Attached files
| file | filename |
|---|---|
| EX-99.3 - EX-99.3 - ImmunityBio, Inc. | d307217dex993.htm |
| EX-99.1 - EX-99.1 - ImmunityBio, Inc. | d307217dex991.htm |
| EX-10.14 - EX-10.14 - ImmunityBio, Inc. | d307217dex1014.htm |
| EX-10.1 - EX-10.1 - ImmunityBio, Inc. | d307217dex101.htm |
| EX-3.1 - EX-3.1 - ImmunityBio, Inc. | d307217dex31.htm |
| 8-K - 8-K - ImmunityBio, Inc. | d307217d8k.htm |
Exhibit 99.2
BUSINESS OF IMMUNITYBIO & NANTKWEST
AS A COMBINED ENTITY
| I. | OUR VISION |
We established ImmunityBio, Inc. “ImmunityBio” (NASDAQ: IBRX) to advance the next generation of immunotherapies and to address unmet needs within oncology and infectious disease. Our platform is designed to overcome limitations of the current standards of T cell-based immunotherapies, including checkpoint inhibitors and CAR-T cells. Fundamentally, our platform activates cytokines, NK cells, T cells and tumoricidal macrophages along with generating CD4+ and CD8+ memory T cells. By doing so, our platform not only harnesses the patient’s own immune response to fight disease shortly after administration of therapy, but can also induce long-term “immunological memory,” or longer-term protection against disease.
Since a subset of patients with solid tumors fail checkpoint therapy alone, there is a great need to develop a next-generation immunotherapy beyond checkpoint inhibitors. We have developed a next-generation immunotherapy platform that stimulates the immune system beyond the current paradigm while seeking to address the limitations of current standards of immunotherapy care. This platform is based on the foundation of four separate modalities: (1) activating NK and T cells using antibody cytokine fusion proteins, (2) activating tumoricidal macrophages using synthetic immunomodulators, (3) generating memory T cells using vaccine candidates developed with our hAd5 technology and yeast vector platform and (4) off-the-shelf natural killer cells from the NK-92 cell line and memory-like cytokine-enhanced natural killer cells (m-ceNK) from allogenic and autologous donors. We have focused our efforts to date on difficult-to-treat oncological and infectious disease indications with unmet need and we believe our platform will also be applicable to other cancers and infectious diseases more broadly.
| II. | HISTORY |
Over the last two decades, our Founder and Executive Chairman, Dr. Patrick Soon-Shiong, investigated mechanisms to activate the immune system and to gain insight into how tumors evade and escape the body’s defense mechanisms. The newly merged ImmunityBio is Dr. Soon-Shiong’s culmination of his decades long quest to orchestrate the innate immune system (natural killer cells, macrophages, and dendritic cells) with the adaptive immune system (CD4+ and CD8+ T cells) to generate immunogenic cell death and transform the treatment for patients afflicted with cancer and life-threatening infectious diseases.
The journey began in the 1990s with the discovery that natural killer cells were key components involved in cancer cell death and that activating macrophages in the tumor microenvironment using albumin-based paclitaxel at low doses could immunomodulate the tumor, thus treating the host as well as the disease. In 2005, a nanoparticle albumin-bound (Nab) paclitaxel, invented by Dr. Soon-Shiong was approved (Abraxane) for the treatment of breast cancer. Today, it is also approved for lung and pancreatic cancer and has reached blockbuster status achieving over a billion dollars in sales globally. By 2010, Dr. Soon-Shiong was instrumental in selling the two publicly traded pharmaceutical companies he founded, American Pharmaceutical Partners (Sold in 2008 to Fresenius) and Abraxis Biosciences (Sold in 2010 to Celgene, now Bristol Meyers-Squibb). In 2011, he began the formation and acquisition of the underlying companies now established as ImmunityBio (NASDAQ: IBRX), including the natural killer cell platform which was launched as NantKwest (NASDAQ: NK). Over the past decade, Dr. Soon-Shiong has acquired and developed the platforms needed to orchestrate the entire immune system including antibody cytokine fusion proteins, albumin-bound chemo-immunomodulators, peptide immunomodulators, and vaccine vectors.
Page 1 of 56
Dr. Soon-Shiong founded ImmunityBio to focus on the next generation of immunotherapies to address life-threatening unmet needs within oncology and infectious diseases which then launched the QUILT trials (QUantum Integrative Lifelong Trials) to explore the safety and early efficacy signals of orchestrating these novel first in human immunotherapy molecules across multiple tumor types. We believe the studies shown below were the first immunotherapy exploratory trials to combine multiple novel-novel agents (QUILT Trials) to explore the safety and early efficacy signals of these combinations in an outpatient setting. The term “Quantum Oncotherapeutics” was coined by Dr. Soon-Shiong to denote the temporal-spatial nature of the treatment regimens to accomplish the sequential induction of DAMPs (damage-associated molecular patterns), activation of natural killer cells, education of dendritic cells and proliferation of killer T cells in the tumor microenvironment.
Encouraging data across multiple tumor types in late-stage advanced cancers showed durable complete remissions in many patients with metastatic disease including pancreatic cancer, triple negative breast cancer, head and neck cancer, merkel cell carcinoma, bladder cancer, indolent non-hodgkin’s lymphoma and multiple myeloma.
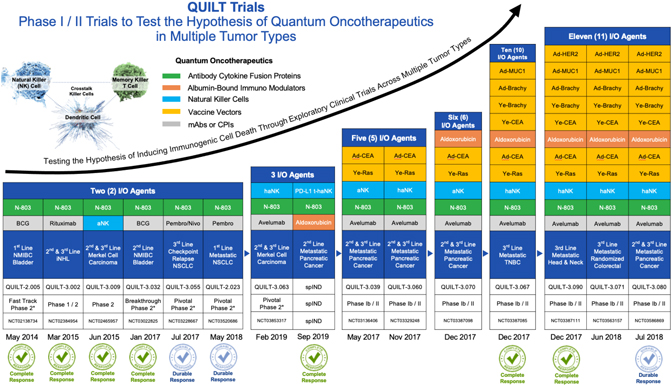
ImmunityBio, together with its merger with NantKwest, has now progressed into a leading late-stage clinical company with a broad immunotherapy clinical-stage pipeline of over 40 clinical trials in Phase I, II and III development (company sponsored and investigator initiated) across 19 indications in solid and liquid cancers and infectious diseases. Our lead antibody cytokine fusion protein, Anktiva, (our IL-15 superagonist, also known as N-803) has received United States Food and Drug Administration, or FDA Breakthrough Therapy designation for BCG unresponsive CIS NMIBC.
The expansive clinical-stage pipeline and intellectual property portfolio with 17 first in human antibody cytokine fusion proteins, chemo immuno-modulators, vaccine vectors and cell therapies in clinical trials, including 25 in Phase II to III clinical trials, as well as a strong global intellectual property portfolio of issued and pending worldwide patent applications with patent life extending to 2035 and beyond, places ImmunityBio in a leading position to transform immunotherapy care beyond current standards of care. In addition, the company has
Page 2 of 56
adopted the strategy of establishing GMP manufacturing capacity at scale and to date, the company has cutting-edge cell manufacturing expertise, ready-to-scale facilities, with extensive and seasoned R&D, clinical trial, and regulatory operations and development teams, which together will occupy over 400,000 square feet of manufacturing and R&D facilities.

