Attached files
| file | filename |
|---|---|
| 8-K - 8-K - HELIUS MEDICAL TECHNOLOGIES, INC. | hsdt-8k_20210105.htm |

Empowering the Healing Process of the Brain Disruptive Technology for Healthcare (NASDAQ:HSDT | TSX:HSM) Exhibit 99.1

Legal Disclaimers This presentation contains forward-looking statements, including statements about: uncertainties regarding the FDA regulatory approval process (including the FDA Breakthrough Designation), uncertainties regarding the regulatory approval process in China and Australia, whether the results of our clinical trials will be sufficient to support an FDA, CE Mark or TGA approval of the PoNS™ device for marketing or whether the agencies may require that the Company conduct future clinical trials; future economic, competitive, reimbursement and regulatory conditions; new product introductions; ability to commercialize its PoNS Treatment™; demographic trends; the intellectual property landscape; financial market conditions; continued availability of capital and financing, including its ability to continue as a going concern; and future business and strategic decisions made by the Company and its competitors. These statements involve substantial known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. Factors that may cause actual results to differ materially from any future results expressed or implied by any forward looking statements include the impact of the COVID-19 pandemic, uncertainties associated with clinical trial enrollments and the results of clinical trials, uncertainties associated with the clinical development process and regulatory submission and approval process, and other risks described in the “Risk Factors” section of Company’s Annual Report on Form 10-K for the year ended December 31, 2019 and the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2020, as well as those set forth from time to time in the