Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Equillium, Inc. | eq-8k_20210309.htm |

Harnessing Novel Immunobiology March 2021 www.equilliumbio.com Exhibit 99.1

Safe Harbor Statement This presentation contains forward-looking statements about Equillium, Inc. (the “Company”). In some cases, you can identify forward-looking statements by the words “will,” “expect,” “intend,” “plan,” “objective,” “believe,” “estimate,” “potential,” “continue” and “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. These statements are based on Company management’s current beliefs and expectations. These statements include but are not limited to statements regarding the Company’s business strategy, the Company’s plans to develop and commercialize its product candidates, the safety and efficacy of the Company’s product candidates, the Company’s plans and expected timing with respect to regulatory filings and approvals, size and growth potential of the markets for the Company’s product candidates and cash runway. These statements involve substantial known and unknown risks, uncertainties and other factors that may cause the Company’s actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. The Company may not actually achieve the plans, intentions or expectations disclosed in its forward-looking statements, and you should not place undue reliance on the Company’s forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed or implied in the forward-looking statements the Company makes due to the risks and uncertainties inherent in the Company’s business, including without limitation, risk described in the Company’s filings with the Securities and Exchange Commission (“SEC”). You are cautioned not to place undue reliance on these forward-looking statements, which represent the Company’s views as of the date of this presentation. The Company’s anticipates that subsequent events and developments will cause the its views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company has no current intention of doing so except to the extent required by applicable law. These and other risks and uncertainties are described more fully under the caption “Risk Factors” and elsewhere in the Company’s filings and reports, which may be accessed for free by visiting EDGAR on the SEC web site at http://www.sec.gov. and on the Company’s website under the heading “Investors.” All forward-looking statements are qualified in their entirety by this cautionary statement. This caution is made under the “safe harbor” provisions of Section 21E of the Private Securities Litigation Reform Act of 1995. 2