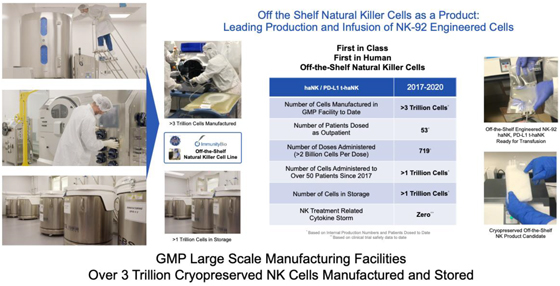
Upon completion of the NantKwest IPO in 2015, the company embarked upon a strategy to develop large-scale manufacturing processes of off-the-shelf natural killer cells which could be cryopreserved and retain activity upon thawing and administering at the bedside in an outpatient setting. To our knowledge, ImmunityBio is the first NK cell therapy based company that has accomplished this scale of manufacture, scale of cryopreserved storage of NK cells, and scale of administration. To date, over 3 trillion off-the-shelf NK cells (haNK, PD-L1 t-haNK) have been manufactured with over a trillion cells in storage and over a trillion cells administered.
Page 3 of 56
| III. | IMMUNITYBIO: A LEADING LATE-STAGE IMMUNOTHERAPY COMPANY WITH LARGE SCALE GMP MANUFACTURING CAPACITY |
ImmunityBio is a leading late-stage immunotherapy company activating both the innate (NK cell and macrophage) and adaptive (T cell) immune system to treat serious unmet needs within oncology and infectious diseases. We are a leading producer of clinical dose forms of off-the-shelf natural killer (NK) cell therapies, autologous/allogenic memory NK cells (m-ceNK), together with a comprehensive platform of antibody cytokine fusion proteins, albumin bound chemo-immunomodulators and vaccine vectors. By combining these NK, macrophage and T cell activation platforms, we believe ImmunityBio leads the field of immunotherapy with late-stage clinical development across multiple tumor types and infectious diseases.
We believe our immunotherapy product candidates have the potential to address both the innate and adaptive immune system in an orchestrated fashion, as follows:
| • | a broad clinical-stage pipeline and intellectual property portfolio with 17 first in human antibody cytokine fusion proteins, chemo immuno-modulators, vaccine vectors and cell therapies in clinical trials, including 25 in Phase II to III clinical trials, as well as a strong global intellectual property portfolio of issued and pending worldwide patent applications with patent life extending to 2035 and beyond; |
| • | differentiated technology and assets including best-in-class combined discovery and development platforms for novel therapies and next-generation early-stage candidates across immunotherapy, neoepitopes and molecules enhancing allogeneic and autologous NK and T-cell therapies; |
| • | being well-positioned to combine expertise, platforms and resources of cell therapy, fusion proteins, vaccine platforms and albumin-bound chemo-immunomodulators to address patients across oncology and infectious disease; |
| • | cutting-edge cell manufacturing expertise and ready-to-scale facilities for cell therapy and adenovirus vectors, with extensive and seasoned R&D, clinical trial, and regulatory operations and development teams, which together will occupy over 400,000 square feet of manufacturing and R&D facilities; and |
| • | the ability to combine cell therapy platforms and immunotherapies, on behalf of patients to drive better outcomes in the fight against oncology and infectious disease. |
| IV. | OUR STRATEGY |
We seek to become the leading global immunological discovery and therapeutics company by creating the next generation of immunotherapies to address serious unmet needs within oncology and infectious diseases. To achieve this goal, the key elements of our strategy include:
| • | Continuously scrutinizing our clinical pipeline and assessing our strategic priorities to maximize opportunities for regulatory approval and to meet unmet medical needs; |
| • | Accelerating our immunotherapy platform and product candidates with registrational intent, to address difficult to treat oncological and infectious disease indications; |
| • | Advancing the approval and commercialization of our lead antibody cytokine fusion protein, Anktiva, which has received FDA Breakthrough Therapy designation, as a backbone of all immunotherapy combinations, including with checkpoint inhibitors; |
Page 4 of 56
| • | Continuing to develop our platform and product candidates across modalities, both as single agent and combination therapies, in order to activate and coordinate the innate and adaptive immune system to generate cell memory against multiple tumor types and infectious diseases; |
| • | Optimizing investment in our discovery, development and manufacturing capabilities for our next generation targeted antibody cytokine fusion proteins and vaccine candidates, as well as for cell therapies including utilizing our just-in-time, decentralized advanced cell therapy GMP-in-a-box manufacturing technologies; and |
| • | Cultivating new and expanding existing collaborations for late-stage programs to efficiently scale globally. |
| V. | BROAD IMMUNOTHERAPY & CELL THERAPY PLATFORMS ACTIVATING THE IMMUNE SYSTEM |
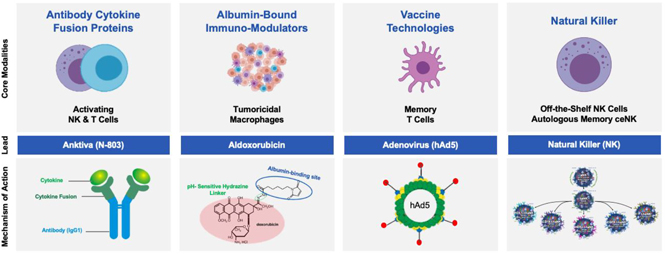
| A. | Antibody Cytokine Fusion Proteins |
Antibody cytokine fusion proteins are a novel class of biopharmaceuticals that have the potential to amplify the therapeutic capability of cytokines and promote lymphocyte infiltration at a site of disease.
Anktiva is our lead antibody cytokine fusion protein, activating NK and T cells through its proprietary IL-15 superagonist, and is in late-stage clinical development. Multiple Phase I and Phase II trials in both liquid and solid tumors have been completed as of March 1, 2021 in over 700 patients. It is currently in late-stage clinical development including in an ongoing registrational Phase II / III NMIBC study. Anktiva has achieved FDA Breakthrough Therapy designation, in addition to Fast Track designation, for the treatment of BCG unresponsive patients with NMIBC CIS as well as Fast Track designation for BCG unresponsive papillary NMIBC and BCG naive CIS NMIBC. However, there can be no assurance that these designations will lead to a faster development or regulatory review process or increase the likelihood of FDA approval. In addition, Anktiva is in late-stage clinical trials for multiple solid tumors, including lung cancer, pancreatic cancer, TNBC, and glioblastoma, in combination with check-point inhibitors, chemotherapy, cell therapy and other immune stimulating agents. Based on patient readout data that was submitted with our application in September 2019 to obtain our Breakthrough Therapy designation, Anktiva achieved its primary endpoint of complete response rate at any time in the ongoing registrational Phase II / III trial.
In addition to Anktiva, we are developing novel antibody cytokine fusion proteins to further enhance NK and T cell activation directed to the infectious disease or tumor microenvironment, and to modulate the
Page 5 of 56
systemic and local immune response to further amplify immunogenic cell death. Prioritized pipeline constructs include antibody cytokine fusion proteins N-820 (targeting CD20), N-809 (targeting PD-L1), N-812 (delivering IL-12 to necrotic tumor cells) and N-830 (delivering TGF-ß Trap to necrotic tumor cells).
| B. | Albumin-Bound Chemo Immunomodulators |
Synthetic immunomodulators target delivery of the chemotherapy agent to the tumor microenvironment, activate tumoricidal macrophages and/or condition the tumor microenvironment towards tumor suppression. Aldoxorubicin is an albumin-linked formulation of doxorubicin which has completed multiple Phase II trials in sarcoma and glioblastoma. Its molecular structure, which includes an acid labile albumin linker, provides favorable properties that distinguishes it from doxorubicin, an anthracycline chemotherapy that has been approved for use in 14 indications including breast cancer, Hodgkin lymphoma and SCLC. Phase II trials have demonstrated that aldoxorubicin crosses the blood-brain barrier, and has an improved cardiotoxicity profile. The improved cardiotoxicity profile allows aldoxorubicin to be given at significantly higher individual and cumulative doses compared with doxorubicin.
| C. | Vaccine Technologies |
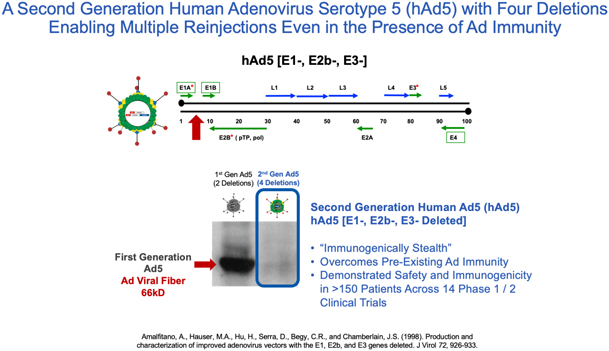
| 1. | Second Generation Human Adenovirus 5 (hAd5) Program |
We have developed vaccine technologies to deliver tumor-associated antigens, or TAAs, and neoepitopes (expressed only by cancer cells), including hAd5, a second-generation adenovirus. Our vaccine technologies have the capability to induce T cell memory due to the activation of both CD4+ and CD8+ T cells along with antibody (or humoral) responses.
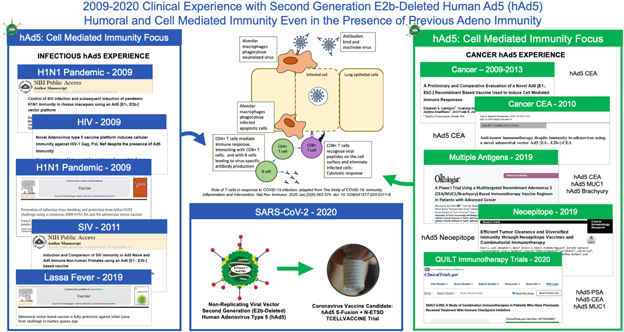
Our hAd5 technology has produced several product candidates, which have been studied in multiple Phase I and Phase II clinical trials as potential vaccines for the treatment of certain cancers. Importantly, these product candidates have shown an ability to overcome previous adenovirus immunity in cancer patients and in preclinical models. The hAd5 technology has also been used with common TAAs to establish memory T cells in multiple clinical trials.
Page 6 of 56
In addition, we are pursuing development of a hAd5 COVID-19 vaccine. While there are a number of vaccine candidates in development, we believe most are limited by their focus on antibody responses to the spike (S) protein. Our candidate uses a combination of S-Fusion and N-ETSD, our novel constructs of COVID-19 spike (S) and nucleocapsid (N) proteins, which has been shown to generate CD4+ and CD8+ T cell mediated immunity and neutralizing antibodies in small animal models.
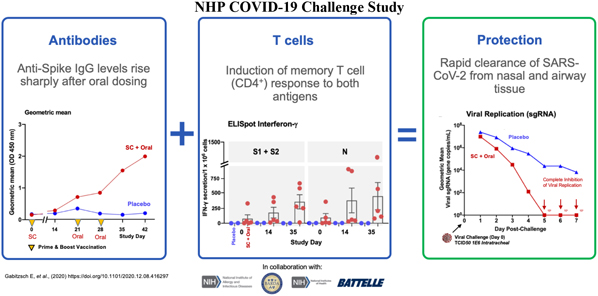
In a pre-clinical non-human primate study funded by BARDA, subcutaneous and oral capsule administration of our vaccine candidate protected rhesus macaques from SARS-CoV-2 live virus challenge, eliciting memory B and T cell responses that resulted in rapid clearance of replicating virus to levels below detection in nasal and lung passages.
Page 7 of 56
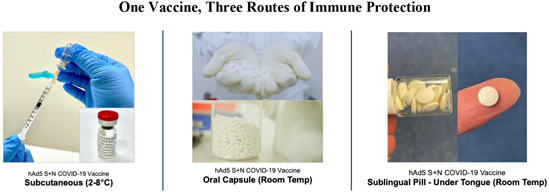
On October 13, 2020, the FDA authorized the Phase I, open-label, dose-finding study to examine the safety, reactogenicity and immunogenicity of the low-dose (5x1010 VP) and intermediate-dose (1x1011 VP) in healthy volunteers. The vaccine was given as a subcutaneous prime on day 1 and a boost on day 22 at Hoag Hospital in Southern California. No significant adverse event, or SAEs, were observed, and the vaccinations elicited Th1-biased T cell and antibody responses in the volunteers. Simultaneously, we are also pursuing development for oral and sublingual administration to provide durable humoral, cell-mediated and mucosal immunity. In addition, the company submitted an amendment to the FDA to our study protocol to add sublingual administration to the subcutaneous shot in various prime and boost combinations. Separately, we submitted an IND for our vaccine candidate in an oral capsule formulation to test it in conjunction with subcutaneous administration. Both of these trials were authorized by the FDA in early 2021 and began enrollment in February 2021.
In addition to these trials in the United States, on March 3rd, 2021 we began a Phase I study of subcutaneous administration of our vaccine candidate in Cape Town, South Africa, setting the stage for further studies in Africa utilizing our sublingual and oral formulations without the cold chain requirements of some of the vaccines currently approved for use.
Page 8 of 56
| 2. | Yeast Program: |
ImmunityBio’s yeast vaccine platform, licensed from our subsidiary GlobeImmune Inc., has been administered to over 800 patients with cancer or infectious diseases in FDA-regulated clinical trials with no SAEs. This platform technology consists of a heat-killed, recombinant S. cerevisiae yeast-based vaccine engineered to express immunogens such as tumor-associated antigens (TAA), pathogen antigens, and tumor-specific neoepitopes. Immunization with this platform elicits CD4+ and CD8+ T-cell responses capable of eliminating tumor cells or pathogen-infected cells.
ImmunityBio recently conducted a first-in-human Phase I clinical trial of a yeast vaccine engineered to express patient and tumor-specific neoepitopes. Patients with cancer were administered yeast vaccines expressing multiple neoepitopes identified through a proprietary algorithm incorporating analysis of tumor vs normal DNA sequence, tumor RNA expression, and predicted human leukocyte antigen (HLA)-neoepitope binding.
Since its licensure, ImmunityBio has improved the potency of the yeast vaccine by further processing the whole cell product into a lysate, thus directly exposing the immunogen and yeast immunogenic proteins to lymphocytes. This new product further accentuates a tumor-suppressive Th1 dominant response, while also potentially furthering the patent life cycle for this platform.
Finally, ImmunityBio has developed a robust, scalable, and economical manufacturing process for the yeast platform, conducive to producing large scale products such as TAA or pathogen vaccines or rapid production of sufficient doses for N of 1 products such as the neoepitope vaccines.
| D. | Off-The-Shelf Natural Killer Cell Platform |
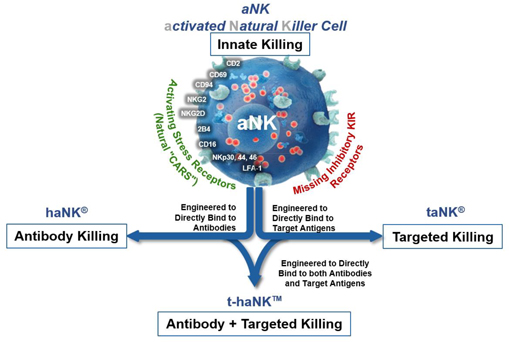
Our NK platforms have demonstrated the ability to induce cell death in cancers and virally infected cells through a variety of concurrent mechanisms including innate killing, antibody-mediated killing, CAR-directed killing and a combination of both antibody-mediated and CAR-directed killing.
Page 9 of 56
| 1. | aNK Platform: Innate Killing |
We have developed a unique NK cell platform, which we believe is capable of being manufactured as a cell-based “off-the-shelf” therapy that can be molecularly engineered in a variety of ways to boost its killing capabilities against cancers and virally infected cells. Unlike normal natural killer cells, our NK cells do not express the key inhibitory receptors that diseased cells often exploit to turn off the killing function of natural killer cells and escape elimination. We have developed a unique aNK cell, which omits key inhibitory receptors, while preserving critical activation receptors that enable selective innate targeting and killing of distressed and diseased cells. They do so through the recognition and binding of stress-proteins that are overexpressed on the surfaces of
| i. | rapidly growing cancer cells due to oxidative and metabolic stress, nutrient deprivation and waste accumulation that typically occurs when cell growth outpaces the capacity of local circulation; and |
| ii. | virally infected cells where the cellular machinery is hijacked to produce an abundance of viral proteins and virions. |
Our aNK cells are also designed to deliver a more lethal blow to their target by delivering a larger payload of lytic enzymes and cytokines responsible for both direct and indirect killing when compared to other natural killer cells isolated from healthy donors. This is due to the higher density of lytic granules and larger cell volume possessed by aNK cells when compared to that of donor derived natural killer cells. We believe that our aNK cells can be produced at commercial scale as a ‘living drug’ using our proprietary manufacturing and distribution processes to adequately address select global cancer markets.
Several Phase I safety studies with aNK cells have been conducted in a variety of bulky hematological cancers and solid tumors, enrolling 49 patients in a range of dose levels and schedules with encouraging evidence of single-agent activity and a durable remission, including some complete responses in liquid tumors. Based on these earlier clinical trials, we have further modified our aNK platform through virus- free molecular engineering designed to leverage additional modes of killing available to aNKs, including antibody-mediated killing, the haNK platform, and both antibody-mediated and CAR-directed antigen targeted killing, the t-haNK platform.
Page 10 of 56
| 2. | haNK Platform: Antibody-Mediated Killing |
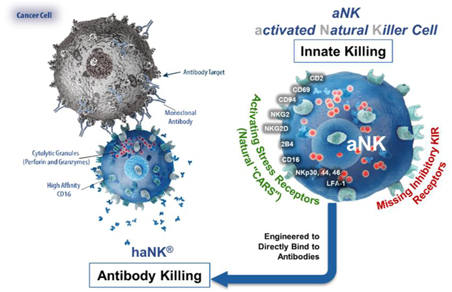
We have genetically engineered our aNK cell platform to overexpress high-affinity CD16 receptors, which bind to antibodies. These antibody-targeted haNK cells are designed to directly bind to IgG1-type antibodies, such as avelumab, trastuzumab, cetuximab and rituximab, with the intention of enhancing the cancer killing efficacy of these antibodies by boosting the population of competent natural killer cells that can kill cancer cells through Antibody Dependent Cellular Cytotoxicity. Antibody products are abundantly utilized to treat cancer. A growing number of studies suggest that clinically meaningful responses to these antibody therapies correlate directly with the overall health of a patient’s natural killer cell population and whether they express the high-affinity variant of the CD16 receptor. Currently available literature estimates that only approximately 10% to 15% of the addressable patient population eligible for antibody therapies carry high-affinity CD16 receptors. This implies that our haNK product candidate may have significant market potential as a combination therapy to potentially address a large number of patients who do not carry high-affinity CD16 receptors and, as a result, exhibit a poorer response to antibody therapies. We therefore intend to develop our haNK product candidate as a combination therapy with widely-used FDA, approved antibody products such as avelumab, trastuzumab, cetuximab and rituximab. Current Good Manufacturing Practice, or cGMP, master and working cell banks of our haNK product candidate have been successfully established and will serve as our source for product for our clinical trials and, if approved, commercialization going forward. We have optimized our manufacturing process partly by designing our haNK product candidate to not require IL-2 cytokine supplementation to the growth media every few days, thereby enabling us to overcome a technically challenging and costly limitation that many other natural killer cell-based therapies face. We have also successfully established processes for large-scale production, cryopreservation and long-term storage of final dose forms, thereby optimizing production efficiencies and allowing for on-demand availability with minimal handling at the infusion sites. Our cryopreserved haNK product candidate has been cleared for clinical testing in several phase Ib/II clinical trials, including our phase II Merkel cell cancer study.
Page 11 of 56
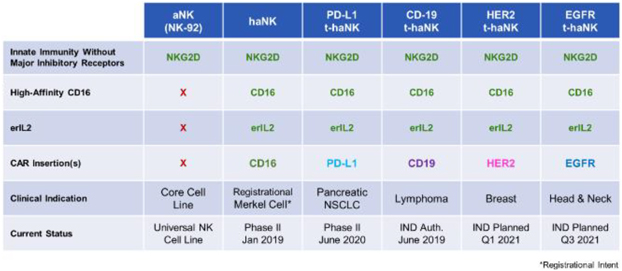
| 3. | t-haNK Platform: CAR-Directed and Antibody-Mediated Killing |
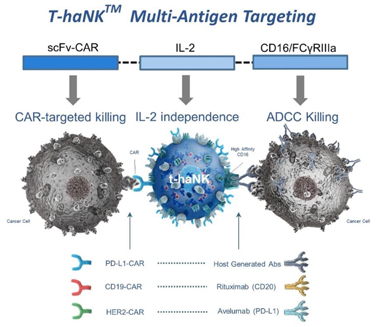
Our newest and most promising platform for the development of therapeutic product candidates is an innovative, bioengineered combination of our haNK and taNK platforms that incorporates all the features of our haNK platform together with a CAR (t-haNK). The resulting line of product candidates under this platform avails itself to all three modes of killing: innate, antibody-mediated and CAR-directed killing. These product candidates also include one or more additional expression elements such as functional cytokines, chemokines and trafficking factors, making them amongst the most versatile in our portfolio. These product candidates are intended to be combined with commercially available therapeutic antibodies to effectively target either two different epitopes of the same cancer specific protein or two entirely different cancer specific proteins. In addition to our two t-haNK product candidates, PD-L1 t-haNK, recently cleared to commence Phase II testing, and CD19 t-haNK, cleared to commence phase I testing, we believe a pipeline of prominent CARs for t-haNK, including HER2, which is nearing IND submission, and EGFR, which is advancing through clinical enabling studies, among others, will enable us to potentially address an even broader range of cancers as part of a chemotherapy-free combination regimen.
In the taNK and t-haNK cell lines, the activation signaling that results from the binding of the CARs to the tumor-specific antigens can be strong enough to overcome both cancer escape mechanisms and suppressive factors present in the tumor microenvironment. These tumor antigens encompass three categories of proteins, all of which can be targeted individually by our engineered taNK products:
| i. | Checkpoint ligands, such as PD-L1; |
| ii. | Well-established tumor proteins such as CD19, HER2 and EGFR; and |
| iii. | Novel surface antigens associated with cancer stem cells. |
Page 12 of 56
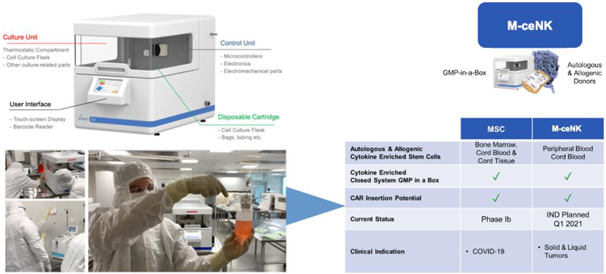
| E. | Memory Cytokine Enriched Natural Killer Cells—M-ceNK (Allogenic / Autologous) in GMP in a Box |
Cytokine-induced memory-like NK cells are a unique set of lymphocytes that differentiate after a brief pre-activation with interleukin-12 (IL-12), IL-15, and IL-18 and exhibit enhanced responses to cytokine re-stimulation that include enhanced interferon-g production and cytotoxicity against leukemic cell lines. These cells have been isolated and characterized by their unique cell-surface marker profile and their highly desirable feature of immune-memory, marked by their pronounced anti-cancer activity for weeks to months in duration, which has made these cells a research focus for more than a decade.
Based on published literature, we believe the ability to generate these memory-like cells at clinically meaningful quantities has been limited to the work performed at Washington University by T.A. Fehniger, et al. Published data so far has been limited to the acute myeloid leukemia patient population in the post-allogeneic, haploidentical stem cell transplantation setting, for which the Fehniger group has generated enough cells to provide a one-time dose of these cytokine-activated, memory-like natural killer cells.
Our cytokine enriched natural killer cell program is based on the ability to enrich and expand donor-sourced natural killer cells in a GMP facility to a clinically relevant scale, which allows for the production of a pure cytokine activated and expanded NK cell population that possesses the unique phenotype we specifically refer to as M-ceNK cells.
We have developed a unique ability to generate a portfolio of distinct ceNK cell products through the application of our proprietary GMP-in-a-Box bioreactors and cytokines and our proprietary methods and overall expertise in scale manufacturing of NK cell-based products.
Page 13 of 56
| F. | Mesenchymal Stem Cell (MSC) Platform. |
Bone marrow-derived allogeneic MSCs are considered to be a prominent cell type to treat degenerative diseases and autoimmune disorders. MSCs have been found to be capable of modulating immune responses, thereby reducing inflammation as well as immunopathology and protecting alveolar epithelial cells during acute respiratory distress syndrome, or ARDS, including that triggered by cytokine storm. More importantly, MSCs demonstrated promising activity in reducing the non-productive inflammation and in promoting lung generation in a phase II clinical trial, as well as in patients with ARDS in clinical practice. As a result, we believe MSCs have the potential to alleviate the SARS-CoV-2-derived cytokine storm and ARDS, and thereby have an effect on the treatment of subsequent chronic respiratory dysfunction and lung fibrosis.
We have developed and optimized procedures and proprietary protocols to generate multiple dose forms of MSC products from a single bone marrow or cord tissue sample, in a scalable format using our GMP-in-a-Box system.
| G. |
GMP-in-a-Box Approach |
We believe we are a pioneer in allogeneic and autologous sourced NK and mesenchymal stem cell, or MSC, therapeutics. We utilize a scalable semi-automated GMP production process using GMP-in-a-Box that combines cytokine expansion and activation reagents such as Anktiva, and unique and simple processing methods, all of which are proprietary.

