Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - RELMADA THERAPEUTICS, INC. | ea129933-8k_relmadathera.htm |
Exhibit 99.1

2020 Corporate Presentation I Targeting Major Advances in Treatment of CNS Disorders November 2020 I Nasdaq: RLMD
2020 Corporate Presentation I Disclosures Certain statements contained in this presentation or in other documents of Relmada Therapeutics, Inc . (the “Company”), along with certain statements that may be made by management of the Company orally in presenting this material, may contain “forward - looking statements . ” These statements can be identified by the fact that they do not relate strictly to historic or current facts . They use words such as “estimate,” “expect,” “intend,” “believe,” “plan,” “anticipate,” “projected” and other words and terms of similar meaning in connection with any discussion of future operating or financial performance or condition . These statements are based upon the current beliefs and expectations of the Company’s management and are subject to significant risks and uncertainties . Statements regarding future action, future performance and/or future results including, without limitation, the proposed offering, those relating to the timing for completion, and results of, scheduled or additional clinical trials and the FDA’s or other regulatory review and/or approval and commercial launch and sales results (if any) of the Company’s formulations and products and regulatory filings related to the same may differ from those set forth in the forward - looking statements . Peak sales and market size estimates have been determined on the basis of market research and comparable product analysis, but no assurances can be given that such sales levels will be achieved, if at all, or that such market size estimates will prove accurate . Because actual results are affected by these and other potential risks, contingencies and uncertainties, the Company cautions investors that actual results may differ materially from those expressed or implied in any forward - looking statement . It is not possible to predict or identify all such risks, contingencies and uncertainties . The Company identifies some of these factors in its Securities and Exchange Commission (“SEC”) filings on Forms 10 - K, 10 - Q and 8 - K, and investors are advised to consult the Company’s filings for a more complete listing of risk factors, contingencies and uncertainties affecting the Company and its business and financial performance . The Company assumes no obligation to update forward - looking statements as circumstances change . Investors are advised to consult further disclosures that the Company makes or has made on related subjects in the Company’s Form 10 - K, 10 - Q and 8 - K reports . 2
2020 Corporate Presentation I • Novel MOA with successful Phase II trial in adjunctive MDD that showed statistically significant, rapid and sustained anti - depressant effects with favorable safety and tolerability profile • Successful EoP2 meeting with the FDA with clear pathway to NDA • Fast track designation from FDA • Strong IP position around REL - 1017 with protection to the mid/late - 2030s • Depression remains the leading cause of ill health and disability worldwide 1 • 50% – 66% of patients with depression do not fully recover on an antidepressant medication 2 • Standard anti - depressants can take 4 - 6 weeks to work, and have significant side - effects • The only FDA - approved adjunctive treatment options are atypical antipsychotics Major Depressive Disorder (MDD): Large potential in underserved markets • 4Q20 – Start of first pivotal Phase III adjunctive MDD trial • 1H21 – Start of second pivotal Phase III adjunctive MDD trial • 1H21 – Start of Phase II monotherapy MDD trial • 2Q21 – Results of human abuse potential studies • 4Q21 – Results of Phase II monotherapy MDD trial • 1H22 – Results of Phase III adjunctive MDD trials Key catalysts expected over next 12 - 18 months Investment Highlights 3 * MDD = Major Depressive Disorder ** Our fiscal year end is December 31. The periods referred to in this slide are calendar years and quarters. 1. https://www.who.int/news - room/fact - sheets/detail/depression 2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3363299/ Highly - compelling de - risked lead product opportunity

2020 Corporate Presentation I 4 as a Potential Treatment for Depression REL - 1017
2020 Corporate Presentation I REL - 1017: A Novel, Selective NMDAR Blocker being developed for MDD ▪ Potential to be the first rapid - acting, oral, once - daily antidepressant treatment ▪ Completed Phase 1 and Phase II trial for Adjunctive treatment of MDD ▪ In a Phase II trial, both doses of REL - 1017 demonstrated statistically significant improvement vs. placebo on all efficacy measures, and: • Rapid onset and sustained antidepressant effects • Only mild and moderate AEs - no serious AEs • No evidence of treatment - induced dissociative and psychotomimetic AEs • No evidence of difference in opiate withdrawal symptoms in treatment groups vs placebo Potentially significantly differentiated profile 5

