Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - FIVE PRIME THERAPEUTICS, INC. | d77467dex991.htm |
| 8-K - 8-K - FIVE PRIME THERAPEUTICS, INC. | d77467d8k.htm |

Exhibit 99.2 A double-blind randomized study of bemarituzumab (bema) plus mFOLFOX6 versus placebo plus mFOLFOX6 as first-line treatment for advanced gastric/gastroesophageal junction cancer (FIGHT) Zev A Wainberg, Peter Enzinger, Yoon-Koo Kang, Kensai Yamaguchi, Shukui Qin, Keun-Wook Lee, Sang Cheul Oh, Jin Li, Haci Mehmet Turk, Alexandra Teixeira, Giovanni Gerardo Cardellino, Rachel Guardeno Sanchez, Siddhartha Mitra, Yingsi Yang, Helen Collins, Daniel V Catenacci 1 2 3 4 University of California, Los Angeles, USA, Dana Farber Cancer Institute, Boston, USA, Asan Medical Center, Seoul, South Korea, The Cancer Institute 5 6 Hospital of JFCR, Koto-Ku , Tokyo, Japan, 81 Hospital Nanjing University of Chinese Medicine, Nanjing, China, Seoul National University College of 7 8 Medicine, Seoul National University Bundang Hospital, Seongnam, Gyeonggi-do, S.Korea, Korea University Guro Hospital, Seoul, South Korea, Shanghai 9 10 East Hospital, Shanghai, China, Bezmialem Vakif Universitesi Tip Fakultesi Hastanesi, Fatih, Turkey, Hospital Senhora Da Oliveira, Guimaraes, Portugal, 11 12 13 Dipartimento di Oncologia, Azienda Ospedaliero Universitaria, Udine, Italy, Institut Catala d Oncologia Girona, Spain, Five Prime Therapeutics, South 14 San Francisco, USA, University of Chicago, Chicago, USA Late Breaking Abstract (LBA160) ASCO Gastrointestinal Cancer Symposium 2021 1Exhibit 99.2 A double-blind randomized study of bemarituzumab (bema) plus mFOLFOX6 versus placebo plus mFOLFOX6 as first-line treatment for advanced gastric/gastroesophageal junction cancer (FIGHT) Zev A Wainberg, Peter Enzinger, Yoon-Koo Kang, Kensai Yamaguchi, Shukui Qin, Keun-Wook Lee, Sang Cheul Oh, Jin Li, Haci Mehmet Turk, Alexandra Teixeira, Giovanni Gerardo Cardellino, Rachel Guardeno Sanchez, Siddhartha Mitra, Yingsi Yang, Helen Collins, Daniel V Catenacci 1 2 3 4 University of California, Los Angeles, USA, Dana Farber Cancer Institute, Boston, USA, Asan Medical Center, Seoul, South Korea, The Cancer Institute 5 6 Hospital of JFCR, Koto-Ku , Tokyo, Japan, 81 Hospital Nanjing University of Chinese Medicine, Nanjing, China, Seoul National University College of 7 8 Medicine, Seoul National University Bundang Hospital, Seongnam, Gyeonggi-do, S.Korea, Korea University Guro Hospital, Seoul, South Korea, Shanghai 9 10 East Hospital, Shanghai, China, Bezmialem Vakif Universitesi Tip Fakultesi Hastanesi, Fatih, Turkey, Hospital Senhora Da Oliveira, Guimaraes, Portugal, 11 12 13 Dipartimento di Oncologia, Azienda Ospedaliero Universitaria, Udine, Italy, Institut Catala d Oncologia Girona, Spain, Five Prime Therapeutics, South 14 San Francisco, USA, University of Chicago, Chicago, USA Late Breaking Abstract (LBA160) ASCO Gastrointestinal Cancer Symposium 2021 1

Fibroblast Growth Factor Receptor 2b (FGFR2b) in Cancer • FGFR2b is a member of the FGFR family (FGFR1-4) and is a splice isoform of FGFR2 1 • FGFR2b overexpression: 3-61% of gastric cancer depending on tumor stage and assay 2 • FGFR tyrosine kinase inhibitors have shown clinical benefit in cancers with FGFR mutations, fusions or translocations 1 Han et al, Pathobiology 2015, Ahn et al, Modern Pathology 2016, Nagatsuma et al, Gastric Cancer 2015, Tokunga et al, Oncotarget 2016 2 Abou-Alfa GK et al, Lancet Onc 2020; Loriot Y et al, NEJM 2019 2Fibroblast Growth Factor Receptor 2b (FGFR2b) in Cancer • FGFR2b is a member of the FGFR family (FGFR1-4) and is a splice isoform of FGFR2 1 • FGFR2b overexpression: 3-61% of gastric cancer depending on tumor stage and assay 2 • FGFR tyrosine kinase inhibitors have shown clinical benefit in cancers with FGFR mutations, fusions or translocations 1 Han et al, Pathobiology 2015, Ahn et al, Modern Pathology 2016, Nagatsuma et al, Gastric Cancer 2015, Tokunga et al, Oncotarget 2016 2 Abou-Alfa GK et al, Lancet Onc 2020; Loriot Y et al, NEJM 2019 2

