Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - ZIOPHARM ONCOLOGY INC | d130474dex992.htm |
| 8-K - 8-K - ZIOPHARM ONCOLOGY INC | d130474d8k.htm |

J.P. Morgan 39th Annual Healthcare Conference January 14, 2021 Exhibit 99.1

Forward Looking Statements This presentation contains certain forward-looking information about Ziopharm Oncology, Inc. that is intended to be covered by the safe harbor for "forward-looking statements" provided by the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts, and in some cases can be identified by terms such as "may," "will," "could," "expects," "plans," "anticipates," and "believes." These statements include, but are not limited to, statements regarding our business and strategic plans, the availability of cash resources, the progress and timing of our research and development programs, including the anticipated dates for the FDA clearance, initiation, patient dosing and data readouts of our clinical trials, the potential market and treatment opportunity of our products, expectations regarding partnership opportunities for our programs and the number of patients in our clinical trials. Although Ziopharm’s management team believes the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Ziopharm, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, changes in our operating plans that may impact our cash expenditures; the uncertainties inherent in research and development, future clinical data and analysis, including whether any of Ziopharm’s product candidates will advance further in the preclinical research or clinical trial process, including receiving clearance from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies to conduct clinical trials and whether and when, if at all, they will receive final approval from the U.S. FDA or equivalent foreign regulatory agencies and for which indication; the strength and