Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Pandion Therapeutics, Inc. | d78635dex991.htm |
| 8-K - 8-K - Pandion Therapeutics, Inc. | d78635d8k.htm |

Nasdaq: pand January 2021 Exhibit 99.2

This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties, including statements regarding the development status of the Company’s product candidates and the timing of availability of clinical trial data. All statements, other than statements of historical facts, contained in this presentation, including statements regarding the Company’s strategy, future operations, future financial position, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in, or implied by, such forward-looking statements. These risks and uncertainties include, but are not limited to, risks associated with Pandion’s ability to obtain and maintain necessary approvals from the FDA and other regulatory authorities; initiate preclinical studies and clinical trials of its product candidates; advance its product candidates in preclinical research and clinical trials; replicate in clinical trials positive results found in preclinical studies; advance the development of its product candidates under the timelines it anticipates in current and future clinical trials; obtain, maintain or protect intellectual property rights related to its product candidates; manage expenses; and raise the substantial additional capital needed to achieve its business objectives. For a discussion of other risks and uncertainties, and other important factors, any of which could cause the Company’s actual results to differ from those contained in the forward-looking statements, see the “Risk Factors” section, as well as discussions of potential risks, uncertainties and other important factors, in the Company’s most recent filings with the Securities and Exchange Commission. In addition, the forward-looking statements included in this presentation represent the Company’s views as of the date hereof and should not be relied upon as representing the Company’s views as of any date subsequent to the date hereof. The Company anticipates that subsequent events and developments will cause the Company’s views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data and estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Neither Pandion nor its affiliates, advisors or representatives makes any representation as to the accuracy or completeness of that data or undertakes to update such data after the date of this presentation. Forward-Looking Statements and Disclaimers

Phase 1a single dose, randomized, blinded clinical trial in 56 healthy volunteers PT101 was well-tolerated; no serious adverse events observed PT101 selectively expanded total regulatory T cells (Treg) to levels exceeding those associated with clinical benefit in third-party clinical trials of low-dose IL-2 At doses 3.5 mg and