Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - RAPT Therapeutics, Inc. | d86412dex992.htm |
| EX-99.1 - EX-99.1 - RAPT Therapeutics, Inc. | d86412dex991.htm |
| 8-K - 8-K - RAPT Therapeutics, Inc. | d86412d8k.htm |

Transforming the Treatment of Cancer and Inflammation FLX475 Clinical Data Update November 16, 2020 Exhibit 99.3

Legal Disclaimers Statements in this Presentation that are not statements of historical fact are forward-looking statements. Such forward-looking statements include, without limitation, statements regarding RAPT Therapeutics, Inc.’s (the "Company," "we," or "us") research and clinical development plans; current and future drug candidates; business strategy and plans; regulatory pathways; and our ability to complete certain milestones. Words such as "believe," "anticipate," "plan," "expect," "will," "may," "upcoming," "milestone," "potential," "target" or the negative of these terms or similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. These forward-looking statements are based on the current beliefs of the Company's management with respect to future events and trends and are subject to known and unknown risks and uncertainties, including those described in the “Risk Factors” section of our most recent Form 10-Q filed with the Securities and Exchange Commission, that may cause our actual performance or achievements to be materially different from any future performance or achievements expressed or implied by the forward-looking statements in this Presentation. These forward-looking statements should not be taken as forecasts or promises nor should they be taken as implying any indication, assurance or guarantee that any assumptions on which such forward-looking statements have been made are correct or exhaustive or, in the case of such assumptions, fully stated in the Presentation. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this Presentation is given. Although we believe that the beliefs and assumptions reflected in the forward-looking statements are reasonable, we cannot guarantee future performance or achievements. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this Presentation. This Presentation discusses drug candidates that are under clinical study and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of any drug candidates for any use for which such drug candidates are being studied.

FLX475: Oral CCR4 Antagonist for Oncology

Key Takeaways Monotherapy responses demonstrate FLX475 is an active agent Combination responses in multiple tumor indications beyond expected from checkpoint inhibition alone Favorable safety profile with broad combinability Biomarker data supports Treg mechanism Multiple cohorts are being expanded Remaining cohorts ongoing/not yet declared FLX475 Tablet
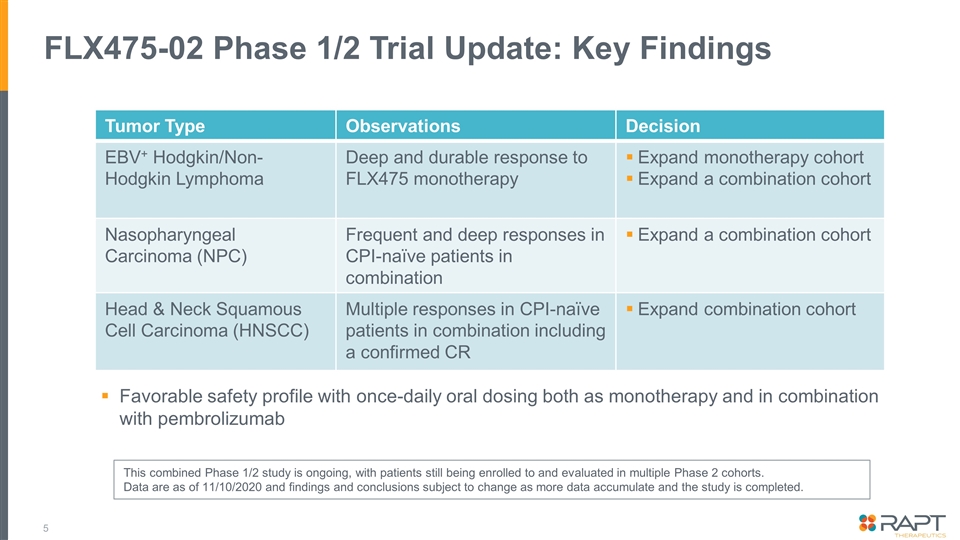
FLX475-02 Phase 1/2 Trial Update: Key Findings Tumor Type Observations Decision EBV+ Hodgkin/Non-Hodgkin Lymphoma Deep and durable response to FLX475 monotherapy Expand monotherapy cohort Expand a combination cohort Nasopharyngeal Carcinoma (NPC) Frequent and deep responses in CPI-naïve patients in combination Expand a combination cohort Head & Neck Squamous Cell Carcinoma (HNSCC) Multiple responses in CPI-naïve patients in combination including a confirmed CR Expand combination cohort Favorable safety profile with once-daily oral dosing both as monotherapy and in combination with pembrolizumab This combined Phase 1/2 study is ongoing, with patients still being enrolled to and evaluated in multiple Phase 2 cohorts. Data are as of 11/10/2020 and findings and conclusions subject to change as more data accumulate and the study is completed.
