Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - LUMOS PHARMA, INC. | lumo-2020q4x8kxex991.htm |
| 8-K - 8-K - LUMOS PHARMA, INC. | lumo-2020q4erx8k.htm |

Full Year 2020 Financial Results and Corporate Update March 9, 2021

This presentation contains forward-looking statements of Lumos Pharma, Inc. that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation are forward-looking statements, within the meaning of The Private Securities Litigation Reform Act of 1995. The words "anticipate," "believe," "estimate," "expect," "intend," "may," "plan," "target," "potential," "will," "could," "should," "seek" or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among others, statements regarding the potential of an orally administered LUM-201 treatment regimen for PGHD and other indications, the projected cash position and its sufficiency to fund the company’s operations through data read-out for the OraGrowtH210 Trial in PGHD and completion of the Pharmacokinetic / Pharmacodynamic OraGrowtH212 Trial in PGHD; expected initiation of the OraGrowtH212 Trial of LUM-201 in PGHD in Q2 2021; the intent to initiate Long-Term Extension OraGrowtH211 Trial; impact of regulatory feedback to clinical timelines and costs, results of its clinical trials for product candidates; its timing of release of data from ongoing clinical studies; its plans related to execution of clinical trials; plans related to moving additional indications into clinical development; milestones or other economic interests, Lumos Pharma’s financial guidance for 2021 and beyond; and any other statements other than statements of historical fact. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements that Lumos Pharma makes due to a number of important factors, including the effects of pandemics or other widespread health problems such as the ongoing COVID-19 pandemic and those risks discussed in "Risk Factors" and elsewhere in Lumos Pharma’s Annual Report on Form 10-K for the year ended December 31, 2019, Form 10-Q for the quarter ended September 30, 2020, and other reports filed with the U.S. Securities and Exchange Commission (SEC). The forward-looking statements in this presentation represent Lumos Pharma’s views as of the date of this presentation. Lumos Pharma anticipates that subsequent events and developments will cause its views to change. However, while it may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. You should, therefore, not rely on these forward-looking statements as representing Lumos Pharma’s views as of any date subsequent to the date of this presentation. 3.9.2021 Forward Looking Statements 2

3 Lumos Pharma Full Year 2020 Conference Call Agenda • Lisa Miller, Director of Investor Relations • Rick Hawkins, CEO • Eugene Kennedy, MD, Outgoing CMO • Carl Langren, CFO • John McKew, PhD, COO & CSO

Overview of Company 4 Novel therapeutic asset, LUM-201, with validating Phase 2b OraGrowtH210 Trial in Pediatric Growth Hormone Deficiency (PGHD) Late-stage Rare Disease Asset Initial indication targeted over $1B*, with potential to disrupt current treatment regimen for significant subset of patients across multiple indications Sizable Target Market Experienced management team with ability to expand pipeline through addition of other rare disease assets Experienced Management Cash balance of $98.7 million at end of Q4 2020 expected to support current operations through OraGrowtH210 Trial read-out anticipated mid-2022 Solid Cash Position Final PRV proceeds received January 2021 plus current cash balance also expected to contribute to expansion of the company’s portfolio of rare disease assets Pipeline Expansion * USA, Germany, France, Italy, Spain, UK, Japan (Grandview Research, Growth Hormone Market Forecast, 2019)

• LUM-201 Trials in PGHD - Phase 2b OraGrowtH210 Trial initiated Q4 2020 - PK/PD OraGrowtH212 Trial to be initiated shortly - Long-term Extension OraGrowtH211 Trial introduced • LUM-201 Opportunity Beyond PGHD - Potential to evaluate LUM-201 in other GHD indications treated by rhGH • Data Publications and Presentations Supportive of LUM-201 Potential in PGHD - Two peer-reviewed publications in the Journal of Endocrine Society, Feb 2021 - Data to be presented at ENDO 2021, March 2021 Clinical Development Highlights 5

• Final tranche of $26 million in proceeds from PRV sale received in January 2021 • PRV proceeds represent non-dilutive funds available for pipeline expansion • Continue pursuit of additional rare disease assets to broaden portfolio Corporate Update 6