2020 Corporate Presentation I ~8M U.S. 50% of MDD patients receive medication treatment 1 ~17M U.S. Individuals in 2017 with at least 1 MDE episode 1 ~65M U.S. Individuals with at least 1 lifetime episode 2 6 Major Depressive Disorder (MDD) Market: Large, Debilitating, Underserved 1. SAMHSA https://www.nimh.nih.gov/health/statistics/major - depression.shtml#:~:text=The%20prevalence%20of%20major%20depressive%20episode%2 0was%20highest,episode%20was%20highest%20among%20individuals%20aged%2018 - 25%20%2813.1%25%29 2. Hasin DS, et al. Epidemiology of Adult DSM - 5 Major Depressive Disorder and Its Specifiers in the United States. JAMA Psychiatry. Published online February 14, 2018.
2020 Corporate Presentation I • ~65% MDD patients do not respond to first antidepressant treatment • ~30% MDD patients do not respond to up to 4 different antidepressant treatments Relmada — Corporate presentation 7 • Sleep disturbance, sexual dysfunction, GI distress and weight gain limit adoption • Atypical anti - psychotics, the only approved adjunctive treatments, have associated AEs including metabolic effects, cognitive impairment, stroke risk, and extrapyramidal symptoms Significant Limitations Characterize Current Standard of Care in MDD Efficacy Onset of Action • Standard antidepressants take 4 - 6 weeks to reach efficacy Safety and Tolerability 1. Source: Am J Psychiatry. 2006 Nov;163(11):1905 - 17, 2. https:// www.samhsa.gov /data/sites/default/files/ cbhsq - reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab8 - 10A
2020 Corporate Presentation I ▪ Traditional MDD therapeutics have been based on MAO hypothesis ▪ Increasingly, NMDA receptor recognized for significance in pathophysiology of depression • NMDA blockade presents potential mechanism for safe and effective MDD treatment with rapid onset ▪ REL - 1017 is a novel, NMDAR channel blocker • Preferentially targets hyperactive channels of particular importance in MDD • Avoids effects on those channels that are associated with normal physiological functions 8 Relmada — Corporate presentation REL - 1017: A Novel NMDAR Channel Blocker with Preferential Activity
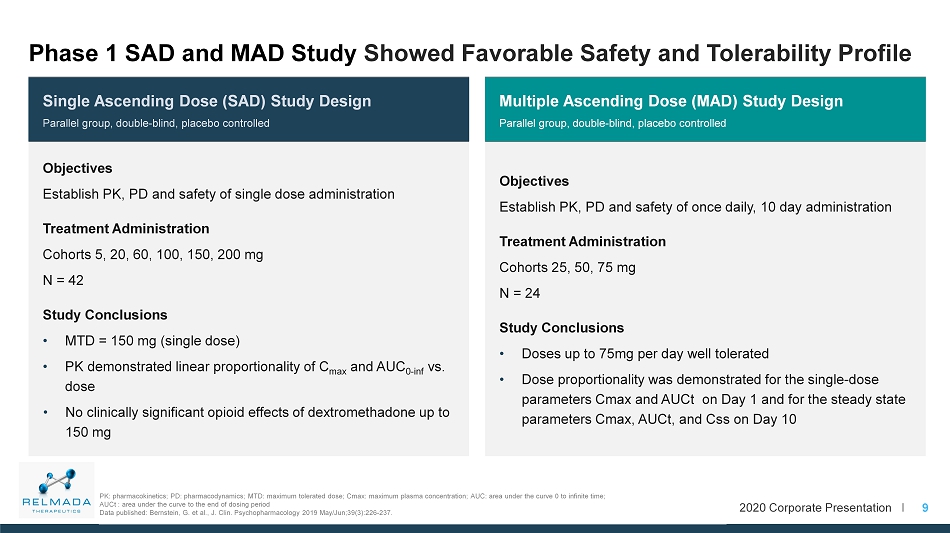