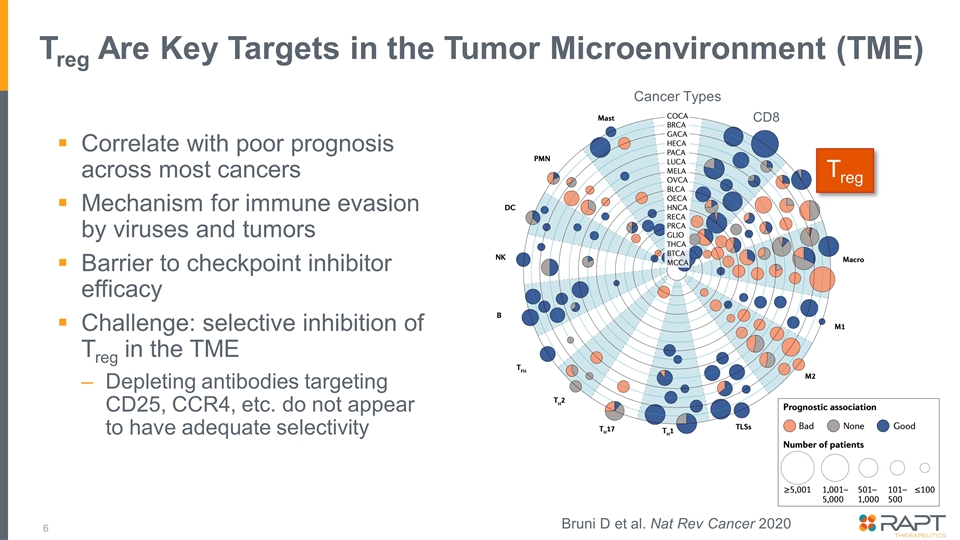
Treg Are Key Targets in the Tumor Microenvironment (TME) Correlate with poor prognosis across most cancers Mechanism for immune evasion by viruses and tumors Barrier to checkpoint inhibitor efficacy Challenge: selective inhibition of Treg in the TME Depleting antibodies targeting CD25, CCR4, etc. do not appear to have adequate selectivity Treg CD8 Cancer Types Bruni D et al. Nat Rev Cancer 2020
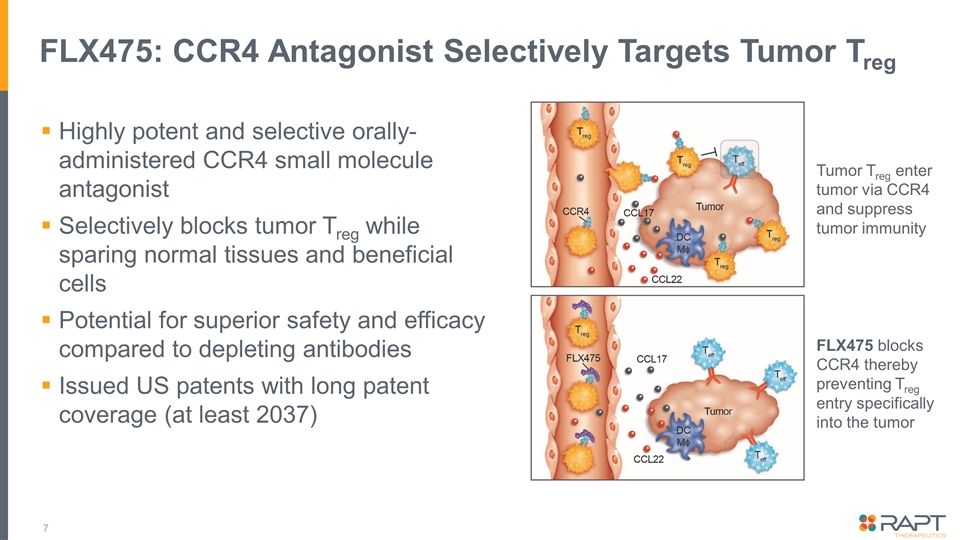
FLX475: CCR4 Antagonist Selectively Targets Tumor Treg Highly potent and selective orally-administered CCR4 small molecule antagonist Selectively blocks tumor Treg while sparing normal tissues and beneficial cells Potential for superior safety and efficacy compared to depleting antibodies Issued US patents with long patent coverage (at least 2037) Tumor Treg enter tumor via CCR4 and suppress tumor immunity FLX475 blocks CCR4 thereby preventing Treg entry specifically into the tumor
