Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Relay Therapeutics, Inc. | d89058dex991.htm |
| 8-K - 8-K - Relay Therapeutics, Inc. | d89058d8k.htm |

Genentech Global Collaboration for RLY-1971 December 2020 Confidential | © 2020 Relay Therapeutics Exhibit 99.2

This presentation contains forward-looking statements and information about our current and future prospects and our operations and financial results, which are based on currently available information. All statements other than statements of historical facts contained in this presentation, including statements regarding our strategy, future financial condition, future operations, projected costs, prospects, plans, objectives of management and expected market growth, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as ‘‘aim,’’ ‘‘anticipate,’’ ‘‘assume,’’ ‘‘believe,’’ ‘‘contemplate,’’ ‘‘continue,’’ ‘‘could,’’ ‘‘design,’’ ‘‘due,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘goal,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘objective,’’ “opportunity,” ‘‘plan,’’ ‘‘predict,’’ ‘‘positioned,’’ ‘‘potential,’’ ‘‘seek,’’ ‘‘should,’’ ‘‘target,’’ ‘‘will,’’ ‘‘would’’ and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward-looking statements include statements about the expected strategic benefits of our collaboration with Genentech; the receipt of upfront and near-term payments and potential milestone and royalty payments under the collaboration; the potential of RLY-1971, including in combination with Genentech’s KRAS G12C and other therapies; the potential therapeutic benefits