Bemarituzumab is an IgG1 antibody specific for the FGFR2b Receptor Bemarituzumab Bemarituzumab Single-agent activity in late-line FGFR2b+ gastric cancer 1 • Confirmed ORR = 18% (n=28) • No dose-limiting toxicities • Corneal adverse events in 3/28 patients • Recommended Phase 2 dose: 15mg/kg Q2W 2 with a single 7.5mg/kg dose on Cycle 1 Day 8 1 Catenacci, et al: JCO 2020 2 Tejani, et al: ASCO GI 2019 3Bemarituzumab is an IgG1 antibody specific for the FGFR2b Receptor Bemarituzumab Bemarituzumab Single-agent activity in late-line FGFR2b+ gastric cancer 1 • Confirmed ORR = 18% (n=28) • No dose-limiting toxicities • Corneal adverse events in 3/28 patients • Recommended Phase 2 dose: 15mg/kg Q2W 2 with a single 7.5mg/kg dose on Cycle 1 Day 8 1 Catenacci, et al: JCO 2020 2 Tejani, et al: ASCO GI 2019 3

FIGHT Trial Design Key Eligibility Criteria • No prior therapy for unresectable locally advanced or metastatic Double blind, placebo controlled gastric/GEJ adenocarcinoma • RECIST v1.1 evaluable disease Bema + mFOLFOX6 Primary endpoint • FGFR2b overexpression by IHC (n = 77) • Investigator-Assessed and/or FGFR2 gene amplification by Progression-Free 1 ctDNA VS Survival R • ECOG 0/1 1:1 Secondary endpoints • HER2 not positive Placebo + mFOLFOX6 • Overall Survival (n = 78) • May receive 1 dose of mFOLFOX6 • Response Rate Stratification Factors 2 Treatment Q2W • Geographic region • Single dose of mFOLFOX6 during Statistical Plan screening Trial initially designed as registrational Phase 3 (n=548) with 2-sided α 0.05 Amended after enrolling n = 155 to a proof-of-concept Phase 2 with pre-specified • Prior adjuvant or neo-adjuvant statistical assumptions of: chemotherapy • Hierarchical sequential testing: PFS, then OS/ORR 1 Central testing: Immunohistochemical stain (Ventana): cut-off any • ≥84 events to demonstrate benefit at a HR≤0.76 for PFS at 2-sided α of 0.2 2+/3+; circulating tumor DNA (PGDx): cut-off 1.5X 2 2 15mg/kg Q2W with a single 7.5mg/kg dose on Cycle 1 Day 8 4FIGHT Trial Design Key Eligibility Criteria • No prior therapy for unresectable locally advanced or metastatic Double blind, placebo controlled gastric/GEJ adenocarcinoma • RECIST v1.1 evaluable disease Bema + mFOLFOX6 Primary endpoint • FGFR2b overexpression by IHC (n = 77) • Investigator-Assessed and/or FGFR2 gene amplification by Progression-Free 1 ctDNA VS Survival R • ECOG 0/1 1:1 Secondary endpoints • HER2 not positive Placebo + mFOLFOX6 • Overall Survival (n = 78) • May receive 1 dose of mFOLFOX6 • Response Rate Stratification Factors 2 Treatment Q2W • Geographic region • Single dose of mFOLFOX6 during Statistical Plan screening Trial initially designed as registrational Phase 3 (n=548) with 2-sided α 0.05 Amended after enrolling n = 155 to a proof-of-concept Phase 2 with pre-specified • Prior adjuvant or neo-adjuvant statistical assumptions of: chemotherapy • Hierarchical sequential testing: PFS, then OS/ORR 1 Central testing: Immunohistochemical stain (Ventana): cut-off any • ≥84 events to demonstrate benefit at a HR≤0.76 for PFS at 2-sided α of 0.2 2+/3+; circulating tumor DNA (PGDx): cut-off 1.5X 2 2 15mg/kg Q2W with a single 7.5mg/kg dose on Cycle 1 Day 8 4
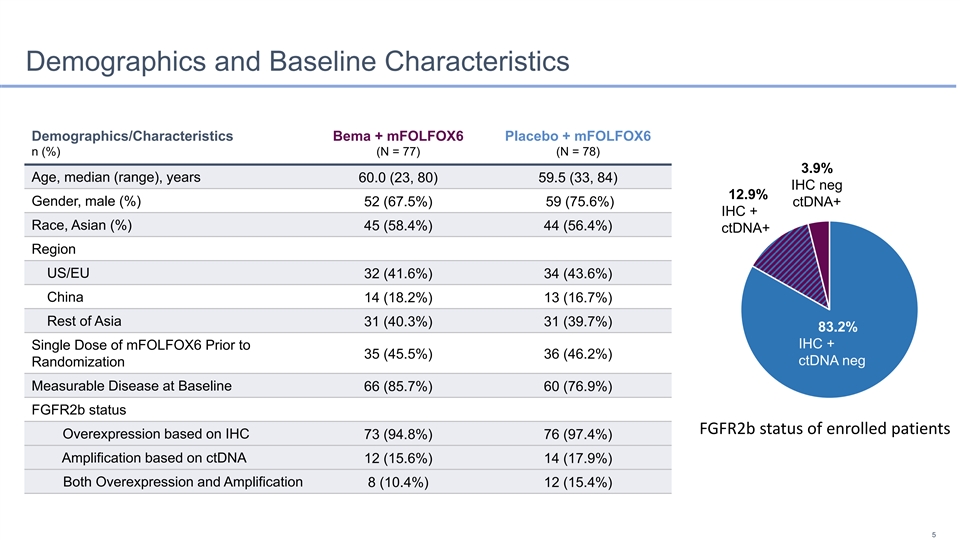
Demographics and Baseline Characteristics Demographics/Characteristics Bema + mFOLFOX6 Placebo + mFOLFOX6 n (%) (N = 77) (N = 78) 3.9% Age, median (range), years 60.0 (23, 80) 59.5 (33, 84) IHC neg 12.9% Gender, male (%) 52 (67.5%) 59 (75.6%) ctDNA+ IHC + Race, Asian (%) 45 (58.4%) 44 (56.4%) ctDNA+ Region US/EU 32 (41.6%) 34 (43.6%) China 14 (18.2%) 13 (16.7%) Rest of Asia 31 (40.3%) 31 (39.7%) 83.2% IHC + Single Dose of mFOLFOX6 Prior to 35 (45.5%) 36 (46.2%) ctDNA neg Randomization Measurable Disease at Baseline 66 (85.7%) 60 (76.9%) FGFR2b status FGFR2b status of enrolled patients Overexpression based on IHC 73 (94.8%) 76 (97.4%) Amplification based on ctDNA 12 (15.6%) 14 (17.9%) Both Overexpression and Amplification 8 (10.4%) 12 (15.4%) 5Demographics and