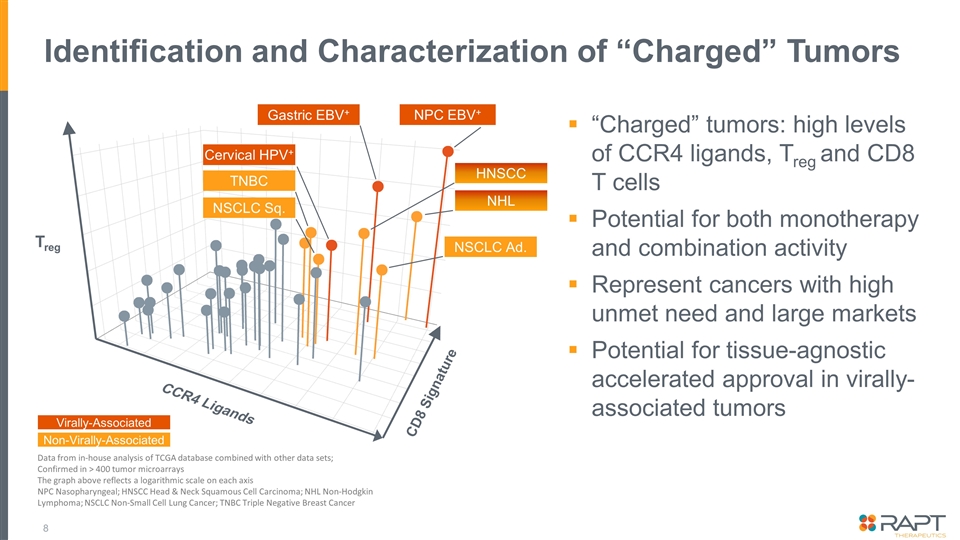
Identification and Characterization of “Charged” Tumors “Charged” tumors: high levels of CCR4 ligands, Treg and CD8 T cells Potential for both monotherapy and combination activity Represent cancers with high unmet need and large markets Potential for tissue-agnostic accelerated approval in virally- associated tumors Data from in-house analysis of TCGA database combined with other data sets; Confirmed in > 400 tumor microarrays The graph above reflects a logarithmic scale on each axis NPC Nasopharyngeal; HNSCC Head & Neck Squamous Cell Carcinoma; NHL Non-Hodgkin Lymphoma; NSCLC Non-Small Cell Lung Cancer; TNBC Triple Negative Breast Cancer Treg CCR4 Ligands CD8 Signature Gastric EBV+ NPC EBV+ NSCLC Sq. TNBC Cervical HPV+ HNSCC NSCLC Ad. Virally-Associated Non-Virally-Associated NHL
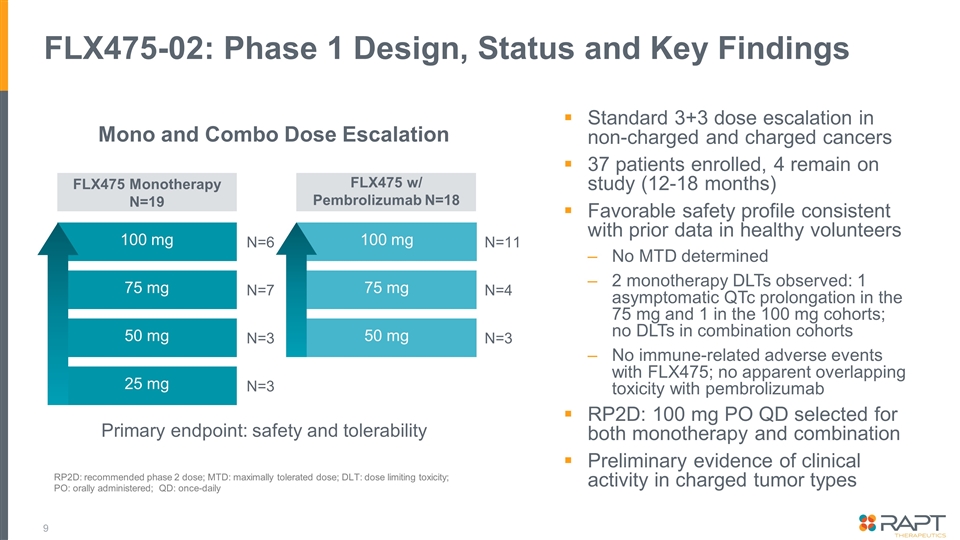