Company’s other filings with the securities and exchange commission and the Canadian securities regulators available at http://www.sec.gov or www.sedar.com The forward-looking statements in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Certain data in this presentation was obtained from various external sources. Neither the Company nor its affiliates, advisers or representatives have verified such data with independent sources. Accordingly, neither the Company nor any of its affiliates, advisers or representatives make any representations as to the accuracy or completeness of that data or commits to update such data after the date of this presentation. Such data involves risks and uncertainties and is subject to change based on various factors. The Company’s first product, PoNS, is an authorized class II, non-implantable medical device authorized for sale in Canada. PoNS is intended as a short term treatment (14 weeks) of gait deficit due to symptoms from multiple sclerosis (“MS”) and balance deficit due to mild-to-moderate traumatic brain injury (“mmTBI”) and is to be used in conjunction with physical therapy (“PoNS Treatment"™). It is an investigational medical device in the United States, the European Union (“EU”), and Australia (“AUS”), and it is currently under review for clearance by the FDA for use in gait dysfunction due to MS and AUS Therapeutic Goods Administration. PoNS Treatment™ is not currently commercially available in the United States, the EU or Australia. 2
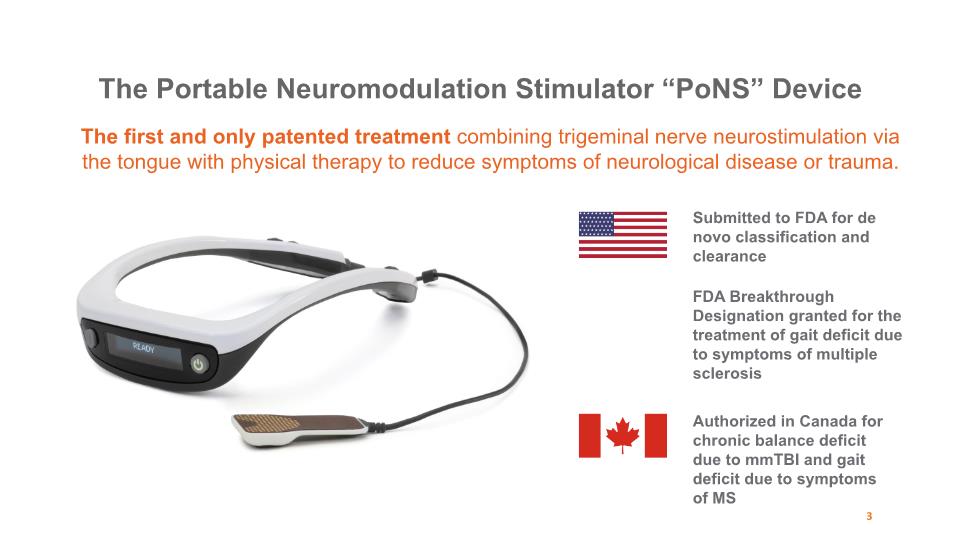
The first and only patented treatment combining trigeminal nerve neurostimulation via the tongue with physical therapy to reduce symptoms of neurological disease or trauma. The Portable Neuromodulation Stimulator “PoNS” Device Authorized in Canada for chronic balance deficit due to mmTBI and gait deficit due to symptoms of MS Submitted to FDA for de novo classification and clearance FDA Breakthrough Designation granted for the treatment of gait deficit due to symptoms of multiple sclerosis 3