2020 Corporate Presentation I Multiple Ascending Dose (MAD) Study Design Parallel group, double - blind, placebo controlled Objectives Establish PK, PD and safety of once daily, 10 day administration Treatment Administration Cohorts 25, 50, 75 mg N = 24 Study Conclusions • Doses up to 75mg per day well tolerated • Dose proportionality was demonstrated for the single - dose parameters Cmax and AUCt on Day 1 and for the steady state parameters Cmax , AUCt , and Css on Day 10 Single Ascending Dose (SAD) Study Design Parallel group, double - blind, placebo controlled Objectives Establish PK, PD and safety of single dose administration Treatment Administration Cohorts 5, 20, 60, 100, 150, 200 mg N = 42 Study Conclusions • MTD = 150 mg (single dose) • PK demonstrated linear proportionality of C max and AUC 0 - inf vs. dose • No clinically significant opioid effects of dextromethadone up to 150 mg 9 Phase 1 SAD and MAD Study Showed Favorable Safety and Tolerability Profile PK: pharmacokinetics; PD: pharmacodynamics; MTD: maximum tolerated dose; Cmax : maximum plasma concentration; AUC: area under the curve 0 to infinite time; AUCt : area under the curve to the end of dosing period Data published: Bernstein, G. et al., J. Clin. Psychopharmacology 2019 May/Jun;39(3):226 - 237.
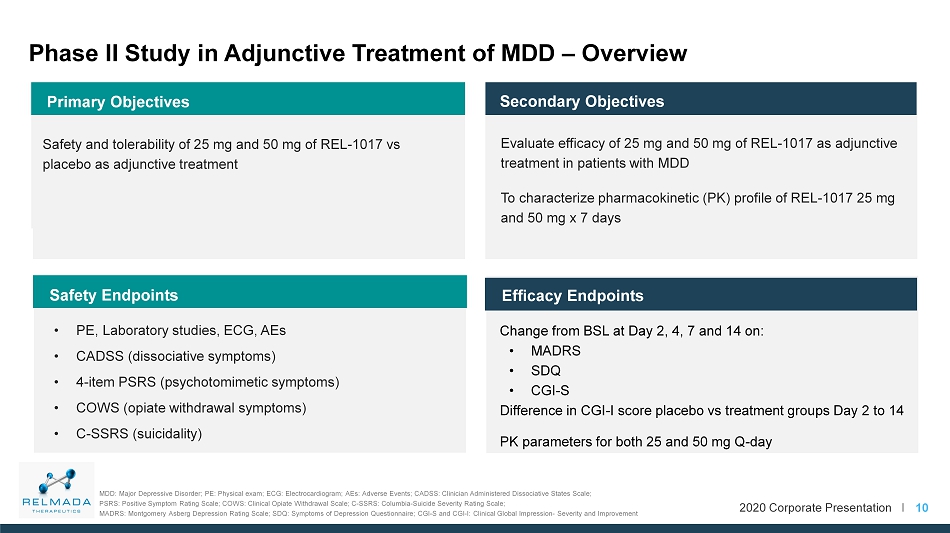
2020 Corporate Presentation I Phase II Study in Adjunctive Treatment of MDD – Overview 10 Change from BSL at Day 2, 4, 7 and 14 on: • MADRS • SDQ • CGI - S Difference in CGI - I score placebo vs treatment groups Day 2 to 14 PK parameters for both 25 and 50 mg Q - day Efficacy Endpoints Secondary Objectives Primary Objectives Safety and tolerability of 25 mg and 50 mg of REL - 1017 vs placebo as adjunctive treatment Evaluate efficacy of 25 mg and 50 mg of REL - 1017 as adjunctive treatment in patients with MDD To characterize pharmacokinetic (PK) profile of REL - 1017 25 mg and 50 mg x 7 days • PE, Laboratory studies, ECG, AEs • CADSS (dissociative symptoms) • 4 - item PSRS (psychotomimetic symptoms) • COWS (opiate withdrawal symptoms) • C - SSRS (suicidality) Safety Endpoints MDD: Major Depressive Disorder; PE: Physical exam; ECG: Electrocardiogram; AEs: Adverse Events; CADSS: Clinician Administered Di ssociative States Scale; PSRS: Positive Symptom Rating Scale; COWS: Clinical Opiate Withdrawal Scale; C - SSRS: Columbia - Suicide Severity Rating Scale; MADRS: Montgomery Asberg Depression Rating Scale; SDQ: Symptoms of Depression Questionnaire; CGI - S and CGI - I: Clinical Global Impression - Severity and I mprovement