of inhibiting KRAS G12C and SHP2 in combination; the initiation, timing, progress and results of our current and future clinical trials and current and future preclinical studies of our product candidates; our ability to successfully establish or maintain collaborations or strategic relationships for our product candidates; and the implementation of our business model and strategic plans for our business, current product candidates and any future product candidates. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make due to a number of risks and uncertainties. These and other risks, uncertainties and important factors are described in the section entitled "Risk Factors" in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2020, as well as any subsequent filings with the Securities and Exchange Commission. Any forward-looking statements represent our views only as of the date of this presentation and we undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, the occurrence of certain events or otherwise. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party studies, publications, surveys and other data to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent source has evaluated the reasonableness or accuracy of our internal estimates or research and no reliance should be made on any information or statements made in this presentation relating to or based on such internal estimates and research. Confidential | © 2020 Relay Therapeutics Disclaimer
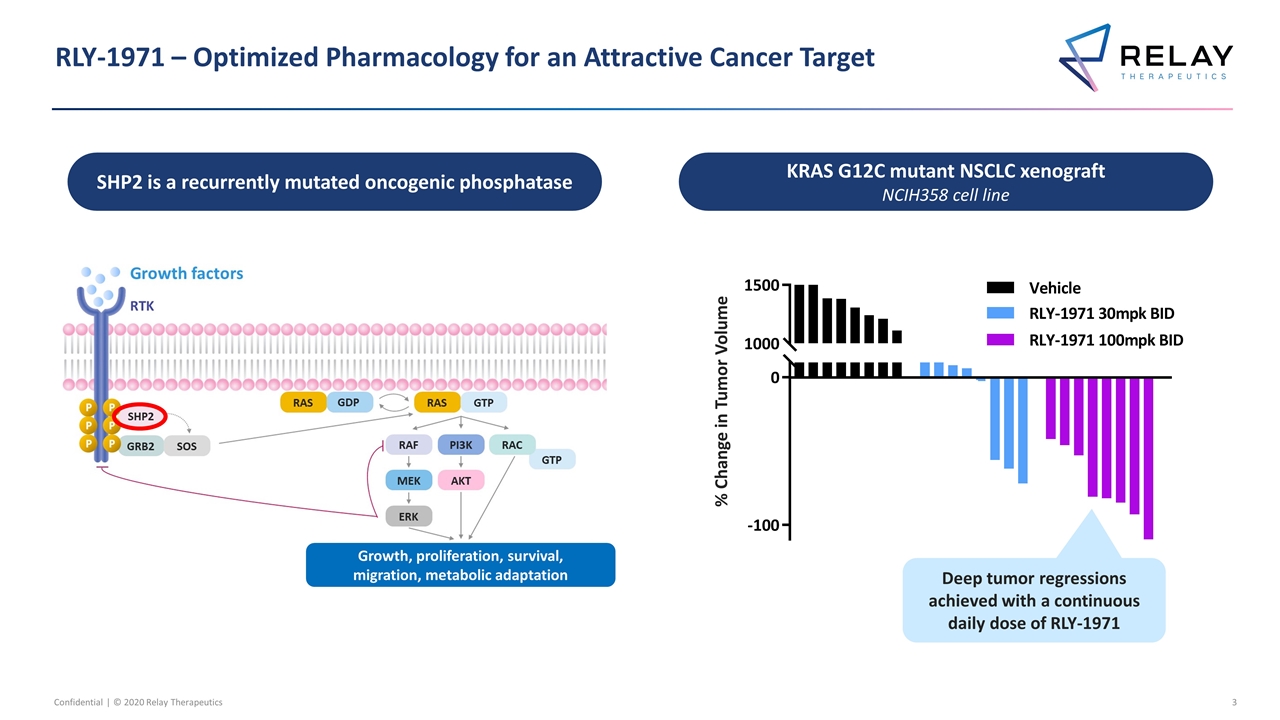