Baseline Characteristics Demographics/Characteristics Bema + mFOLFOX6 Placebo + mFOLFOX6 n (%) (N = 77) (N = 78) 3.9% Age, median (range), years 60.0 (23, 80) 59.5 (33, 84) IHC neg 12.9% Gender, male (%) 52 (67.5%) 59 (75.6%) ctDNA+ IHC + Race, Asian (%) 45 (58.4%) 44 (56.4%) ctDNA+ Region US/EU 32 (41.6%) 34 (43.6%) China 14 (18.2%) 13 (16.7%) Rest of Asia 31 (40.3%) 31 (39.7%) 83.2% IHC + Single Dose of mFOLFOX6 Prior to 35 (45.5%) 36 (46.2%) ctDNA neg Randomization Measurable Disease at Baseline 66 (85.7%) 60 (76.9%) FGFR2b status FGFR2b status of enrolled patients Overexpression based on IHC 73 (94.8%) 76 (97.4%) Amplification based on ctDNA 12 (15.6%) 14 (17.9%) Both Overexpression and Amplification 8 (10.4%) 12 (15.4%) 5

Study Disposition FGFR2b+ (N = 275) Pre-screen (N=910) 30.2% pre-screened FGFR2b+ Screened (N = 191) Randomized (N = 155) Bema (n = 77) Placebo (n = 78) 1 never treated 1 never treated • 8 ongoing on placebo • 14 ongoing on Bema • 70 off Bema • 63 off Bema • 39 Radiographic PD • 26 Radiographic PD • 7 Consent withdrawal • 3 Consent withdrawal • 4 Death • 5 Death • 20 AE • 3 AE • 17 Other • 9 Other • 42 ongoing on study • 27 ongoing on study Enrollment period: 28 September 2018 • 35 off study • 51 off study to 12 May 2020 • 28 Death • 40 Death Data cutoff date 23 September 2020, • 10 Consent withdrawal • 6 Consent withdrawal median follow up is 10.9 months • 1 Other • 1 Other (range, 0.03-22) 6Study Disposition FGFR2b+ (N = 275) Pre-screen (N=910) 30.2% pre-screened FGFR2b+ Screened (N = 191) Randomized (N = 155) Bema (n = 77) Placebo (n = 78) 1 never treated 1 never treated • 8 ongoing on placebo • 14 ongoing on Bema • 70 off Bema • 63 off Bema • 39 Radiographic PD • 26 Radiographic PD • 7 Consent withdrawal • 3 Consent withdrawal • 4 Death • 5 Death • 20 AE • 3 AE • 17 Other • 9 Other • 42 ongoing on study • 27 ongoing on study Enrollment period: 28 September 2018 • 35 off study • 51 off study to 12 May 2020 • 28 Death • 40 Death Data cutoff date 23 September 2020, • 10 Consent withdrawal • 6 Consent withdrawal median follow up is 10.9 months • 1 Other • 1 Other (range, 0.03-22) 6

Progression-Free Survival and Overall Survival: Intent to Treat PFS Primary Endpoint OS Key Secondary Endpoint Bema Placebo Bema Placebo N = 77 N = 78 N = 77 N = 78 NR 12.9 9.5 7.4 Median OS, mo Median PFS, mo (13.8, NR) (9.1, 15.0) (7.3, 12.9) (5.8, 8.4) (95% CI) (95% CI) P=0.0268 P=0.0727 HR (95% CI) 0.58 (0.35, 0.95) HR (95% CI) 0.68 (0.44, 1.04) 7Progression-Free Survival and Overall Survival: Intent to Treat PFS Primary Endpoint OS Key Secondary Endpoint Bema Placebo Bema Placebo N = 77 N = 78 N = 77 N = 78 NR 12.9 9.5 7.4 Median OS, mo Median PFS, mo (13.8, NR) (9.1, 15.0) (7.3, 12.9) (5.8, 8.4) (95% CI) (95% CI) P=0.0268 P=0.0727 HR (95% CI) 0.58 (0.35, 0.95) HR (95% CI) 0.68 (0.44, 1.04) 7

Progression-Free Survival Benefit Increased with Higher Levels of FGFR2b Overexpression N = 118 N = 96 Intent to Treat (ITT)* N = 155 Bema Placebo Bema Placebo Bema Placebo N = 77 N = 78 N = 58 N = 60 N = 44 N = 52 9.5 7.4 mPFS, mo 10.2 7.3 mPFS, mo 14.1 7.3 mPFS, mo (7.3, 12.9) (5.8, 8.4) (95% CI) (6.8, 14.1) (5.5, 8.2) (95% CI) (6.8, NR) (5.4, 8.2) (95% CI) P=0.0727 HR (95% CI) 0.54 (0.33, 0.87) HR (95% CI) 0.44 (0.25, 0.77) HR (95% CI) 0.68 (0.44, 1.04) *ITT = 149 with IHC 2+/3+ and 6 pts with IHC <2+ or not available who were enrolled based on ctDNA alone 8Progression-Free Survival Benefit Increased with Higher Levels of FGFR2b Overexpression N = 118 N = 96 Intent to Treat (ITT)* N = 155 Bema Placebo Bema Placebo Bema Placebo N = 77 N = 78 N = 58 N = 60 N = 44 N = 52 9.5 7.4 mPFS, mo 10.2 7.3 mPFS, mo 14.1 7.3 mPFS, mo (7.3, 12.9) (5.8, 8.4) (95% CI) (6.8, 14.1) (5.5, 8.2) (95% CI) (6.8, NR) (5.4, 8.2) (95% CI) P=0.0727 HR (95% CI) 0.54 (0.33, 0.87) HR (95% CI) 0.44 (0.25, 0.77) HR (95% CI) 0.68 (0.44, 1.04) *ITT = 149 with IHC 2+/3+ and 6 pts with IHC <2+ or not available who were enrolled based on ctDNA alone 8

