Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Ocugen, Inc. | ocgn-20210105x8k.htm |
Our Mission is to Develop Gene Therapies to Cure Blindness Diseases and Develop a Vaccine to fight COVID-19 NASDAQ: OCGN Corporate Deck: January 2021
1©2021 Ocugen. All Rights Reserved. Forward Looking Statement This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our business strategy, future results of operations and financial position, prospective products, product approvals, research and development costs, timing and likelihood of success, estimated market size or growth, and plans and objectives of management for future operations, are forward-looking statements. When used in this presentation, the words “anticipate,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “would,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Forward-looking statements involve known and unknown risks, uncertainties and other factors, including those risks set forth in the Company’s filings with the Securities and Exchange Commission, which are available at www.sec.gov, that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Forward-looking statements are based on our management’s beliefs and assumptions and on information available to management as of the date of this presentation. Our actual future results may be materially different from what we expect. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. This presentation includes estimates by us of statistical data relating to market size and growth and other estimated data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. This presentation also includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. While we believe these industry publications and third-party research, surveys and studies are reliable, we have not independently verified such data. This communication shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended.

2©2021 Ocugen. All Rights Reserved. Developing transformative therapies for blindness diseases and a vaccine for COVID-19 OCUGEN’S BREAKTHROUGH MODIFIER GENE THERAPY PLATFORM NOVEL BIOLOGIC Potential for one product to treat many diseases & multi-factor approach (POC study results published in Nature) OCU400 (AAV-NR2E3): 4 FDA Orphan Drug Designations with the potential to treat broad Retinitis Pigmentosa (RP), which has over 150 gene mutations, in lieu of developing separate therapies for each mutation under traditional gene therapy – initiation of Phase 1/2a within a year OCU410 (AAV-RORA): Potential to treat dry age-related macular degeneration (Dry AMD) through multi-factor treatment approach – initiation of Phase 1/2 in 2022 Strategic manufacturing partnership with CanSinoBio (~$7B market cap) – sets clear path for critical manufacturing OCU200: Targeting major retinal diseases: Diabetic Macular Edema (DME), Diabetic Retinopathy (DR), and Wet Age- Related Macular Degeneration (Wet AMD) (estimated global market size over $10B) – initiation of Phase 1/2 in 2022 Novel MoA: Potential to initially treat non-responders to anti-VEGF/ therapies (~50% of patients) Ocugen Overview COVID-19 VACCINE COVAXIN™: Whole-virion inactivated COVID-19 vaccine candidate (with adjuvant). Co-develop with Bharat Biotech for the US market (currently received EUA in India). Standard vaccine storage condition (2-8°C) Promising safety and immunogenicity demonstrated by the Phase 1 and Phase 2 trials in India. Currently in Phase 3 trials in India involving about 26,000 volunteers Potential coverage against multiple protein antigens of the virus and potentially applicable to broader population

3©2021 Ocugen. All Rights Reserved. Shankar Musunuri, PhD, MBA Chairman, CEO and Co-Founder Sanjay Subramanian, MBA CFO and Head of Corporate Development Vijay Tammara, PhD SVP, Regulatory & Quality Jessica Crespo, CPA Corporate Controller Arun Upadhyay, PhD Head of Research & Development Mohamed Genead, MD Acting CMO and Chair of SAB Leadership Team Ophthalmology and Vaccine-Focused Expertise

4©2021 Ocugen. All Rights Reserved. Mohamed Genead, MD Chair Retina Scientific Advisory Board David Boyer, MD Carl D. Regillo, MD, FACS Mark Pennesi, MD, PhD Geeta Lalwani, MD Scientific Advisory Boards Vaccine Scientific Advisory Board Satish Chandran PhD David Fajgenbaum, MD, MBA, MSc, FCPP Bruce Forrest, MD, MBA, MB, BS Catharine Pachuck, PhD Harvey Rubin, MD, PhD Susan Weiss, PhD

