonable terms, or at all, or may be non-exclusive. If we are unable to obtain and maintain such licenses, we may need to cease the development, manufacture and commercialization of one or more of the product candidates we may develop. In addition, if we or our licensors are unsuccessful in any inventorship disputes to which we or they are subject, we may lose valuable intellectual property rights, such as exclusive ownership of, or the exclusive right to use, our owned or in-licensed patents. The loss of exclusivity or the narrowing of our owned and licensed patent claims could limit our ability to stop others from using or commercializing similar or identical technology and products. Any of the foregoing could result in a material adverse effect on our business, financial condition, results of operations or prospects. Even if we are successful in any of the foregoing disputes, it could result in substantial costs and be a distraction to management and other employees. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation or proceeding.
Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Most of our competitors are larger than we are and have substantially greater resources. They are, therefore, more likely to be able to sustain the costs of complex patent litigation or proceedings than we can because of their greater financial resources and more mature and developed intellectual property portfolios. Accordingly, despite our efforts, we may not be able to prevent third parties from infringing, misappropriating or otherwise violating our intellectual property. Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims could result in substantial costs and diversion of management resources, which could harm our business. In addition, the uncertainties associated with litigation could compromise our ability to raise the funds necessary to continue our clinical trials, continue our internal research programs or in-license needed technology or other product candidates. There could also be public announcements of the results of the hearing, motions or other interim proceedings or developments. If securities analysts or investors perceive those results to be negative, it could cause the price of shares of our common stock to decline. Any of the foregoing events could harm our business, financial condition, results of operations and prospects.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting, maintaining, defending and enforcing patents and other intellectual property rights on our product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the U.S. can be less extensive than those in the U.S. As such, we may choose not to seek to protect our intellectual property in certain jurisdictions which could leave us without recourse to prevent competitive products from being manufactured or commercialized in such jurisdictions. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the U.S.. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the U.S., or from selling or importing products made using our inventions in and into the U.S. or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection or other intellectual property rights to develop their own products
75
Table of Contents
and may export otherwise infringing, misappropriating or violating products to territories where we have patent or other intellectual property protection, but enforcement rights are not as strong as those in the U.S.. These products may compete with our product candidates, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of some countries do not favor the enforcement of patents and other intellectual property rights, which could make it difficult for us to stop the infringement, misappropriation or other violation of our intellectual property rights generally. Proceedings to enforce our intellectual property rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful.
Many countries, including European Union countries, India, Japan and China, have compulsory licensing laws under which a patent owner may be compelled under specified circumstances to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In those countries, we may have limited remedies if patents are infringed or if we are compelled to grant a license to a third party, which could materially diminish the value of those patents. This could limit our potential revenue opportunities. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license, which could adversely affect our business, financial condition, results of operations and prospects.
We may not identify relevant third-party patents or pending patent applications or may incorrectly interpret the relevance, scope or expiration of a third-party patent which might adversely affect our ability to develop and market our product candidates.
We are developing certain product candidates in highly competitive areas and cannot guarantee that any patent searches or analyses that we may conduct, including the identification of relevant patents or pending patent applications, the scope of patent claims or the expiration of relevant patents, are complete or thorough, nor can we be certain that we have identified each and every third-party patent and pending patent application in the U.S. and abroad that is or may be relevant to or necessary for the commercialization of our product candidates in any jurisdiction. For example, U.S. patent applications filed before November 29, 2000 and certain U.S. patent applications filed after that date that will not be filed outside the U.S. remain confidential until patents issue. Patent applications in the U.S. and elsewhere are published approximately 18 months after the earliest filing for which priority is claimed, with such earliest filing date being commonly referred to as the priority date. Therefore, patents or pending patent applications covering our product candidates could have been or may be filed in the future by third parties without our knowledge. Additionally, patents and pending patent applications that have been published can, subject to certain limitations, be later amended in a manner that could cover our product candidates or the manufacturing or use of our product candidates. The scope of a patent claim is determined by an interpretation of the law, the written disclosure in a patent and the patent’s prosecution history. Our interpretation of the relevance or the scope of a patent or a pending patent application may be incorrect, which may negatively impact our ability to market our product candidates. We may incorrectly determine that our product candidates are not covered by a third-party patent or pending patent application or may incorrectly predict whether a third party’s pending application will issue with claims of relevant scope. Our determination of the expiration date of any patent in the U.S. or abroad that we consider relevant may be incorrect, which may negatively impact our ability to develop and market our product candidates. Our failure to identify and correctly interpret relevant patents or pending patent applications may negatively impact our ability to develop and market our product candidates.
76
Table of Contents
If we fail to identify or correctly interpret relevant patents or pending patent applications or if we are unable to obtain licenses to relevant patents or pending patent applications, we may be subject to infringement claims. we cannot guarantee that we will be able to successfully settle or otherwise resolve such infringement claims. If we fail in any such dispute, in addition to being forced to pay damages, potentially including in the form of future royalties, which may be significant, we may be temporarily or permanently prohibited from commercializing any of our product candidates that are held to be infringing. We might, if possible, also be forced to redesign product candidates so that we no longer infringe the third-party intellectual property rights. Any of these events, even if we were ultimately to prevail, could require us to divert substantial financial and management resources that we would otherwise be able to devote to our business and could adversely affect our business, financial condition, results of operations and prospects.
If we are unable to obtain licenses from third parties on commercially reasonable terms or fail to comply with our obligations under such agreements, our business could be harmed.
It may be necessary for us to use the patented or other proprietary technology of third parties to commercialize our products, in which case we would be required to obtain a license or ownership from these third parties. The licensing or acquisition of third-party intellectual property rights is a competitive area, and more established companies may pursue strategies to license or acquire third-party intellectual property rights that we may consider attractive or necessary. These established companies may have a competitive advantage over us due to their size, capital resources or greater clinical development and commercialization capabilities. In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. If we are unable to license or acquire such intellectual property or technology, or if we are forced to in-license such intellectual property or technology, on unfavorable terms, our business could be materially harmed. If we are unable to obtain a necessary license, we may be unable to develop or commercialize the affected product candidates, or the cost of development, manufacture or commercialization may be materially increased, which could materially harm our business, and the third parties owning such intellectual property rights could seek either an injunction prohibiting our sales, or, with respect to our sales, an obligation on our part to pay royalties and/or other forms of compensation. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us.
If we fail to comply with our obligations under any future license agreements, such counterparties may have the right to terminate these agreements, in which event we might not be able to develop, manufacture or commercialize, or may be forced to cease developing, manufacturing or marketing, any product that is covered by these agreements or may face other penalties under such agreements. Such an occurrence could materially adversely affect the value of the product candidate being developed under any such agreement. Termination of these agreements or reduction or elimination of our rights under these agreements may result in our having to negotiate new or reinstated agreements with less favorable terms, cause us to lose our rights under these agreements, including our rights to important intellectual property or technology, or impede, delay or prohibit the further development or commercialization of one or more product candidates that rely on such agreements. If we were to lose our rights to licensed intellectual property, we may not be able to continue developing or commercializing our product candidates, if approved. If we breach any of the agreements under which we license the use, development and commercialization rights to our product candidates or technology from third parties or, in certain cases, we fail to meet certain development deadlines, we could lose license rights that are important to our business.
Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time.
Patents have a limited lifespan. In the U.S., if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates are obtained, once the patent life has expired for a product candidate, we may be open to competition
77
Table of Contents
from competitive products, including generic medications. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such product candidates might expire before or shortly after such product candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing product candidates similar or identical to ours.
Depending upon the timing, duration and conditions of any FDA marketing approval of our product candidates, one or more of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Amendments, and one or more of our foreign patents may be eligible for patent term extension under similar legislation, for example, in the European Union. In the U.S., the Hatch-Waxman Amendments permit a patent term extension of up to five years for a patent covering an approved product as compensation for effective patent term lost during product development and the FDA regulatory review process provided other requirements are met. However, there are no assurances that the FDA, USPTO or any comparable foreign regulatory authority or national patent office will grant such extensions, in whole or in part and the length of any available extension may vary based on a number of factors. For example, we may not receive an extension if we fail to exercise due diligence during the testing phase or regulatory review process, fail to apply within applicable deadlines, fail to apply prior to expiration of relevant patents or otherwise fail to satisfy applicable requirements. Moreover, the length of the extension could be less than we request. Only one patent per approved product can be extended, the extension cannot extend the total patent term beyond 14 years from approval, and only those claims covering the approved drug, a method for using it or a method for manufacturing it may be extended. If we are unable to obtain patent term extension or the term of any such extension is less than we request, the period during which we can enforce our patent rights for the applicable product candidate will be shortened, and our competitors may obtain approval to market competing products sooner. As a result, our revenue from applicable products could be reduced. Further, if this occurs, our competitors may take advantage of our investment in development and trials by referencing our clinical and preclinical data and launch their product earlier than might otherwise be the case, and our competitive position, business, financial condition, results of operations and prospects could be adversely affected.
Changes in patent law could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
Obtaining and enforcing patents in the pharmaceutical industry is inherently uncertain, due in part to ongoing changes in the patent laws. Depending on decisions by Congress, the federal courts, and the USPTO and equivalent institutions in other jurisdictions, the laws and regulations governing patents, and interpretation thereof, could change in unpredictable ways that could weaken our and our licensors’ or collaborators’ ability to obtain new patents or to enforce existing or future patents. For example, in recent years the U.S. Supreme Court has ruled on several patent cases that have been interpreted to have either narrowed the scope of patent protection or weakened the rights of patent owners in certain situations. Therefore, there is increased uncertainty with regard to our and our licensors’ or collaborators’ ability to obtain patents in the future, as well as uncertainty with respect to the value of patents once obtained.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our and our licensors’ or collaborators’ patent applications and the enforcement or defense of our or our licensors’ or collaborators’ issued patents. Assuming that other requirements for patentability are met, prior to March 2013, in the U.S., the first to invent the claimed invention was entitled to the patent, while outside the U.S., the first to file a patent application was entitled to the patent. After March 2013, under the Leahy-Smith America Invents Act enacted in September 2011 (the “Leahy-Smith Act”), the U.S. transitioned to a first inventor to file system in which, assuming that other requirements for patentability are met, the first inventor to file a patent application will be entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. The Leahy-Smith Act also includes a number of significant changes that affect the
78
Table of Contents
way patent applications are prosecuted and may also affect patent litigation. These include allowing third party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by USPTO-administered post-grant proceedings, including post-grant review, inter partes review and derivation proceedings. The USPTO recently developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, particularly the first inventor-to-file provisions. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our or our licensors’ patent applications and the enforcement or defense of our or our licensors’ issued patents, all of which could harm our business, financial condition, results of operations and prospects.
Obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated if we fail to comply with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other government fees on any issued patents and certain pending patent applications are required to be paid to the USPTO or foreign patent agencies in several stages over the lifetime of a patent. In certain circumstances, we rely on our licensors to pay these fees. The USPTO and various foreign patent agencies also require compliance with a number of procedural, documentary, fee payment and other similar requirements during the patent application and prosecution process. Noncompliance events that could result in abandonment or lapse of a patent or patent application include failure to respond to official communications within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which non-compliance can result in irrevocable abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. If we or our licensors or collaborators fail to maintain the patents and patent applications covering our product candidates, our competitors might be able to enter the market with similar or identical products or technology, which would harm our business, financial condition, results of operations and prospects.
Third parties may initiate legal proceedings alleging that we are infringing, misappropriating or otherwise violating their intellectual property rights, the outcome of which would be uncertain and could negatively impact the success of our business.
Our commercial success depends upon our ability and the ability of our collaborators to develop, manufacture, market and sell our product candidates and use our proprietary technologies without infringing, misappropriating or otherwise violating the intellectual property and other proprietary rights of third parties. There is considerable intellectual property litigation in the biotechnology and pharmaceutical industries. We may become party to, or be threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our product candidates and technology, including re-examination, interference, post-grant review, inter partes review or derivation proceedings, or other similar proceedings, before the USPTO, a federal court or an equivalent foreign body. Numerous U.S. and foreign issued patents and pending patent applications owned by third parties exist in the fields in which we are developing our product candidates. In the event that any of these patents were asserted against us, we believe that we would have defenses against any such action, including that such patents are not valid, that our product candidates do not infringe such patents, or that we would be able to replace such technology with alternative, non-infringing technology. However, if any such patents were to be asserted against us and our defenses to such assertion were unsuccessful and such alternative technology was not available or technologically or commercially practical, unless we obtain a license to such patents, we could be liable for damages, which could be significant and include treble damages and attorneys’ fees if we are found to willfully infringe such patents, and we could be precluded from commercializing any product candidates that were ultimately held to infringe such patents. Any potential future legal proceedings relating to these patents could cause us to incur significant expenses, and could distract our technical and
79
Table of Contents
management personnel from their normal responsibilities. If we are unsuccessful in our challenges to these patents and become subject to litigation or are unable to obtain a license on commercially reasonable terms with respect to these patents, it could harm our business, financial condition, results of operations and prospects.
Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future, regardless of their merit. Even if we believe third-party intellectual property claims are without merit, there is no assurance that a court would find in our favor on questions of infringement, validity, enforceability or priority. A court of competent jurisdiction could hold that third-party patents asserted against us are valid, enforceable and infringed, which could materially and adversely affect our ability to commercialize any product candidates we may develop and any other product candidates or technologies covered by the asserted third-party patents. In order to successfully challenge the validity of any such U.S. patent in federal court, we would need to overcome a presumption of validity. As this burden is a high one requiring us to present clear and convincing evidence as to the invalidity of any such U.S. patent claim, there is no assurance that a court of competent jurisdiction would invalidate the claims of any such U.S. patent. If we are found to infringe, misappropriate or otherwise violate a third party’s intellectual property rights, and we are unsuccessful in demonstrating that such rights are invalid or unenforceable, we could be required to obtain a license from such a third party in order to continue developing and marketing our products and technology. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us, and it could require us to make substantial licensing and royalty payments. We could be forced, including by court order, to cease commercializing the infringing technology or product candidates. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties and other fees, redesign our infringing drug or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business. Any of the foregoing events would harm our business, financial condition, results of operations and prospects.
We may be subject to claims by third parties asserting that we or our employees have infringed upon, misappropriated or otherwise violated their intellectual property rights, or claiming ownership of what we regard as our own intellectual property.
Many of our employees were previously employed at other biotechnology or pharmaceutical companies. Although we try to ensure that our employees, consultants and advisors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these individuals have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such individual’s former employer. Litigation may be necessary to defend against these claims.
In addition, we or our licensors may be subject to claims that former employees, collaborators or other third parties have an interest in our owned or in-licensed patents or other intellectual property as an inventor or co-inventor. While it is our policy to require our employees and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact conceives, develops or reduces to practice intellectual property that we regard as our own. Our and their assignment agreements may not be self-executing or may be breached, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property.
If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in litigating such claims, litigation could result in substantial costs, delay development of our product candidates and be a distraction to management. Any of the foregoing events would harm our business, financial condition, results of operations and prospects.
80
Table of Contents
Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could compromise our ability to compete in the marketplace, including compromising our ability to raise the funds necessary to continue our clinical trials, continue our research programs, license necessary technology from third parties or enter into development collaborations that would help us commercialize our product candidates, if approved. Any of the foregoing events would harm our business, financial condition, results of operations and prospects.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
We rely on trade secrets and agreements containing confidentiality obligations to protect our unpatented know-how, technology and other proprietary information and to maintain our competitive position. With respect to our research and development programs, we consider trade secrets and know-how to be one of our important sources of intellectual property, including our extensive knowledge of certain drug delivery techniques and drug conjugation. Trade secrets and know-how can be difficult to protect. We seek to protect these trade secrets and other proprietary technology, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, collaborators, CROs, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants. We cannot guarantee that we have entered into such agreements with each party that may have or has had access to our trade secrets or proprietary technology and processes. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the U.S. are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor or other third party, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor or other third party, our competitive position would be materially and adversely harmed.
We may not be able to protect and enforce our trademarks and trade names, or build name recognition in our markets of interest thereby harming our competitive position.
We intend to rely on both registered and common law rights for our trademarks. We have applied to register certain of our trademarks with the USPTO and certain other countries and may in the future seek to register additional trademarks in the U.S. or other countries. Our current and future trademark applications may not be allowed for registration in a timely fashion or at all, and our registered trademarks may not be maintained or enforced. In addition, the registered or unregistered trademarks or trade names that we own may be challenged, infringed, circumvented, declared generic, lapsed or determined to be infringing on or dilutive of other marks. We may not be able to protect our rights in these trademarks and trade names or may be forced to stop using these names, which we need for name recognition by potential partners or customers in our markets of interest. In
81
Table of Contents
addition, third parties have filed, and may in the future file, for registration of trademarks similar or identical to our trademarks, thereby impeding our ability to build brand identity and possibly leading to market confusion. If they succeed in registering or developing common law rights in such trademarks, and if we are not successful in challenging such rights, we may not be able to use these trademarks to develop brand recognition of our technologies, products or services. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names.
During the trademark registration process, we may receive office actions from the USPTO or from comparable agencies in foreign jurisdictions objecting to the registration of our trademark. Although we would be given an opportunity to respond to those objections, we may be unable to overcome such rejections. In addition, in the USPTO and in comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and/or to seek the cancellation of registered trademarks. Opposition or cancellation proceedings may in the future be filed against our trademark applications or registrations, and our trademark applications or registrations may not survive such proceedings. In addition, third parties may file first for our trademarks in certain countries. If they succeed in registering such trademarks, and if we are not successful in challenging such third party rights, we may not be able to use these trademarks to market our products in those countries. If we do not secure registrations for our trademarks, we may encounter more difficulty in enforcing them against third parties than we otherwise would. If we are unable to establish name recognition based on our trademarks and trade names, we may not be able to compete effectively, which could have an adverse effect on our business, financial condition, results of operations and prospects.
Intellectual property rights do not necessarily address all potential threats.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business or permit us to maintain our competitive advantage. For example:
| • | others may be able to make products that are similar to any product candidates we may develop or utilize similar technology but that are not covered by the claims of the patents that we own or license now or in the future; |
| • | we, or our current or future licensors, might not have been the first to make the inventions covered by the issued patent or pending patent application that we own or license now or in the future; |
| • | we, or our current or future licensors, might not have been the first to file patent applications covering certain of our or their inventions; |
| • | others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our owned or licensed intellectual property rights; |
| • | it is possible that our pending owned or licensed patent applications or those that we may own or license in the future will not lead to issued patents; |
| • | issued patents that we hold rights to may be held invalid or unenforceable, including as a result of legal challenges by other persons; |
| • | our competitors might conduct research and development activities in the U.S. under FDA-related safe harbor patent infringement exemptions and/or in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets; |
| • | we may not develop additional proprietary technologies that are patentable; |
| • | the patents or pending patent applications of others may harm our business; and |
| • | we may choose not to file a patent in order to maintain certain trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property. |
82
Table of Contents
Should any of these events occur, they could harm our business, financial condition, results of operations and prospects.
Risks Related to Our Business Operations, Employee Matters and Managing Growth
Our business, operations and clinical development plans and timelines and supply chain could be adversely affected by the effects of health epidemics, including the ongoing COVID-19 pandemic, on the manufacturing, clinical trial and other business activities performed by us or by third parties with whom we conduct business, including our CMOs, CROs, shippers and others.
Our business could be adversely affected by health epidemics wherever we have clinical trial sites or other business operations. In addition, health epidemics could cause significant disruption in the operations of CMOs, CROs and other third parties upon whom we rely. For example, the COVID-19 pandemic has presented a substantial public health and economic challenge around the world and is affecting employees, patients, communities and business operations, as well as the U.S. economy and financial markets. Many geographic regions have imposed, or in the future may impose, “shelter-in-place” orders, quarantines or similar orders or restrictions to control the spread of COVID-19. Our headquarters are located in the New York, New York and San Francisco, California areas and at present, we have implemented work-from-home policies for all employees. The effects of the executive order and our work-from-home policies may negatively impact productivity, disrupt our business and delay our clinical programs and timelines, the magnitude of which will depend, in part, on the length and severity of the restrictions and other limitations on our ability to conduct our business in the ordinary course. These and similar, and perhaps more severe, disruptions in our operations could negatively impact our business, operating results and financial condition.
We are dependent on a worldwide supply chain for products to be used in our clinical trials and, if approved by the regulatory authorities, for commercialization. Quarantines, shelter-in-place and similar government orders, or the expectation that such orders, shutdowns or other restrictions could occur, whether related to COVID-19 or other infectious diseases, could impact personnel at third-party manufacturing facilities in the U.S. and other countries, or the availability or cost of materials or supplies, which could disrupt our supply chain or our ability to enroll patients in or perform testing for our clinical trials. In addition, closures of transportation carriers and modal hubs could materially impact our clinical development and any future commercialization timelines.
If our relationships with our suppliers or other vendors are terminated or scaled back as a result of the COVID-19 pandemic or other health epidemics, we may not be able to enter into arrangements with alternative suppliers or vendors or do so on commercially reasonable terms or in a timely manner. Switching or adding additional suppliers or vendors involves substantial cost and requires management time and focus. In addition, there is a natural transition period when a new supplier or vendor commences work. As a result, delays generally occur, which could adversely impact our ability to meet our desired clinical development and any future commercialization timelines. Although we carefully manage our relationships with our suppliers and vendors, there can be no assurance that we will not encounter challenges or delays in the future or that these delays or challenges will not have an adverse impact on our business, financial condition and prospects. See “Risk Factors—Risks Related to Our Dependence on Third Parties.”
In addition, our clinical trials may be affected by the COVID-19 pandemic. In the future, clinical site initiation and patient enrollment may be delayed due to prioritization of hospital resources toward the COVID-19 pandemic or concerns among patients about participating in clinical trials during a pandemic and public health measures imposed by the respective national governments of countries in which the clinical sites are located. Some patients may have difficulty following certain aspects of clinical trial protocols if quarantines impede patient movement or interrupt healthcare services. Similarly, our inability to successfully recruit and retain patients and principal investigators and site staff who, as healthcare providers, may have heightened exposure to COVID-19 or experience additional restrictions by their institutions, city or state governments could adversely impact our clinical trial operations.
The spread of COVID-19 has also led to disruption and volatility in the global capital markets, which increases the cost of, and adversely impacts access to, capital and increases economic uncertainty. The trading
83
Table of Contents
prices for the common stock of other biopharmaceutical companies have, at times, been highly volatile as a result of COVID-19. To the extent the COVID-19 pandemic adversely affects our business, financial results and value of our common stock, it may also affect our ability to access capital, which could in the future negatively affect our liquidity.
The global pandemic of COVID-19 continues to evolve rapidly. The ultimate impact of the COVID-19 pandemic or a similar health epidemic is highly uncertain and subject to change. We do not yet know the full extent of potential delays or impacts on our business, our clinical trials, healthcare systems or the global economy as a whole. However, these effects could have a material impact on our operations, and we will continue to monitor the COVID-19 situation closely.
Our future success depends on our ability to retain Dr. Hung and our other key employees, consultants and advisors and to attract, retain and motivate qualified personnel.
We are highly dependent on the management, research and development, clinical, financial and business development expertise of Dr. Hung and our executive officers, as well as the other members of our scientific and clinical teams. Although we have employment offer letters with each of our executive officers, each of them may terminate their employment with us at any time. We do not maintain “key person” insurance for any of our executives or employees.
Recruiting and retaining qualified scientific and clinical personnel and, if we are successful in obtaining marketing approval for our product candidates, sales and marketing personnel, is critical to our success. The loss of the services of our executive officers or other key employees could impede the achievement of our research, development and commercialization objectives and seriously harm our ability to successfully implement our business strategy.