Overall Survival Benefit Increased with Higher Levels of FGFR2b Overexpression Intent to Treat (ITT)* N = 155 N = 96 N = 118 Bema Placebo Bema Placebo Bema Placebo N = 44 N = 52 N = 58 N = 60 N = 77 N = 78 mOS, mo NR 11.1 NR 12.9 mOS, mo NR 12.5 mOS, mo (95% CI) (13.8, NR) (8.4, 13.8) (13.8, NR) (9.1, 15.0) (95% CI) (13.8, NR) (8.8, 15.0) (95% CI) HR (95% CI) 0.41 (0.22, 0.79) P=0.0268 HR (95% CI) 0.52 (0.30, 0.91) HR (95% CI) 0.58 (0.35, 0.95) *ITT = 149 with IHC 2+/3+ and 6 pts with IHC <2+ or not available who were enrolled based on ctDNA alone 9Overall Survival Benefit Increased with Higher Levels of FGFR2b Overexpression Intent to Treat (ITT)* N = 155 N = 96 N = 118 Bema Placebo Bema Placebo Bema Placebo N = 44 N = 52 N = 58 N = 60 N = 77 N = 78 mOS, mo NR 11.1 NR 12.9 mOS, mo NR 12.5 mOS, mo (95% CI) (13.8, NR) (8.4, 13.8) (13.8, NR) (9.1, 15.0) (95% CI) (13.8, NR) (8.8, 15.0) (95% CI) HR (95% CI) 0.41 (0.22, 0.79) P=0.0268 HR (95% CI) 0.52 (0.30, 0.91) HR (95% CI) 0.58 (0.35, 0.95) *ITT = 149 with IHC 2+/3+ and 6 pts with IHC <2+ or not available who were enrolled based on ctDNA alone 9

Best % Change in Target Lesions from Baseline Bema + Placebo + mFOLFOX6 mFOLFOX6 ORR (ITT) n/N, % 36/77, 47% 26/78, 33% ORR (Measurable Disease at 35/66, 53% 24/60, 40% Baseline) n/N, % Best %change in tumor size, -41.7 (33.76) -29.9 (30.49) mean (StD) Median TTR (mon), (range) 1.84 (1.7, 7.6) 1.87 (1.6, 7.3) Median DOR (mon), (95% CI) 12.2 (5.5,15.6) 7.1 (4.3,11.7) Only subjects with measurable disease at baseline and at least 1 evaluable scan postbaseline are included in the waterfall plot. DOR = Duration of response; TTR = Time to response ^: estimated among subjects with measurable disease at baseline 10Best % Change in Target Lesions from Baseline Bema + Placebo + mFOLFOX6 mFOLFOX6 ORR (ITT) n/N, % 36/77, 47% 26/78, 33% ORR (Measurable Disease at 35/66, 53% 24/60, 40% Baseline) n/N, % Best %change in tumor size, -41.7 (33.76) -29.9 (30.49) mean (StD) Median TTR (mon), (range) 1.84 (1.7, 7.6) 1.87 (1.6, 7.3) Median DOR (mon), (95% CI) 12.2 (5.5,15.6) 7.1 (4.3,11.7) Only subjects with measurable disease at baseline and at least 1 evaluable scan postbaseline are included in the waterfall plot. DOR = Duration of response; TTR = Time to response ^: estimated among subjects with measurable disease at baseline 10

Summary of Adverse Events Bema (N = 76) Placebo (N = 77) Adverse Events All Treatment-Emergent Adverse Events (TEAE) 76 (100.0%) 76 (98.7%) Grade ≥ 3 63 (82.9%) 57 (74.0%) Leading to Death (Grade 5) 5 (6.6%) 4 (5.2%) Serious Adverse Events 24 (31.6%) 28 (36.4%) Leading to any component of mFOLFOX6 35 (46.1%) 28 (36.4%) discontinuation Leading to Bema/placebo discontinuation 26 (34.2%) 4 (5.2%) Exposure Bema (N = 76) Placebo (N = 77) Duration of Exposure to mFOLFOX6 (weeks), median 29.80 26.47 (range) (2.1, 73.0) (2.1, 66.7) Duration of Exposure to bema/placebo (weeks), 24.00 26.00 median (range) (2.0, 71.6) (2.0, 73.6) 11Summary of Adverse Events Bema (N = 76) Placebo (N = 77) Adverse Events All Treatment-Emergent Adverse Events (TEAE) 76 (100.0%) 76 (98.7%) Grade ≥ 3 63 (82.9%) 57 (74.0%) Leading to Death (Grade 5) 5 (6.6%) 4 (5.2%) Serious Adverse Events 24 (31.6%) 28 (36.4%) Leading to any component of mFOLFOX6 35 (46.1%) 28 (36.4%) discontinuation Leading to Bema/placebo discontinuation 26 (34.2%) 4 (5.2%) Exposure Bema (N = 76) Placebo (N = 77) Duration of Exposure to mFOLFOX6 (weeks), median 29.80 26.47 (range) (2.1, 73.0) (2.1, 66.7) Duration of Exposure to bema/placebo (weeks), 24.00 26.00 median (range) (2.0, 71.6) (2.0, 73.6) 11