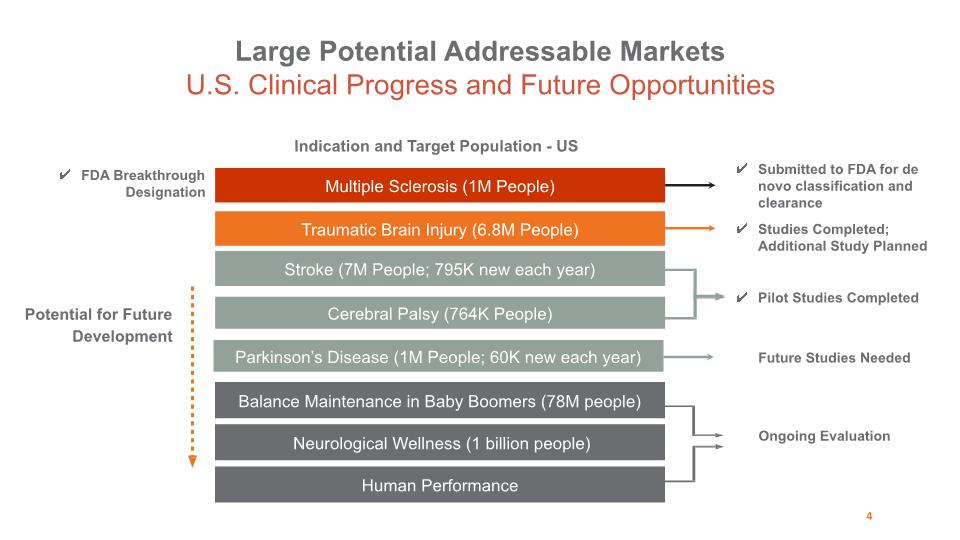
Stroke (7M People; 795K new each year) Cerebral Palsy (764K People) Indication and Target Population - US Potential for Future Development Submitted to FDA for de novo classification and clearance Ongoing Evaluation Multiple Sclerosis (1M People) Pilot Studies Completed Studies Completed; Additional Study Planned Traumatic Brain Injury (6.8M People) Parkinson’s Disease (1M People; 60K new each year) Future Studies Needed FDA Breakthrough Designation Large Potential Addressable Markets U.S. Clinical Progress and Future Opportunities 4

Breakthrough Designation Program Helius is working interactively with the FDA under the program Advantages Focused on addressing high unmet needs in gait deficit for MS patients Prioritized review of the submission under Breakthrough Designation Interacting with FDA to efficiently address topics as they arise during the premarket review phase Interacting and utilizing leading industry experts 5

Potential first 4 years reimbursement under the pending CMS policy for companies with a breakthrough designation and FDA clearance. 70 million people in the US are covered under Medicare and Medicaid, which PoNS will have access to if qualified. 6
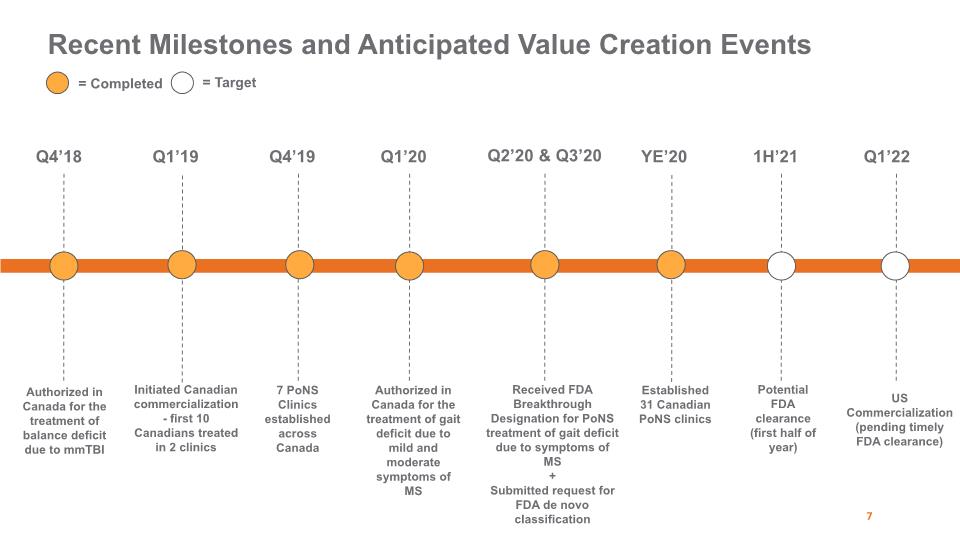
Authorized in Canada for the treatment of balance deficit due to mmTBI Recent Milestones and Anticipated Value Creation Events Q4’18 Q1’19 Initiated Canadian commercialization - first 10 Canadians treated in 2 clinics Q4’19 7 PoNS Clinics established across Canada Q1’20 Authorized in Canada for the treatment of gait deficit due to mild and moderate symptoms of MS Q2’20 & Q3’20 Received FDA Breakthrough Designation for PoNS treatment of gait deficit due to symptoms of MS + Submitted request for FDA de novo classification YE’20 Established 31 Canadian PoNS clinics 1H’21 Potential FDA clearance (first half of year) 7 = Completed = Target US Commercialization (pending timely FDA clearance) Q1’22