In particular, in light of Dr. Hung’s central role in the discovery of all of our current product candidates, our ongoing discovery activities and development programs, the recruitment of our other executives and key employees and all other aspects of our strategy and operations, we believe our loss of Dr. Hung’s services for any reason would severely impair our business and prospects. Replacing executive officers and key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to successfully develop, gain regulatory approval for and commercialize our product candidates.
Competition to hire qualified personnel in our industry is intense, and we may be unable to hire, train, retain or motivate these key personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. Furthermore, to the extent we hire personnel from competitors, we may be subject to allegations that they have been improperly solicited or that they have divulged proprietary or other confidential information, or that their former employers own their research output. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us. If we are unable to continue to attract and retain high quality personnel, our ability to pursue our growth strategy will be limited, and could harm our business, financial condition, results of operations and prospects.
We expect to expand our development and regulatory capabilities and potentially implement sales, marketing and distribution capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
As of February 28, 2021, we had 36 employees. As our preclinical and clinical development progresses, we expect to experience growth in the number of our employees and the scope of our operations, particularly in the
84
Table of Contents
areas of research, clinical operations, regulatory affairs, general and administrative and, if any of our product candidates receives marketing approval, sales, marketing and distribution. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
Our employees, independent contractors, consultants, commercial collaborators, principal investigators, CROs and vendors may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements.
We are exposed to the risk that our employees, independent contractors, consultants, commercial collaborators, principal investigators, CROs and vendors may engage in fraudulent conduct or other illegal activity. Misconduct by these parties could include intentional, reckless or negligent conduct or unauthorized activities that violates (1) the laws and regulations of the FDA, the EMA, the MHRA and other similar regulatory authorities, including those laws requiring the reporting of true, complete and accurate information to such authorities, (2) manufacturing standards, (3) federal and state data privacy, security, fraud and abuse and other healthcare laws and regulations in the U.S. and abroad and (4) laws that require the true, complete and accurate reporting of financial information or data. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Misconduct by these parties could also involve the improper use of individually identifiable information, including information obtained in the course of clinical trials, creating fraudulent data in our preclinical studies or clinical trials or illegal misappropriation of product candidates, which could result in regulatory sanctions and serious harm to our reputation.
We intend to adopt a code of business conduct and ethics, but it is not always possible to identify and deter misconduct by employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. Additionally, we are subject to the risk that a person or government could allege such fraud or other misconduct, even if none occurred. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant civil, criminal and administrative penalties, including damages, fines, disgorgement, imprisonment, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, contractual damages, reputational harm and the delay, reduction, termination or restructuring of our operations.
International operations may expose us to business, regulatory, political, operational, financial, pricing and reimbursement risks associated with doing business outside of the U.S.
Our business will be subject to risks associated with conducting business internationally. Some of our suppliers, industry partners and clinical study centers are located outside of the U.S. Furthermore, our business strategy incorporates potential international expansion as we seek to obtain regulatory approval for, and commercialize, our product candidates in patient populations outside the U.S. If approved, we may hire sales representatives and conduct physician and patient association outreach activities outside of the U.S. Doing business internationally involves a number of risks, including but not limited to:
| • | multiple, conflicting and changing laws and regulations such as privacy regulations, tax laws, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits and licenses; |
85
Table of Contents
| • | failure by us to obtain and maintain regulatory approvals for the use of our products in various countries; |
| • | rejection or qualification of foreign clinical trial data by the competent authorities of other countries; |
| • | delays or interruptions in the supply of clinical trial materials resulting from any events affecting raw material supply or manufacturing capabilities abroad, including those that may result from the ongoing COVID-19 pandemic; |
| • | additional potentially relevant third-party patent and other intellectual property rights; |
| • | complexities and difficulties in obtaining, maintaining, protecting and enforcing our intellectual property; |
| • | difficulties in staffing and managing foreign operations; |
| • | complexities associated with managing multiple payor reimbursement regimes, government payors or patient self-pay systems; |
| • | limits in our ability to penetrate international markets; |
| • | financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our product candidates and exposure to foreign currency exchange rate fluctuations; |
| • | natural disasters, political and economic instability, including wars, terrorism and political unrest, outbreak of disease, including COVID-19 and related shelter-in-place orders, travel, social distancing and quarantine policies, boycotts, curtailment of trade and other business restrictions; |
| • | certain expenses including, among others, expenses for travel, translation and insurance; and |
| • | regulatory and compliance risks that relate to anti-corruption compliance and record-keeping that may fall within the purview of the U.S. Foreign Corrupt Practices Act, its accounting provisions or its anti-bribery provisions or provisions of anti-corruption or anti-bribery laws in other countries. |
Any of these factors could harm our future international expansion and operations and, consequently, our results of operations.
Our internal computer systems, or those used by our CROs or other contractors or consultants, may fail or experience security breaches or other unauthorized or improper access.
Despite the implementation of security measures, our internal computer systems, and those of our CROs and other third parties on which we rely, are vulnerable to privacy and information security incidents, such as data breaches, damage from computer viruses and unauthorized access, malware, natural disasters, fire, terrorism, war and telecommunication, electrical failures, cyber-attacks or cyber-intrusions over the Internet, attachments to emails, persons inside our organization or persons with access to systems inside our organization. The risk of a security breach or disruption, particularly through cyber-attacks or cyber intrusion, including by computer hackers, foreign governments and cyber terrorists, has generally increased as the number, intensity and sophistication of attempted attacks and intrusions from around the world have increased. While we have not experienced any such material system failure or security breach to our knowledge to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and our business operations. For example, the loss of clinical trial data from completed, ongoing or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we rely on third parties for the manufacture of our product candidates and to conduct clinical trials, and similar events relating to their computer systems could also have a material adverse effect on our business.
86
Table of Contents
Unauthorized disclosure of sensitive or confidential data, including personally identifiable information, whether through a breach of computer systems, systems failure, employee negligence, fraud or misappropriation, or otherwise, or unauthorized access to or through our information systems and networks, whether by our employees or third parties, could result in negative publicity, legal liability and damage to our reputation. Unauthorized disclosure of personally identifiable information could also expose us to sanctions for violations of data privacy laws and regulations around the world. To the extent that any disruption or security breach resulted in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development of our product candidates could be delayed.
As we become more dependent on information technologies to conduct our operations, cyber incidents, including deliberate attacks and attempts to gain unauthorized access to computer systems and networks, may increase in frequency and sophistication. These threats pose a risk to the security of our systems and networks, the confidentiality and the availability and integrity of our data and these risks apply both to us, and to third parties on whose systems we rely for the conduct of our business. Because the techniques used to obtain unauthorized access, disable or degrade service or sabotage systems change frequently and often are not recognized until launched against a target, we and our partners may be unable to anticipate these techniques or to implement adequate preventative measures. Further, we do not have any control over the operations of the facilities or technology of our cloud and service providers, including any third party vendors that collect, process and store personal data on our behalf. Our systems, servers and platforms and those of our service providers may be vulnerable to computer viruses or physical or electronic break-ins that our or their security measures may not detect. Individuals able to circumvent such security measures may misappropriate our confidential or proprietary information, disrupt our operations, damage our computers or otherwise impair our reputation and business. We may need to expend significant resources and make significant capital investment to protect against security breaches or to mitigate the impact of any such breaches. There can be no assurance that we or our third party providers will be successful in preventing cyber attacks or successfully mitigating their effects. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our future product candidates could be delayed.
Risks Related to Ownership of Our Securities
The market price of our securities may be volatile and fluctuate substantially, which could result in substantial losses for our investors and may subject us to securities litigation suits.
The market price of our securities may be volatile. The stock market in general and the market for pharmaceutical companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, investors may not be able to sell their securities or above the price they paid. The market price for our securities may be influenced by many factors, including:
| • | adverse regulatory decisions; |
| • | any delay in our regulatory filings for our product candidates and any adverse development or perceived adverse development with respect to the applicable regulatory authority’s review of such filings, including without limitation the FDA’s issuance of a “refusal to file” letter or a request for additional information; |
| • | the impact of the continued effects of and responses to the ongoing COVID-19 pandemic; |
| • | the commencement, enrollment or results of any future clinical trials we may conduct, or changes in the development status of our product candidates; |
| • | adverse results from, delays in or termination of clinical trials; |
| • | unanticipated serious safety concerns related to the use of our product candidates; |
| • | lower than expected market acceptance of our product candidates following approval for commercialization; |
87
Table of Contents
| • | changes in financial estimates by us or by any securities analysts who might cover our securities; |
| • | conditions or trends in our industry; |
| • | changes in the market valuations of similar companies; |
| • | stock market price and volume fluctuations of comparable companies and, in particular, those that operate in the pharmaceutical industry; |
| • | publication of research reports about us or our industry or positive or negative recommendations or withdrawal of research coverage by securities analysts; |
| • | announcements by us or our competitors of significant acquisitions, strategic partnerships or divestitures; |
| • | announcements of investigations or regulatory scrutiny of our operations or lawsuits filed against us; |
| • | investors’ general perception of our company and our business; |
| • | recruitment or departure of key personnel; |
| • | overall performance of the equity markets; |
| • | trading volume of our securities; |
| • | disputes or other developments relating to intellectual property rights, including patents, litigation matters and our ability to obtain, maintain, defend, protect and enforce patent and other intellectual property rights for our technologies; |
| • | significant lawsuits, including patent or stockholder litigation; |
| • | proposed changes to healthcare laws in the U.S. or foreign jurisdictions, or speculation regarding such changes; |
| • | general political and economic conditions; and |
| • | other events or factors, many of which are beyond our control. |
In addition, in the past, stockholders have initiated class action lawsuits against pharmaceutical and biotechnology companies following periods of volatility in the market prices of these companies’ stock. Such litigation, if instituted against us, could cause us to incur substantial costs and divert management’s attention and resources from our business.
The dual-class structure of our common stock has the effect of concentrating voting power with our Chief Executive Officer, which limits other stockholders’ ability to influence the outcome of important transactions, including a change in control.
Dr. Hung holds all of the outstanding shares of our Class B common stock and 27.2% of our Class A and Class B common stock outstanding. In addition to voting together with the Class A common stock (with one vote per share) on all matters, the holders of Class B common stock have (i) the right to elect and remove without cause three of our directors plus at least 50% of all directors in excess of seven and (ii) an approval right over any acquisition (whether by merger, sale of shares or sale of assets) or our liquidation. Accordingly, Dr. Hung has the ability to control or exert substantial influence over all matters submitted to our stockholders for approval, including the election of directors and amendments of our organizational documents, and an approval right over any acquisition or liquidation of our company. Dr. Hung may have interests that differ from those of the other stockholders and may vote in a way with which the other stockholders disagree and which may be adverse to their interests. This concentrated control may have the effect of delaying, preventing or deterring a change in control, could deprive our stockholders of an opportunity to receive a premium for their capital stock as part of a sale of our company, and might ultimately affect the market price of shares of our Class A common stock. For information about our dual-class structure, see the section titled “Description of Securities” in this proxy statement.
88
Table of Contents
We cannot predict the impact our dual-class structure may have on the market price of our Class A common stock.
We cannot predict whether our dual-class structure, combined with the concentrated voting power of Dr. Hung by virtue of his ownership of 100% of the outstanding shares of our Class B common stock, will result in a lower or more volatile market price of our Class A common stock in the future, or in adverse publicity or other adverse consequences. Certain index providers have announced restrictions on including companies with multi-class share structures in certain of their indices. For example, in July 2017, FTSE Russell and Standard & Poor’s announced that they would cease to allow most newly public companies utilizing dual or multi-class capital structures to be included in their indices. Under the announced policies, our dual-class capital structure makes us ineligible for inclusion in any of these indices. Given the sustained flow of investment funds into passive strategies that seek to track certain indices, exclusion from stock indices would likely preclude investment by many of these funds and could make our securities less attractive to other investors. As a result, the market price of our Class A common stock could be adversely affected.
There can be no assurance that we will be able to comply with the continued listing standards of the NYSE.
Our Class A common stock and Public Warrants are listed on the NYSE under the symbols “NUVB” and “NUVBW,” respectively. Our continued eligibility for listing will depend on our compliance with the continued listing standards of the NYSE and may depend on the number of our shares that are redeemed. If the NYSE delists our securities from trading on its exchange for failure to meet the listing standards, we and our stockholders could face significant negative consequences including:
| • | limited availability of market quotations for our securities; |
| • | a determination that our common stock is a “penny stock” which will require brokers trading in New our common stock to adhere to more stringent rules, possibly resulting in a reduced level of trading activity in the secondary trading market for shares of our common stock; |
| • | a limited amount of analyst coverage; and |
| • | a decreased ability to issue additional securities or obtain additional financing in the future. |
Future sales, or the perception of future sales, by us or our stockholders in the public market following the merger could cause the market price for our securities to decline.
The sale of our securities in the public market, or the perception that such sales could occur, could harm the prevailing market price of our securities. These sales, or the possibility that these sales may occur, also might make it more difficult for us to sell equity securities in the future at a time and at a price that we deems appropriate.
As of the consummation of the Business Combination, we had a total of approximately 217,650,055 shares of common stock outstanding, consisting of approximately 216,650,055 shares of Class A common stock and 1,000,000 shares of Class B common stock. All shares issued in the merger are freely tradable without registration under the Securities Act, and without restriction by persons other than our “affiliates” (as defined under Rule 144 of the Securities Act, “Rule 144”), including our directors, executive officers and other affiliates.
On October 20, 2020, Legacy Nuvation Bio entered into certain agreements restricting the transfer of our securities held by such contracting parties immediately following the closing date, including agreements with (i) the Sponsor and Dr. Hung (the “Sponsor and Founder Lock-Up Agreement”), (ii) purchasers under the forward purchase agreement (the “FPA Lock-Up Agreement”) and (iii) certain of Legacy Nuvation Bio stockholders (the “Stockholder Lock-Up Agreements”). In the case of the Sponsor and Founder Lock-Up Agreement and the FPA Lock-Up Agreement, such restrictions begin at the closing date and end on the earlier of (i) the date that is 365 days after the closing date, (ii) the closing date of a merger, liquidation, stock exchange, reorganization or other similar transaction after the closing date that results in all of our public stockholders having the right to
89
Table of Contents
exchange their shares of common stock for cash securities or other property, or (iii) the day after the date on which the closing price of the our Class A common stock equals or exceeds $12.00 per share for any 20 trading days within any 30-trading day period commencing at least 150 days after the closing date. In the case of the Stockholder Lock-Up Agreements, such restrictions begin at the closing date and end on the earlier of (i) the date that is 180 days after the closing date, (ii) the closing of a merger, liquidation, stock exchange, reorganization or other similar transaction after the closing date that results in all of our public stockholders having the right to exchange their shares of common stock for cash securities or other property, or (iii) the day after the date on which the closing price of our Class A common stock equals or exceeds $12.00 per share for any 20 trading days within any 30-trading day period commencing at least 150 days after the closing of the merger.
The shares covered by registration rights represent approximately 28.0% of the outstanding shares of common stock.
In addition, the shares of Class A common stock reserved for future issuance under our equity incentive plans will become eligible for sale in the public market once those shares are issued, subject to provisions relating to various vesting agreements, lock-up agreements and, in some cases, limitations on volume and manner of sale applicable to affiliates under Rule 144, as applicable. A total of approximately 19.2% of the fully diluted shares of common stock and Class B common stock are expected to be reserved for future issuance under our equity incentive plans. Our compensation committee of our board of directors may determine the exact number of shares to be reserved for future issuance under our equity incentive plans at its discretion. We are expected to file one or more registration statements on Form S-8 under the Securities Act to register shares of Class A common stock or securities convertible into or exchangeable for shares of Class A common stock issued pursuant to our equity incentive plans. Any such Form S-8 registration statements will automatically become effective upon filing. Accordingly, shares registered under such registration statements will be available for sale in the open market.
In the future, we may also issue its securities in connection with investments or acquisitions. The amount of shares of Class A common stock issued in connection with an investment or acquisition could constitute a material portion of our then-outstanding shares of Class A common stock. Any issuance of additional securities in connection with investments or acquisitions may result in additional dilution to our stockholders.
Because we do not anticipate paying any cash dividends on our Class A common stock in the foreseeable future, capital appreciation, if any, will be your sole source of gains and you may never receive a return on your investment.
We may retain future earnings, if any, for future operations, expansion and debt repayment and have no current plans to pay any cash dividends for the foreseeable future. Any decision to declare and pay dividends as a public company in the future will be made at the discretion of our board of directors and will depend on, among other things, our results of operations, financial condition, cash requirements, contractual restrictions and other factors that our board of directors may deem relevant. In addition, our ability to pay dividends may be limited by covenants of any existing and future outstanding indebtedness we or our subsidiaries incur. As a result, you may not receive any return on an investment in our securities unless you sell your securities for a price greater than that which you paid for it.
There is no guarantee that our warrants will be in the money at the time they become exercisable, and they may expire worthless.
The exercise price for our warrants, including our Public Warrants, is $11.50 per share of Class A common stock. There is no guarantee that any of our warrants will be in the money following the time they become exercisable and prior to their expiration, and as such, the warrants may expire worthless.
90
Table of Contents
We may issue additional shares securities without your approval, which would dilute your ownership interests and may depress the market price of our securities.
We have options outstanding to purchase approximately 9,571,976 shares of Class A common stock. Pursuant to the 2021 Plan and the Employee Stock Purchase Plan (“ESPP”), we may issue an aggregate of up to 20.5% of the fully diluted shares of Class A common stock and Class B common stock, which amount will be subject to increase from time to time. For additional information about the plan, please see “Executive Compensation — Employee Benefit Plans.” We may also issue additional shares of Class A common stock or other equity securities of equal or senior rank in the future in connection with, among other things, future acquisitions or repayment of outstanding indebtedness, without stockholder approval, in a number of circumstances.
The issuance of additional shares or other equity securities of equal or senior rank would have the following effects:
| • | existing stockholders’ proportionate ownership interest in our company will decrease; |
| • | the amount of cash available per share, including for payment of dividends in the future, may decrease; |
| • | the relative voting strength of each previously outstanding common stock may be diminished; and |
| • | the market price of our securities may decline. |
Anti-takeover provisions in our amended and restated certificate of incorporation and under Delaware law could make an acquisition of our company, which may be beneficial to our stockholders, more difficult, and may prevent attempts by our stockholders to replace or remove our current management.
Our amended and restated certificate of incorporation contains provisions that may delay or prevent an acquisition of the company or change in our management in addition to the significant rights of Dr. Hung as the holder of 100% of the outstanding shares of our Class B common stock. These provisions may make it more difficult for stockholders to replace or remove members of our board of directors. Because the board of directors is responsible for appointing the members of the management team, these provisions could in turn frustrate or prevent any attempt by its stockholders to replace or remove our current management. In addition, these provisions could limit the price that investors might be willing to pay in the future for shares of our Class A common stock. Among other things, these provisions include:
| • | the limitation of the liability of, and the indemnification of, our directors and officers; |
| • | a prohibition on actions by our stockholders except at an annual or special meeting of stockholders; |
| • | a prohibition on actions by our stockholders by written consent; and |
| • | the ability of the board of directors to issue preferred stock without stockholder approval, which could be used to institute a “poison pill” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by the board of directors. |
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law (the “DGCL”), which prohibits a person who owns 15% or more of its outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired 15% or more of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner. This could discourage, delay or prevent a third party from acquiring or merging with us, whether or not it is desired by, or beneficial to, its stockholders. This could also have the effect of discouraging others from making tender offers for our Class A common stock, including transactions that may be in our stockholders’ best interests. Finally, these provisions establish advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings. These provisions would apply even if the offer may be considered beneficial by some stockholders. For more information, see the section titled “Description of Securities”.
91
Table of Contents
Our amended and restated certificate of incorporation provides that the Court of Chancery of the State of Delaware and the federal district courts of the United States of America are the exclusive forums for substantially all disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our amended and restated certificate of incorporation provides that the Court of Chancery of the State of Delaware will be the exclusive forum for the following types of actions or proceedings under Delaware statutory or common law:
| • | any derivative action or proceeding brought on our behalf; |
| • | any action asserting a breach of fiduciary duty; |
| • | any action asserting a claim against us arising under the Delaware General Corporation Law, our amended and restated certificate of incorporation or our amended and restated bylaws; and |
| • | any action asserting a claim against us that is governed by the internal-affairs doctrine or otherwise related to our internal affairs. |
To prevent having to litigate claims in multiple jurisdictions and the threat of inconsistent or contrary rulings by different courts, among other considerations, our amended and restated certificate of incorporation further provides that the federal district courts of the United States of America will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act. This provision would not apply to suits brought to enforce a duty or liability created by the Exchange Act. Furthermore, Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all such Securities Act actions. Accordingly, both state and federal courts have jurisdiction to entertain such claims. While the Delaware courts have determined that such choice of forum provisions are facially valid, a stockholder may nevertheless seek to bring a claim in a venue other than those designated in the exclusive forum provisions. In such instance, we would expect to vigorously assert the validity and enforceability of the exclusive forum provisions of our amended and restated certificate of incorporation. This may require significant additional costs associated with resolving such action in other jurisdictions and there can be no assurance that the provisions will be enforced by a court in those other jurisdictions.
These exclusive forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage lawsuits against us and our directors, officers and other employees. If a court were to find either exclusive-forum provision in our amended and restated certificate of incorporation to be inapplicable or unenforceable in an action, we may incur further significant additional costs associated with resolving the dispute in other jurisdictions, all of which could harm our business.
We are an “emerging growth company” and a “smaller reporting company,” and as a result of the reduced reporting requirements applicable to “emerging growth companies” and “smaller reporting companies,” our securities may be less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. For as long as we continue to be an “emerging growth company,” we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies,” including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. As an “emerging growth company,” we are required to report only two years of financial results and selected financial data compared to three and five years, respectively, for comparable data reported by other public companies. We may take advantage of these exemptions until we are no longer an “emerging growth company.” We could be an “emerging growth company” until December 31, 2025, although circumstances could cause us to lose that status earlier, including if the aggregate market value of our securities held by non-affiliates exceeds $700 million as of any June 30 (the end of
92
Table of Contents
our second quarter) before that time, in which case we would no longer be an “emerging growth company” as of the following December 31 (our year-end). Even after we no longer qualify as an “emerging growth company,” we may still qualify as a “smaller reporting company,” which would allow us to continue to take advantage of many of the same exemptions from disclosure requirements, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We cannot predict if investors will find our securities less attractive because we may rely on these exemptions. If some investors find our securities less attractive as a result, there may be a less active trading market for our securities and the price of our securities may be more volatile.
General Risk Factors
If we fail to maintain proper and effective internal controls, our ability to produce accurate financial statements on a timely basis could be impaired.
We are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, the Sarbanes-Oxley Act and the rules and regulations of the NYSE. Section 302 of the Sarbanes-Oxley Act requires, among other things, that public companies report on the effectiveness of its disclosure controls and procedures in our quarterly and annual reports and, beginning with our annual report for the year ending 2021, Section 404 of the Sarbanes-Oxley Act requires that we perform system and process evaluation and testing of our internal control over financial reporting to allow management to report on the effectiveness of our internal control over financial reporting in our Form 10-K filing for that year. This will require that we incur substantial additional professional fees and internal costs to expand our accounting and finance functions and that we expend significant management efforts. To date, we have not been required to test our internal control within a specified period, and, as a result, we may experience difficulty in meeting these reporting requirements in a timely manner. In addition, our independent registered public accounting firm will be required to attest to the effectiveness of our internal control over financial reporting in our first annual report required to be filed with the SEC following the date we are no longer an emerging growth company or a smaller reporting company.
