Attached files
| file | filename |
|---|---|
| 8-K - Oncotelic Therapeutics, Inc. | form8-k.htm |
Exhibit 99.1
 |
 |
ARTIVedaTM positioned to address the COVID-19 pandemic in India.
Introduction: Mateon Therapeutics, Inc. is a US oncology company focusing on TGF-β inhibitors as therapies against cancers and infectious diseases. Mateon will launch an Ayurvedic therapeutic for COVID-19, with its India partner Windlas Biotech Private Ltd., in late-December 2020. The product, ARTIVedaTM, is a formulated plant extract of the indigenous plant Artemisia, known in Sanskrit texts as Damanaka and Davana. ARTIVedaTM is the first Ayurvedic drug against COVID-19 through TGF-β inhibition. It is broadly active against diseases of aging and infectious diseases including COVID-19.
 |
ARTIVedaTM is expected to be effective through the entire infection cycle of COVID-19.
1. Prevention of COVID-19: Inhibition of viral binding to ACE2 receptor and inhibition of viral replication make this a prophylactic agent for prevention of COVID-19 [1-6].
2. Treatment of COVID-19: Clinical data demonstrated improvement in COVID-19 symptoms following treatment with ARTIVeda [7].
3. Recovery enhancer: Inhibition of TGF-β reduces scarring and reduces post-COVID-19 symptoms. Liver, lung, renal, and other organs fibrosis are inhibited [8] |
| 4. | Treatment of respiratory diseases in general. Inhibition of TGF-β has been shown to be effective against multiple viruses including influenza [9] and respiratory disorders such as allergic rhinitis [10]. |
Manufacturing: The active component of ARTIVeda™ has been identified as artemisinin. Through proprietary GMP quality extraction and manufacturing processes, the Artemisia extract was rendered active against SARS-CoV-2 with robust Safety Index (SI) greater than 100 (ratio of nonspecific cell kill versus viral kill). Other extracts have SI <10. Testing was performed at the US NIAID core viral laboratory. The product is protected by a patent portfolio of over 15 international patents by Mateon’s R&D. The mechanism of action against COVID-19 has been confirmed in numerous international scientific/medical publications as listed below.
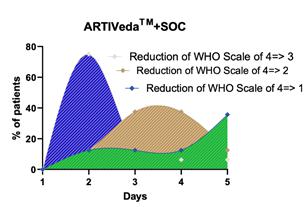 |
Preliminary Data Clinical Study: ARTI-19: Mateon and Windlas have initiated a clinical trial scaling to 300 patients at 6 sites in India (including the prestigious AIIMS) with CTRI. Early read from ARTI-19 clinical trial suggests potent efficacy against COVID-19. The clinical study uses WHO scale from 1-asymptomatic to 8-death. In the treatment arm, 75% of patients who were moderate (WHO-scale-4) exhibited a drop to WHO-scale-3 by day “two” which discharged them from hospital. Majority (>80%) patients dropped to asymptomatic WHO-scale-1 by completion of one cycle of treatment (5 days-on, 5 days-off). Overall, the temporal sequence of the treatment demonstrated linear progression to asymptotic stage versus the control arm which exhibited high degree of randomness. The randomness can be explained by non-specific cocktail of anti-inflammatory and antibiotic drugs, many proven to be ineffective, including Remdesivir and Hydrochloroquinine [7]. This trial is supported by a recent independent study published in the International Journal of Antimicrobial Agents, which showed that the combination of Artemisinin + Quinoline (Piperaquine) versus Quinoline (Hydroxychloroquine or HCQ) cut the time to virus-free by 50% and time to hospital discharge by 40%. |
| contact: saran.saund@oncotelic.com | Page 1 of 2 |
 |
 |
Bottomline: Deployed broadly ARTIVedaTM has the ability to stop the pandemic in India by cutting infectivity rate (R0) by at least 50%.
References:
| 1. | Sehailia M, Chemat S. In-silico Studies of Antimalarial-agent Artemisinin and Derivatives Portray More Potent Binding to Lys353 and Lys31-Binding Hotspots of SARS-CoV-2 Spike Protein than Hydroxychloroquine: Potential Repurposing of Artenimol for COVID-19. ChemRxiv. 2020. Preprint. https://doi.org/10.26434/chemrxiv.12098652.v1. |
| 2. | Alazmi M, Motwalli O. Molecular basis for drug repurposing to study the interface of the S protein in SARS-CoV-2 and human ACE2 through docking, characterization, and molecular dynamics for natural drug candidates. J Mol Model. 2020;26:338. |
| 3. | Cao Y, Feng YH, Gao LW, et al. Artemisinin enhances the anti-tumor immune response in 4T1 breast cancer cells in vitro and in vivo. Int Immunopharmacol. 2019;70:110-116. |
| 4. | Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271. |
| 5. | Cao R, Hu H, Li Y, et al. Anti-SARS-CoV-2 Potential of Artemisinins In Vitro. ACS Infect Dis. 2020;6(9):2524-2531. |
| 6. | Gilmore K, Zhou Y, Ramirez S, et al. In vitro efficacy of Artemisinin-based treatments against SARS-CoV-2. bioRxiv. 2020. 10.05.326637; doi: https://doi.org/10.1101/2020.10.05.326637. |
| 7. | Li G, Yuan M, Li H, et al. Safety and efficacy of artemisinin-piperaquine for treatment of COVID-19: an open-label, non-randomised and controlled trial. Int J Antimicrob Agents. 2020;106216. |
| 8. | Dolivo David et al., Artemisinin and artemisinin derivatives as anti-fibrotic therapeutics, Acta Pharmaceutica Sinica B, https://doi.org/10.1016/j.apsb.2020.09.001 |
| 9. | Denney L, Branchett W, Gregory LG, Oliver RA, Lloyd CM. Epithelial-derived TGF-β1 acts as a pro-viral factor in the lung during influenza A infection. Mucosal Immunology. 2018;11(2):523-535. |
| 10. | J. Li, et al., Effect of artemisinin and neurectomy of pterygoid canal in ovalbumin-induced allergic rhinitis mouse model, Allergy Asthma Clin. Immunol. 14 (2018) 22. |
| contact: saran.saund@oncotelic.com | Page 2 of 2 |
