Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Enochian Biosciences Inc | e2498_8k.htm |
Exhibit 99.1

Enochian Biosciences Elegant Simplicity: Integrating Diverse Scientific Approaches to Develop Cures Dr. Mark Dybul, Executive Vice Chairman March 9-10, 2021 HC Wainwright Global Life Sciences Conference NASDAQ: ENOB

Forward-Looking Statements 2 Statements in this presentation that are not strictly historical in nature are forward-looking statements.Thesestatementsareonlypredictionsbasedoncurrentinformationandexpectations andinvolveanumberofrisksanduncertainties,includingbutnotlimitedtothesuccessorefficacy ofourpipeline.Allstatementsotherthanhistoricalfactsareforward-lookingstatements,whichcan beidentifiedbytheuseofforward-lookingterminologysuchas“believes,”plans,”“expects,”“aims,” “intends”orsimilarexpressions.Actualeventsorresultsmaydiffermateriallyfromthoseprojected inanyofsuchstatementsduetovariousuncertainties,includingassetforthinEnochian’smost recentAnnualReportonForm10-KfiledwiththeSEC.Readersarecautionednottoplaceundue relianceontheseforward-lookingstatements,whichspeakonlyasofthedatehereof.Allforward- looking statementsare qualified in their entirety by this cautionary statementand Enochian undertakesnoobligationtoreviseorupdatethispressreleasetoreflecteventsorcircumstances afterthedatehereof.

Strategy: Leverage Scientific Approaches to Create “Multiple Shots on Goal” & New Intellectual Property 3 Combining novel cell, gene and immunotherapy to build a diverse pipeline across and within diseases to allow for better business decisions to sell, sub-license, partner or manufacture and maximize patient impact and shareholder value. Develop pipeline of Hepatitis B (HBV) treatments/cures Develop pipeline of HIV treatments/cures Sell or sub-license to maximize impact and value Develop pipeline of oncology treatments/cures Enochian Grounding Breaking Science

Experienced Leadership: Management Team 4 Mark Dybul, MD Executive Vice Chair Luisa Puche Chief Financial Officer Tung Nguyen Director of Molecular Biology Lu Chen, PhD Director of Product Development & CMC Bios available at: www.enochianbio.com

Experienced Leadership: Board of Directors 5 Mark Dybul, MD Executive Vice Chair Rene Sindlev Chairman Evelyn D’An Director James Sapirstein, RPh, MBA Director Carol Brosgart, MD Director Gregg Alton, JD Director Carl Sandler Director Henrik Grønfeldt –Sørensen Director Bios available at: www.enochianbio.com

Deep Scientific Expertise: HIV Scientific Advisory Board 6 Strong expertise in the field of gene therapy, HIV and immuno-oncology focusing on developing transformative, multi-indication, platform drugs to change the standard of care of HIV/AIDS and cancer Bios available at: www.enochianbio.com Professor of Medicine in Residence, University of California, San Francisco; Faculty member, Division of HIV, Infectious Diseases and Global Medicine, Zuckerberg San Francisco General Hospital Steven Deeks, MD Executive Vice Chair; Professor, Department of Medicine, Georgetown University Medical Center; Faculty Co-Director of Center for Global Health and Quality Mark Dybul, MD Chairperson; Senior Director of Evidence-based Practices and HIV/primary care provider, Whitman- Walker Health; Adjunct Professor of Medicine, Johns Hopkins University School of Medicine David Hardy, MD Director, Stem Cell & Gene Therapy Program, Fred Hutchinson Cancer Research Center, Seattle, WA; Vice- Chairperson of the American Society for Gene and Cell Therapy and American Society of Hematology Hans Peter Kiem, MD, PhD

Deep Scientific Expertise: Hepatitis B (HBV) Scientific Advisory Board 7 Strong expertise in the field of HBV focusing on developing transformative therapies, or a cure, for HBV Bios available at: www.enochianbio.com Chairperson; Member of the Enochian BioSciences Board of Directors; Clinical Professor of Medicine, Biostatistics and Epidemiology, University of California, San Francisco Carol Brosgart, MD Clinical Professor of Medicine at Lyon University; Medical Director, Hepatology Department, Hospices Civils de Lyon; and Scientific Director, Department of Immunology and Virology, INSERM Unit 1052, France Fabien Zoulim, MD, PhD Senior Medical Scientist, Victorian Diseases Reference Laboratory, Royal Melbourne Hospital, Peter Doherty Institute for Infection and Immunity, Melbourne, Australia Peter Revill, PhD