Dane Andreeff Interim CEO and Member, Board of Directors Dr. Jonathan Sackier Chief Medical Officer Joyce LaViscount Chief Financial Officer & Chief Operating Officer Mark Leno VP, General Manager, Canadian Operations Executive Team Experienced Leadership With Healthcare and Commercialization Expertise 20+ years at Maple Leaf Partners as the General Partner and Portfolio manager, a value-based hedge fund which grew to over $2b in assets Board member and advisor to Helius for over 3 years, Myocardial Solutions for 4 years and HDL Therapeutics, Inc. for over 15 years ~6.3% ownership of the company 30+ years in the health sciences industry Trained surgeon and pioneer of new medical technologies Has helped build several companies including medical technology, research and product-design and medical contract sales organizations 30+ years in the health sciences industry Accomplished pharmaceutical/healthcare public company CAO Former COO and CFO at MM Pharmaceutical Solutions Former Executive Director/Group Controller at Aptalis Pharmaceuticals 17+ years in the medical devices industry Sales and Marketing Director, Boston Scientific Canada National Sales Manager, Canada Johnson & Johnson Former Media Relations and Marketing executive for Blue Jays & NHL

Blane Walter Chairman of the Board Ed Straw Director Jeff Mathiesen, CPA Director Mitch Tyler Director Board of Directors Experienced Leadership With Healthcare and Commercialization Expertise Partner, Talisman Capital Partners Vice Chair of InVentive Health Chair of the Governor of Ohio’s Executive Workforce Board Former CEO of InVentive Health Former Founder of InChord Communications Founder, Managing Partner of