Attached files
| file | filename |
|---|---|
| 8-K - 8-K - CHIASMA, INC | d42549d8k.htm |
| EX-99.2 - EX-99.2 - CHIASMA, INC | d42549dex992.htm |

Phase 3 MPOWEREDTM Trial Top-line Results November 18, 2020 Exhibit 99.1

These slides and the accompanying presentation contain forward-looking statements and information. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify forward-looking statements. These statements include, without limitation, those statements regarding the data from the MPOWERED trial and whether the data will support the submission of a marketing authorization application (MAA) to the European Medicines Agency (EMA) for MYCAPSSA in the European Union and ultimately regulatory approval, statements regarding the timing of an MAA submission and regulatory review, statements regarding the company’s plans for the presentation of the full trial results, statements regarding the company’s expectations relating to MYCAPSSA for the long-term maintenance therapy in acromegaly patients who have responded to and tolerated treatment with octreotide or lanreotide, statements concerning the therapeutic potential of MYCAPSSA, including its ability to become a standard of care, statements regarding the commercialization of MYCAPSSA, including plans and strategies for potentially commercializing in the European Union and in other jurisdictions, and statements concerning the utilization of TPE platform to develop new therapeutic agents. All forward-looking statements are based on estimates and assumptions by Chiasma’s management that, although Chiasma believes them to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that Chiasma expects. Management’s expectations and, therefore, any forward-looking statements in these slides and the accompanying presentation could be affected by risks and uncertainties relating to a number of factors, including the following: the content and timing of decisions made by the EMA, the sufficiency of the data collected from the company’s clinical trials to obtain regulatory approval in the European Union or elsewhere, and the impact the ongoing COVID-19 pandemic may have on the company’s business, including its expected development, manufacturing, regulatory and commercialization timelines for MYCAPSSA. These and other potential risks, uncertainties and other important factors are described under the heading “Risk Factors” in our Form 10-Q for the quarter ended September 30, 2020 filed with the Securities and Exchange Commission, or SEC, as well as in Chiasma’s subsequent filings with the SEC. Undue reliance should not be placed on any forward-looking statement, which speak only as of the date on which it was made. Chiasma undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. Unless otherwise noted, all references to acromegaly market sizes are Chiasma internal estimates. This presentation is intended only for communications with investors. MYCAPSSA has been approved by the FDA for the long-term maintenance treatment in patients with acromegaly who have responded to and tolerated treatment with octreotide or lanreotide, but remains an investigational drug outside the U.S. Forward-Looking Statements
MYCAPSSA is FDA Approved and is Commercially Available in the U.S. Full prescribing information available at www.MYCAPSSA.com MYCAPSSA is a somatostatin analog indicated for long-term maintenance treatment in acromegaly patients who have responded to and tolerated treatment with octreotide or lanreotide.