Our mission is to dramatically improve the lives of patients through the development of novel immunotherapies Leaders in CD6 biology developing itolizumab, a first-in-class anti-CD6 antibody targeting the CD6-ALCAM pathway Itolizumab is a validated therapeutic with differentiated mechanism of action (MoA) inhibiting the activity and trafficking of Teff We are advancing development in three severe autoimmune and inflammatory disorders Acute Graft-versus-Host Disease (aGVHD) Lupus/Lupus Nephritis Uncontrolled Asthma Novel Treatments for Severe Immuno-inflammatory Disorders 3
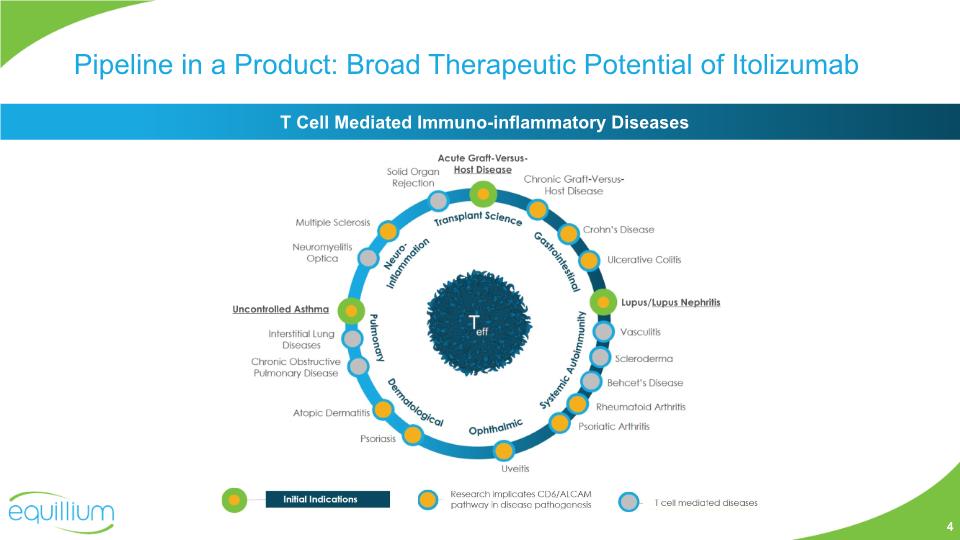
Pipeline in a Product: Broad Therapeutic Potential of Itolizumab T Cell Mediated Immuno-inflammatory Diseases 4

Accomplished Management Team Bruce Steel, CFA Chief Executive Officer Steve Connelly, PhD Chief Scientific Officer Jason Keyes Chief Financial Officer Dolca Thomas, MD EVP Research & Development, Chief Medical Officer Christine Zedelmayer Chief Operating Officer Joel Rothman Chief Development Officer 5
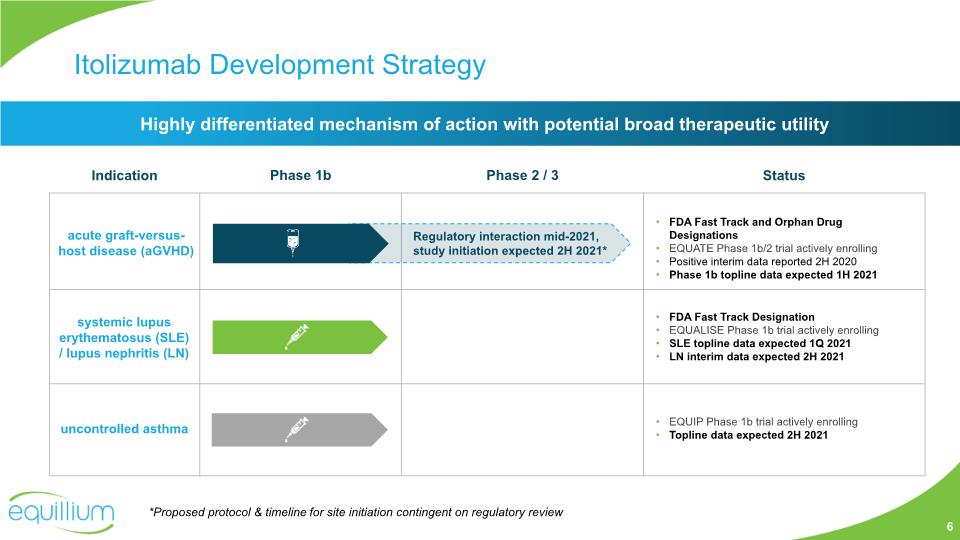
Highly differentiated mechanism of action with potential broad therapeutic utility Itolizumab Development Strategy 6

Strategic partnership with Biocon Limited (Biocon): exclusive rights to commercialize itolizumab in the U.S., Canada, Australia, New Zealand Includes commercial-scale manufacturing drug product supply agreement Three clinical development programs ongoing; initial data promising Strong cash balance and core institutional shareholders Complete proof-of-concept (PoC) for initial indications Accelerate development on path to approval in first-line aGVHD Broaden asset pipeline to complement itolizumab Potential to be first to market in first-line treatment of aGVHD Build Company through pipeline expansion Demonstrably improve patient lives by bringing novel immunotherapeutics to market Our Journey to Improve Patient Lives... 7

Itolizumab & CD6-ALCAM Pathway
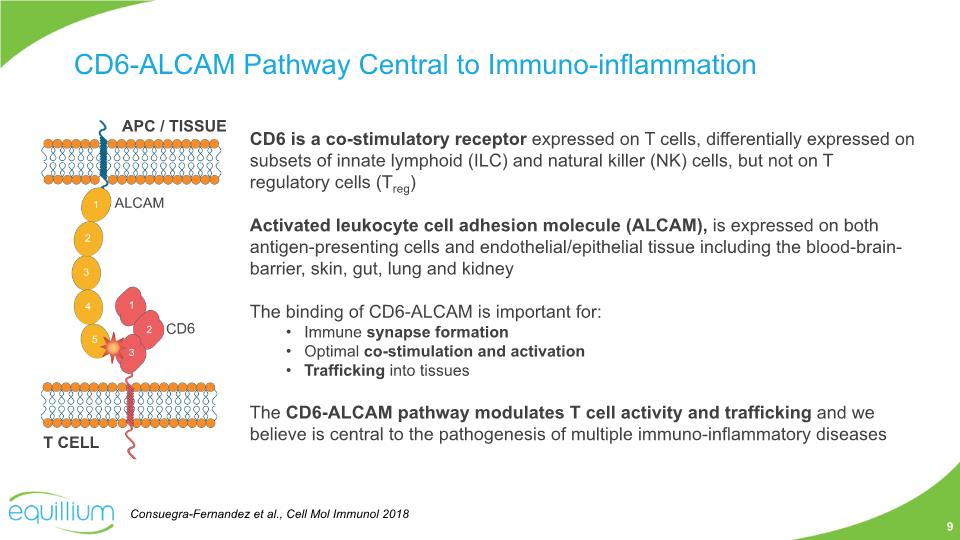
CD6 is a co-stimulatory receptor expressed on T cells, differentially expressed on subsets of innate lymphoid (ILC) and natural killer (NK) cells, but not on T regulatory cells (Treg) Activated leukocyte cell adhesion molecule (ALCAM), is expressed on both antigen-presenting cells and endothelial/epithelial tissue including the blood-brain-barrier, skin, gut, lung and kidney The binding of CD6-ALCAM is important for: Immune synapse formation Optimal co-stimulation and activation Trafficking into tissues The CD6-ALCAM pathway modulates T cell activity and trafficking and we believe is central to the pathogenesis of multiple immuno-inflammatory diseases CD6-ALCAM Pathway Central to Immuno-inflammation Consuegra-Fernandez et al., Cell Mol Immunol 2018 9
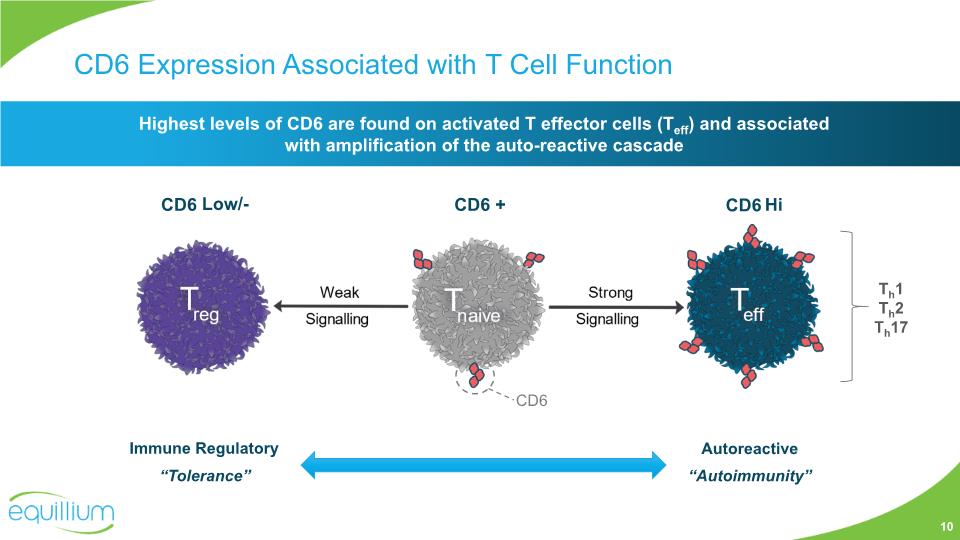
CD6 Expression Associated with T Cell Function Highest levels of CD6 are found on activated T effector cells (Teff) and associated with amplification of the auto-reactive cascade CD6 Low/- CD6 + CD6 Hi CD6 Th1 Th2 Th17 10
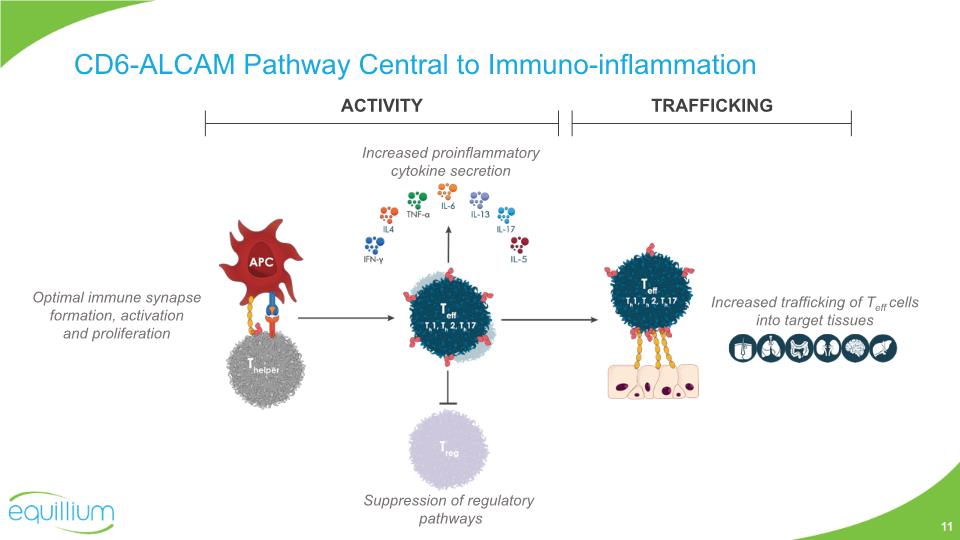