We have optimized processes for generating both fresh and cryopreserved clinical dose forms of memory-like NK cells with 100% purity (in the allogeneic setting) from a variety of sources, including cord blood and allogeneic and autologous peripheral blood. We have also optimized processes for generating fresh and cryopreserved clinical dose forms of MSCs from cord tissue and allogeneic bone marrow sources. We avoid the use of both feeder-layers for activation as well as other commonly applied additives that frequently create downstream issues in achieving a high-quality releasable final dose form and have been able to generate multiple dose forms from each donor product, both of which are critical features in achieving scalability.
Page 14 of 56
| VI. | BROAD PIPELINE ACROSS ONCOLOGY AND INFECTIOUS DISEASE AT LATE-STAGE CLINICAL DEVELOPMENT |
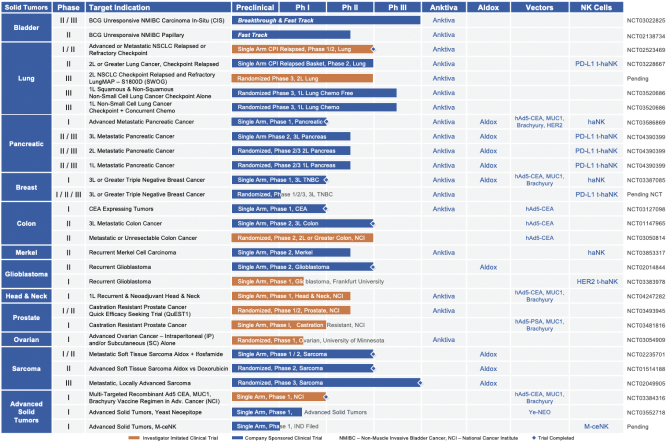
| A. | Solid Tumors: Late-Stage Clinical Pipeline |
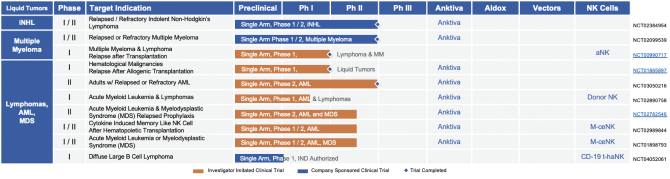
| B. | Liquid Tumors: Clinical Pipeline |
| C. | Infectious Diseases: Clinical Pipeline |

Page 15 of 56
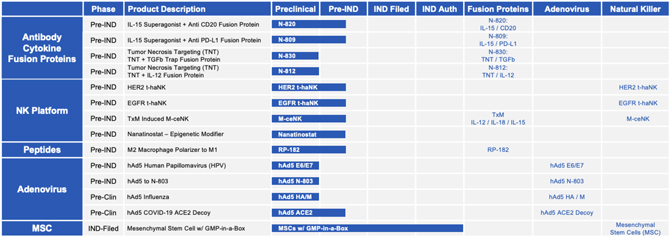
| D. | Pre-Clinical & Pre-IND Pipeline |
| VII. | OUR SOLID TUMOR DEVELOPMENT PROGRAM |
| 1. | Bladder Cancer |
CIS & Papillary Non-Muscle Invasive Bladder Cancer (NMIBC)
Opportunity
In the United States, bladder cancer is ranked the sixth most prevalent cancer and one of the most expensive (approximately $4 billion a year) cancers to treat. Bladder cancer is a common malignancy, with approximately 81,000 new patients diagnosed each year in the United States. The high recurrence rate and ongoing invasive monitoring requirement of bladder cancers are the key contributors to the economic and human toll of this disease. The median age at diagnosis is approximately 73 years. NMIBC makes up 75% of all bladder cancers in the United States. NMIBC is divided into two forms, papillary and CIS, of which approximately 10% of diagnoses are CIS disease. CIS disease is always classified as high-grade, whereas papillary tumors can range from low malignant potential to high-grade carcinoma. A substantial proportion of patients with intermediate and high-risk disease are at a significant risk for metastasis and death. Overall, 45% of all NMIBC incidence is classified as high-grade carcinoma.
Standard of Care
There is a significant unmet medical need in NMIBC, with one of the worst patient experiences among common cancers. A treatment approved in 1989, BCG immunotherapy, has been the primary standard of care for nearly 40 years. Intravesical administration of BCG causes the release of antigen presenting molecules and cytokines thereby inducing an immune response against the tumor cells.
After first line treatment with BCG, patients who progress (80-85% at one year) typically either undergo radical cystectomy (removal of the bladder) or face cancer progression. Radical cystectomy for bladder cancer is a high-risk procedure, with morbidity and mortality rates ranging from 28-64% and 3-6%, respectively. The high rates of morbidity and mortality reflect the fact that the majority of patients undergoing radical cystectomy are elderly patients with multiple comorbidities.
Page 16 of 56
We are targeting patients with BCG unresponsive high-risk NMIBC. Patients with CIS NMIBC who recur within one year after receiving two courses of BCG are considered BCG unresponsive and patients with high-risk papillary disease who recur within nine months after receiving adequate BCG are considered BCG unresponsive. Anktiva is expected to enhance the immunostimulatory effects of BCG, by causing proliferation and activation of cytotoxic NK and T cells critical for killing bladder tumor cells. Our initial target market includes the approximately 17,000 of these patients diagnosed annually, including those patients who have previously failed BCG and have refused cystectomy.
We would expect that, if Anktiva for the treatment of high-risk NMIBC is approved by the FDA, patients would receive treatment until the earlier of up to two years or disease recurrence and would receive treatment in order to avoid cystectomy and delay disease recurrence.
Late-Stage Clinical Experience
Anktiva has been administered intravesically to more than 300 subjects across one Phase I clinical trial and one ongoing Phase II / III clinical trial, as well as several spINDs. The Phase I trial, completed in 2018, was a dose finding study of intravesical BCG in combination with Anktiva in patients with NMIBC in the BCG naïve setting. As can be seen in the table below, durable complete responses were noted in nine out of nine patients who also demonstrated durable responses at 24 months for both CIS and papillary disease. As a point of reference, the historical response rate for BCG naïve alone (standard of care) is 58-81% at 3-6 months post BCG alone. No serious adverse events or dose limiting toxicities were reported in any subjects and the maximum tolerated dose was not reached.
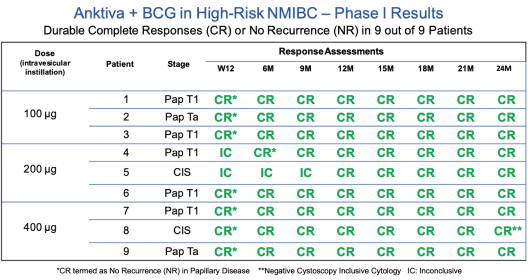
A. BCG Unresponsive CIS NMIBC
Anktiva has achieved FDA Breakthrough Therapy designation (in addition to Fast Track designation) for the treatment of BCG unresponsive patients with CIS NMIBC, as well as Fast Track designation for BCG unresponsive papillary NMIBC and BCG naive CIS NMIBC.
In our Phase II / III, open-label multicenter trial of BCG unresponsive high grade CIS NMIBC patients, the patients are receiving BCG plus Anktiva weekly for six consecutive weeks during induction. The patients also receive additional treatment including three weekly maintenance instillations every three months for up to 12 months and then at month 18. Patients with no disease or low-grade Ta disease at months 24, 30, and 36 are eligible for continued BCG plus Anktiva (Cohorts A and B) or Anktiva alone (Cohort C) treatment (3 weekly instillations), by PI discretion.
Page 17 of 56
The primary endpoint of the BCG unresponsive CIS NMIBC trial is complete response rate at any time equal to or greater than 30% and the lower bound of the 95% confidence interval must be greater than or equal to 20% for success. Complete response, or the disappearance of measurable disease in response to treatment, is evaluated at three months or nine months following initial administration of Anktiva plus BCG (and every three months thereafter until 24 months). This endpoint would be achieved once at least 24 of the 80 patients in the study achieve complete response.
In February 2021, we presented the following data at ASCO GU 2021 regarding our Phase II/III clinical results of IL-15RaFc superagonist Anktiva with BCG in BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) carcinoma in situ (CIS) patients: 80 patients have enrolled in cohort A of this phase II/III trial. Evaluable analysis at this time shows complete response (CR) rate at any time of 71% (N=51/72); for patients achieving CR, the probability of maintaining a CR for 12 months is 56%, with a median duration of complete response of 19.2 (7.6, 26.4) months. Low-grade treatment related adverse events (AE) include dysuria, hematuria, and pollakiuria (all £18%), urgency and fatigue (each 8%), chills (6%), and bladder spasm and pyrexia (each 5%), all other AEs were seen at 4% or less. The serious adverse event (SAE) rate is 1% for any given AE. No immune related SAEs have been observed. To date, 70/80 (87.5%) patients have avoided cystectomy in this BCG unresponsive population.
The investigator concluded that with a complete response rate of 71%, Anktiva has met its primary endpoint with a 56% probability of CR patients maintaining CR for at least 12 months. With the observed strong efficacy and an SAE rate of 1%, Anktiva represents a novel treatment option for BCG unresponsive CIS with a favorable benefit to risk ratio in a therapeutically challenging disease.
As of February 2021, our NMIBC CIS data as of Feb 2021 are as follows:
| • | QUILT 3.032 demonstrated excellent tolerability profile of Anktiva + BCG with no treatment related serious adverse events, 0% immune related AE, 0% treatment related discontinuation, and no grade 4 or 5 adverse events. |
| • | CR rate at any time of 71% with median follow up 10.7 months, with cystectomy avoidance rate 88%. |
| • | In responding patients, the estimated median duration of CR is 19.2 months based on Kaplan-Meier analysis methods, with probability of 56% maintaining CR at least 12 months |
| • | Favorable and familiar administration profile to urologists. |
All patients enrolled in the NMIBC BCG unresponsive CIS trial have been treated with the recommended number of full-strength doses of BCG on study during our trial. We have enrolled patients who have received a lower dosage of BCG therapy before enrollment in our trial as a result of BCG shortages. No less than 90% of patients enrolled in the trial as of December, 2020 have received the number of doses and amount of BCG recommended by the American Urological Association before enrolling in the trial. In the 51 patients with a complete response, 46 patients (90%) received a full dose of BCG prior to study entry. The FDA allowed our modification of the study design to allow enrollment of such patients, and definition of these patients may require further discussions with the FDA upon review. A published meta-analysis of six relevant randomized controlled trials and two quasi-randomized controlled trials in NMIBC concluded that low-dose BCG instillation significantly reduces the incidence of overall side effects, especially severe and systemic
Page 18 of 56
symptoms in patients with NMIBC, while the oncological control efficacy of low-dose BCG is not inferior to standard-dose BCG. While there can be no assurance that the FDA will agree with this conclusion, we believe that this study may be relevant to the FDA’s consideration for our label.
For BCG Unresponsive CIS NMIBC we expect an initial readout in the first half of 2021, a BLA filing in the second half of 2021 and a decision on our BLA filing by the FDA in 2022.
| B. | BCG Unresponsive Papillary NMIBC |
As discussed, we are also pursuing approval in BCG unresponsive papillary NMIBC, for which we have also received Fast Track designation. In our Phase II, open-label multicenter trial of BCG unresponsive high grade papillary NMIBC patients, the patients are receiving BCG plus Anktiva weekly for six consecutive weeks during induction. The patients also receive additional treatment including three weekly maintenance instillations every three months for up to 12 months and then every nine months for up to 24 months. The primary endpoint of the trial is a 12-month disease free rate greater than or equal to 30% and the lower bound of the 95% confidence interval must be greater than or equal to 20% for success. To meet the primary endpoint, 24 out of 80 patients must be disease free at 12 months.
As of March 1, 2021, 26 sites are active in the United States, and 60 of the planned 80 patients with BCG unresponsive papillary NMIBC have been enrolled. We expect full accrual in Q1 2022 and an initial readout anticipated in Q2 2022.
| 2. | Lung Cancer |
Non-Small Cell Lung Cancer
Opportunity
Lung cancer is the second most common cancer in the United States. In 2018, in the United States alone it is estimated that 228,820 new cases of lung cancer will be diagnosed, and 135,720 deaths will be attributed to the disease. Lung cancer is divided into two forms, NSCLC and SCLC, with NSCLC comprising 85-90% of lung cancer cases in the United States. Approximately 55% of NSCLC cases are metastatic, and we estimate that 20% of those metastatic cases involve relapsed or refractory cancers.
Standard of Care
The development of checkpoint inhibitors, such as nivolumab, pembrolizumab and atezolizumab, targeting PD-1 and its ligand PD-L1, or PD-L1, have provided an improvement in the survival of treated patients. PD-1 and PD-L1 are proteins referred to as checkpoints that exist on the surface of cells and act to suppress the adaptive immune system. PD-1 and PD-L1 checkpoint inhibitor drugs bind to the proteins and interfere with their suppressive mechanisms. The aforementioned therapies are FDA-approved for patients with metastatic NSCLC. The application of these new therapies to NSCLC has been revolutionary, doubling the median overall survival in some settings; however, patient response may be short lived, due to late response and/or progression after achieving an initial response.
As with bladder cancer, Anktiva enhances the proliferation and activation of NK and T cells critical for targeting and killing of lung cancer cells. There is therefore a strong rationale to evaluate Anktiva in addition to an anti–PD-1 or anti–PD-L1 checkpoint inhibitor for patients with NSCLC who have relapsed after achieving an initial response to PD-1 or PD-L1 checkpoint inhibitor therapy.
Page 19 of 56
Lung Cancer Program
| 1. | Development in First Line Lung Cancer |

We are enrolling patients in a randomized Phase III trial (NCT03520686) to evaluate Anktiva plus checkpoint inhibitor combinations versus other checkpoint inhibitor combinations in the first line setting for NSCLC. Patients will be treated either in cohort A (immunotherapy for either squamous or nonsquamous NSCLC with PD-L1 TPS >1%), cohort B (chemoimmunotherapy for squamous NSCLC), or cohort C (chemoimmunotherapy for nonsquamous NSCLC).
Each study cohort will be analyzed separately:
| • | Cohort A stratifies patients based on squamous or non-squamous NSCLC types and PD-L1 expression. Patients are randomized 1:1 into a control arm where patients receive single agent pembrolizumab or an experimental arm where patients receive pembrolizumab plus Anktiva. |
| • | Cohort B will randomize squamous NSCLC patients 1:1 into a control arm where patients receive an induction phase of carboplatin plus taxane plus pembrolizumab and a maintenance phase of pembrolizumab or into an experimental arm where patients receive the same treatment with the addition of Anktiva in both the induction and maintenance phases. |
| • | Cohort C will randomize nonsquamous NSCLC patients 1:1 into a control arm where patients receive an induction phase of platinum-based chemotherapy plus pemetrexed plus pembrolizumab and a maintenance phase of pembrolizumab or into an experimental arm where patients receive the same treatment with the addition of Anktiva in both the induction and maintenance phases. Progression free survival is the primary outcome of all three cohorts. |
| 2. | Development in Second Line or Greater Lung Cancer |
Analysis of the pooled data from a Phase I / II study (NCT02523469) conducted from January 2016 to June 2017 in 23 patients, and a subsequent investigator-initiated Phase II trial conducted by the Medical University of South Carolina yielded confirmation of efficacy of the combination of checkpoint and Anktiva in relapsed NSCLC. In 15 patients with PD-L1 greater than 50%, the overall response rate was 38% and the median
Page 20 of 56
overall survival rate was 17.1 months (4.6, ongoing). These preliminary findings are favorable relative to the historical response rate seen in this patient population in the first line setting with checkpoint inhibitor therapy.
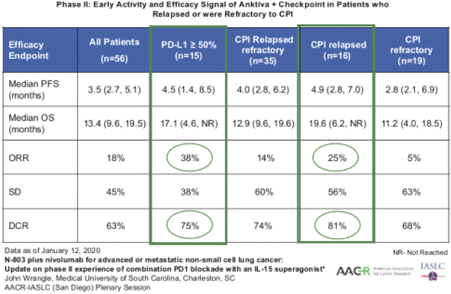
On the basis of these findings, we initiated a single-arm Phase IIb (NCT03228667) multicohort basket trial of Anktiva and checkpoint inhibitor combinations in patients who have previously received treatment with PD-1/PD-L1 immune checkpoint inhibitors per an FDA-approved indication. We are leveraging this trial to advance our program in second line or greater NSCLC. Enrollment is ongoing, and through March 1, 2021, 135 patients have been enrolled in the following cohorts:
| Phase IIb: Multi-Cohort Basket Trial of CPI Failures |
Enrolled Patients | |||
| Lung Cancer: Non-Small Cell | 18 / 18 Enrolling | |||
| Lung Cancer: Small Cell | 10 / 10 Enrolled | |||
| Cohort 1 | Head & Neck Squamous Cell Carcinoma | 8 / 18 Enrolling | ||
| Third Line Patients | Melanoma | 15 / 18 Enrolling | ||
| Checkpoint Failures Alone | Renal Cell Carcinoma | 7 / 18 Enrolling | ||
| Gastric | 3 / 18 Enrolling | |||
| Urothelial | 1 / 18 Enrolling | |||
| Cervical | 2 / 18 Enrolling | |||
| Cohort 2 Second Line Checkpoint Failures Alone |
High PD-L1 NSCLC | 10 / 20 Enrolling | ||
| Cohort 3 Second Line Concurrent Chemo + CPI Failures |
NSCLC | 19 / 19 Completed Enrollment | ||
Page 21 of 56
| Cohort 4 Second or Third Line Concurrent Chemo + CPI Stable Disease |
NSCLC, Urothelial, HNSCC, RCC | (34 lung cancer patients) 42 / 41 Completed Enrollment |
Preliminary Results for NSCLC from Ongoing Phase IIb Multi Cohort Trial
91 out of a total of 135 patients who have been enrolled and assigned to a cohort in the Phase IIb trial as of March 1 2021 are lung cancer patients, with 81 having NSCLC and 10 having SCLC.
Patients enrolling into this trial were eligible if actively progressing on checkpoint therapy. Upon enrollment (target lesion “0” in the following spider plot below, representing ongoing progression), patients continued on the same checkpoint but with the addition of Anktiva. Despite progressing on checkpoint therapy upon entry into the trial, the majority of patients reverted to stable disease and demonstrated durability of stable disease, some extending as long as nine months in this ongoing study as seen in the spider plot below. The spider plot below shows preliminary evidence of conversion from active progression (target lesion “0” baseline) to long-term stable disease and disease control in second- and third-line NSCLC patients who were actively progressing on checkpoint therapy upon study entry.
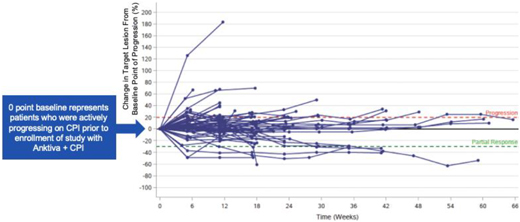
Based on the promising Phase II results of the Wrangle study and confirmatory basket trial data of Anktiva activity in rescuing patients who progressed on checkpoint therapy, a randomized registration trial in second and third line lung cancer patients is being planned. We expect complete data for the lung cancer cohorts in Q2 2021.
Based on the above data, Lung-MAP and ImmunityBio began discussions in July 2020 for inclusion in the multi-institutional Lung-MAP nationwide master protocol as a sub-study of NSCLC patients. We are currently working with Lung-MAP on the design of this Anktiva plus checkpoint inhibitor sub-study.
| 3. | Pancreatic Cancer |
Metastatic Pancreatic Cancer
Pancreatic cancer is the third leading cause of cancer-related death in the United States, with an estimated 47,050 deaths and an estimated 57,600 new cases expected in 2020.
Surgery and subsequent adjuvant chemotherapy are the preferred treatment option for pancreatic cancer today. Approximately 82-89% of pancreatic cancer cases are recurrent or metastatic, and 80% of pancreatic cancer patients relapse. For the majority of patients who present with more advanced disease, treatment
Page 22 of 56
typically consists of chemotherapy alone or supportive care for metastatic patients, and chemotherapy with or without radiation for those with locally advanced disease. Conventional immunotherapy is not part of the standard of care for these patients and the prognosis is not promising, with a five-year survival rate of 3%.
Exploratory Phase Ib/II trials and spINDs in patients with second line or greater metastatic pancreatic cancer in which Anktiva and aldoxorubicin were combined with off-the-shelf NK, or haNK, cells and other agents showed a durable complete remission in patients with advanced disease. The primary endpoints of the Phase Ib and II portions of the study were safety and objective response rate, respectively. In aggregate, 82% of patients (14 / 17) with advanced pancreatic cancer achieved disease control following combination therapy including Anktiva and aldoxorubicin. A single patient demonstrated an ongoing complete response, over nine months in duration through August 2020 with a significant and rapid decline of their cancer antigen 19-9, or CA19-9, levels. There were no Anktiva-related grade 3 or 4 adverse events reported.
On the basis of these exploratory studies in metastatic pancreatic cancer, together with the pre-clinical findings that PD-L1 t-haNK is as effective as haNK + anti-PD-L1 monoclonal antibody, the ongoing clinical development in first, second- and third-line pancreatic cancer utilizes PD-L1 t-haNK as described below.
Development in First, Second and Third Line Metastatic Pancreatic Cancer