Summary of Selected Treatment-Emergent Adverse Events Any Grade Grade ≥ 3 Selected Adverse Events Bema (N = 76) Placebo (N = 77) Bema (N = 76) Placebo (N = 77) Preferred Term 76 (100.0%) 76 (98.7%) 63 (82.9%) 57 (74.0%) Nausea 36 (47.4%) 41 (53.2%) 0 3 (3.9%) Vomiting 22 (28.9%) 24 (31.2%) 2 (2.6%) 2 (2.6%) Diarrhoea 31 (40.8%) 24 (31.2%) 2 (2.6%) 1 (1.3%) Stomatitis 24 (31.6%) 10 (13.0%) 7 (9.2%) 1 (1.3%) Peripheral sensory neuropathy 15 (19.7%) 15 (19.5%) 4 (5.3%) 3 (3.9%) Neutrophil count decreased 31 (40.8%) 33 (42.9%) 23 (30.3%) 27 (35.1%) Platelet count decreased 14 (18.4%) 21 (27.3%) 1 (1.3%) 0 Aspartate aminotransferase 23 (30.3%) 15 (19.5%) 4 (5.3%) 2 (2.6%) increased Alanine aminotransferase 22 (28.9%) 11 (14.3%) 2 (2.6%) 1 (1.3%) increased Dry eye 20 (26.3%) 5 (6.5%) 2 (2.6%) 0 12Summary of Selected Treatment-Emergent Adverse Events Any Grade Grade ≥ 3 Selected Adverse Events Bema (N = 76) Placebo (N = 77) Bema (N = 76) Placebo (N = 77) Preferred Term 76 (100.0%) 76 (98.7%) 63 (82.9%) 57 (74.0%) Nausea 36 (47.4%) 41 (53.2%) 0 3 (3.9%) Vomiting 22 (28.9%) 24 (31.2%) 2 (2.6%) 2 (2.6%) Diarrhoea 31 (40.8%) 24 (31.2%) 2 (2.6%) 1 (1.3%) Stomatitis 24 (31.6%) 10 (13.0%) 7 (9.2%) 1 (1.3%) Peripheral sensory neuropathy 15 (19.7%) 15 (19.5%) 4 (5.3%) 3 (3.9%) Neutrophil count decreased 31 (40.8%) 33 (42.9%) 23 (30.3%) 27 (35.1%) Platelet count decreased 14 (18.4%) 21 (27.3%) 1 (1.3%) 0 Aspartate aminotransferase 23 (30.3%) 15 (19.5%) 4 (5.3%) 2 (2.6%) increased Alanine aminotransferase 22 (28.9%) 11 (14.3%) 2 (2.6%) 1 (1.3%) increased Dry eye 20 (26.3%) 5 (6.5%) 2 (2.6%) 0 12

Corneal-Related Adverse Events 1 Trial required corneal evaluation at baseline and every 8 weeks until the end of treatment Bema (N = 76) Placebo (N = 77) 2 3 Corneal Adverse Events (SMQ) All Grade 51 (67.1%) 8 (10.4%) 4 Corneal Adverse Events (SMQ) Grade 3 18 (23.7%) 0 16.1 11.6 Median time to onset to any grade, weeks (range) (0.1, 41.0) (6.0, 29.0) Corneal AE leading to bema/placebo 20 (26.3%) 0 5 discontinuation AE resolved 12 (60.0%) 0 AE not resolved as of 23 Sept 2020 8 (40.0%) 0 27.0 Median time to resolution, weeks (95%CI) NA (18.9, NR) 1 If any event reported, examinations were to continue every 8W until resolution, even if drug discontinued 2 SMQ = Standardised MedDRA Query 3 Most common: dry eye (26.3%), keratitis (15.8%), punctate keratitis (14.5%), vision blurred (15.0%), corneal epithelium defect (10.5%) 4 No ≥ grade 4 event reported 5 Most common: dry eye (n=4), keratitis (n=4), corneal disorder (n=2), eye disorder (n=2) limbal stem cell deficiency (n=2), punctate keratitis (n=2) 13Corneal-Related Adverse Events 1 Trial required corneal evaluation at baseline and every 8 weeks until the end of treatment Bema (N = 76) Placebo (N = 77) 2 3 Corneal Adverse Events (SMQ) All Grade 51 (67.1%) 8 (10.4%) 4 Corneal Adverse Events (SMQ) Grade 3 18 (23.7%) 0 16.1 11.6 Median time to onset to any grade, weeks (range) (0.1, 41.0) (6.0, 29.0) Corneal AE leading to bema/placebo 20 (26.3%) 0 5 discontinuation AE resolved 12 (60.0%) 0 AE not resolved as of 23 Sept 2020 8 (40.0%) 0 27.0 Median time to resolution, weeks (95%CI) NA (18.9, NR) 1 If any event reported, examinations were to continue every 8W until resolution, even if drug discontinued 2 SMQ = Standardised MedDRA Query 3 Most common: dry eye (26.3%), keratitis (15.8%), punctate keratitis (14.5%), vision blurred (15.0%), corneal epithelium defect (10.5%) 4 No ≥ grade 4 event reported 5 Most common: dry eye (n=4), keratitis (n=4), corneal disorder (n=2), eye disorder (n=2) limbal stem cell deficiency (n=2), punctate keratitis (n=2) 13

Summary • The FIGHT trial is the first study to evaluate targeting overexpression of FGFR2b • ~ 30% of 1L advanced non-HER2+ GC/GEA overexpress FGFR2b+ by a centrally performed IHC test • Bemarituzumab, added to mFOLFOX6 chemotherapy, led to clinically meaningful and statistically significant improvements in PFS, OS and ORR • Bemarituzumab was associated with an increase in corneal adverse events and stomatitis, the majority of which were reversible • The FIGHT trial results support • A prospective randomized phase 3 study in gastric/gastroesophageal adenocarcinoma • The evaluation of bemarituzumab to treat other FGFR2b+ tumor types 14Summary • The FIGHT trial is the first study to evaluate targeting overexpression of FGFR2b • ~ 30% of 1L advanced non-HER2+ GC/GEA overexpress FGFR2b+ by a centrally performed IHC test • Bemarituzumab, added to mFOLFOX6 chemotherapy, led to clinically meaningful and statistically significant improvements in PFS, OS and ORR • Bemarituzumab was associated with an increase in corneal adverse events and stomatitis, the majority of which were reversible • The FIGHT trial results support • A prospective randomized phase 3 study in gastric/gastroesophageal adenocarcinoma • The evaluation of bemarituzumab to treat other FGFR2b+ tumor types 14