If we are not able to comply with the requirements of Section 404 of the Sarbanes-Oxley Act in a timely manner, or if we are unable to maintain proper and effective internal controls, we may not be able to produce timely and accurate financial statements. If that were to happen, the market price of our stock could decline and we could be subject to sanctions or investigations by the NYSE, the SEC or other regulatory authorities. In addition, our securities may not be able to remain listed on the NYSE or any other securities exchange.
We will incur costs and demands upon our management as a result of complying with the laws and regulations affecting public companies in the U.S., which may harm our business.
As a public company listed in the U.S., we will incur significant additional legal, accounting and other expenses. In addition, changing laws, regulations and standards relating to corporate governance and public disclosure, including regulations implemented by the SEC and the NYSE may increase legal and financial compliance costs and make some activities more time consuming. These laws, regulations and standards are subject to varying interpretations and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management’s time and attention from regular business activities to compliance activities. If, notwithstanding our efforts, we fail to comply with new laws, regulations and standards, regulatory authorities may initiate legal proceedings against us and our business may be harmed.
Failure to comply with these rules might also make it more difficult for us to obtain certain types of insurance, including director and officer liability insurance, and we might be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these events could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, on committees of our board of directors or as members of senior management.
93
Table of Contents
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they change their recommendations regarding our securities adversely, the price and trading volume of our securities could decline.
We do not currently have and may never obtain research coverage by equity research analysts. Equity research analysts may elect not to provide research coverage of our securities after the closing of this Business Combination, and such lack of research coverage may adversely affect the market price of our securities. In the event we do have equity research analyst coverage, we will not have any control over the analysts or the content and opinions included in their reports. The price of our stock could decline if one or more equity research analysts downgrade our stock or issue other unfavorable commentary or research. If one or more equity research analysts ceases coverage of our company or fails to publish reports on us regularly, demand for our stock could decrease, which in turn could cause our securities’ prices or trading volume to decline.
| Item 1B. | Unresolved Staff Comments. |
None.
| Item 2. | Properties. |
Our principal executive office is located in New York, New York, where we lease approximately 7,900 square feet of office space under a lease that terminates in 2027. We also occupy approximately 8,200 square feet of office space in San Francisco, California, under a lease that terminates in 2022. We believe that these existing facilities will be adequate for our current needs and that suitable additional or alternative space will be available in the future on commercially reasonable terms, if required.
| Item 3. | Legal Proceedings. |
From time to time, we may become involved in legal proceedings or be subject to claims arising in the ordinary course of our business. We are not currently a party to any material legal proceedings. Regardless of outcome, such proceedings or claims can have an adverse impact on us because of defense and settlement costs, diversion of resources and other factors, and there can be no assurances that favorable outcomes will be obtained.
| Item 4. | Mine Safety Disclosures. |
Not applicable.
94
Table of Contents
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. |
Market Information
On February 10, 2021, Panacea and Legacy Nuvation Bio completed the Business Combination. Following the Business Combination, we changed the name of the combined company to Nuvation Bio Inc.
Our Class A common stock and warrants to purchase Class A common stock originally began trading as units on The New York Stock Exchange on July 1, 2020. Prior to July 1, 2020, there was no public market for our securities. Following the Business Combination, beginning February 11, 2021, our Class A common stock and warrants to purchase Class A common stock continued trading on The New York Stock Exchange under the symbols “NUVB” and “NUVB.WS,” respectively.
Holders of Record
As of March 9, 2021, there were approximately 141 holders of record of our Class A common stock and five holders of record of our warrants to purchase shares of our Class A common stock.
Dividend Policy
We have never declared or paid cash dividends on our capital stock. We intend to retain all available funds and any future earnings, if any, to fund the development and expansion of our business and we do not anticipate paying any cash dividends in the foreseeable future. Any future determination related to dividend policy will be made at the discretion of our board of directors.
Recent Sales of Unregistered Securities
On July 6, 2020, we consummated our initial public offering (the “Initial Public Offering” or “Panacea IPO”) of 14,375,000 Units, which includes the full exercise by the underwriters of their over-allotment option in the amount of 1,875,000. The Units sold in the Initial Public Offering were sold at an offering price of $10.00 per unit, generating total gross proceeds of $143,750,000. Cowen and Company, LLC acted as sole book-running manager. The securities in the offering were registered under the Securities Act on a registration statement on Form S-1 (No. 333-239138). The Securities and Exchange Commission declared the registration statement effective on June 30, 2020.
Simultaneous with the consummation of the Initial Public Offering and the closing of the over-allotment option, we consummated the private placement of an aggregate of 487,500 units at a price of $10.00 per Private Placement Unit, generating total proceeds of $4,875,000. The issuance was made pursuant to the exemption from registration contained in Section 4(a)(2) of the Securities Act.
Of the gross proceeds received from the Initial Public Offering, the closing of the over-allotment option and the Private Placement Units, $143,750,000 was placed in the Trust Account.
We paid a total of $2,875,000 in underwriting discounts and commissions and $515,063 for other offering costs related to the Initial Public Offering.
On February 10, 2021, in connection with the closing of the Business Combination, a number of purchasers purchased from the Company an aggregate of 47,655,000 shares of Class A Common Stock (the “PIPE Shares”), for a purchase price of $10.00 per share and an aggregate purchase price of approximately $476.6 million, pursuant to separate subscription agreements entered into concurrently with the Business Combination Agreement. The sale was made pursuant to the exemption from registration contained in Section 4(a)(2) of the Securities Act.
Additionally, on February 10, 2021, in connection with the closing of the Business Combination, certain purchasers purchased 2,500,000 shares of Class A common stock and 833,333 forward purchase warrants (the “Forward Purchase Securities”) in a private placement at a price of $10.00 per share for an aggregate purchase price of $25.0 million pursuant to the terms of the forward purchase agreement that Panacea entered into in connection with the Initial Public Offering. Each whole warrant entitles the holder to purchase one share of Class A common stock at an exercise price of $11.50 per share. The sale was made pursuant to the exemption from registration contained in Section 4(a)(2) of the Securities Act. The sales of the PIPE Shares and the Forward Purchase Securities were consummated concurrently with the closing of the Business Combination.
On March 2, 2021, we, Legacy Nuvation Bio, GiraF (as defined below) and Dr. Hung entered into an Agreement Regarding Subsequent Shares under which (i) Nuvation Bio agreed to issue 368,408 shares of Class A common stock in satisfaction of Legacy Nuvation Bio’s obligations with respect to the Additional GiraF Shares (as defined below) and (ii) Dr. Hung agreed to surrender for cancellation an equal number of shares of Class A common stock. GiraF contributed to Legacy Nuvation Bio all of its intellectual property rights with respect to specified drug development programs to be pursued by Legacy Nuvation Bio in exchange for, among other things, Legacy Nuvation Bio’s agreement to issue the Additional GiraF Shares. This issuance will be made pursuant to the exemption from registration contained in Section 4(a)(2) of the Securities Act.
| Item 6. | Selected Financial Data. |
Not applicable.
95
Table of Contents
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with our consolidated financial statements and related notes included in Part II, Item 8 of this report.
Unless otherwise indicated, references in this section to the terms “Panacea,” the “Company,” “we,” “our” and “us” refer to Panacea Acquisition Corp. prior to the Business Combination. The term “Legacy Nuvation Bio” refers to privately-held Nuvation Bio Inc. prior to its merger with Panacea Merger Subsidiary Corp., a wholly owned subsidiary of Panacea Acquisition Corp. The term “Panacea” refers to Panacea Acquisition Corp. prior to the Business Combination.
The financial information included in this Management’s Discussion and Analysis of Financial Condition and Results of Operations is that of Panacea prior to the Business Combination because the Business Combination was consummated after the period covered by the financial statements included in this Annual Report on Form 10-K. Accordingly, the historical financial information included in this Annual Report on Form 10-K, unless otherwise indicated or as the context otherwise requires, is that of Panacea prior to the Business Combination.
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Exchange Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. Forward-looking statements are identified by words such as “believe,” “will,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “expect,” “predict,” “could,” “potentially” or the negative of these terms or similar expressions. You should read these statements carefully because they discuss future expectations, contain projections of future results of operations or financial condition, or state other “forward-looking” information. These statements relate to our future plans, objectives, expectations, intentions and financial performance and the assumptions that underlie these statements. These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from those anticipated in the forward-looking statements. Factors that might cause such a difference include, but are not limited to, those discussed in this report in Part I, Item 1A — “Risk Factors,” and elsewhere in this report. Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. These statements, like all statements in this report, speak only as of their date, and we undertake no obligation to update or revise these statements in light of future developments. We caution investors that our business and financial performance are subject to substantial risks and uncertainties. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based on information available to us as of the date of this Annual Report on Form 10-K. While we believe that information provides a reasonable basis for these statements, that information may be limited or incomplete. Our statements should not be read to indicate that we have conducted an exhaustive inquiry into or review of, all relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely on these statements.
96
Table of Contents
Introduction and Recent Developments
As of December 31, 2020, we were a blank check company formed under the laws of the State of Delaware on April 24, 2020 for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or other similar business combination with one or more businesses.
On October 20, 2020, we entered into the Agreement and Plan of Merger with Panacea Merger Subsidiary Corp. and Legacy Nuvation Bio, pursuant to which Merger Sub merged with and into Legacy Nuvation Bio, with Legacy Nuvation Bio surviving the merger and as our wholly owned subsidiary. On February 10, 2021, upon consummation of the Business Combination, we changed our name to Nuvation Bio Inc. The discussion below relates to Panacea prior to the Business Combination and, except as otherwise specifically stated, does not reflect the Business Combination.
Results of Operations
We have neither engaged in any operations (other than searching for a Business Combination after our Initial Public Offering) nor generated any revenues to date. Our only activities from April 24, 2020 (inception) through December 31, 2020 were organizational activities, those necessary to prepare for the Initial Public Offering, described below. We did not generate any operating revenues until after the completion of our Business Combination. We generated non-operating income in the form of interest earned on investments held after the Initial Public Offering. We incurred expenses as a result of being a public company (for legal, financial reporting, accounting and auditing compliance), as well as for due diligence expenses.
For the period from April 24, 2020 (inception) through December 31, 2020, we had a net loss of $3,054,441, which consists of operating costs of $3,061,452, offset by interest income on investments held in the Trust Account of $7,011.
Liquidity and Capital Resources
On July 6, 2020, we consummated the Initial Public Offering of 14,375,000 Units at a price of $10.00 per Unit, which included the full exercise by the underwriter of their over-allotment option in the amount of 1,875,000, generating gross proceeds of $143,750,000. Simultaneously with the closing of the Initial Public Offering, we consummated the sale of 487,500 Private Placement Units at a price of $10.00 per Private Placement Unit in a private placement to certain stockholders, generating gross proceeds of $4,875,000.
Following the Initial Public Offering, the full exercise of the over-allotment option by the underwriters and the sale of the Private Placement Units, a total of $143,750,000 was placed in the Trust Account. We incurred $3,390,063 in transaction costs, including $2,875,000 of underwriting fees and $515,063 of other offering costs.
For the period from April 24, 2020 (inception) through December 31, 2020, cash used in operating activities was $700,826. Net loss of $3,054,441 was reduced by interest earned on investments held in the Trust Account of $7,011, offset by net changes in operating assets and liabilities of $2,360,626.
As of December 31, 2020, we had investments held in the Trust Account of $143,757,011. We used substantially all of the funds held in the Trust Account, including any amounts representing interest earned on the Trust Account to complete our Business Combination. During the period ended December 31, 2020, we did not withdraw any interest income from the Trust Account. The remaining proceeds held in the Trust Account will be used as working capital to finance the operations of the target business or businesses, make other acquisitions and pursue our growth strategies.
As of December 31, 2020, we had $908,111 of cash held outside of the Trust Account. We used the funds held outside the Trust Account primarily to identify and evaluate target businesses, perform business due diligence on prospective target businesses, travel to and from the offices, plants or similar locations of prospective target businesses or their representatives or owners, review corporate documents and material agreements of prospective target businesses, and structure, negotiate and complete a Business Combination.
In order to fund working capital deficiencies or finance transaction costs in connection with a Business Combination, the Sponsors, PA Co-Investments LLC or an affiliate of the Sponsor or PA Co-Investments LLC, or certain of the Company’s officers and directors or their affiliates could, but were not obligated to, loan us funds as may have been required. The Working Capital Loans would either be repaid upon consummation of a Business Combination, without interest, or, at the lender’s discretion, up to $1,500,000 of such Working Capital Loans may have been convertible into units of the post Business Combination entity. The units would be identical to the Private Placement Units. Except for the foregoing, the terms of such Working Capital Loans, if any, were not determined and no written agreements exist with respect to such loans. As of December 31, 2020, there were no amounts outstanding under such Working Capital Loans.
97
Table of Contents
Off-Balance Sheet Financing Arrangements
We have no obligations, assets or liabilities, which would be considered off-balance sheet arrangements as of December 31, 2020. We did not participate in transactions that created relationships with unconsolidated entities or financial partnerships, often referred to as variable interest entities, which would have been established for the purpose of facilitating off-balance sheet arrangements. We have not entered into any off-balance sheet financing arrangements, established any special purpose entities, guaranteed any debt or commitments of other entities, or purchased any non-financial assets.
Contractual Obligations
As of December 31, 2020, we did not have any long-term debt, capital lease obligations, operating lease obligations or long-term liabilities, other than an agreement to pay an affiliate of the Sponsor a monthly fee of $10,000 for office space, administrative and support services to us. We began incurring these fees on July 1, 2020.
We engaged the underwriters as an advisor in connection with a Business Combination to assist us in holding meetings with its stockholders to discuss the potential Business Combination and the target business’ attributes, introduce us to potential investors that are interested in purchasing our securities in connection with a Business Combination, assist us in obtaining stockholder approval for the Business Combination and assist us with its press releases and public filings in connection with the Business Combination. We paid the underwriters a cash fee for such services upon the consummation of the Business Combination in an amount equal to, in the aggregate, 3.5% of the gross proceeds of the Initial Public Offering, or $5,031,250, which included any proceeds from the full or partial exercise of the over-allotment option.
In addition, on June 30, 2020, we entered into a forward purchase agreement with funds affiliated with EcoR1 Capital, LLC that will provide for the purchase by such funds of an aggregate of 2,500,000 shares of Class A common stock and 833,333 redeemable warrants, for an aggregate purchase price of $25,000,000, or $10.00 per one share of Class A common stock and one-third of one redeemable warrant, in a private placement to close substantially concurrently with the closing of a Business Combination. The obligations under the forward purchase agreement did not depend on whether any shares of Class A common stock were redeemed by the Public Stockholders. The shares of Class A common stock and redeemable warrants issued pursuant to the forward purchase agreement were identical to the shares of Class A common stock and redeemable warrants included in the units being sold in the Initial Public Offering, respectively, except that the holders thereof have certain registration rights. On the closing date of the Business Combination, the forward purchase securities were purchased in full.
Critical Accounting Policies
The preparation of consolidated financial statements and related disclosures in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and income and expenses during the periods reported. Actual results could materially differ from those estimates. We have identified the following critical accounting policies:
Class A Common Stock Subject to Possible Redemption
We account for our Class A common stock subject to possible redemption in accordance with the guidance in the Financial Accounting Standard Board’s (“FASB”) Accounting Standards Codification (“ASC”) Topic 480 “Distinguishing Liabilities from Equity.” Shares of Class A common stock subject to mandatory redemption are classified as a liability instrument and are measured at fair value. Conditionally redeemable common stock (including common stock that features redemption rights that is either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within our control) is classified as temporary equity. At all other times, common stock is classified as stockholders’ equity. Our Class A common stock features certain redemption rights that are considered to be outside of our control and subject to occurrence of uncertain future events. Accordingly, shares of Class A common stock subject to possible redemption are presented as temporary equity, outside of the stockholders’ equity section of our consolidated balance sheet.
98
Table of Contents
Net Income (Loss) per Common Share
We apply the two-class method in calculating earnings per share. Net income per common share, basic and diluted for Class A redeemable common stock is calculated by dividing the interest income earned on the Trust Account, net of applicable franchise and income taxes, by the weighted average number of Class A redeemable common stock outstanding for the period. Net loss per common share, basic and diluted for Class B non-redeemable common stock is calculated by dividing the net income, less income attributable to Class A redeemable common stock, by the weighted average number of Class B non-redeemable common stock outstanding for the period presented.
Recent Accounting Standards
Management does not believe that any other recently issued, but not yet effective, accounting standards, if currently adopted, would have a material effect on our consolidated financial statements.
| Item 7A. | Quantitative and Qualitative Disclosures about Market Risk |
As of December 31, 2020, we were not subject to any market or interest rate risk. Following the consummation of our Initial Public Offering, the net proceeds of our Initial Public Offering, including amounts in the Trust Account, have been invested in U.S. government treasury obligations with a maturity of 185 days or less or in certain money market funds that invest solely in U.S. treasuries. Due to the short-term nature of these investments, we believe there will be no associated material exposure to interest rate risk.
| Item 8. | Financial Statements and Supplementary Data |
This information appears following Item 15 of this report and is included herein by reference.
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
As previously reported on Form 8-K, as filed with the SEC on February 12, 2021, the audit committee of our board of directors approved the engagement of KPMG LLP (“KPMG”) as our independent registered public accounting firm to audit our consolidated financial statements for the year ending December 31, 2021 on February 10, 2021. KPMG served as the independent registered public accounting firm of Legacy Nuvation Bio prior to the Business Combination. Accordingly, WithumSmith+Brown, PC, Panacea’s independent registered public accounting firm prior to the Business Combination, was informed on February 12, 2021 that it would be replaced by KPMG as our independent registered public accounting firm following (i) completion of our audit of the year ended December 31, 2020, which consists only of the accounts of the pre-Business Combination special purpose acquisition company, Panacea, and (ii) the filing of this Annual Report on Form 10-K for the fiscal year ended December 31, 2020.
| Item 9A. | Controls and Procedures. |
Evaluation of Disclosure Controls and Procedures
Disclosure controls are procedures that are designed with the objective of ensuring that information required to be disclosed in our reports filed under the Exchange Act, such as this report, is recorded, processed, summarized, and reported within the time period specified in the SEC’s rules and forms. Disclosure controls are also designed with the objective of ensuring that such information is accumulated and communicated to our management, including the chief executive officer and chief financial officer, as appropriate to allow timely decisions regarding required disclosure. Our management evaluated, with the participation of our current chief executive officer and chief financial officer (our “Certifying Officers”), the effectiveness of our disclosure controls and procedures as of December 31, 2020, pursuant to Rule 13a-15(b) under the Exchange Act. Based upon that evaluation, our Certifying Officers concluded that, as of December 31, 2020, our disclosure controls and procedures were effective.
We do not expect that our disclosure controls and procedures will prevent all errors and all instances of fraud. Disclosure controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the disclosure controls and procedures are met. Further, the design of disclosure controls and procedures must reflect the fact that there are resource constraints, and the benefits must be considered relative to their costs. Because of the inherent limitations in all disclosure controls and procedures, no evaluation of disclosure controls and procedures can provide absolute assurance that we have detected all our control deficiencies and instances of fraud, if any. The design of disclosure controls and procedures also is based partly on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions.
99
Table of Contents
Management’s Report on Internal Controls Over Financial Reporting
This Annual Report on Form 10-K does not include a report of management’s assessment regarding internal control over financial reporting or an attestation report of our independent registered public accounting firm due to a transition period established by rules of the SEC for newly public companies.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act) during the most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. | Other |
None.
100
Table of Contents
| Item 10. | Directors, Executive Officers and Corporate Governance. |
As used in this section, “our” refers to Legacy Nuvation Bio prior to the closing of the Business Combination and Nuvation Bio Inc. after the closing of the Business Combination. Upon the closing of the Business Combination, the executive officers of Legacy Nuvation Bio became executive officers of Nuvation Bio Inc.
Our current directors and executive officers are as follows:
| Name |
Age | Position | ||||
| Executive Officers |
||||||
| David Hung, M.D. |
63 | Founder, President, Chief Executive Officer and Director | ||||
| Jennifer Fox |
49 | Chief Financial Officer | ||||
| Sergey Yurasov, M.D., Ph.D. |
52 | Chief Medical Officer | ||||
| Gary Hattersley, Ph.D. |
54 | Chief Scientific Officer | ||||
| Thomas Templeman, Ph.D. |
61 | Senior Vice President, Pharmaceutical Operations and Quality | ||||
| Stacy Markel |
56 | Senior Vice President, Human Resources | ||||
| Non-Employee Directors |
||||||
| Daniel G. Welch(1)(2) |
63 | Chair of the Board | ||||
| Robert B. Bazemore Jr.(3) |
53 | Director | ||||
| Kim Blickenstaff(1)(3) |
68 | Director | ||||
| Michelle Doig |
47 | Director | ||||
| Kathryn E. Falberg(1)(2) |
60 | Director | ||||
| Oleg Nodelman |
44 | Director | ||||
| W. Anthony Vernon(2)(3) |
65 | Director | ||||
| (1) | Member the Audit Committee. |
| (2) | Member of the Nominating and Corporate Governance Committee. |
| (3) | Member of the Compensation Committee. |
Executive Officers
David Hung, M.D. is our founder and has served as our President, Chief Executive Officer and member of the board of directors since inception (April 2018). From April 2017 to February 2018, Dr. Hung served as Chief Executive Officer and a member of the board of directors of Axovant Sciences Ltd. Prior to that, in 2003, Dr. Hung founded Medivation, Inc., a pharmaceutical company, and served as its President and Chief Executive Officer, and a member of the board of directors until its acquisition by Pfizer Inc. in 2016. Prior to founding Medivation, from 1998 until 2001, Dr. Hung was employed by ProDuct Health, Inc., a privately held medical device company, as Chief Scientific Officer from 1998 to 1999 and as president and Chief Executive Officer from 1999 to 2001 until its acquisition by Cytyc Corporation. Dr. Hung also serves on the boards of directors of two public medical device companies: NovoCure Ltd., since May 2018, and Establishment Labs Holdings, since February 2016. Dr. Hung received an A.B. in Biology from Harvard College and an M.D. from the University of California, San Francisco, School of Medicine.
We believe that Dr. Hung is qualified to serve on our board of directors because of his deep knowledge of our company, history leading life sciences companies and his industry experience.
Jennifer Fox has served as our Chief Financial Officer since October 2020. Prior to this role, Ms. Fox served as Managing Director, Co-Head of North America Healthcare Corporate and Investment Banking Group at Citigroup from June 2015 to October 2020. From February 2006 to June 2015, Ms. Fox served as Managing Director at Deutsche Bank, and most recently also as Co-Head of Life Sciences Investment Banking Group. Prior to that, Ms. Fox served as Senior Managing Director Healthcare Investment Banking at Bear Stearns, Vice President Healthcare Investment Banking at Bank of America and Financial Analyst, Investment Banking Analyst, Associate, Vice President, Health Care Investment Banking at Prudential Vector Healthcare Group and Prudential Securities Incorporated. Ms. Fox received B.S. degrees in Finance and Marketing from Manhattan College.