Based on this encouraging data, we are enrolling patients in a Phase II trial.
| • | First Line Advanced pancreatic cancer. We are evaluating the combination of Anktiva with aldoxorubicin and low dose chemotherapy with or without PDL1 t-haNK versus Gemcitabine/Abraxane as the standard of care control arm in this randomized trial. |
| • | Second Line Advanced pancreatic cancer. We are evaluating the combination of Anktiva with aldoxorubicin and low dose chemotherapy + PDL1 t-haNK versus 5FU/Onivyde as the standard of |
Page 23 of 56
care control arm in this randomized trial.
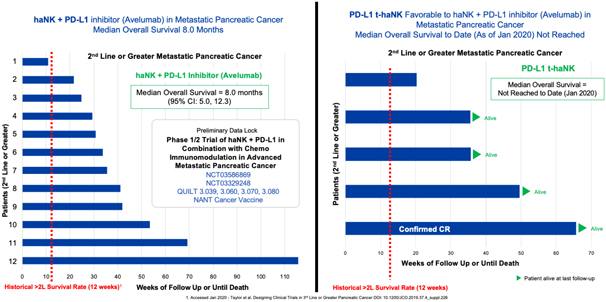
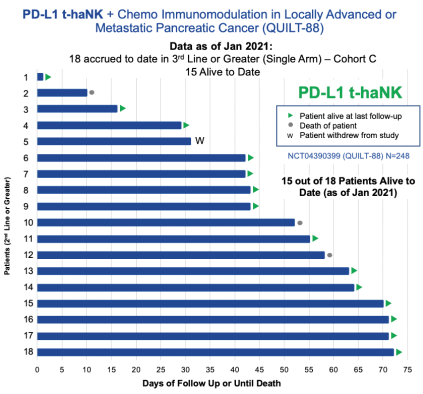
| • | Third Line and Beyond, we are evaluating the combination of Anktiva with aldoxorubicin and low dose chemotherapy + PDL1 t-haNK in a single arm cohort of this trial. The primary endpoint is overall survival and as of January 2021, 15 out of 18 (83%) of patients enrolled remain alive to date. We plan to meet with the FDA for an end of Phase II meeting and special protocol assessment during the second half of 2021 to discuss the adequacy of this trial design for the approval of combination therapies for pancreatic cancer. |
| 4. | Breast Cancer |
Triple Negative Breast Cancer
Breast cancer is the fourth leading cause of cancer-related death in the United States, with an estimated 42,690 deaths from the disease and an estimated 279,100 new cases expected in 2020. TNBC is an aggressive subtype of breast cancer with limited treatment options and a poor prognosis that accounts for approximately 10-20% of all breast cancer types. 27% of cases are metastatic and recurrent. TNBC tumors frequently present at an advanced stage, are very heterogeneous (not all types and subgroups have been defined) and are associated with a higher risk of early relapse. They are characterized by a lack of hormonal receptor expression (estrogen receptor, or ER, and progesterone receptor, or PR), and an absence of human epidermal growth factor receptor 2, or HER2, protein expression or ERBB2 gene overexpression and/or amplification, which makes ER, PR and HER2 targeted therapies ineffective at treating TNBC. The checkpoint inhibitor atezolizumab has become the new standard of care for patients with advanced TNBC who are PD-L1 positive.
Additionally, the Merck-sponsored KEYNOTE-086 Phase II clinical trial that led to approval of the checkpoint inhibitor pembrolizumab in previously treated metastatic TNBC patients reported two out of 170 patients with a complete response and showed disease control in 13 of the 170 patients. Recently
Page 24 of 56
Immunomedics received approval for third line TNBC for their drug sacituzumab govitecan-hziy, in which the overall response rate was 33.3% and the median duration of response was 7.7 months.
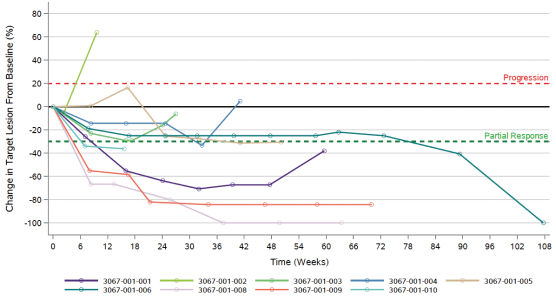
We have treated nine patients in a Phase Ib / II trial of heavily pre-treated, metastatic TNBC with a combination immunotherapy that included Anktiva and aldoxorubicin, along with several other immunotherapy agents. Two out of the nine patients had complete responses to the combination therapy with eight of the nine patients having disease control. The primary endpoints of the Phase Ib and II portions of the study were safety and objective response rate, respectively. Serious adverse events reported in the trial included disease progression, pyrexia, mastitis, pneumonia, nausea, cholecystitis and pain in extremity, each of which was reported only once. The exploratory Phase Ib/II trial in the patients with advanced TNBC showed a disease control rate of 89% (n=9) with a complete or partial response of 67% (6 / 9). The median progression-free survival was 14.3 months with median overall survival of 20.2 months as of December 2020.
| Exploratory Trial in Advanced TNBC: Aldox + Anktiva + haNK | ||||
| Subjects with Complete or Partial Overall Response |
6 / 9 (67%) | |||
| Subjects with Complete Response (irRC) |
2 / 9 (22%) | |||
| Subjects with Disease Control (irRC) |
8 / 9 (89%) | |||
| Median Duration of Response (irRC) |
12.7 months | |||
| Median Progression-Free Survival (irRC) |
13.7 months | |||
| Average Overall Survival to date (median not yet reached) |
19.2 months | |||
The preliminary findings of our study in advanced metastatic (third-line or greater) TNBC where modalities 1, 2, and 3 were combined, showed 13.7 months progression-free survival and 19.2 months overall survival, as seen below. The Phase III IMpassion130 study of atezolizumab plus nab-paclitaxel in first line metastatic TNBC patients reported progression-free survival of 7.2 months and an overall survival of 21.3 months. We have not conducted a head-to-head clinical trial with atezolizumab.
Page 25 of 56
Development in Third Line Triple Negative Breast Cancer

An open label Phase I, II and III randomized study comparing Sacituzumab Govitecan-Hziy versus Sacituzumab Plus Anktiva and PD-L1 t-haNK for the treatment of subjects with advanced Triple-Negative Breast Cancer after prior therapy, is being designed for submission in Q1 2021.
| 5. | Colon Cancer |
Colorectal cancer is the third leading cause of cancer-related deaths in the United States when men and women are considered separately, and the second leading cause when both sexes are combined. The American Cancer Society estimates that there will be over 104,000 new cases of colon cancer and 45,000 new cases of colorectal cancer in the United States in 2021.
ImmunityBio’s colorectal cancer immunotherapy targets carcinoembryonic antigen, or CEA expressing colorectal cancer cells. CEA represents an attractive target antigen for immunotherapy since it is over-expressed in nearly all colorectal cancers and many pancreatic cancers, 70% of non-small cell lung cancers and approximately 50% of breast cancers. ImmunityBio’s colorectal cancer immunotherapeutic product candidate has completed Phase I/II clinical trials at Duke University Medical Center in Durham, North Carolina and Medical Oncology Associates, in Spokane, Washington, funded by the National Cancer Institute (NCI).
Development in Metastatic Colon Cancer

A Phase I/II clinical trial evaluating dosing, safety, immunogenicity, and overall survival on metastatic colorectal cancer (mCRC) patients after immunotherapy with an advanced generation hAd5 [E1-, E2b-]-CEA(6D) vaccine candidate was performed (NCT01147965). Extended observations on long-term overall survival and further immune analyses on a subset of treated patients including assessment of cytolytic T cell responses, T-regulatory (Treg) to T-effector (Teff) cell ratios, flow cytometry on peripheral blood mononuclear cells (PBMC), and determination of HLA-A2 status was performed.
Overall median survival of 11 months was observed during long-term follow-up and no long-term adverse effects were reported. Cytolytic T cell responses increased after immunizations and cell-mediated immune (CMI) responses were induced whether or not patients were HLA-A2 positive or Ad5 immune. Preliminary results revealed that activated CD4+ and CD8+ T cells were detected in a post-immunization sample exhibiting high CMI activity.
Page 26 of 56
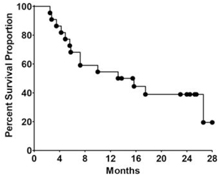
Kaplan-Meier survival plot on long-term overall survival of metastatic colorectal cancer patients immunized 3 times with the highest doses of our vaccine candidate, demonstrating a median survival of 13 months, with 19% of patients surviving 28-months. While no head-to-head studies have been performed, this data compares favorably to historical controls of patients with late-stage metastatic colorectal cancer.
In light of these favorable results, we are exploring a trial in late-stage colorectal cancer patients
| 6. | Merkel Cell Carcinoma |
Merkel cell carcinoma, or MCC, is a rare and aggressive skin cancer that arises from uncontrolled growth of cells in the skin. Increasing in incidence, over 2,000 new cases are reported in the United States each year. Patients with metastatic or locally advanced MCC have an extremely poor prognosis, with less than 20% of patients surviving longer than five years. Typically, these patients are treated with a range of drugs, including chemotherapy, which can result in significant side effects. Although new immune therapies have the potential to improve survival, MCC is still fatal for a majority of patients who have progressed on or after treatment with a checkpoint inhibitor and represents an unmet medical need.
Development in Recurrent Merkel Cell Carcinoma

QUILT 3.063 (NCT03853317) is our Phase II, open-label, single-arm trial evaluating the novel triple combination of “off-the-shelf” haNK cell therapy with Anktiva and avelumab, without chemotherapy in subjects that have progressed after treatment with a checkpoint inhibitor for MCC. This trial is currently open at multiple centers across the United States. As a rare disease, with approximately 2,000 patients being diagnosed in the United States each year, MCC patients often require regional referral and additional travel to a clinical trial site. The ongoing COVID-19 pandemic has continued to have an impact on enrollment due in part to limitations in travel and study accessibility as well as a significant reduction in patient referrals. In response to this, we will continue to implement measures to increase local community awareness of the trial as we add new sites. While we remain cautiously optimistic, it may require additional time to reach our interim data readout.
Page 27 of 56
| 7. | Glioblastoma |
Recurrent Glioblastoma
Glioblastoma has an incidence of two to three per 100,000 adults per year, accounting for about 52% of all primary tumors to the brain and about 17% of all primary and metastatic brain tumors. It is an incurable disease based on current approved therapies. Relapsed patients usually receive bevacizumab with an objective response rate of approximately 20%, and overall survival of approximately 31 weeks. To date, clinical trials assessing novel therapies for recurrent glioblastoma have resulted in only modest increases in progression-free survival and minimal increases in overall survival.
Development in Recurrent Glioblastoma

We are developing a protocol to treat recurrent glioblastoma using aldoxorubicin. Unlike doxorubicin, aldoxorubicin appears to penetrate the blood-brain barrier in humans and is associated with objective tumor responses, stable disease and prolonged survival.
A single arm Phase II trial (NCT02014844) was completed in 2016 that assessed the preliminary efficacy and safety of aldoxorubicin administered to recurrent glioblastoma patients who progressed after first line therapy. The primary endpoint of the trial was objective response rate. Out of 28 subjects, investigator assessment of best overall tumor response reported one patient with a partial response and 11 patients with stable disease. Treatment-related grade 3 or 4 adverse events included: neutropenia; thrombocytopenia; febrile neutropenia; lymphopenia; anemia; decreased white blood cell count, neutrophil count or lymphocyte count; fatigue; mucosal inflammation; somnolence; hemiparesis; intestinal perforation; oral candidiasis; decreased appetite; and hypertension.
In addition, an investigator-initiated trial (NCT03383978) is ongoing exploring the safety and efficacy signals of HER2 t-haNK in patients with recurrent glioblastoma.
| 8. | Head & Neck Cancer |
Human papilloma virus, or HPV, is estimated to cause approximately 95% of cervical and 30-60% of oropharyngeal carcinoma cases. High-risk HPV type 16 is involved in more than 50% of cervical cancers worldwide and is the primary viral driver of esophageal, anal cancers, and head and neck squamous cell carcinomas, or HNSCC. HPV E6DD and E7DD genes expressed in squamous cell cancers are considered to be an attractive target for tumor specific immunotherapy because the cancer cells require E6 and E7 for progression.
We have developed our proprietary hAd5 technology to deliver a proprietary modified/fused non-oncogenic HPV E6/E7 gene (E6D/E7D) to treat cancer patients with HPV-expressing cancer. The addition of a proprietary localization signal (ETSD) to the E6/E7 construct further distinguishes this vaccine by allowing for trafficking of the antigens to specific cellular compartments presentable and recognizable by CD4+ and CD8+ T cells, potentiating immunological memory against HPV-bearing tumor cells. This product candidate, in an earlier iteration, has been granted orphan-drug designation by the FDA for the treatment of HPV-associated HNSCC, and we intend to seek this designation for the ETSD modified product candidate.
Page 28 of 56
Development in Head & Neck Cancer

| 9. | Prostate Cancer |
Prostate cancer is the second most common cancer in the United States in men with close to 200,000 new cases and over 34,000 deaths annually. One in eight men will be diagnosed with prostate cancer during their lifetime. Although localized or regional prostate cancer is highly treatable, the 5 year survival rate for distant metastatic prostate cancer is 30%.
Development in Castration Resistant Prostate Cancer
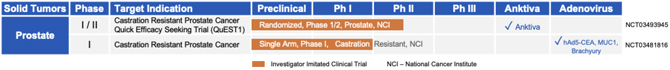
18 patients were enrolled in a Phase I/II trial to examine Ad-PSA, Ad-MUC1, and Ad-Brachyury as a therapy for patients with prostate cancer. The vaccine candidate was well tolerated with no dose limiting toxicities or grade three or higher treatment related adverse events. A Phase II trial in patients with castration resistant prostate cancer was initiated by the NCI.
| 10. | Ovarian Cancer |
There are over 20,000 new ovarian cancer cases diagnosed and approximately 14,000 deaths in the United States annually. Although ovarian cancer may occur at any age, it is more common in patients older than 50 years. Treatment often includes surgical debulking followed by chemotherapy. Prognosis is highly dependent on stage and grade and composition. Epithelial ovarian cancer is the most common type of ovarian cancer, and because 70 percent of cases are diagnosed at stage III or IV, it is associated with a poor prognosis
Allogeneic natural killer (NK) cell transfer is a potential immunotherapy to eliminate and control cancer. A promising source are CD34+hematopoietic progenitor cells (HPCs), since large numbers of cytotoxic NK cells can be generated. Effective boosting of NK cell function can be achieved by interleukin (IL)-15. However, its in vivo half-life is short and potent transpresentation by IL-15 receptor α (IL-15Rα) is absent.
ImmunityBio developed IL-15 superagonist N-803 (Anktiva), which combines IL-15 with an activating mutation, an IL-15Rα sushi domain for trans-presentation, and IgG1-Fc for increased half-life. Here, we investigated whether and how Anktiva improves HPC-NK cell functionality in leukemia and ovarian cancer (OC) models in vitro and in vivo in OC-bearing immunodeficient mice. We used flow cytometry-based assays, enzyme linked immunosorbent assay, microscopy-based serial killing assays, and bioluminescence imaging, for in vitro and in vivo experiments. Anktiva increased HPC-NK cell proliferation and interferon (IFN)g production. On leukemia cells, co-culture with HPC-NK cells and Anktiva increased ICAM-1 expression. Furthermore, Anktiva improved HPC-NK cell-mediated (serial) leukemia killing. Treating OC spheroids with HPC-NK cells and Anktiva increased IFNg-induced CXCL10 secretion, and target killing after prolonged exposure. In immunodeficient mice bearing human OC, Anktiva supported HPC-NK cell persistence in combination with total human immunoglobulins to prevent Fc-mediated HPC-NK cell depletion. Moreover, this combination treatment decreased tumor growth.
Page 29 of 56
Anktiva is a promising IL-15-based compound that boosts HPC-NK cell expansion and functionality in vitro and in vivo. Adding Anktiva to HPC-NK cell therapy could improve cancer immunotherapy.
Development in Advanced Ovarian Cancer