enforceability of Ziopharm’s intellectual property rights; competition from other pharmaceutical and biotechnology companies as well as risk factors discussed or identified in the public filings with the Securities and Exchange Commission made by Ziopharm, including those risks and uncertainties listed in Ziopharm’s quarterly report on Form 10-Q for the three months ended September 30, 2020 filed by Ziopharm with the Securities and Exchange Commission. Readers are cautioned not to place undue reliance on these forward-looking statements that speak only as of the date of the presentation, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events.
01 02 03 04 Agenda Where We Are Entering The Year TCR-T Program: Our Top Priority And Opportunity Approach For Our CAR-T And IL-12 Programs 2021 Expectations
01 02 03 04 Agenda Where We Are Entering The Year TCR-T Program: Our Top Priority And Opportunity Approach For Our CAR-T And IL-12 Programs 2021 Expectations
Ziopharm Oncology Today Mission We develop innovative T cell-based therapies for the treatment of solid tumors, treating cancer on time, one cell at a time Long-Term Vision Distinctive commercial and clinical portfolio of immunotherapies transforming patient lives, supported by a growing body of compelling data Well positioned with distinctive platforms in clinic Evolved strategy and disciplined capital allocation
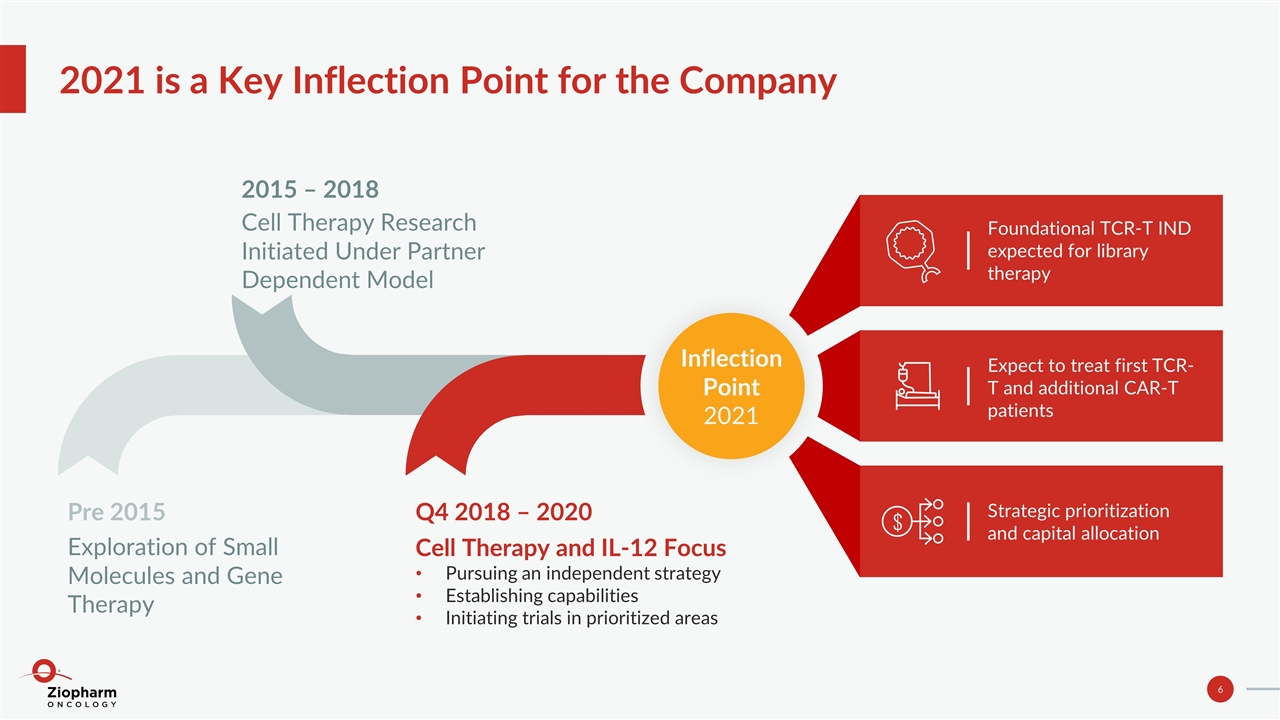
2021 is a Key Inflection Point for the Company Q4 2018 – 2020 Cell Therapy and IL-12 Focus Pursuing an independent strategy Establishing capabilities Initiating trials in prioritized areas 2015 – 2018 Cell Therapy Research Initiated Under Partner Dependent Model Pre 2015 Exploration of Small Molecules and Gene Therapy Inflection Point 2021 Foundational TCR-T IND expected for library therapy Expect to treat first TCR-T and additional CAR-T patients Strategic prioritization and capital allocation