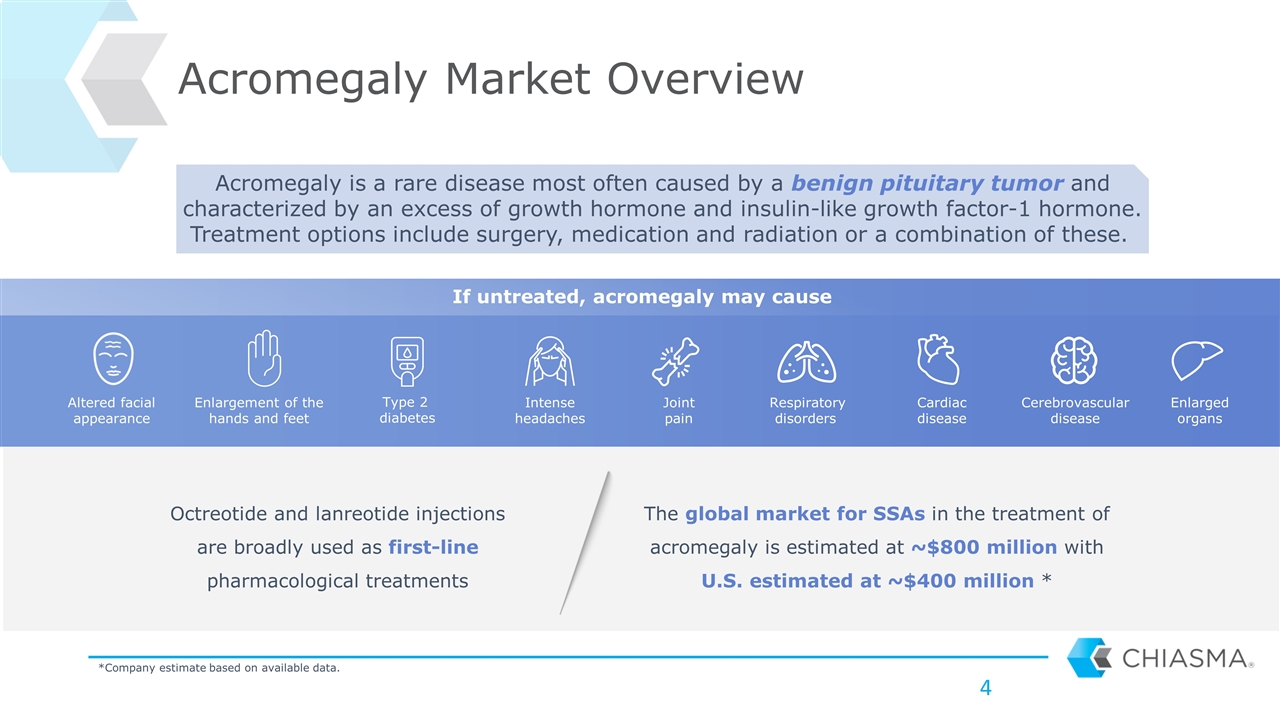
Acromegaly Market Overview Octreotide and lanreotide injections are broadly used as first-line pharmacological treatments Acromegaly is a rare disease most often caused by a benign pituitary tumor and characterized by an excess of growth hormone and insulin-like growth factor-1 hormone. Treatment options include surgery, medication and radiation or a combination of these. If untreated, acromegaly may cause *Company estimate based on available data. Enlarged organs Intense headaches Altered facial appearance Enlargement of the hands and feet Type 2 diabetes Respiratory disorders Cardiac