FLX475-02: Phase 1 Design, Status and Key Findings N=6 N=7 N=3 N=3 N=11 N=4 N=3 100 mg 100 mg 75 mg 50 mg 25 mg 75 mg 50 mg FLX475 Monotherapy N=19 FLX475 w/ Pembrolizumab N=18 Standard 3+3 dose escalation in non-charged and charged cancers 37 patients enrolled, 4 remain on study (12-18 months) Favorable safety profile consistent with prior data in healthy volunteers No MTD determined 2 monotherapy DLTs observed: 1 asymptomatic QTc prolongation in the 75 mg and 1 in the 100 mg cohorts; no DLTs in combination cohorts No immune-related adverse events with FLX475; no apparent overlapping toxicity with pembrolizumab RP2D: 100 mg PO QD selected for both monotherapy and combination Preliminary evidence of clinical activity in charged tumor types Mono and Combo Dose Escalation Primary endpoint: safety and tolerability RP2D: recommended phase 2 dose; MTD: maximally tolerated dose; DLT: dose limiting toxicity; PO: orally administered; QD: once-daily
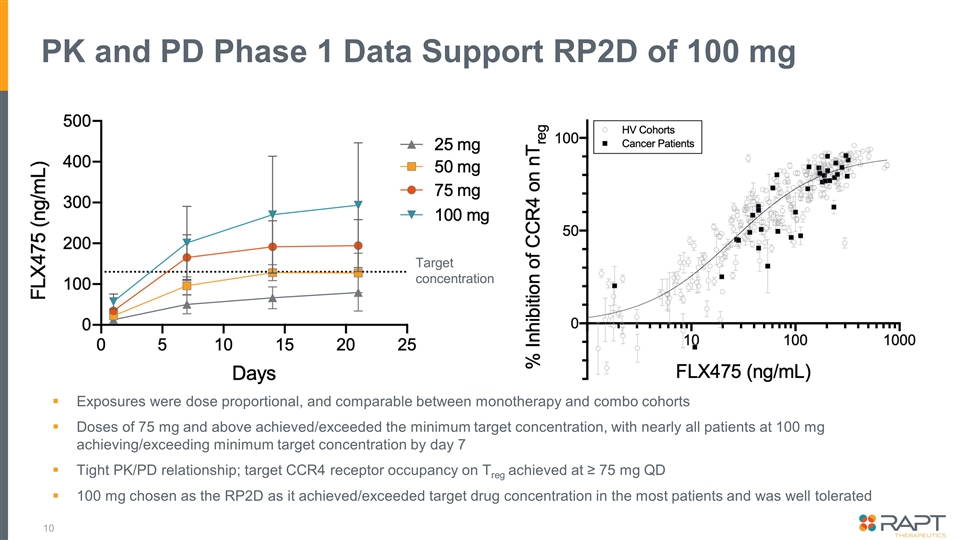