5©2021 Ocugen. All Rights Reserved. Pipeline Overview * No approved therapies exist https://www.aao.org/eye-health/diseases/retinitis-pigmentosa-treatment https://www.aao.org/eye-health/diseases/amd-treatment * * * * * Program Indication Prevalence (US) Discovery Preclinical IND-Enabling Phase 1/2 NR2E3 Mutation - Associated Retinal Degeneration 500 - 600 RHO Mutation - Associated Retinal Degeneration 10,400 - 12,700 CEP290 Mutation - Associated Retinal Degeneration 2,500 - 3,000 PDE6B Mutation - Associated Retinal Degeneration 1800 - 2800 OCU410 AAV-hRORA Dry Age Related Macular Degeneration (Dry AMD) 9M - 10M Diabetic Macular Edema 0.75M Diabetic Retinopathy 7.7M Wet Age Related Macular Degeneration (Wet AMD) 1.1M OCU400 AAV-hNR2E3 OCU200 Transferrin- Tumstatin Modifier Gene Therapy Platform Novel Biologic Orphan US Orphan US Orphan US Orphan US Vaccine COVAXIN™ Whole-Virion Inactivated Vaccine COVID-19 • EUA in India • Phase-3 in progress in India • US EUA pathway in development

6 COVAXIN™ Whole-Virion Inactivated COVID-19 Vaccine Being Co-Developed for the US Market

7©2021 Ocugen. All Rights Reserved. COVID-19 Vaccine in Development for US Market Evaluated in about 1,000 subjects in Phase 1 & 2 clinical trials in India. Phase 3 trial underway in India involving about 26,000 volunteers Promising safety and immunogenicity demonstrated by the Phase 1 and Phase 2 data The adjuvanted inactivated virus vaccine candidate elicited strong IgG responses against spike (S1) protein, receptor-binding domain (RBD), and the nucleocapsid (N) protein of SARS-CoV-2 along with strong cellular responses Highly purified and inactivated vaccine, manufactured in a vero cell manufacturing platform with an excellent safety track record Vaccine scientific advisory board (SAB) established with esteemed thought leaders from industry and academia Evaluating clinical and regulatory path for EUA in US COVAXIN™ has received Emergency Use Authorization (EUA) in India

Ocugen’s Modifier Gene Therapy Platform Breakthrough Technology Designed to Address Multiple Diseases with One Product Approach Complex Diseases Through Multiple Factors

9©2021 Ocugen. All Rights Reserved. Novel approach that targets nuclear hormone genes (NHRs), which regulate multiple functions within the retina Smoother regulatory pathway due to ability to target multiple diseases with one product Ability to recoup development costs over multiple therapeutic indications Gene Augmentation: Transfer functional version of a non-functional gene into the target cells. Modifier Gene Therapy: Introduce a functional gene to modify the expression of many genes, gene-networks and regulate basic biological processes in retina Traditional approach that targets one individual gene mutation at a time Regulatory pathway focused on specific product for one disease Longer time to recoup development costs Cell with mutated/ nonfunctioning gene X Cell with mutated/nonfunctioning gene(s) other than modifier gene Cell with normal function GENE X GENE X GENE X GENE X GENE X GENE X Normal gene X GENE X GENE M Modifier gene M Cell Cell Cell Cell GENE M GENE X Cell Cell We can address a number of diseases using the same Modifier Gene product. Traditional Gene Therapy ONE Disease Traditional Approach vs. Ocugen’s Novel Platform Rhodopsin Mutation-Associated Retinal Disease OCU400 Broad Spectrum Therapy for RPCEP290 Mutation-Associated Retinal Disease PDE6B Mutation-Associated Retinal Disease NR2E3 Mutation-Associated Retinal Disease

10©2021 Ocugen. All Rights Reserved. Why Target Nuclear Hormone Receptor Genes (NHRs)? Modulators of retinal development & function Act as “master genes” in the retina Molecular reset of key transcription factors and associated gene networks – retinal homeostasis Gene modifier concept including impact on clinical phenotypes is well known in other disease areas, CF and SMA * Inflammation & Cell Survival Phototransduction Metabolism Photoreceptor Development Cone Cell Development NR2E3 NR1D1 RORA Key Mutations: RGR, RHO, PDE6 Key Mutations: PRP16, OTX Key Mutations: GNB3, RP78, GNAT Key Mutations: PEX7 Key Mutations: NR2E3, RP68 * References: https://pubmed.ncbi.nlm.nih.gov/28556246/ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5409218/ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4339951/ https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0183526