CD6-ALCAM Pathway Central to Immuno-inflammation ACTIVITY TRAFFICKING Increased proinflammatory cytokine secretion Suppression of regulatory pathways Increased trafficking of Teff cells into target tissues Optimal immune synapse formation, activation and proliferation 11
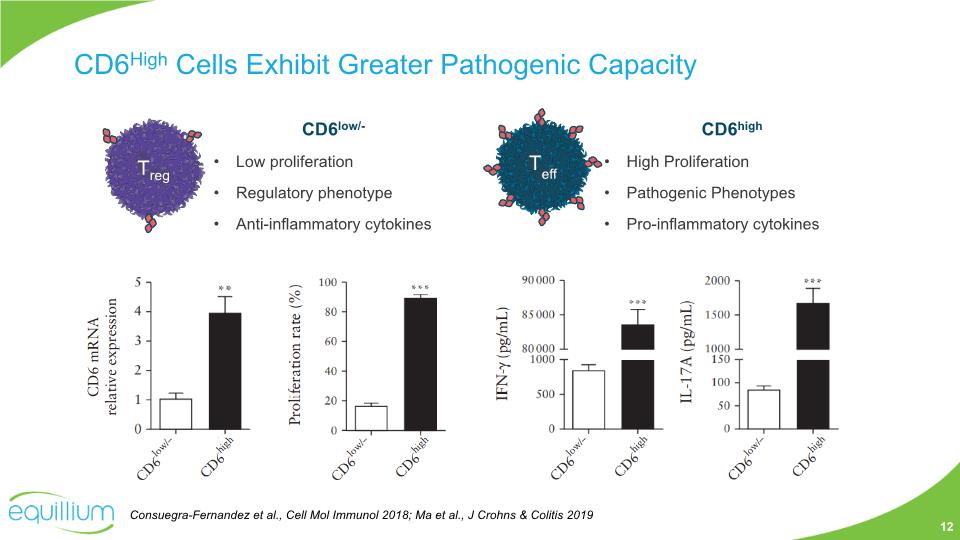
CD6high High Proliferation Pathogenic Phenotypes Pro-inflammatory cytokines CD6low/- Low proliferation Regulatory phenotype Anti-inflammatory cytokines CD6High Cells Exhibit Greater Pathogenic Capacity Consuegra-Fernandez et al., Cell Mol Immunol 2018; Ma et al., J Crohns & Colitis 2019 12
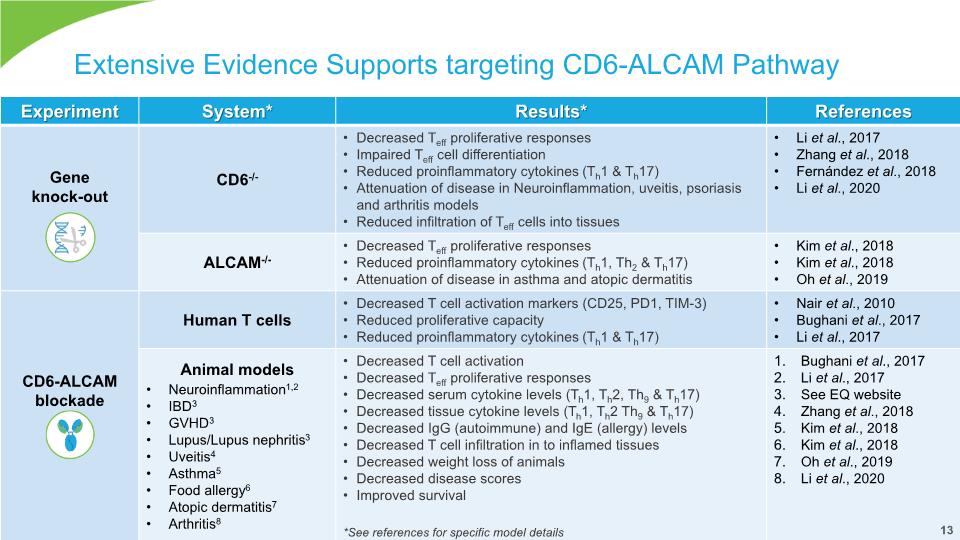
Extensive Evidence Supports targeting CD6-ALCAM Pathway 13 13
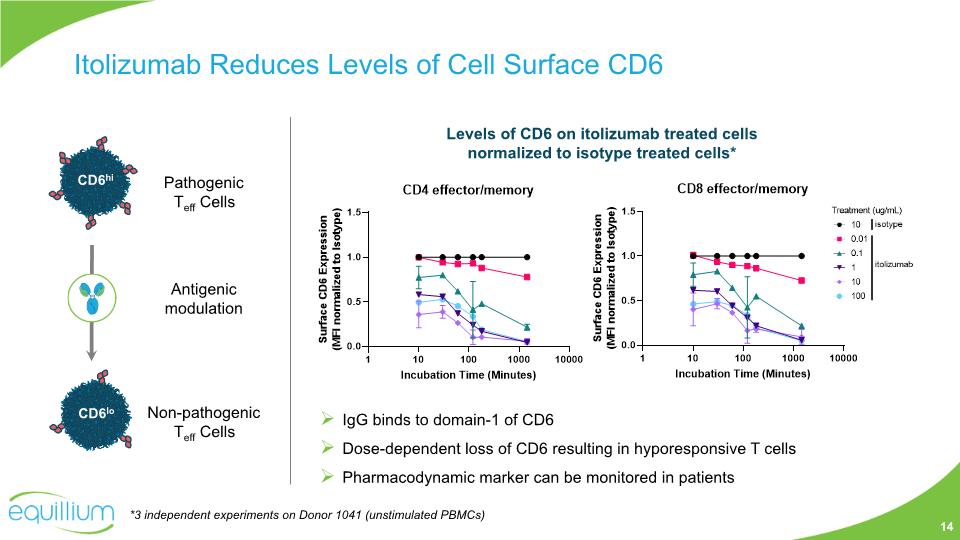
Itolizumab Reduces Levels of Cell Surface CD6 Levels of CD6 on itolizumab treated cells normalized to isotype treated cells* *3 independent experiments on Donor 1041 (unstimulated PBMCs) IgG binds to domain-1 of CD6 Dose-dependent loss of CD6 resulting in hyporesponsive T cells Pharmacodynamic marker can be monitored in patients Pathogenic Teff Cells Non-pathogenic Teff Cells Antigenic modulation 14
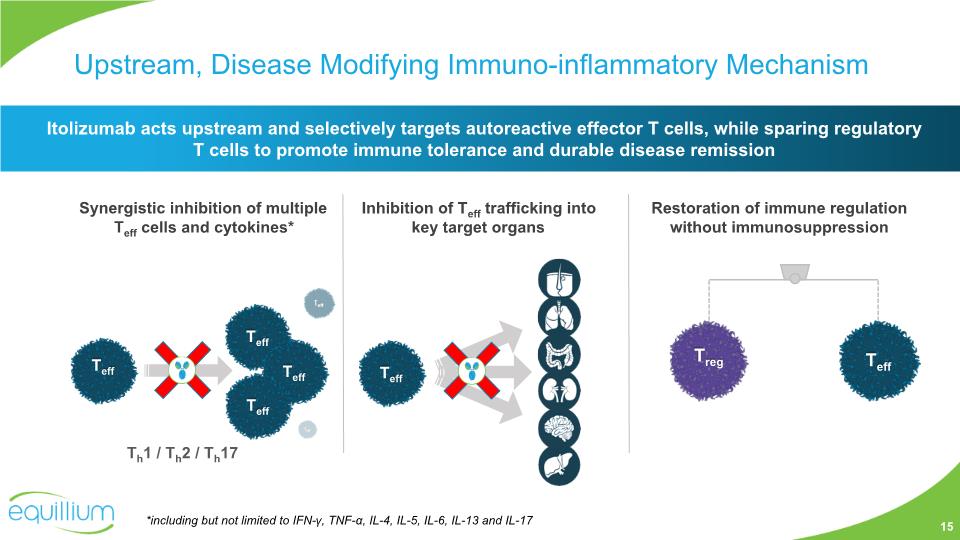
Upstream, Disease Modifying Immuno-inflammatory Mechanism Restoration of immune regulation without immunosuppression Inhibition of Teff trafficking into key target organs Synergistic inhibition of multiple Teff cells and cytokines* Itolizumab acts upstream and selectively targets autoreactive effector T cells, while sparing regulatory T cells to promote immune tolerance and durable disease remission *including but not limited to IFN-γ, TNF-α, IL-4, IL-5, IL-6, IL-13 and IL-17 Th1 / Th2 / Th17 15

Clinical Strategy
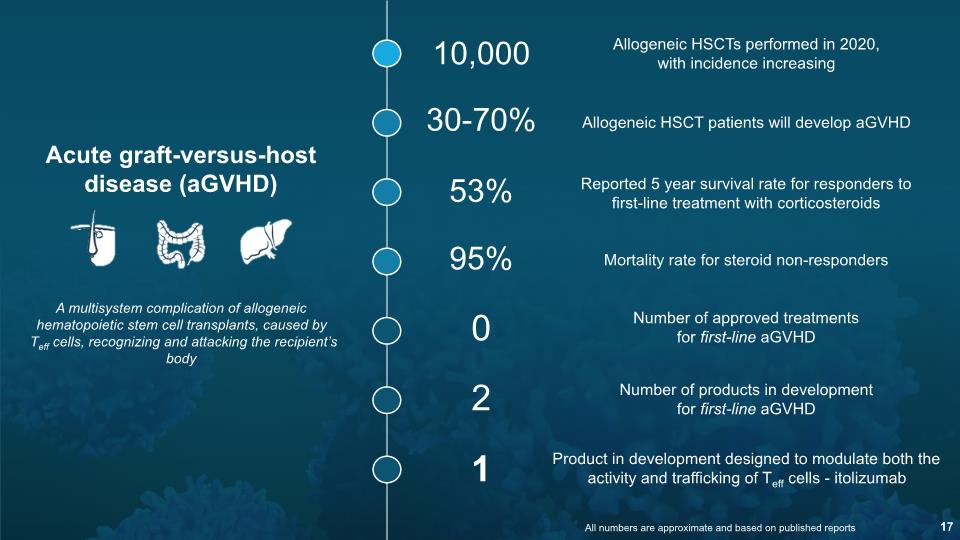
Acute graft-versus-host disease (aGVHD) 10,000 30-70% 53% 0 Allogeneic HSCTs performed in 2020, with incidence increasing Allogeneic HSCT patients will develop aGVHD Reported 5 year survival rate for responders to first-line treatment with corticosteroids Number of approved treatments for first-line aGVHD Number of products in development for first-line aGVHD 2 95% Mortality rate for steroid non-responders A multisystem complication of allogeneic hematopoietic stem cell transplants, caused by Teff cells, recognizing and attacking the recipient’s body Product in development designed to modulate both the activity and trafficking of Teff cells - itolizumab 1 All numbers are approximate and based on published reports 17
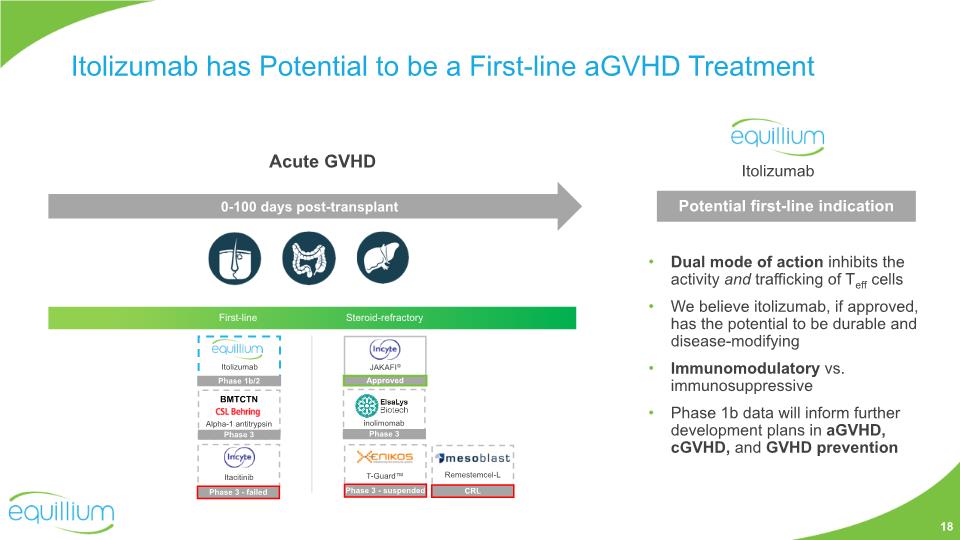
Itolizumab has Potential to be a First-line aGVHD Treatment JAKAFI® First-line Steroid-refractory Itolizumab Phase 1b/2 CRL Remestemcel-L Acute GVHD 0-100 days post-transplant Dual mode of action inhibits the activity and trafficking of Teff cells We believe itolizumab, if approved, has the potential to be durable and disease-modifying Immunomodulatory vs. immunosuppressive Phase 1b data will inform further development plans in aGVHD, cGVHD, and GVHD prevention Potential first-line indication Itolizumab Approved Itacitinib Phase 3 - failed Alpha-1 antitrypsin Phase 3 BMTCTN 18 inolimomab Phase 3

