Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Osmotica Pharmaceuticals plc | tm219123d1_8k.htm |
Exhibit 99.1

Corporate Presentation Barclays Global Healthcare Conference March 2021

Forward Looking Statements This presentation contains forward - looking statements. You should not rely upon forward - looking statements as predictions of future events . These forward - looking statements generally can be identified by the use of words such as “anticipate,” “expect,” “plan,” “could,” “may,” “will,” “believe,” “estimate,” “forecast,” “goal,” “project,” and other words of similar me ani ng. These forward - looking statements address various matters including information concerning the timing of our commercial development and launch plans with respect to our products and product candidates; information concerning the commercial opportunity for UPNEEQ, including our ability to build an addressable patient audience for UPNEEQ and our estimates regarding the proportion of the U.S. population that self identifies as having droopy eyelid; and information concerning our commercial strategy for UPNEEQ, including planned enhancements to our sales and marketing efforts and our focus on the aesthetic market and consumer activation. Each forward - looking statement contained in this presentation is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statement. Applicable risks and uncertainties include, among others: that our estimates regarding the commercial opportunity and addressable patient audience for UPNEEQ are inaccurate; that the intended effects of UPNEEQ are not experienced by patients or consumers; that we are unable to realize our planned sales and marketing enhancements in order execute on our commercial strategy for UPNEEQ, as well as the other factors that are described in the “Risk Factors” section of our Annual Report on Form 10 - K for the year ended December 31, 2019, our Quarterly Reports on Form 10 - Q for the quarters ended March 31, 2020 and June 30, 2020, and in our other filings with the Securities and Exchange or Commission . We caution investors not to place considerable reliance on the forward - looking statements contained in this presentation. You are encouraged to read our filings with the SEC, available at www.sec.gov , for a discussion of these and other risks and uncertainties. The forward - looking statements in this presentation speak only as of the date of this document, and we undertake no obligation to update or revise any of these statements. Our business is subject to substantial risks and uncertainties, including those referenced above. Investors, potential investors, and others should give careful consideration to these risks and uncertainties . 1

• 2020 was a transformational year for Osmotica highlighted by the approval of Upneeq®, the first and only FDA - approved treatment for acquired blepharoptosis in adults (“droopy eyelid”) • US market introduction in Q320 with expanded launch underway across eye care specialties • Executed Santen licensing partnership in Japan, China, other Asian countries, and the EMEA which marks the beginning of the globalization of Upneeq • Osmotica’s in - line business comprising generics and non - promoted brands performed well throughout 2020 with strong uninterrupted manufacturing supply and customer service despite COVID - 19 pandemic • Recent Type - A meeting with the FDA regarding Arbaclofen ER (Phase III candidate for treatment of spasticity associated with Multiple Sclerosis) was constructive and the Company is evaluating a number of options • Strategic Review initiated 4Q20 is underway and robust 2 Corporate Update

Upneeq® Investor Update March 2021

Upneeq: The First & Only Approved Treatment for Droopy Eyelid With Large Aesthetic Potential Administration 1 One drop administered topically to the ptotic eye(s) once daily Patients should not touch the tip of the single patient - use container to their eye or to any surface, in order to avoid eye injury or contamination of the solution 1* Description 1 Oxymetazoline hydrochloride ophthalmic solution, 0.1% Preservative - free; formulated with a balanced salt solution and the hydrophilic polymer hypromellose – Hypromellose crosslinks upon contact with the surface of the eye, acting to coat and protect the ocular surface 2 Indication 1 Upneeq is indicated for the treatment of acquired blepharoptosis (“droopy eyelid”) in adults * Patients should discard the single patient - use container immediately after dosing; contact lenses should be removed prior to installation of Upneeq and may be reinserted 15 minutes after administration; if more than one topical ophthalmic drug is being used, the drugs should be administered at least 15 minutes before applications. 1. Upneeq Œ (oxymetazoline hydrochloride ophthalmic solution), 0.1%. RVL Pharmaceuticals, Inc. 2020. 2. Larson T. Artificial tears: a primer. Available at: http://webeye.ophth.uiowa.edu/eyeforum/tutorials/artificial - tears.htm. Ac cessed October 22, 2020. November 23, 2016. BEFORE AFTER Upneeq: the drop that lifts 4