11©2021 Ocugen. All Rights Reserved. Efficacy results shown in 5 unique mouse models of RP Technology developed at Harvard Medical School, Dr. Neena Haider’s Lab Study demonstrates potency of modifier gene therapy to elicit broad-spectrum therapeutic benefits in early and advanced stages of RP Results show evidence of vision rescue in Early & Advanced Stages of disease https://www.nature.com/articles/s41434-020-0134-z Preclinical POC Data for Nr2e3 Published in Nature Gene Therapy Important milestone for development of therapy; demonstrated proof of principle Protection elicited in multiple animal models of degeneration caused by different mutations Potential to represent first broad-spectrum therapy and to provide rescue even after disease onset Nature Gene Therapy Publication

12©2021 Ocugen. All Rights Reserved. Human Diseases: Control PDE6β associated RP Rhodopsin associated adRP Leber Congenital Amaurosis Enhanced S-cone Syndrome • P0 single subretinal injection, evaluation 3-4 months post injection • rd1 evaluated one-month post injection ONL: Outer Nuclear Layer • P21 subretinal injection, evaluation 2–3 months post injection • Restored ONL photoreceptors morphology in rd7 • ONL cell layer change in rd7 model doesn’t progress until 4-5 mos. of age Fundus images and ONL count show how single product recuses vision in multiple mutations https://www.nature.com/articles/s41434-020-0134-z OCU400 – Rescue in Early & Advanced Stage of Disease Early Stage Rescue Advanced Stage Rescue

13©2021 Ocugen. All Rights Reserved. ERG response: P0 single subretinal injection, evaluation 3-4 months post injection Human vision is enabled by three primary modes: • Photopic vision: Vision under well-lit conditions, which provides for color perception and functions primarily due to cone cells in the eye • Mesopic vision: A combination of photopic vision and scotopic vision in low lighting, which functions due to a combination of rod and cone cells in the eye • Scotopic vision: Monochromatic vision in very low light, which functions primarily due to rod cells in the eye https://www.nature.com/articles/s41434-020-0134-z OCU400 – Demonstrates Improved Vision Signals in Retina Electroretinogram (ERG) Response Reveals Rescue under Both Scotopic (dim-lit) as well as Photopic (well-lit) Conditions