RLY-1971 – Optimized Pharmacology for an Attractive Cancer Target Confidential | © 2020 Relay Therapeutics SHP2 is a recurrently mutated oncogenic phosphatase KRAS G12C mutant NSCLC xenograft NCIH358 cell line Deep tumor regressions achieved with a continuous daily dose of RLY-1971 % Change in Tumor Volume Growth, proliferation, survival, migration, metabolic adaptation
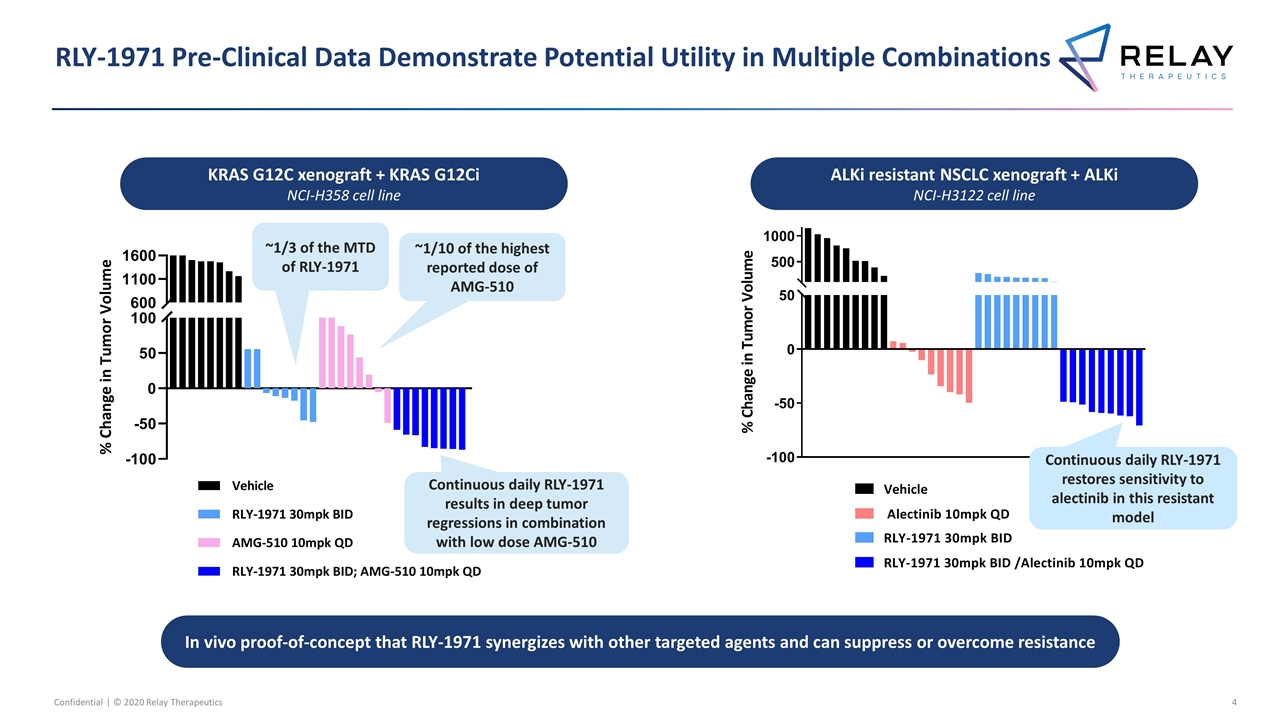
RLY-1971 Pre-Clinical Data Demonstrate Potential Utility in Multiple Combinations Confidential | © 2020 Relay Therapeutics KRAS G12C xenograft + KRAS G12Ci NCI-H358 cell line ALKi resistant NSCLC xenograft + ALKi NCI-H3122 cell line In vivo proof-of-concept that RLY-1971 synergizes with other targeted agents and can suppress or overcome resistance ~1/3 of the MTD of RLY-1971 ~1/10 of the highest reported dose of AMG-510 Continuous daily RLY-1971 results in deep tumor regressions in combination with low dose AMG-510 Continuous daily RLY-1971 restores sensitivity to alectinib in this resistant model

Genentech Global Collaboration for RLY-1971 Confidential | © 2020 Relay Therapeutics Potential for multiple combinations with Genentech’s pipeline, including its clinical stage KRAS G12C inhibitor, GDC-6036 Genentech’s global footprint and deep expertise in oncology make them the perfect partner Relay Tx retains ability to combine RLY-1971 with its lead assets, RLY-4008 and RLY-PI3K1047 program Increases scale, scope, and speed of globally developing and commercializing RLY-1971 Provides meaningful economics on RLY-1971, including option for US cost-profit share RLY-1971’s potent, continuous once daily profile optimally positions it to unlock value via combinations