Ptosis is a Common Disorder of the Upper Eyelid and is Typically Age - Related and Progressive 1. Forman WM, Leatherbarrow B, Sridharan GV, Tallis RC. A community survey of ptosis of the eyelid and pupil size of elderly people. Age Ageing. 1995;24:21 - 24. 2. Hashemi H, Khabazkhoob M, Emamian MH, et al. The prevalence of ptosis in an Iranian adult population. J Curr Ophthalmol . 2016;28:142 - 145. 3. Kim MH, Cho J, Zhao D, et al. Prevalence and associated factors of blepharoptosis in Korean adult population: the Korea Na tio nal Health and Nutrition Examination Survey. Eye ( Lond ). 2017;31:940 - 946. 4. US Census Bureau, Population Division. 2017 National Population Projections Tables: Detailed age and sex composition of th e p opulation, 2017 - 2060. Available at: https://www.census.gov/data/tables/2017/demo/popproj/2017 - summary - tables.html. Accessed Sept ember 1, 2020. 5. Richards, HS, Jenkinson E, Rumsey N, et al. The psychological well - being and appearance concerns of patients presenting with ptosis. Eye. 2014;28(3):296 - 302. 6. Finsterer J. Ptosis: causes, presentation, and management. Aesthetic Plast Surg. 2003;27:193 - 204. unilateral ptosis and avoiding contralateral ptosis. Aesthet Surg J. 2010;30:320 - 328. in the US ≥40 years of age are at risk for developing acquired blepharoptosis . 1,2 Millions of People … and prevalence increases with age 1 - 3 The US ‘silver tsunami’ 4 means a growing number of patients who may develop age - related ptosis † 50 65 80 95 110 125 140 2020 2025 2030 2035 People (millions) 35 - 64 years old 0 - 19 years old 20 - 34 years old ≥65 years old 135 million 86 million 70 million 79 million By 2035: Ptosis affects individuals of all ages… Aesthetic 5 Medical 5,6 Cosmetic concerns “Sleepy” or “bored” appearance Asymmetrical ocular appearance Visual disturbance Visual field deficits Neck pain Brow ache Headaches MRD - 1 (mm) Mild - to - moderate ptosis (with little functional impairment) often goes undiagnosed and untreated due to limited treatment options Both functional and non - functional patients may benefit from treatment Normal Mild Moderate Severe 5.0 0.0 4.0 2.0 5
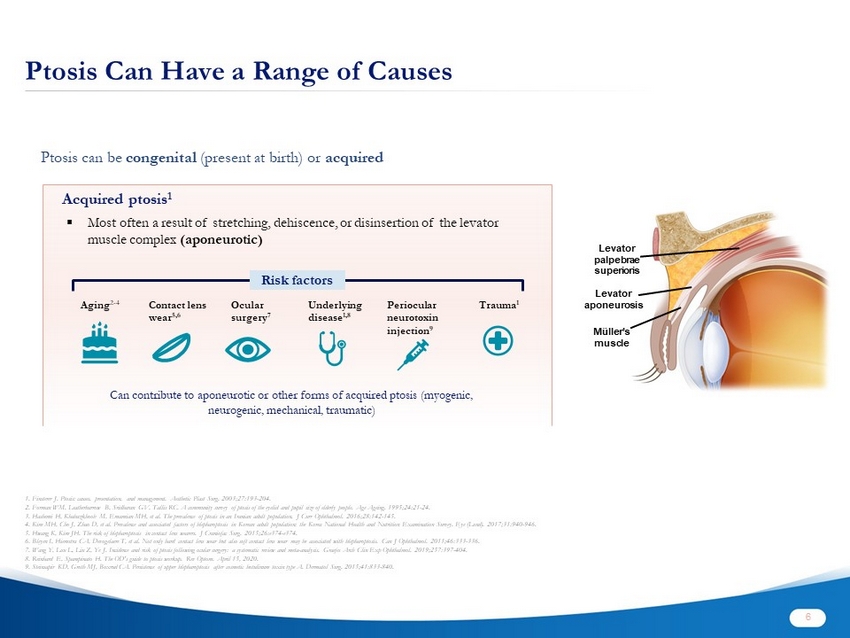
Ptosis Can Have a Range of Causes 1. Finsterer J. Ptosis: causes, presentation, and management. Aesthetic Plast Surg. 2003;27:193 - 204. 2. Forman WM, Leatherbarrow B, Sridharan GV, Tallis RC. A community survey of ptosis of the eyelid and pupil size of elderly people. Age Ageing. 1995;24:21 - 24. 3. Hashemi H, Khabazkhoob M, Emamian MH, et al. The prevalence of ptosis in an Iranian adult population. J Curr Ophthalmol . 2016;28:142 - 145. 4. Kim MH, Cho J, Zhao D, et al. Prevalence and associated factors of blepharoptosis in Korean adult population: the Korea Na tio nal Health and Nutrition Examination Survey. Eye ( Lond ). 2017;31:940 - 946. 5. Hwang K, Kim JH. The risk of blepharoptosis in contact lens wearers. J Craniofac Surg. 2015;26:e374 - e374. 6. Bleyen I, Hiemstra CA, Devogelaere T, et al. Not only hard contact lens wear but also soft contact lens wear may be associated with blepharoptosis. Can J Ophthalmol . 2011;46:333 - 336. 7. Wang Y, Lou L, Liu Z, Ye J. Incidence and risk of ptosis following ocular surgery: a systematic review and meta - analysis. Graefes Arch Clin Exp Ophthalmol . 2019;257:397 - 404. 8. Reinhard E, Spampinato H. The OD's guide to ptosis workup. Rev Optom . April 15, 2020. 9. Steinsapir KD, Groth MJ, Boxrud CA. Persistence of upper blepharoptosis after cosmetic botulinum toxin type A. Dermatol Surg. 2015;41:833 - 840. Acquired ptosis 1 ▪ Most often a result of stretching, dehiscence, or disinsertion of the levator muscle complex (aponeurotic) Ptosis can be congenital (present at birth) or acquired Levator palpebrae superioris Levator aponeurosis Müller’s muscle Aging 2 - 4 Ocular surgery 7 Contact lens wear 5,6 Underlying disease 1,8 Periocular neurotoxin injection 9 Trauma 1 Risk factors Can contribute to aponeurotic or other forms of acquired ptosis (myogenic, neurogenic, mechanical, traumatic) 6