EQUATE Study for First-line Treatment in aGVHD Assess the safety and tolerability of IV dosing of itolizumab Determine optimal IV dose level(s) of itolizumab Primary Objectives Secondary Objectives Characterize the pharmacokinetics (PK) and pharmacodynamics (PD) of itolizumab Assess the clinical activity of itolizumab: GVHD ORR, NRM, cGVHD, durability Study Population: First-line treatment of newly diagnosed aGVHD patients Study Design Open-label, 3x3, dose escalation 5 doses, bi-weekly – intravenous (IV) Concomitant w/ steroids (2mg/kg) Topline Phase 1b data expected 1H 2021, informs further development strategy in aGHVD, cGVHD, and GVHD prevention Phase 1b N~24 (Grade III – IV / Grade II AA2 and 3) 19 Cohort 1 0.4 mg/kg Cohort 2 0.8 mg/kg Cohort 3 1.6 mg/kg Cohort 4 2.4 mg/kg
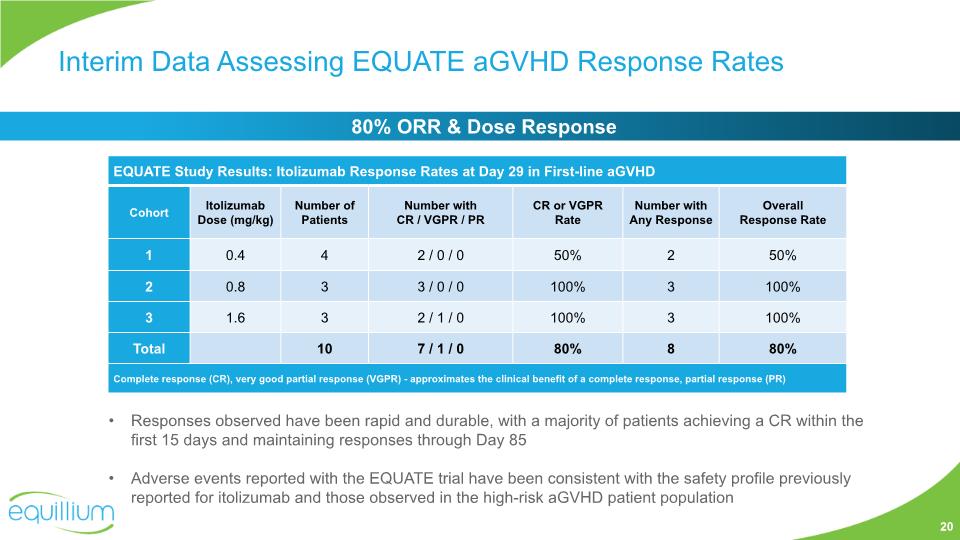
Interim Data Assessing EQUATE aGVHD Response Rates Responses observed have been rapid and durable, with a majority of patients achieving a CR within the first 15 days and maintaining responses through Day 85 Adverse events reported with the EQUATE trial have been consistent with the safety profile previously reported for itolizumab and those observed in the high-risk aGVHD patient population 20 80% ORR & Dose Response
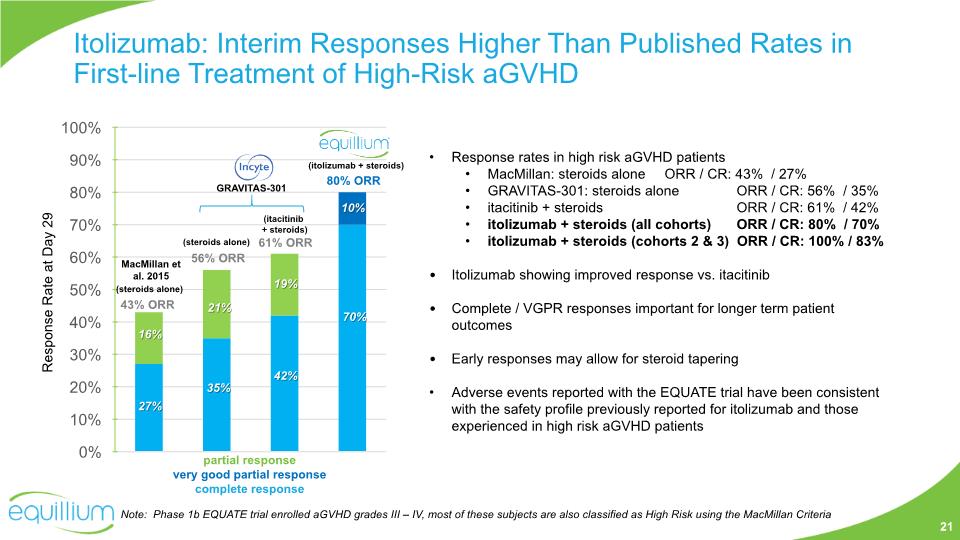
Itolizumab: Interim Responses Higher Than Published Rates in First-line Treatment of High-Risk aGVHD 80% ORR 61% ORR 27% 16% partial response very good partial response complete response Response Rate at Day 29 Response rates in high risk aGVHD patients MacMillan: steroids alone ORR / CR: 43% / 27% GRAVITAS-301: steroids alone ORR / CR: 56% / 35% itacitinib + steroids ORR / CR: 61% / 42% itolizumab + steroids (all cohorts) ORR / CR: 80% / 70% itolizumab + steroids (cohorts 2 & 3) ORR / CR: 100% / 83% Itolizumab showing improved response vs. itacitinib Complete / VGPR responses important for longer term patient outcomes Early responses may allow for steroid tapering Adverse events reported with the EQUATE trial have been consistent with the safety profile previously reported for itolizumab and those experienced in high risk aGVHD patients Note: Phase 1b EQUATE trial enrolled aGVHD grades III – IV, most of these subjects are also classified as High Risk using the MacMillan Criteria 42% 19% 70% 10% MacMillan et al. 2015 43% ORR (steroids alone) (itacitinib + steroids) (itolizumab + steroids) 35% 21% 56% ORR (steroids alone) GRAVITAS-301 21
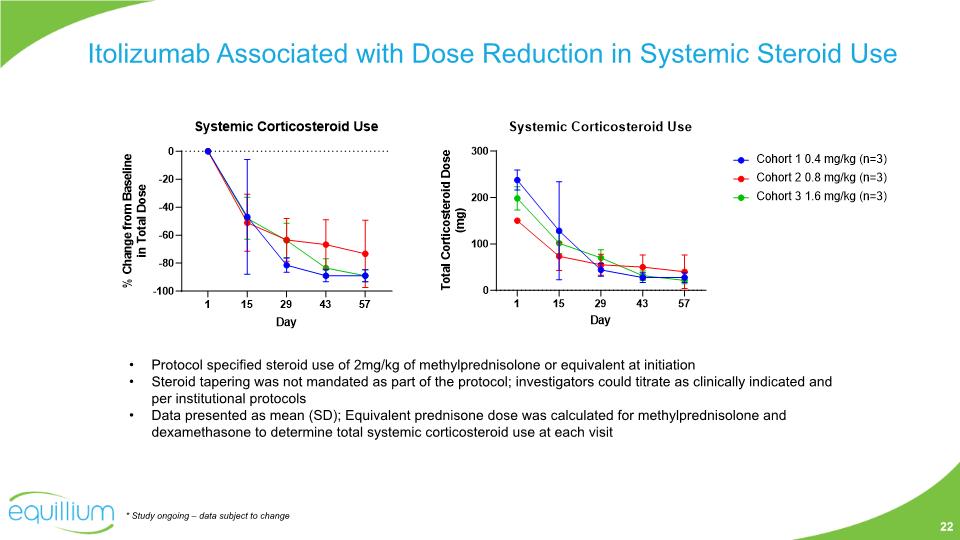