above, >80% of subjects achieved 2-fold or greater Treg expansion Mean maximum total Tregs expanded up to 3.6-fold above baseline Mean maximum CD25bright subset of Tregs expanded up to 72.5-fold above baseline Treg expansion profile supports every four-week dosing PT101 was selective for Tregs; no evidence of expansion of natural killer (NK) cells or pro-inflammatory conventional T cells (Tconv) at any dose level Top-Line Data from Phase 1a Clinical Trial of PT101
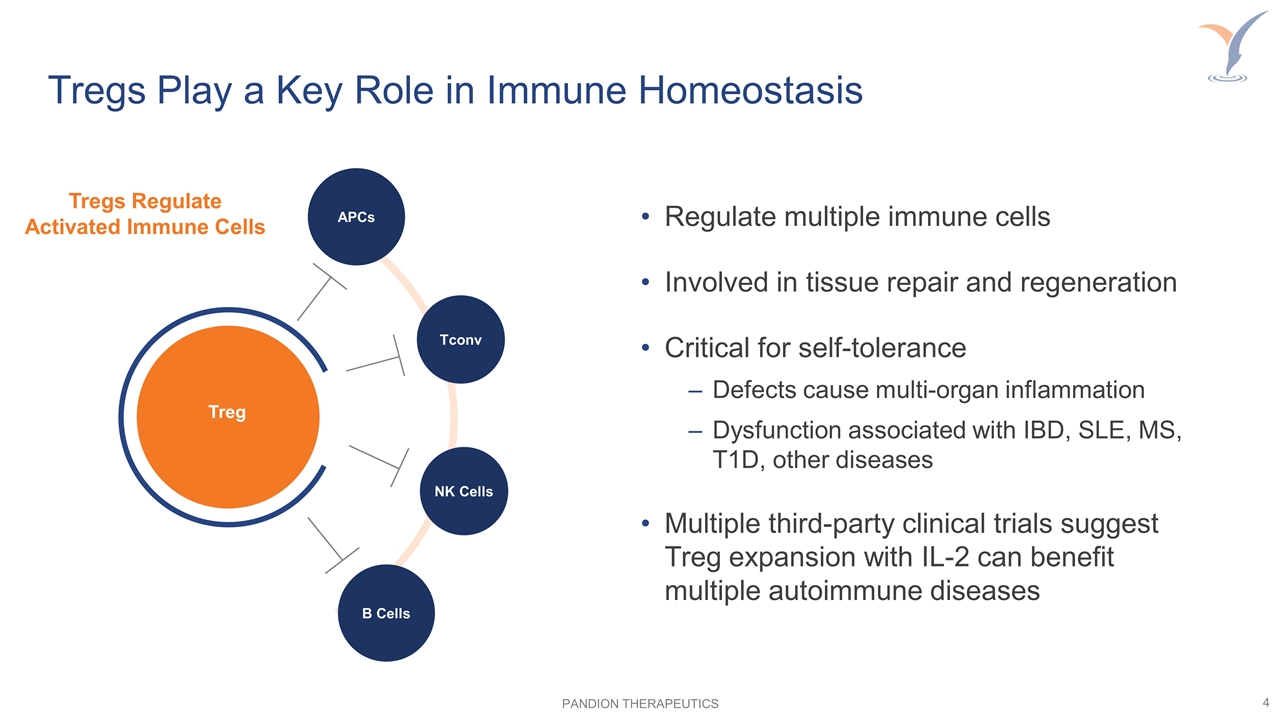
Regulate multiple immune cells Involved in tissue repair and regeneration Critical for self-tolerance Defects cause multi-organ inflammation Dysfunction associated with IBD, SLE, MS, T1D, other diseases Multiple third-party clinical trials suggest Treg expansion with IL-2 can benefit multiple autoimmune diseases Tregs Play a Key Role in Immune Homeostasis Tregs Regulate Activated Immune Cells Treg Tconv APCs NK Cells B Cells
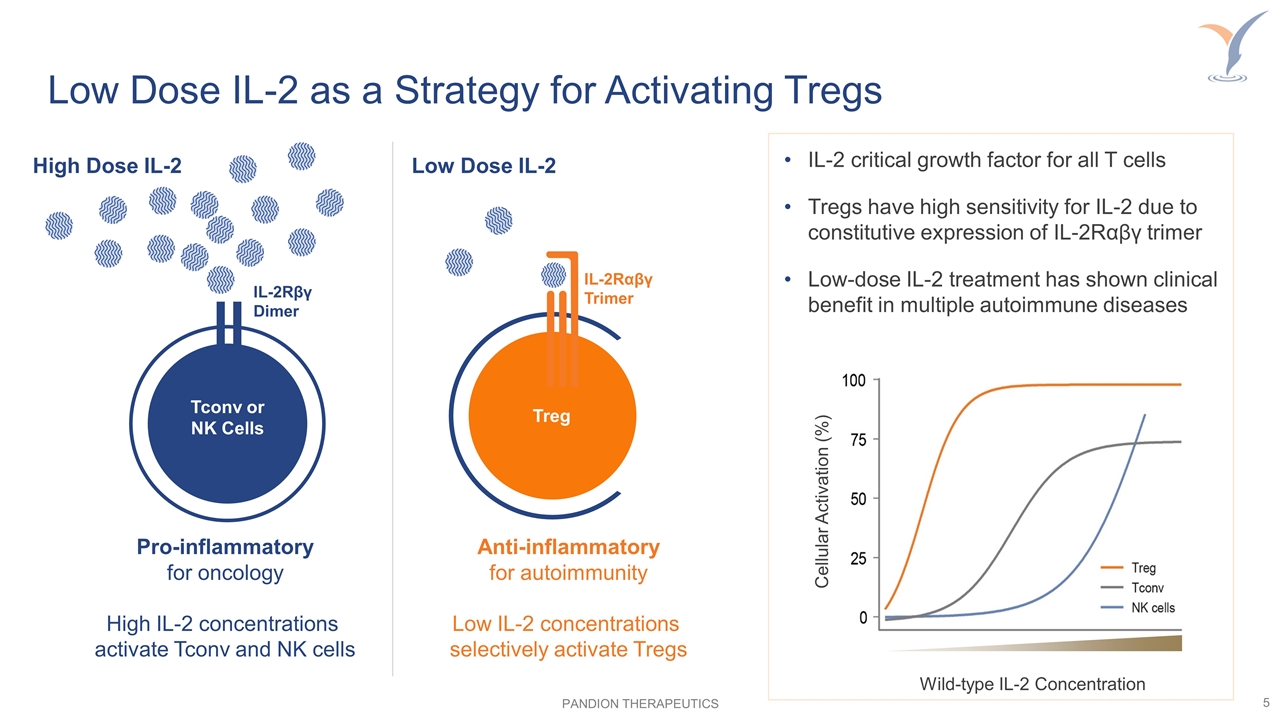
Low Dose IL-2 as a Strategy for Activating Tregs IL-2 critical growth factor for all T cells Tregs have high sensitivity for IL-2 due to constitutive expression of IL-2Rαβγ trimer Low-dose IL-2 treatment has shown clinical benefit in multiple autoimmune diseases High Dose IL-2 Pro-inflammatory for oncology High IL-2 concentrations activate Tconv and NK cells Anti-inflammatory for autoimmunity Low IL-2 concentrations selectively activate Tregs Treg IL-2Rαβγ Trimer IL-2Rβγ Dimer Tconv or NK Cells Wild-type IL-2 Concentration Cellular Activation (%) Low Dose IL-2