PK and PD Phase 1 Data Support RP2D of 100 mg Exposures were dose proportional, and comparable between monotherapy and combo cohorts Doses of 75 mg and above achieved/exceeded the minimum target concentration, with nearly all patients at 100 mg achieving/exceeding minimum target concentration by day 7 Tight PK/PD relationship; target CCR4 receptor occupancy on Treg achieved at ≥ 75 mg QD 100 mg chosen as the RP2D as it achieved/exceeded target drug concentration in the most patients and was well tolerated Target concentration
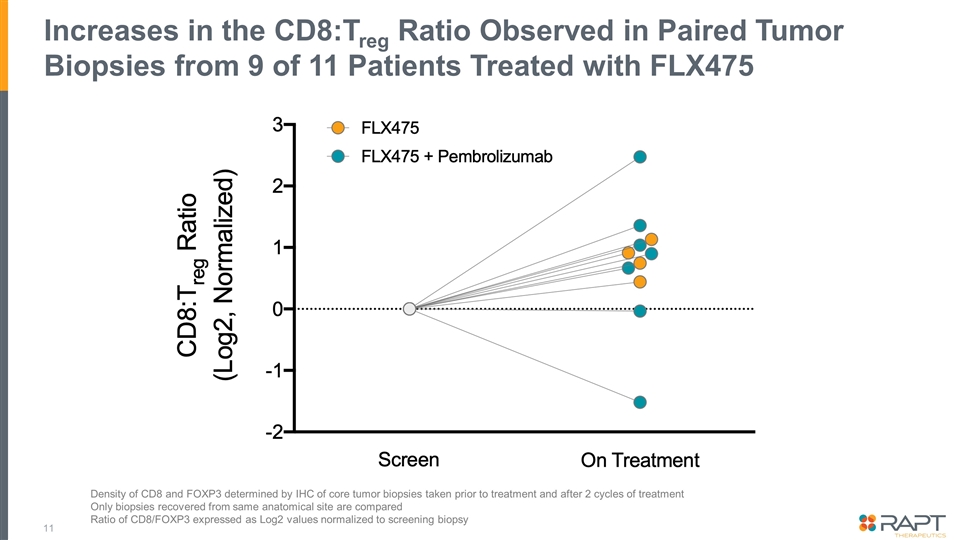
Increases in the CD8:Treg Ratio Observed in Paired Tumor Biopsies from 9 of 11 Patients Treated with FLX475 Density of CD8 and FOXP3 determined by IHC of core tumor biopsies taken prior to treatment and after 2 cycles of treatment Only biopsies recovered from same anatomical site are compared Ratio of CD8/FOXP3 expressed as Log2 values normalized to screening biopsy
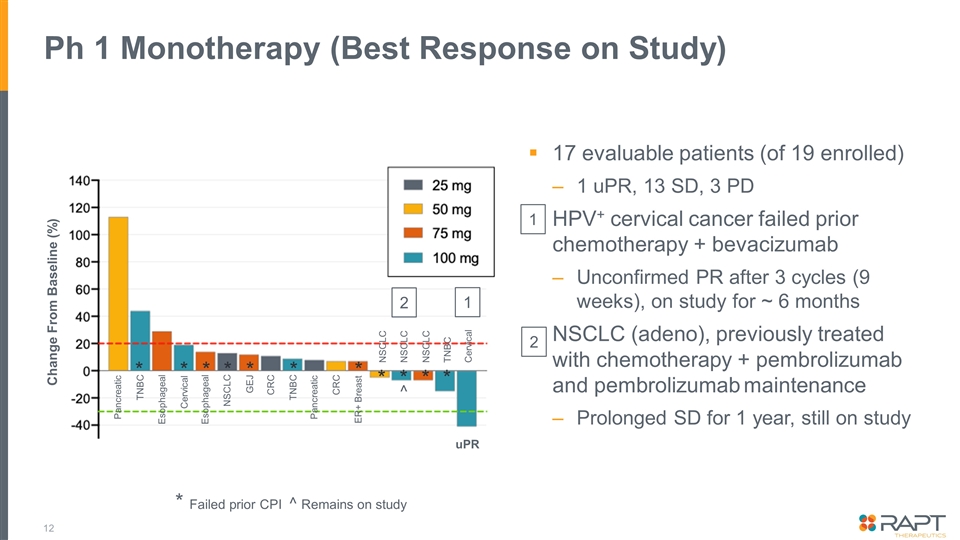