Sergey Yurasov, M.D., Ph.D. has served as our Chief Medical Officer since September 2019. Prior to this role, Dr. Yurasov served as Senior Vice President, Clinical Development and Chief Medical Officer at Immune Design Corp., from October 2016 to July 2019. From August 2014 until September 2016, Dr. Yurosov held positions of increasing responsibility at Clovis Oncology, Inc., most recently serving as Senior Vice President, Clinical Development. From August 2010 until August 2014, Dr. Yurasov held positions of increasing responsibility at ImClone Systems Incorporated, a subsidiary of Eli Lilly & Co., most recently serving as Associate Vice-President, Global Medicine Science. Prior to that, he served as Clinical Director of Oncology, Pharma Research and Early Development at Hoffman-La Roche. Prior to his industry experience, Dr. Yurasov was Assistant Professor of Clinical Investigation at the Rockefeller University and Clinical Instructor in the Department of Pediatrics at Memorial Sloan-Kettering Cancer Center, where he was an attending physician. Dr. Yurasov received an M.D. from the Russian State Medical University and a Ph.D. in medical sciences from the Research Institute for Pediatric Oncology in Moscow, Russia.
101
Table of Contents
Gary Hattersley, Ph.D. has served as our Chief Scientific Officer since June 2019. Prior to this role, December 2003 to November 2018, Dr. Hattersley held roles of increasing seniority, including Senior Vice President of Preclinical Development, Vice President of Biology, and most recently as Chief Scientific Officer at Radius Health Inc. Prior to that, Dr. Hattersley was a Senior Scientist at Millennium Pharmaceuticals, Inc. from 2000 to 2003. He also held positions at Genetics Institute from 1992 to 2000, including Principle Scientist. Dr. Hattersley received a Ph.D. from St. George’s Hospital Medical School in London and a BSc from the University of Hull.
Thomas Templeman, Ph.D. has served as our Senior Vice President, Pharmaceutical Operations and Quality since July 2019, prior to which he served as the Senior Vice President, Chemistry, Manufacturing and Controls from April 2019 to July 2019. Since February 2018, he has also served as a consultant at Templeman Consulting. Prior to that, he served as the Senior Vice President, Pharmaceutical Operations and Quality at Axovant Sciences Ltd. from June 2017 to February 2018. From January 2017 to July 2017, he served as Chief Operating Officer of Greybug Vision. Dr. Templeman also served as Senior Vice President of Pharmaceutical Operations and Quality at Medivation, Inc. from September 2015 to November 2016, Vice President of Manufacturing Science and Technology at Hospira from 2011 until its acquisition by Pfizer in 2015, and Senior Vice President, Integrated Supply Chain at Liquidia Technologies from 2009 to 2011. Dr. Templeman received a B.S. in Biology from the University of Santa Clara, a Ph.D. in Biological Sciences from Dartmouth College, and was a post-doctoral fellow at Harvard University.
Stacy Markel has served as our Senior Vice President, Human Resources since October 2019. From March 2018 to September 2019, she served as Executive Vice President, Human Resources, at Rigel Pharmaceuticals, Inc. Prior to Rigel, from March 2015 to March 2018, Ms. Markel served as Senior Vice President of Human Resources at Portola Pharmaceuticals, Inc. Ms. Markel also served in various roles, most recently as Senior Vice President of Human Resources and Professional Development at Actelion Pharmaceuticals, Ltd. from 2005 to 2015. Ms. Markel received a B.A. from the University of California, Davis.
Non-Employee Directors
Daniel G. Welch has served as Chair of the our board since July 2020. From January 2015 to February 2018, Mr. Welch served as an Executive Partner of Sofinnova Ventures, a venture capital firm. From September 2003 until its acquisition by Roche Holdings in September 2014, Mr. Welch served as Chief Executive Officer and President of InterMune, Inc., a biotechnology company. Mr. Welch also served as Chairman of InterMune from May 2008 to September 2014. From 2002 to 2003, Mr. Welch served as Chairman and Chief Executive Officer of Triangle Pharmaceuticals, Inc., a pharmaceutical company that was acquired by Gilead Sciences. From 2000 to 2002, Mr. Welch served as President of Biopharmaceuticals at Elan Corporation. From 1987 to 2000, Mr. Welch served in various senior management roles at Sanofi-Synthelabo, now Sanofi, including Vice President of Worldwide Marketing and Chief Operating Officer of the U.S. business. From 1980 to 1987, Mr. Welch was with American Critical Care, a division of American Hospital Supply. Mr. Welch serves on the boards of directors of Intercept Pharmaceuticals, Inc., a public biopharmaceutical company, since November 2015, SeaGen Inc., a public biotechnology company, since June 2007 and Ultragenyx Pharmaceutical Inc., a public biotechnology company, since April 2015. Mr. Welch also serves on the board of directors of several private companies. Mr. Welch received a B.S. from the University of Miami and an MBA from the University of North Carolina.
We believe that Mr. Welch is a strong operating executive with operational and strategic expertise in the global pharmaceutical market, whose experience contributes valuable insight to our board of directors.
Robert B. Bazemore Jr. has served as a member of our board of directors since July 2020. Since September 2015, Mr. Bazemore has served as the President, Chief Executive Officer and member of the board of directors of Epizyme, Inc., a biopharmaceutical company. Prior to that, from September 2014 to June 2015, Mr. Bazemore served as the Chief Operating Officer of Synageva BioPharma Corp., a biopharmaceutical company. Prior to joining Synageva, Mr. Bazemore served in increasing levels of responsibility at Johnson & Johnson, a healthcare company, including Vice President of Centocor Ortho Biotech Sales & Marketing from 2008 to 2010, President of Janssen Biotech from 2010 to 2013, and Vice President of Global Surgery at Ethicon from 2013 to 2014. Prior to Johnson & Johnson, Mr. Bazemore worked at Merck & Co., Inc. from 1991 to 2013, where he served in a variety of roles in medical affairs, sales and marketing. Mr. Bazemore also serves on the board of directors of Ardelyx, Inc., a public biopharmaceutical company, since June 2016. Mr. Bazemore received a B.S. in Biochemistry from the University of Georgia.
We believe that Mr. Bazemore’s extensive experience in the pharmaceutical industry, his experience as an executive, and his past service on the board of directors of a life sciences industry group, qualify him to serve as a member of our board of directors.
Kim Blickenstaff has served as a member of our board of directors since August 2019. From September 2007 to March 2019, Mr. Blickenstaff served as the President and Chief Executive Officer of Tandem Diabetes Care, Inc., a medical device manufacturer. Mr. Blickenstaff has served on Tandem’s board of directors since September 2007, serving as the Executive Chairman of the Tandem board since March 2019 and the Chairman of the Tandem board since March 2020. Mr. Blickenstaff served as Chairman and Chief Executive Officer of Biosite Incorporated, or Biosite, a provider of medical diagnostic products, from 1988 until its acquisition by Inverness Medical Innovations, Inc. in June 2007. Mr. Blickenstaff previously served as a director of Medivation, Inc., a biotechnology company, from 2005 to 2016, until its acquisition by Pfizer, and as a director of DexCom, Inc., a provider of continuous glucose monitoring systems, from June 2001 to September 2007. Mr. Blickenstaff was formerly a certified public accountant and has more than 20 years of experience overseeing the preparation of financial statements. He holds a B.A. in Political Science from Loyola University, Chicago, and an M.B.A. from the Graduate School of Business, Loyola University, Chicago.
102
Table of Contents
We believe that Mr. Blickenstaff’s extensive experience at the board level of various healthcare companies, as well as leadership skills, industry experience and knowledge, qualify him to serve as a member of our board of directors.
Michelle Doig has served as a member of our board of directors since July 2019. Since December 2016, Ms. Doig has served as a Partner and the Head of Corporate Development at Omega Funds, a leading investment firm. Prior to that, she was Director of Corporate Finance at Third Rock Ventures from December 2013 to December 2016, where she supported numerous portfolio companies. From 2004 to 2013, Ms. Doig was a Principal, Corporate Finance at Abingworth. Prior to that, she was a life sciences investment banker with multiple investment firms, including Lehman Brothers International, J.P. Morgan and Morgan Stanley. Ms. Doig received a degree in Business Administration from Richard Ivey School of Business at the University of Western Ontario in London.
We believe that Ms. Doig’s industry experience investing, including in biopharmaceutical companies, qualifies her to serve as a member of our board of directors.
Kathryn E. Falberg has served as a member of our board of directors since October 2020. Ms. Falberg served as Executive Vice President and Chief Financial Officer of Jazz Pharmaceuticals plc, a public biopharmaceutical company, from March 2012 to March 2014 after serving as Senior Vice President and Chief Financial Officer since December 2009. From 1995 to 2001, Ms. Falberg served as Senior Vice President, Finance and Strategy and Chief Financial Officer at Amgen Inc., and prior to that as Vice President Chief Accounting Officer, and Vice President, Treasurer. Ms. Falberg also serves as a member of the board of directors for the public biopharmaceutical companies, UroGen Pharma, Arcus Biosciences, Inc. and Tricida, Inc., as well as The Trade Desk, Inc., a public technology company. Ms. Falberg also served on the board of directors of private companies, including Medivation, Inc. from 2013 to 2016 and Halozyme Therapeutics, Inc. from 2007 to 2016. Ms. Falberg received a B.A. in Economics and an MBA in Finance from the University of California, Los Angeles.
We believe that Ms. Falberg’s experience in the biopharmaceutical industry qualifies her to serve as a member of our board of directors.
Oleg Nodelman has served as a member of our board of directors since February 2021. Mr. Nodelman was the Chief Executive Officer and Chairman of Panacea’s board of directors from April 2020 to February 2021. Mr. Nodelman has been the Portfolio Manager of EcoR1, a biotech-focused investment advisory firm that invests in companies at all stages of research and development, since he founded it in 2013. Before founding EcoR1, Mr. Nodelman was an analyst and portfolio manager from 2001 to 2012 at Biotechnology Value Fund (BVF). Prior to BVF, Mr. Nodelman worked in strategic consulting and organizational management at Mercer Management Consulting (now Oliver Wyman). He also serves on the board of directors for Prothena Corporation, a public clinical-stage neuroscience company. Mr. Nodelman received a B.S. in Foreign Service with a concentration in Science and Technology from Georgetown University.
We believe that Mr. Nodelman’s industry experience investing, including in biopharmaceutical companies, qualifies him to serve as a member of our board of directors.
W. Anthony Vernon, has served as a member of our board of directors since June 2019. Mr. Vernon served as senior advisor to Kraft Foods Group, Inc. from January 2015 through May 2015, and Chief Executive Officer for Kraft Foods Group, Inc. from October 2012 to December 2014. Mr. Vernon previously served as Executive Vice President and President at Kraft Foods of North America from 2009 to October 2012. From 2006 to 2009, Mr. Vernon was the Healthcare Industry Partner at Ripplewood Holdings, Inc., a private equity firm. Mr. Vernon previously led a number of Johnson & Johnson’s largest franchises during a 23-year career at Johnson & Johnson, a public company engaged in the research and development, manufacture and sale of products in the healthcare field. From 2004 until 2005, Mr. Vernon was employed as Company Group Chairman of Depuy Inc., an orthopedics company, which is a subsidiary of Johnson & Johnson. From 2001 until 2004, Mr. Vernon served as President and Chief Executive Officer of Centocor, Inc., a biomedicines company, a division of Johnson & Johnson. He has also served as President of McNeil Consumer Products and Nutritionals, Worldwide President of The Johnson & Johnson-Merck Joint Venture and as a member of Johnson & Johnson’s Group Operating Committees for Consumer Healthcare and Nutritionals, Biopharmaceuticals, and Medical Devices and Diagnostics. Mr. Vernon serves on the boards of directors of NovoCure Ltd., a public medical device company, since 2006, Intersect ENT, Inc., a public medical device company, since 2015 and McCormick & Co., a global food company, since 2017. He formerly served as a director of Medivation, Inc. and Kraft Foods Group, Inc. Mr. Vernon received a B.A. from Lawrence University and an MBA from the Northwestern University Kellogg Graduate School of Management.
We believe that Mr. Vernon’s business and investment experience, as an executive in various industries and as the former chief executive officer of a global Fortune 500 company, qualify him to serve as a member of our board of directors.
Family Relationships
There are no family relationships among any of our directors or executive officers.
103
Table of Contents
Board Composition
Our business and affairs is managed under the direction of our board of directors. Daniel G. Welch serves as the Chair of our board of directors. The primary responsibilities of the our board of directors is to provide oversight, strategic guidance, counseling and direction to our management. The board of directors will meet on a regular basis and additionally as required.
In accordance with the terms of our amended and restated bylaws, the board of directors may establish the authorized number of directors from time to time by resolution. Our board of directors consists of eight members and are divided into three classes, Class I, Class II and Class III, with members of each class serving staggered three-year terms. The board of directors are divided into the following classes:
| • | Class I directors are Kathryn E. Falberg, David Hung, M.D. and Oleg Nodelman, whose terms will expire at our annual meeting of stockholders to be held in 2022; |
| • | Class II directors are Robert B. Bazemore Jr., Kim Blickenstaff and Michelle Doig, whose terms will expire at our annual meeting of stockholders to be held in 2023; and |
| • | Class III directors are W. Anthony Vernon and Daniel G. Welch, whose terms will expire at our annual meeting of stockholders to be held in 2024. |
Director Independence
Our board of directors has reviewed of the independence of each director. Based on information provided by each director concerning her or his background, employment and affiliations, our board of directors determined that none of our directors, other than Dr. Hung and Mr. Nodelman, has any current or prior relationships that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of the directors, other than Dr. Hung and Mr. Nodelman, is “independent” as that term is defined under the NYSE listing standards. In making these determinations, our board of directors considered the current and prior relationships that each non-employee director has with our company and all other facts and circumstances our board of directors deems relevant in determining their independence, including the beneficial ownership of securities of our company by each non-employee director and the transactions described in the section titled “Certain Relationships and Related Party Transactions” in this prospectus.
Role of the Board in Risk Oversight
One of the key functions of our board of directors is the informed oversight of our risk management process. Our board of directors does not have a standing risk management committee, but rather administers this oversight function directly through our board of directors as a whole, as well as through various standing committees of our board that address risks inherent in their respective areas of oversight. In particular, our board is responsible for monitoring and assessing strategic risk exposure and the audit committee has the responsibility to consider and discuss our major financial risk exposures and the steps our management will take to monitor and control such exposures, including guidelines and policies to govern the process by which risk assessment and management is undertaken. The audit committee will also monitor compliance with legal and regulatory requirements. The compensation committee assesses and monitors whether our compensation plans, policies and programs comply with applicable legal and regulatory requirements.
Board Committees
Our board of directors has reconstituted our audit committee, compensation committee, and nominating and corporate governance committee and adopted a new charter for each of these committees, which comply with the applicable requirements of current SEC and NYSE rules. Copies of the charters for each committee are available on the investor relations portion of our website at www.nuvationbio.com.
Audit Committee
Our audit committee consists of Ms. Falberg, Mr. Blickenstaff and Mr. Welch, each of whom our board of directors has determined satisfies the independence requirements under NYSE listing standards and Rule 10A- 3(b)(1) of the Exchange Act. The chair of our audit committee is Ms. Falberg, who our board of directors has determined is an “audit committee financial expert” within the meaning of SEC regulations. Each member of our audit committee can read and understand fundamental financial statements in accordance with applicable requirements. In arriving at these determinations, our board of directors has examined each audit committee member’s scope of experience and the nature of their employment in the corporate finance sector.
The primary purpose of our audit committee is to discharge the responsibilities of our board of directors with respect to our corporate accounting and financial reporting processes, systems of internal control and financial statement audits, and to oversee the independent registered public accounting firm. Specific responsibilities of our audit committee include:
104
Table of Contents
| • | helping our board of directors oversee corporate accounting and financial reporting processes; |
| • | managing the selection, engagement, qualifications, independence and performance of a qualified firm to serve as the independent registered public accounting firm to audit the financial statements; |
| • | discussing the scope and results of the audit with the independent registered public accounting firm, and reviewing, with management and the independent accountants, the interim and year-end operating results; |
| • | developing procedures for employees to submit concerns anonymously about questionable accounting or audit matters; |
| • | reviewing related person transactions; |
| • | obtaining and reviewing a report by the independent registered public accounting firm at least annually that describes internal quality control procedures, any material issues with such procedures and any steps taken to deal with such issues when required by applicable law; and |
| • | approving or, as permitted, pre-approving, audit and permissible non-audit services to be performed by the independent registered public accounting firm. |
Compensation Committee
The compensation committee consists of Mr. Vernon, Mr. Bazemore Jr. and Mr. Blickenstaff. The chair of the compensation committee is Mr. Vernon. Our board of directors has determined that each member of the compensation committee is independent under the NYSE listing standards and a “non-employee director” as defined in Rule 16b-3 promulgated under the Exchange Act.
The primary purpose of the compensation committee is to discharge the responsibilities of our board of directors in overseeing our compensation policies, plans and programs and to review and determine the compensation to be paid to executive officers, directors and other senior management, as appropriate. Specific responsibilities of our compensation committee include:
| • | reviewing and approving the compensation of our chief executive officer, other executive officers and senior management; |
| • | administering our equity incentive plans and other benefit programs; |
| • | reviewing, adopting, amending and terminating incentive compensation and equity plans, severance agreements, profit sharing plans, bonus plans, change-of-control protections and any other compensatory arrangements for our executive officers and other senior management; and |
| • | reviewing and establishing general policies relating to compensation and benefits of our employees, including the overall compensation philosophy. |
Nominating and Corporate Governance Committee
Our nominating and corporate governance committee consists of Mr. Welch, Mr. Vernon and Ms. Falberg. The chair of our nominating and corporate governance committee is Mr. Welch. We expect that all members of our nominating and corporate governance committee is independent under the NYSE listing standards. Specific responsibilities of the nominating and corporate governance committee include:
| • | identifying and evaluating candidates, including the nomination of incumbent directors for reelection and nominees recommended by stockholders, to serve on our board of directors; |
| • | considering and making recommendations to our board of directors regarding the composition and chairmanship of the committees of our board of directors; |
| • | reviewing and recommending to the board the compensation paid to our directors; |
| • | instituting plans or programs for the continuing education of our board of directors and orientation of new directors; |
| • | reviewing, evaluating and recommending to our board of directors succession plans for our executive officers; |
| • | developing and making recommendations to our board of directors regarding corporate governance guidelines and matters, including in relation to corporate social responsibility; and |
| • | overseeing periodic evaluations of the performance of our board of directors, including our individual directors and committees. |
Compensation Committee Interlocks
None of the members of our compensation committee has ever been an executive officer or employee of our company. None of our executive officers currently serve, or has served during the last completed fiscal year, on our compensation committee or board of directors of any other entity that has one or more executive officers that serve as a member of our board of directors or compensation committee.
105
Table of Contents
Limitation on Liability and Indemnification of Directors and Officers
Our certificate of incorporation limits a directors’ liability to the fullest extent permitted under the DGCL. The DGCL provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability:
| • | for any transaction from which the director derives an improper personal benefit; |
| • | for any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| • | for any unlawful payment of dividends or redemption of shares; or |
| • | for any breach of a director’s duty of loyalty to the corporation or its stockholders. |
If the DGCL is amended to authorize corporate action further eliminating or limiting the personal liability of directors, then the liability of the directors will be eliminated or limited to the fullest extent permitted by the DGCL, as so amended.
Delaware law and our amended and restated bylaws provide that we will, in certain situations, indemnify our directors and officers and may indemnify other employees and other agents, to the fullest extent permitted by law. Any indemnified person is also entitled, subject to certain limitations, to advancement, direct payment, or reimbursement of reasonable expenses (including attorneys’ fees and disbursements) in advance of the final disposition of the proceeding.
In addition, we have entered into separate indemnification agreements with our directors and officers. These agreements, among other things, require us to indemnify our directors and officers for certain expenses, including attorneys’ fees, judgments, fines, and settlement amounts incurred by a director or officer in any action or proceeding arising out of their services as one of our directors or officers or any other company or enterprise to which the person provides services at our request.
We also maintain a directors’ and officers’ insurance policy pursuant to which our directors and officers are insured against liability for actions taken in their capacities as directors and officers. We believe these provisions in the certificate of incorporation and amended and restated bylaws and these indemnification agreements are necessary to attract and retain qualified persons as directors and officers.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers, or control persons, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires the Company’s directors and officers subject to Section 16 filing obligations, as well as any persons beneficially owning more than 10 percent of its outstanding shares of common stock, to file reports concerning the beneficial ownership of the Company’s equity securities with the SEC. Based solely on the Company’s review of copies of Forms 3, 4, and 5 available to it, or written representations that no Forms 5 were required, the Company believes that all required Forms concerning beneficial ownership for the year ended December 31, 2020 were filed on time by all directors and applicable officers.
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics, or the Code of Conduct, applicable to all of our employees, executive officers and directors. The Code of Conduct is available at the investors section of our website at www.nuvationbio.com. Information contained on or accessible through this website is not a part of this prospectus, and the inclusion of such website address in this prospectus is an inactive textual reference only. Any amendments to the Code of Conduct, or any waivers of its requirements, will be disclosed on our website to the extent required by applicable rules and exchange requirements.
106
Table of Contents
| Item 11. | Executive Compensation. |
We did not pay compensation of any kind, including finder’s and consulting fees, to holders of the founder shares, executive officers and directors, or any of their respective affiliates, for services rendered prior to or in connection with the consummation of an initial business combination other than (i) repayment of loans made to us by the Sponsor to cover initial public offering-relating and organization expenses, (ii) repayment of loans that the Sponsor, members of our management team or any of their respective affiliates or other third parties made to finance transaction costs in connection with an intended initial business combination, (iii) payments to the Sponsor or its affiliate of a total of $10,000 per month for office space, administrative and support services, (iv) to reimburse for any out-of-pocket expenses related to identifying, investigating and completing an initial business combination and (v) Cowen was entitled to a portion of the fee payable pursuant to a Business Combination Marketing Agreement and placement fees in connection with the PIPE investment and was reimbursed for any out-of-pocket expenses incurred by it in connection with the performance of such services. Our audit committee reviewed on a quarterly basis all payments that were made to our Sponsor, officers, directors or our or any of their affiliates.