Protocol specified steroid use of 2mg/kg of methylprednisolone or equivalent at initiation Steroid tapering was not mandated as part of the protocol; investigators could titrate as clinically indicated and per institutional protocols Data presented as mean (SD); Equivalent prednisone dose was calculated for methylprednisolone and dexamethasone to determine total systemic corticosteroid use at each visit Itolizumab Associated with Dose Reduction in Systemic Steroid Use * Study ongoing – data subject to change 22
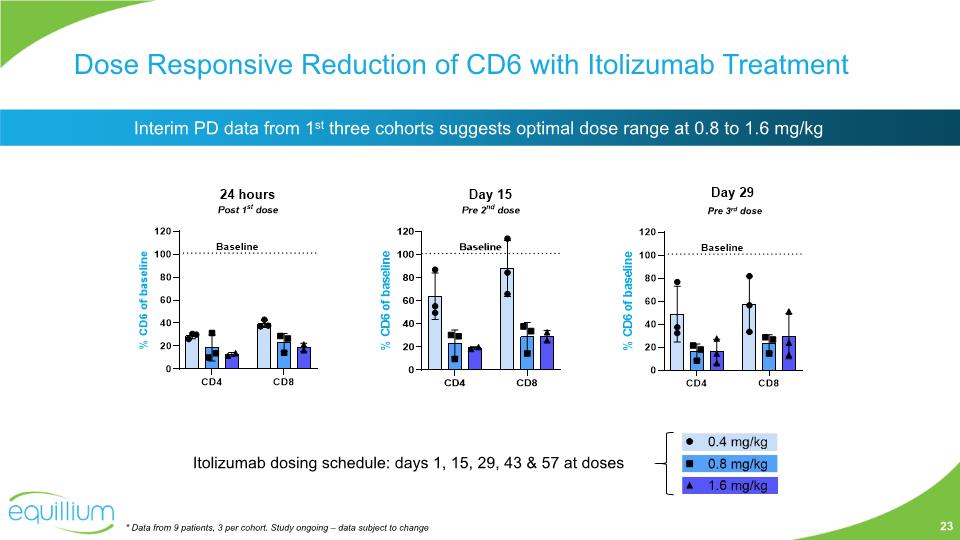
Dose Responsive Reduction of CD6 with Itolizumab Treatment * Data from 9 patients, 3 per cohort. Study ongoing – data subject to change Interim PD data from 1st three cohorts suggests optimal dose range at 0.8 to 1.6 mg/kg 23 Pre 3rd dose
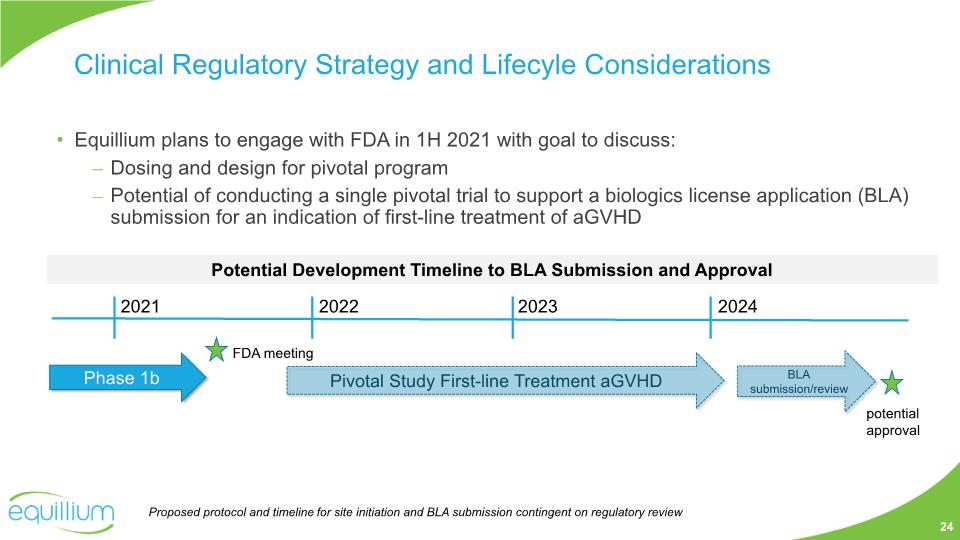
Equillium plans to engage with FDA in 1H 2021 with goal to discuss: Dosing and design for pivotal program Potential of conducting a single pivotal trial to support a biologics license application (BLA) submission for an indication of first-line treatment of aGVHD Potential Development Timeline to BLA Submission and Approval 2021 2022 2023 Phase 1b FDA meeting Pivotal Study First-line Treatment aGVHD 2024 BLA submission/review potential approval Clinical Regulatory Strategy and Lifecyle Considerations 24 Proposed protocol and timeline for site initiation and BLA submission contingent on regulatory review

Lupus nephritis 100,000 50%-75% 40% 2 13 1 A heterogeneous disease that is the most frequent and serious manifestation of systemic lupus erythematosus (SLE) All numbers are approximate and based on published reports Product in development designed to modulate both the activity and trafficking of Teff cells - itolizumab 25
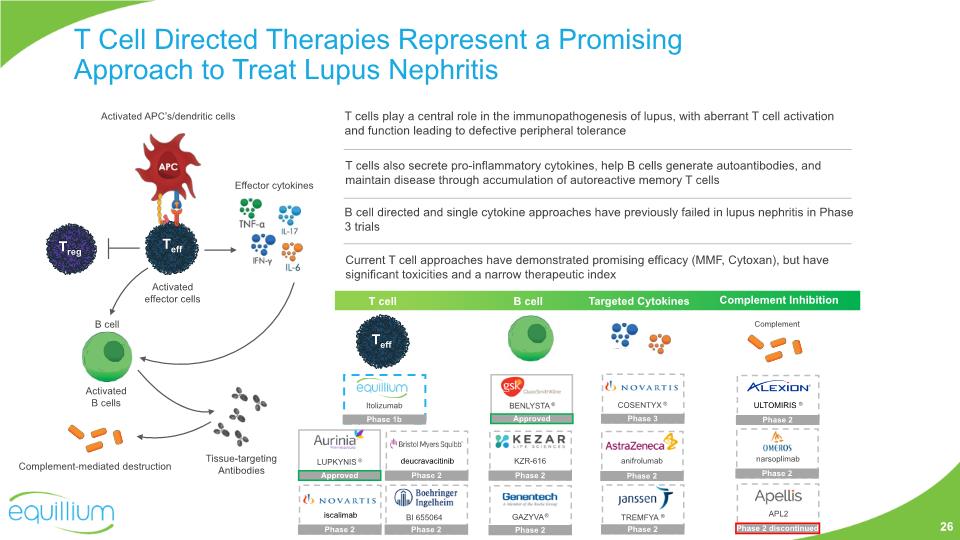
LUPKYNIS ® Teff Current T cell approaches have demonstrated promising efficacy (MMF, Cytoxan), but have significant toxicities and a narrow therapeutic index B cell directed and single cytokine approaches have previously failed in lupus nephritis in Phase 3 trials B cell T cells also secrete pro-inflammatory cytokines, help B cells generate autoantibodies, and maintain disease through accumulation of autoreactive memory T cells Targeted Cytokines T cell B cell Complement Inhibition Complement T cells play a central role in the immunopathogenesis of lupus, with aberrant T cell activation and function leading to defective peripheral tolerance Itolizumab Phase 1b Teff GAZYVA® BENLYSTA® KZR-616 Phase 2 Approved Phase 2 anifrolumab Phase 2 APL2 Phase 2 discontinued Treg T Cell Directed Therapies Represent a Promising Approach to Treat Lupus Nephritis narsoplimab Phase 2 Complement-mediated destruction Activated APC’s/dendritic cells Tissue-targeting Antibodies Activated effector cells Activated B cells Effector cytokines 26 Approved COSENTYX ® Phase 3 TREMFYA ® Phase 2 deucravacitinib Phase 2 iscalimab Phase 2 ULTOMIRIS ® Phase 2