An investigator at the University of Minnesota is also conducting a single site, Phase II trial (NCT03054909) to study patients with late-stage ovarian cancer with single agent Anktiva via subcutaneous or intraperitoneal administration. The data demonstrated intraperitoneal (IP) or subcutaneous administered Anktiva, increases NK cell cytotoxicity in ovarian cancer.
| 11. | Sarcoma |
Soft tissue sarcomas arise in any of the mesodermal tissues of the extremities, trunk, retroperitoneum, or head and neck. There will be an estimated 13,130 new cases of soft tissue sarcoma in the United States in 2020 and 5,350 deaths resulting from the disease. The five-year survival rate for localized soft tissue sarcoma is approximately 81%, which drops 57% and 16% for regional and distant metastatic disease, respectively. Treatment for Stage I-III soft tissue sarcoma includes surgery which can be followed by radiation and chemotherapy. Stage IV disease is rarely curable, with surgery, radiation and chemotherapy (with drugs doxorubicin and Ifosfamide) being the most common therapeutic approach.
Development in Sarcoma:

Aldoxorubicin Single Agent Studies
Aldoxorubicin has been extensively studied in Phase I, II and III as a single agent and in combination with Ifosfamide/Mesna (Phase Ib/II). The furthest development has been in soft tissue sarcomas where Phase I, II and III studies have been completed. There have also been Phase II trials in lung cancer, pancreatic cancer, glioblastoma and Kaposi’s sarcoma in HIV positive patients.
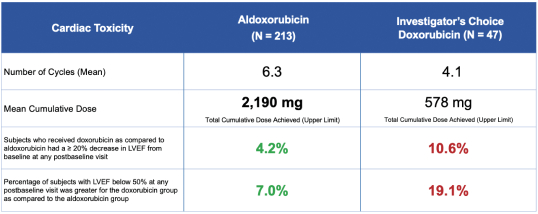
In 2014, a Phase II trial (NCT01514188) conducted by CytRx compared the safety and efficacy of aldoxorubicin to doxorubicin in patients with metastatic, locally advanced, unresectable soft tissue sarcoma. In this randomized study, patients received aldoxorubicin at 350 mg/m2 or doxorubicin at 75 mg/m2, with the lower dose of doxorubicin set due to the association between the cumulative dose of doxorubicin and cardiotoxicity. Aldoxorubicin showed lower rates of cardiac events than doxorubicin, measured as a drop in left ventricular ejection fraction, or LVEF. The rate of patients with ³10% drop in LVEF was 8% for aldoxorubicin versus 35% for doxorubicin after four cycles of treatment. The drop in LVEF persisted at two months after the end of treatment in 3.7% of patients treated with aldoxorubicin versus 33% of those treated with doxorubicin.
Page 30 of 56
The Phase II trial results also showed that aldoxorubicin had a higher rate of response than doxorubicin. The median progression free survival, the primary efficacy endpoint, was significantly longer for aldoxorubicin versus doxorubicin. The results of the study are shown below.
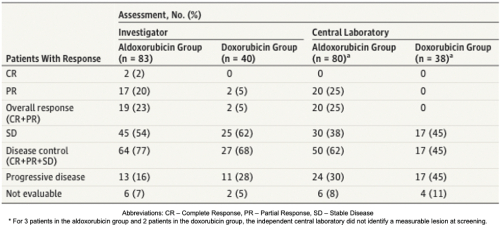
The figure below shows the rates of progression-free survival for aldoxorubicin versus doxorubicin based on Central Radiology Review.
In addition, in 2017, a Phase III trial (NCT02049905) of aldoxorubicin versus physician’s choice of treatment was completed. The trial was found to be underpowered to meet the primary efficacy endpoint, however, aldoxorubicin was shown to have a significantly lower cardiotoxicity compared to doxorubicin, even at nearly four times the cumulative dose of doxorubicin.
Page 31 of 56
| 12. | Advanced Solid Tumors |
Clinical Development in Advanced Solid Tumors

| A. | Multi-Targeted Recombinant hAd5 CEA, MUC1, Brachyury Vaccine Regimen in Advanced Solid Tumors |
In advanced solid tumors, ImmunityBio uses a second-generation human adenovirus (hAd5) to administer CEA, MUC1 and Brachyury concurrently to patients with advanced cancer. We performed an open-label, Phase I trial (NCT03384316) that evaluated concurrent administration of three therapeutic vaccines (ETBX-011 = CEA, ETBX-061 = MUC1 and ETBX-051 = brachyury). All three vaccines used the same modified adenovirus 5 (hAd5) vector backbone and were administered at a single dose level (DL) of 5 × 1011 viral particles (VP) per vector. The vaccine regimen consisting of all three vaccines was given every 3 weeks for three doses then every 8 weeks for up to 1 year. Clinical and immune responses were evaluated.
All patients developed CD4+ and/or CD8+ T-cell responses after vaccination to at least one tumor-associated antigen (TAA) encoded by the vaccine; 5/6 patients (83%) developed MUC1-specific T cells, 4/6 (67%) developed CEA-specific T cells, and 3/6 (50%) developed brachyury-specific T cells. The presence of adenovirus 5-neutralizing antibodies did not prevent the generation of TAA-specific T cells.
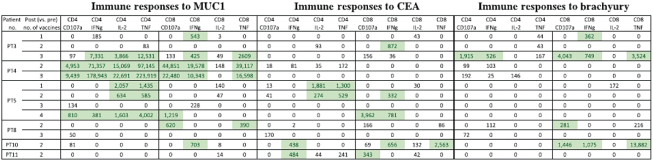
Table: Tumor-associated antigen T cell responses developed after treatment with the TriAdeno vaccine regimen. Immune responses reported in this table are calculated by comparing the absolute number of CD4+ or CD8+ T cells producing cytokine (IFN, IL-2, TNFα) or positive for CD107a per 1 × 106 PBMCs plated at the start of the in vitro stimulation at the specified time points after vaccine. Background (obtained with the negative control peptide pool, human leukocyte antigen [HLA]) and any response prior to vaccine are subtracted: [TAA after vaccine – HLA after vaccine] – [TAA before vaccine – HLA before vaccine]. Positive immune responses are defined as >250 (highlighted). Abbreviations: IFNg, interferon gamma; IL-2, interleukin-2; PT, patient; TNF, tumor necrosis factor.
Ten patients enrolled on trial (DL1 = 6 with 4 in the DL1 expansion cohort). All treatment-related adverse events were temporary, self-limiting, grade 1/2 and included injection site reactions and flu-like symptoms. Antigen-specific T cells to MUC1, CEA, and/or brachyury were generated in all patients. There was no evidence of antigenic competition. The administration of the vaccine regimen produced stable disease as the best clinical response.
Page 32 of 56
In conclusion, concurrent CEA, MUC1, and Brachyury can be administered to patients with advanced cancer with little to no risk of SAEs. Further studies of the vaccine regimen in combination with other agents, including immune checkpoint blockade, are planned.
| B. | Yeast Neoepitope |
ImmunityBio’s yeast vaccine platform, licensed from IB subsidiary GlobeImmune Inc., has been administered to over 800 patients with cancer or infectious diseases in FDA-regulated clinical trials with no SAEs. This platform technology consists of a heat-killed, recombinant S. cerevisiae yeast-based vaccine engineered to express immunogens such as tumor-associated antigens (TAA), pathogen antigens, and tumor-specific neoepitopes. Immunization with this platform elicits CD4+ and CD8+ T-cell responses capable of eliminating tumor cells or pathogen-infected cells. ImmunityBio recently conducted a first-in-human Phase I clinical trial of a yeast vaccine engineered to express patient and tumor-specific neoepitopes. Patients with cancer were administered yeast vaccines expressing multiple neoepitopes identified through a proprietary algorithm incorporating analysis of tumor vs normal DNA sequence, tumor RNA expression, and predicted human leukocyte antigen (HLA)-neoepitope binding. Since its licensure, ImmunityBio has improved the potency of the yeast vaccine by further processing the whole cell product into a lysate, thus directly exposing the immunogen and yeast immunogenic proteins to lymphocytes. This new product further accentuates a tumor-suppressive Th1 dominant response, while also potentially furthering the patent life cycle for this platform. Finally, ImmunityBio has developed a robust, scalable, and economical manufacturing process for the yeast platform, conducive to producing large scale products such as TAA or pathogen vaccines or rapid production of sufficient doses for N of 1 products such as the neoepitope vaccines.
| C. | M-ceNK - Memory Cytokine Enriched Natural Killer Cell Platform. |
Cytokine-induced memory-like NK cells are a unique set of lymphocytes that differentiate after a brief pre-activation with interleukin-12 (IL-12), IL-15, and IL-18 and exhibit enhanced responses to cytokine re-stimulation that include enhanced interferon-g production and cytotoxicity against leukemic cell lines. These cells have been isolated and characterized by their unique cell-surface marker profile and their highly desirable feature of immune-memory, marked by their pronounced anti-cancer activity for weeks to months in duration, which has made these cells a research focus for more than a decade. Published data so far has been limited to the acute myeloid leukemia patient population in the post-allogeneic, haploidentical stem cell transplantation setting.
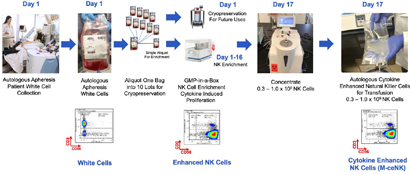
Our cytokine enriched natural killer cell program is based on the ability to enrich and expand donor sourced NK cells in a GMP facility to a clinically relevant scale which allows for the production of a pure cytokine activated and expanded NK cell population that possesses the unique phenotype we call M-CENK cells. Phase I M-ceNK clinical trials in subjects with locally advanced or metastatic solid tumors is anticipated to begin in the second half of 2021.
Page 33 of 56
| VIII. | OUR LIQUID TUMOR CLINICAL DEVELOPMENT PROGRAM |
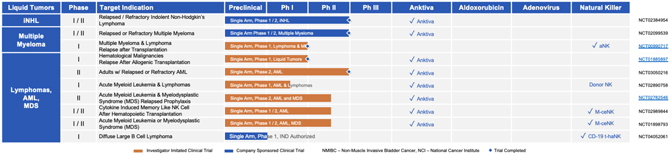
| 1. | iNHL – Indolent Non-Hodgkin’s Lymphoma |
Incidence of iNHL
Over 80,000 adults and children will be diagnosed with Non-Hodgkin’s Lymphoma, or NHL, and close to 20,000 with die each year in the United States. Slow-growing or indolent subtypes represent about 40 percent of all NHL cases annually. Indolent lymphomas commonly have fewer signs and symptoms when first diagnosed as they are slow growing and moving relative to aggressive lymphomas. Follicular lymphoma (FL) is the most common subtype of indolent NHL.
Immunotherapy is a rising modality in NHL cancer therapeutics, harnessing concepts from immunology to enhance, trigger, or rescue immune responses against malignant targets. Established immunotherapies include engineered therapeutic monoclonal antibodies (mAbs), such as the anti-CD20 mAb rituximab, which targets endogenous Fc-receptor bearing immune cells, including natural killer (NK) cells, to B cell malignancies.
IL-15 is a key cytokine for the development, survival, and function of NK cells. Recent in vitro and in vivo work demonstrated the ability of IL-15 to prime non-cytotoxic CD56bright NK cells to become anti-tumor effectors, a function previously believed to be largely restricted to the CD56dim NK cell subset. IL-15 also plays a key role in supporting memory CD8+ T cells, thus augmenting two types of anti-tumor effector lymphocytes. In vivo, IL-15 is trans-presented by IL-15Rα from accessory cells such as monocytes/macrophages and dendritic cells to ligate the IL-2/15Rgb heterodimer expressed on NK and T cells, resulting in activation of multiple signaling pathways, and hence multiple anti-tumor functions. Although IL-15 and IL-2 share signaling components, including the beta (CD122) and gamma chain (CD132), they have divergent effects, with IL-2 promoting Treg expansion. Initial pioneering studies tested E. coli-derived recombinant human (rh)-IL-15, which demonstrated immune modulation in patients. However, rhIL-15 has a short half-life and dose levels that modulated immune cells were limited by unacceptable adverse events (AE). N-803 (Anktiva) is an IL-15R super agonist complex with a prolonged in vivo half-life, physiologic trans-presentation, and accumulation in secondary lymphoid tissues.
Page 34 of 56
Clinical Development in Relapsed / Refractory iNHL:

In a multicenter Phase I study (NCT02384954), we tested the hypothesis that combining Anktiva with the lymphoma-targeting anti-CD20 mAb rituximab safely augments the immune system and response against indolent non-Hodgkin lymphoma (iNHL). This immunotherapy combination was well-tolerated, with no observed SAEs, and resulted in durable clinical responses including in rituximab-refractory patients. Subcutaneous Anktiva plus rituximab induced sustained proliferation, expansion, and activation of peripheral blood NK cells and CD8 T cells in vivo.
Overall response rate (ORR) was 67% (8 of 12) in the SQ cohort. The majority of patients experienced reductions in the size of their lymph nodes. For patients with anti-CD20 mAb sensitive disease, the ORR in the SQ cohort was 78% (7 of 9). In the SQ cohort, 7 of 7 (100%) responses were complete remissions (CR).
Patients in the SQ cohort had prolonged stable disease (SD) and conversion of SD and/or partial response (PR) to CR with a prolonged duration without progression (8 of 12 patients without progression at 18-24 months). In the SQ cohort, 6/7 CRs occurred at either the first (11 weeks) or second evaluation (40 weeks). In the IV cohort, CR responses occurred following the second evaluation and as late as 18 months follow-up. For the 5 patients with anti-CD20 mAb refractory disease in both IV and SQ cohorts, the ORR was 2 of 5 (40%) with 1 CR, 1 PR, 1 SD, and 2 PD. The PR and SD are ongoing in the SQ patients at > 18 months. The SQ cohort patient with PD was highly refractory, having received 5 prior lines of therapy.
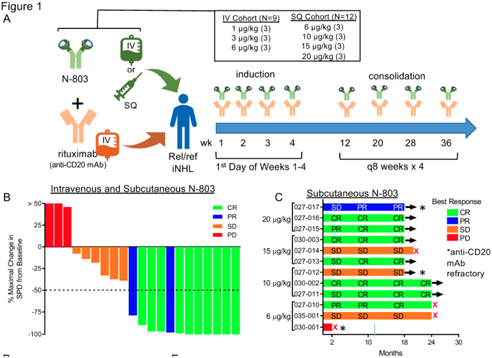
Based on these encouraging findings of 100% complete remission (7 out of 7) in patients receiving subcutaneous Anktiva, ImmunityBio will explore the development of subcutaneous Anktiva in combination with rituximab +/- CD-19 t-haNK in this indication.
Page 35 of 56
Liquid Tumors: Multiple Myeloma, Lymphomas, AML, MDS
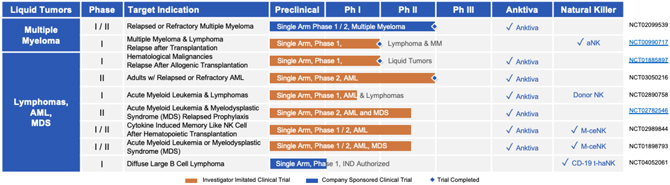
Development in Multiple Myeloma, Lymphomas, AML and MDS:
Early in the development history of NK-92, we conducted a single-center, non-randomized, non-blinded, open-label, Phase I (NCT00990717) dose-escalation trial of irradiated NK-92 cells in adults with refractory hematological malignancies who relapsed after autologous hematopoietic cell transplantation (AHCT). The objectives were to determine safety, feasibility and evidence of activity. Patients were treated at one of three dose levels (1 × 109 cells/m2, 3 × 109 cells/m2 and 5 × 109 cells/m2), given on day 1, 3 and 5 for a planned total of six-monthly cycles.
Twelve (12) patients with lymphoma or multiple myeloma who relapsed after AHCT for relapsed/refractory disease were enrolled in this trial. The treatment was well tolerated, with minor toxicities restricted to acute infusion events, including fever, chills, nausea and fatigue. Two patients achieved a complete response (Hodgkin lymphoma and multiple myeloma), two patients had minor responses and one had clinical improvement on the trial.
Irradiated NK-92 cells can be administered at very high doses with minimal toxicity in patients with refractory blood cancers, who had relapsed after AHCT. We conclude that high dose NK-92 therapy shows some evidence of safety and efficacy in patients with refractory blood cancers and warrants further clinical investigation.
Since 2016, ImmunityBio has pursued two paths to develop natural killer cells for the treatment of liquid tumors:
| • | Off-the-shelf CD19 t-haNK |
| • | Allogeneic and allogenic memory cytokine enhanced natural killer cells (M-ceNK) |
It is anticipated that these cellular therapies, combined with Anktiva which has been shown to enhance in-vivo NK and T cell proliferation, will provide next-generation approaches to liquid tumors.
Page 36 of 56
| A. | Off-The-Shelf CD19 t-haNK |
Our newest and one of our most promising platforms for the development of therapeutic product candidates is an innovative, bioengineered combination of our haNK and taNK platforms that incorporates all the features of our haNK platform together with a CAR. The resulting line of product candidates under this platform avails itself to all three modes of killing: innate, antibody-mediated and CAR-directed killing. These product candidates also include one or more additional expression elements such as functional cytokines, chemokines and trafficking factors, making them amongst the most versatile in our portfolio.
These product candidates are intended to be combined with commercially available therapeutic antibodies to effectively target either two different epitopes of the same cancer specific protein or two entirely different cancer specific proteins. In addition to our two t-haNK product candidates, PD-L1.t-haNK, recently cleared to commence Phase II trials, and CD19.t-haNK, cleared to commence Phase I trials. Based on the encouraging data of Anktiva in combination with rituximab, we anticipate combining CD19 t-haNK to this chemotherapy free protocol for the treatment of liquid tumors including diffuse large b-cell lymphoma and non-Hodgins’s lymphoma.
| B. | M-ceNK - Memory Cytokine Enriched Natural Killer Cell Platform. |
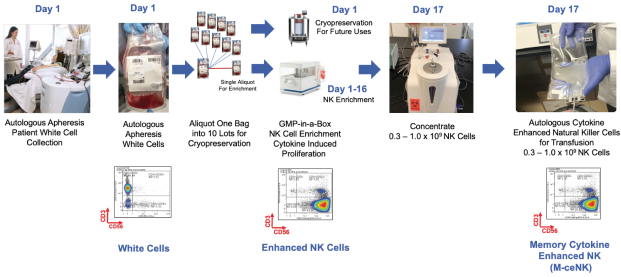
Cytokine-induced memory-like NK cells are a unique set of lymphocytes that differentiate after a brief pre-activation with interleukin-12 (IL-12), IL-15, and IL-18 and exhibit enhanced responses to cytokine re-stimulation that include enhanced interferon-g production and cytotoxicity against leukemic cell lines. These cells have been isolated and characterized by their unique cell-surface marker profile and their highly desirable feature of immune-memory, marked by their pronounced anti-cancer activity for weeks to months in duration, which has made these cells a research focus for more than a decade.
Published data so far has been limited to the acute myeloid leukemia patient population in the post-allogeneic, haploidentical stem cell transplantation setting.
Our cytokine enriched natural killer cell program is based on the ability to enrich and expand donor sourced NK cells in a GMP facility to a clinically relevant scale which allows for the production of a pure cytokine activated and expanded NK cell population that possesses the unique phenotype we call M-CENK cells. Phase I M-ceNK clinical trials in subjects with locally advanced or metastatic solid tumors is anticipated to begin in the second half of 2021.
Page 37 of 56
| C. |
GMP-in-a-Box Approach |
We are a leading company in the efforts to generate allogeneic and autologous sourced NK and mesenchymal stem cell, or MSC, therapeutics. We utilize a scalable GMP production process that combines the use of our semi-automated manufacturing equipment, cytokine expansion and activation reagents such as Anktiva and TxM, and unique and simple processing methods, all of which are proprietary. We have optimized processes for generating both fresh and cryopreserved clinical dose forms of memory-like NK cells with 100% purity (in the allogeneic setting) from a variety of sources, including cord blood and allogeneic and autologous peripheral blood. We avoid the use of both feeder-layers for activation as well as other commonly applied additives that frequently create downstream issues in achieving a high-quality releasable final dose form and have been able to generate multiple dose forms from each donor product, both of which are critical features in achieving scalability.
Page 38 of 56
| IX. | OUR INFECTIOUS DISEASE CLINICAL DEVELOPMENT PROGRAM |
| 1. | HIV |
Anktiva is being evaluated in subjects infected with HIV and multiple investigator-initiated Phase I clinical trials at the University of Minnesota and the University of California San Francisco, and a national multi-site trial with the AIDS Clinical Trial Group, or the ACTG, are in development.
The current strategy for curing HIV is known as the “kick and kill” approach. The “kick” is to induce HIV out of its latent resting state in T cells and the “kill” is to remove or kill the infected cells via an immune response or immunotherapy. Anktiva is a molecule capable of both kick and kill in this strategy because of its ability to activate viral transcription in CD4+ T cells (kick) while strongly activating CD8+ effector memory cells and NK cells important for recognizing and killing HIV infected cells (kill), as well as directing these cells to sites of viral reservoirs.
In multiple non-human primate experiments, Anktiva has been shown to activate CD8+ and NK cells and home these cells to lymphoid organs including normally T cell protected areas such as B cell follicles, reducing the amount of virus in these tissues at the same time. In these animal studies a significant reduction of plasma viremia was observed in NHPs infected with Simian Immunodeficiency Virus, or SIV, for over one year, who were given Anktiva weekly for four weeks.
Recently, Anktiva has also shown strong activation of SIV from latency in NHP that are also CD8+-depleted, indicating an additional mechanism for shocking HIV out of hiding and perturbing the viral reservoir ultimately necessary in HIV cure strategies.
We have observed that Anktiva plus one or two anti-HIV broadly neutralizing antibodies, or bNAbs, has allowed long term suppression of simian/human immunodeficiency virus replication in the absence of anti-retroviral therapy in nine of the 13 animals.
To follow on from these preclinical experiments, we filed an IND in December 2020 to conduct a Phase I clinical trial (N=46) of Anktiva plus two bNAbs sponsored by National Institute of Allergy and Infectious Diseases (NIAID) and conducted by the ACTG. This trial will explore whether Anktiva in combination with bNAbs can result in long term viral remission in the absence of anti-retroviral drugs, functionally curing patients from HIV infection.
Clinical Evidence of Viremia Control in HIV Patients
With respect to HIV, a Phase I clinical dose escalation study with the aim to determine the safety and tolerability of Anktiva in HIV-infected patients has been completed. The study has mirrored preclinical results in non-human primates in that Anktiva induces significant activation and proliferation of T and NK cells, shows evidence for activating virus transcription, suggests reservoir reduction in peripheral blood mononuclear cells, or PBMC, and produces no evidence of production of IL-15 antibodies or cytokine side effects, as shown in the figure below. The figure below shows a decrease over time in the number of cells with measurable HIV.
Page 39 of 56
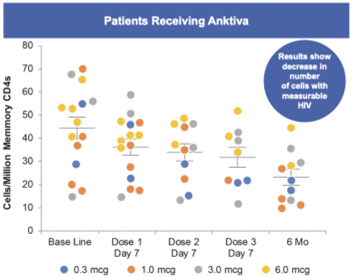
Clinical Development in HIV:

A second Phase I trial (NCT04340596) with total enrollment of eight patients is currently evaluating Anktiva in combination with adoptive transfer of haplo-identical NK cells. The primary and secondary objectives of this trial are to determine the safety of adoptive transfer of haploidentical NK cells when given with Anktiva therapy to HIV infected subjects who are on fully suppressive HIV therapy and to determine if adoptive transfer of haploidentical NK cells will decrease HIV virus reservoirs, respectively. HIV-infected individuals
Page 40 of 56
who have been maintained on suppressive antiretroviral therapy for a minimum of 12 months with a CD4 count >500 cells/µl will be eligible for the therapy.
An additional Phase II (NCT04505501) protocol, anticipated to enroll 15 patients, in development with the Thai Red Cross and the United States Military HIV Research Program, is designed to investigate the safety, tolerability and immunostimulatory effects of administering Anktiva during acute HIV infection. Anktiva will be administered subcutaneously at weeks zero, three and six (for a total of three doses) and will be initiated together with antiretroviral therapy in order to determine if the immunostimulatory effects of Anktiva will reduce the amount of HIV present during acute infection. The study duration for individual participants will be approximately 12 weeks. It is hypothesized that Anktiva initiated with antiretroviral therapy during acute HIV infection will not result in complications or additional toxicities compared with anti-retroviral therapy alone, and may result in a reduced viral load in these subjects.
| 2. | COVID-19 |
We have developed vaccine technologies to deliver tumor-associated antigens, or TAAs, and neoepitopes (expressed only by cancer cells), including hAd5, a second-generation adenovirus. Our vaccine technologies have the capability to induce T cell memory due to the activation of both CD4+ and CD8+ T cells along with antibody (or humoral) responses. Our hAd5 technology has produced several product candidates, which have been studied in multiple Phase I and Phase II clinical trials as potential vaccines for the treatment of certain cancers. Importantly, these product candidates have shown an ability to overcome previous adenovirus immunity in cancer patients and in preclinical models. The hAd5 technology has also been used with common TAAs to establish memory T cells in multiple clinical trials.
First-Generation Versus Second-Generation Adenovirus in a COVID-19 Context
First-generation adenovirus vectors feature the deletion of E1 and/or E3 genes normally responsible for producing viral structural proteins that generate anti-adenovirus-specific immune responses [E1- E3-]. Because adenoviruses are among the causes of the common cold, adenovirus immunity can be as high as 40-70% of the population. Therefore, using these first-generation adenovirus vectors as vaccines faces an immediate hurdle in needing to overcome this pre-existing immunity against the vector itself. Current developers of adenovirus-based COVID-19 vaccines and vaccine candidates appear to be challenged with reduced immunogenicity in patients with pre-existing adenovirus immunity. To address this issue, we have established a second-generation adenovirus vaccine candidate, leveraging our hAd5 technology, with the capability of being administered in the presence of this pre-existing adenovirus immunity for the delivery of tumor associated antigens or SARS-CoV-2 antigens in the fight against COVID-19, as a primary vaccine and/or as a booster to other vaccines.