2020 Corporate Presentation I 60 patients three arm placebo - controlled trial Two doses tested 25mg and 50mg once a day versus placebo 7 days daily treatment in clinic + 7 days observation as outpatient Follow up at day 14 for efficacy and safety Follow up at day 21 for safety only 11 REL - 1017 Phase II Study Design

2020 Corporate Presentation I Inpatient Days - 1 to 9 12 REL - 1017 Phase II Trial Design MDD = Major Depressive Disorder; RDPC = randomized double - blind placebo controlled; MADRS = Montgomery - Asberg Depression Rating Scale; SDQ = Symptoms of Depression Questionnaire; CGIs = Clinical Global Impression scales Placebo REL - 1017 25mg REL - 1017 50mg SAFER interview done by CTNI - MGH Dosing Days 1 to 7 Screening Days - 30 to - 2 Observation Days 8 to 14 Follow Up Days 15 - 21
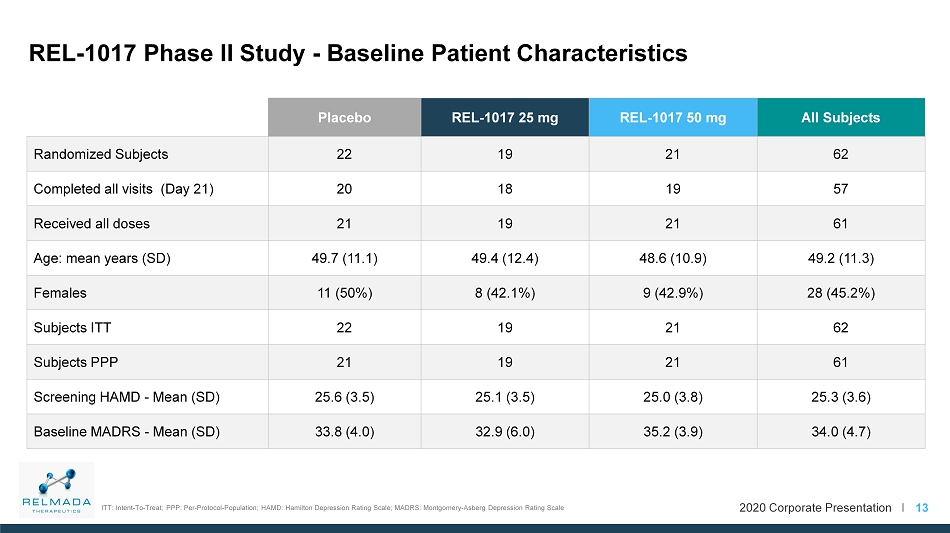
2020 Corporate Presentation I Placebo REL - 1017 25 mg REL - 1017 50 mg All Subjects Randomized Subjects 22 19 21 62 Completed all visits (Day 21) 20 18 19 57 Received all doses 21 19 21 61 Age: mean years (SD) 49.7 (11.1) 49.4 (12.4) 48.6 (10.9) 49.2 (11.3) Females 11 (50%) 8 (42.1%) 9 (42.9%) 28 (45.2%) Subjects ITT 22 19 21 62 Subjects PPP 21 19 21 61 Screening HAMD - Mean (SD) 25.6 (3.5) 25.1 (3.5) 25.0 (3.8) 25.3 (3.6) Baseline MADRS - Mean (SD) 33.8 (4.0) 32.9 (6.0) 35.2 (3.9) 34.0 (4.7) 13 REL - 1017 Phase II Study - Baseline Patient Characteristics ITT: Intent - To - Treat; PPP: Per - Protocol - Population; HAMD: Hamilton Depression Rating Scale; MADRS: Montgomery - Asberg Depression Rating Scale