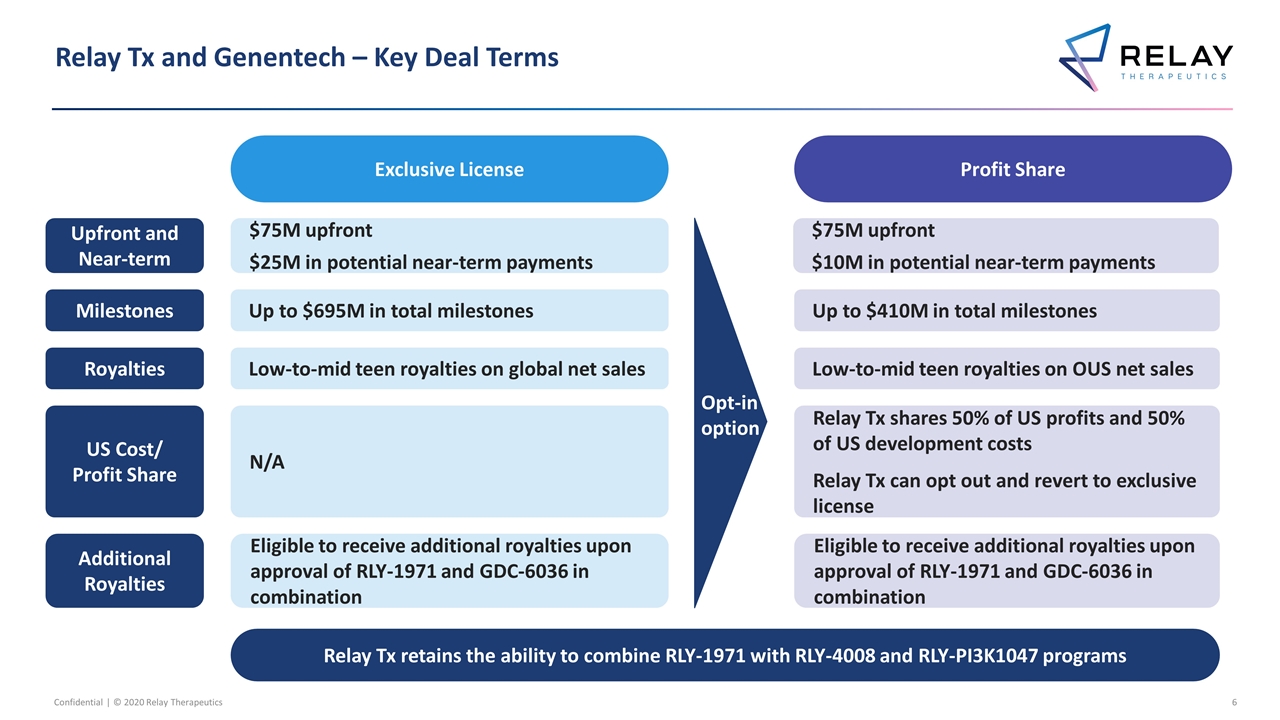
Relay Tx and Genentech – Key Deal Terms Confidential | © 2020 Relay Therapeutics Exclusive License Profit Share Low-to-mid teen royalties on global net sales Royalties Low-to-mid teen royalties on OUS net sales Up to $695M in total milestones Milestones Up to $410M in total milestones Relay Tx shares 50% of US profits and 50% of US development costs Relay Tx can opt out and revert to exclusive license US Cost/ Profit Share N/A Opt-in option Eligible to receive additional royalties upon approval of RLY-1971 and GDC-6036 in combination Eligible to receive additional royalties upon approval of RLY-1971 and GDC-6036 in combination Additional Royalties $75M upfront $25M in potential near-term payments Upfront and Near-term $75M upfront $10M in potential near-term payments Relay Tx retains the ability to combine RLY-1971 with RLY-4008 and RLY-PI3K1047 programs
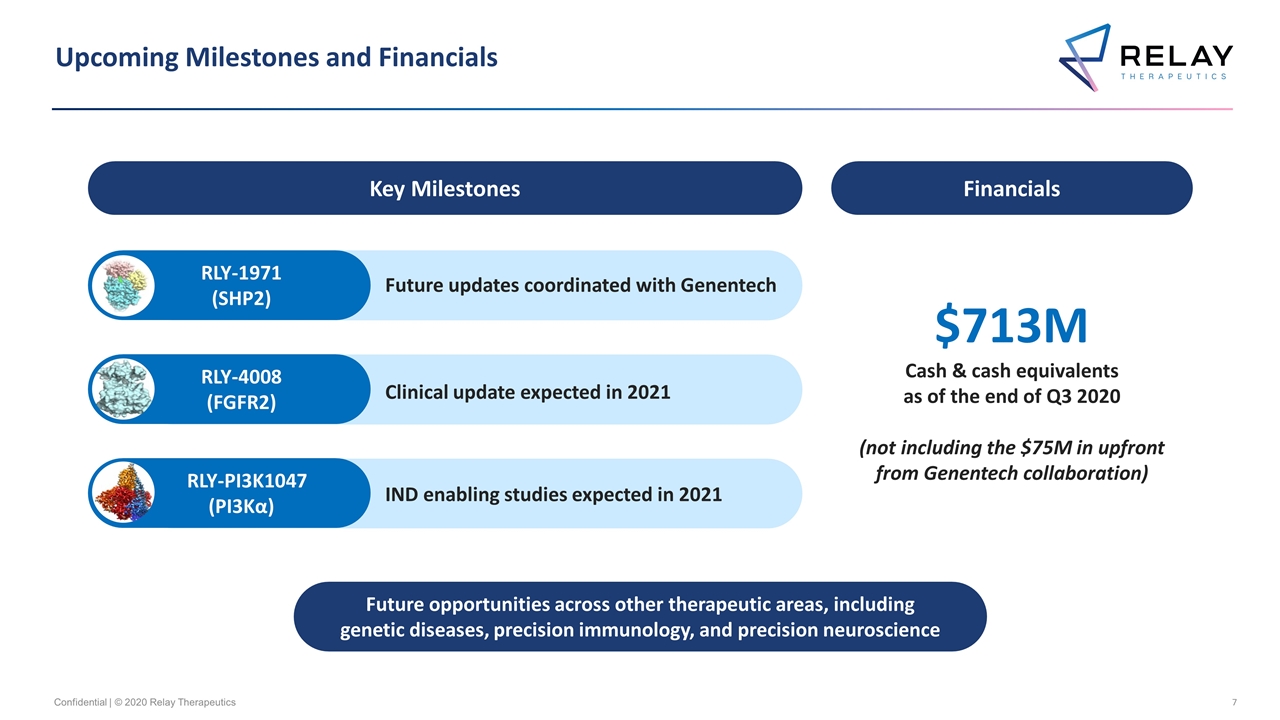
Upcoming Milestones and Financials Confidential | © 2020 Relay Therapeutics Future opportunities across other therapeutic areas, including genetic diseases, precision immunology, and precision neuroscience Key Milestones Future updates coordinated with Genentech Financials RLY-1971 (SHP2) RLY-4008 (FGFR2) RLY-PI3K1047 (PI3Kα) Clinical update expected in 2021 IND enabling studies expected in 2021 $713M Cash & cash equivalents as of the end of Q3 2020 (not including the $75M in upfront from Genentech collaboration)

Confidential | © 2020 Relay Therapeutics