Deep Scientific Expertise Strategic Research Collaborations Strong expertise in the field of gene therapy, HIV and immuno-oncology focusing on developing transformative, multi-indication, platform drugs to change the standard of care of HIV/AIDS and cancer Dong Sung An, MD, PhD Professor, UCLA, Los Angeles, CA KathrynBar,MD Assistant Professor and Director, Virus Reservoir Core of the Center for AIDS Research, University of Pennsylvania TimothyBlock,PhD Co-Founder & President, Hepatitis B Foundation & Baruch S. Blumberg Institute, Doylestown, PA Tae-WookChun,PhD Principal Investigator, NIAID, NIH, Bethesda, MD Philippe Gallay, PhD Professor of Immunology, the Scripps Research Institute, San Diego, CA Hans-Peter Kiem, MD, PhD Director, Stem Cell & Gene Therapy Program, Fred Hutchinson Cancer Research Center, Seattle, WA; Vice- Chairperson of the American Society for Gene and Cell Therapy and American Society of Hematology AnaJewett,PhD Professor & Director of Tumor Immunology, UCLA, Los Angeles, CA BiosAvailable At: www.Enochianbio.com 8

HIV, HBV & Oncology Pipeline 9 in vivo

HIV Program ENOB-HV-01: Autologous Human Peripheral Stem Cell Transplantation for Potential Cure of HIV ENOB-HV-11/12: Preventive (HV-11) and Therapeutic (HV-12)Vaccines ENOB-HV-31: “Hijacking” HIV RNA

Why Do We Need a Cure and Vaccine For HIV? 11 Short Term Market ~1.2 MILLION People living with HIV in the U.S. ~2.2 MILLION People living with HIV in Europe ~7.5 MILLION People living with HIV in South Africa ~40 Thousand Diagnosed with HIV in the U.S.in 2018 ~$36,000 to $48,000 Yearly cost of ART in the US in 2018 ~Half Trillion $ spent between 2000-2015 ~ $30 billion/year USA Antiretroviral Therapy (ART) • Standard of Care for HIV Today • Causes Bone and Kidney Damage ~38 MILLION Living with HIV worldwide in 2019 ~1.7 MILLION Infected with HIV worldwide in 2019 ~690 Thousand AIDS related deaths worldwide in 2019 HIV can be controlled with antiretroviral therapy or ART with taxing side effects & at significant cost to healthcare system; no effective cure currently exists for HIV/AIDS