Ph 1 Monotherapy (Best Response on Study) 17 evaluable patients (of 19 enrolled) 1 uPR, 13 SD, 3 PD HPV+ cervical cancer failed prior chemotherapy + bevacizumab Unconfirmed PR after 3 cycles (9 weeks), on study for ~ 6 months NSCLC (adeno), previously treated with chemotherapy + pembrolizumab and pembrolizumab maintenance Prolonged SD for 1 year, still on study * Failed prior CPI ^ Remains on study NSCLC Pancreatic TNBC Esophageal Cervical Esophageal NSCLC GEJ CRC TNBC Pancreatic CRC ER+ Breast NSCLC NSCLC TNBC Cervical uPR Change From Baseline (%) 1 2 * * * * * * * * * * * ^ 1 2
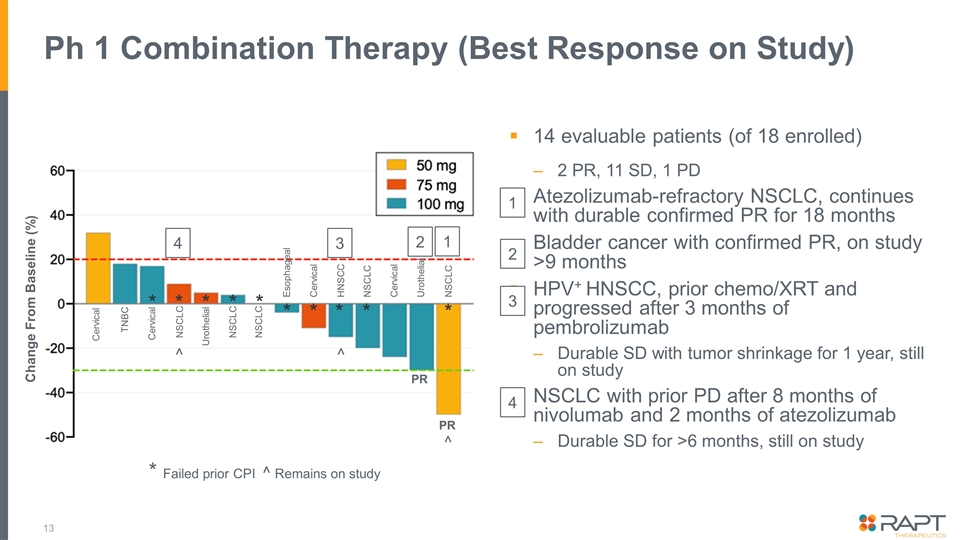
Ph 1 Combination Therapy (Best Response on Study) 14 evaluable patients (of 18 enrolled) 2 PR, 11 SD, 1 PD Atezolizumab-refractory NSCLC, continues with durable confirmed PR for 18 months Bladder cancer with confirmed PR, on study >9 months HPV+ HNSCC, prior chemo/XRT and progressed after 3 months of pembrolizumab Durable SD with tumor shrinkage for 1 year, still on study NSCLC with prior PD after 8 months of nivolumab and 2 months of atezolizumab Durable SD for >6 months, still on