0 0.5 1 1.5 2 2.5 Day 1 (6 hours post-instillation) Day 14 (2 hours post-instillation) ** ** Mean (SD) change from baseline MRD - 1 Upneeq ® Acts at Müller’s Muscle to Raise the Upper Eyelid * Receptor binding affinity is defined via in vitro binding assays. ** Pooled data from two 6 - week, randomized, double - masked, placebo controlled clinical trials; p<0.0001 vs. vehicle, from an ANC OVA model with study and treatment as fixed factors and baseline score as a covariate. Patients with congenital ptosis, Horn er syndrome, myasthenia gravis, mechanical ptosis, pseudoptosis or substantial dermatochalasis (redundant eyelid skin occurring wit hin 3 mm of the upper eyelid margin), or visual field loss from any cause other than ptosis were excluded from both trials. 1. Haenisch B, Walstab J, Herberhold S, et al. Alpha - adrenoceptor agonistic activity of oxymetazoline and xylometazoline. Fundam Clin Pharmacol . 2010;24(6):729 - 739. 2. Sugden D, Anwar N, Klein D. Rat pineal α1 - adrenoceptor subtypes: studies using radioligand binding and reverse transcription - polymerase chain reaction analysis. Br J Pharmacol . 1996;118(5):1246 - 1252. 3. Hosten LO, Snyder C. Over - the - counter ocular decongestants in the United States - mechanisms of action and clinical utility for manage ment of ocular redness. Clin Optom . 2020;12:95 - 105. 4. Esmaeli - Gutstein B, Hewlett B, Pashby R, Oestreicher J, Harvey J. Distribution of adrenergic receptor subtypes in the retractor muscles of the upper eyelid. Ophthalmic Plast Reconstr Surg. 1999;15(2):92 – 99. 5. Skibell BC, Harvey JH, Oestreicher JH, et al. Adrenergic receptors in the ptotic human eyelid: correlation with phenylephrine testing and surgical success in pt os is repair. Ophthalmic Plast Reconstr Surg. 2007;23:367 - 371. 6. Park SJ, Jang SY, Baek JS, et al. Distribution of adrenergic receptor subtypes and responses to topical 0.5% apraclonidine in patients with blepharo pt osis. Ophthalmic Plast Reconstr Surg. 2018;34:547 - 551. 7. Slonim CB, Foster S, Jaros M, et al. Association of oxymetazoline hydrochloride, 0.1%, solution administration with visual field in acquired ptosis: a p oo led analysis of 2 randomized clinical trials. JAMA Ophthalmol . 2020;Epub ahead of print. The active ingredient oxymetazoline: Is a potent, direct - acting α - adrenergic receptor agonist 1,2 Has ≈5 - fold greater affinity for α 2 receptors 3 * Targets receptors in Müller’s muscle , causing contraction and raising the eyelid Levator palpebrae superioris ↑ β 1 adrenergic receptor expression 4 Levator aponeurosis Müller’s muscle ↑ α 1/2 adrenergic receptor expression 4 - 6 Selectively activates receptors in Müller’s muscle Upneeq (n=203) Vehicle (n=101) Marginal Reflex Distance 1 (MRD - 1) is a measure of upper eyelid lift In two clinical trials, once - daily Upneeq significantly increased upper eyelid lift 1 MRD - 1 Average lift with Upneeq: 1 mm 7 (max. ≥3.5 mm on both days) 7