disease Joint pain Cerebrovascular disease The global market for SSAs in the treatment of acromegaly is estimated at ~$800 million with U.S. estimated at ~$400 million *
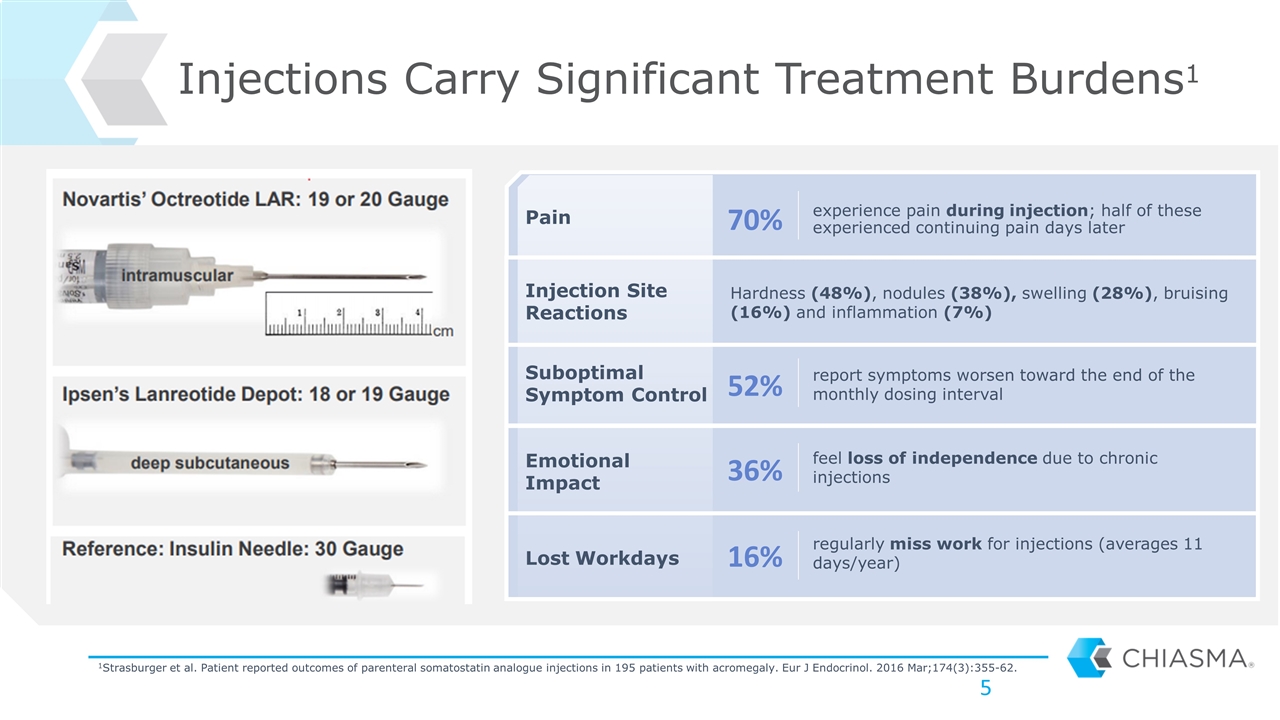
experience pain during injection; half of these experienced continuing pain days later Injections Carry Significant Treatment Burdens1 Pain Suboptimal Symptom Control Lost Workdays Emotional Impact Injection Site Reactions Hardness (48%), nodules (38%), swelling (28%), bruising (16%) and inflammation (7%) report symptoms worsen toward the end of the monthly dosing interval feel loss of independence due to chronic injections