PGHD • Inadequate secretion of growth hormone by pituitary gland during childhood • Hereditary or acquired, although majority of cases are idopathic • Lack of physical growth and numerous metabolic processes also affected • Incidence ≈ 1:3500 PGHD and Standard of Care 2 Rosenfeld 2008 Endocrine Practice 3 Cutfield 2011 PLOS ONE7 versus Robust, established market primed for an oral alternative SOC • Daily, subcutaneous injections of recombinant human growth hormone (rhGH) • Weekly injections may enter the market Need • Injections can be painful Missed doses and sub-optimal growth2,3 possible • Daily injections ≈2500 injections over years of treatment

• Oral LUM-201 is a growth hormone (GH) secretagogue • Acts as an agonist of GH Secretagogue Receptor (GHSR1a) to stimulate GH release1 • LUM-201 has been observed to increase the amplitude of endogenous pulsatile GH secretion2,3 • LUM-201’s stimulatory effect is regulated by GH/IGF-1 feedback LUM-201 Mechanism of Action 1 Howard 1996 Science 2 Nass 2008 Ann Intern Med 3 Chapman 1997 J Clin Endocrinol Metab8 Pituitary IGF-1 LUM- 201 Hypothalamus G H S R 1 a G H S R 1 a GH SST GHRH SST somatostatin GHRH growth hormone-releasing hormone IGF-1 insulin-like growth factor-1 GHSR1a GH secretagogue receptor 1a

Targeted PGHD Population HP-GH hypothalamic-pituitary-growth hormone 1 Blum, et al, Corroboration of Height Velocity Prediction Markers for rhGH with an Oral GH Secretagogue Treatment with Children with GHD, Journal of Endocrine Society, Feb 2021. bvab029, https://doi.org/10.1210/jendso/bvab0299 PEM-Positive: Included PEM-Negative: Excluded IGF-1 Pituitary LUM- 201 Hypothalamus G H S R 1 a G H S R 1 a GH SST GHRH Predictive Enrichment Markers (PEMs): GH response to single LUM-201 dose and baseline IGF-1 have potential to distinguish these populations • Functional but reduced HP-GH axis - Able to secrete some, but insufficient, GH - Expected to respond to LUM-201 - Represents ~60% of PGHD patients1 IGF-1 • Non-functional HP-GH axis - Unable to secrete GH - Not expected to respond to LUM-201 - Represents ~40% of PGHD patients Pituitary LUM- 201 Hypothalamus G H S R 1 a G H S R 1 a GH SST GHRH

• Journal of the Endocrine Society Publications, February 2021 - Development of a Predictive Enrichment Marker for Oral GH Secretagogue LUM-201 in Children with Growth Hormone Deficiency, by Bright, G., et al; bvab029, https://doi.org/10.1210/jendso/bvab029 - Corroboration Between Predictive Enrichment Markers for Height Velocity to rhGH and an Oral GH Secretagogue Treatment in Children with Moderate GHD, by Blum, W., et al; bvab030, https://doi.org/10.1210/jendso/bvab030 • ENDO 2021 Poster to be Presented, March 2021 - LUM-201 Elicits Greater GH Response than Standard GH Secretagogues in Pediatric Growth Hormone Deficiency, by Bright, G., et al; (Abstract 7102) Journal Publications and ENDO 2021 Presentation 10

Main Objectives for OraGrowth210 Trial: • Prospectively confirm utility of PEM strategy • Confirm repeatability of PEM classification • Determine optimal dose for Phase 3 trial OraGrowtH210 Trial Sites: • 40-50 trial sites US & International • Academic centers & private clinics OraGrowtH210 Trial Read-Out: • Anticipated mid-2022 11 Generate safety and efficacy data to move on to Phase 3 study 80 randomized PEM Positive PGHD patients Treatment naive, age matched cohorts, 6-month dosing 20- LUM-201: 0.8 mg/kg/day 20- LUM-201: 1.6 mg/kg/day 20-LUM-201: 3.2 mg/kg/day 20 Daily rhGH treatment arm Primary outcome measure: Annualized Height Velocity (AHV) Anticipate OraGrowtH210 Trial data read-out mid-2022 OraGrowtH210 Trial in PGHD: Clinical Development Outline