Dissatisfaction with engagement and Board/management flux Concerns regarding the capital requirements of carrying three platforms forward Delays in program progress (e.g., NCI TCR-T Personalized Phase 2, CAR-T) Share price erosion Addressing Shareholder Feedback is Critically Important Shareholder Feedback How we are Addressing 03 04 01 02 Refreshing Board with active shareholder participation and input; renewed IR approach Strategically prioritizing our capital allocation expenditure and making the tough decisions Controlling our own destiny through building operational capabilities and infrastructure Unlocking under-appreciated value through focus, transparency (e.g., R&D / Investor Day in Q1) and execution
We Have the Core Technology and Infrastructure to be Leaders in Cell Therapy 01 02 03 04 Pioneers in non-viral gene transfer Cytokine biology expertise Strong SAB and established partnership network bring external strength Building momentum in TCR-T clinical programs Establishing infrastructure and capabilities for clinical programs Eden Biocell advancing CAR-T in Asia Leadership and innovation in T-cell immunobiology 05 06 07
2021 is a Year of Disciplined Strategic Focus Strategic Filters Our distinct capabilities Balance of de-risked feasibility and innovation Direct line of sight to patient data and unmet need Assessment of resource and capital constraints Strategic Positioning TCR-T Programs: Advance clinical program for library as top internal priority (and expand library); plan for personalized / next gen program(s); leverage NCI where possible CD19 CAR-T Programs: Cost effectively advance program to generate clinical data. Evaluate partnership opportunities for future development and commercialization. Evaluate cross-over potential of CAR-T technology to the TCR program Controlled IL-12 Program: Seek partner(s) that can optimize the potential of the asset for patients and monetize / return value to Ziopharm shareholders 01 02 03 04 Strategy entails transparent prioritization and directed capital allocation