Panacea was not party to any agreements with its executive officers and directors that provided for benefits upon termination of employment.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
The following table sets forth information regarding the beneficial ownership of shares of Company Common Stock as of the Closing Date, by:
| • | each person known by the Company to be the beneficial owner of more than 5% of any class of Company Common Stock; |
| • | each of the Company’s named executive officers and directors; and |
| • | all of the Company’s executive officers and directors as a group. |
Beneficial ownership is determined according to the rules of the SEC, which generally provide that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power over that security, including options and warrants that are currently exercisable or exercisable within 60 days. This table is based upon information supplied by officers, directors and principal stockholders and Schedules 13G filed with the SEC. Unless otherwise indicated in the footnotes to this table and subject to community property laws where applicable, the Company believes that all persons named in the table have sole voting and investment power with respect to all shares of the Company’s common stock beneficially owned by them. The beneficial ownership percentages set forth in the table below are based upon approximately 216,650,055 shares of Class A Common Stock and 1,000,000 shares of Class B Common Stock issued and outstanding as of the Closing Date and do not take into account the issuance of any shares of Common Stock upon the exercise of Warrants to purchase up to
107
Table of Contents
approximately 5,787,500 shares of Class A Common Stock that remain outstanding but which are not exercisable until the date that is twelve (12) months from the date of the Panacea IPO.
| Name and Address of Beneficial Owner(1) |
Number of Shares of Class A Common Stock |
Percentage of Class A Common Stock Outstanding |
Number of Shares of Class B Common Stock |
Percentage of Class B Common Stock Outstanding |
||||||||||||
| 5% or Greater Stockholders: |
||||||||||||||||
| David Hung, M.D. |
59,645,012 | (2) | 27.4 | 1,000,000 | 100 | |||||||||||
| Entities affiliated with FMR LLC(3) |
32,283,985 | (4) | 14.9 | — | — | |||||||||||
| Omega Fund V, L.P.(5) |
28,212,376 | 13.0 | — | — | ||||||||||||
| EcoR1 Panacea Holdings, LLC(6) |
13,247,017 | (7) | 6.1 | — | — | |||||||||||
| Named Executive Officers and Directors: |
||||||||||||||||
| David Hung, M.D. |
59,645,012 | (2) | 27.4 | 1,000,000 | 100 | |||||||||||
| Jennifer Fox |
— | — | — | |||||||||||||
| Sergey Yurasov |
149,600 | (8) | * | — | — | |||||||||||
| Gary Hattersley |
174,534 | (9) | * | — | — | |||||||||||
| Thomas Templeman |
174,534 | (10) | * | — | — | |||||||||||
| Stacy Markel |
95,554 | (11) | * | — | — | |||||||||||
| Robert B. Bazemore Jr. |
37,739 | (12) | * | — | — | |||||||||||
| Kim Blickenstaff |
80,744 | (13) | * | — | — | |||||||||||
| Michelle Doig(5) |
28,212,376 | (14) | 13.0 | — | — | |||||||||||
| Kathryn E. Falberg |
24,933 | (15) | * | — | — | |||||||||||
| Oleg Nodelman(6) |
13,247,017 | (7) | 6.1 | — | — | |||||||||||
| W. Anthony Vernon |
392,663 | (16) | * | — | — | |||||||||||
| Daniel G. Welch |
62,898 | (17) | * | — | — | |||||||||||
| All current directors and executive officers as a group (13 persons) |
102,297,604 | 47.0 | 1,000,000 | 100 | ||||||||||||
| * | Less than one percent. |
| (1) | Unless otherwise noted, the business address of each of the following entities or individuals is c/o Nuvation Bio Inc., 1500 Broadway, Suite 1401, New York, NY 10036. |
108
Table of Contents
| (2) | Interests shown include 1,000,000 shares of Class A common stock issuable upon conversion of Class B Common Stock. |
| (3) | These accounts are managed by direct or indirect subsidiaries of FMR LLC. Abigail P. Johnson is a director, the chairman, the chief executive officer and the president of FMR LLC. Members of the Johnson family, including Abigail P. Johnson, are the predominant owners, directly or through trusts, of Series B voting common shares of FMR LLC, representing 49% of the voting power of FMR LLC. The Johnson family group and all other Series B shareholders of FMR LLC have entered into a shareholders’ voting agreement under which all Series B voting common shares will be voted in accordance with the majority vote of Series B voting common shares. Accordingly, through their ownership of voting common shares and the execution of the shareholders’ voting agreement, members of the Johnson family may be deemed, under the Investment Company Act of 1940, to form a controlling group with respect to FMR LLC. Neither FMR LLC nor Abigail P. Johnson has the sole power to vote or direct the voting of the shares owned directly by the various investment companies registered under the Investment Company Act of 1940 (the “Fidelity Funds”) advised by Fidelity Management & Research Company, a wholly owned subsidiary of FMR LLC, which power resides with the Fidelity Funds’ Boards of Trustees. Fidelity Management & Research Company carries out the voting of the shares under written guidelines established by the Fidelity Funds’ Boards of Trustees. The principal business address for each person and entity named in this footnote is 245 Summer Street, Boston, MA 02110. |
| (4) | Interests shown include (i) 1,102,137 shares of Class A Common Stock owned of record by Fidelity Mt. Vernon Street Trust: Fidelity Series Growth Company Fund, (ii) 4,681,845 shares of Class A Common Stock owned of record by Fidelity Mt. Vernon Street Trust: Fidelity Growth Company Fund, (iii) 4,008,665 shares of Class A Common Stock owned of record by Fidelity Growth Company Commingled Pool, (iv) 10,588,266 shares of Class A Common Stock owned of record by Fidelity Contrafund: Fidelity Contrafund, (v) 2,593,012 shares of Class A Common Stock owned of record by Fidelity Contrafund Commingled Pool, (vi) 942,518 shares of Class A Common Stock owned of record by Fidelity Contrafund: Fidelity Contrafund K6, (vii) 1,408,991 shares of Class A Common Stock owned of record by Fidelity Select Portfolios: Biotechnology Portfolio, (viii) 119,094 shares of Class A Common Stock owned of record by Fidelity Hastings Street Trust: Fidelity Growth Discovery Fund, (ix) 186,501 shares of Class A Common Stock owned of record by Fidelity Advisor Series I: Fidelity Advisor Equity Growth Fund, (x) 56,254 shares of Class A Common Stock owned of record by Fidelity Advisor Series I: Fidelity Advisor Series Equity Growth Fund, (xi) 326,843 shares of Class A Common Stock owned of record by Variable Insurance Products Fund: Growth Portfolio, (xii) 78,128 shares of Class A Common Stock owned of record by Variable Insurance Products Fund III: Growth Opportunities Portfolio, (xiii) 243,417 shares of Class A Common Stock owned of record by Variable Insurance Products Fund III: Growth Opportunities Portfolio, (xiv) 360,498 shares of Class A Common Stock owned of record by Fidelity Advisor Series I: Fidelity Advisor Growth Opportunities Fund, (xv) 1,599,372 shares of Class Common Stock owned of record by Fidelity Advisor Series I: Fidelity Advisor Growth Opportunities Fund, (xvi) 102,770 shares of Class A Common Stock owned of record by Fidelity Advisor Series I: Fidelity Advisor Series Growth Opportunities Fund, (xvii) 2,712,871 shares of Class A Common Stock owned of record by Fidelity Securities Fund: Fidelity Blue Chip Growth Fund, (xviii) 94,047 shares of Class A Common Stock owned of record by Fidelity Blue Chip Growth Commingled Pool, (xix) 3,442 shares of Class A Common Stock owned of record by Fidelity Securities Fund: Fidelity Flex Large Cap Growth Fund, (xx) 249,129 shares of Class A Common Stock owned of record by Fidelity Securities Fund: Fidelity Blue Chip Growth K6 Fund, (xxi) 10,761 shares of Class A Common Stock owned of record by Fidelity Blue Chip Growth Institutional Trust, (xxii) 268,094 shares of Class A Common Stock owned of record by FIAM Target Date Blue Chip Growth Commingled Pool, (xxiii) 14,132 shares of Class A Common Stock owned of record by Fidelity U.S. Growth Opportunities Investment Trust, (xxiv) 77,785 shares of Class A Common Stock owned of record by Fidelity NorthStar Fund, (xxv) 142,573 shares of Class A Common Stock owned of record by Fidelity Securities Fund: Fidelity Series Blue Chip Growth Fund and (xxvi) 312,840 shares of Class A Common Stock owned of record by Fidelity Mt. Vernon Street Trust: Fidelity Growth Company K6 Fund. |
109
Table of Contents
| (5) | Omega Fund V GP, L.P. (“Omega V GP LP”) is the general partner of Omega Fund V, L.P. (“Omega V”). Omega Fund V GP Manager, Ltd. (“Omega V GP Ltd”) is the general partner of Omega V GP LP. Otello Stampacchia, Claudio Nessi and Anne-Mari Paster are directors of Omega V GP Ltd and have shared voting and investment power over the shares held by Omega V. Otello Stampacchia, Claudio Nessi and Anne-Mari Paster each disclaim beneficial ownership of the shares held by Omega V, except to the extent of his or her pecuniary interest therein. The address of Omega V, Omega V GP LP, Omega V GP Ltd and the above-mentioned persons is 888 Boylston Street, Suite 1111, Boston MA 02199. |
| (6) | Other than as described in note 7 to this table, EcoR1 Panacea Holdings, LLC, a Delaware limited liability company (the “Sponsor”) is a record holder of shares reported herein. EcoR1 Capital Fund, L.P., EcoR1 Capital Fund Qualified, L.P. and EcoR1 Venture Opportunity Fund, L.P. are the Members of the Sponsor. EcoR1 Capital, LLC is the general partner of EcoR1 Capital Fund, L.P. and EcoR1 Capital Fund Qualified, L.P., and the investment adviser to EcoR1 Venture Opportunity Fund, L.P. Biotech Opportunity GP, LLC is the general partner of EcoR1 Venture Opportunity Fund, L.P. Oleg Nodelman is the control person of EcoR1 Capital, LLC and Biotech Opportunity GP, LLC. As such, Mr. Nodelman may be deemed to have beneficial ownership of the common stock held directly by the Sponsor and the various funds. Each of the Sponsor’s independent directors is, directly or indirectly, a non-managing member of the Sponsor. |
| (7) | Interests shown include (i) 6,584,246 shares of Class A Common Stock owned of record by EcoR1 Capital Fund Qualified, L.P., (ii) 1,297,144 shares of Class A Common Stock owned of record by EcoR1 Capital Fund, L.P., (iii) 2,200,627 shares of Class A Common Stock owned of record by EcoR1 Venture Opportunity Fund, LP and (iv) 3,165,000 shares of Class A Common Stock owned of record by EcoR1 Panacea Holdings, LLC. Interests shown do not include warrants. |
| (8) | Consists of 149,600 shares of Class A Common Stock issuable upon the exercise of options within 60 days of February 10, 2021. |
| (9) | Consists of 174,534 shares of Class A Common Stock issuable upon the exercise of options within 60 days of February 10, 2021. |
| (10) | Consists of 174,534 shares of Class A Common Stock issuable upon the exercise of options within 60 days of February 10, 2021. |
| (11) | Consists of 95,554 shares of Class A Common Stock issuable upon the exercise of options within 60 days of February 10, 2021. |
| (12) | Consists of 37,739 shares of Class A Common Stock issuable upon the exercise of options within 60 days of February 10, 2021. |
| (13) | Consists of 80,744 shares of Class A Common Stock issuable upon the exercise of options within 60 days of February 10, 2021. |
| (14) | Consists of 28,212,376 shares of Class A Common Stock held directly by Omega V. Ms. Doig is a partner of Omega V and may be deemed to have shared voting, investment and dispositive power with respect to the shares held by Omega V. Ms. Doig disclaims beneficial ownership of these shares except to the extent of her pecuniary interest, if any, therein. |
| (15) | Consists of 24,933 shares of Class A Common Stock issuable upon the exercise of options within 60 days of February 10, 2021. |
| (16) | Includes 88,563 shares of Class A Common Stock issuable upon the exercise of options within 60 days of February 10, 2021. |
| (17) | Consists of 62,898 shares of Class A Common Stock issuable upon the exercise of options within 60 days of February 10, 2021. |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence. |
Below is a description of transactions since our inception in March 2018 to which we were a party or will be a party, in which:
| • | the amounts involved exceeded or will exceed the lesser of (1) $120,000, or (2) 1% of the average of our total assets for the last two completed fiscal years; and |
| • | any of our directors, executive officers or holders of more than 5% of any class of our capital stock, or any member of the immediate family of, or person sharing the household with, the foregoing persons, had or will have a direct or indirect material interest. |
110
Table of Contents
Amended and Restated Registration Rights Agreement
In connection with the closing, we entered into the Registration Rights Agreement with certain securityholders, pursuant to which such holders of Registrable Securities (as defined therein), subject to certain conditions, will be entitled to registration rights. Pursuant to the Registration Rights Agreement, we agreed that, within 15 business days following the closing, we would file with the SEC (at our sole cost and expense) a registration statement registering the resale of such Registrable Securities, and that we would use our commercially reasonable efforts to have such registration statement declared effective by the SEC as soon as reasonably practicable after the filing thereof. Certain of such stockholders have been granted demand underwritten offering registration rights and all of such stockholders will be granted piggyback registration rights. The Registration Rights Agreement does not provide for any cash penalties by us if we fail to satisfy any of our obligations under the Registration Rights Agreement. The stockholders may not exercise their registration rights after the seven year anniversary of the closing.
Panacea Related Agreements
Founder Shares
On May 7, 2020, EcoR1 Panacea Holdings, LLC (the “Sponsor”) and an affiliate of PA Co-Investment LLC (“Cowen Investments” and collectively, the “Founders”) paid an aggregate of $25,000 to cover certain offering costs of Panacea in consideration for the founder shares. In May 2020, the Sponsor transferred 25,000 founder shares to each independent director of Panacea, or an aggregate of 100,000 founder shares, at their original purchase price. An affiliate of Cowen Investments subsequently transferred all of its founder shares to Cowen Investments. The founder shares included an aggregate of up to 468,750 shares subject to forfeiture to the extent that the underwriters’ over-allotment option was not exercised in full or in part, so that the number of founder shares would equal 20% of Panacea’s issued and outstanding shares after Panacea’s IPO (not including the private placement shares). As a result of the underwriters’ election to fully exercise their over-allotment option, no founder shares are currently subject to forfeiture.
Private Placements
Simultaneous with the consummation of the Initial Public Offering and the closing of the over-allotment option, the Founders purchased an aggregate of 487,500 private placement units for a purchase price of $10.00 per unit generating total proceeds of $4,875,000. Among the private placement units, 390,000 were purchased by the Sponsor and/or its designees and 97,500 were purchased by Cowen Investments and/or its designees.
Promissory Notes
Prior to the commencement of the Panacea IPO, the Founders agreed to loan Panacea up to $300,000 to be used for a portion of the expenses of the Panacea IPO, the Sponsor up to $240,000 and Cowen Investments up to $60,000. As of December 31, 2020, there were no amounts outstanding under such promissory notes.
111
Table of Contents
Related Party Loans
In order to finance transaction costs in connection with a business combination, the Sponsor or its affiliates, or certain of our officers and directors could, but were not obligated to, provide working capital loans to us as may have been required. If we completed a business combination, we would repay the working capital loans out of the proceeds of the Trust Account released to us. Otherwise, the working capital loans would be repaid only out of funds held outside the Trust Account. In the event that a business combination did not close, we may have used a portion of proceeds held outside the Trust Account to repay the working capital loans but no proceeds held in the Trust Account would be used to repay the working capital loans, other than the interest on such proceeds that may have been released for working capital purposes. Except for the foregoing, the terms of such working capital loans, if any, were not determined and no written agreements exist with respect to such loans. The working capital loans would either be repaid upon consummation of a business combination, without interest, or, at the lender’s discretion, up to $1.5 million of such working capital loans may have been convertible into warrants of the post business combination entity at a price of $10.00 per warrant. The warrants would be identical to the private placement units. There were no working capital loans outstanding as of the date of this report.
Administrative Support Agreement
Panacea entered into an agreement whereby, commencing on July 6, 2020, Panacea agreed to pay an
affiliate of the Sponsor a total of $10,000 per month for office space and administrative support. Upon completion of the Business Combination or Panacea’s liquidation, Panacea ceased paying these monthly fees. For the period from inception to December 31, 2020, the Company incurred and paid $60,000 in fees for these services.
Underwriters Fee
Panacea also paid to Cowen, one of the underwriters of the Panacea IPO and an affiliate of one of the Founders, an underwriting discount of $0.20 per unit purchased by it in the Panacea IPO. Panacea also engaged Cowen as an advisor in connection with its proposed business combination, pursuant to the Business Combination Marketing Agreement. Panacea paid Cowen a $5.0 million Marketing Fee for such services upon the consummation of the Business Combination.
Reimbursements
The Founders, officers and directors or any of their respective affiliates were reimbursed for any out-of-pocket expenses incurred in connection with activities on Panacea’s behalf such as identifying potential target businesses and performing due diligence on suitable business combinations. Panacea’s audit committee reviewed on a quarterly basis all payments made by Panacea to the Sponsor, officers, directors or our or any of their respective affiliates and determined which expenses and the amount of expenses that would be reimbursed. There was no cap or ceiling on the reimbursement of out-of-pocket expenses incurred by such persons in connection with activities on Panacea’s behalf.
Forward Purchase Agreement
On June 30, 2020, Panacea entered into a forward purchase agreement with funds affiliated with the Sponsor that provide for the purchase by such funds of an aggregate of 2,500,000 shares of Panacea Class A common stock and 833,333 redeemable warrants, for an aggregate purchase price of $25.0 million, or $10.00 per one share of Class A common stock and one-third of one redeemable warrant, in a private placement that closed concurrently with the closing of the Business Combination. The obligations under the forward purchase agreement did not depend on whether any shares of Panacea Class A common stock were redeemed by the public stockholders. The shares of Panacea Class A common stock and redeemable warrants issued pursuant to the forward purchase agreement are identical to the shares of Panacea Class A common stock and redeemable warrants included in the units sold in the Panacea IPO, respectively, except that the holders thereof had certain registration rights.
Panacea Letter Agreement
Panacea entered into a letter agreement with Panacea’s initial stockholders, officers and directors, pursuant to which the initial stockholders, officers and directors agreed to waive: (a) their redemption rights with respect to any founder shares, private placement shares and any public shares held by them in connection with the completion of Panacea’s initial business combination, (b) their redemption rights with respect to any founder shares, private placement shares and public shares held by them in connection with a stockholder vote to approve an amendment to the amended and restated certificate of incorporation (A) to modify the substance or timing of Panacea’s obligation to allow redemptions in connection with its initial business combination or to redeem 100% of its public shares if it did not consummate its initial business combination within 24 months from the closing of Panacea’s IPO or (B) with respect to any other provision relating to stockholders’ rights or pre-initial business combination activity; and (c) their rights to liquidating distributions from the trust account with respect to any founder shares and private placement shares held by them if Panacea failed to complete its initial business
112
Table of Contents
combination within 24 months from the closing of Panacea’s IPO or during any extension period (although they were entitled to liquidating distributions from the trust account with respect to any public shares they held if Panacea failed to complete its initial business combination within the prescribed time frame); (3) the founder shares are subject to certain transfer restrictions, as described in more detail below; (4) the founder shares are automatically convertible into shares of our Class A common stock at the time of the initial business combination, or earlier at the option of the holder, on a one-for-one basis, subject to adjustment pursuant to certain anti-dilution rights, as described herein; and (5) the holders of founder shares are entitled to registration rights. Panacea’s initial stockholders, officers and directors agreed (and their permitted transferees, as applicable, agreed) to vote any founder shares, private placement shares and any public shares held by them in favor of the Business Combination.
Sponsor Support Agreement
The Founders, Legacy Nuvation Bio, and Panacea entered into a support agreement, in substantially the form attached to the Business Combination Agreement (the “Sponsor Support Agreement”). Under the Sponsor Support Agreement, the Sponsor agreed to, among other things, (i) vote in favor of the Business Combination Agreement and the transactions contemplated thereby, (ii) waive any adjustment to the conversion ratio set forth in the governing documents of Panacea or any other anti-dilution or similar protection with respect to the Panacea Class A common stock and (iii) be bound by certain other covenants and agreements related to the Business Combination, and (iv) be bound by certain transfer restrictions with respect to its shares in Panacea prior to the closing of the Business Combination, in each case, on the terms and subject to the conditions set forth in the Sponsor Support Agreement.
Lock-Up Agreements
Panacea entered into a lock-up agreement with the Founders, in substantially the form attached to the Business Combination Agreement (the “Sponsor and Founder Lock-Up Agreement”). Under the Sponsor and Founder Lock-Up Agreement, each party to the agreement agreed that it will not, without the prior written consent of Panacea, during the period commencing on the closing date of the merger and ending on the date that is 365 days after the closing date (i) lend, offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any founder shares (and the shares of Panacea Class A common stock issued upon the conversion thereof) or any securities convertible into or exercisable or exchangeable for Panacea Class A common stock issued or issuable to such party pursuant to the Business Combination Agreement (collectively, the “Sponsor and Founder Lock-Up Shares”) or (ii) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the Sponsor and Founder Lock-Up Shares.
Notwithstanding the foregoing, if, at any time beginning 365 days after the closing date (the “Earliest Release Date”), the closing price of the our Class A common stock equals or exceeds $12.00 per share (as adjusted for share splits, share capitalizations, reorganizations, recapitalizations and the like) for any twenty trading days within any thirty-trading day period commencing at least 150 days after the closing date of the merger, then each party’s Sponsor and Founder Lock-Up Shares (which, for purposes of holders of options, shall only include options that have vested as of such date) will be automatically released from the lock-up restrictions as of the last day of such thirty-trading day period. The lock-up restrictions contain customary exceptions, including for estate planning transfers, affiliates transfers, and transfers upon death or by will. Panacea has entered into a lock-up agreement with certain stockholders of Legacy Nuvation Bio, including the directors, officers, and 1% holders of Legacy Nuvation Bio, in substantially the form attached to the Business Combination Agreement (the “Stockholder Lock-Up Agreement”). Under the Stockholder Lock-Up Agreement, each party to the agreement agreed that it will not, without the prior written consent of Panacea, during the period commencing on the closing date of the merger and ending on the date that is 180 days after the closing date (i) lend, offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any shares of
113
Table of Contents
Panacea Class A common stock, founder shares (and the shares of Panacea Class A common stock issued upon the conversion thereof), or any securities convertible into or exercisable or exchangeable for Panacea Class A common stock or founder shares (and the shares of Panacea Class A common stock issued upon the conversion thereof) issued or issuable to such party held by it immediately after the effective time (collectively, the “Stockholder Lock-Up Shares”) or (ii) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the Stockholder Lock-Up Shares. Notwithstanding the foregoing, if, at the Earliest Release Date, the closing price of our Class A common stock equals or exceeds $12.00 per share (as adjusted for share splits, share capitalizations, reorganizations, recapitalizations and the like) for any twenty trading days within any thirty-trading day period commencing at least 150 days after the closing date of the merger, then each party’s Stockholder Lock-Up Shares (which, for purposes of holders of options, shall only include options that have vested as of such date) will be automatically released from the lock-up restrictions as of the last day of such thirty-trading day period. The lock-up restrictions contain customary exceptions, including for estate planning transfers, affiliates transfers, and transfers upon death or by will.
Panacea entered into a lock-up agreement with each of the purchasers under the forward purchase agreement, in substantially the form attached to the Business Combination Agreement (the “FPA Lock-Up Agreement”). Under the FPA Lock-Up Agreement, each party to the agreement agreed that it will not, without the prior written consent of Panacea, during the period commencing on the closing date of the merger and ending on the date that is 365 days after the closing date (i) lend, offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any shares of Panacea Class A common stock under the forward purchase agreement (collectively, the “FPA Lock-Up Shares”) or (ii) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the FPA Lock-Up Shares. Notwithstanding the foregoing, if, at the Earliest Release Date, the closing price of our Class A common stock equals or exceeds $12.00 per share (as adjusted for share splits, share capitalizations, reorganizations, recapitalizations and the like) for any twenty trading days within any thirty-trading day period commencing at least 150 days after the closing date of the merger, then each party’s FPA Lock-Up Shares (which, for purposes of holders of options, shall only include options that have vested as of such date) will be automatically released from the lock-up restrictions as of the last day of such thirty-trading day period. The lock-up restrictions contain customary exceptions, including for estate planning transfers, affiliates transfers, and transfers upon death or by will.
PIPE Subscription Agreements
On October 20, 2020, concurrently with the execution of the Business Combination Agreement, Panacea and Legacy Nuvation Bio entered into subscription agreements with certain investors, including funds affiliated with the Sponsor, (collectively, the “PIPE Investors”), pursuant to, and on the terms and subject to the conditions of which, the PIPE Investors collectively subscribed for 47,655,000 shares of Panacea Class A common stock for an aggregate purchase price equal to $476.6 million (the “PIPE Investment”), a portion of which was funded by Dr. Hung, which coupled with the $25.0 million in proceeds from the forward purchase agreement entered into in connection with Panacea’s IPO resulted in equity financings totaling $501.6 million.