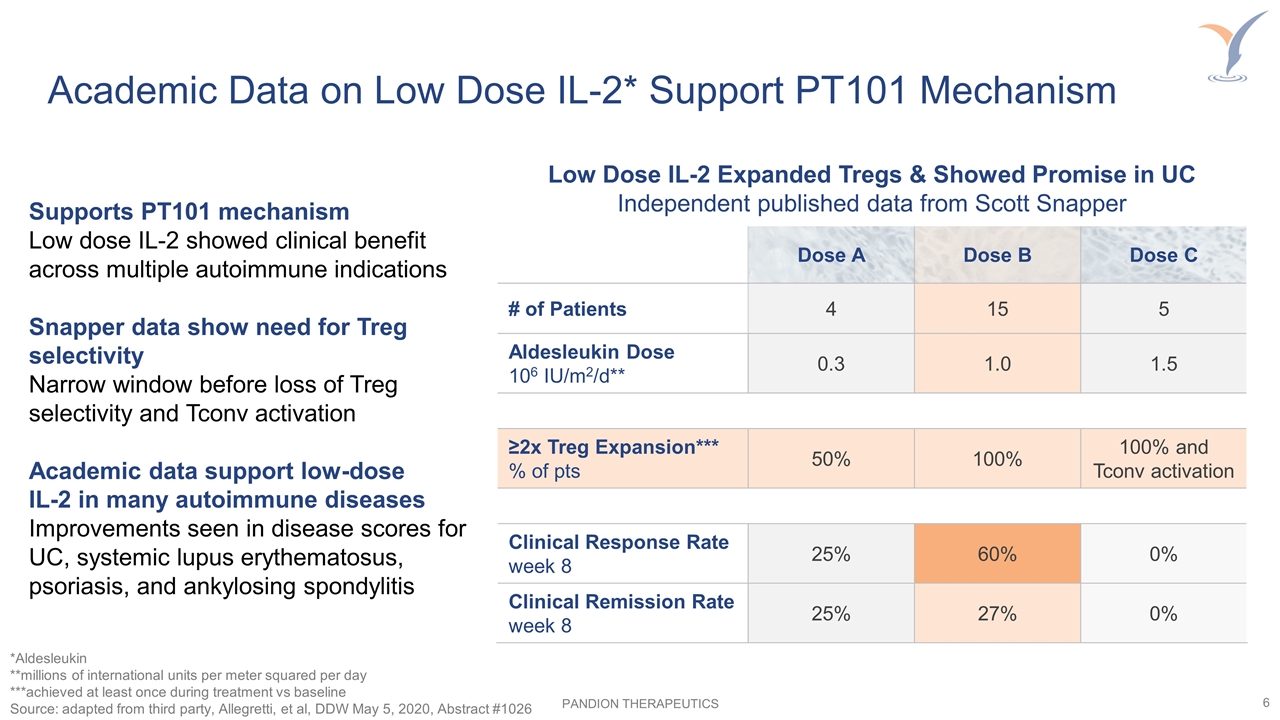
Academic Data on Low Dose IL-2* Support PT101 Mechanism Dose A Dose B Dose C # of Patients 4 15 5 Aldesleukin Dose 106 IU/m2/d** 0.3 1.0 1.5 ≥2x Treg Expansion*** % of pts 50% 100% 100% and Tconv activation Clinical Response Rate week 8 25% 60% 0% Clinical Remission Rate week 8 25% 27% 0% *Aldesleukin **millions of international units per meter squared per day ***achieved at least once during treatment vs baseline Source: adapted from third party, Allegretti, et al, DDW May 5, 2020, Abstract #1026 Supports PT101 mechanism Low dose IL-2 showed clinical benefit across multiple autoimmune indications Snapper data show need for Treg selectivity Narrow window before loss of Treg selectivity and Tconv activation Academic data support low-dose IL-2 in many autoimmune diseases Improvements seen in disease scores for UC, systemic lupus erythematosus, psoriasis, and ankylosing spondylitis Low Dose IL-2 Expanded Tregs & Showed Promise in UC Independent published data from Scott Snapper
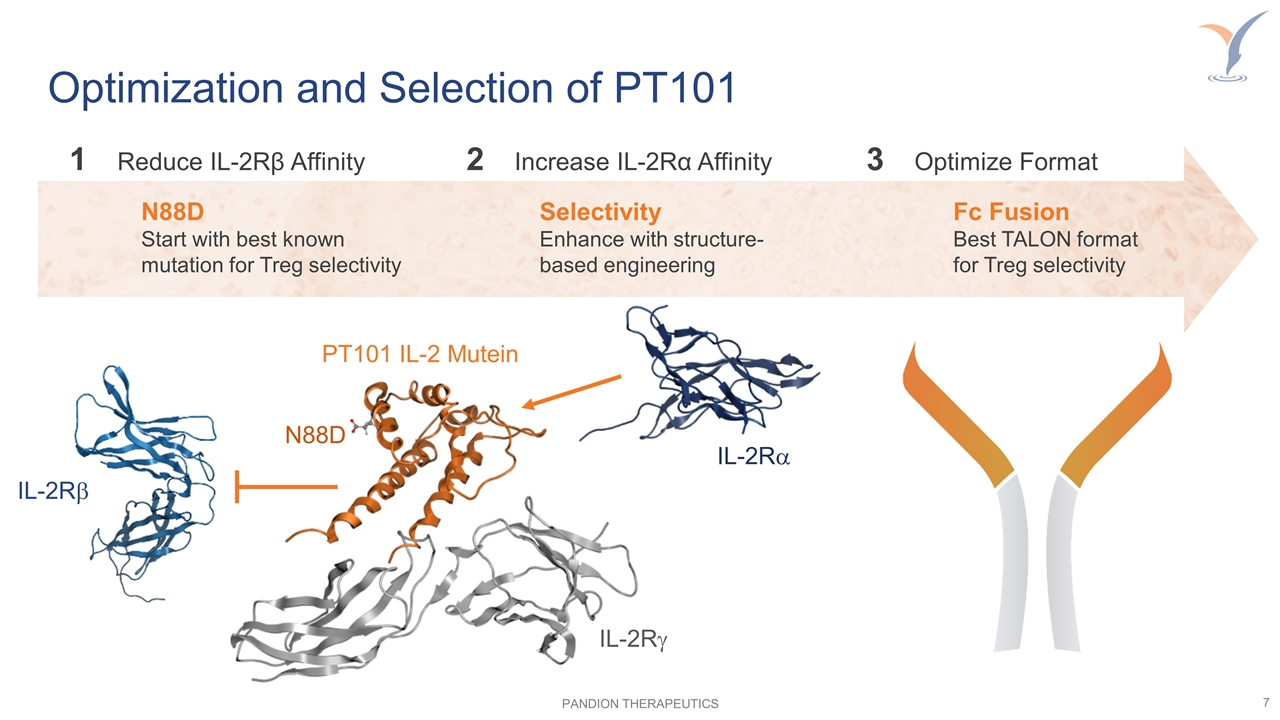
Optimization and Selection of PT101 Selectivity Enhance with structure-based engineering Fc Fusion Best TALON format for Treg selectivity 1Reduce IL-2Rβ Affinity 2Increase IL-2Rα Affinity 3Optimize Format IL-2Rb IL-2Ra IL-2Rg N88D PT101 IL-2 Mutein N88D Start with best known mutation for Treg selectivity