01 02 03 04 Agenda Where We Are Entering The Year TCR-T Program: Our Top Priority And Opportunity Approach For Our CAR-T And IL-12 Programs 2021 Expectations
Neoantigens are the Blueprint for Targeting Solid Tumors The Right Targets Clinical Foundation Treatment Opportunity We believe over half of all cancer patients could potentially be treated by this type of immunotherapy meaning 7M+ patients suffering today could benefit in the US alone (plus 700-800K new patients every year), with potential for global expansion All cancer cells contain mutated genes which may be translated into neoantigens and presented to T cells through the natural immune system processes. There is no expression on normal tissues of the mutated genes Established, metastatic tumors have been eliminated by infusion of neoantigen-reactive T cells resulting in objective clinical benefit Can be addressed by “off-the-shelf” library or personalized approaches
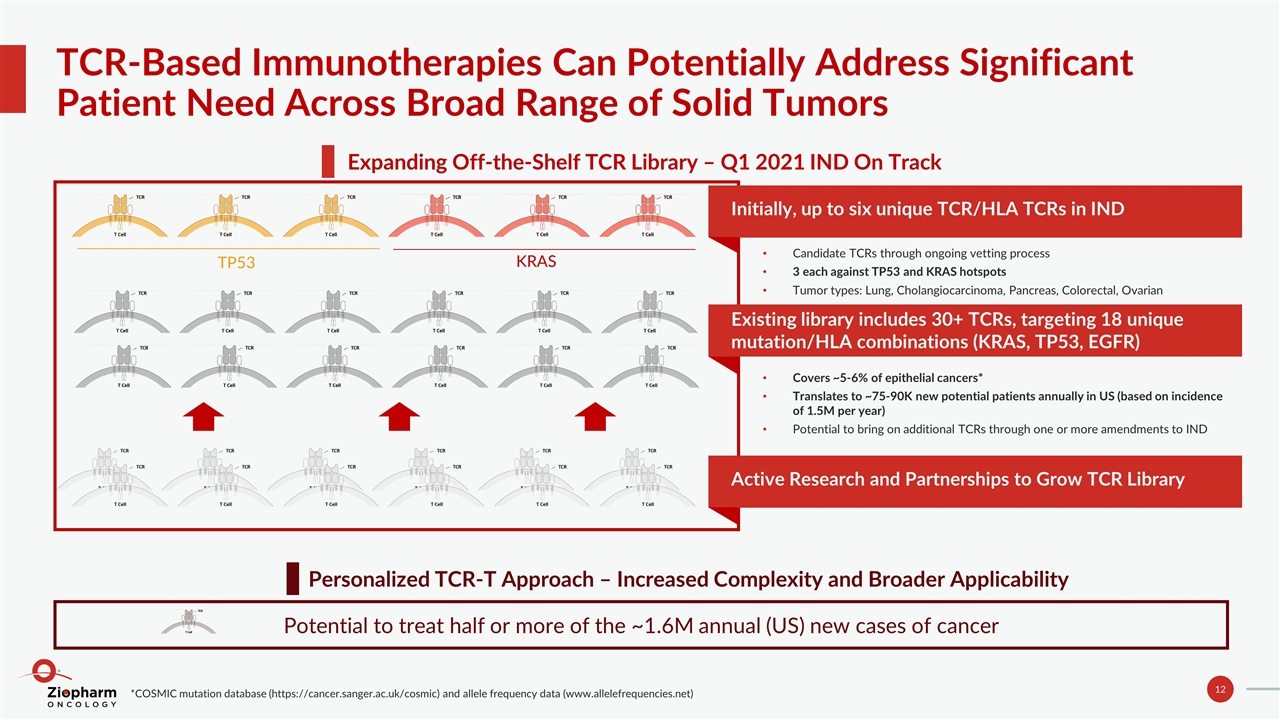
TCR-Based Immunotherapies Can Potentially Address Significant Patient Need Across Broad Range of Solid Tumors Expanding Off-the-Shelf TCR Library – Q1 2021 IND On Track Personalized TCR-T Approach – Increased Complexity and Broader Applicability Potential to treat half or more of the ~1.6M annual (US) new cases of cancer TP53 KRAS Initially, up to six unique TCR/HLA TCRs in IND Candidate TCRs through ongoing vetting process 3 each against TP53 and KRAS hotspots Tumor types: Lung, Cholangiocarcinoma, Pancreas, Colorectal, Ovarian Existing library includes 30+ TCRs, targeting 18 unique mutation/HLA combinations (KRAS, TP53, EGFR) Covers ~5-6% of epithelial cancers* Translates to ~75-90K new potential patients