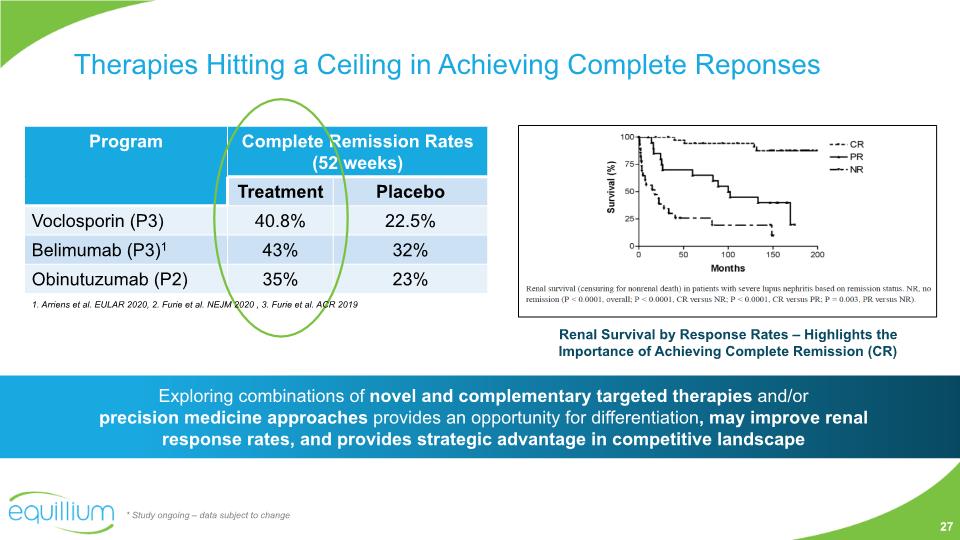
Therapies Hitting a Ceiling in Achieving Complete Reponses Renal Survival by Response Rates – Highlights the Importance of Achieving Complete Remission (CR) * Study ongoing – data subject to change Exploring combinations of novel and complementary targeted therapies and/or precision medicine approaches provides an opportunity for differentiation, may improve renal response rates, and provides strategic advantage in competitive landscape 1. Arriens et al. EULAR 2020, 2. Furie et al. NEJM 2020 , 3. Furie et al. ACR 2019 27
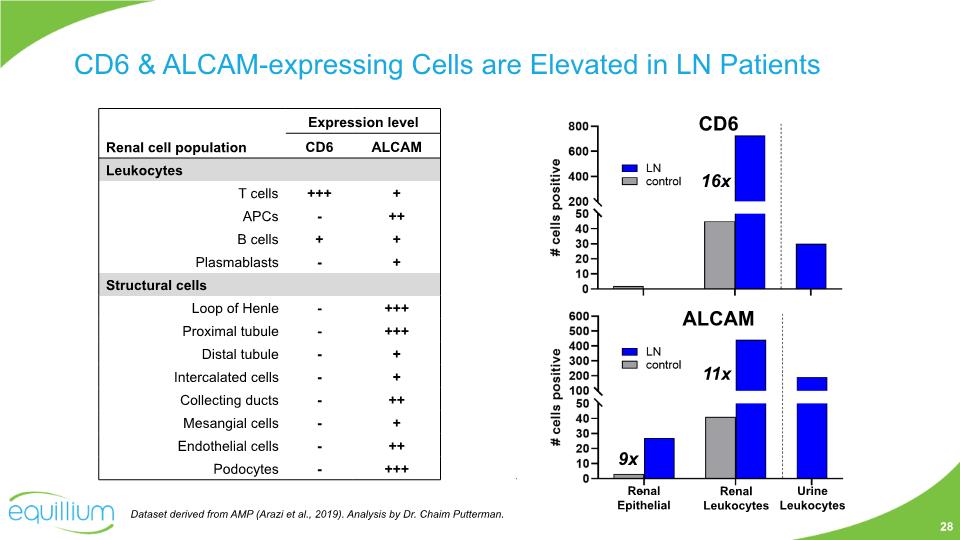
CD6 & ALCAM-expressing Cells are Elevated in LN Patients Renal Epithelial Renal Leukocytes Urine Leukocytes CD6 ALCAM 9x 16x 11x Dataset derived from AMP (Arazi et al., 2019). Analysis by Dr. Chaim Putterman. 28
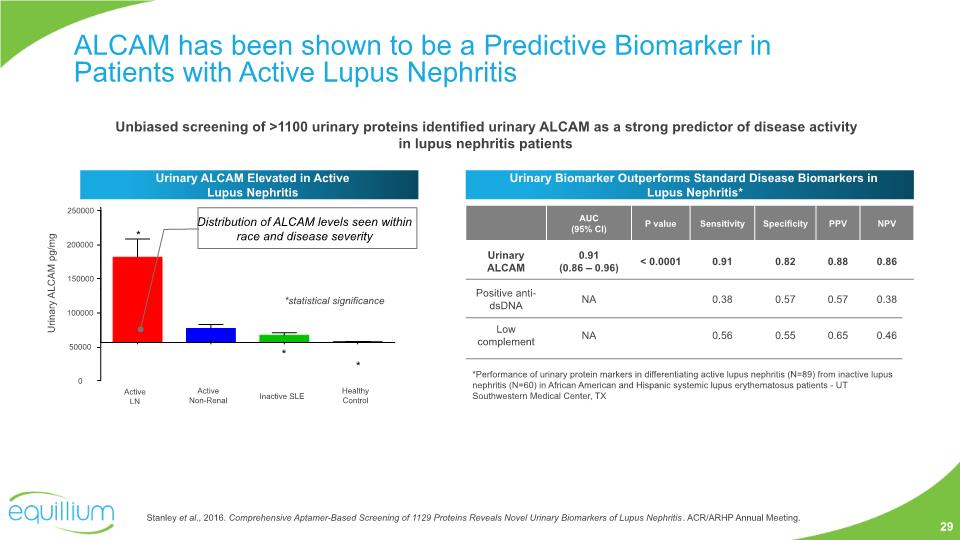
Stanley et al., 2016. Comprehensive Aptamer-Based Screening of 1129 Proteins Reveals Novel Urinary Biomarkers of Lupus Nephritis. ACR/ARHP Annual Meeting. ALCAM has been shown to be a Predictive Biomarker in Patients with Active Lupus Nephritis Unbiased screening of >1100 urinary proteins identified urinary ALCAM as a strong predictor of disease activity in lupus nephritis patients Urinary ALCAM Elevated in Active Lupus Nephritis Urinary Biomarker Outperforms Standard Disease Biomarkers in Lupus Nephritis* *Performance of urinary protein markers in differentiating active lupus nephritis (N=89) from inactive lupus nephritis (N=60) in African American and Hispanic systemic lupus erythematosus patients - UT Southwestern Medical Center, TX Urinary ALCAM pg/mg * * * * Distribution of ALCAM levels seen within race and disease severity 29
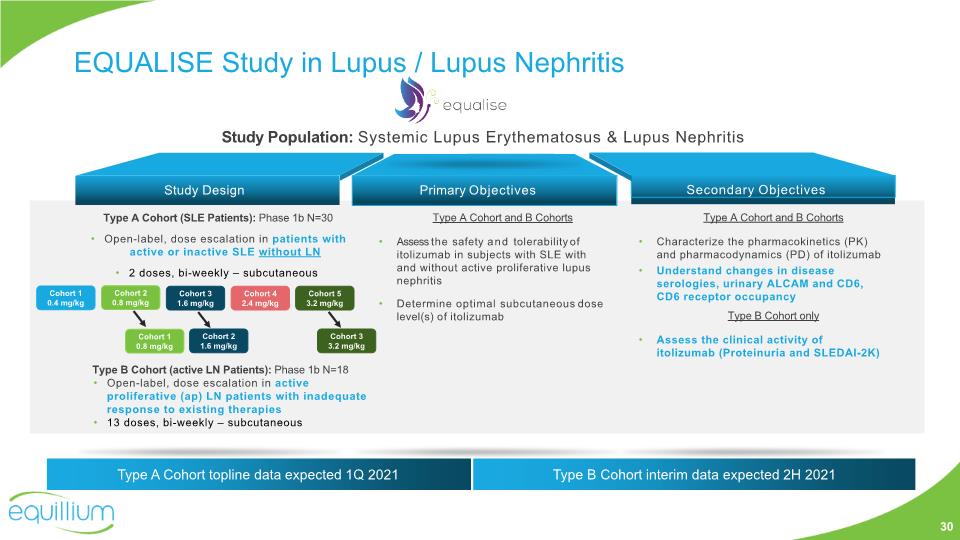
Type A Cohort and B Cohorts Assess the safety and tolerability of itolizumab in subjects with SLE with and without active proliferative lupus nephritis Determine optimal subcutaneous dose level(s) of itolizumab Secondary Objectives Type A Cohort and B Cohorts Characterize the pharmacokinetics (PK) and pharmacodynamics (PD) of itolizumab Understand changes in disease serologies, urinary ALCAM and CD6, CD6 receptor occupancy Type B Cohort only Assess the clinical activity of itolizumab (Proteinuria and SLEDAI-2K) Study Design Primary Objectives Type A Cohort (SLE Patients): Phase 1b N=30 Open-label, dose escalation in patients with active or inactive SLE without LN 2 doses, bi-weekly – subcutaneous Re-initiation planned for Q2/Q3 2020 Study Population: Systemic Lupus Erythematosus & Lupus Nephritis Cohort 1 0.4 mg/kg Cohort 2 0.8 mg/kg Cohort 3 1.6 mg/kg Cohort 4 2.4 mg/kg Cohort 1 0.8 mg/kg Cohort 2 1.6 mg/kg Type B Cohort (active LN Patients): Phase 1b N=18 Open-label, dose escalation in active proliferative (ap) LN patients with inadequate response to existing therapies 13 doses, bi-weekly – subcutaneous Cohort 5 3.2 mg/kg Cohort 3 3.2 mg/kg Type A Cohort topline data expected 1Q 2021 Type B Cohort interim data expected 2H 2021 EQUALISE Study in Lupus / Lupus Nephritis 30