University of Rochester Wilmot Cancer Institute Seoul National University Hospital Szpital Specjalistyczny w Brzozowie Podkarpacki Os The University of Chicago S.G. Moscati Hospital Ajou University Hospital SP ZOZ MSWiA z W-M CO w Olsztynie Dana-Farber Cancer Institute Seoul National University Bundang Hospital IRCSS Casa Sollievo Della Sofferenza MRUKMED. Lekarz Beata Madej Mruk i Partner. Sp. p. The University of Arizona Cancer Center Korea University Guro Hospital AOU Careggi Centro Hospitalar do Baixo Vouga, E. P. E. Innovated Clinical Research Institute Chonbuk National University Hospital Az. Osp. Valtellina e Valchiavenna Hospital Senhora da Oliveira – Guimarães Summary Karmanos Cancer Institute Korea University Anam Hospital Centrum Onkologii Instituto Português de Oncologia do Porto Francisc Kyungpook National University Chilgok Hospital White Plains Hospital Unidade Local de Saude de Matosinhos E.P.E. Samodzielny Publiczny Szpital Kliniczny Nr 1 w Lub The Catholic University of Korea, Seoul St. Mary's Northwestern Medicine Cancer Center in Warrenville Institutul Clinic Fundeni BCO-SM Hallym University Sacred Heart Hospital Northwestern Medicine Delnor S.C. Medisprof S.R.L Szpital Specjalistyczny w Brzozowie Podkarpacki Os Kangbuk Samsung Hospital Ochsner Clinic Foundation S.C. Centrul de Oncologie Sf. Nectarie S.R.L. SP ZOZ MSWiA z W-M CO w Olsztynie Chungnam National University Hospital (CNUH) Utah Cancer Specialists S.C. Oncolab SRL MRUKMED. Lekarz Beata Madej Mruk i Partner. Sp. p. Dong-A University Hospital, Henry Ford Health System Clin. Emergency County Hospital -Sf.Apostol Andrei Gangnam Severance Hospital Centro Hospitalar do Baixo Vouga, E. P. E. St Luke's University Health Network PUERTA DE HIERRO ASAN Medical Center Hospital Senhora da Oliveira - Guimarães Innovated Clinical Research Institute Hospital General de Catalunya King Chulalongkorn Memorial Hospital University of Kansas Medical Center Instituto Português de Oncologia do Porto Francisc Institut Català d'Oncologia Townsville Hospital Virginia Mason Unidade Local de Saude de Matosinhos E.P.E. Institut Catala De La Salut (Ics) Sydney Adventist Hospital Tennessee Cancer Specialists Institutul Clinic Fundeni HOSPITAL UNIVERSITARIO FUNDACIÓN ALCORCÓN Harbin Medical University Cancer Hospital UCLA Department of Medicine - Hematology/Oncology S.C. Medisprof S.R.L Hospital Del Mar Thank you to the patients, their Fudan Universisity Shanghai Cancer Center Yale Cancer Center S.C. Centrul de Oncologie Sf. Nectarie S.R.L. Complejo Hospitalario Universitario A Coruna Zhejiang Cancer Hospital Beth Israel Deaconess Medical Center S.C. Oncolab SRL UNIV HOSP ARNAU DE VILANOVA The First Bethune Hospital of Jilin University Stony Brook Medicine families, the study site investigators HGUG MARAÑÓN Clin. Emergency County Hospital -Sf.Apostol Andrei Liaoning Cancer Hospital CHU Morvan-Institut de Cancerologie Hospital Madrid Norte Sanchinarro PUERTA DE HIERRO Wuhan Union Hospital Clinique Sainte Anne Hospital Parc Taulí Taihe Hospital Hospital General de Catalunya and their staff CHRU Besancon Complejo Hospitalario de Navarra Hunan Cancer Hospital(The Affiliated Cancer Hospit Azienda Ospedaliera Spedali Civili di Brescia- Hôpital Nord Franche-Comté Hospital Mutua De Terrassa Shanghai East Hospital A.O.U. Ospedali Riuniti - Umberto I Klinikum Ludwigsburg Clinica Universidad de Navarra The 4th Hospital of Hebei Medical University S.C. Oncologia, Istituti Ospitalieri di Cremona Medizinische Universitatsklinik Mannheim Istanbul