Osprey Venture Partners Chairman of Odyssey Logistics Member of the Board of Directors of Performance Equity Management, Capital Teas and Document Capture Technologies, Inc. Former President, Global Operations, Estee Lauder Former SVP, Global Manufacturing and Supply Chain Management at Compaq Computer Corporation Distinguished 3-star Admiral, US Navy Vice Chair, Lead Independent Director, and Audit Committee Chair of Panbela Therapeutics, Inc. (Nasdaq: PBLA) Director and Audit Committee Chair of NeuroOne Medical Technologies Corporation (OTCQB: NMTC) Former Board Member and Audit Committee Chair of eNeura, Inc. Former CFO at Gemphire Therapeutics and Sunshine Heart Founder and Co-Inventor of PoNS™ technology Co-founder of Wicab, Inc and former VP, Research and Development Lead Inventor of the BrainPort Balance Device Former University of Wisconsin Biomedical Engineering faculty member MS, Biomedical Engineering and Registered Professional Engineer

PoNS™ Device Empowering the brain and recovery during PoNS Treatment PoNS controller and mouthpiece are connected by a cord Mouthpiece electrodes stimulate the tongue surface, sending signals to the brain 10
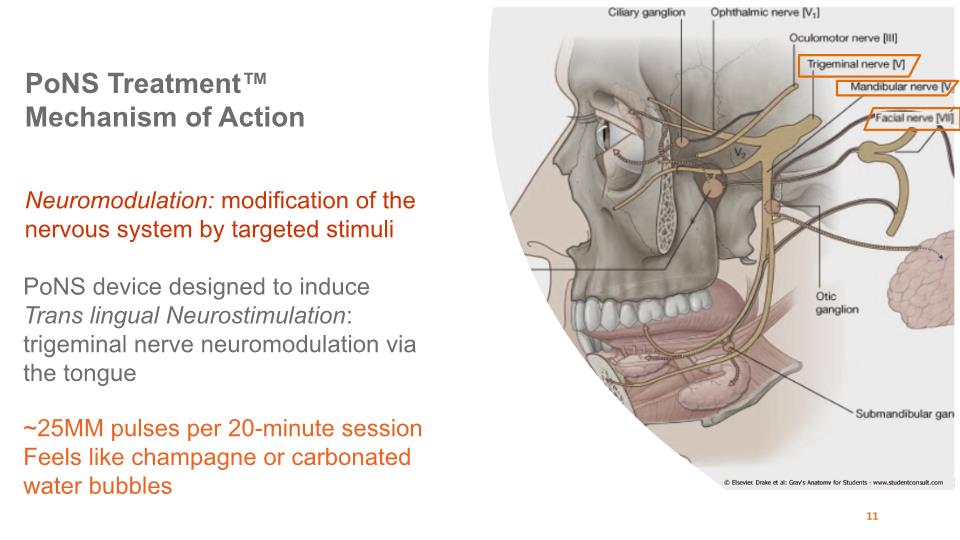
PoNS Treatment™ Mechanism of Action 11
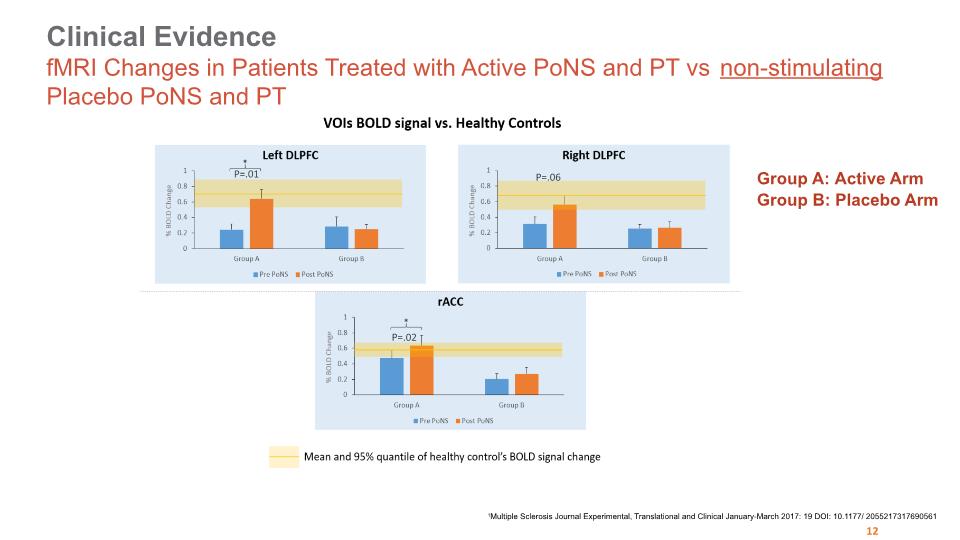
Clinical Evidence fMRI Changes in Patients Treated with Active PoNS and PT vs non-stimulating Placebo PoNS and PT 26 Group A: Active Arm Group B: Placebo Arm Active Arm Placebo Arm Active Arm Active Arm Placebo Arm 1Multiple Sclerosis Journal Experimental, Translational and Clinical January-March 2017: 19 DOI: 10.1177/ 2055217317690561 P=.01 P=.02 P=.06 12
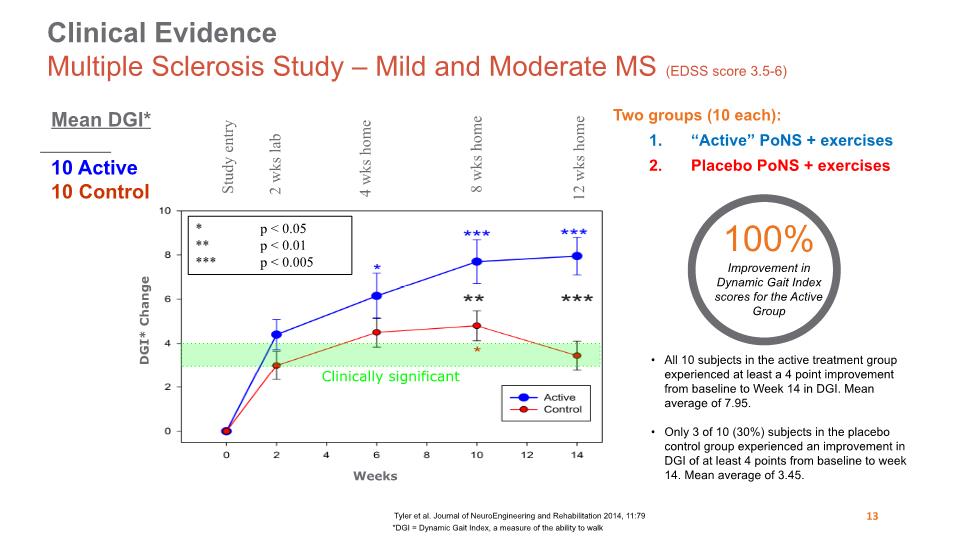