14©2021 Ocugen. All Rights Reserved. https://www.nature.com/articles/s41434-020-0134-z Study Results Confirm Overexpression of Nr2e3 by subretinal AAV8-Nr2e3 Injection Is Not Detrimental to Retina – No Off-Target Effects OCU400 – Demonstrated Safety in Mouse Model
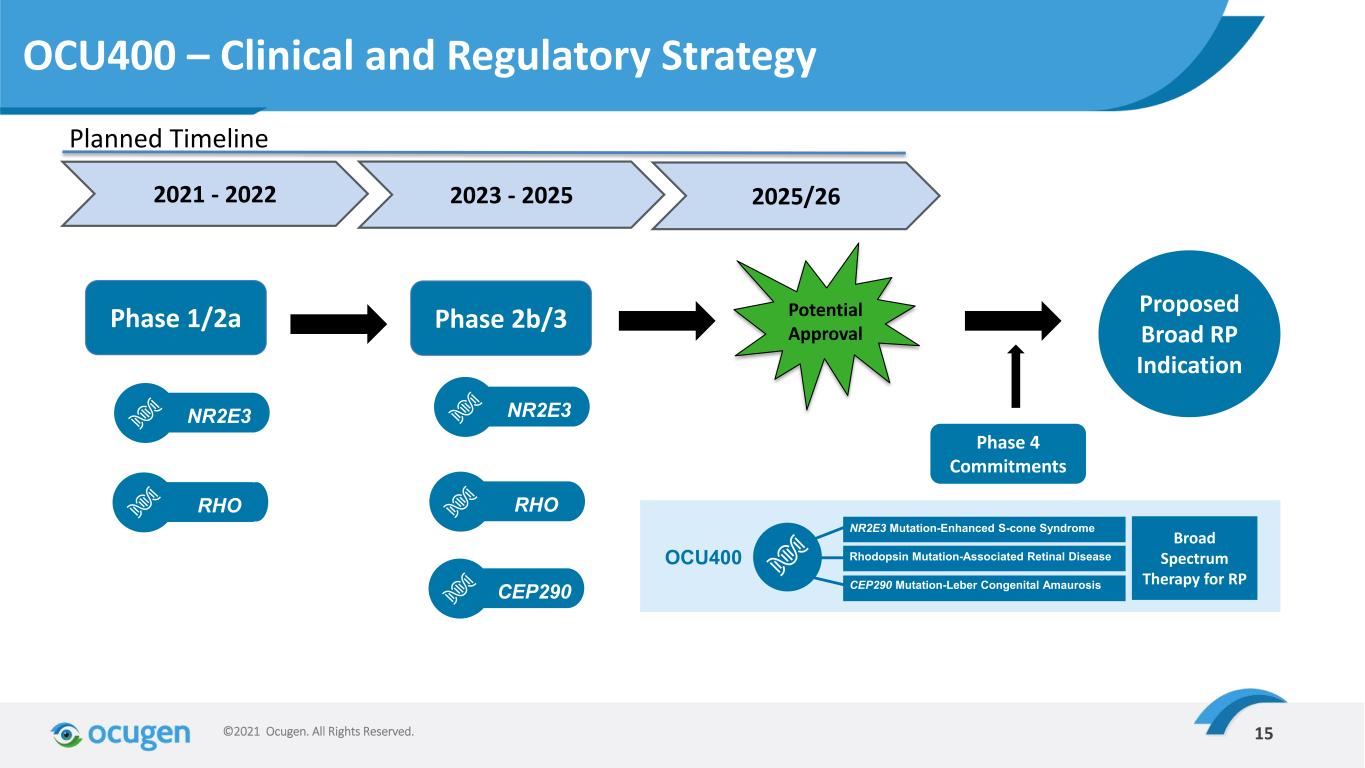
15©2021 Ocugen. All Rights Reserved. Phase 2b/3Phase 1/2a NR2E3 RHO NR2E3 RHO CEP290 Potential Approval Proposed Broad RP Indication Phase 4 Commitments OCU400 NR2E3 Mutation-Enhanced S-cone Syndrome Rhodopsin Mutation-Associated Retinal Disease CEP290 Mutation-Leber Congenital Amaurosis Broad Spectrum Therapy for RP 2021 - 2022 2023 - 2025 2025/26 OCU400 – Clinical and Regulatory Strategy Planned Timeline
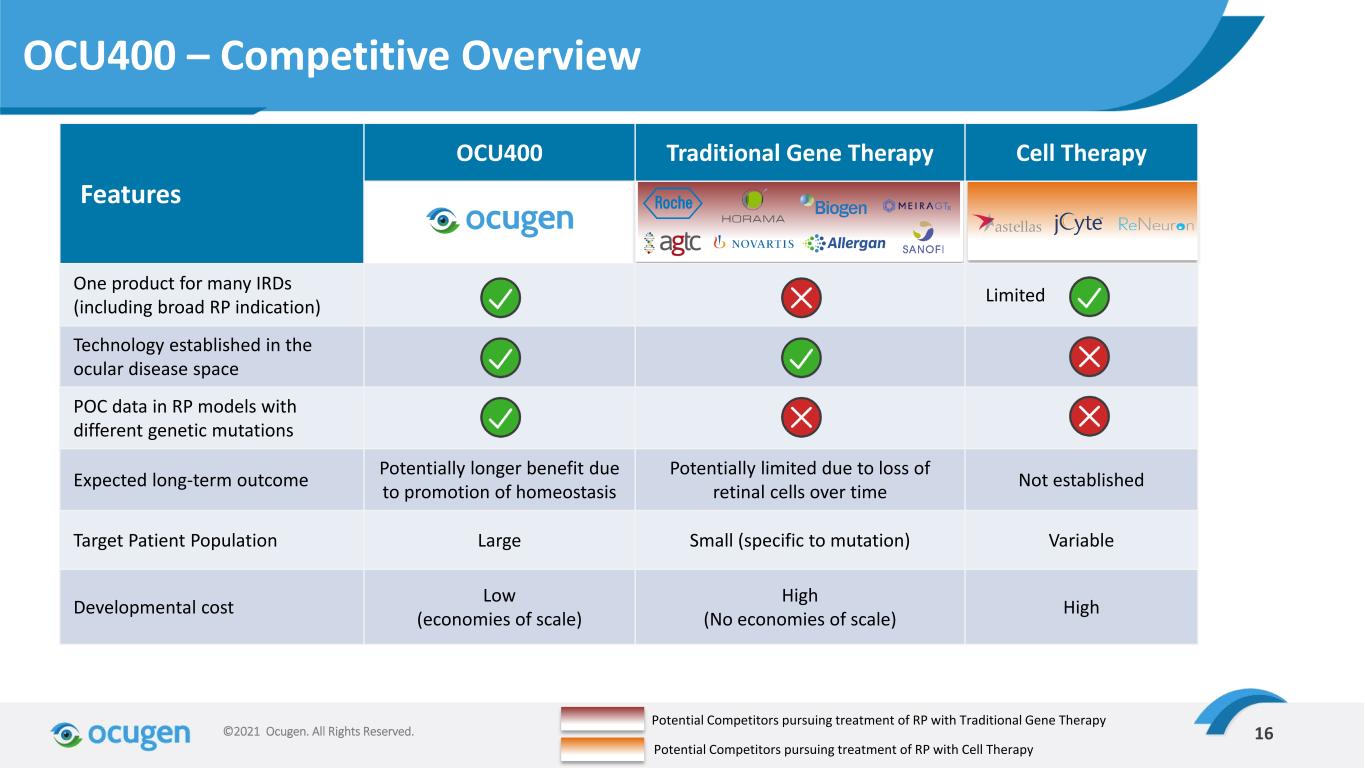
16©2021 Ocugen. All Rights Reserved. Features OCU400 Traditional Gene Therapy Cell Therapy One product for many IRDs (including broad RP indication) Limited Technology established in the ocular disease space POC data in RP models with different genetic mutations Expected long-term outcome Potentially longer benefit due to promotion of homeostasis Potentially limited due to loss of retinal cells over time Not established Target Patient Population Large Small (specific to mutation) Variable Developmental cost Low (economies of scale) High (No economies of scale) High OCU400 – Competitive Overview Potential Competitors pursuing treatment of RP with Traditional Gene Therapy Potential Competitors pursuing treatment of RP with Cell Therapy