Multiple Pathways Should Increase Likelihood of Successful HIV Product Candidates 12 Each pathway could be curative on its own, or be used in potent combination 1 2 3 ENOB-HV-01: To genetically modify autologous T cells to prevent HIV infection ENOB-HV-12: To boost the host immune system in order to seek, and kill HIV infected cells ENOB-HV-31: To cause apoptosis in HIV infected cells (cells “self-destruct”)
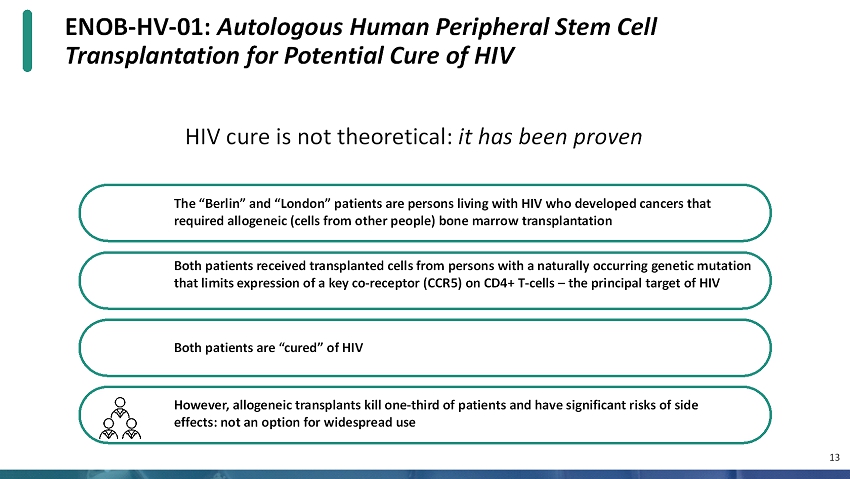
ENOB-HV-01: Autologous Human Peripheral Stem Cell Transplantation for Potential Cure of HIV 13 HIV cure is not theoretical: it has been proven The “Berlin” and “London” patients are persons living with HIV who developed cancers that required allogeneic (cells from other people) bone marrow transplantation Both patients received transplanted cells from persons with a naturally occurring genetic mutation that limits expression of a key co-receptor (CCR5) on CD4+ T-cells –the principal target of HIV Both patients are “cured” of HIV However, allogeneic transplants kill one-third of patients and have significant risks of side effects: not an option for widespread use
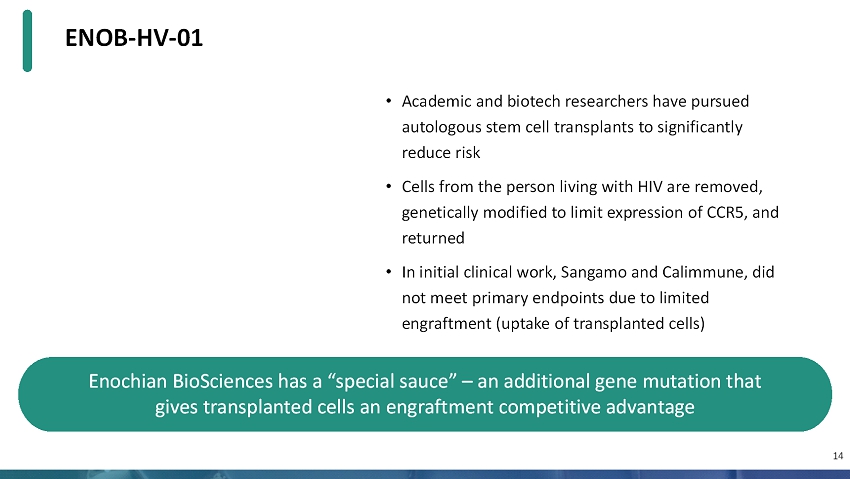
ENOB-HV-01 • Academic and biotech researchers have pursued autologous stem cell transplants to significantly reduce risk • Cells from the person living with HIV are removed, genetically modified to limit expression of CCR5, and returned • In initial clinical work, Sangamo and Calimmune, did not meet primary endpoints due to limited engraftment (uptake of transplanted cells) 14 Enochian BioSciences has a “special sauce” –an additional gene mutation that gives transplanted cells an engraftment competitive advantage

Summary of Key ENOB-HV-01 Results and Next Steps 15 • INTERACT meeting with FDA was held in June 2020 • In vivoincreased genetically modified cells in peripheral blood by 90% • In vivo163% increase in engrafted genetically modified cells in bone marrow For context: Studies of genetically modified cells in peripheral or bone marrow cells are generally considered to be successful with an increase of 10% • IND-enabling in vitroand in vivostudies are underway • Potentially beginning studies in humans in 2022 Next Steps Results

ENOB-HV-11/12: Preventative (11) & Therapeutic (12) Vaccines 16 PreventivevaccinesareforHIVuninfectedpeople–thepotentialmarketisestimatedatabillionpeople globally TherapeuticvaccinesareusedinpeoplelivingwithHIVtohyperstimulatetheirownimmuneresponse beyondwhattheycandoontheirownwithintenttopotentiallycurethepatient–toallowthemtostop treatmentforextendedperiodsoftime MosteffortstodatehavefocusedoneithertheB-cell(humoral)immuneresponseortheT-cell(cellular) immuneresponse Enochianusesaproprietarycombinationofcell-,gene-andimmunotherapytostimulateapotentially overwhelmingBandTcellresponse Preventive vaccines are for HIV uninfected people –the potential market is estimated at a billion people globally

Non-Human Primate Study: Fred Hutchinson Cancer Research Center 17 Non-human primates are considered the gold standard to study the potential of preventive and therapeutic vaccination Enochian BioSciences is collaborating with leaders in the field to conduct the preventive and therapeutic vaccine studies: Dr. Hans-Peter Kiem will oversee the studies being conducted at the animal research facilities of the Fred Hutchinson Cancer Research Center Dr. Kathryn Bar has provided the virus to be used