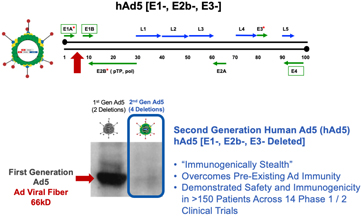
Page 41 of 56
Our advanced second-generation adenovirus technology, with two additional deletions in the E2b region [E1-, E2b-, E3-], confers advantageous immune properties to the vaccine candidate by eliciting potent immune responses to inserted viral (e.g. COVID-19) antigens while minimizing the immune responses to the adenovirus vector itself. Neutralizing antibodies and cytotoxic T cells recognizing adenovirus are directed to the adenovirus fiber protein and other “late” proteins whose expression is muted in our second-generation adenovirus technology. Thus, in individuals with pre-existing adenovirus immunity, we have demonstrated the generation of immunogenic responses to inserted antigens in multiple vaccinations over many months. Because of these modifications, we believe that our hAd5 [E1, E2b-, E3-] vectors have the potential to be superior to adenovirus [E1-] vectors in terms of immunogenicity and safety profile, and are a compelling technology to develop a COVID-19 vaccine in a rapid and efficient manner.
Our Next Generation COVID-19 Vaccine Candidate
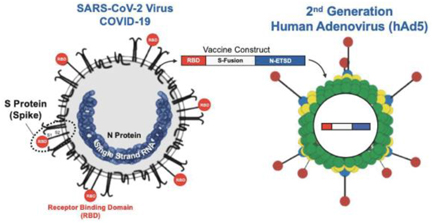
The Differentiated Approach and Current Status of Our COVID-19 Vaccine Candidate Development
To address the ongoing COVID-19 pandemic, particularly in the face of mutations in Spike protein and the high efficiency of SARS-CoV-2 transmission that puts vulnerable persons and front-line workers at risk, we have developed a vaccine candidate to protect individuals from and prevent transmission of SARS-CoV-2 that elicits not only robust humoral responses but also activates T cells. This bivalent hAd5 S-Fusion + N-ETSD vaccine candidate expresses both an optimized viral spike (S) protein (S-Fusion) and a nucleocapsid protein with an Enhanced T-cell Stimulation Domain (N-ETSD) that directs N to the endo/lysosomal subcellular compartment to enhance MHC class II responses. The vaccine antigens are delivered by the second-generation adenovirus serotype 5 [E1-, E2b-, E3-] platform that appear to be safe and effective even in the presence of pre-existing adenovirus immunity. We previously developed this attenuated hAd5 viral vector platform that can be used to rapidly generate vaccines against multiple agents, allowing production of high numbers of doses in a minimal time frame. The hAd5 platform has unique deletions in the early 1 (E1), early 2 (E2b) and early 3 (E3) regions (hAd5 [E1-, E2b-, E3-]), which distinguishes it from other adenoviral vaccine platform technologies under development, and not only allows it to be effective in the presence of pre-existing adenovirus immunity but has a very low risk of generating de novo vector-directed immunity. Genes encoding target antigens are cloned into the viral genome, which once administered in vivo infect antigen presenting cells that express the inserted antigen gene and induce immune responses to the pathogenic target. The platform induces both antibodies and cell mediated immunity (CMI).
We have utilized this platform to produce vaccines candidates against viral antigens such as Influenza, HIV-1 and Lassa fever and COVID-19. In 2009, we employed the hAd5 [E1-, E2b-, E3-] vector platform to express hemagglutinin (HA) and neuraminidase (NA) genes from the H1N1 pandemic viruses (see Figure below). Inserts were consensus sequences designed from viral isolate sequences and the vaccine was rapidly constructed and
Page 42 of 56
produced. Vaccination induced H1N1 immune responses in mice, which afforded protection from lethal virus challenge. In ferrets, vaccination protected from disease development and significantly reduced viral titers in nasal washes. H1N1 CMI as well as antibody induction correlated with the prevention of disease symptoms and reduction of viral replication.
The overwhelming majority of other SARS-CoV-2 vaccines and vaccine candidates in development target only the S antigen (see Figure below) and are expected to elicit SARS-CoV-2 neutralizing antibody responses. In the development of our vaccine candidate, we have paid specific attention to the generation of T cells which is predicted to enhance the breath and duration of the protective immune response against the two antigens; the addition of N in particular affords a greater opportunity for T cell responses. Importantly, we have previously shown that the hAd5 S-Fusion + N-ETSD vaccine candidate elicits T helper cell 1 (Th1) dominant antibody responses to both S and N as well as T-cell activation after vaccination of a murine (CD1) pre-clinical animal model. We have also shown that the SARSCoV-2 antigens expressed by the hAd5 S-Fusion + N-ETSD construct are recognized by T cells from previously SARS-CoV-2 infected individuals when expressed by autologous monocyte derived dendritic cells. These studies provide evidence that vaccination with the hAd5 S-Fusion + N-ETSD vaccine candidate (see Figure below) will re-capitulate natural infection that will then generate protective antibodies and memory T cells. As described in Canete and Venuesa’s “COVID-19 makes B cells forget, but T cells remember”, T cells provide protection even in the absence of antibody responses. This is supported by Sekine et al., who characterized T cell immunity in COVID-19 convalescent patients, finding SARS-CoV-2-specific T cells in most convalescent individuals (including asymptomatic cases) with undetectable antibody responses.
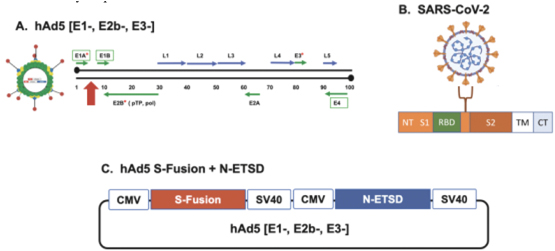
Figure: The hAd5 platform, SARS-CoV-2, spike, and the hAd5 S-Fusion + N-ETSD vaccine. (A) The human adenovirus serotype 5 vaccine platform with E1, E2b, and E3 regions deleted (hAd5 [E1-, E2b-, E3-]) is shown. (B) The SARS-CoV-2 virus displays spike (S) protein as a trimer on the viral surface. S protein comprises the N-terminal (NT), the S1 region including the Receptor Binding Domain (RBD), the S2 and transmembrane (TM) regions, and the C-terminal (CT); other function regions not labeled. (C) The bivalent vaccine comprises both S-Fusion and N-ETSD under control of cytomegalovirus (CMV) promoters delivered by the hAd5 [E1-, E2b-, E3-] platform.
Pre-Clinical & Non-Human Primate Challenge Studies to Date
Our preclinical studies have shown that the bivalent S-Fusion + N-ETSD hAd5 vaccine candidate resulted in robust T cell and humoral immune responses against SARS-CoV-2 S-Fusion and N-ETSD antigens. Immunogenicity in CD1 mice was assessed after two doses given 21 days apart (Day 0 and Day 21). Immune responses measured on Day 28 showed that the vaccinations elicited robust T cell responses to SARS-CoV-2. Importantly, a statistically significant CD4+ T cell response to N protein was generated in all five mice. This is consistent with studies in patients who have recovered from SARS-CoV who show memory T cells to N
Page 43 of 56
protein. Four out of five mice generated an S-specific antibody response with evidence of Th1 dominance. Two of these mice demonstrated potent neutralizing antibodies against the spike protein. Analysis of both T cell cytokine responses and antibody isotypes demonstrated that the overall immune response was highly skewed towards T helper, or Th1, cell dominance important for potentially mitigating the risk of antibody-dependent enhancement of infection. In addition, we have shown that the vaccine antigens, when expressed in normal human dendritic cells, are recognized by COVID-19 patient antibodies in their convalescent plasma samples and that these dendritic cells can also activate the patients’ S and N specific T cells. These results are summarized below:
| • | The S-Fusion and N-ETSD optimizations to S and N, respectively, generate antigen specific B cell, CD4+ and CD8+ T cell responses in mouse models. |
| • | Characterization of this immune response demonstrated a Th1 bias in both T cell and antibody responses against S and N. |
| • | The vaccine candidate induces neutralizing antibodies in these models verified by two independent SARS-CoV-2 neutralization assays. |
| • | The vaccine antigens are expressed by normal donor dendritic cells and recognized by convalescent patient plasma via anti-SARS-CoV-2 antibodies. |
| • | The vaccine antigens are processed and presented by patient dendritic cells to activate S and N specific T cells. |
Complete Protection of Nasal and Lung Airways Against SARS-CoV-2 Challenge by Antibody Plus Th1 Dominant N- and S-Specific T-Cell Responses to Subcutaneous Prime and Thermally-Stable Oral Boost Bivalent hAd5 Vaccination in an NHP Study
In addition to the preclinical mouse data summarized above, preliminary findings from the non-human primate (NHP) COVID-19 challenge study conducted at Battelle Biomedical Research Center (“Battelle”) and sponsored by Biomedical Advanced Research & Development Authority (BARDA, ASPR, DHHS) are noted below. The objective of this study was to evaluate the efficacy of novel SARS-CoV-2 vaccine candidates in NHPs. Vaccine-treated NHPs consisted of two groups (n=5/group) of male and female rhesus macaques that were administered three vaccinations of hAd5 S-Fusion + N-ETSD through a combination of subcutaneous injection (SC) and enteric-coated capsule delivery (Oral). Control NHP (n=4) were administered placebo equivalent of the treatment arm. Vaccinations occurred on study Days 0, 14 and 28. Twenty-eight days after the final vaccination (Day 56), all groups were administered virulent SARS-CoV-2 in the upper respiratory tract. Efficacy of the vaccine candidate was assessed by clinical monitoring, testing of sera in the cPass assay for inhibition of S RBD:angiotensin converting enzyme 2 (ACE2; the natural receptor for S during the initiation of infection) and viral burden reduction (genomic and sub-genomic RNA) in the NHP.
This study provided evidence for the efficacy of subcutaneous (prime) followed by oral (boost) of the hAd5 S+N vaccine candidate to provide protection against SARS-CoV-2 challenge. The results showed that immunization with the hAd5-COVID-19 vaccine candidate inhibited SARS-CoV-2 virus replication in 100% (10 of 10) of Rhesus macaques, with a drop in viral replication starting on the first day of vaccine administration, and undetectable viral levels as early as three to five days post-challenge in most of the animals. The vaccine candidate targeted both the inner nucleocapsid (N) and the outer spike (S) proteins of the virus to maximize the immune response. The goal of targeting both S and N was to both activate virus-specific T cells and generate anti-SARS-CoV-2-neutralizing antibodies. The study showed this broad immune response led to the complete clearance of the virus in a matter of days after infection of previously-vaccinated primates. This blocking of viral replication was observed in both the lung and nasal passages. By protecting the nasal passages (the primary point of entry for the virus), the vaccine candidate has the potential to reduce reinfection. Clearing replicating viruses from nasal passages is critical for reducing transmission of the virus from immunized recipients to others.
Page 44 of 56
Rapid Clearance in Nasal Passages and Lung following Challenge
Viral Replication from Nasal Swab and Lung samples (sgRNA): Immunization with hAd5 S-Fusion+N-ETSD, both as subcutaneous and oral forms, successfully cleared the viruses from lung and nasal airways with zero viral replication detected within days after challenge. A comparison of the geometric means of the vaccinated and placebo-treated macaques revealed dramatic reduction of sub-genomic SARS-CoV-2 RNA (sgRNA), indicating vaccination dramatically reduced viral replication in the nasal and lung passages (Fig. 2) These results indicate protection of both the upper and lower respiratory tract by hAd5 S-Fusion + N-ETSD vaccination and suggest the vaccination could prevent transmission as well as COVID-related morbidity and mortality.
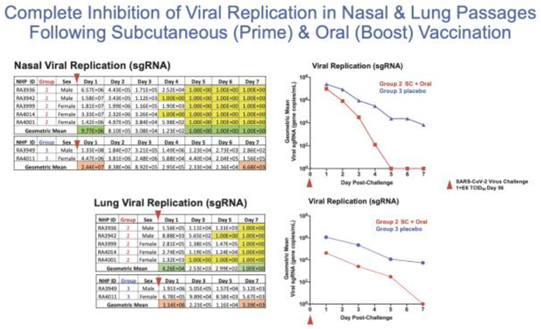
Group 2 viral replicating virus in the nasal and lung passages post-challenge.
Evidence of Memory B Cells Induced by Vaccination
Results from a microneutralization assay demonstrated potent antibody response immediately following the virus challenge in both regimens of subcutaneous prime and boost followed by a single oral boost (group 1) as well as the regimen of subcutaneous prime with two subsequent oral boosts (group 2). Group 3 & 6 are placebo controls. As can be seen, neutralizing antibodies as high as 1:9000 dilution was achieved in the vaccinated animals.
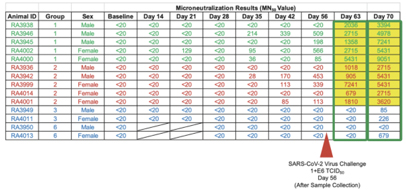
Page 45 of 56
Summary of Rapid Viral Clearance in NHP Challenge Based on T Cell & B Cell Activation Following Subcutaneous & Oral hAd5 S+N Vaccination
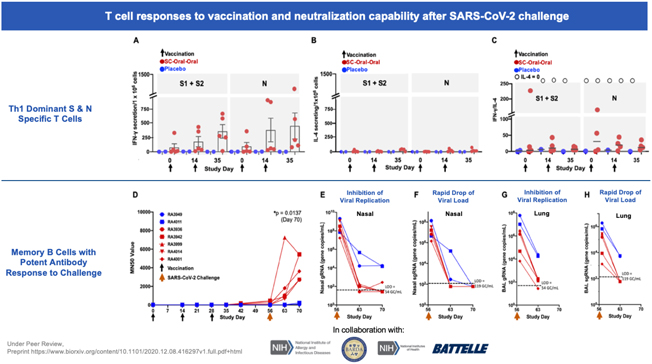
Based on these positive preclinical findings, we are advancing this next generation hAd5 COVID-19 vaccine candidate initially as a subcutaneous administration as our lead clinical candidate to test for its ability to potentially provide robust, durable cell-mediated and humoral immunity against SARS-CoV-2 infection. In addition to this route of administration, we are also developing our vaccine candidate into an oral and sublingual delivery formulation, which we refer to as AdenoCap. We believe AdenoCap can overcome the cold chain, global delivery and universal access challenges of an injectable vaccine and meet all the WHO preferred Target Product Profile requirements for a COVID-19 vaccine, including rapid scalability and low cost.
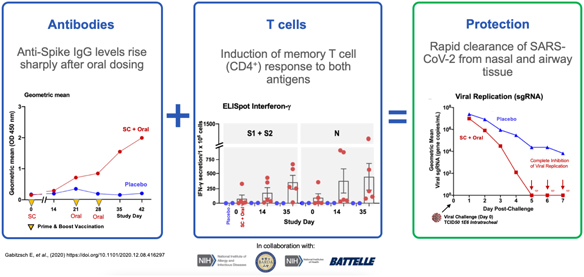
Page 46 of 56
Current Clinical Status in COVID-19 Vaccine Candidate