Legacy Nuvation Bio Related Agreements
Asset Acquisition Agreement
In January 2019, Legacy Nuvation Bio, GiraFpharma LLC (“GiraF”) and Dr. Hung entered into an Asset Acquisition Agreement (the “GiraF Agreement”) pursuant to which GiraF agreed that it would, concurrently with the initial closing of Legacy Nuvation Bio’s Series A preferred stock financing, contribute to Legacy Nuvation Bio all of its intellectual property rights with respect to specified drug development programs to be pursued by Legacy Nuvation Bio, together with other related assets, in consideration of Legacy Nuvation Bio’s payment to it of $5.0 million of cash and issuance to it of a number of shares of Legacy Nuvation Bio’s common stock determined under a specified formula. Dr. Hung also agreed in the GiraF Agreement that he would, substantially
114
Table of Contents
concurrently with GiraF’s asset transfer, contribute to Legacy Nuvation Bio all of his intellectual property rights with respect to the specified drug development programs and certain other assets in consideration of Legacy Nuvation Bio’s issuance to him of shares of its common stock. Under the GiraF Agreement, Legacy Nuvation Bio also agreed to issue to GiraF a number of additional shares of its common stock determined under a formula based on Legacy Nuvation Bio’s valuation at the time of the first underwritten public offering of its common stock (the “Additional GiraF Shares”). Pursuant to the GiraF Agreement, concurrently with the June 2019 initial closing of its Series A preferred stock financing, GiraF transferred to Legacy Nuvation Bio the specified intellectual property rights and other assets and Legacy Nuvation Bio paid GiraF $5.0 million of cash and issued to GiraF 12,963,780 shares of its common stock. Concurrently, Dr. Hung contributed to Legacy Nuvation Bio the specified intellectual property rights and other assets in consideration of Legacy Nuvation Bio’s issuance to him of shares of its common stock as described below under “Common Stock Purchase Agreement.” On March 2, 2021, we, Legacy Nuvation Bio, GiraF and Dr. Hung entered into an Agreement Regarding Subsequent Shares under which (i) Nuvation Bio agreed to issue 368,408 shares of Class A common stock in satisfaction of Legacy Nuvation Bio’s obligations with respect to the Additional GiraF Shares and (ii) Dr. Hung agreed to surrender for cancellation an equal number of shares of Class A common stock. The shares to be issued to GiraF under this agreement will be subject to the Stockholder Lock-up Agreement described above under “Lock-Up Agreements.”
Convertible Promissory Notes
In February 2019, Legacy Nuvation Bio entered into a Note Purchase Agreement with two investors, including Omega Fund V, LP (“Omega”), which subsequently became the beneficial owner of more than 5% of Legacy Nuvation Bio’s capital stock, providing for the issuance to such investors of convertible promissory notes in the aggregate principal amount of $15.0 million. Pursuant to the Note Purchase Agreement, Legacy Nuvation Bio issued to Omega convertible promissory notes in the principal amounts of $4.5 million in February 2019 and $6.8 million in May 2019. These two convertible promissory notes converted into an aggregate of 17,157,943 shares of Legacy Nuvation Bio’s Series A preferred stock in June 2019. The convertible promissory notes accrued interest at a rate of 8.0%; however, the investors waived the payment of interest upon the conversion of the convertible promissory notes.
Common Stock Purchase Agreement
In February 2019, Legacy Nuvation Bio entered into a Common Stock Purchase Agreement with Dr. Hung. Pursuant to the Common Stock Purchase Agreement, concurrently with the June 2019 initial closing of its Series A preferred stock financing, Legacy Nuvation Bio issued to Dr. Hung 171,130,898 shares of its common stock in consideration of Dr. Hung’s assignment to Legacy Nuvation Bio of patent applications and various other intellectual property rights and assets with respect to specified drug development programs to be pursued by Legacy Nuvation Bio, as well as $5.0 million of cash.
Series A Preferred Stock Financing
In two equal tranches in June 2019 and October 2020, Legacy Nuvation Bio issued and sold an aggregate of 337,509,640 shares of its Series A preferred stock at a purchase price of $0.77138 per share, for an aggregate purchase price of $260.3 million, and issued an aggregate of 22,877,257 shares of Legacy Nuvation Bio Series A preferred stock upon conversion of an aggregate of $15.0 million of convertible promissory notes at a conversion price of $0.65567 per share.
Each share of Legacy Nuvation Bio Series A preferred stock converted into a number of shares of our Class A common stock equal to the exchange ratio upon the completion of the Business Combination.
115
Table of Contents
The table below sets forth the number of shares of Series A preferred stock purchased by our related parties:
| Stockholder | Shares of Series A Preferred Stock |
Total Cash Purchase Price |
Conversion of Convertible Promissory Note |
|||||||||
| David Hung, M.D. |
12,963,780 | $ | 10,000,001 | — | ||||||||
| Omega Fund V, L.P.(1) |
41,465,030 | 18,750,001 | $ | 11,250,000 | ||||||||
| W. Anthony Vernon |
1,296,378 | 1,000,000 | — | |||||||||
| Entities Affiliated with EcoR1 Panacea Holdings, LLC(2) |
25,927,560 | 20,000,001 | — | |||||||||
| Entities Affiliated with FMR LLC(3) |
90,744,100 | 69,998,184 | — | |||||||||
| (1) | Michelle Doig, a member of our board of directors, is a partner of Omega Fund Management, LLC, the general manager of Omega Fund V, LP, a beneficial owner of more than 5% of our capital stock. |
| (2) | Shares are held by the following funds associated with EcoR1 Panacea Holdings, LLC, an owner of more than 5% of our outstanding capital stock: EcoR1 Capital Fund Qualified, L.P.; EcoR1 Capital Fund, L.P.; and EcoR1 Venture Opportunity Fund, LP. |
| (3) | Shares are held by the following funds associated with FMR LLC, an owner of more than 5% of our outstanding capital stock: Fidelity Mt. Vernon Street Trust: Fidelity Series Growth Company Fund; Fidelity Mt. Vernon Street Trust: Fidelity Growth Company Fund; Fidelity Growth Company Commingled Pool; Fidelity Contrafund: Fidelity Contrafund; Fidelity Contrafund Commingled Pool; Fidelity Contrafund: Fidelity Contrafund K6; Fidelity Select Portfolios: Biotechnology Portfolio; Fidelity Hastings Street Trust: Fidelity Growth Discovery Fund; Fidelity Advisor Series I: Fidelity Advisor Equity Growth Fund; Fidelity Advisor Series I: Fidelity Advisor Series Equity Growth Fund; Variable Insurance Products Fund: Growth Portfolio; Variable Insurance Products Fund III: Growth Opportunities Portfolio; Fidelity Advisor Series I: Fidelity Advisor Growth Opportunities Fund; Fidelity Advisor Series I: Fidelity Advisor Series Growth Opportunities Fund; Fidelity Securities Fund: Fidelity Blue Chip Growth Fund; Fidelity Blue Chip Growth Commingled Pool; Fidelity Securities Fund: Fidelity Flex Large Cap Growth Fund; Fidelity Securities Fund: Fidelity Blue Chip Growth K6 Fund; Fidelity Blue Chip Growth Institutional Trust; and FIAM Target Date Blue Chip Growth Commingled Pool. |
Stock Restriction Agreement
In June 2019, Legacy Nuvation Bio entered into a Stock Restriction Agreement with Dr. Hung pursuant to which Dr. Hung granted Legacy Nuvation Bio (a) a repurchase right, lapsing ratably over 36 months, with respect to 66% of his shares of Legacy Nuvation Bio’s common stock, and (b) a cancellation right entitling Legacy Nuvation Bio to cancel, without consideration, a number of his shares of Legacy Nuvation Bio’s common stock equal to the number, if any, of Additional GiraF Shares that Legacy Nuvation Bio became obligated to issue pursuant to the GiraF Agreement. The terms of the restriction agreement remain in place.
Business Combination—Private Placement
In connection with the execution of the Business Combination Agreement, Panacea entered into Subscription Agreements with the PIPE subscribers, pursuant to which the PIPE subscribers agreed to purchase, and Panacea agreed to sell to the PIPE subscribers, an aggregate of 47,655,000 shares of Panacea Class A common stock, for a purchase price of $10.00 per share and an aggregate purchase price of $476.6 million, in the PIPE Investment.
116
Table of Contents
The table below sets forth the number of shares of Panacea Class A common stock purchased by our related parties:
| Stockholder | Shares of Panacea Class A Common Stock |
Total Cash Purchase Price |
||||||
| David Hung, M.D. |
2,000,000 | $ | 20,000,000 | |||||
| Omega Fund V, LP(1)(2) |
500,000 | 5,000,000 | ||||||
| W. Anthony Vernon |
50,000 | 500,000 | ||||||
| Entities Affiliated with EcoR1 Panacea Holdings, LLC(2) |
2,500,000 | 25,000,000 | ||||||
| Entities Affiliated with FMR, LLC(3) |
14,500,000 | 145,000,000 | ||||||
| (1) | Michelle Doig, a member of our board of directors, is a partner of Omega Fund Management, LLC, the general manager of Omega Fund V, LP, a beneficial owner of more than 5% of our capital stock. |
| (2) | Subscribers are the following funds associated with EcoR1 Panacea Holdings, LLC: EcoR1 Capital Fund Qualified, L.P. and EcoR1 Capital Fund, L.P. |
| (3) | Subscribers are the following funds associated with Fidelity Management & Research Company, LLC, an owner of more than 5% of our outstanding capital stock: Fidelity Mt. Vernon Street Trust: Fidelity Growth Company K6 Fund; FIAM Target Date Blue Chip Growth Commingled Pool; Variable Insurance Products Fund III: Growth Opportunities Portfolio; Fidelity Securities Fund: Fidelity Blue Chip Growth Fund; Fidelity Blue Chip Growth Commingled Pool; Fidelity Securities Fund: Fidelity Flex Large Cap Growth Fund; Fidelity Securities Fund: Fidelity Blue Chip Growth K6 Fund; Fidelity Select Portfolios: Biotechnology Portfolio; Fidelity Contrafund: Fidelity Contrafund; Fidelity Contrafund Commingled Pool; Fidelity Contrafund: Fidelity Contrafund K6; Fidelity Advisor Series I: Fidelity Advisor Growth Opportunities Fund; Fidelity Advisor Series I: Fidelity Advisor Series Growth Opportunities Fund; Fidelity U.S. Growth Opportunities Investment Fund; Fidelity Blue Chip Growth Institutional Trust; Fidelity NorthStar Fund; Fidelity Securities Fund: Fidelity Series Blue Chip Growth Fund; Fidelity Mt. Vernon Street Trust: Fidelity Series Growth Company Fund; Fidelity Growth Company Commingled Pool. |
Stockholder Support Agreement
In October 2020, Panacea, Legacy Nuvation Bio and certain Legacy Nuvation Bio stockholders, including holders affiliated with members of the Legacy Nuvation Bio board of directors and beneficial owners of more than 5% of a class of Legacy Nuvation Bio’s capital stock, entered into support agreements, in substantially the form attached to the merger agreement (the “Stockholder Support Agreements”), whereby such Legacy Nuvation Bio stockholders agreed to vote all of their shares of Legacy Nuvation Bio’s capital stock in favor of the approval and adoption of the proposed transactions. Additionally, such stockholders agreed, among other things, not to transfer any of their shares of Legacy Nuvation Bio capital stock (or enter into any arrangement with respect thereto), subject to certain customary exceptions, or enter into any voting arrangement that is inconsistent with the Stockholder Support Agreement.
Share Exchange Transaction
In November 2020, pursuant to a Share Exchange Agreement dated as of October 5, 2020, between Dr. Hung and Legacy Nuvation Bio, Dr. Hung surrendered all of his holdings of Legacy Nuvation Bio capital stock (consisting of 12,963,780 shares of Series A Preferred Stock and 281,130,898 shares of Class A common stock) to Legacy Nuvation Bio in exchange for the issuance to him of 294,094,678 shares of Legacy Nuvation Bio Class B common stock.
Indemnification Agreements
Our amended and restated certificate of incorporation contains provisions limiting the liability of directors, and our amended and restated bylaws provide that we will indemnify each of our directors and officers to the fullest
117
Table of Contents
extent permitted under Delaware law. Our amended and restated certificate of incorporation and amended and restated bylaws also provide our board of directors with discretion to indemnify our employees and other agents when determined appropriate by the board. In addition, we have entered into an indemnification agreement with each of our directors and executive officers, which requires us to indemnify them. For more information regarding these agreements, see the section titled “Executive Compensation—Limitations on Liability and Indemnification Matters.”
Related Person Transactions Policy Following the Business Combination
Following the Business Combination, our board adopted a new written related person transactions policy that sets forth our policies and procedures regarding the identification, review, consideration and oversight of “related person transactions.” For purposes of our policy only, a “related person transaction” is a transaction, arrangement or relationship (or any series of similar transactions, arrangements or relationships) in which we or any of our subsidiaries are participants involving an amount that exceeds the lesser of (a) $120,000 or (b) 1% of the average of our total assets for the last two completed fiscal years, in which any “related person” has a material interest.
Transactions involving compensation for services provided to us as an employee, consultant or director will not be considered related person transactions under this policy. A related person is any executive officer, director, nominee to become a director or a holder of more than 5% of any class of our voting securities (including our common stock), including any of their immediate family members and affiliates, including entities owned or controlled by such persons.
Under this policy, the related person in question or, in the case of transactions with a holder of more than 5% of any class of our voting securities, an officer with knowledge of a proposed transaction, must present information regarding the proposed related person transaction to our audit committee (or, where review by our audit committee would be inappropriate, to another independent body of our Board) for review. To identify related person transactions in advance, we will rely on information supplied by our executive officers, directors and certain significant stockholders. In considering related person transactions, our audit committee will take into account the relevant available facts and circumstances, which may include, but are not limited to:
| • | the risks, costs, and benefits to us; |
| • | the impact on a director’s independence in the event the related person is a director, immediate family member of a director or an entity with which a director is affiliated; |
| • | the terms of the transaction; |
| • | the availability of other sources for comparable services or products; and |
| • | the terms available to or from, as the case may be, unrelated third parties. |
| • | Our audit committee will approve only those transactions that it determines are fair and in our best interests. All of the transactions described above were entered into prior to the adoption of such policy. |
118
Table of Contents
| Item 14. | Principal Accountant Fees and Services. |
The firm of WithumSmith+Brown, PC, or Withum, acts as our independent registered public accounting firm. The following is a summary of fees paid to Withum for services rendered.
Audit Fees. For the period from April 24, 2020 (inception) through December 31, 2020, fees incurred for our independent registered public accounting firm were approximately $ 121,000, for the services Withum performed in connection with our Initial Public Offering, review of quarterly Form 10-Q’s, audit related consents and the audit of our December 31, 2020 financial statements included in this Annual Report on Form 10-K.
Audit-Related Fees. For the period from April 24, 2020 (inception) through December 31, 2020, our independent registered public accounting firm did not render assurance and related services related to the performance of the audit or review of consolidated financial statements.
Tax Fees. For the period from April 24, 2020 (inception) through December 31, 2020, our independent registered public accounting firm did not render services to us for tax compliance, tax advice and tax planning.
All Other Fees. For the period from April 24, 2020 (inception) through December 31, 2020, there were no fees billed for products and services provided by our independent registered public accounting firm other than those set forth above.
Pre-Approval Policy
Our audit committee was formed upon the consummation of our Initial Public Offering. As a result, the audit committee did not pre-approve all of the foregoing services, although any services rendered prior to the formation of our audit committee were approved by our board of directors. Since the formation of our audit committee, and on a going-forward basis, the audit committee has and will pre-approve all auditing services and permitted non-audit services to be performed for us by our auditors, including the fees and terms thereof (subject to the de minimis exceptions for non-audit services described in the Exchange Act which are approved by the audit committee prior to the completion of the audit).
119
Table of Contents
| Item 15. | Exhibits, Financial Statement Schedules. |
(a) The following documents are filed as part of this report:
1. Consolidated Financial Statements
| Page | ||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
2. Consolidated Financial Statement Schedules
None.
3. Exhibits
The exhibits listed in the accompanying index to exhibits are filed or incorporated by reference as part of this Annual Report on Form 10-K.
120
Table of Contents
121
Table of Contents
| Incorporated by Reference | ||||||||||
| Exhibit Number |
Description |
Schedule/ |
File No. |
Exhibit |
Filing Date | |||||
| 10.14† | Collaboration Agreement by and among Legacy Nuvation Bio, RePharmation Limited and Sparcbio LLC, dated as of January 21, 2019 | S-4/A | 333-250036 | 10.10 | December 18, 2020 | |||||
| 10.15 | Agreement of Lease by and between Zapco 1500 Investment, L.P. and Legacy Nuvation Bio, dated June 30, 2019 | S-4/A | 333-250036 | 10.17 | December 18, 2020 | |||||
| 10.16 | Standard Industrial/Commercial Multi-Tenant Lease-Gross by and between 585 Howard Street Partners and the Legacy Nuvation Bio, dated June 7, 2019, as amended | S-4 | 333-250036 | 10.19 | November 12, 2020 | |||||
| 10.17† | Asset Acquisition Agreement by and between RePharmation Inc., GIRAFPHARMA LLC and David Hung, dated January 21, 2019 | S-4/A | 333-250036 | 10.19 | December 18, 2020 | |||||
| 10.18 | Stock Restriction Agreement by and between the Legacy Nuvation Bio and David Hung, dated June 17, 2019 | S-4 | 333-250036 | 10.21 | November 12, 2020 | |||||
| 10.19 | Form of Lock-Up Agreement | 8-K | 001-39351 | 10.6 | October 21, 2020 | |||||
| 14.1 | Code of Business Conduct and Ethics | 8-K | 001-39351 | 14.1 | February 12, 2021 | |||||
| 16.1 | Letter from Withum | 8-K |
001-39351 |
16.1 |
February 12, 2021 | |||||
| 21.1 | List of Subsidiaries | 8-K | 001-39351 | 21.1 | February 12, 2021 | |||||
| 31.1 | ||||||||||
| 31.2 | ||||||||||
| 32.1 | ||||||||||
| 101.INS | XBRL Instance Document | |||||||||
| 101.SCH | XBRL Taxonomy Extension Schema Document | |||||||||
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | |||||||||
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | |||||||||
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document | |||||||||
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | |||||||||
| + | Certain of the exhibits and schedules to this Exhibit have been omitted in accordance with Regulation S-K Item 601. The Registrant agrees to furnish a copy of all omitted exhibits and schedules to the SEC upon its request. | |
| # | Indicates a management contract or compensatory plan, contract or arrangement. | |
| † | Portions of this exhibit, as marked by asterisks, have been omitted in accordance with Regulation S-K Item 601. |
122
Table of Contents
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| NUVATION BIO INC. | ||||||
| Date: March 11, 2021 | By: | /s/ David Hung, M.D. | ||||
| David Hung, M.D. | ||||||
| President and Chief Executive Officer | ||||||
POWER OF ATTORNEY
Each person whose individual signature appears below hereby authorizes and appoints David Hung, M.D. and Jennifer Fox, and each of them, with full power of substitution and resubstitution and full power to act without the other, as his or her true and lawful attorney-in-fact and agent to act in his or her name, place and stead and to execute in the name and on behalf of each person, individually and in each capacity stated below, and to file any and all amendments to this Annual Report on Form 10-K and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing, ratifying and confirming all that said attorneys-in-fact and agents or any of them or their or his or her substitute or substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /S/ DAVID HUNG, M.D. |
Chief Executive Officer (Principal Executive Officer) |
March 11, 2021 | ||
| David Hung, M.D. | ||||
| /S/ JENNIFER FOX |
Chief Financial Officer (Principal Financial and Accounting Officer) |
March 11, 2021 | ||
| Jennifer Fox | ||||
| /S/ DANIEL G. WELCH |
Chair of the Board of Directors | March 11, 2021 | ||
| Daniel G. Welch | ||||
| /S/ ROBERT B. BAZEMORE, JR. |
Director | March 11, 2021 | ||
| Robert B. Bazemore, Jr. | ||||
| /S/ KIM BLICKENSTAFF |
Director | March 11, 2021 | ||
| Kim Blickenstaff | ||||
| /S/ MICHELLE DOIG |
Director | March 11, 2021 | ||
| Michelle Doig | ||||
| /S/ KATHRYN E. FALBERG |
Director | March 11, 2021 | ||
| Kathryn E. Falberg | ||||
| /S/ OLEG NODELMAN |
Director | March 11, 2021 | ||
| Oleg Nodelman | ||||
| /S/ W. ANTHONY VERNON |
Director | March 11, 2021 | ||
| W. Anthony Vernon | ||||
124
Table of Contents
INDEX TO FINANCIAL STATEMENTS
| F-2 | ||||
| Financial Statements: |
||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 to F-17 |
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of
Panacea Acquisition Corp.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheet of Panacea Acquisition Corp. (the “Company”) as of December 31, 2020, the related consolidated statements of operations, changes in stockholders’ equity and cash flows for the period from April 24, 2020 (inception) through December 31, 2020, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020, and the results of its operations and its cash flows for the period from April 24, 2020 (inception) through December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ WithumSmith+Brown, PC
We have served as the Company’s auditor since 2020.
New York, New York
March 11, 2021
F-2
Table of Contents
PANACEA ACQUISITION CORP.
DECEMBER 31, 2020
| ASSETS |
||||
| Current assets |
||||
| Cash |
$ | 908,111 | ||
| Prepaid expenses |
339,089 | |||
|
|
|
|||
| Total Current Assets |
1,247,200 | |||
| Investments held in trust account |
143,757,011 | |||
|
|
|
|||
| Total Assets |
$ | 145,004,211 | ||
|
|
|
|||
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
||||
| Current liabilities |
||||
| Accounts payable and accrued expenses |
$ | 2,699,715 | ||
| Accrued offering costs |
99,000 | |||
|
|
|
|||
| Total Liabilities |
2,798,715 | |||
|
|
|
|||
| Commitments and contingencies |
||||
| Class A common stock subject to possible redemption, 13,720,549 shares at $10.00 per share redemption value |
137,205,490 | |||
| Stockholders’ Equity |
||||
| Preferred stock, $0.0001 par value; 5,000,000 shares authorized; none issued or outstanding |
— | |||
| Class A common stock, $0.0001 par value; 500,000,000 shares authorized; 1,141,951 shares issued and outstanding (excluding 13,720,549 shares subject to possible redemption) |
114 | |||
| Class B common stock, $0.0001 par value; 20,000,000 shares authorized; 3,593,750 shares issued and outstanding |
359 | |||
| Additional paid-in capital |
8,053,974 | |||
| Accumulated deficit |
(3,054,441 | ) | ||
|
|
|
|||
| Total Stockholders’ Equity |
5,000,006 | |||
|
|
|
|||
| Total Liabilities and Stockholders’ Equity |
$ | 145,004,211 | ||
|
|
|
The accompanying notes are an integral part of the consolidated financial statements.
F-3
Table of Contents
PANACEA ACQUISITION CORP.
CONSOLIDATED STATEMENT OF OPERATIONS
FOR THE PERIOD FROM APRIL 24, 2020 (INCEPTION) THROUGH DECEMBER 31, 2020
| General and administrative expenses |
$ | 3,061,452 | ||
|
|
|
|||
| Loss from operations |
(3,061,452 | ) | ||
| Other income: |
||||
| Interest earned on investments held in Trust Account |
7,011 | |||
|
|
|
|||
| Net loss |
$ | (3,054,441 | ) | |
|
|
|
|||
| Weighted average shares outstanding of Class A redeemable common stock |
14,375,000 | |||
|
|
|
|||
| Basic and diluted income per share, Class A redeemable common stock |
$ | — | ||
|
|
|
|||
| Weighted average shares outstanding of Class A and Class B non-redeemable common stock |
3,840,179 | |||
|
|
|
|||
| Basic and diluted net loss per share, Class A and Class B non-redeemable common stock |
$ | (0.80 | ) | |
|
|
|
The accompanying notes are an integral part of the consolidated financial statements.