• Dose response to 5.6 mg/kg PGHD dose equivalent* • PD plateau possible ≥ 2.8 mg/kg PGHD dose equivalent* PK/PD Response Supports Proposed Doses in PGHD Merck Study 001 in healthy adult subjects *Dose equivalence is based on AUC12 Pharmacokinetics Pharmacodynamics 0 2 4 6 8 0 20 40 60 80 Time (hr) P la s m a G H C o n c e n tr a ti o n ( n g /m l) 200 mg adult (5.6 mg/kg PGHD) 100 mg adult (2.8 mg/kg PGHD) 50 mg adult (1.4 mg/kg PGHD) 25 mg adult (0.7 mg/kg PGHD) 10 mg adult (0.3 mg/kg PGHD) 5 mg adult (0.1 mg/kg PGHD) Pbo 1 mg adult (0.03 mg/kg PGHD) 0 2 4 6 8 0 20 40 60 80 Time (hr) P la s m a G H C o n c e n tr a ti o n ( n g /m l) 200 mg adult (5.6 mg/kg PGHD) 100 mg adult (2.8 mg/kg PGHD) 50 mg adult (1.4 mg/kg PGHD) 25 mg adult (0.7 mg/kg PGHD) 10 mg adult (0.3 mg/kg PGHD) 5 mg adult (0.1 mg/kg PGHD) Pbo 1 mg adult (0.03 mg/kg PGHD) 0 6 12 18 24 0.1 1 10 100 1000 Time (hr) P la s m a L U M -2 0 1 C o n c e n tr a ti o n ( n g /m l)
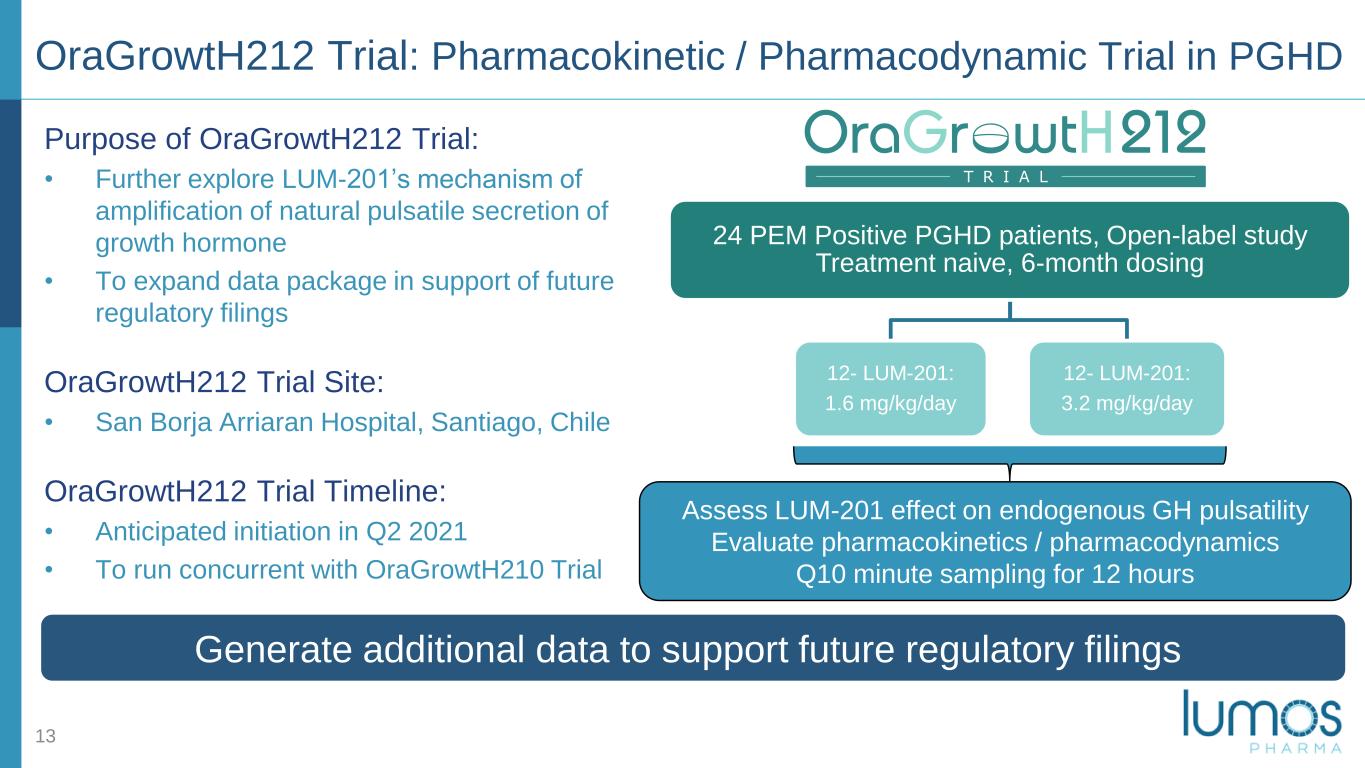
Purpose of OraGrowtH212 Trial: • Further explore LUM-201’s mechanism of amplification of natural pulsatile secretion of growth hormone • To expand data package in support of future regulatory filings OraGrowtH212 Trial Site: • San Borja Arriaran Hospital, Santiago, Chile OraGrowtH212 Trial Timeline: • Anticipated initiation in Q2 2021 • To run concurrent with OraGrowtH210 Trial 13 Generate additional data to support future regulatory filings 24 PEM Positive PGHD patients, Open-label study Treatment naive, 6-month dosing 12- LUM-201: 1.6 mg/kg/day 12- LUM-201: 3.2 mg/kg/day Assess LUM-201 effect on endogenous GH pulsatility Evaluate pharmacokinetics / pharmacodynamics Q10 minute sampling for 12 hours OraGrowtH212 Trial: Pharmacokinetic / Pharmacodynamic Trial in PGHD