17©2021 Ocugen. All Rights Reserved. Dry AMD Leads to irreversible blindness due to degeneration of the retina ~9-10M patients in the U.S. Currently no approved treatment for Dry AMD Contributing Factors Aging Genetics Environmental Factors Normal Retina Dry AMD Oxidative Stress RORA Inflammation Lipid Peroxidation References: https://www.brightfocus.org/macular/article/age-related-macular-facts-figures https://www.uniprot.org/uniprot/P35398#function https://pubmed.ncbi.nlm.nih.gov/21998696/ https://pubmed.ncbi.nlm.nih.gov/19786043/ OCU410 (AAV-RORA) – Dry Age-Related Macular Degeneration We Believe OCU410 Has the Potential to Address this Disease through its Multi-Factor Approach

18©2021 Ocugen. All Rights Reserved. Ocugen Partnership with CanSino Biologics Inc. (CanSinoBIO) CanSinoBIO to perform CMC development & manufacturing of clinical supplies for OCU400 Publicly-listed (6185.HK) with market cap of ~$7B State-of-the-art facilities with world class team Provides scalable GMP cell lines (such as HEK293 suspension culture adopted) for commercial manufacturing Responsible for all associated costs (typical costs until BLA filing ~$25M-$35M) Manufacturing at commercial scale (200L) for Phase 1/2 for product consistency CanSinoBIO has rights to develop, manufacture and commercialize OCU400 for Greater China Market Ocugen to receive mid to high single-digit royalties on Greater China sales CanSinoBio to receive low to mid single-digit royalties on all other global sales Source: Manufacturing Cures: Infrastructure Challenges Facing Cell And Gene Therapy Developers In Vivo June 2019 invivo.pharmaintelligence.informa.com Bloomberg: How a Chinese Firm Jumped to the Front of the Virus Vaccine Race Gene Therapy Manufacturing Partnership Helps Advance OCU400 into the Clinic with Significantly Reduced Capital and Resources

19 OCU200: Diabetic Macular Edema (DME) Diabetic Retinopathy (DR) Wet Age-Related Macular Degeneration (Wet AMD) Novel Biologic Offering Benefits Beyond Anti-VEGF