ENOB-HV-12 Update & Next Steps Animals infected and on ART Intervention planned for October 2021 Preliminary results possible by end 2021 18
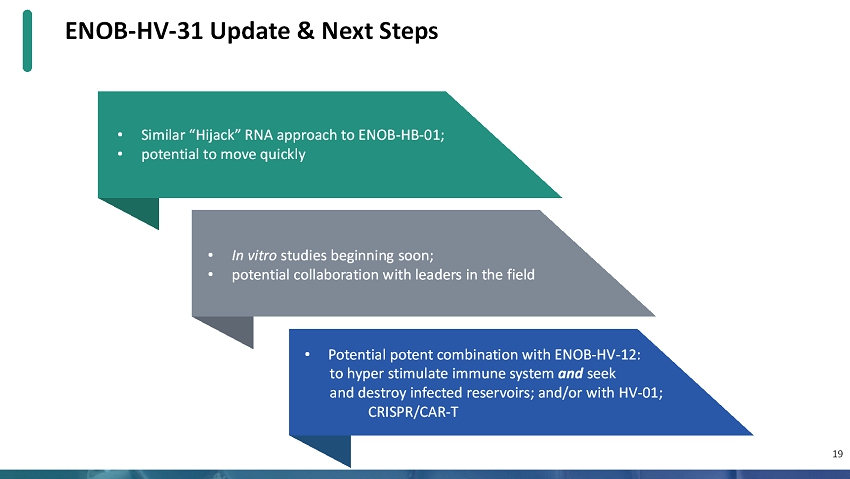
• Similar “Hijack” RNA approach to ENOB-HB-01; • potential to move quickly ENOB-HV-31 Update & Next Steps • In vitro studies beginning soon; • potential collaboration with leaders in the field • Potential potent combination with ENOB-HV-12: to hyper stimulate immune system andseek and destroy infected reservoirs; and/or with HV-01; CRISPR/CAR-T 19

HBV & Oncology Programs HBV: Jan. 2020. Established exclusive license with Seraph Research Institute (SRI) for the life of any products related to HBV involving certain scientific approaches Oncology: At the cutting edge

Why Do We Need a Cure For HBV? 21 Short Term Market ~2.2 MILLION People living with chronic HBV in the U.S. ~15 MILLION People living with chronic HBV in Europe ~1.2 MILLION People living with chronic HBV in Middle East ~86 MILLION People living with chronic HBV in India and China ~350 million Living with chronic HBV worldwide ~30 MILLION Infected with HBV worldwide ~1 MILLION HBV related deaths worldwide Despite the existence of a highly effective vaccine, there are: Gilead purchased global rights for Hepatitis C virus (HCV) cure for $11.9 billion; 177 million people are living with HCV globally; half as many as HBV; peak sales $33 billion
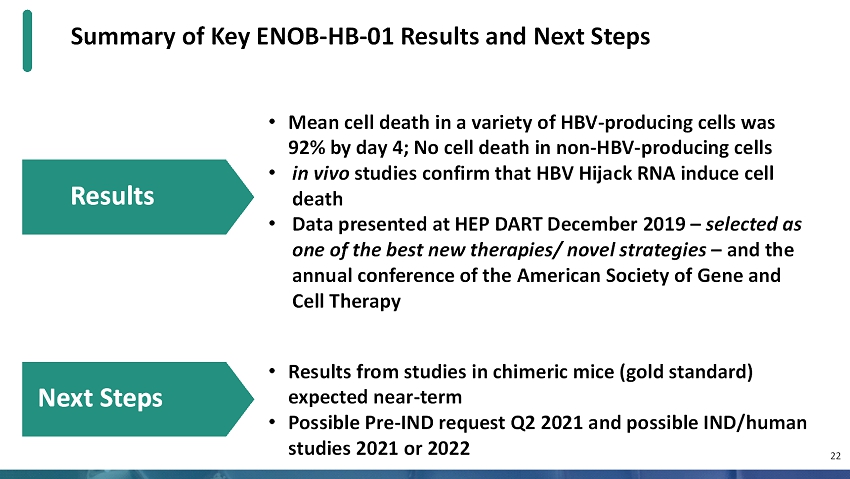
Summary of Key ENOB-HB-01 Results and Next Steps 22 Results • Additional in vivo studies are being conducted • Mean cell death in a variety of HBV-producing cells was 92% by day 4; No cell death in non-HBV-producing cells • in vivo studies confirm that HBV Hijack RNA induce cell death • Data presented at HEP DART December 2019 –selected as one of the best new therapies/ novel strategies –and the annual conference of the American Society of Gene and Cell Therapy • Results from studies in chimeric mice (gold standard) expected near-term • Possible Pre-IND request Q2 2021 and possible IND/human studies 2021 or 2022 Next Steps Results