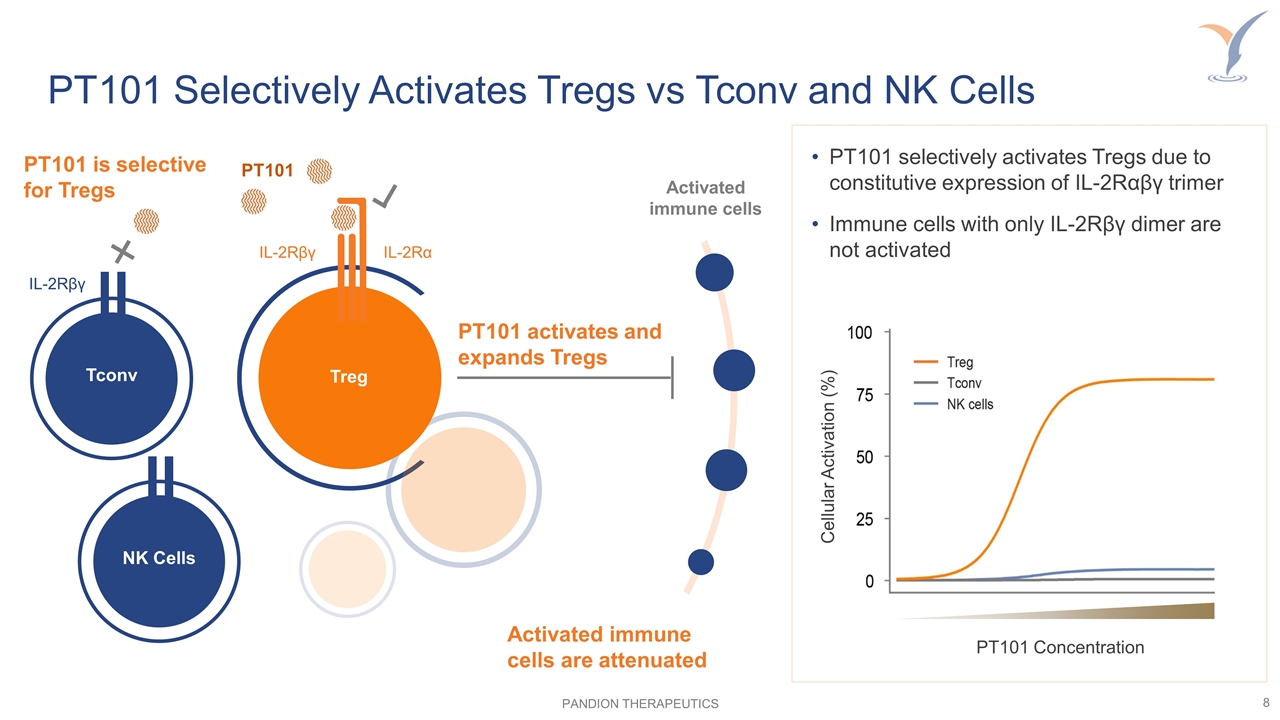
Activated immune cells PT101 is selective for Tregs IL-2Rβγ PT101 Treg IL-2Rβγ IL-2Rα Tconv PT101 activates and expands Tregs NK Cells PT101 Selectively Activates Tregs vs Tconv and NK Cells PT101 Concentration Cellular Activation (%) PT101 selectively activates Tregs due to constitutive expression of IL-2Rαβγ trimer Immune cells with only IL-2Rβγ dimer are not activated Activated immune cells are attenuated
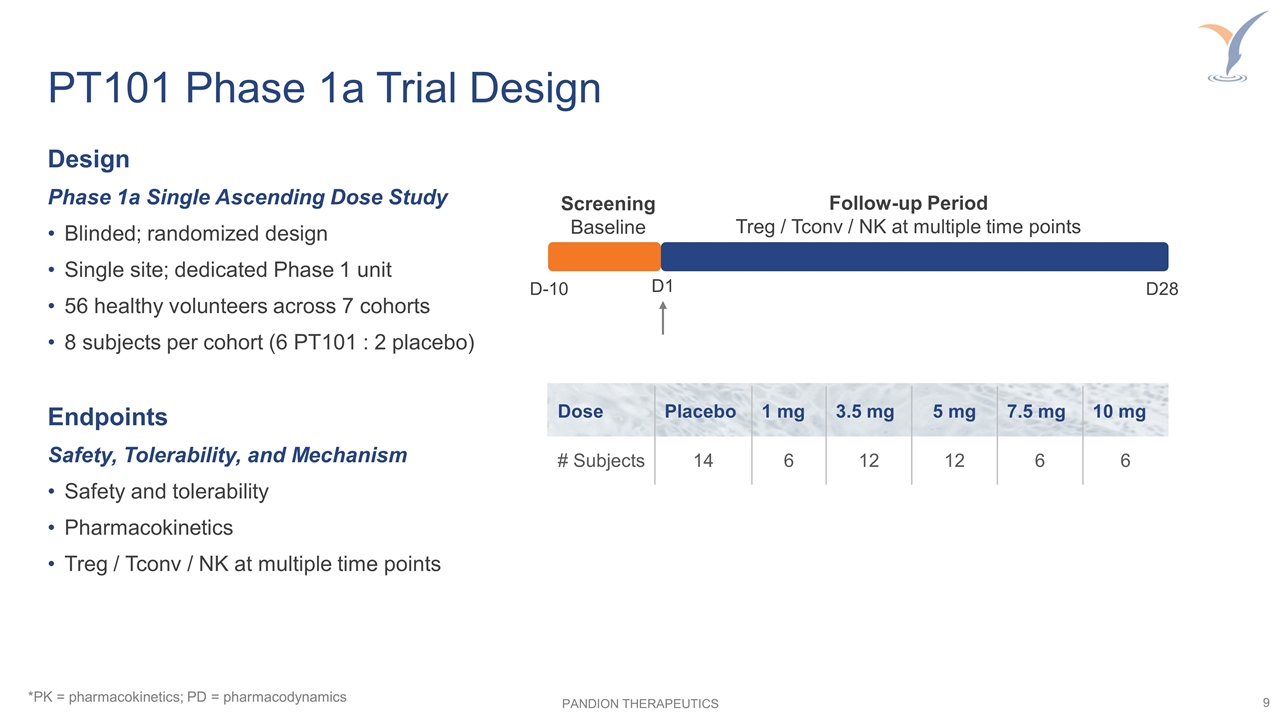
PT101 Phase 1a Trial Design *PK = pharmacokinetics; PD = pharmacodynamics Design Phase 1a Single Ascending Dose Study Blinded; randomized design Single site; dedicated Phase 1 unit 56 healthy volunteers across 7 cohorts 8 subjects per cohort (6 PT101 : 2 placebo) Endpoints Safety, Tolerability, and Mechanism Safety and tolerability Pharmacokinetics Treg / Tconv / NK at multiple time points Screening Baseline Follow-up Period Treg / Tconv / NK at multiple time points D-10 D1 D28 Dose Placebo 1 mg 3.5 mg 5 mg 7.5 mg 10 mg # Subjects 14 6 12 12 6 6
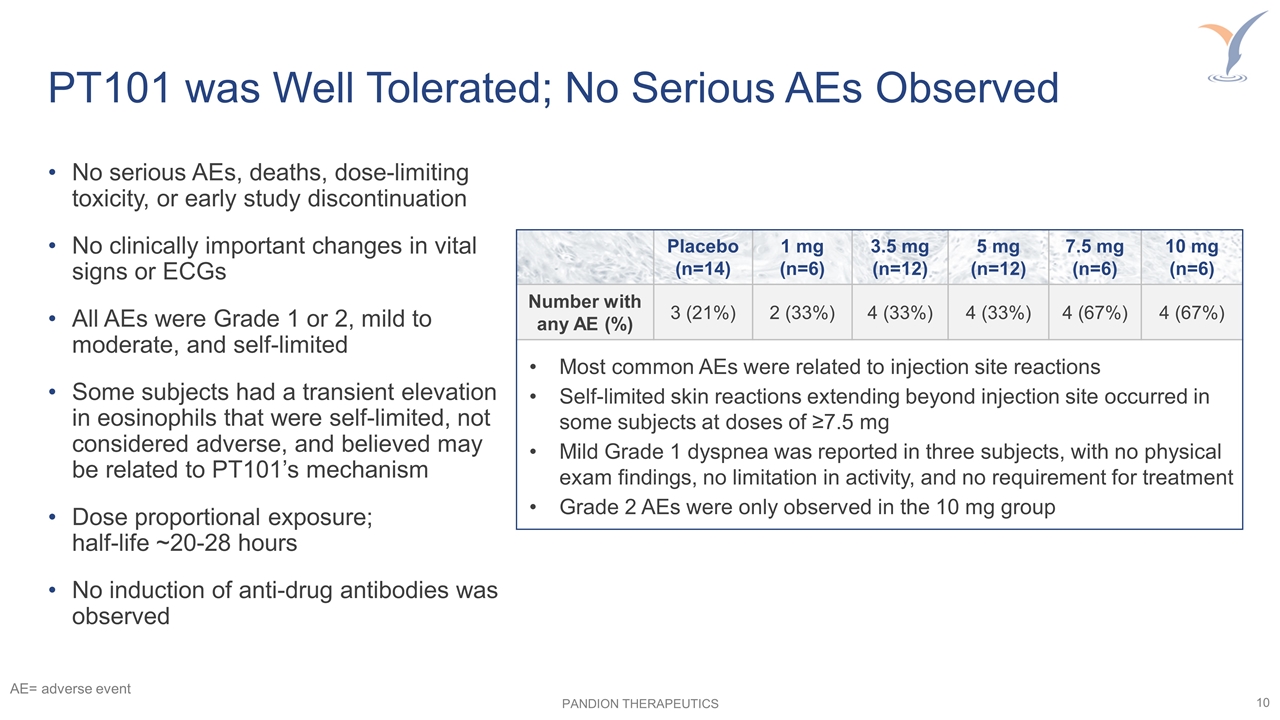
No serious AEs, deaths, dose-limiting toxicity, or early study discontinuation No clinically important changes in vital signs or ECGs All AEs were Grade 1 or 2, mild to moderate, and self-limited Some subjects had a transient elevation in eosinophils that were self-limited, not considered adverse, and