Uncontrolled moderate to severe asthma 2.6M 1.3M ~50% 0 Severe asthma patients in the U.S. Patients uncontrolled by standard-of-care treatments (long-acting beta-agonists, inhaled corticosteroids, oral corticosteroids) Of uncontrolled patients do not respond to existing biologic treatments Approved products covering the full spectrum of disease (Th2 High to Non-Th2 asthma) Target categories in development that target Non-Th2 asthma 3 A heterogeneous disease characterized by different Teff cell subtypes and other innate immune cells driving both allergic and autoimmune mechanisms, leading to chronic airway inflammation 1 All numbers are approximate and based on published reports Product in development designed to modulate both the activity and trafficking of Teff cells - itolizumab 31
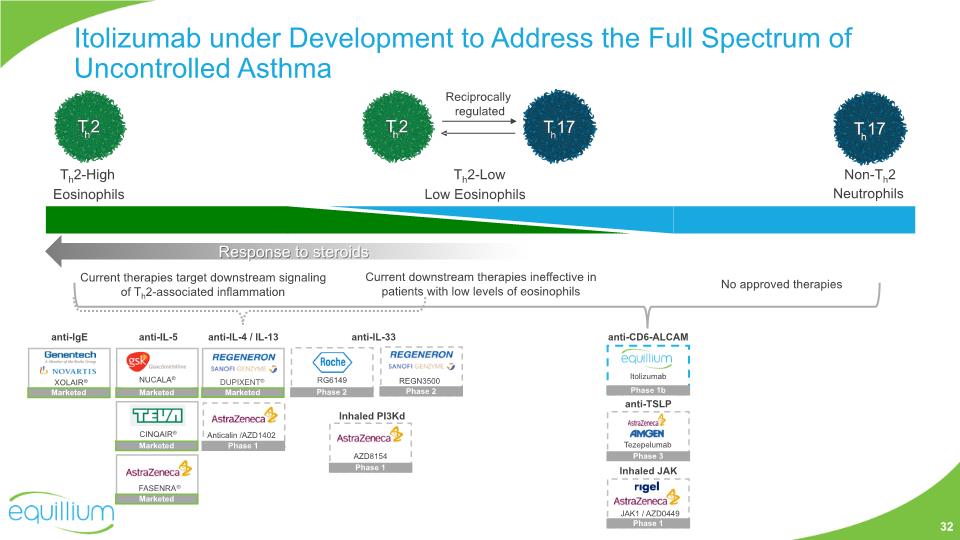
Itolizumab under Development to Address the Full Spectrum of Uncontrolled Asthma Current therapies target downstream signaling of Th2-associated inflammation Current downstream therapies ineffective in patients with low levels of eosinophils No approved therapies anti-TSLP anti-IgE anti-IL-5 CINQAIR® anti-IL-4 / IL-13 anti-IL-33 XOLAIR® FASENRA® DUPIXENT® RG6149 Tezepelumab Th2-High Eosinophils Non-Th2 Neutrophils Th2-Low Low Eosinophils Reciprocally regulated Response to steroids Marketed Marketed Marketed Marketed NUCALA® Marketed Phase 2 Phase 3 anti-CD6-ALCAM Itolizumab Phase 1b REGN3500 Phase 2 Inhaled JAK Anticalin /AZD1402 Phase 1 JAK1 / AZD0449 Phase 1 Inhaled PI3Kd AZD8154 Phase 1 32
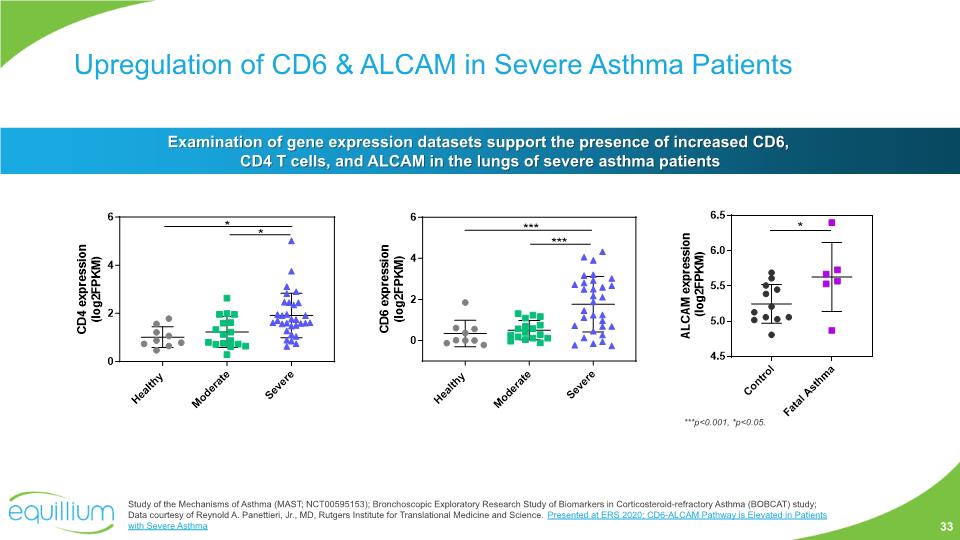
Upregulation of CD6 & ALCAM in Severe Asthma Patients ***p<0.001, *p<0.05. Examination of gene expression datasets support the presence of increased CD6, CD4 T cells, and ALCAM in the lungs of severe asthma patients Study of the Mechanisms of Asthma (MAST; NCT00595153); Bronchoscopic Exploratory Research Study of Biomarkers in Corticosteroid-refractory Asthma (BOBCAT) study; Data courtesy of Reynold A. Panettieri, Jr., MD, Rutgers Institute for Translational Medicine and Science. Presented at ERS 2020; CD6-ALCAM Pathway is Elevated in Patients with Severe Asthma 33
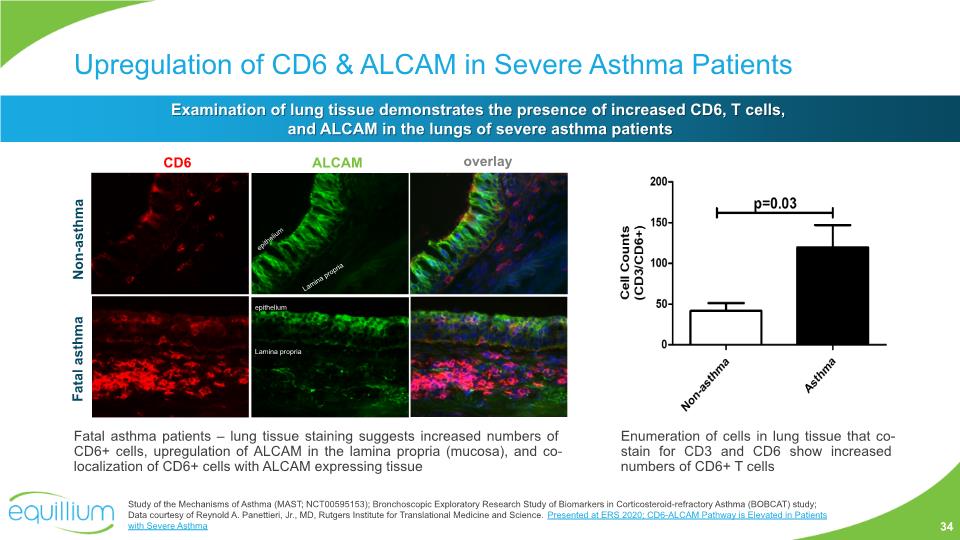