F-4
Table of Contents
PANACEA ACQUISITION CORP.
CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS’ EQUITY
| Class A Common Stock |
Class B Common Stock |
Additional Paid-in |
Accumulated | Total Stockholders’ |
||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | Equity | ||||||||||||||||||||||
| Balance – April 24, 2020 (Inception) |
— | $ | — | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||
| Issuance of Class B common stock to initial stockholders |
— | — | 3,593,750 | 359 | 24,641 | — | 25,000 | |||||||||||||||||||||
| Sale of 14,375,000 Units, net of underwriting discounts |
14,375,000 | 1,438 | — | — | 140,358,499 | — | 140,359,937 | |||||||||||||||||||||
| Sale of 487,500 Private Placement Units |
487,500 | 48 | — | — | 4,874,952 | — | 4,875,000 | |||||||||||||||||||||
| Common stock subject to possible redemption |
(13,720,549 | ) | (1,372 | ) | — | — | (137,204,118 | ) | — | (137,205,490 | ) | |||||||||||||||||
| Net loss |
— | — | — | — | — | (3,054,441 | ) | (3,054,441 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance – December 31, 2020 |
1,141,951 | $ | 114 | 3,593,750 | $ | 359 | $ | 8,053,974 | $ | (3,054,441 | ) | $ | 5,000,006 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-5
Table of Contents
PANACEA ACQUISITION CORP.
CONSOLIDATED STATEMENT OF CASH FLOWS
FOR THE PERIOD FROM APRIL 24, 2020 (INCEPTION) THROUGH DECEMBER 31, 2020
| Cash Flows from Operating Activities: |
||||
| Net loss |
$ | (3,054,441 | ) | |
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||
| Interest earned on investments held in Trust Account |
(7,011 | ) | ||
| Changes in operating assets and liabilities: |
||||
| Prepaid expenses |
(339,089 | ) | ||
| Accounts payable and accrued expenses |
2,699,715 | |||
|
|
|
|||
| Net cash used in operating activities |
(700,826 | ) | ||
|
|
|
|||
| Cash Flows from Investing Activities: |
||||
| Investment of cash into Trust Account |
(143,750,000 | ) | ||
|
|
|
|||
| Net cash used in investing activities |
(143,750,000 | ) | ||
|
|
|
|||
| Cash Flows from Financing Activities: |
||||
| Proceeds from sale of Units, net of underwriting discounts paid |
140,875,000 | |||
| Proceeds from sale of Private Placement Units |
4,875,000 | |||
| Proceeds from promissory note – related party |
80,000 | |||
| Repayment of promissory note – related party |
(80,000 | ) | ||
| Payment of offering costs |
(391,063 | ) | ||
|
|
|
|||
| Net cash provided by financing activities |
145,358,937 | |||
|
|
|
|||
| Net Change in Cash |
908,111 | |||
| Cash – Beginning of period |
— | |||
|
|
|
|||
| Cash – End of period |
$ | 908,111 | ||
|
|
|
|||
| Non-Cash financing activities: |
||||
| Initial classification of Class A common stock subject to possible redemption |
$ | 140,258,930 | ||
|
|
|
|||
| Change in value of Class A common stock subject to possible redemption |
$ | (2,828,440 | ) | |
|
|
|
|||
| Offering costs paid directly by Sponsor in consideration for the issuance of Class B common stock |
$ | 25,000 | ||
|
|
|
|||
| Offering costs included in accrued offering costs |
$ | 99,000 | ||
|
|
|
The accompanying notes are an integral part of the consolidated financial statements.
F-6
Table of Contents
PANACEA ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2020
NOTE 1. DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS
Panacea Acquisition Corp. (the “Company”) was incorporated in Delaware on April 24, 2020. The Company was formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses (the “Business Combination”). The Company is not limited to a particular industry or sector for purposes of consummating a Business Combination. The Company is an early stage and emerging growth company and, as such, the Company is subject to all of the risks associated with early stage and emerging growth companies.
The Company has one subsidiary, Panacea Merger Subsidiary Corp., a wholly owned subsidiary of the Company incorporated in Delaware on October 16, 2020 (“Merger Sub”).
As of December 31, 2020, the Company had not commenced any operations. All activity for the period from April 24, 2020 (inception) through December 31, 2020 relates to the Company’s formation, the initial public offering (“Initial Public Offering”), which is described below, and activities in connection with the proposed acquisition of Nuvation Bio Inc., a Delaware corporation (“Nuvation Bio”). The Company will not generate any operating revenues until after the completion of its initial Business Combination, at the earliest. The Company will generate non-operating income in the form of interest income from the proceeds derived from the Initial Public Offering.
The registration statement for the Company’s Initial Public Offering was declared effective on June 30, 2020. On July 6, 2020, the Company consummated the Initial Public Offering of 14,375,000 units (the “Units” and, with respect to the shares of Class A common stock included in the Units sold, the “Public Shares”), which includes the full exercise by the underwriters of the over-allotment option to purchase an additional 1,875,000 Units, at $10.00 per Unit, generating gross proceeds of $143,750,000, which is described in Note 3.
Simultaneously with the closing of the Initial Public Offering, the Company consummated the sale of 487,500 units (each, a “Private Placement Unit” and collectively, the “Private Placement Units”) at a price of $10.00 per Private Placement Unit in a private placement to EcoR1 Panacea Holdings, LLC, a Delaware limited liability company (the “Sponsor”), and PA Co-Investments LLC, an affiliate of one of the underwriters (“PA Co-Investments LLC”), generating gross proceeds of $4,875,000, which is described in Note 4.
Transaction costs amounted to $3,390,063, consisting of $2,875,000 of underwriting fees and $515,063 of other offering costs.
Following the closing of the Initial Public Offering on July 6, 2020, an amount of $143,750,000 ($10.00 per Unit) from the net proceeds of the sale of the Units in the Initial Public Offering and the sale of the Private Placement Units was placed in a trust account (the “Trust Account”) located in the United States and that will invest only in U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act of 1940, as amended (the “Investment Company Act”), with a maturity of 185 days or less or in any open-ended investment company that holds itself out as a money market fund selected by the Company meeting certain conditions of Rule 2a-7 of the Investment Company Act, as determined by the Company, until the earlier of: (i) the completion of a Business Combination and (ii) the distribution of the funds held in the Trust Account, as described below.
The Company’s management has broad discretion with respect to the specific application of the net proceeds of the Initial Public Offering and the sale of Private Placement Units, although substantially all of the net proceeds are intended to be applied generally toward consummating a Business Combination. There is no assurance that the Company will be able to complete a Business Combination successfully. The Company must complete one or more initial Business Combinations with one or more operating businesses or assets with a fair market value equal to at least 80% of the net assets held in the Trust Account (net of amounts disbursed to management for working capital purposes, if permitted, and excluding the amount of any deferred underwriting discount). The Company will only complete a Business Combination if the post-transaction company owns or acquires 50% or more of the outstanding voting securities of the target or otherwise acquires a controlling interest in the target business sufficient for it not to be required to register as an investment company under the Investment Company Act.
The Company will provide the holders of the outstanding Public Shares (the “Public Stockholders”) with the opportunity to redeem all or a portion of their Public Shares upon the completion of a Business Combination either (i) in connection with a stockholder meeting called to approve the Business Combination or (ii) by means of a tender offer. The decision as to whether the Company will seek stockholder approval of a Business Combination or conduct a tender offer will be made by the Company. The Public Stockholders will be entitled to redeem their Public Shares for a pro rata portion of the amount then in the Trust Account (initially anticipated to be $10.00 per Public Share, plus any pro rata interest then in the Trust Account, net of taxes payable). There will be no redemption rights upon the completion of a Business Combination with respect to the Company’s warrants.
F-7
Table of Contents
PANACEA ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2020
The Company will only proceed with a Business Combination if the Company has net tangible assets of at least $5,000,001 following any related redemptions and, if the Company seeks stockholder approval, a majority of the shares voted are voted in favor of the Business Combination. If a stockholder vote is not required by applicable law or stock exchange listing requirements and the Company does not decide to hold a stockholder vote for business or other reasons, the Company will, pursuant to its Amended and Restated Certificate of Incorporation (the “Certificate of Incorporation”), conduct the redemptions pursuant to the tender offer rules of the U.S. Securities and Exchange Commission (“SEC”) and file tender offer documents with the SEC prior to completing a Business Combination. If, however, stockholder approval of the transaction is required by applicable law or stock exchange listing requirements, or the Company decides to obtain stockholder approval for business or other reasons, the Company will offer to redeem shares in conjunction with a proxy solicitation pursuant to the proxy rules and not pursuant to the tender offer rules. If the Company seeks stockholder approval in connection with a Business Combination, the Company’s Sponsor, PA Co-Investments LLC and any other holders of the Company’s common stock prior to the Initial Public Offering (the “initial stockholders”) have agreed to vote their Founder Shares (as defined in Note 5), Private Placement Shares (as defined in Note 4) and any Public Shares purchased during or after the Initial Public Offering in favor of approving a Business Combination. Additionally, each Public Stockholder may elect to redeem their Public Shares without voting, and if they do vote, irrespective of whether they vote for or against the proposed transaction.
If the Company seeks stockholder approval of a Business Combination and it does not conduct redemptions pursuant to the tender offer rules, the Certificate of Incorporation will provide that a Public Stockholder, together with any affiliate of such stockholder or any other person with whom such stockholder is acting in concert or as a “group” (as defined under Section 13 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”)), will be restricted from redeeming its shares with respect to more than an aggregate of 15% of the Public Shares, without the prior consent of the Company.
The initial stockholders have agreed (a) to waive their redemption rights with respect to their Founder Shares, Private Placement Shares and Public Shares held by them in connection with the completion of a Business Combination and (b) not to propose an amendment to the Certificate of Incorporation (i) to modify the substance or timing of the Company’s obligation to allow redemptions in connection with a Business Combination or to redeem 100% of its Public Shares if the Company does not complete a Business Combination within the Combination Period (as defined below) or (ii) with respect to any other provision relating to stockholders’ rights or pre-business combination activity, unless the Company provides the Public Stockholders with the opportunity to redeem their Public Shares in conjunction with any such amendment.
The initial stockholders have agreed to waive their liquidation rights with respect to the Founder Shares and Private Placement Shares if the Company fails to complete a Business Combination within the Combination Period. However, if the initial stockholders acquire Public Shares in or after the Initial Public Offering, such Public Shares will be entitled to liquidating distributions from the Trust Account if the Company fails to complete a Business Combination within the Combination Period.
If the Company has not completed a Business Combination by July 6, 2022 (as it may be extended, the “Combination Period”), the Company will (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the Public Shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account, including interest (which interest shall be net of taxes payable, and less up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding Public Shares, which redemption will completely extinguish Public Stockholders’ rights as stockholders (including the right to receive further liquidating distributions, if any), and (iii) as promptly as reasonably possible following such redemption, subject to the approval of the Company’s remaining stockholders and the Company’s board of directors, dissolve and liquidate, subject in each case to the Company’s obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. There will be no redemption rights or liquidating distributions with respect to the Company’s warrants, which will expire worthless if the Company fails to complete a Business Combination within the Combination Period.
In order to protect the amounts held in the Trust Account, the Sponsor has agreed to be liable to the Company if and to the extent any claims by a third party for services rendered or products sold to the Company, or a prospective target business with which the Company has discussed entering into a transaction agreement, reduce the amount of funds in the Trust Account to below (i) $10.00 per Public Share or (ii) such lesser amount per Public Share held in the Trust Account as of the date of the liquidation of the Trust Account due to reductions in the value of the trust assets, in each case net of the amount of interest which may be withdrawn to pay taxes, except as to any claims by a third party who executed a waiver of any and all rights to seek access to the Trust Account and except as to any claims under the Company’s indemnity of the underwriters of the Initial Public Offering against certain liabilities, including liabilities under the Securities Act of 1933, as amended (the “Securities Act”). Moreover, in the event that an executed waiver is deemed to be unenforceable against a third party, the Sponsor will not be responsible to the extent of any liability for such third-party claims. The Company will seek to reduce the possibility that the Sponsor will have to indemnify the Trust Account due to claims of creditors by endeavoring to have all vendors, service providers (except for the Company’s independent registered public accounting firm), prospective target businesses and other entities with which the Company does business, execute agreements with the Company waiving any right, title, interest or claim of any kind in or to monies held in the Trust Account.
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and pursuant to the rules and regulations of the SEC.
F-8
Table of Contents
PANACEA ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2020
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of the Company and its wholly owned subsidiary. All significant intercompany balances and transactions have been eliminated in consolidation.
Emerging Growth Company
The Company is an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and it may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the independent registered public accounting firm attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s consolidated financial statements with another public company which is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires the Company’s management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period.
Making estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the consolidated financial statements, which management considered in formulating its estimate, could change in the near term due to one or more future confirming events. Accordingly, the actual results could differ significantly from those estimates.
Class A Common Stock Subject to Possible Redemption
The company accounts for its Class A common stock subject to possible redemption in accordance with the guidance in the Financial Accounting Standard Board’s (“FASB”) Accounting Standards Codification (“ASC”) Topic 480 “Distinguishing Liabilities from Equity.” Shares of Class A common stock subject to mandatory redemption are classified as a liability instrument and are measured at fair value. Conditionally redeemable common stock (including common stock that feature redemption rights that is either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within the Company’s control) is classified as temporary equity. At all other times, common stock is classified as stockholders’ equity. The Company’s Class A common stock features certain redemption rights that are considered to be outside of the Company’s control and subject to occurrence of uncertain future events. Accordingly, shares of Class A common stock subject to possible redemption are presented as temporary equity, outside of the stockholders’ equity section of the Company’s consolidated balance sheet.
Offering Costs
Offering costs consist of legal, accounting, underwriting fees and other costs incurred through the balance sheet date that are directly related to the Initial Public Offering. Offering costs amounting to $3,390,063 were charged to stockholders’ equity upon the completion of the Initial Public Offering.
F-9
Table of Contents
PANACEA ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2020
Income Taxes
The Company follows the asset and liability method of accounting for income taxes under ASC 740, “Income Taxes.” Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the consolidated financial statements carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that included the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
ASC 740 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities. The Company recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense. There were no unrecognized tax benefits and no amounts accrued for interest and penalties as of December 31, 2020. The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation from its position. The Company is subject to income tax examinations by major taxing authorities since inception.
Net Income (Loss) per Common Share
Net income (loss) per common share is computed by dividing net income (loss) by the weighted average number of common shares outstanding for the period. The Company has not considered the effect of warrants sold in the Initial Public Offering and private placement to purchase 4,954,167 shares of Class A common stock in the calculation of diluted income per share, since the exercise of the warrants are contingent upon the occurrence of future events and the inclusion of such warrants would be anti-dilutive.
The Company’s consolidated statement of operations includes a presentation of income (loss) per share for common shares subject to possible redemption in a manner similar to the two-class method of income (loss) per share. Net income per common share, basic and diluted, for Class A redeemable common stock is calculated by dividing the interest income earned on the Trust Account, by the weighted average number of Class A redeemable common stock outstanding since original issuance. Net loss per share, basic and diluted, for Class A and B non-redeemable common stock is calculated by dividing the net loss, adjusted for income attributable to Class A redeemable common stock, net of applicable franchise and income taxes, by the weighted average number of Class A and B non-redeemable common stock outstanding for the period. Class A and B non-redeemable common stock includes the Founder Shares as these shares do not have any redemption features and do not participate in the income earned on the Trust Account.
The following table reflects the calculation of basic and diluted net income (loss) per common share (in dollars, except per share amounts):
| For the Period From April 24, 2020 (inception) Through December 31, 2020 |
||||
| Redeemable Class A Common Stock |
||||
| Numerator: Earnings allocable to Redeemable Class A Common Stock |
||||
| Interest Income |
$ | 7,011 | ||
| Income and Franchise Tax |
(7,011 | ) | ||
|
|
|
|||
| Net Earnings |
$ | — | ||
| Denominator: Weighted Average Redeemable Class A Common Stock |
||||
| Redeemable Class A Common Stock, Basic and Diluted |
14,375,000 | |||
| Earnings/Basic and Diluted Redeemable Class A Common Stock |
$ | — | ||
| Non-Redeemable Class A and B Common Stock |
||||
| Numerator: Net Loss minus Redeemable Net Earnings |
||||
| Net Loss |
$ | (3,054,441 | ) | |
| Redeemable Net Earnings |
— | |||
|
|
|
|||
| Non-Redeemable Net Loss |
$ | (3,054,441 | ) | |
| Denominator: Weighted Average Non-Redeemable Class A and B Common Stock |
||||
| Non-Redeemable Class A and B Common Stock, Basic and Diluted (1) |
3,840,179 | |||
| Loss/Basic and Diluted Non-Redeemable Class A and B Common Stock |
$ | (0.80 | ) | |
Note: As of December 31, 2020, basic and diluted shares are the same as there are no non-redeemable securities that are dilutive to the Company’s stockholders.
| (1) | The weighted average non-redeemable common stock for the year ended December 31, 2020 includes the effect of 487,500 Private Placement Units, which were issued in conjunction with the initial public offering on July 6, 2020. |
F-10
Table of Contents
PANACEA ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2020
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist of a cash account in a financial institution, which, at times, may exceed the Federal Depository Insurance Coverage of $250,000. The Company has not experienced losses on this account and management believes the Company is not exposed to significant risks on such account.
Fair Value of Financial Instruments
The fair value of the Company’s assets and liabilities, which qualify as financial instruments under ASC 820, “Fair Value Measurement,” approximates the carrying amounts represented in the accompanying consolidated balance sheet, primarily due to their short-term nature.
Recent Accounting Standards
Management does not believe that any recently issued, but not yet effective, accounting standards, if currently adopted, would have a material effect on the Company’s consolidated financial statements.
NOTE 3. INITIAL PUBLIC OFFERING
Pursuant to the Initial Public Offering, the Company sold 14,375,000 Units, which includes the full exercise by the underwriters of their option to purchase an additional 1,875,000 Units, at a purchase price of $10.00 per Unit. Each Unit consists of one share of Class A common stock and one-third of one redeemable warrant (“Public Warrant”). Each whole Public Warrant entitles the holder to purchase one share of Class A common stock at a price of $11.50 per share, subject to adjustment (see Note 7).
NOTE 4. PRIVATE PLACEMENT
Simultaneously with the closing of the Initial Public Offering, the Sponsor and PA Co-Investments LLC purchased an aggregate of 487,500 Private Placement Units at a price of $10.00 per Private Placement Unit, for an aggregate purchase price of $4,875,000. The Sponsor purchased 390,000 Private Placement Units and PA Co-Investments LLC purchased 97,500 Private Placement Units. Each Private Placement Unit consists of one share of Class A common stock (“Private Placement Share” or, collectively, “Private Placement Shares”) and one-third of one warrant (each, a “Private Placement Warrant”). Each whole Private Placement Warrant is exercisable to purchase one share of Class A common stock at a price of $11.50 per share, subject to adjustment. A portion of the proceeds from the Private Placement Units were added to the proceeds from the Initial Public Offering held in the Trust Account. If the Company does not complete a Business Combination within the Combination Period, the proceeds from the sale of the Private Placement Units will be used to fund the redemption of the Public Shares (subject to the requirements of applicable law), and the Private Placement Units and all underlying securities will expire worthless.
NOTE 5. RELATED PARTY TRANSACTIONS
Founder Shares
On May 7, 2020, the Sponsor and Cowen Investments II LLC paid an aggregate of $25,000 to cover certain offering costs of the Company in consideration for 3,593,750 shares of the Company’s Class B common stock (the “Founder Shares”). In May 2020, the Sponsor transferred 25,000 Founder Shares to each of its directors, or an aggregate of 100,000 Founder Shares, at their original purchase price. Cowen Investments II LLC subsequently transferred all of its Founder Shares to PA Co-Investments LLC. The Founder Shares included an aggregate of up to 468,750 shares subject to forfeiture to the extent that the underwriters’ over-allotment option was not exercised in full or in part, so that the number of Founder Shares would equal 20% of the Company’s issued and outstanding shares after the Initial Public Offering (not including the Private Placement Shares). As a result of the underwriters’ election to fully exercise their over-allotment option, no Founder Shares are currently subject to forfeiture.
The initial stockholders have agreed, subject to limited exceptions, not to transfer, assign or sell any of the Founder Shares until the earlier to occur of: (A) one year after the completion of a Business Combination and (B) subsequent to a Business Combination, (x) if the last reported sale price of the Class A common stock equals or exceeds $12.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-trading day period commencing at least 150 days after a Business Combination, or (y) the date on which the Company completes a liquidation, merger, capital stock exchange or other similar transaction that results in all of the Public Stockholders having the right to exchange their shares of common stock for cash, securities or other property.
F-11
Table of Contents
PANACEA ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2020
Administrative Support Agreement
The Company entered into an agreement, commencing on July 1, 2020 through the earlier of the Company’s consummation of a Business Combination or its liquidation, to pay an affiliate of the Sponsor a total of $10,000 per month for office space, administrative and support services. For the period from April 24, 2020 (inception) through December 31, 2020, the Company incurred $60,000 in fees for these services, of which such amount is included in accounts payable and accrued expenses in the accompanying consolidated balance sheet.
Promissory Notes — Related Parties
On May 15, 2020, the Sponsor and an affiliate of PA Co-Investments LLC issued unsecured promissory notes to the Company (the “Promissory Notes”), pursuant to which the Company could borrow up to an aggregate principal amount of $300,000. The Promissory Notes were non-interest bearing and payable on the earlier of December 31, 2020 or the consummation of the Initial Public Offering. The outstanding balance under the Promissory Notes of $80,000 was repaid upon the consummation of the Initial Public Offering on July 6, 2020.
Related Party Loans
In addition, in order to fund working capital deficiencies or finance transaction costs in connection with a Business Combination, the Sponsor, PA Co-Investments LLC or an affiliate of the Sponsor or PA Co-Investments LLC, or certain of the Company’s officers and directors may, but are not obligated to, loan the Company funds as may be required (“Working Capital Loans”). If the Company completes a Business Combination, the Company may repay the Working Capital Loans out of the proceeds of the Trust Account released to the Company. Otherwise, the Working Capital Loans may be repaid only out of funds held outside the Trust Account. In the event that a Business Combination does not close, the Company may use a portion of proceeds held outside the Trust Account to repay the Working Capital Loans but no proceeds held in the Trust Account would be used to repay the Working Capital Loans. The Working Capital Loans would either be repaid upon consummation of a Business Combination, without interest, or, at the lender’s discretion, up to $1,500,000 of such Working Capital Loans may be convertible into units upon consummation of the Business Combination at a price of $10.00 per unit. The units would be identical to the Private Placement Units. Except for the foregoing, the terms of such Working Capital Loans, if any, have not been determined and no written agreements exist with respect to such loans. As of December 31, 2020, no amounts were outstanding under the Working Capital Loans.