2020 Corporate Presentation I -25 -20 -15 -10 -5 0 Day 2 Day 4 Day 7 Day 14 Least - Square Mean Change Placebo REL-1017 25mg REL-1017 50mg P = 0.0087; d = 0.9 P = 0.0096; d = 0.8 P = 0.0122; d = 0.8 P = 0.0308; d = 0.7 P = 0.0103; d = 0.9 P = 0.0039; d = 1.0 14 REL - 1017 Phase II Study Efficacy MADRS Scores for Achieved Statistically Significant Difference vs Placebo for Days 4 - 14 MADRS: Montgomery - Asberg Depression Rating Scale; ITT: Intent - To - Treat; Error Bars: Standard Errors; P and d values as Treatment vs Placebo

2020 Corporate Presentation I 15 REL - 1017 Phase II Study Efficacy Remission Rates (MADRS <10 Points) Percent Subjects per ITT Group 14% 5% 32% 31% 19% 33% 0% 10% 20% 30% 40% 50% 60% Day 7 Day 14 24% 20% 42% 50% 57% 44% 0% 10% 20% 30% 40% 50% 60% Day 7 Day 14 Response Rates (>50% Change vs. Baseline) PBO REL 25 mg REL 50 mg PBO REL 25 mg REL 50 mg PBO REL 25 mg REL 50 mg PBO REL 25 mg REL 50 mg

2020 Corporate Presentation I ▪ Only Mild and Moderate AEs - no SAEs ▪ No increased prevalence of specifically relevant organ group AEs in treatment groups vs placebo ▪ No evidence of treatment induced dissociative symptoms in the treatment groups vs placebo ▪ No evidence of treatment induced psychotomimetic symptoms in treatment groups vs placebo ▪ No evidence of opiate withdrawal symptoms in treatment groups vs placebo 16 REL - 1017 Phase II Study Indicated Favorable Safety & Tolerability, Consistent with Phase I

2020 Corporate Presentation I Indication: Studies will assess REL - 1017 as adjunctive treatment in MDD patients who have failed at least one prior treatment in current depression episode Two Pivotal Studies: Two sister two - arm, placebo - controlled clinical studies Primary Endpoint: Change from baseline on MADRS at Day 28 for REL - 1017 vs. placebo and collection of sufficient safety data to support use as chronic treatment Key Secondary Endpoints: Change from baseline on 7 - Day MADRS to evaluate time to onset of treatment effect; achieved by Day 4 in Phase II 17 End of Phase II Meeting Outcome REL - 1017 can advance into Phase 3 registration studies w/o additional clinical studies. FDA and Relmada are aligned on all key aspects of Phase 3 program to be initiated in Q4 ’20.

2020 Corporate Presentation I Dosing: 25 mg dose of REL - 1017 to be evaluated. PD relationship in Phase II supports equivalence of 25 mg and 50 mg doses Tablet formulation Equivalence Established: No PK bridging studies required to support transition from powder - in - solution formulation of REL - 1017 utilized in Phase II to tablet formulation to be used in Phase 3 Abuse Liability Testing: Studies to determine scheduling not required prior to starting Phase 3 and will be conducted pre - NDA 18 End of Phase II Meeting Outcome REL - 1017 can advance into Phase 3 registration studies w/o additional clinical studies. FDA and Relmada are aligned on all key aspects of Phase 3 program to be initiated in Q4 ’20.