Upneeq ® Provides a Rapid Effect and Improves Field of Vision * Data from 6 - week, randomized, double - masked, placebo - controlled clinical trial (RVL - 1201 - 202); p<0.05 vs. vehicle, from an ANC OVA model with treatment as a fixed factor and baseline score as a covariate. * *Pooled data from two 6 - week, randomized, double - masked, placebo controlled clinical trials; p<0.0001 vs. vehicle, from an ANC OVA model with study and treatment as fixed factors and baseline score as a covariate. 1. Osmotica data on file, 2019. 2. Slonim CB, Foster S, Jaros M, et al. Association of oxymetazoline hydrochloride, 0.1%, solution administration with visual field in acquired ptosis: a p oo led analysis of 2 randomized clinical trials. JAMA Ophthalmol . 2020; Epub ahead of print. 0.0 0.5 1.0 1.5 2.0 2.5 Mean (SD) change from baseline in MRD - 1 (mm) * Upneeq (n=109) Vehicle (n=55) 5 min 15 min 2 hours 6 hours 5 min 15 min 2 hours 6 hours 5 min 15 min * * * * * * * * * Day 1 Day 14 Day 42 In its second pivotal clinical trial, once - daily Upneeq significantly increased upper eyelid lift compared to vehicle as soon as 5 minutes post - instillation 1 (x 40, y −10) (x −40, y −5) (x −50, y 50) (x 50, y 10) Inferior visual field (14 points) Superior visual field (35 points) The Leicester Peripheral Field Test (LPFT) measures the superior visual field 0 2 4 6 8 10 12 14 Day 1 (6 hours post-instillation) Day 14 (2 hours post-instillation) ** * * Mean (SD) change from baseline in points seen in top 4 rows on the LPFT In two clinical trials, once - daily Upneeq significantly improved superior visual field deficits 2 Upneeq (n=203) Vehicle (n=101) In clinical trials, Upneeq’s safety was comparable to placebo and caused “no discomfort” in 95.5% and 92.0% of subjects using it for 6 and 12 weeks, respectively 8

Launch Strategy & Current Progress — Upneeq Stage 1 Stage 2 Stage 3 Stage 4 • KOL/Market Development to drive awareness, uptake and advocacy • Execute UP (“Uncovering Ptosis”) early experience program with leading ECPs demonstrating PoC and Brand Potential • Introduce RVL Pharma, RVL Pharmacy, and develop sales relationships with eye care customers • Expand reach and frequency across eye care • Enhance relationships with key accounts with value - share programs • Initiate dermatology/aesthetics channel planning and KOL development • Explore additional clinical studies that may support broader consumer opportunity (aesthetic, QoL , etc.), expand labeling and / or OTC pathway • Optimize eye care footprint • Scaled - up DTC marketing investments • Advance clinical studies that will support broader consumer opportunity (aesthetic, QoL, etc.) • Finalize preparation for dermatology/aesthetics launch • Build depth of prescribing within optimized ECP footprint • Full - scale DTC marketing campaign • Launch into dermatology channel; office - dispensing • Begin leveraging additional clinical data to bolster messaging for broader audience t - time <1M > 50M (1) Controlled launch with ~65 territories, 650 ECP’s; small fraction of ptosis population presenting to eye care The Company believes a significant proportion of the US female population is estimated to self - identify as having droopy eyelid, but haven’t discussed with an HCP 1. Company estimate of addressable patient audience COMPLETE Q4 2020 – Q2 2021 2H 2021 + 9