believed may be related to PT101’s mechanism Dose proportional exposure; half-life ~20-28 hours No induction of anti-drug antibodies was observed PT101 was Well Tolerated; No Serious AEs Observed Placebo (n=14) 1 mg (n=6) 3.5 mg (n=12) 5 mg (n=12) 7.5 mg (n=6) 10 mg (n=6) Number with any AE (%) 3 (21%) 2 (33%) 4 (33%) 4 (33%) 4 (67%) 4 (67%) Most common AEs were related to injection site reactions Self-limited skin reactions extending beyond injection site occurred in some subjects at doses of ≥7.5 mg Mild Grade 1 dyspnea was reported in three subjects, with no physical exam findings, no limitation in activity, and no requirement for treatment Grade 2 AEs were only observed in the 10 mg group AE= adverse event
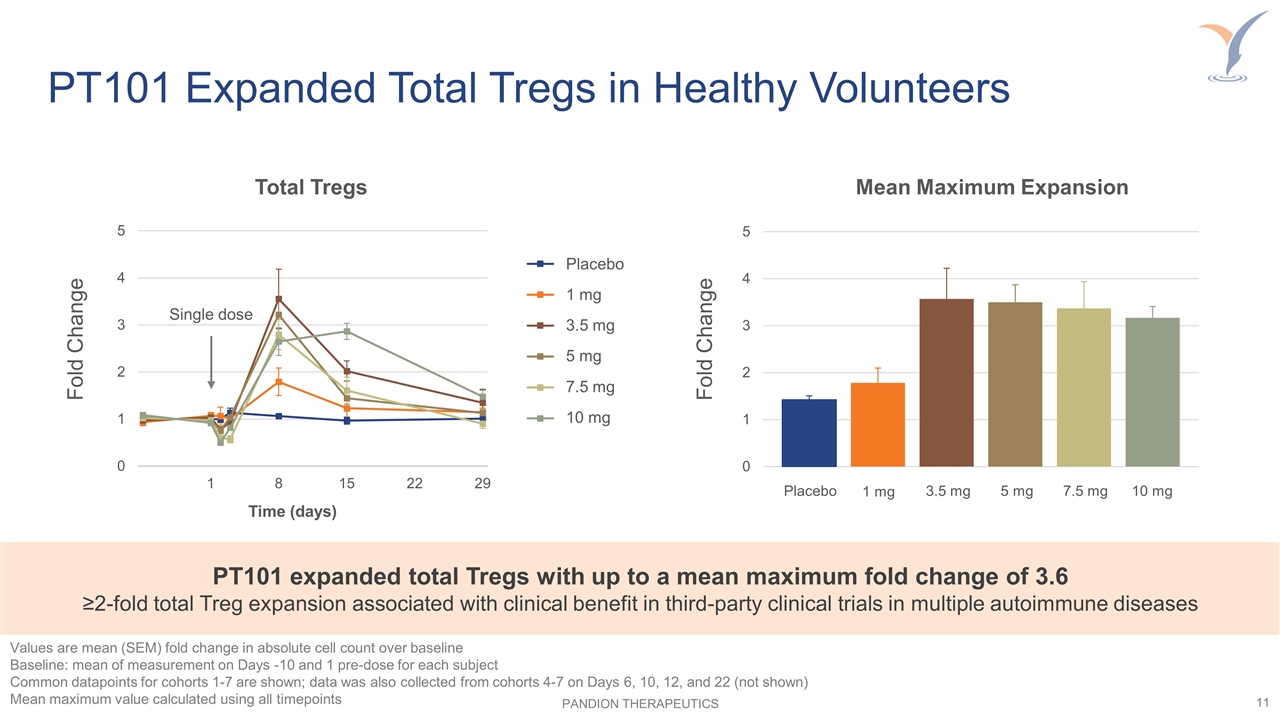
PT101 Expanded Total Tregs in Healthy Volunteers Mean Maximum Expansion Placebo 1 mg 3.5 mg 5 mg 7.5 mg 10 mg Fold Change PT101 expanded total Tregs with up to a mean maximum fold change of 3.6 ≥2-fold total Treg expansion associated with clinical benefit in third-party clinical trials in multiple autoimmune diseases Total Tregs Time (days) Fold Change Single dose Values are mean (SEM) fold change in absolute cell count over baseline Baseline: mean of measurement on Days -10 and 1 pre-dose for each subject Common datapoints for cohorts 1-7 are shown; data was also collected from cohorts 4-7 on Days 6, 10, 12, and 22 (not shown) Mean maximum value calculated using all timepoints Placebo 1 mg 3.5 mg 5 mg 7.5 mg 10 mg