Upregulation of CD6 & ALCAM in Severe Asthma Patients Examination of lung tissue demonstrates the presence of increased CD6, T cells, and ALCAM in the lungs of severe asthma patients Fatal asthma patients – lung tissue staining suggests increased numbers of CD6+ cells, upregulation of ALCAM in the lamina propria (mucosa), and co-localization of CD6+ cells with ALCAM expressing tissue Study of the Mechanisms of Asthma (MAST; NCT00595153); Bronchoscopic Exploratory Research Study of Biomarkers in Corticosteroid-refractory Asthma (BOBCAT) study; Data courtesy of Reynold A. Panettieri, Jr., MD, Rutgers Institute for Translational Medicine and Science. Presented at ERS 2020; CD6-ALCAM Pathway is Elevated in Patients with Severe Asthma Enumeration of cells in lung tissue that co-stain for CD3 and CD6 show increased numbers of CD6+ T cells 34
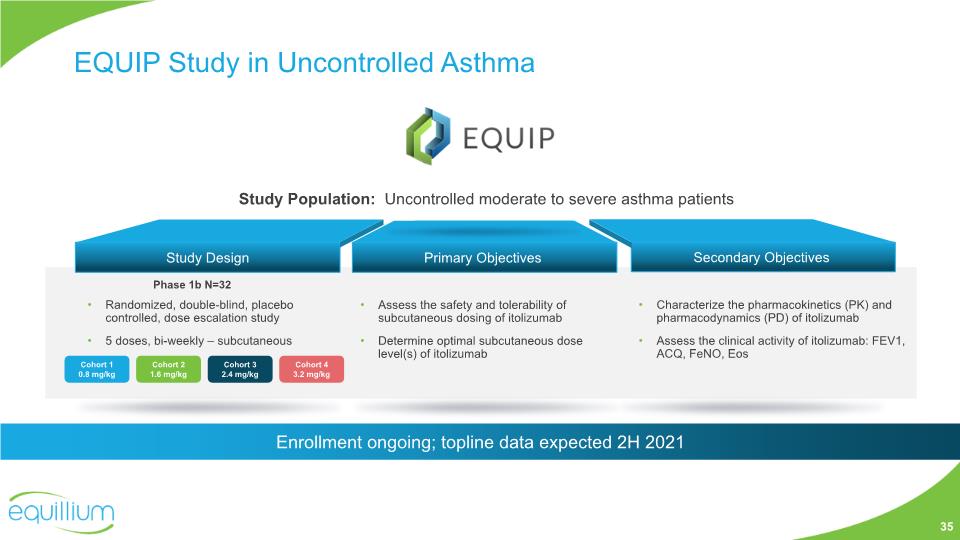
EQUIP Study in Uncontrolled Asthma Assess the safety and tolerability of subcutaneous dosing of itolizumab Determine optimal subcutaneous dose level(s) of itolizumab Primary Objectives Secondary Objectives Characterize the pharmacokinetics (PK) and pharmacodynamics (PD) of itolizumab Assess the clinical activity of itolizumab: FEV1, ACQ, FeNO, Eos Phase 1b N=32 Study Design Randomized, double-blind, placebo controlled, dose escalation study 5 doses, bi-weekly – subcutaneous Study Population: Uncontrolled moderate to severe asthma patients 35 Enrollment ongoing; topline data expected 2H 2021 Cohort 1 0.8 mg/kg Cohort 2 1.6 mg/kg Cohort 3 2.4 mg/kg Cohort 4 3.2 mg/kg

Corporate
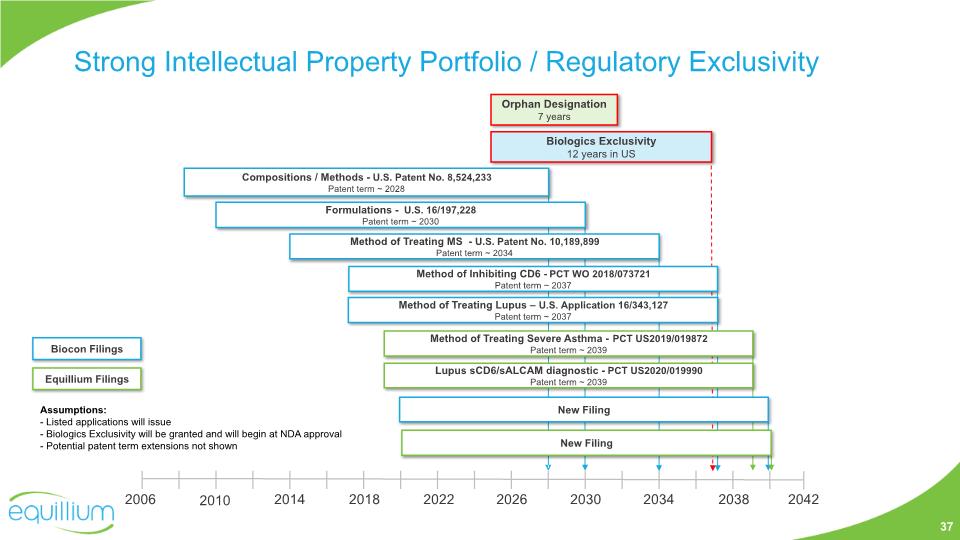
Strong Intellectual Property Portfolio / Regulatory Exclusivity Orphan Designation 7 years Biologics Exclusivity 12 years in US 2006 2014 2022 2030 2010 2018 2026 2034 2038 2042 Compositions / Methods - U.S. Patent No. 8,524,233 Patent term ~ 2028 Method of Treating Lupus – U.S. Application 16/343,127 Patent term ~ 2037 Method of Treating MS - U.S. Patent No. 10,189,899 Patent term ~ 2034 Formulations - U.S. 16/197,228 Patent term ~ 2030 Method of Treating Severe Asthma - PCT US2019/019872 Patent term ~ 2039 Lupus sCD6/sALCAM diagnostic - PCT US2020/019990 Patent term ~ 2039 New Filing Assumptions: - Listed applications will issue - Biologics Exclusivity will be granted and will begin at NDA approval - Potential patent term extensions not shown New Filing 37 Biocon Filings Equillium Filings Method of Inhibiting CD6 - PCT WO 2018/073721 Patent term ~ 2037
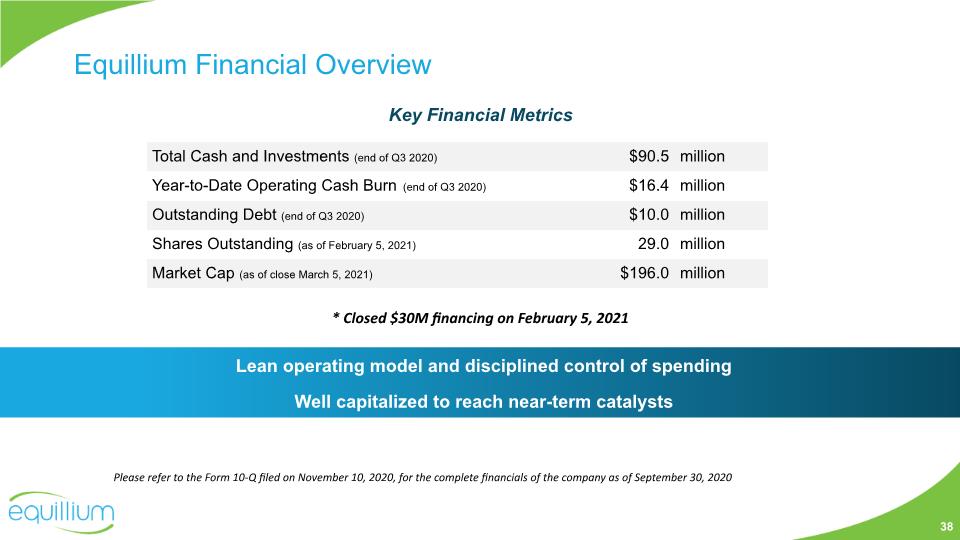
Equillium Financial Overview Lean operating model and disciplined control of spending Well capitalized to reach near-term catalysts Key Financial Metrics Please refer to the Form 10-Q filed on November 10, 2020, for the complete financials of the company as of September 30, 2020 38 * Closed $30M financing on February 5, 2021
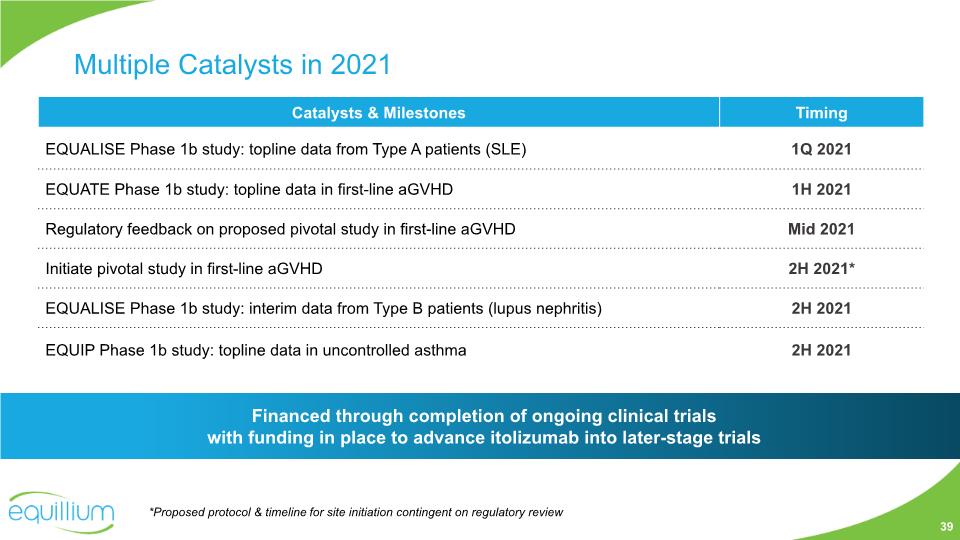
Multiple Catalysts in 2021 Financed through completion of ongoing clinical trials with funding in place to advance itolizumab into later-stage trials 39 *Proposed protocol & timeline for site initiation contingent on regulatory review

Equillium, Inc. 2223 Avenida de la Playa / Suite 105 La Jolla, CA 92037 www.equilliumbio.com ir@equilliumbio.com