annually in US (based on incidence of 1.5M per year) Potential to bring on additional TCRs through one or more amendments to IND Active Research and Partnerships to Grow TCR Library *COSMIC mutation database (https://cancer.sanger.ac.uk/cosmic) and allele frequency data (www.allelefrequencies.net)
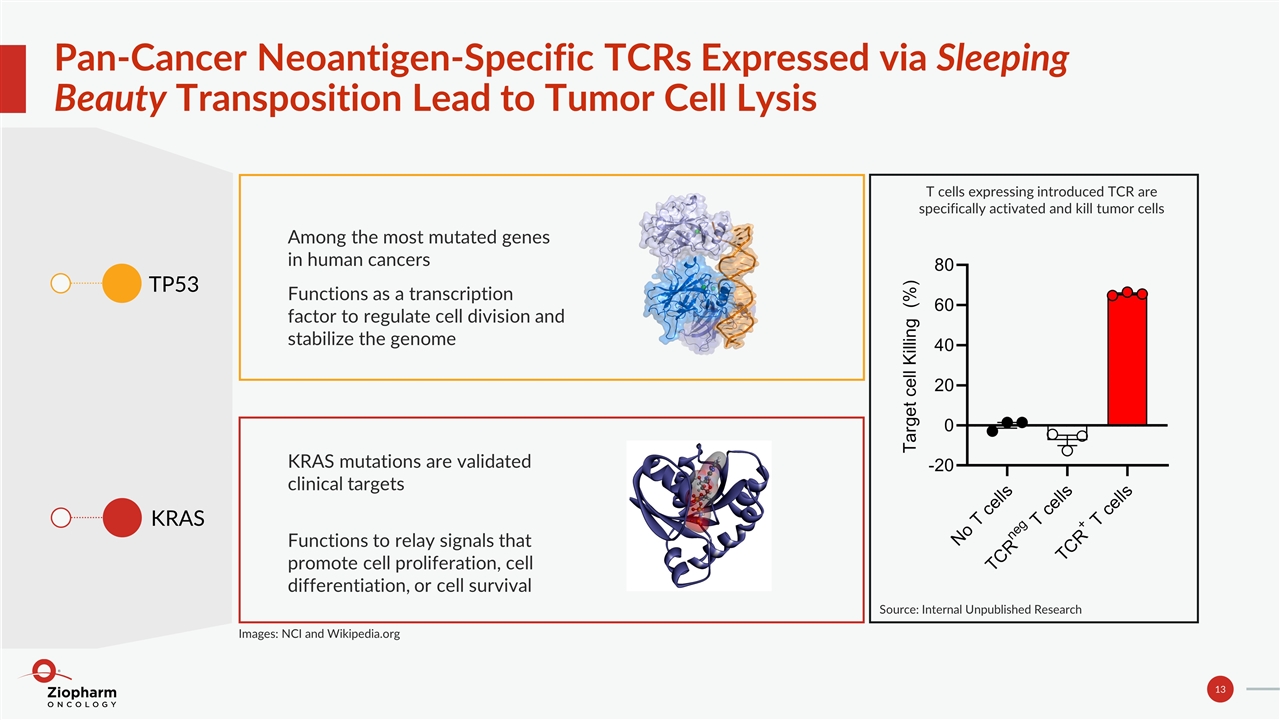
Pan-Cancer Neoantigen-Specific TCRs Expressed via Sleeping Beauty Transposition Lead to Tumor Cell Lysis TP53 KRAS Among the most mutated genes in human cancers Functions as a transcription factor to regulate cell division and stabilize the genome KRAS mutations are validated clinical targets Functions to relay signals that promote cell proliferation, cell differentiation, or cell survival T cells expressing introduced TCR are specifically activated and kill tumor cells Images: NCI and Wikipedia.org Source: Internal Unpublished Research
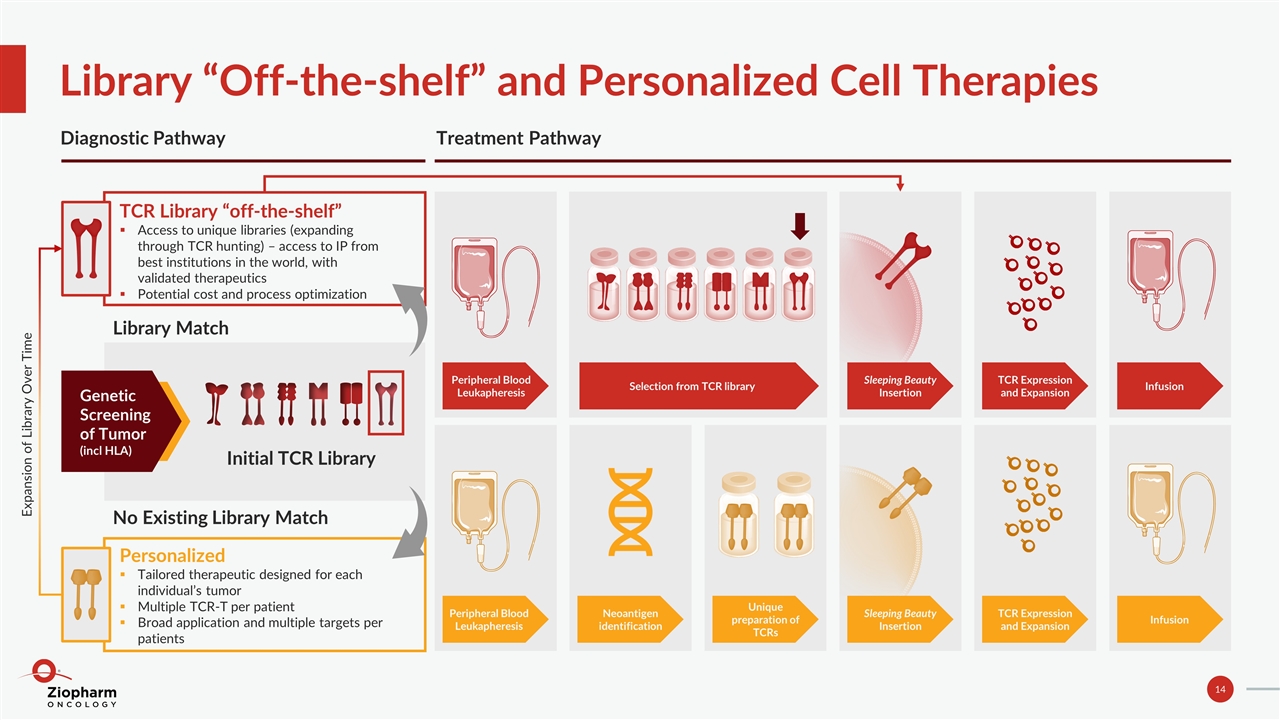
Library “Off-the-shelf” and Personalized Cell Therapies Diagnostic Pathway Treatment Pathway Genetic Screening of Tumor (incl HLA) Initial TCR Library Library Match No Existing Library Match TCR Library “off-the-shelf” Access to unique libraries (expanding through TCR hunting) – access to IP from best institutions in the world, with validated therapeutics Potential cost and process optimization Personalized Tailored therapeutic designed for each individual’s tumor Multiple TCR-T per patient Broad application and multiple targets per patients Peripheral Blood Leukapheresis Neoantigen identification Unique preparation of TCRs Sleeping Beauty Insertion TCR Expression and Expansion Infusion Peripheral Blood Leukapheresis Sleeping Beauty Insertion TCR Expression and Expansion Infusion Selection from TCR library Expansion of Library Over Time
01 02 03 04 Agenda Where We Are Entering The Year TCR-T Program: Our Top Priority And Opportunity Approach For Our CAR-T And IL-12 Programs 2021 Expectations