20©2021 Ocugen. All Rights Reserved. DME ~0.7M patients in the US* DR ~7.7M patients in the US* Wet AMD ~1.1M patients in the US* OCU200 is a Transferrin-Tumstatin Fusion Protein • Tumstatin: Multiple MOAs for treatment and prevention of macular degeneration and neovascularization • Transferrin: Targets the site of action and improves uptake (better target engagement) Integrin Targeting provides hope to these patients who are non-responders to current therapies Distinct MOA through targeting Integrin pathways can potentially also help reduce number of injections for patients who do respond to Anti-VEGF & corticosteroids therapies Significant global market potential ~50% of Patients DO NOT Respond to Anti-VEGF/Corticosteroids Therapies OCU200 – Potential to Treat DME, DR & Wet AMD OCU200 Provides Hope to All patients with DME, DR or Wet AMD https://www.gene.com/stories/retinal-diseases-fact-sheet https://www.brightfocus.org/macular/article/age-related-macular-facts-figures (*)

21©2021 Ocugen. All Rights Reserved. Data expressed as percentage of CNV lesions on Day 10 after treatment. Laser induction & treatment start on Day 0 Wet AMD In-Vivo Laser-Induced Mouse CNV Model * indicates p<0.05 when compared to PBS and/or tumstatin treatment † indicates p<0.05 when compared to Avastin; CNV lesions measured on day 14 after treatment Wet AMD In-Vivo Laser-Induced Rat CNV Model AvastinOCU200TumstatinPBS V e h i c le E y le a O C U 2 0 0 O C U 2 0 0 + E y le a 4 0 6 0 8 0 L e a k y C N V l e s io n s (% ) DME/DR Oxygen-Induced Retinopathy (OIR) Mouse Model Effect of OCU200 intravitreal treatments on Neovascularization (NV). Data are presented as mean± SD. Filled circles represent data points from individual eyes * P < 0.05, ** P < 0.01 (n = 9-10 eyes per group) • Inhibits new blood vessel formation • Anti-inflammatory • Anti-oxidative OCU200 –Transferrin-Tumstatin Fusion Protein OCU200 Demonstrated Superior Efficacy Compared to Existing Anti-VEGF Therapies -50 0 50 100 150 Neovascular area (normalized) N V (% o f c on tro l r et in a N V ) * ** **

22©2021 Ocugen. All Rights Reserved. Features OCU200 Anti-VEGF Anti-Integrin Reduces VEGF level/Fluid Selectively works on active endothelial cells (Neovascular) Activates native anti-angiogenic response Enhanced effective delivery through Transferrin Pro-apoptotic and anti-oxidative Dosing Frequency Expected once in 3 months 1-3 months 1-3 months (1) Approved (1) (1) (1) OCU200 – Distinct Mechanism of Action Potential Competitors pursuing treatment using Anti-VEGF approach Potential Competitors pursuing treatment using Anti-Integrin approach We believe OCU200 has the potential to become a disease modifying therapeutic for broader patient population

23©2021 Ocugen. All Rights Reserved. Near & Mid-Term Milestones: Planned Timeline 1H 2021 2H 2021 1H 2022 2H 2022 Expected -Orphan Drug Designation in EMA IND Filing Phase 1/2a start-US Phase 1/2a start-EU Safety & Efficacy signal Tox study initiation IND Filing Start Ph 1/2a Safety & Efficacy signal FDA pre- IND mtg Tox study initiation IND Filing Start Ph 1/2a OCU400 (AAV-NR2E3) IRDs (gene therapy) OCU410 (AAV-RORA) Dry AMD (gene therapy) OCU200 DME, DR, Wet-AMD (novel biologic)
24©2021 Ocugen. All Rights Reserved. Investment Highlights Modifier Gene Therapy Platform has the potential for one product to treat many diseases OCU200 novel biologic has the potential to treat anti-VEGF /corticosteroids non-responders (~50% of the patients) Multiple near and mid-term milestones with plan to initiate four Phase 1/2 trials within 1-2 years COVAXIN™ - Vaccine candidate for the US market

A Bold Vision to Cure Blindness Diseases and Offer a Differentiated Vaccine for COVID-19 in the US For more information, contact: IR@ocugen.com