2020 Corporate Presentation I REL - 1017 ( dextromethadone ) • REL - 1017 is dextromethadone , the dextro - isomer of parent, racemic methadone – NCE with unique pharmacology – Per DEA, distinct from parent and levo - isomer responsible for opioid effects of methadone – Phase II assessments during inpatient period and days after treatment discontinuation showed AE, COW profile ~ placebo • Abuse potential assessments for NDA submission – Preclinical program with 3 studies (drug discrimination, self administration, physical dependence) – Clinical program with 2 studies (vs. ketamine and vs. oxycodone), data expected H1 2021 Background & Planned Assessments re: Abuse Potential 19 “ Analgesic activity of racemic methadone is entirely due to its l - isomer, 8 to 50 times more potent than d - isomer. The d - isomer lacks significant respiratory depressant action and addiction liability , but possesses antitussive activity. “ - Methadone Statement, July 2019

2020 Corporate Presentation I 20 REL - 1017: Anticipated Development Timeline* Q4 2020 H1 2021 H2 2021 H1 2022 Adjunctive Treatment of Major Depressive Disorder (MDD) Major Depressive Disorder (MDD) 1 st Pivotal Adjunctive MDD trial MDD Monotherapy Phase II *subject to FDA feedback Indication 2 nd Pivotal Adjunctive MDD trial

2020 Corporate Presentation I 21 Potential Competitive Advantages of Dextromethadone Compound (Company) Mechanism of Action Delivery Current Clinical Stage Dosing Regimen Dextromethadone (Relmada) Non - competitive NMDA channel blocker Oral Completed Phase II Once Daily Esketamine/ Spravato (Janssen/J&J) Nasal ( in - clinic administration) Approved Biweekly AXS - 05 DM 45 mg + BUP 105 mg (Axsome) Multimodal (NMDA+others) Oral Pre - NDA¹ Twice daily Sage - 217 (Sage) GABA receptor allosteric modulator Oral Phase 3² Once Daily 1 First P3 study met primary endpoint 2 First P3 study did not meet primary endpoint

2020 Corporate Presentation I 22 Corporate Information
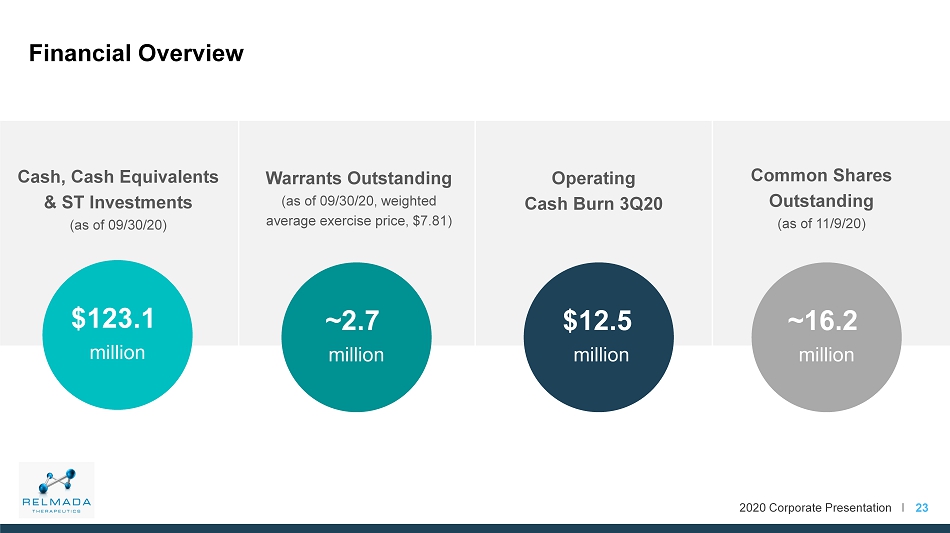
2020 Corporate Presentation I 23 Financial Overview Cash, Cash Equivalents & ST Investments (as of 09/30/20) $123.1 million ~2.7 million $12.5 million ~16.2 million Warrants Outstanding (as of 09/30/20, weighted average exercise price, $7.81) Operating Cash Burn 3Q20 Common Shares Outstanding (as of 11/9/20)

2020 Corporate Presentation I Management Team and Key Scientific Advisors Management Sergio Traversa Chief Executive Officer Paolo Manfredi, MD Acting Chief Scientific Officer Marco Pappagallo, MD Acting Chief Medical Officer Tom Wessel, MD, Ph.D Head of Research & Development Maged Shenouda Chief Financial Officer Marc de Somer, MD, MBA, ScD, MPH, MSc SVP, Clinical Development and Safety Chuck Ence Chief Accounting and Compliance Officer Molly Harper Executive Vice President of Operations 24