Study entry 2 wks lab 4 wks home 8 wks home 12 wks home Weeks DGI* Change * p < 0.05 ** p < 0.01 *** p < 0.005 Clinically significant Two groups (10 each): “Active” PoNS + exercises Placebo PoNS + exercises Clinical Evidence Multiple Sclerosis Study – Mild and Moderate MS (EDSS score 3.5-6) Tyler et al. Journal of NeuroEngineering and Rehabilitation 2014, 11:79 All 10 subjects in the active treatment group experienced at least a 4 point improvement from baseline to Week 14 in DGI. Mean average of 7.95. Only 3 of 10 (30%) subjects in the placebo control group experienced an improvement in DGI of at least 4 points from baseline to week 14. Mean average of 3.45. Mean DGI* 10 Active 10 Control *DGI = Dynamic Gait Index, a measure of the ability to walk 13

Extensive IP Portfolio Exclusively licensed from inventors (4% royalty): 9 US Medical Method Patents Issued Patents expire between 2029 and 2031 Patents owned by Helius (no royalty): 29 US Patents Issued 41 Foreign Patents Issued Patents expire between 2026 and 2040 Helius Patents Transferred to China Medical System Holdings (CMS): 3 Chinese Design Patents Independent Verification of Patents and Freedom to Operate Opinion September 2017 14
Current Strategies for Managing Neurological Disorders Prescription Drugs Therapy Surgery Medical Devices 15
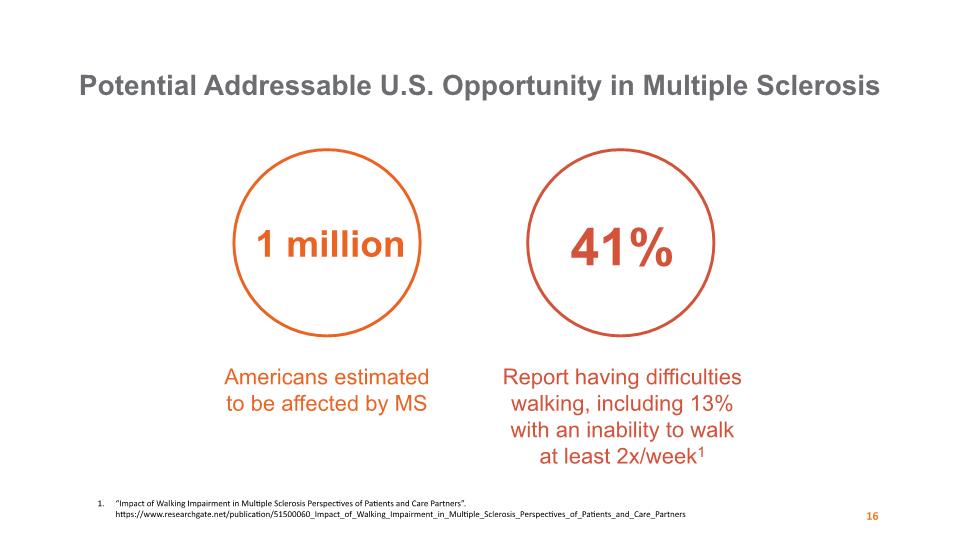
1 million 41% Americans estimated to be affected by MS Report having difficulties walking, including 13% with an inability to walk at least 2x/week1 Potential Addressable U.S. Opportunity in Multiple Sclerosis 16 “Impact of Walking Impairment in Multiple Sclerosis Perspectives of Patients and Care Partners”. https://www.researchgate.net/publication/51500060_Impact_of_Walking_Impairment_in_Multiple_Sclerosis_Perspectives_of_Patients_and_Care_Partners
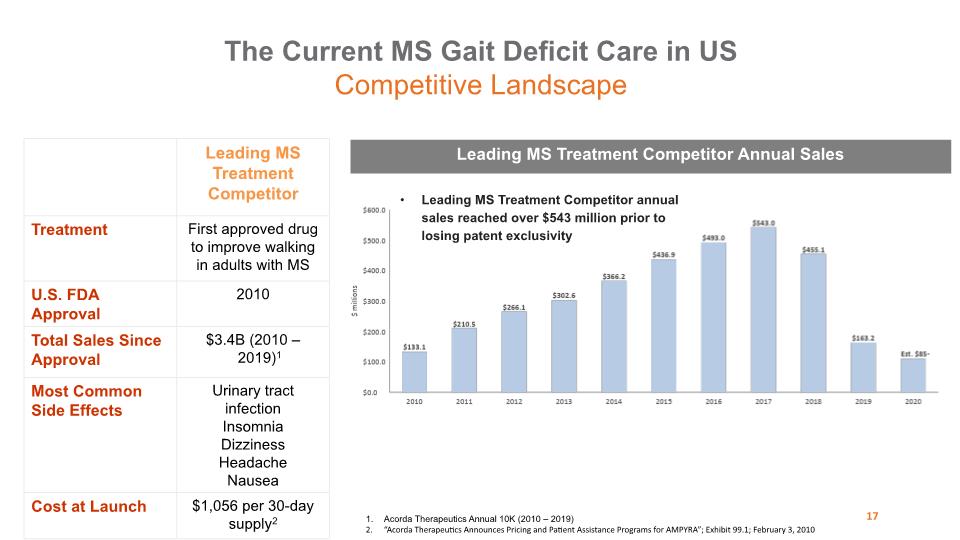
Leading MS Treatment Competitor annual sales reached over $543 million prior to losing patent exclusivity Leading MS Treatment Competitor Annual Sales The Current MS Gait Deficit Care in US Competitive Landscape 17 Acorda Therapeutics Annual 10K (2010 – 2019) “Acorda Therapeutics Announces Pricing and Patient Assistance Programs for AMPYRA”; Exhibit 99.1; February 3, 2010