First in Class Commercial Model 10 Rich Engagement with Providers & Patients • Independent subsidiary d edicated to the commercialization of Upneeq • Marketing • Sales • Medical / R&D • Supply Chain • Shared services from Osmotica • Key advantages: • Flexibility to form unique partnerships with prescribers • HCP dispensed optionality • Removes traditional barriers to access for both providers and patients • Private / cash pay free from insurance constraints • Wholly owned pharmacy dedicated to the distribution of Upneeq • Exclusive access point for all Upneeq prescriptions • Compatible with the modern consumer lifestyle (e.g., convenient home delivery) • Removes cost adding intermediaries such as PBMs, wholesalers, retail pharmacies • Consistent and seamless experience from Rx to fulfillment for providers and patients • Additional patient discounting flexibility for refill /auto - refill • Flexibility to meet future demands (i.e., telemedicine) • Direct line of sight into key factors like duration of use, abandonment, and prescriber behavior RVL PHARMACEUTICALS RVL PHARMACY

Upneeq ® Launch x Validated market opportunity through real world patient and prescriber early experience Executed early experience program targeting <5% of eye care to test adapt, learn, and design tactics prior to full - scale rollout ( new product, new indication) Overwhelming enthusiasm from eye care providers (“ECPs”) and patients upon learning about and experiencing Upneeq Diagnosing ptosis is a new treatment paradigm that necessitates a change in behavior Establishing a sound foundation of medical safety and efficacy within eye care setting, which is critical to the consumerization of Upneeq Focus on accelerating growth across channels (eye care, consumer, aesthetics) Broadening field force footprint from ~60 territories covering <5,000 ECP’s to ~100 territories covering >13,000 ECP’s Initiating patient awareness campaign through digital media Ongoing medical education programs for ECPs Deploying value - share programs for HCPs (sell - through at office or loyalty/rewards) Preparing for expansion into dermatology and aesthetics (engaging with KoL advisors) Preparing for introduction of telemedicine functionality 11 Progress following Controlled Launch 0 500 1,000 1,500 2,000 2,500 3,000 3,500 Cumulative Prescribers (Launch - to - Date) (1) Adding >100 new writers weekly 1. Reflects prescribers submitting new prescriptions to RVL Pharmacy

Building Towards the Future Today Market Building Transformative Opportunity Lies in Upneeq’s Broad Utility & Appeal Eye Care: ~ 5,000 ECP’s Eye Care: ~ 55,000 ECPs Dermatology /Aesthetics: ~15,000 HCPs Consumers: >50 Million Today Ptosis is recognized by eye care experts as a condition that generally affects the elderly ▪ Perceived as a relatively common cosmetic nuisance that can escalate toward functional impairment Executing on step - wise approach that positions Upneeq for long term success ▪ Establish “buy - in” and medical foundation of efficacy and safety in eye care ▪ Expanding the breadth of prescribing specialties and increasing awareness paves way for consumer activation to accelerate growth 12