LUM-201 Program Pipeline *In a subset of patients selected by Predictive Enrichment Markers14 Company plans to target other indications for LUM-201 beyond PGHD and pursue acquisitions and collaborations to expand pipeline beyond LUM-201 Pre-Clinical Phase 1 Phase 2 Phase 3 Status LUM-201 (Ibutamoren) in PGHD* Ongoing Phase 2b trial with data read-out expected mid-2022 Long-term extension study for OraGrowtH210, OraGrowtH212, and future OraGrowtH Trials Pharmacokinetic/Pharmacodynamic (PK/PD) trial expected to commence in Q2 2021

Metric Position Cash balance December 31, 2020 $98.7 million1 Additional non-dilutive resources Final tranche of $26 million proceeds from PRV sale received in January 2021 Projected cash use per quarter through 2021 ~ $8 to $9 million Shares outstanding as of December 31, 2020 ~ 8.3 million Secure Cash Position 1 Excludes final tranche proceeds of $26m, less fees, from PRV sale15 Cash balance plus additional PRV proceeds to support current operations through OraGrowtH210 Trial read-out, OraGrowtH212 Trial completion, and contribute to pipeline expansion

• Lead program, LUM-201, with potential to be the first oral growth hormone secretagogue therapy for PGHD • Opportunity to disrupt established and sizable injectable market in PGHD and other indications • Management team with extensive experience in the clinical advancement of rare disease therapeutics • Strong cash position with additional funds from PRV sale to support current operations through Phase 2b OraGrowtH210 Trial read-out mid- 2022 and contribute to pipeline expansion Lumos Pharma: Summary of Investment Thesis 16 Lumos Pharma (NASDAQ:LUMO) Transforming Lives with Rare Focus Potential to significantly increase shareholder value