NOTE 6. COMMITMENTS
Risks and Uncertainties
Management continues to evaluate the impact of the COVID-19 pandemic and has concluded that while it is reasonably possible that the virus could have a negative effect on the Company’s financial position, results of its operations and/or search for a target company, the specific impact is not readily determinable as of the date of these consolidated financial statements. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Registration Rights
Pursuant to a registration rights agreement entered into on June 30, 2020, the holders of the Founder Shares, Private Placement Units, Private Placement Shares, Private Placement Warrants, certain forward purchase securities and units that may be issued upon conversion of Working Capital Loans and the shares and warrants included therein (and any shares of common stock issuable upon the exercise of the Private Placement Warrants, forward purchase warrants or warrants included in the units issued upon conversion of Working Capital Loans) will be entitled to registration rights requiring the Company to register such securities for resale (in the case of the Founder Shares, only after conversion to Class A common stock). The holders of these securities will be entitled to make up to three demands, excluding short form demands, that the Company register such securities. In addition, the holders have certain “piggy-back” registration rights with respect to registration statements filed subsequent to the completion of a Business Combination and rights to require the Company to register for resale such securities pursuant to Rule 415 under the Securities Act. Notwithstanding the foregoing, PA Co-Investments LLC may not exercise its demand or “piggyback” registration rights after five and seven years, respectively, after the effective date of the registration statement related to the Initial Public Offering and may not exercise its demand rights on more than one occasion. The Company will bear the expenses incurred in connection with the filing of any such registration statements.
Underwriting Agreement
On July 6, 2020, the underwriters were paid a cash underwriting discount of $0.20 per Unit, or $2,875,000 in the aggregate.
F-12
Table of Contents
PANACEA ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2020
Business Combination Marketing Agreement
The Company has engaged the underwriters as an advisor in connection with a Business Combination to assist the Company in holding meetings with its stockholders to discuss the potential Business Combination and the target business’ attributes, introduce the Company to potential investors that are interested in purchasing the Company’s securities in connection with a Business Combination, assist the Company in obtaining stockholder approval for the Business Combination and assist the Company with its press releases and public filings in connection with the Business Combination. The Company will pay the underwriters a cash fee for such services upon the consummation of a Business Combination in an amount equal to, in the aggregate, 3.5% of the gross proceeds of Initial Public Offering, or $5,031,250, including any proceeds from the full or partial exercise of the over-allotment option. At the closing of the Business Combination this amount was paid.
Forward Purchase Agreement
On June 30, 2020, the Company entered into a forward purchase agreement with funds affiliated with EcoR1 Capital, LLC that will provide for the purchase by such funds of an aggregate of 2,500,000 shares of Class A common stock and 833,333 redeemable warrants, for an aggregate purchase price of $25,000,000, or $10.00 per one share of Class A common stock and one-third of one redeemable warrant, in a private placement to close substantially concurrently with the closing of a Business Combination. The obligations under the forward purchase agreement will not depend on whether any shares of Class A common stock are redeemed by the Public Stockholders. The shares of Class A common stock and redeemable warrants issuable pursuant to the forward purchase agreement will be identical to the shares of Class A common stock and redeemable warrants included in the units being sold in the Initial Public Offering, respectively, except that the holders thereof will have certain registration rights.
On February 10, 2021, certain purchasers purchased 2,500,000 shares of Class A Common Stock and 833,333 forward purchase warrants in a private placement at a price of $10.00 per share for an aggregate purchase price of $25.0 million pursuant to the terms of the forward purchase. The sales of the PIPE Shares and the Forward Purchase Securities were consummated concurrently with the closing of the Business Combination (see Note 10).
Merger Agreement
On October 20, 2020, the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Merger Sub and Nuvation Bio.
Pursuant to the transactions contemplated by the terms of the Merger Agreement (the “Closing”), and subject to the satisfaction or waiver of certain conditions set forth therein, Merger Sub will merge with and into Nuvation Bio, with Nuvation Bio surviving the merger and as a wholly owned subsidiary of the Company (the “Merger”) (the transactions contemplated by the Merger Agreement and the related ancillary agreements, the “Nuvation Bio Business Combination”).
As a result of the merger, among other things,
| (i) | each share of Nuvation Bio Class A common stock and each share of Nuvation Bio Series A preferred stock issued and outstanding immediately prior to the Effective Time will be canceled and converted into the right to receive the number of shares of the Company’s Class A common stock equal to the Exchange Ratio (as defined below). The Company’s Class A common stock will have one vote per share; | |
| (ii) | each share of Nuvation Bio Class B common stock issued and outstanding immediately prior to the Effective Time (all of which is owned by David Hung (the “Founder”)) will be canceled and converted into the right to receive the number of shares of the Company’s Class B common stock equal to the Exchange Ratio. The Company’s Class B common stock will have veto rights over business combinations and liquidations, one vote on all other matters and the right to appoint three directors (including the seat occupied by the Chief Executive Officer) plus at least 50% of any directors beyond the initial seven. The Company’s Class B common stock will automatically convert into the Company’s Class A common stock upon the occurrence of certain events, including upon transfers to a non-authorized holder or if the Founder ceases to be Chief Executive Officer of Nuvation Bio, with limited exceptions; | |
| (iii) | any shares of Nuvation Bio capital stock held in the treasury of Nuvation Bio or owned by the Company, Merger Sub or Nuvation Bio immediately prior to the Effective Time (each, an “Excluded Share”) will be canceled without any conversion thereof and no payment or distribution shall be made with respect thereto; | |
| (iv) | each issued and outstanding share of common stock of Merger Sub will be converted into and become one validly issued, fully paid and nonassessable share of common stock of the surviving corporation; and | |
| (v) | each option to purchase Nuvation Bio Class A common stock (each, a “Nuvation Bio Option”) that is outstanding under Nuvation Bio’s 2019 Equity Incentive Plan immediately prior to the Closing, whether vested or unvested, will be assumed by the Company and converted into an option to purchase shares of the Company’s Class A common stock (each, a “Converted Option”) equal to the product (rounded down to the nearest whole number) of (a) the number of shares of Nuvation Bio common stock subject to such Nuvation Bio Option immediately prior to the Effective Time and (b) the Exchange Ratio, at an exercise price per share (rounded up to the nearest whole cent) equal to (i) the exercise price per share of such Nuvation Bio Option immediately prior to the Effective Time divided by (ii) the Exchange Ratio; provided, however, that the exercise price and the number of shares of the Company’s common stock purchasable pursuant to the Converted Options shall be determined in a manner consistent with the requirements of Section 409A of the Code; provided, further, however, that in the case of any Converted Option to which Section 422 of the Code applies, the exercise price and the number of shares of the Company’s common stock purchasable pursuant to such option shall be determined in accordance with the foregoing, subject to such adjustments in a manner consistent with Treasury Regulation Section 1.424-1, such that the Converted Option will not constitute a modification of such Nuvation Bio Option for purposes of Section 409A or Section 424 of the Code. Except as specifically provided above, following the Effective Time, each Converted Option shall continue to be governed by the same terms and conditions (including vesting and exercisability terms) as were applicable to the corresponding former Nuvation Bio Option immediately prior to the Effective Time. At or prior to the Effective Time, the Company shall take any actions that are necessary to effectuate the treatment of the Nuvation Bio Options pursuant to this paragraph. | |
F-13
Table of Contents
PANACEA ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2020
The “Exchange Ratio” means the quotient of (i) 150,000,000; divided by (ii) Nuvation Bio’s “fully diluted company shares” (as defined in the Merger Agreement).
The Nuvation Bio Business Combination was consummated on February 10, 2021 as further described in Note 10.
NOTE 7. STOCKHOLDERS’ EQUITY
Preferred Stock — On June 30, 2020, the Company amended its Certificate of Incorporation such that the Company is authorized to issue 5,000,000 shares of preferred stock with a par value of $0.0001 per share with such designations, voting and other rights and preferences as may be determined from time to time by the Company’s board of directors. At December 31, 2020, there were no shares of preferred stock issued or outstanding.
Class A Common Stock — The Company is authorized to issue 500,000,000 shares of Class A common stock with a par value of $0.0001 per share. Holders of Class A common stock are entitled to one vote for each share. At December 31, 2020, there was 1,141,951 shares of Class A common stock issued and outstanding, excluding 13,720,549 shares of Class A common stock subject to possible redemption.
Class B Common Stock — The Company is authorized to issue 20,000,000 shares of Class B common stock with a par value of $0.0001 per share. Holders of Class B common stock are entitled to one vote for each share. At December 31, 2020, there were 3,593,750 shares of Class B common stock issued and outstanding.
Prior to the Company’s initial Business Combination, holders of the Class B common stock will have the right to elect all of the Company’s directors and remove members of the Company’s board of directors for any reason. On any other matter submitted to a vote of our stockholders, holders of Class A common stock and Class B common stock will vote together as a single class on all other matters submitted to a vote of stockholders except as required by applicable law or stock exchange rule.
The shares of Class B common stock will automatically convert into shares of Class A common stock at the time of a Business Combination, or earlier at the option of the holder, on a one-for-one basis, subject to adjustment. In the case that additional shares of Class A common stock, or equity-linked securities, are issued or deemed issued in excess of the amounts issued in the Initial Public Offering and related to the closing of a Business Combination, other than the forward purchase securities described in the prospectus, the ratio at which shares of Class B common stock shall convert into shares of Class A common stock will be adjusted (unless the holders of a majority of the outstanding shares of Class B common stock agree to waive such anti-dilution adjustment with respect to any such issuance or deemed issuance) so that the number of shares of Class A common stock issuable upon conversion of all shares of Class B common stock will equal, in the aggregate, on an as-converted basis, 20% of the sum of the total number of all shares of common stock outstanding upon the completion of the Initial Public Offering (not including the shares of Class A common stock underlying the Private Placement Units) plus all shares of Class A common stock and equity-linked securities issued or deemed issued in connection with a Business Combination (net of the number of shares of Class A common stock redeemed in connection with a Business Combination), excluding forward purchase securities and any shares or equity-linked securities issued, or to be issued, to any seller in a Business Combination.
Warrants — Public Warrants may only be exercised for a whole number of shares. No fractional warrants will be issued upon separation of the Units and only whole warrants will trade. The Public Warrants will become exercisable on the later of (a) 30 days after the completion of a Business Combination and (b) 12 months from the closing of the Initial Public Offering. The Public Warrants will expire five years after the completion of a Business Combination or earlier upon redemption or liquidation.
The Company will not be obligated to deliver any shares of Class A common stock pursuant to the exercise of a warrant and will have no obligation to settle such warrant exercise unless a registration statement under the Securities Act covering the issuance of the shares of Class A common stock issuable upon the exercise of the warrants is then effective and a current prospectus relating to those shares of Class A common stock is available, subject to the Company satisfying its obligations with respect to registration. No warrant will be exercisable and the Company will not be obligated to issue shares of Class A common stock upon exercise of a warrant unless Class A common stock issuable upon such warrant exercise has been registered, qualified or deemed to be exempt under the securities laws of the state of residence of the registered holder of the warrants.
F-14
Table of Contents
PANACEA ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2020
The Company has agreed that as soon as practicable, but in no event later than 20 business days after the closing of a Business Combination, the Company will use its commercially reasonable efforts to file with the SEC, and within 60 business days following a Business Combination to have declared effective, a registration statement covering the issuance of the shares of Class A common stock issuable upon exercise of the warrants and to maintain a current prospectus relating to those shares of Class A common stock until the warrants expire or are redeemed. Notwithstanding the above, if the Class A common stock is at the time of any exercise of a warrant not listed on a national securities exchange such that it satisfies the definition of a “covered security” under Section 18(b)(1) of the Securities Act, the Company may, at its option, require holders of Public Warrants who exercise their warrants to do so on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act and, in the event the Company so elects, the Company will not be required to file or maintain in effect a registration statement, but will use its commercially reasonable efforts to register or qualify the shares under applicable blue sky laws to the extent an exemption is not available.
Redemptions of warrants when the price of Class A common stock equals or exceeds $18.00 — Once the warrants become exercisable, the Company may redeem the Public Warrants:
| • in whole and not in part; |
| • at a price of $0.01 per warrant; |
| • upon not less than 30 days’ prior written notice of redemption, or the 30-day redemption period, to each warrant holder; and |
| • if, and only if, the reported last sale price of the Company’s Class A common stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within a 30-trading day period ending on the third trading day prior to the date on which the Company sends the notice of redemption to the warrant holders. |
If and when the warrants become redeemable by the Company, the Company may exercise its redemption right even if it is unable to register or qualify the underlying securities for sale under all applicable state securities laws.
Redemption of warrants when the price per share of Class common stock equals or exceeds $10.00 — Commencing ninety days after the warrants become exercisable, the Company may redeem the outstanding Public Warrants:
| • in whole and not in part; |
| • at a price of $0.10 per warrant provided that holders will be able to exercise their warrants prior to redemption and receive that number of shares of Class A common stock determined based on the redemption date and the “fair market value” of the Company’s Class A common stock; |
| • upon a minimum of 30 days’ prior written notice of redemption; |
| • if, and only if, the last reported sale price of the Company’s Class A common stock equals or exceeds $10.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) on the trading day prior to the date on which the Company sends the notice of redemption to the warrant holders; |
| • if, and only if, there is an effective registration statement covering the issuance of the shares of Class A common stock issuable upon exercise of the warrants and a current prospectus relating thereto is available throughout the 30-day period after the written notice of redemption is given. |
If the Company calls the Public Warrants for redemption as described above under “— Redemptions of warrants when the price of Class A common stock equals or exceeds $18.00,” the Company’s management will have the option to require all holders that wish to exercise the Public Warrants to do so on a “cashless basis,” as described in the warrant agreement. The exercise price and number of shares of Class A common stock issuable upon exercise of the warrants may be adjusted in certain circumstances including in the event of a stock dividend, or recapitalization, reorganization, merger or consolidation. However, except as described below, the warrants will not be adjusted for issuance of Class A common stock at a price below its exercise price. Additionally, in no event will the Company be required to net cash settle the warrants. If the Company is unable to complete a Business Combination within the Combination Period and the Company liquidates the funds held in the Trust Account, holders of warrants will not receive any of such funds with respect to their warrants, nor will they receive any distribution from the Company’s assets held outside of the Trust Account with the respect to such warrants. Accordingly, the warrants may expire worthless.
In addition, if (x) the Company issues additional shares of Class A common stock or equity-linked securities for capital raising purposes in connection with the closing of an initial Business Combination at an issue price or effective issue price of less than $9.20 per share of Class A common stock (with such issue price or effective issue price to be determined in good faith by the Company’s board of directors, and, in the case of any such issuance to the Sponsor or PA Co-Investments LLC or their affiliates, without taking into account any Founder Shares held by the Sponsor or PA Co-Investments LLC or their affiliates, as applicable, prior to such issuance) (the “Newly Issued Price”), (y) the aggregate gross proceeds from such issuances represent more than 60% of the total equity proceeds, and interest thereon, available for the funding of a Business Combination on the date of the completion of a Business Combination (net of redemptions), and (z) the volume weighted average trading price of our Class A common stock during the 20 trading day period starting on the trading day prior to the day on which the Company completes their initial business combination (such price, the “Market Value”) is below $9.20 per share, the exercise price of the warrants will be adjusted (to the nearest cent) to be equal to 115% of the higher of the Market Value and the Newly Issued Price, and the $18.00 per share redemption trigger price will be adjusted (to the nearest cent) to be equal to 180% of the higher of the Market Value and the Newly Issued Price.
F-15
Table of Contents
PANACEA ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2020
The Private Placement Warrants are identical to the Public Warrants underlying the Units sold in the Initial Public Offering, except that (1) the Private Placement Warrants and the Class A common stock issuable upon the exercise of the Private Placement Warrants will not be transferable, assignable or saleable until 30 days after the completion of a Business Combination, subject to certain limited exceptions, (2) the Private Placement Warrants will be exercisable on a cashless basis, (3) the Private Placement Warrants will be non-redeemable so long as they are held by the initial purchasers or their permitted transferees, (4) the holders of the Private Placement Warrants and the Class A common stock issuable upon the exercise of the Private Placement Warrants will have certain registration rights and (5) Private Placement Warrants held by PA Co-Investments LLC will not be exercisable more than five years from the effective date of the registration statement related to the Initial Public Offering in accordance with FINRA Rule 5110(f)(2)(G)(i). If the Private Placement Warrants are held by someone other than the initial purchasers or their permitted transferees, the Private Placement Warrants will be redeemable by the Company and exercisable by such holders on the same basis as the Public Warrants.
NOTE 8. INCOME TAX
The Company’s net deferred tax asset is summarized as follows as of December 31, 2020:
| Deferred tax asset |
||||
| Net operating loss carryforward |
$ | 28,198 | ||
| Organizational costs/startup expenses |
883,247 | |||
|
|
|
|||
| Total deferred tax assets |
911,445 | |||
| Valuation allowance |
(911,445 | ) | ||
|
|
|
|||
| Deferred tax asset, net of allowance |
$ | — | ||
|
|
|
The income tax provision consists of the following for the period April 24, 2020 (inception) through December 31, 2020:
| Federal |
||||
| Current |
$ | — | ||
| Deferred |
(641,453 | ) | ||
| State |
||||
| Current |
$ | — | ||
| Deferred |
(270,013 | ) | ||
| Change in valuation allowance |
911,446 | |||
|
|
|
|||
| Income tax provision |
$ | — | ||
|
|
|
As of December 31, 2020, the Company had $94,497 of U.S. federal and state net operating loss carryovers available to offset future taxable income.
In assessing the realization of the deferred tax assets, management considers whether it is more likely than not that some portion of all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which temporary differences representing net future deductible amounts become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment. After consideration of all of the information available, management believes that significant uncertainty exists with respect to future realization of the deferred tax assets and has therefore established a full valuation allowance. For the period from April 24, 2020 (inception) through December 31, 2020, the change in the valuation allowance was $911.446.
F-16
Table of Contents
PANACEA ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2020
A reconciliation of the federal income tax rate to the Company’s effective tax rate at December 31, 2020 is as follows:
| Statutory federal income tax rate |
21.0 | % | ||
| State taxes, net of federal tax benefit |
8.8 | % | ||
| Change in valuation allowance |
(29.8 | )% | ||
|
|
|
|||
| Income tax provision |
0.00 | % | ||
|
|
|
The Company files income tax returns in the U.S. federal jurisdiction in various state and local jurisdictions and is subject to examination by the various taxing authorities.
NOTE 9. FAIR VALUE MEASUREMENTS
The fair value of the Company’s financial assets and liabilities reflects management’s estimate of amounts that the Company would have received in connection with the sale of the assets or paid in connection with the transfer of the liabilities in an orderly transaction between market participants at the measurement date. In connection with measuring the fair value of its assets and liabilities, the Company seeks to maximize the use of observable inputs (market data obtained from independent sources) and to minimize the use of unobservable inputs (internal assumptions about how market participants would price assets and liabilities). The following fair value hierarchy is used to classify assets and liabilities based on the observable inputs and unobservable inputs used in order to value the assets and liabilities:
| Level 1: |
Quoted prices in active markets for identical assets or liabilities. An active market for an asset or liability is a market in which transactions for the asset or liability occur with sufficient frequency and volume to provide pricing information on an ongoing basis. | |
| Level 2: |
Observable inputs other than Level 1 inputs. Examples of Level 2 inputs include quoted prices in active markets for similar assets or liabilities and quoted prices for identical assets or liabilities in markets that are not active. | |
| Level 3: |
Unobservable inputs based on our assessment of the assumptions that market participants would use in pricing the asset or liability. | |
At December 31, 2020, assets held in the Trust Account were comprised of $143,757,011 in money market funds which are invested primarily in U.S. Treasury Securities. Through December 31, 2020, the Company did not withdraw any of interest earned on the Trust Account to pay for its franchise and income tax obligations.
The following table presents information about the Company’s assets that are measured at fair value on a recurring basis at December 31, 2020 and indicates the fair value hierarchy of the valuation inputs the Company utilized to determine such fair value:
| Description |
Level | |||||||
| Assets: |
||||||||
| Investments held in Trust Account – U.S. Treasury Securities Money Market Fund |
1 | $ | 143,757,011 | |||||
NOTE 10. SUBSEQUENT EVENTS
The Company evaluated subsequent events and transactions that occurred after the balance sheet date up to the date that the consolidated financial statements were issued. Based upon this review, other than as described below, the Company did not identify any subsequent events that would have required adjustment or disclosure in the consolidated financial statements.
On February 10, 2021 (the “Closing Date”), Nuvation Bio Inc. (“Legacy Nuvation Bio”), Panacea Acquisition Corp. (“Panacea”) and Panacea Merger Subsidiary Corp, a wholly owned subsidiary of Panacea (“Merger Sub”), consummated the transactions contemplated by the Agreement and Plan of Merger among them, dated October 20, 2020. Pursuant to the terms of the Merger Agreement, a business combination of Panacea and Legacy Nuvation Bio was effected through the merger of Merger Sub with and into Legacy Nuvation Bio, with Legacy Nuvation Bio surviving as a wholly owned subsidiary of Panacea (the “Merger” and, collectively with the other transactions described in the Merger Agreement, the “Business Combination”). On the Closing Date, Legacy Nuvation Bio changed its name to Nuvation Bio Operating Company Inc. and Panacea changed its name from Panacea Acquisition Corp. to Nuvation Bio Inc. (the “Company”).
In connection with Special Meeting and the Business Combination, holders of 3,350 shares of Panacea Class A common stock, par value $.0001 per share (“Panacea Class A Common Stock”), or approximately 0.02% of the shares with redemption rights, exercised their right to redeem their shares for cash at a redemption price of approximately $10.00 per share, for an aggregate redemption amount of $33,502.
At the effective time of the Merger (the “Effective Time”), each share of Legacy Nuvation Bio Class A common stock, par value $0.0001 per share (“Legacy Nuvation Bio Class A Common Stock”), and each share of Legacy Nuvation Bio Series A preferred stock, par value $0.0001 per share (“Legacy Nuvation Bio Preferred Stock”), was converted into and exchanged for approximately 0.196 shares (the “Exchange Ratio”) of the Company’s Class A common stock, par value $0.0001 per share (“Class A Common Stock”). Additionally, each share of Legacy Nuvation Bio Class B common stock, par value $0.0001 (“Legacy Nuvation Bio Class B Common Stock” and together with Legacy Nuvation Bio Class A Common Stock, the “Legacy Nuvation Bio Common Stock”) (all of which were owned by David Hung, M.D., the founder, President and Chief Executive Officer of Legacy Nuvation Bio) was canceled and converted into and exchanged for approximately 0.196 shares of the Company’s Class B common stock, par value $0.0001 per share (“Class B Common Stock” and together with the Class A Common Stock, the “Company Common Stock”).
On the Closing Date, a number of purchasers (each, a “Subscriber”) purchased from the Company an aggregate of 47,655,000 shares of Class A Common Stock (the “PIPE Shares”), for a purchase price of $10.00 per share and an aggregate purchase price of approximately $476.6 million, pursuant to separate subscription agreements (each, a “Subscription Agreement”) entered into concurrently with the Merger Agreement, effective as of October 20, 2020.
Additionally, on the Closing Date, certain purchasers purchased 2,500,000 shares of Class A Common Stock and 833,333 forward purchase warrants (the “Forward Purchase Securities”) in a private placement at a price of $10.00 per share for an aggregate purchase price of $25.0 million (the “Forward Purchase”) pursuant to the terms of the forward purchase agreement (the “Forward Purchase Agreement”) that Panacea entered into in connection with Panacea’s initial public offering. The sales of the PIPE Shares and the Forward Purchase Securities were consummated concurrently with the closing of the Business Combination (the “Closing”).
As of the Closing Date and following the completion of the Business Combination, the Company had the following outstanding securities:
| • | 216,650,055 shares of Class A Common Stock; |
| • | 1,000,000 shares of Class B Common Stock; |
| • | 5,787,500 warrants, each exercisable for one share of Class A Common Stock at a price of $11.50 per share; and |
| • | 9,571,976 shares of Class A Common Stock issuable upon exercise of Exchanged Options with a weighted average exercise price of $4.41 per share. |
F-17