regularly miss work for injections (averages 11 days/year) 70% 52% 36% 16% 1Strasburger et al. Patient reported outcomes of parenteral somatostatin analogue injections in 195 patients with acromegaly. Eur J Endocrinol. 2016 Mar;174(3):355-62.
MPOWERED Trial Positive: Met Primary Non-inferiority Endpoint MYCAPSSA (octreotide capsules) was shown to be non-inferior to long-acting injectable somatostatin analog injections (SSAs)* in maintenance of biochemical response for acromegaly patients. * Octreotide LAR and lanreotide autogel
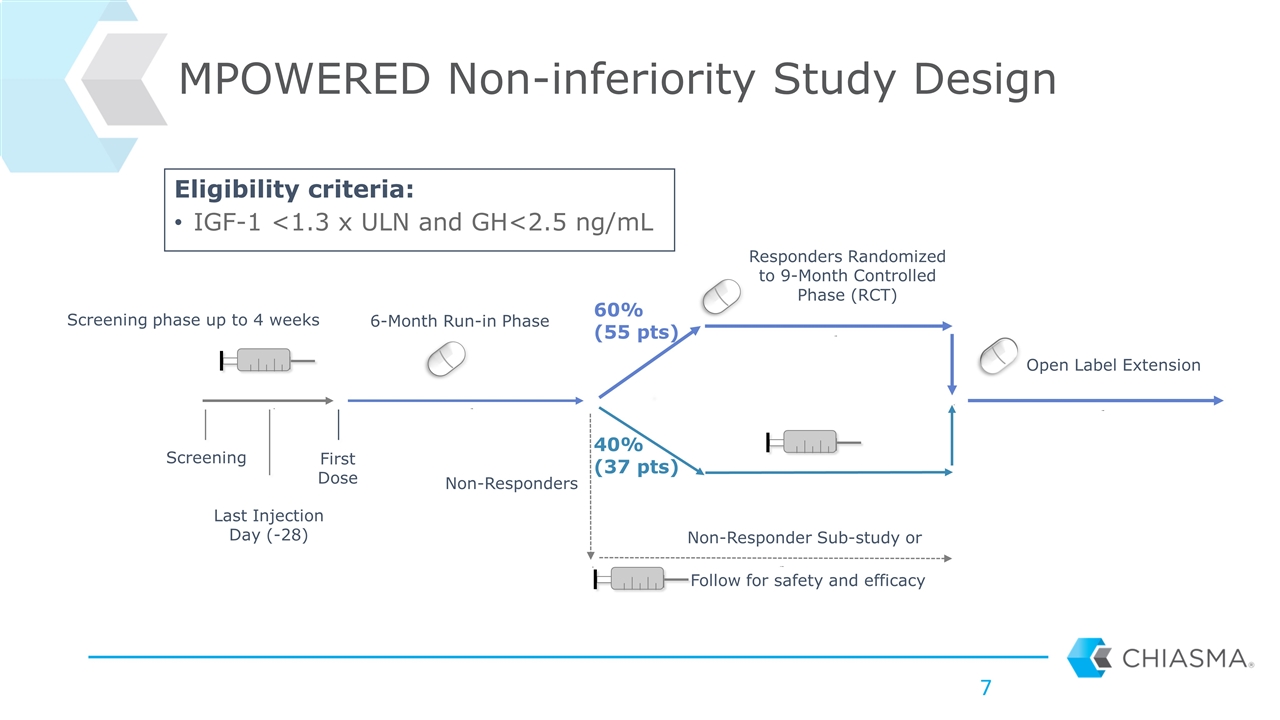