While there are a number of vaccines with Emergency Use Authorization (EUA) and other candidates in development, we believe most are limited by their focus on antibody responses to the spike (S) protein. Our candidate uses a combination of S-Fusion and N-ETSD, our novel constructs of COVID-19 spike (S) and nucleocapsid (N) proteins, which has been shown to generate CD4+ and CD8+ T cell mediated immunity and
Page 47 of 56
neutralizing antibodies in small animal models and inhibition of viral replication in nasal and lung passages in a non-human primate challenge study.
On October 13, 2020, the FDA authorized a Phase I, open-label, dose-finding study to examine the safety, reactogenicity and immunogenicity of the low-dose (5x1010 VP) and intermediate-dose (1x1011 VP) regimens of our vaccine candidate in healthy volunteers.
Notably, in our current cancer vaccine Phase I/II clinical trials, the doses of our hAd5 vaccine candidate were administered at (5x1011 VP) and (1.5x1012 VP) when administered for three tumor associated antigens simultaneously. The full dose for our hAD5 COVID-19 vaccine candidate is being determined and will be identified following the completion of the Phase I trials, including awaiting authorization from FDA to combine sublingual and oral formulation with a chosen subcutaneous dose. In the current Phase I study, the vaccine was given as a prime on day 1 and a boost on day 22, subcutaneously.
On Nov 10, 2020, enrollment was completed for both low and intermediate dose cohorts. Volunteers tolerated both doses remarkably well with no reports of any grade 3 or grade 4 adverse events in either group, and the grade 1 and grade 2 adverse events were mild in nature. On the basis of these preliminary findings, we filed an IND protocol amendment for a Phase II / III placebo-controlled, randomized, clinical trial observer-blind study to evaluate the safety, tolerability, immunogenicity, and efficacy of our hAd5 COVID-19 vaccine candidate to be administered subcutaneously at the intermediate dose.
In Q1 2021, the following clinical trials were authorized and initiated:
USA Phase I: The first two cohorts of the Phase I, open label, dose-ranging study (NCT04591717) of the vaccine candidate received two different dose levels (0.5 and 1.0ml). Participants received two subcutaneous injections 21 days apart. An additional group of 40 subjects will be enrolled to evaluate safety, reactogenicity, and immunogenicity of the combination of hAd5 in four different cohorts receiving sublingual and subcutaneous formulations to select an optimal combination dose for future studies.
USA Phase Ib: The second Phase 1b trial (NCT04732468) is designed to assess the safety, reactogenicity, and immunogenicity of the combination of hAd5 in oral capsule and subcutaneous formulations; and to select an optimal combination dose for future studies. Up to 40 subjects will be enrolled in the four-cohort study, which is anticipated to begin in Q1.
South Africa Phase I: Recruitment has begun in March 2021 in Cape Town, South Africa for a trial (NCT04710303) of subcutaneous administration to be followed by amendments using sublingual delivery and room temperature-stable oral capsules.
One Vaccine, Three Routes of Immune Protection
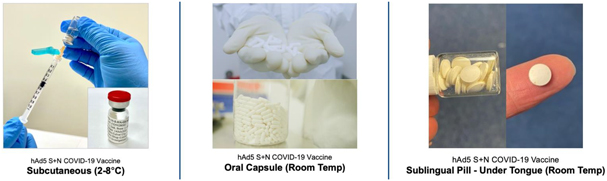
Page 48 of 56
| XI. | Preclinical & Pre-IND Pipeline |
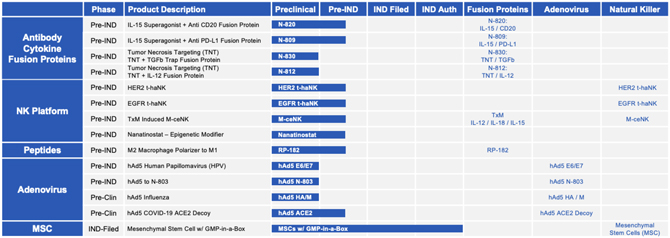
| 1. | Antibody Cytokine Fusion Proteins |
Antibody Cytokine Fusion Proteins in Development
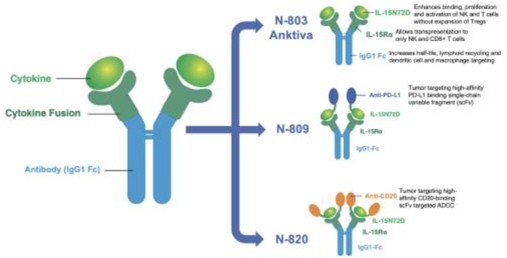
In addition to Anktiva, we are developing novel cytokine fusion proteins to further enhance NK and T cell activation directed to the tumor microenvironment and modulate the systemic and local immune response to further amplify tumor destruction.
| A. | N-820: IL-15 / CD20 |
N-820 is a novel bifunctional protein, fusing Anktiva with anti-CD20. The N-820 molecule allows for CD20-targeted antibody-dependent cellular cytotoxicity, or ADCC, while targeting IL-15 activity to areas expressing CD20, such as B cells, lymphomas and leukemias, particularly non-Hodgkin lymphoma and chronic lymphocytic leukemia, respectively.
Page 49 of 56
In preclinical studies, N-820 depleted B cells in the blood, lymph nodes and spleen of non-human primates, and directly targeted CD20-expressing B cell lymphoma cells akin to rituximab. N-820 was selectively targeted to the lymph nodes and other organs such as the liver, whereas rituximab persisted in blood, and simultaneously activated NK cells to enhance ADCC to induce cell death of B-lymphoma cells. This directed localization of IL-15 activity to the targeted tumor cells may allow for precise induction of a local immunological response and a robust cell death outcome within a lesion or cancerous tissue (e.g., a cancerous lymph node).
| B. | N-809: IL-15 / Anti-PDL1 |
N-809 is a fusion of Anktiva with a proprietary anti-PD-L1. In collaborative in vivo studies with the NCI, we observed that N-809 has the same ability to bind PD-L1 as an anti-PD-L1 monoclonal antibody, N-809 tripled proliferation and doubled activation of T cells in tumor-bearing mice, and effected clearance of human bladder cancer cells that express PD-L1 in which localization of N-809 to the tumor site was witnessed for 24 hours. Accumulation to the site of PD-L1 expressing tumor cells was specific to N-809, which cured six out of ten tumor-bearing mice. N-809 also increased the cytotoxic potential of NK cells, effecting lysis of several tumor cell types. Similarly, the highest level of ADCC was seen when N-809 was added to patient-derived NK cells. In a subsequent study, N-809 enhanced NK and CD8+ T-cell activation and function when compared with an Anktiva and anti-PD-L1 combination. Overall, N-809 increased survival rate in preclinical animal cancer models when compared to the combination of Anktiva and anti-PD-L1.
Necrotic Tumor Cell Targeting
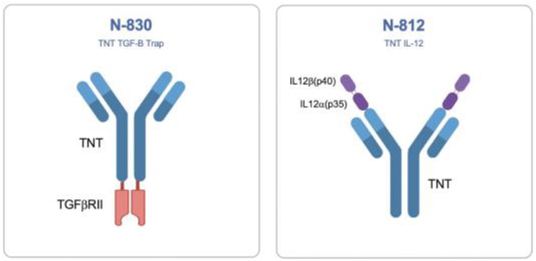
| C. | N-830: TNT / TGF-ß Trap |
Necrotic areas appear in cancer lesions when rapidly dividing cancer cells die (known as necrosis) due to insufficiencies in available nutrients, growth factors, and/or oxygen supply or due to patient treatment with radiation or other cancer therapies. Necrosing cells in these areas lyse and reveal their intracellular DNA to extracellular factors. Using exposed DNA as a target, we are developing an antibody-based fusion protein, N-830, which recognizes single and double-stranded DNA through the tumor necrosis targeting, or TNT, antibody and serves as a decoy receptor, or trap, for secreted TGF-ß. An earlier version of the TNT antibody that was radiolabeled and used as a diagnostic demonstrated selective targeting to and prolonged retention in tumors of clinical trial patients. TNT antibody administration following cytoreductive therapies demonstrated increased localization of the antibody to tumors in animal models and enhanced median survival in patients.
Page 50 of 56
TGF-ß plays a pivotal role in fibrosis in a variety of clinical diseases including cancer, autoimmunity, and infectious disease. By creating the TNT-TGFß trap fusion protein, N-830, we are developing this potential therapeutic to decrease fibrosis in naturally fibrotic cancers (e.g. pancreatic cancer, hepatocellular carcinoma, breast cancer, etc.) or for coupling with radio- or chemotherapy regimens which are known to induce large populations of necrotic cell bodies at the tumor site.
| D. | N-812: TNT / IL-12 |
In addition to using the TNT antibody as a means of targeting tumors for the removal of cytokines like TGF-ß using the “trap” mechanism, the TNT strategy also enables tumor-specific delivery of other fusion partners, such as immune-activating cytokines. IL-12 stimulates the cytotoxic activity of CD8+ T and NK cells against cancer cells and diminishes the activity of inhibitory cytokines like IL-4. However, due to its potency, IL-12 is particularly toxic when administered systemically, which has significantly limited its clinical development. Here, by fusing IL-12 to TNT, we have developed a potential therapeutic to direct the localization of IL-12 to the necrotic tumor cells via TNT, thus activating cytotoxic immunity at the tumor microenvironment, while avoiding potential systemic toxicity. In collaborative in vivo studies with the NCI, six out of ten tumor-bearing mice had complete responses by the combination of an IL-12 fused to a different tumor necrosis targeting antibody with Anktiva and our hAd5 vaccine candidate immunizing for mutations expressed only by tumor cells (neoepitopes). Further analysis of these experiments demonstrated that this treatment regimen effected cytotoxic T cell infiltration into the tumor microenvironment and the formation of immunological memory in cured animals. Our lead TNT / IL-12 fusion product (N-812) candidate is entering pre-IND development.
| 2. | t-haNK Platform: CAR-Directed and Antibody-Mediated Killing |
Our newest and most promising platform for the development of therapeutic product candidates is an innovative, bioengineered combination of our haNK and taNK platforms that incorporates all the features of our haNK platform together with a CAR. The resulting line of product candidates under this platform avails itself to all three modes of killing: innate, antibody-mediated and CAR-directed killing. These product candidates also include one or more additional expression elements such as functional cytokines, chemokines and trafficking factors, making them amongst the most versatile in our portfolio. These product candidates are intended to be combined with commercially available therapeutic antibodies to effectively target either two different epitopes of the same cancer specific protein or two entirely different cancer specific proteins.
In addition to our two t-haNK product candidates, PD-L1.t-haNK, recently cleared to commence Phase II testing, and CD19.t-haNK, cleared to commence Phase I testing, we believe a pipeline of prominent CARs for t-haNK, including HER2, which is nearing IND submission, and EGFR, which is advancing through clinical enabling studies, among others, will enable us to potentially address an even broader range of cancers as part of a chemotherapy-free combination regimen.
| • | EGFR t-haNK: Advancing through clinical enabling studies |
| • | HER2 t-haNK: IND submission |
Page 51 of 56
| 3. | Peptides & Immunomodulators |
| A. | RP-182 |
Under the tumoricidal macrophage modality, we are developing the small, easy-to-manufacture synthetic peptide RP-182, to induce tumor-associated macrophages, or TAM, to become phagocytic in the tumor microenvironment and facilitate therapeutic activity.
Various TAM subpopulations can co-exist within tumor microenvironment facilitating angiogenesis, metabolism and immune suppression/evasion.
RP-182, an activator of tumoricidal macrophages, effects tumor suppression, induces anti-tumor immunity and extends the survival of preclinical pancreatic animal models. We believe that in orchestration with our other immunomodulatory agents, such as our adenovirus and yeast vaccine technologies and engineered cytokines, it will produce positive tumoricidal and immunological responses in tough-to-treat cancers, especially those prone to fibrosis.
| B. | Cynviloq |
Cynviloq, an alternative to nab-paclitaxel (AbraxaneTM, a standard of care for pancreatic cancer) and a co-polymer nanoparticle micelle paclitaxel with certain rights owned by NANTibody, a joint venture between Sorrento and us, in which we have majority ownership, will continue Phase Ib/II trials for advanced metastatic pancreatic carcinoma to evaluate safety upon receipt of additional manufacturing, drug product and safety information from the drug’s manufacturer, Samyang Biopharmaceuticals. Cynviloq is approved in South Korea for metastatic breast cancer, NSCLC and ovarian cancer.
| 4. | Adenovirus |
| A. | hAd5 Human Papillomavirus (HPV) |
In April 2017, we entered into a license agreement with Sanford Health pursuant to which we obtained a worldwide, exclusive license under Sanford’s applicable patent and know-how rights to use, make, have made, sell, offer to sell, export and import products for all uses and applications of polynucleotides encoding mutant E16 antigen (mutant HPV16 E6 antigen + mutant HPV16 E7 antigen) and the encoded mutant E16 antigen
Head & Neck Cancer
Human papilloma virus, or HPV, is known to cause approximately 95% of cervical and 30-60% of oropharyngeal carcinoma cases. High-risk HPV type 16 is involved in more than 50% of cervical cancers worldwide and is the primary viral driver of esophageal, anal cancers, and head and neck squamous cell carcinomas, or HNSCC. HPV E6 and E7 genes expressed in squamous cell cancers are considered to be an attractive target for tumor specific immunotherapy because the cancer cells require E6 and E7 for progression.
We have developed our proprietary hAd5 technology to deliver a proprietary modified/fused non-oncogenic HPV E6/E7 gene (E6D/E7D) to treat cancer patients with HPV-expressing cancer. The addition of a proprietary localization signal (ETSD) to the E6/E7 construct further distinguishes this vaccine by allowing for trafficking of the antigens to specific cellular compartments presentable and recognizable by CD4+ and CD8+ T cells, potentiating immunological memory against HPV-bearing tumor cells. This product candidate,
Page 52 of 56
in an earlier iteration, has been granted orphan-drug designation by the FDA for the treatment of HPV-associated HNSCC, and we intend to seek this designation for the ETSD modified product candidate.
| B. | hAd5 Influenza |
Conventional influenza vaccines take at least 12 to 18 months to prepare and distribute, due to their production using the 1950s technology of chicken eggs and complicated cell lines. The inability to quickly respond to an outbreak, as exemplified during the 2009 H1N1 pandemic, has accelerated the need to develop novel technologies that minimize vaccine creation, preparation, testing, manufacturing, regulatory approval and distribution time. In response to the pandemic H1N1 of 2009, we utilized the hAd5 [E1, E2b-, E3-] platform to quickly create a vaccine against the disease that could induce both humoral immunity and cell mediated responses.
Vaccination with hAd5 [E1-, E2b-,E3]-H1N1 induced robust CMI and humoral immune responses to H1N1, which translated into significant protection from disease development and death following H1N1 challenge in mice. In challenge studies, mice and ferrets were protected from intranasal challenge with virulent H1N1 influenza virus following immunization with hAd5 [E1, E2b-, E3-] platform expressing HA and NA. Vaccinated ferrets had minimal or no clinical symptoms of H1N1 infection and nasal washes revealed a complete blockade of the virus production in the upper respiratory tract. Reducing the clinical symptoms of influenza such as coughing and sneezing as well as potentially blocking H1N1 virus shedding can greatly reduce horizontal transmission, an important aspect in containing pandemic infectious diseases.
Conventional influenza vaccines function by inducing antibodies (Abs) against the highly variable surface glycoprotein hemagglutinin (HA). Humoral responses against influenza viruses are important, but increasing data indicate that induction of a potent cell-mediated immune response will increase the protective effects. ImmunityBio has constructed and evaluated several new Influenza vaccines containing conserved and consensus influenza proteins. To develop complete immunologic memory against influenza, especially heterosubtypic immunity, it has been reported that the vaccination must induce both B-cell and CD4 immunity.9 Thus, vaccines inducing both humoral and cellular immune responses would be of greater benefit than current vaccines. To this end and to address the critical need, IB has developed a rapidly amenable, multiple use vaccine delivery platform in a single backbone. The capacity of CMI to respond to heterologous influenza viruses holds tremendous potential in the development of a universal influenza vaccine. We have utilized our hAd5 [E1-, E2b-, E3-] as a platform for a universal vaccine.
We are evaluating and continue to develop “Universal” Influenza vaccine candidates that will induce broad immune responses against influenza resulting a wide breadth of protection against various strains of influenza viruses.
| C. | hAd5 COVID-19 ACE2 Decoy |
The highly-transmissible SARS-CoV-2 variants now replacing the first wave strain pose an increased threat to human health by their ability, in some instances, to escape existing humoral protection conferred by previous infection, neutralizing antibodies, and possibly vaccination. Thus, other therapeutic options are necessary. One such therapeutic option that leverages SARS-CoV-2 initiation of infection by binding of its spike receptor binding domain (S RBD) to surface expressed host cell angiotensin-converting enzyme 2 (ACE2) is an ACE2 ‘decoy’ that would trap the virus by competitive binding and thus inhibit propagation of infection. Here, we used Molecular Dynamic (MD) simulations to predict ACE2 mutations that might increase its affinity for S RBD and screened these candidates for binding affinity in vitro. A double mutant ACE2(T27Y/H34A)-IgG1FC fusion protein was found to have very high affinity for S RBD and to show greater neutralization of SARS-CoV-2 in a live virus assay as compared to wild type ACE2.
Page 53 of 56
We further modified the double mutant ACE2 decoy by addition of an H374N mutation to inhibit ACE2 enzymatic activity while maintaining high S RBD affinity. We then confirmed the potential efficacy of our ACE2(T27Y/H34A/H374N)-IgG1FC Triple Decoy against S RBD expressing variant-associated E484K, K417N, N501Y, and L452R mutations and found that our ACE2 Triple Decoy not only maintains its high affinity for S RBD expressing these mutations, but shows enhanced affinity for S RBD expressing the N501Y or L452R mutations and the highest affinity for S RBD expressing both the E484K and N501Y mutations. The ACE2 Triple Decoy also demonstrates the ability to compete with wild type ACE2 in the cPass™ surrogate virus neutralization in the presence of S RBD with these mutations. Additional MD simulation of ACE2 interactions using mutated S RBD provides some insight into the enhanced affinity of our ACE2 Triple Decoy for mutated S RBD. The ACE2 Triple Decoy is now undergoing continued assessment, including expression by a human adenovirus serotype 5 (hAd5) construct to facilitate delivery in vivo.
SARS-CoV-2 variants have rapidly swept the globe and very recent investigations reveal that several of these variants have shown the ability to escape neutralization by convalescent antibodies in recovered COVID-19 patients and recombinant neutralizing antibodies (nAbs) developed as therapeutics. There are also fears that current vaccines may not be as effective against some of the variants and early evidence suggests that for some vaccines, this risk may exist. The latter is a particular concern, as the massive vaccine efforts currently underway employ vaccines designed to elicit immune responses against first-wave sequence SARS-CoV-2 spike (S) protein and specifically the S receptor binding domain (S RBD) that binds to angiotensin converting enzyme 2 (ACE2) on the surface of human cells in the airway and gut that initiates viral entry and infection. While one response to the threat of loss of vaccine efficacy might be to continually re-design vaccines to target specific new variants, this would be an ongoing game of catch-up because it can be expected that further novel variants will emerge, particularly since several recent reports have shown that antibodies elicited by infection and vaccination act as evolutionary forces that result in the predominance of viral variants that escape these immune defenses. While efforts to adapt vaccines should be encouraged, in parallel, new therapeutic approaches to neutralize viral infection that are not undermined by the presence of mutations should be advanced.
To address the need for a therapeutic and potentially prophylactic approach that has a low likelihood of being adversely affected by variant mutations, we have designed and tested ACE2 ‘decoys’ that leverage the binding of the S RBD to ACE2. This is an approach that is also being pursued by others using a variety of fusion proteins and delivery methods. Our ACE2 decoys under development are recombinant ACE2-IgG1FC or -IgAFC fusion proteins, with the ACE2 sequence optimized for binding affinity to S RBD. The ACE2 decoy would be given to a patient infected with SARS-CoV-2 to prevent binding of virus to host cell ACE2 by competing with endogenous ACE2 for spike binding, and allow clearance of the virus. To successfully compete, an efficacious ACE2 decoy would ideally have significantly higher affinity for S RBD than endogenous, host-cell expressed ACE2. To identify ACE2 mutations with a high probability of increasing affinity, we utilized our in silico Molecular Dynamic (MD) simulation capabilities.
Because the ACE2 decoy concept is based on interaction of ACE2 with S RBD, its binding affinity and thus efficacy may also be vulnerable to changes in the SARS-CoV-2 S RBD sequence. We therefore assessed the affinity of our ACE2 decoy, as compared to wild type (WT) ACE2, for S RBD with a variety of single or multiple mutations associated with the currently predominant variants, including the B.1.351 variant expressing E484K, K417N, and N501Y mutations, the B.1.1.7 variant (N501Y), and the Cal.20.C L452R variant.
Our findings show that the N27Y and H34A mutations of ACE2 conferred the greatest increase in affinity for S RBD of the ACE2 variants tested. Our final ACE2 Triple Decoy also included an H374N mutation to
Page 54 of 56
abrogate ACE2 enzymatic activity. This ACE2 Triple Decoy not only maintained affinity for variant S RBD, it showed an increased affinity for S RBD expressing N501Y or L452R mutations.
| 5. | Mesenchymal Stem Cells (MSCs) Platform |
Bone marrow-derived allogeneic MSCs are considered to be a prominent cell type to treat degenerative diseases and autoimmune disorders. MSCs are reported to be immunoprivileged, allowing for transplantation of allogeneic MSCs without the risk of being rejected by the host immune system. MSCs have been found to be capable of modulating immune responses, thereby reducing inflammation as well as immunopathology and protecting alveolar epithelial cells during acute respiratory distress syndrome, or ARDS, including that triggered by cytokine storm. More importantly, MSCs demonstrated promising activity in reducing the non-productive inflammation and in promoting lung generation in a phase II clinical trial, as well as in patients with ARDS in clinical practice. As a result, we believe MSCs have the potential to alleviate the SARS-CoV-2-derived cytokine storm and ARDS, and thereby have an effect on the treatment of subsequent chronic respiratory dysfunction and lung fibrosis.
We have developed and optimized procedures and proprietary protocols to generate multiple dose forms of MSC products from a single bone marrow or cord tissue sample, in a scalable format using our GMP-in-a-Box system.
| 6. | Memory Cytokine Enriched Natural Killers (M-ceNK) |
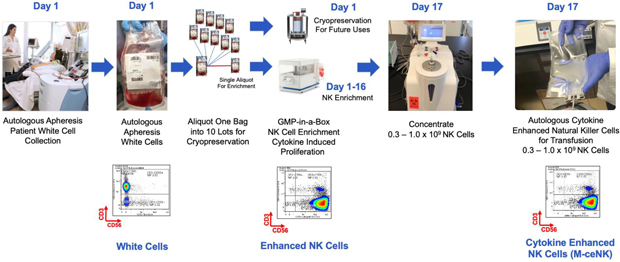
Cytokine-induced memory-like NK cells are a unique set of lymphocytes that differentiate after a brief pre-activation with interleukin-12 (IL-12), IL-15, and IL-18 and exhibit enhanced responses to cytokine re-stimulation that include enhanced interferon-g production and cytotoxicity against leukemic cell lines. These cells have been isolated and characterized by their unique cell-surface marker profile and their highly desirable feature of immune-memory, marked by their pronounced anti-cancer activity for weeks to months in duration, which has made these cells a research focus for more than a decade.
Published data so far has been limited to the acute myeloid leukemia patient population in the post-allogeneic, haploidentical stem cell transplantation setting.
Our cytokine enriched natural killer cell program is based on the ability to enrich and expand donor sourced NK cells in a GMP facility to a clinically relevant scale which allows for the production of a pure cytokine
Page 55 of 56
activated and expanded NK cell population that possesses the unique phenotype we call M-CENK cells. Phase I M-ceNK clinical trials in subjects with locally advanced or metastatic solid tumors is anticipated to begin in the second half of 2021.
CAR-T cell therapy requires isolating and modifying a patient’s own cells, which requires time and which may not be feasible if rapid treatment is required. In addition, some patients may have already endured multiple rounds of therapy, depleting their own supplies of CAR-T cells and preventing generation of a useful dose. CAR-T therapies have shown high remission rates in patients with B-cell precursor ALL and B-cell lymphomas; however, approximately 30% to 50% of patients who achieve disease remission at one month with CD19 CAR-T eventually relapse, usually within one year of treatment. Practical limitations of CAR-T therapy include cost barriers (including the difficulty of applicable insurance coverage), challenges in manufacturing high-quality, effective CAR-T therapies, and toxicity.
| 7. |
GMP-in-a-Box: A Next Generation CAR-T Therapy |
In response to the limitations currently faced by CAR-T cell therapies, we have developed a novel automated closed system bioreactor for T cell proliferation called GMP-in-a-Box, which is less expensive, labor intensive and cumbersome than current methods of manufacturing. This bioreactor has received CE marking in the European Economic Area, which allows products to be sold that have met high health, safety and environmental requirements. GMP-in-a-Box has demonstrated the capability to manufacture multiple cell types from cord blood, bone marrow, adipose tissue, and peripherical blood from both allogenic and autologous sources. We have established proprietary manufacturing techniques utilizing fusion-proteins, including Anktiva, to generate cytokine enriched T cells, or M-ceNK. We believe autologous and allogeneic M-ceNK cells will serve as the basis for next generation CAR-T cells.
GMP-in-a-Box, a proprietary closed system advanced cell manufacturing bioreactor, as shown below, provides just-in-time and repeat dosing if needed, while avoiding complex logistics associated with current CAR-T cell generation. The manufacturing process is automated, scalable and efficient, reducing costs and increasing speed and reliability. There is also the potential to provide multiple doses of cryopreserved cytokine enriched CAR-T cells from a single apheresis.
Page 56 of 56