2020 Corporate Presentation I Advisors Maurizio Fava, MD, Chair Stephen M. Stahl, MD Luca Pani, MD Thomas Laughren, MD Dan Iosifescu , MD, MSc Sanjay Johan Mathew, MD Charles Inturrisi , PhD Scientific Advisors 25

2020 Corporate Presentation I • 4Q20 – Start of first pivotal Phase III adjunctive MDD trial • 1H21 – Start of second pivotal Phase III adjunctive MDD trial • 1H21 – Start of Phase II monotherapy MDD trial • 2Q21 – Results of human abuse potential studies • 4Q21 – Results of Phase II monotherapy MDD trial • 1H22 – Results of Phase III adjunctive MDD trials 26 Key Catalysts Expected Over Next 12 - 18 Months * MDD = Major Depressive Disorder ** Our fiscal year end is December 31. The periods referred to in this slide are calendar years and quarters. 1. https://www.who.int/news - room/fact - sheets/detail/depression 2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3363299/

2020 Corporate Presentation I • Novel MOA with successful Phase II trial in adjunctive MDD that showed statistically significant, rapid and sustained anti - depressant effects with favorable safety and tolerability profile • Successful EoP2 meeting with the FDA with clear pathway to NDA • Fast track designation from FDA • Strong IP position around REL - 1017 with protection to the mid/late - 2030s • Depression remains the leading cause of ill health and disability worldwide 1 • 50% – 66% of patients with depression do not fully recover on an antidepressant medication 2 • Standard anti - depressants can take 4 - 6 weeks to work, and have significant side - effects • The only FDA - approved adjunctive treatment options are atypical antipsychotics Major Depressive Disorder (MDD): Large potential in underserved markets • 4Q20 – Start of first pivotal Phase III adjunctive MDD trial • 1H21 – Start of second pivotal Phase III adjunctive MDD trial • 1H21 – Start of Phase II monotherapy MDD trial • 2Q21 – Results of human abuse potential studies • 4Q21 – Results of Phase II monotherapy MDD trial • 1H22 – Results of Phase III adjunctive MDD trials Key catalysts expected over next 12 - 18 months Investment Highlights 27 * MDD = Major Depressive Disorder ** Our fiscal year end is December 31. The periods referred to in this slide are calendar years and quarters. 1. https://www.who.int/news - room/fact - sheets/detail/depression 2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3363299/ Highly - compelling de - risked lead product opportunity

2020 Corporate Presentation I BACK UP Sept 2020 I Nasdaq: RLMD

2020 Corporate Presentation I Strong Anti - Depressant Effects Observed in Three Animal Models of Depression Improved performance on the rat forced swim test 24 hours after d - methadone treatment 29 Forced Swim Test * = p<0.05 compared to placebo group

2020 Corporate Presentation I Strong Anti - Depressant Effects Observed in Three Depression Animal Models Improved performance on the rat FUST and the NSFT 24 hours after d - methadone treatment 30 * * * * Female Urine Smell Test Novelty Suppressed Feeding Test
2020 Corporate Presentation I Study REL - 1017 Phase II Key Efficacy Findings 31 REL - 1017 25 mg and 50 mg show rapid onset and sustained antidepressant effects with statistically significant differences compared to placebo on all efficacy measures • Solid effects observed on MADRS with P values < 0.03 and large effect sizes (0.7 - 1.0) from Day 4 to Day 14 • CGI - S and CGI - I solid findings consistent with MADRS results with P - values and effect sizes of similar magnitude • SDQ scores with moderate effect size differences (d = 0.4 and 0.5) from Day 4 to Day 7 and with both statistically significant differences and large effect size for both 25 mg (P = 0.0066; d = 0.9) and 50 mg (P= 0.0014; d = 1.1) arms at Day 14 • Study demonstrates rapid onset and long - lasting antidepressant effects • Findings support continuing clinical development and larger pivotal study