MPOWERED Non-inferiority Study Design Last Injection Day (-28) Screening First Dose 6-Month Run-in Phase Responders Randomized to 9-Month Controlled Phase (RCT) Open Label Extension Follow for safety and efficacy 60% (55 pts) 40% (37 pts) Non-Responder Sub-study or Non-Responders Screening phase up to 4 weeks Eligibility criteria: IGF-1 <1.3 x ULN and GH<2.5 ng/mL
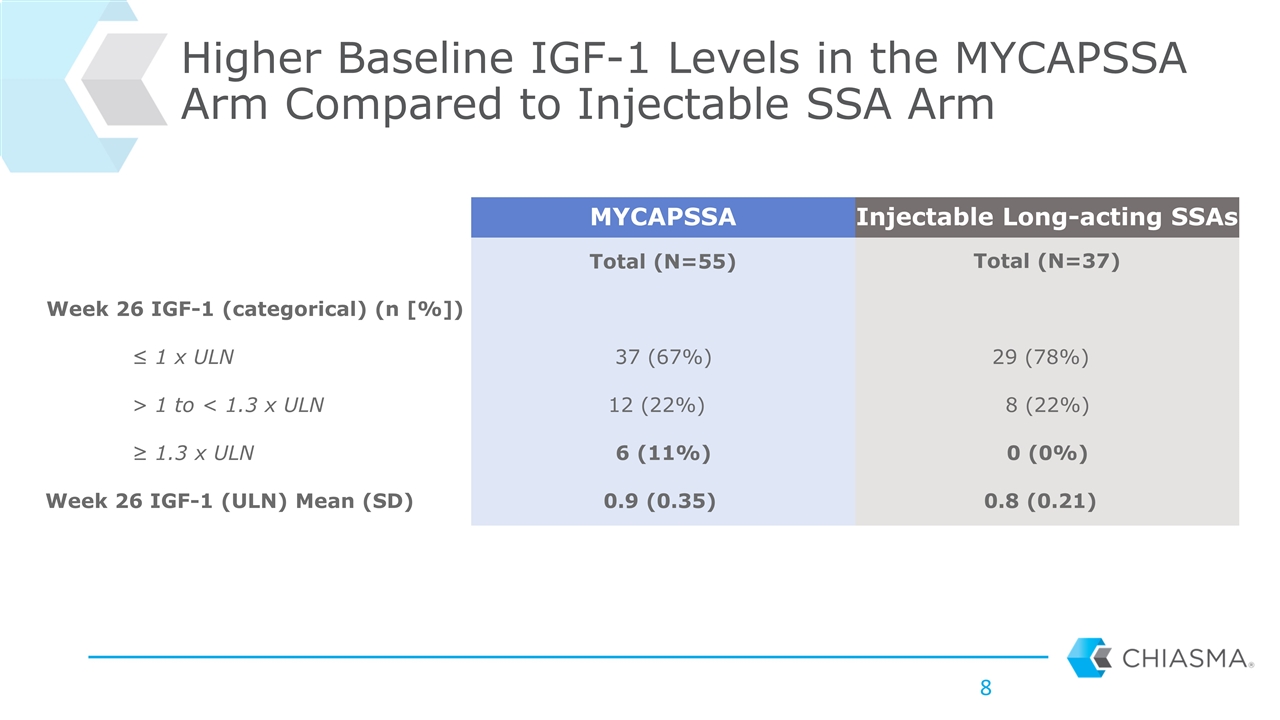
Higher Baseline IGF-1 Levels in the MYCAPSSA Arm Compared to Injectable SSA Arm MYCAPSSA Injectable Long-acting SSAs Total (N=55) Total (N=37) Week 26 IGF-1 (categorical) (n [%]) ≤ 1 x ULN 37 (67%) 29 (78%) > 1 to < 1.3 x ULN 12 (22%) 8 (22%) ≥ 1.3 x ULN 6 (11%) 0 (0%) Week 26 IGF-1 (ULN) Mean (SD) 0.9 (0.35) 0.8 (0.21)