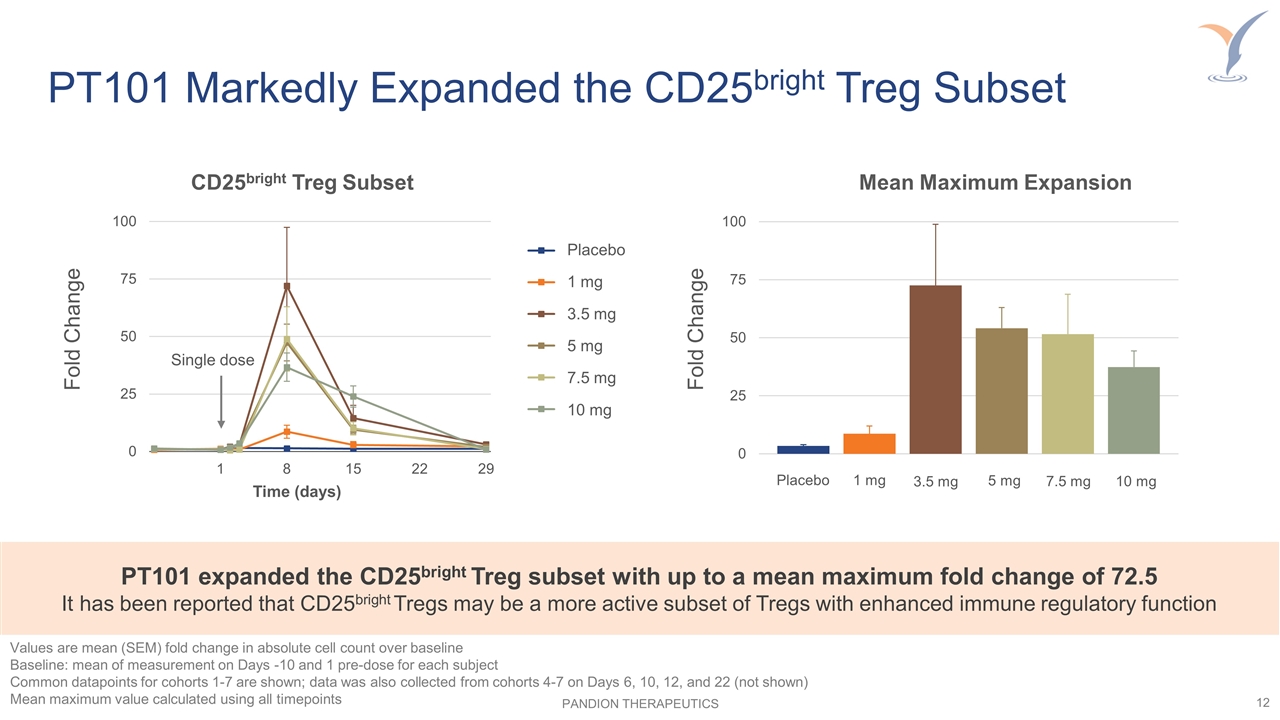
PT101 Markedly Expanded the CD25bright Treg Subset Mean Maximum Expansion PT101 expanded the CD25bright Treg subset with up to a mean maximum fold change of 72.5 It has been reported that CD25bright Tregs may be a more active subset of Tregs with enhanced immune regulatory function Placebo 1 mg 3.5 mg 5 mg 7.5 mg 10 mg Fold Change CD25bright Treg Subset Time (days) Fold Change Values are mean (SEM) fold change in absolute cell count over baseline Baseline: mean of measurement on Days -10 and 1 pre-dose for each subject Common datapoints for cohorts 1-7 are shown; data was also collected from cohorts 4-7 on Days 6, 10, 12, and 22 (not shown) Mean maximum value calculated using all timepoints Single dose Placebo 1 mg 3.5 mg 5 mg 7.5 mg 10 mg
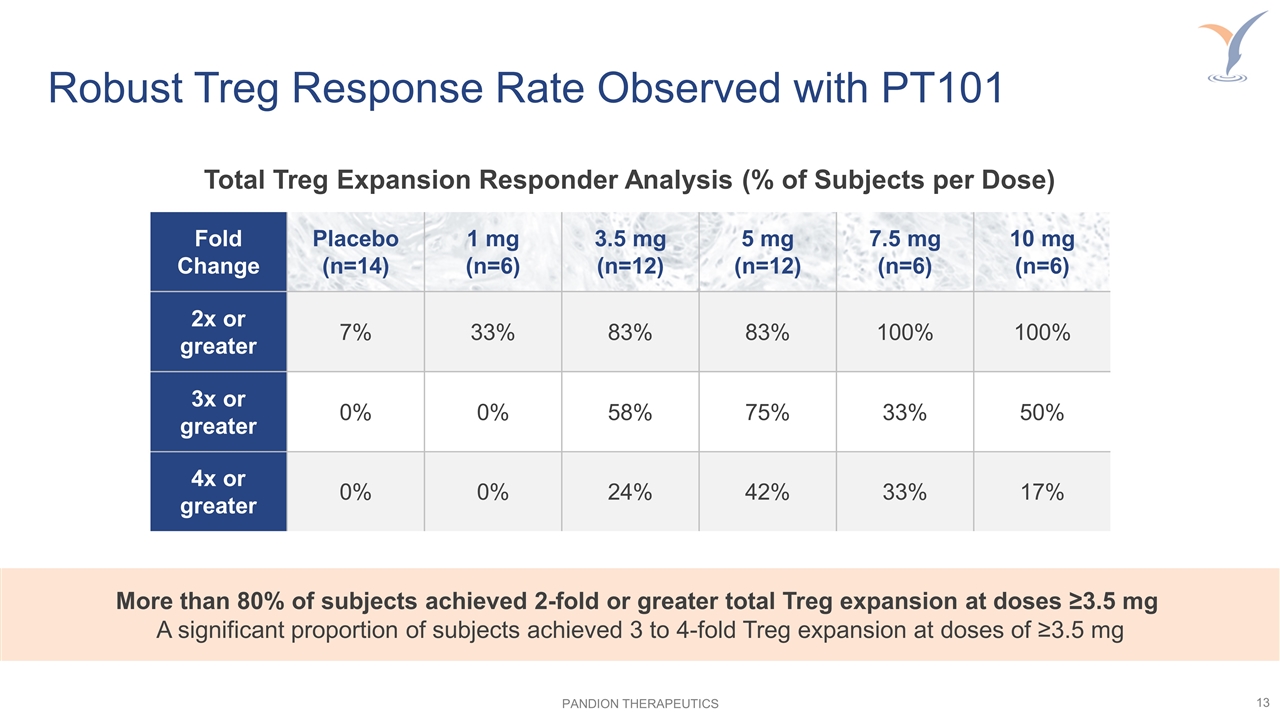
Robust Treg Response Rate Observed with PT101 Fold Change Placebo (n=14) 1 mg (n=6) 3.5 mg (n=12) 5 mg (n=12) 7.5 mg (n=6) 10 mg (n=6) 2x or greater 7% 33% 83% 83% 100% 100% 3x or greater 0% 0% 58% 75% 33% 50% 4x or greater 0% 0% 24% 42% 33% 17% More than 80% of subjects achieved 2-fold or greater total Treg expansion at doses ≥3.5 mg A significant proportion of subjects achieved 3 to 4-fold Treg expansion at doses of ≥3.5 mg Total Treg Expansion Responder Analysis (% of Subjects per Dose)
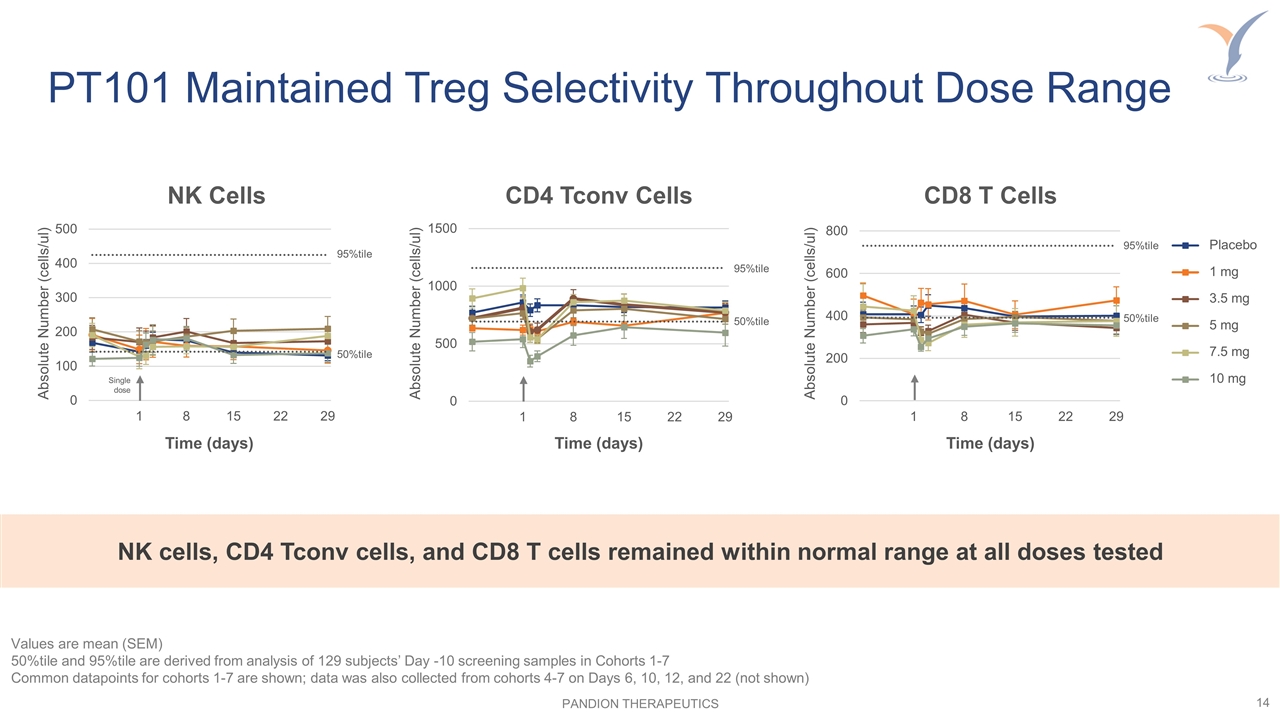