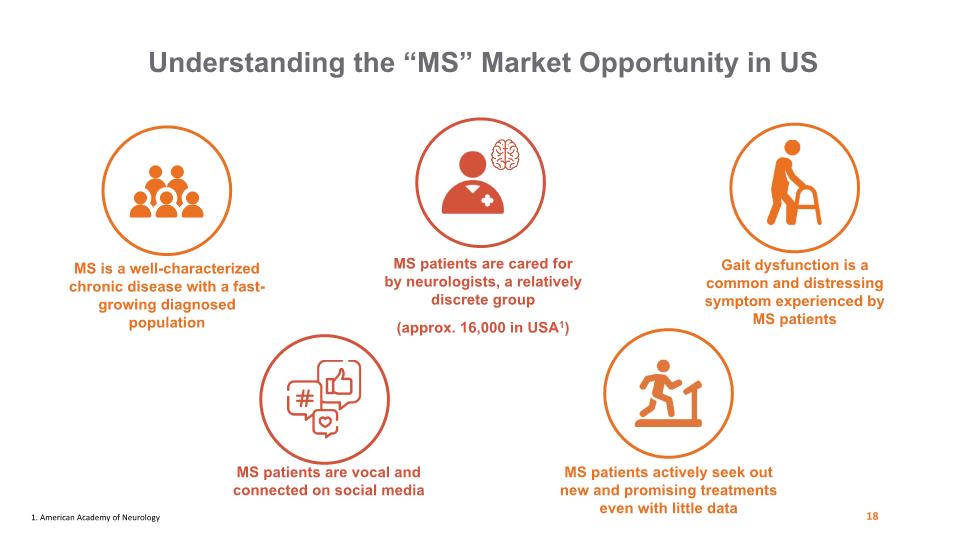
Understanding the “MS” Market Opportunity in US MS is a well-characterized chronic disease with a fast-growing diagnosed population MS patients are vocal and connected on social media MS patients are cared for by neurologists, a relatively discrete group (approx. 16,000 in USA1) Gait dysfunction is a common and distressing symptom experienced by MS patients MS patients actively seek out new and promising treatments even with little data 18 1. American Academy of Neurology
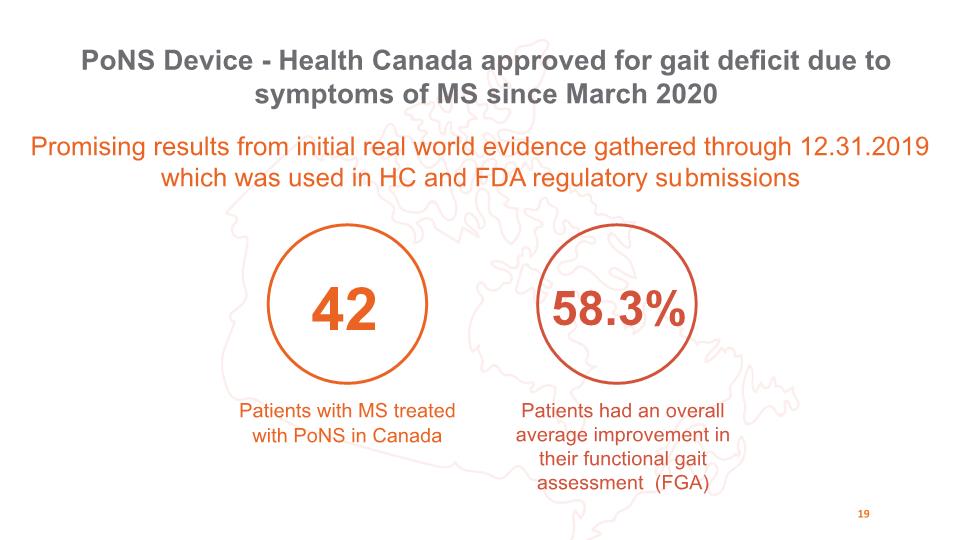
42 Patients with MS treated with PoNS in Canada 58.3% Patients had an overall average improvement in their functional gait assessment (FGA) PoNS Device - Health Canada approved for gait deficit due to symptoms of MS since March 2020 19 Promising results from initial real world evidence gathered through 12.31.2019 which was used in HC and FDA regulatory submissions
20
Multiple Sclerosis “MS” Opportunity 93,500 patients MS Society of Canada 1,000,000 patients National MS Society MS patients are highly motivated and linked on social media High urgency to treat High Patient awareness Specialty clinic system BOTH 21

Helius MS Scientific Advisory Board 22

CHINA: Strategic Agreement with China Medical Systems (“CMS”) for development and commercialization of PoNS in China plus 4 territories Transferred ownership of Asian patents, patent applications and granted exclusive license to market, promote distribute and sell the technology CMS assumed all development, patent (both application and defense), future manufacturing, clinical trial, and regulatory clearance costs for the territories Upon clearance in the U.S., CMS can use relevant documentation used for U.S. approval to apply for Chinese approval AUSTRALIA: Awaiting decision from Therapeutic Goods Administration (TGA) International Strategy 23