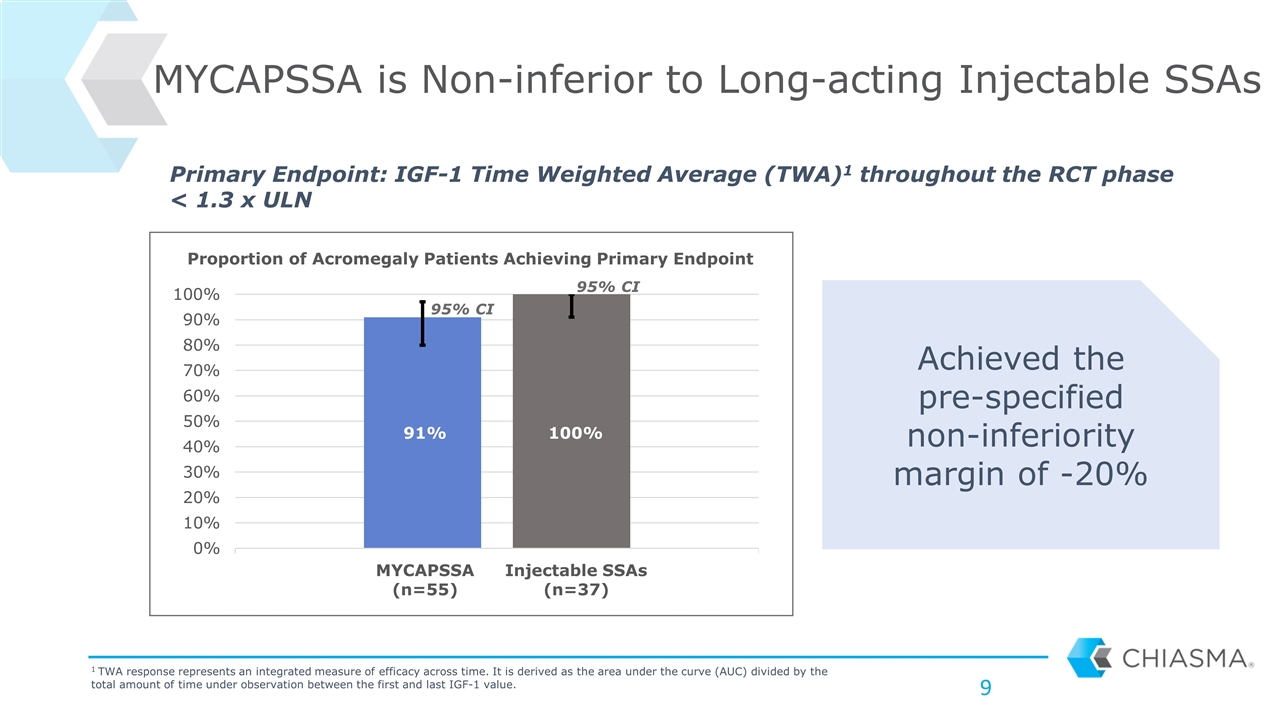
MYCAPSSA is Non-inferior to Long-acting Injectable SSAs Achieved the pre-specified non-inferiority margin of -20% Primary Endpoint: IGF-1 Time Weighted Average (TWA)1 throughout the RCT phase < 1.3 x ULN 1 TWA response represents an integrated measure of efficacy across time. It is derived as the area under the curve (AUC) divided by the total amount of time under observation between the first and last IGF-1 value. MYCAPSSA (n=55) Injectable SSAs (n=37) 95% CI 95% CI Proportion of Acromegaly Patients Achieving Primary Endpoint 91% 100%
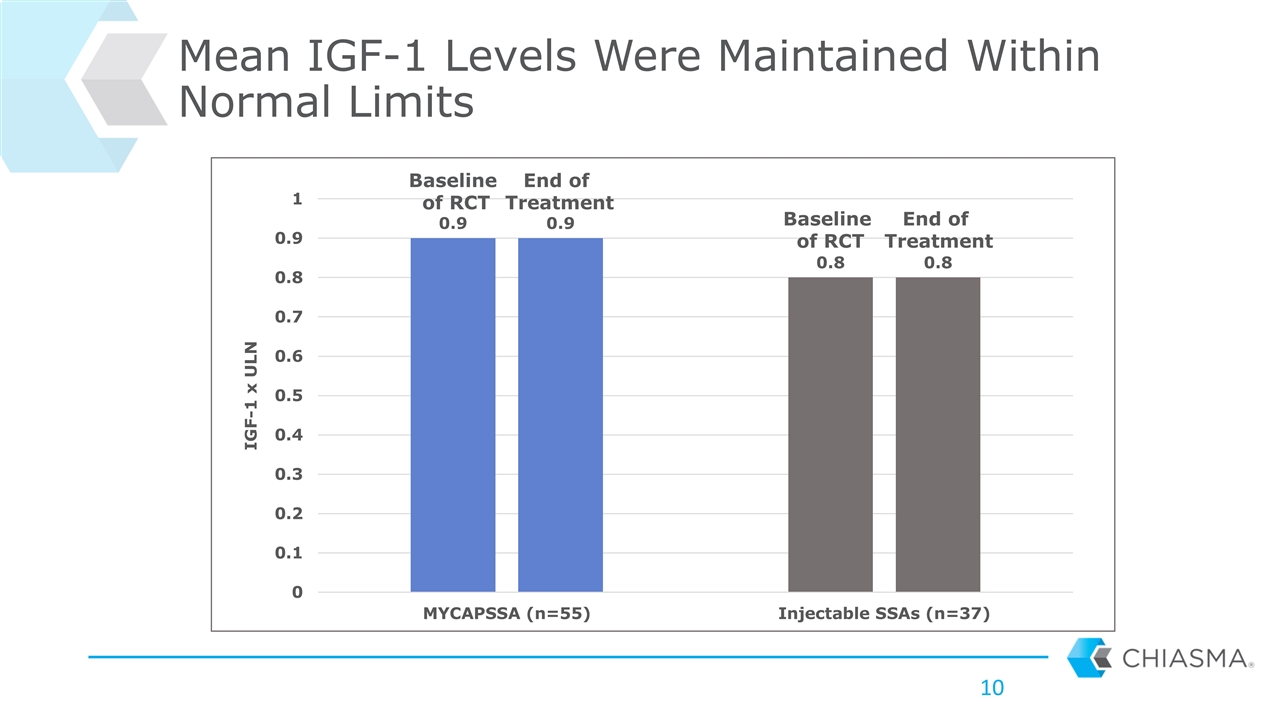