Medeniyet Universitesi Anhui Provincial Cancer A.O. Universitaria Molinette San Giovanni Battista Städtisches Klinikum Braunschweig gGmbH Ankara Oncology Education and Research Hospital Chongqing Daping Hospital B-A-Z. Country Hospital P. O. U. S.Maria della Misericordia Fujian Cancer Hospital Inonu Universitesi Turgut Ozal Tip Merkezi SzSzBMK Josa Andras Teaching Hospital IRCCS Policlinico San Matteo Ondokuz Mayas University Henan Cancer Hospital National Institute of Oncology IRCCS A.O.U.San Martino Ozel Medical Park Izmir Hastanesi Jiangsu Province Hospital Hetenyi Geza Hospital Azienda Ospedaliero-Universitaria Pisana Istanbul Yeni Yuzyil Universitesi Cancer Hospital of Shantou University Medical Coll Del-pesti Centrumkorhaz – OHII St. Bortolo Hospital - AULSS 8 Berica Adnan Menderes Universitesi Hastanesi Azienda Ospedaliera Spedali Civili di Brescia- The First Affiliated Hospital, Zhejiang University Ospedale Mater Salutis di Legnago - UOC Oncologia Ege University hospital A.O.U. Ospedali Riuniti - Umberto I Sir Run Run Shaw Hospital Seconda Università Napoli Bezmialem Vakif Universitesi Tip Fakultesi Hastane S.C. Oncologia, Istituti Ospitalieri di Cremona The 81st Hospital of Chinese PLA Hacettepe Universitesi Tip Fakultesi S.G. Moscati Hospital A.O. Universitaria Molinette San Giovanni Battista Kocaeli Universitesi Tip Fakultesi IRCSS Casa Sollievo Della Sofferenza Xiangya Hospital Central South University P. O. U. S.Maria della Misericordia Uludag Universitesi Tip Fakultesi AOU Careggi Tianjin Cancer Institute and Hospital IRCCS Policlinico San Matteo Gaziantep Universitesi Tip Fakultesi Az. Osp. Valtellina e Valchiavenna IRCCS A.O.U.San Martino Beijing Cancer Hospital Ninewells Hospital Azienda Ospedaliero-Universitaria Pisana Centrum Onkologii Second Affiliated Hospital of Soochow University Gachon University Gil Medical Center St. Bortolo Hospital - AULSS 8 Berica National Cancer Center Hospital East Samodzielny Publiczny Szpital Kliniczny Nr 1 w Lub Yonsei University Health System, Ospedale Mater Salutis di Legnago - UOC Oncologia Niigata Cancer Center Hospital BCO-SM Osaka Medical College Hospital Seconda Università Napoli The Cancer Institute Hospital Of JFCR Osaka General Medical Center 15University of Rochester Wilmot Cancer Institute Seoul National University Hospital Szpital Specjalistyczny w Brzozowie Podkarpacki Os The University of Chicago S.G. Moscati Hospital Ajou University Hospital SP ZOZ MSWiA z W-M CO w Olsztynie Dana-Farber Cancer Institute Seoul National University Bundang Hospital IRCSS Casa Sollievo Della Sofferenza MRUKMED. Lekarz Beata Madej Mruk i Partner. Sp. p. The University of Arizona Cancer Center Korea University Guro Hospital AOU Careggi Centro Hospitalar do Baixo Vouga, E. P. E. Innovated Clinical Research Institute Chonbuk National University Hospital Az. Osp. Valtellina e Valchiavenna Hospital Senhora da Oliveira – Guimarães Summary Karmanos Cancer Institute Korea University Anam Hospital Centrum Onkologii Instituto Português de Oncologia do Porto Francisc Kyungpook National University Chilgok Hospital White Plains Hospital Unidade Local de Saude de Matosinhos E.P.E. Samodzielny Publiczny Szpital Kliniczny Nr 1 w Lub The Catholic University of Korea, Seoul St. Mary's Northwestern Medicine Cancer Center in Warrenville Institutul Clinic Fundeni BCO-SM Hallym University Sacred Heart Hospital Northwestern Medicine Delnor S.C. Medisprof S.R.L Szpital Specjalistyczny w Brzozowie Podkarpacki Os Kangbuk Samsung Hospital Ochsner Clinic Foundation S.C. Centrul de Oncologie Sf. Nectarie S.R.L. SP ZOZ MSWiA z W-M CO w Olsztynie Chungnam National University Hospital (CNUH) Utah Cancer Specialists S.C. Oncolab SRL MRUKMED. Lekarz Beata Madej Mruk i Partner. Sp. p. Dong-A University Hospital, Henry Ford Health System Clin. Emergency County Hospital -Sf.Apostol Andrei Gangnam Severance Hospital Centro Hospitalar do Baixo Vouga, E. P. E. St Luke's University Health Network PUERTA DE HIERRO ASAN Medical Center Hospital