Oncology Program 23 Many cancers are caused by immune dysregulation The immune system plays an important role in targeting and destroying cancer cells. Our strategy is to combine our gene modified cellular therapy expertise with our extensive knowledge of dendritic cells to develop a novel proprietary immuno-oncology technology platform for the treatment of cancer The program will focus on solid tumors with limited or poor first-line therapies, e.g. renal cell, glioblastoma, triple-negative breast and pancreatic cancers, to accelerate the clinical pipeline through regulatory processes and capture key cancer markets The program is in early pre-clinical design

~450,000 Cases per year worldwide ~60,000 Cases per year in the U.S. ~48,000 Deaths per year in the U.S. ENOB-DC-11: First Target –Pancreatic cancer 24 No Good Treatment Options Beyond Stage I Bristol-Myers Squibb’s-wholly owned subsidiary Celgene’s Abraxane has ~$1B+ in sales per year ~4% Survival rate at 5 years
ENOB-DC-11 Update & Next Steps • Partnership with Dr. Anahid Jewett, UCLA • Promising in vitroexperiments • Proof of concept in vitro &in vivo experimental plan completed; results expected in 2021 25

Oncology: Targeting High Unmet Needs 26 Enochian plans to initially target pancreatic cancer followed by glioblastoma, triple negative breast cancer, and renal cell carcinoma Cancer is among the leading causes of death worldwide -In 2018-17 million new cases and 9.6 million cancer-related deaths worldwide. Most deadly form of human cancer, median survival of ~ 1 year & 5-year survival rates below 10%; 2018 -Approximately 23,000 incident cases (classified as a rare disease & qualifies for FDA Fast Track Designation) & $200M in sales US/EU Glioblastoma Survival rates tend to be lower with TNBC compared to other forms of breast cancer; sales of key triple-negative breast cancer drugs across the US, Japan, & five major EU markets will grow from $293m in 2015 to $1.0bn in 2024 Triple Negative Breast Cancer 2018 incidence cases of over 110,000 and expected to continue to grow; currently a $3B market in sales Renal Cell Cancer

Key Financials

Key Financials •Current 10Q filed on February 16, 2021 •July 8, 2020, $20 million funding commitment from an institutional investor 28 •July 13, 2020, $50 million S-3 shelf registration statement

Enochian Biosciences Elegant Simplicity: Integrating Diverse Scientific Approaches to Develop Cures Dr. Mark Dybul, Executive Vice Chairman March 9-10, 2021 HC Wainwright Global Life Sciences Conference NASDAQ: ENOB
Enochian Biosciences Inc Reports
- 07/17/2012 - 10-K
- 07/16/2013 - 10-K
- 03/31/2015 - 10-K
- 09/28/2016 - 10-K
- 09/29/2017 - 10-K
- 09/28/2018 - 10-K
- 09/23/2020 - 10-K
- 11/25/2011 - 10-Q
- 02/21/2012 - 10-Q
- 08/20/2012 - 10-Q
- 11/14/2012 - 10-Q
- 02/14/2013 - 10-Q
- 08/14/2013 - 10-Q
- 11/15/2013 - 10-Q
- 02/11/2014 - 10-Q
- 05/14/2014 - 10-Q
- 08/14/2014 - 10-Q
- 11/13/2014 - 10-Q
- 05/13/2015 - 10-Q
- 11/13/2015 - 10-Q
- 02/12/2016 - 10-Q
- 05/13/2016 - 10-Q
- 02/14/2017 - 10-Q
- 05/15/2017 - 10-Q
- 11/20/2017 - 10-Q
- 02/08/2018 - 10-Q
- 02/10/2020 - 10-Q
- 05/11/2020 - 10-Q
- 11/12/2020 - 10-Q
- 02/16/2021 - 10-Q
- 05/17/2021 - 10-Q