Ptosis Impacts a Patient’s Vision, Appearance and Overall Daily Well - Being 1. Ho SF, Morawski A, Sampath R, Burns J. Modified visual field test for ptosis surgery (Leicester Peripheral Field Test). Eye. 2011;25:365 - 369. 2. Meyer DR, Stern JH, Jarvis JM, Lininger LL. Evaluating the visual field effects of blepharoptosis using automated static p eri metry. Ophthalmology. 1993;100:651 - 658. 3. Alniemi ST, Pang NK, Woog JJ, Bradley EA. Comparison of automated and manual perimetry in patients with blepharoptosis. Ophthal Plast Reconstr Surg. 2013;29:361 - 363. 4. Fowler BT, Pegram TA, Cutler - Peck C, et al. Contrast sensitivity testing in functional ptosis and dermatochalasis surgery. Op hthalmic Plast Reconstr Surg. 2015;31(4):272 - 274. 5. Zheng X, Yamada H, Mitani A, et al. Improvement of visual function and ocular and systemic symptoms following blepharoptosis surgery. Orbit. 2020; Epub ahead of print. 6. Finsterer J. Ptosis: causes, presentation, and management. Aesthetic Plast Surg. 2003;27:193 - 204. 7. Zoumalan CI, Lisman RD. Evaluation and management of unilateral ptosis and avoiding contralateral ptosis. Aesthet Surg J. 2010;30:320 - 328. 8. Cahill KV, Bradley EA, Meyer DR, et al. Functional indications for upper eyelid ptosis and blepharoplasty surgery: a repor t b y the American Academy of Ophthalmology. Ophthalmol . 2011;118(12):2510 - 2517. 9. Richards, HS, Jenkinson E, Rumsey N, et al. The psychological well - being and appearance concerns of patients presenting with ptosis. Eye. 2014;28(3):296 - 302. 10. McKean - Cowdin R, Varma R, Wu J, et al. Severity of visual field loss and health - related quality of life. Am J Ophthalmol . 2007;143:1013 - 1023. Ptosis can have broad negative effects across a patient’s life: Visual function ▪ Eyelid droop can cause pupil obstruction, superior peripheral visual field deficits 1 - 3 ▪ Impairs vision quality by reducing contrast sensitivity and increasing higher - order aberrations 4,5 Appearance ▪ Asymmetric or sleepy look 6,7 Activities of daily living and psychosocial well - being ▪ Reduced independence, increased appearance - related distress, anxiety, and depression, similar to levels in patients with other appearance - altering ocular conditions (e.g., strabismus) 8 - 10 13
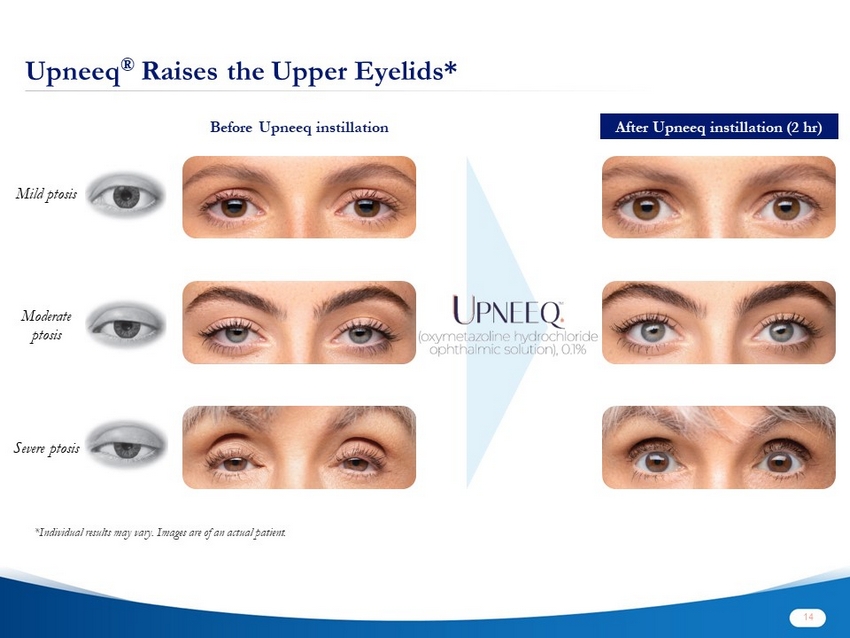
Upneeq ® Raises the Upper Eyelids* Before Upneeq instillation After Upneeq instillation (2 hr) Mild ptosis *Individual results may vary. Images are of an actual patient. Moderate ptosis Severe ptosis 14

Intellectual Property US Issued Method of Use (Expiry August 2031) US Issued Formulation (Expiry December 2039) Ptosis Aesthetic / Broad Treatment of ptosis with oxymetazoline without causing pupil dilation (any strength) Treatment of ptosis with 0.10% oxymetazoline (covers commercial formulation of RVL - 1201) Treatment with oxymetazoline to increase the vertical separation between eyelids without causing pupil dilation Latest Patent Expiry 2039 Latest Patent Expiry 2032 Additional IP protection in: Australia, Brazil, Canada, Korea, Mexico, New Zealand, Russia, Singapore, South Africa and Vietnam Aqueous pharmaceutically stable ophthalmic preservative - free comprising oxymetazoline 0.1% and other substances (1) Single use container comprising an aqueous pharmaceutically stable ophthalmic formulation containing oxymetazoline 0.1% and other substances (1) A n ophthalmic, sterile, preservative - free formulation comprising oxymetazoline 0.1% and other substances (1) (1) See issued patents 10,799,481 B1, 10,814,001 B1, and 10,898,573 B1 for further details Strong and growing IP portfolio with additional Orange Book patents pending 15