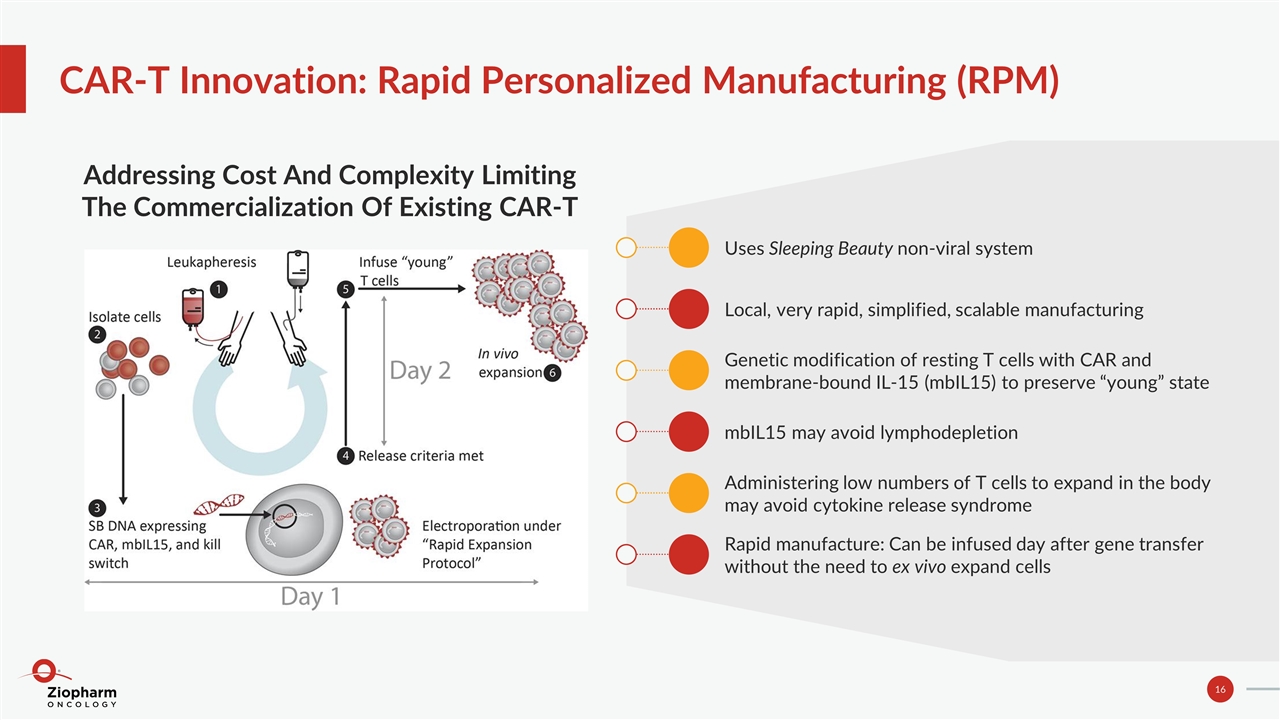
CAR-T Innovation: Rapid Personalized Manufacturing (RPM) Addressing Cost And Complexity Limiting The Commercialization Of Existing CAR-T Uses Sleeping Beauty non-viral system Local, very rapid, simplified, scalable manufacturing Genetic modification of resting T cells with CAR and membrane-bound IL-15 (mbIL15) to preserve “young” state mbIL15 may avoid lymphodepletion Administering low numbers of T cells to expand in the body may avoid cytokine release syndrome Rapid manufacture: Can be infused day after gene transfer without the need to ex vivo expand cells

CD19 CAR Rapid Personalized Manufacturing (RPM) – Clinical Programs A solution to cost and complexity of commercial CAR-T today with one continuous program Phase 1 Trial Initiated To Evaluate Allogeneic CD19 CAR-T Investigational treatment for patients with CD19+ leukemias and lymphomas who have relapsed after allogeneic bone marrow transplantation Strategic purpose to validate Ziopharm’s RPM technology, potential commercial opportunity Infuse as soon as day after gene transfer Trial to be conducted at MD Anderson; Initiation announced in July 2020 Ziopharm & Eden BioCell pursuing autologous CD19 CAR-T 50-50 joint venture with TriArm Therapeutics Taiwan: Eden BioCell IND cleared in Q4 2020 for Phase 1 trial Mainland China: Infusion of several patients with encouraging data
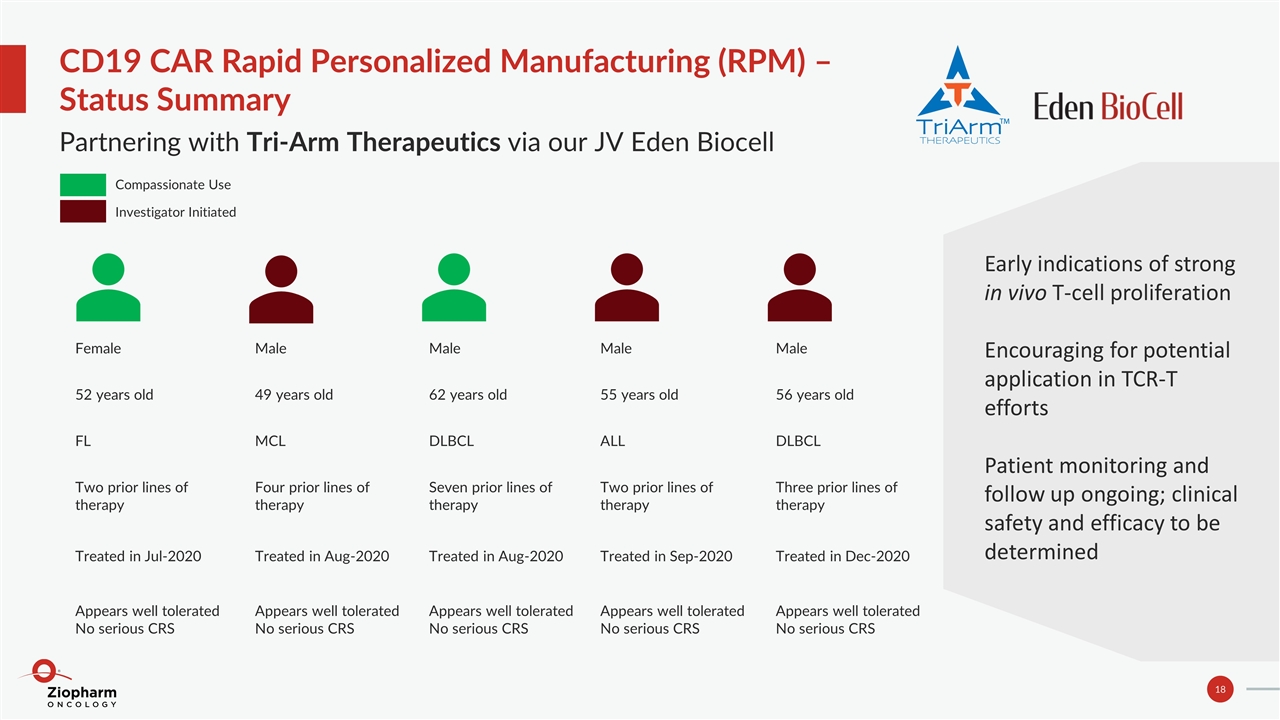
CD19 CAR Rapid Personalized Manufacturing (RPM) – Status Summary Partnering with Tri-Arm Therapeutics via our JV Eden Biocell Female 52 years old FL Two prior lines of therapy Treated in Jul-2020 Appears well tolerated No serious CRS Male 49 years old MCL Four prior lines of therapy Treated in Aug-2020 Appears well tolerated No serious CRS Male 62 years old DLBCL Seven prior lines of therapy Treated in Aug-2020 Appears