795k US individuals have a stroke each year1 50% of stroke survivors 65 and over suffer from reduced mobility2 Walking is the most frequently reported activity at the time of a fall in stroke survivors3 In post-stroke patients, the cerebral cortex becomes impaired while the spinal cord is preserved. The ability to generate information from the spinal cord required for walking can be utilized through specific movements to reorganize the cortex (neuroplasticity) Falls are the leading cause of injury related deaths among elderly people in the U.S. Next Neurological Market Opportunity Stroke Center for Disease Control and Prevention: Stroke Facts “Walking Adaptability after a Stroke and Its Assessment in Clinical Settings”; Stroke Res Treat.; August 28, 2014 Verma R, Arya KN, Sharma P, Garg RK: Understanding gait control in post-stroke: Implications for management. Journal of Bodywork and Movement Therapies 2010, 1-8 24

Next Neurological Market Opportunity Continued Mild-to-Moderate TBI 6.8m CerebralPalsy 764k Baby Boomers Balance 78m Neurological Wellness 1b 25 Parkinson’s Disease 1m
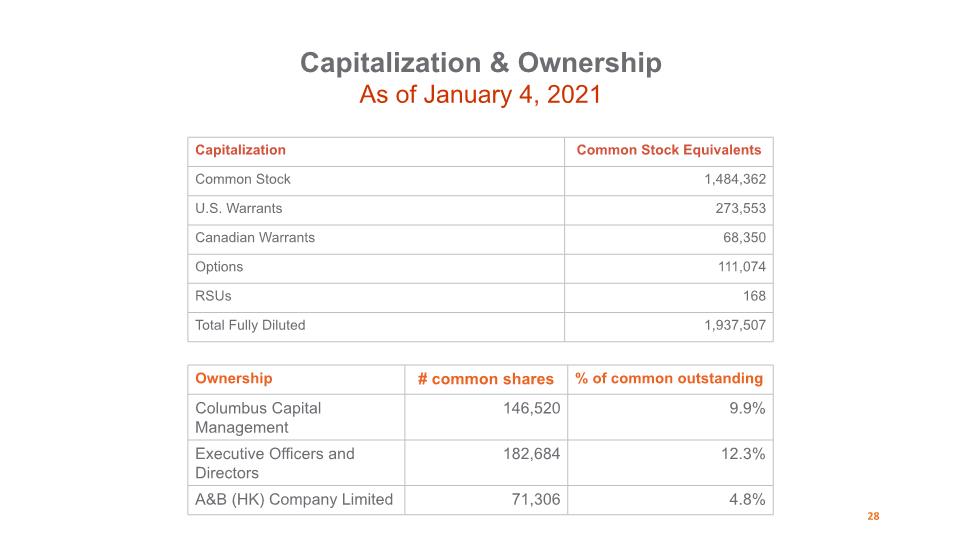
Capitalization & Ownership As of January 4, 2021 28

We are a neurotech company in the medical device industry focused on neurological wellness. FDA Breakthrough Designation and pending US clearance in MS Potential to obtain CMS reimbursement for 4 years – 70M covered lives Building on success/knowledge/infrastructure developed in two years of commercialization in Canada for mild-to-moderate TBI and one year for MS US commercialization would leverage existing Canadian commercialization infrastructure First and only treatment to restore lost function by stimulating cranial nerves via the tongue, supported by an extensive IP portfolio 27

Thank you (NASDAQ:HSDT | TSX:HSM)