Senhora da Oliveira - Guimarães Innovated Clinical Research Institute Hospital General de Catalunya King Chulalongkorn Memorial Hospital University of Kansas Medical Center Instituto Português de Oncologia do Porto Francisc Institut Català d'Oncologia Townsville Hospital Virginia Mason Unidade Local de Saude de Matosinhos E.P.E. Institut Catala De La Salut (Ics) Sydney Adventist Hospital Tennessee Cancer Specialists Institutul Clinic Fundeni HOSPITAL UNIVERSITARIO FUNDACIÓN ALCORCÓN Harbin Medical University Cancer Hospital UCLA Department of Medicine - Hematology/Oncology S.C. Medisprof S.R.L Hospital Del Mar Thank you to the patients, their Fudan Universisity Shanghai Cancer Center Yale Cancer Center S.C. Centrul de Oncologie Sf. Nectarie S.R.L. Complejo Hospitalario Universitario A Coruna Zhejiang Cancer Hospital Beth Israel Deaconess Medical Center S.C. Oncolab SRL UNIV HOSP ARNAU DE VILANOVA The First Bethune Hospital of Jilin University Stony Brook Medicine families, the study site investigators HGUG MARAÑÓN Clin. Emergency County Hospital -Sf.Apostol Andrei Liaoning Cancer Hospital CHU Morvan-Institut de Cancerologie Hospital Madrid Norte Sanchinarro PUERTA DE HIERRO Wuhan Union Hospital Clinique Sainte Anne Hospital Parc Taulí Taihe Hospital Hospital General de Catalunya and their staff CHRU Besancon Complejo Hospitalario de Navarra Hunan Cancer Hospital(The Affiliated Cancer Hospit Azienda Ospedaliera Spedali Civili di Brescia- Hôpital Nord Franche-Comté Hospital Mutua De Terrassa Shanghai East Hospital A.O.U. Ospedali Riuniti - Umberto I Klinikum Ludwigsburg Clinica Universidad de Navarra The 4th Hospital of Hebei Medical University S.C. Oncologia, Istituti Ospitalieri di Cremona Medizinische Universitatsklinik Mannheim Istanbul Medeniyet Universitesi Anhui Provincial Cancer A.O. Universitaria Molinette San Giovanni Battista Städtisches Klinikum Braunschweig gGmbH Ankara Oncology Education and Research Hospital Chongqing Daping Hospital B-A-Z. Country Hospital P. O. U. S.Maria della Misericordia Fujian Cancer Hospital Inonu Universitesi Turgut Ozal Tip Merkezi SzSzBMK Josa Andras Teaching Hospital IRCCS Policlinico San Matteo Ondokuz Mayas University Henan Cancer Hospital National Institute of Oncology IRCCS A.O.U.San Martino Ozel Medical Park Izmir Hastanesi Jiangsu Province Hospital Hetenyi Geza Hospital Azienda Ospedaliero-Universitaria Pisana Istanbul Yeni Yuzyil Universitesi Cancer Hospital of Shantou University Medical Coll Del-pesti Centrumkorhaz – OHII St. Bortolo Hospital - AULSS 8 Berica Adnan Menderes Universitesi Hastanesi Azienda Ospedaliera Spedali Civili di Brescia- The First Affiliated Hospital, Zhejiang University Ospedale Mater Salutis di Legnago - UOC Oncologia Ege University hospital A.O.U. Ospedali Riuniti - Umberto I Sir Run Run Shaw Hospital Seconda Università Napoli Bezmialem Vakif Universitesi Tip Fakultesi Hastane S.C. Oncologia, Istituti Ospitalieri di Cremona The 81st Hospital of Chinese PLA Hacettepe Universitesi Tip Fakultesi S.G. Moscati Hospital A.O. Universitaria Molinette San Giovanni Battista Kocaeli Universitesi Tip Fakultesi IRCSS Casa Sollievo Della Sofferenza Xiangya Hospital Central South University P. O. U. S.Maria della Misericordia Uludag Universitesi Tip Fakultesi AOU Careggi Tianjin Cancer Institute and Hospital IRCCS Policlinico San Matteo Gaziantep Universitesi Tip Fakultesi Az. Osp. Valtellina e Valchiavenna IRCCS A.O.U.San Martino Beijing Cancer Hospital Ninewells Hospital Azienda Ospedaliero-Universitaria Pisana Centrum Onkologii Second Affiliated Hospital of Soochow University Gachon University Gil Medical Center St. Bortolo Hospital - AULSS 8 Berica National Cancer Center Hospital East Samodzielny Publiczny Szpital Kliniczny Nr 1 w Lub Yonsei University Health System, Ospedale Mater Salutis di Legnago - UOC Oncologia Niigata Cancer Center Hospital BCO-SM Osaka Medical College Hospital Seconda Università Napoli The Cancer Institute Hospital Of JFCR Osaka General Medical Center 15