well tolerated No serious CRS Male 55 years old ALL Two prior lines of therapy Treated in Sep-2020 Appears well tolerated No serious CRS Male 56 years old DLBCL Three prior lines of therapy Treated in Dec-2020 Appears well tolerated No serious CRS Compassionate Use Investigator Initiated Early indications of strong in vivo T-cell proliferation Encouraging for potential application in TCR-T efforts Patient monitoring and follow up ongoing; clinical safety and efficacy to be determined
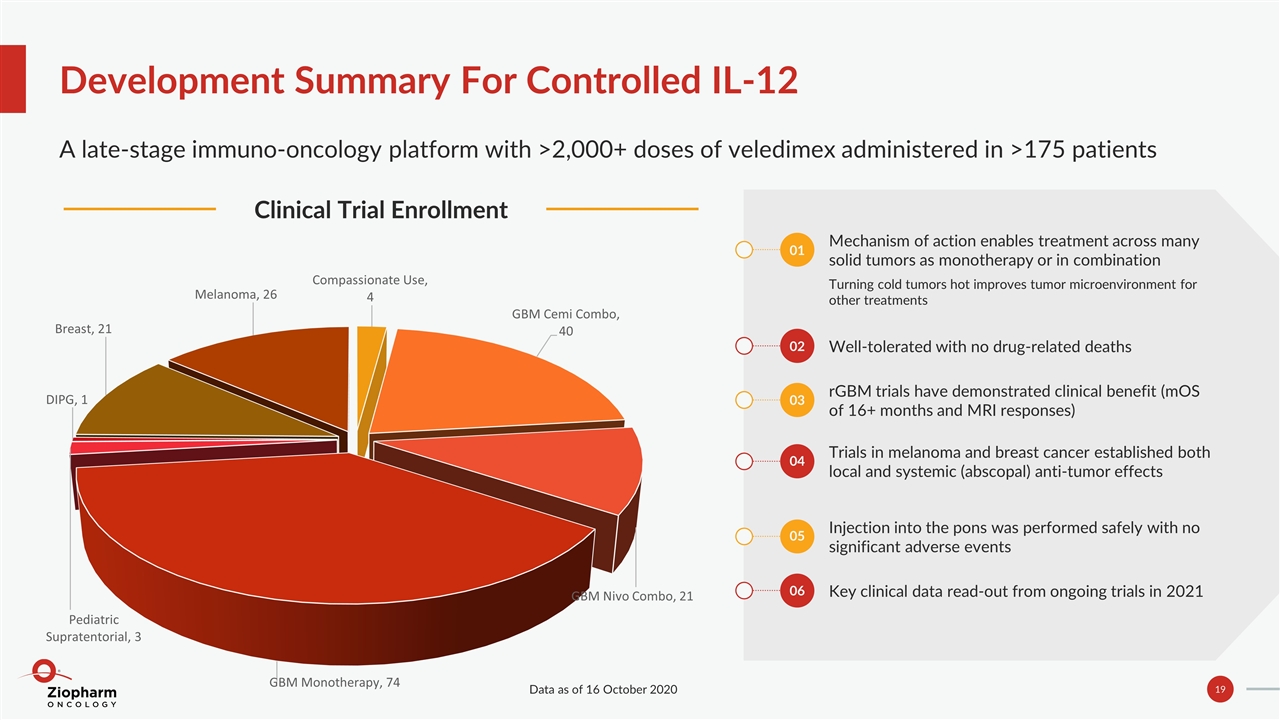
Development Summary For Controlled IL-12 A late-stage immuno-oncology platform with >2,000+ doses of veledimex administered in >175 patients Clinical Trial Enrollment Mechanism of action enables treatment across many solid tumors as monotherapy or in combination Turning cold tumors hot improves tumor microenvironment for other treatments Well‐tolerated with no drug‐related deaths rGBM trials have demonstrated clinical benefit (mOS of 16+ months and MRI responses) Trials in melanoma and breast cancer established both local and systemic (abscopal) anti-tumor effects Injection into the pons was performed safely with no significant adverse events Key clinical data read-out from ongoing trials in 2021 01 02 03 04 05 06 Data as of 16 October 2020
01 02 03 04 Agenda Where We Are Entering The Year TCR-T Program: Our Top Priority And Opportunity Approach For Our CAR-T And IL-12 Programs 2021 Expectations
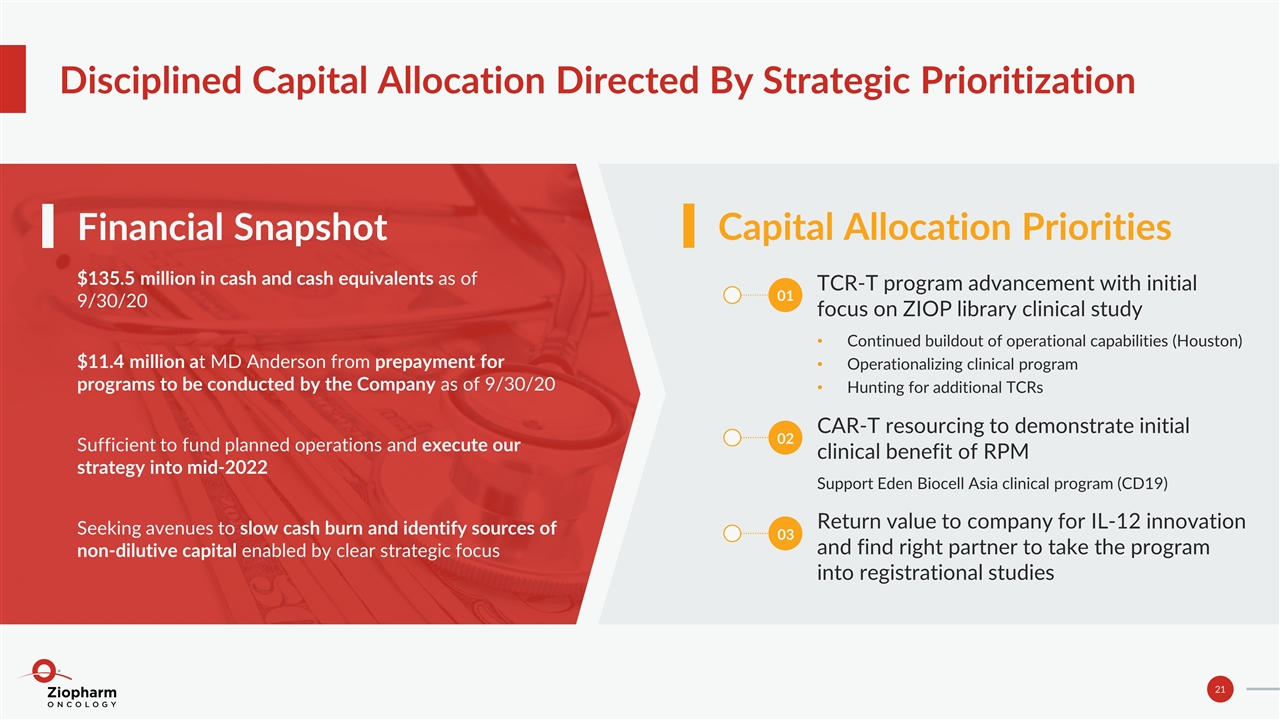
Disciplined Capital Allocation Directed By Strategic Prioritization $135.5 million in cash and cash equivalents as of 9/30/20 $11.4 million at MD Anderson from prepayment for programs to be conducted by the Company as of 9/30/20 Sufficient to fund planned operations and execute our strategy into mid-2022 Seeking avenues to slow cash burn and identify sources of non-dilutive capital enabled by clear strategic focus