PT101 Maintained Treg Selectivity Throughout Dose Range Values are mean (SEM) 50%tile and 95%tile are derived from analysis of 129 subjects’ Day -10 screening samples in Cohorts 1-7 Common datapoints for cohorts 1-7 are shown; data was also collected from cohorts 4-7 on Days 6, 10, 12, and 22 (not shown) CD8 T Cells Time (days) Absolute Number (cells/ul) 95%tile 50%tile NK Cells Time (days) Absolute Number (cells/ul) 95%tile 50%tile CD4 Tconv Cells Time (days) Absolute Number (cells/ul) 95%tile 50%tile Single dose NK cells, CD4 Tconv cells, and CD8 T cells remained within normal range at all doses tested Placebo 1 mg 3.5 mg 5 mg 7.5 mg 10 mg
PT101 was well-tolerated; no serious adverse events observed PT101 selectively expanded total Tregs to levels exceeding those associated with clinical benefit in third-party clinical trials of low-dose IL-2 At doses 3.5 mg and above, >80% of subjects achieved 2-fold or greater Treg expansion Mean maximum total Tregs expanded up to 3.6-fold above baseline Mean maximum CD25bright subset of Tregs expanded up to 72.5-fold over baseline Treg expansion profile supports every four-week dosing PT101 was selective for Tregs; no evidence of expansion of NK cells or pro-inflammatory conventional T cells at any dose level Expect to initiate Phase 1b/2a clinical trial in patients with ulcerative colitis in mid-2021 and a Phase 2 clinical trial in patients with systemic lupus erythematosus in 2H 2021 Conclusion