Mean IGF-1 Levels Were Maintained Within Normal Limits Baseline of RCT Baseline of RCT End of Treatment End of Treatment
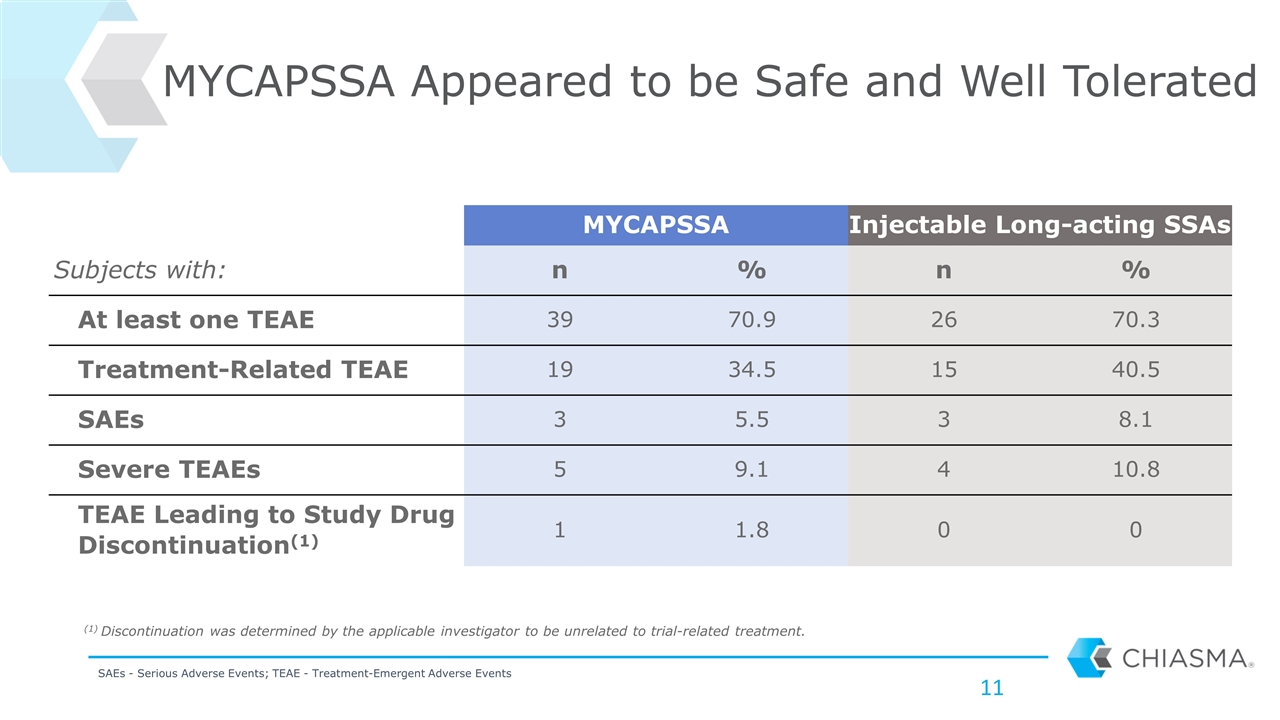
MYCAPSSA Appeared to be Safe and Well Tolerated MYCAPSSA Injectable Long-acting SSAs Subjects with: n % n % At least one TEAE 39 70.9 26 70.3 Treatment-Related TEAE 19 34.5 15 40.5 SAEs 3 5.5 3 8.1 Severe TEAEs 5 9.1 4 10.8 TEAE Leading to Study Drug Discontinuation(1) 1 1.8 0 0 SAEs - Serious Adverse Events; TEAE - Treatment-Emergent Adverse Events (1) Discontinuation was determined by the applicable investigator to be unrelated to trial-related treatment.