Financial Snapshot Capital Allocation Priorities TCR-T program advancement with initial focus on ZIOP library clinical study Continued buildout of operational capabilities (Houston) Operationalizing clinical program Hunting for additional TCRs CAR-T resourcing to demonstrate initial clinical benefit of RPM Support Eden Biocell Asia clinical program (CD19) Return value to company for IL-12 innovation and find right partner to take the program into registrational studies 01 02 03
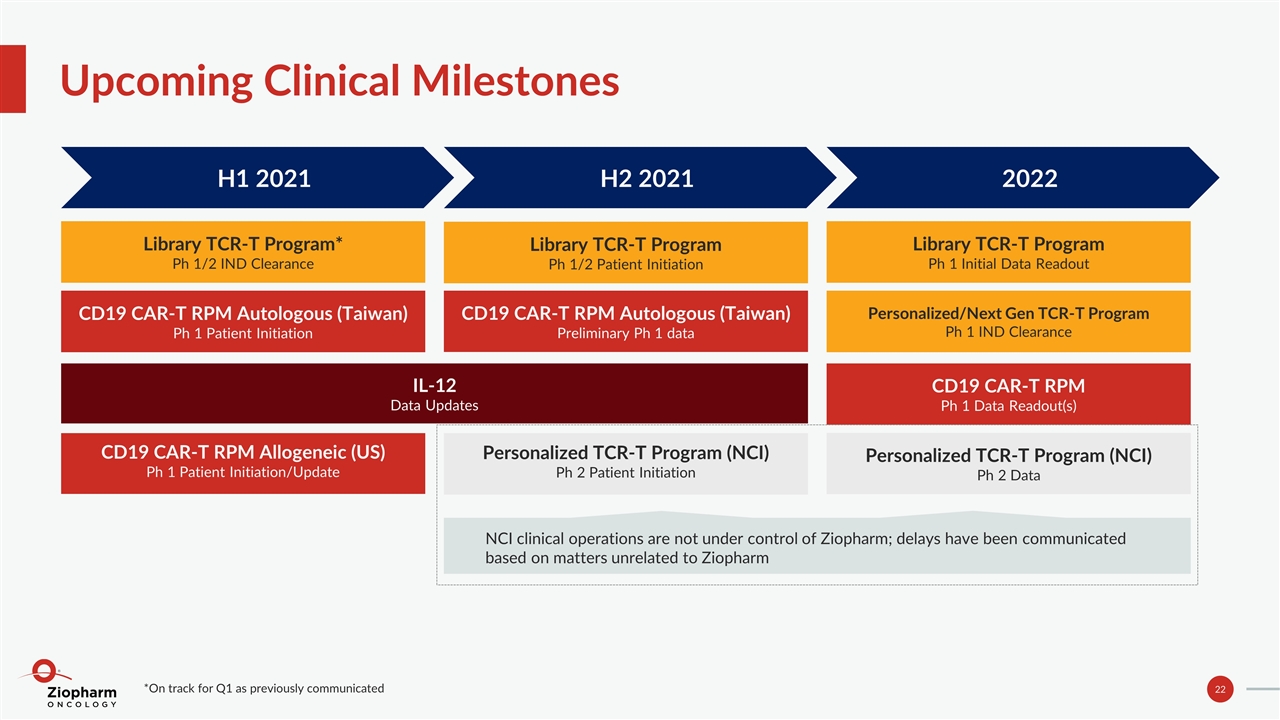
Upcoming Clinical Milestones Library TCR-T Program* Ph 1/2 IND Clearance H1 2021 CD19 CAR-T RPM Autologous (Taiwan) Preliminary Ph 1 data H2 2021 Library TCR-T Program Ph 1 Initial Data Readout 2022 Personalized TCR-T Program (NCI) Ph 2 Patient Initiation Personalized/Next Gen TCR-T Program Ph 1 IND Clearance IL-12 Data Updates CD19 CAR-T RPM Autologous (Taiwan) Ph 1 Patient Initiation CD19 CAR-T RPM Ph 1 Data Readout(s) Personalized TCR-T Program (NCI) Ph 2 Data *On track for Q1 as previously communicated CD19 CAR-T RPM Allogeneic (US) Ph 1 Patient Initiation/Update Library TCR-T Program Ph 1/2 Patient Initiation NCI clinical operations are not under control of Ziopharm; delays have been communicated based on matters unrelated to Ziopharm
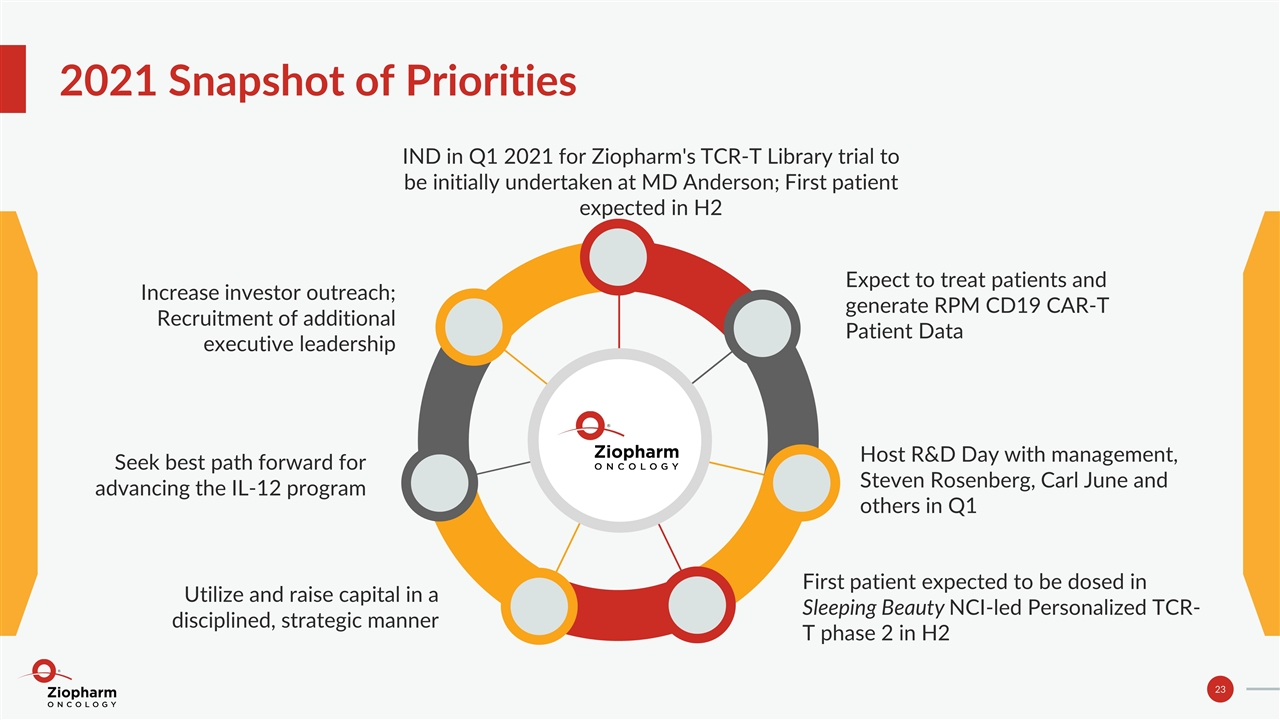
2021 Snapshot of Priorities Host R&D Day with management, Steven Rosenberg, Carl June and others in Q1 Expect to treat patients and generate RPM CD19 CAR-T Patient Data IND in Q1 2021 for Ziopharm's TCR-T Library trial to be initially undertaken at MD Anderson; First patient expected in H2 First patient expected to be dosed in Sleeping Beauty NCI-led Personalized TCR-T phase 2 in H2 Increase investor outreach; Recruitment of additional executive leadership Seek best path forward for advancing the IL-12 program Utilize and raise capital in a disciplined, strategic manner