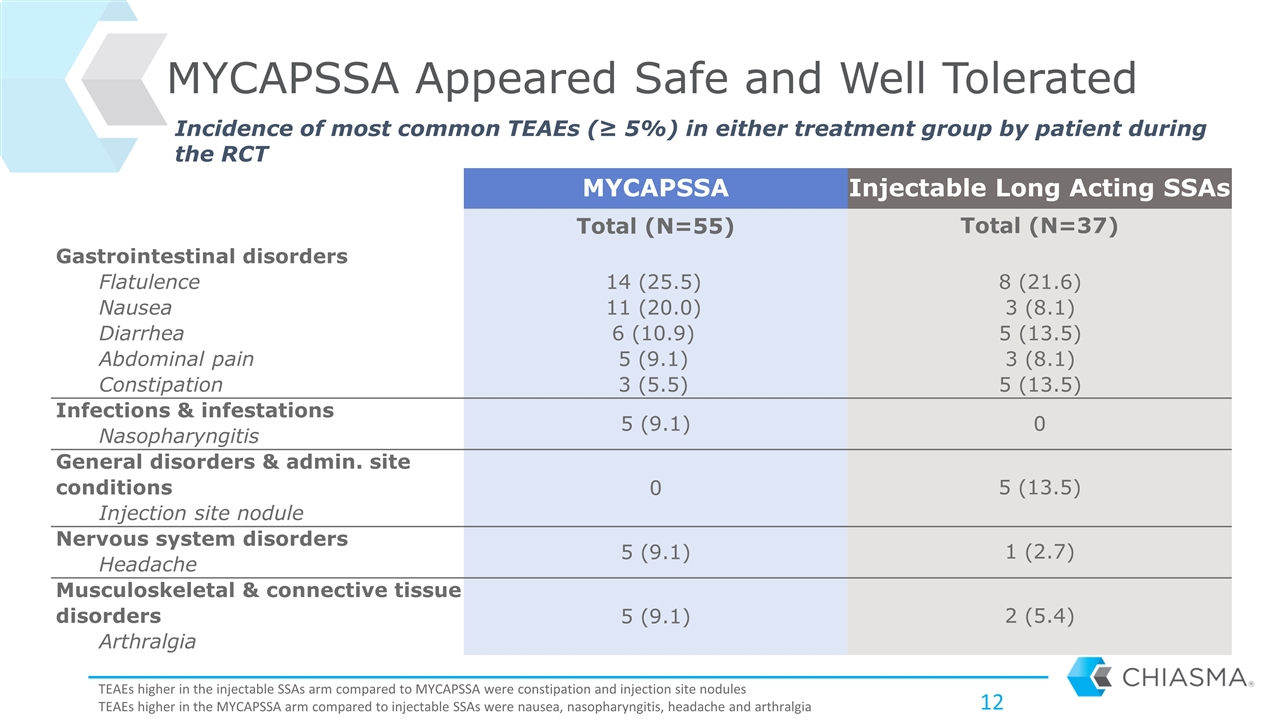
MYCAPSSA Appeared Safe and Well Tolerated MYCAPSSA Injectable Long Acting SSAs Total (N=55) Total (N=37) Gastrointestinal disorders Flatulence 14 (25.5) 8 (21.6) Nausea 11 (20.0) 3 (8.1) Diarrhea 6 (10.9) 5 (13.5) Abdominal pain 5 (9.1) 3 (8.1) Constipation 3 (5.5) 5 (13.5) Infections & infestations 5 (9.1) 0 Nasopharyngitis General disorders & admin. site conditions 0 5 (13.5) Injection site nodule Nervous system disorders 5 (9.1) 1 (2.7) Headache Musculoskeletal & connective tissue disorders 5 (9.1) 2 (5.4) Arthralgia Incidence of most common TEAEs (≥ 5%) in either treatment group by patient during the RCT TEAEs higher in the injectable SSAs arm compared to MYCAPSSA were constipation and injection site nodules TEAEs higher in the MYCAPSSA arm compared to injectable SSAs were nausea, nasopharyngitis, headache and arthralgia