study * Failed prior CPI ^ Remains on study Cervical TNBC Cervical NSCLC Urothelial NSCLC NSCLC Esophageal Cervical HNSCC NSCLC Cervical Urothelial NSCLC PR PR Change From Baseline (%) 1 2 3 4 * * * * * * * * * * ^ ^ ^ 2 1 3 4
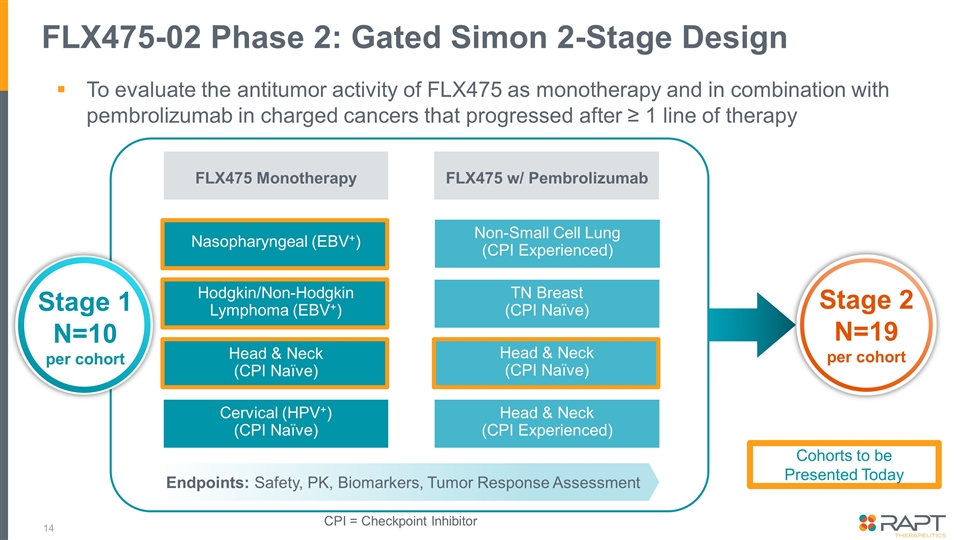
FLX475-02 Phase 2: Gated Simon 2-Stage Design FLX475 Monotherapy FLX475 w/ Pembrolizumab Nasopharyngeal (EBV+) Non-Small Cell Lung (CPI Experienced) Hodgkin/Non-Hodgkin Lymphoma (EBV+) Head & Neck (CPI Naïve) Cervical (HPV+) (CPI Naïve) TN Breast (CPI Naïve) Head & Neck (CPI Naïve) Head & Neck (CPI Experienced) Stage 2 N=19 per cohort Stage 1 N=10 per cohort Endpoints: Safety, PK, Biomarkers, Tumor Response Assessment CPI = Checkpoint Inhibitor To evaluate the antitumor activity of FLX475 as monotherapy and in combination with pembrolizumab in charged cancers that progressed after ≥ 1 line of therapy Cohorts to be Presented Today

Phase 2 Stage 1 Goals Initial evaluation of clinical activity in selected charged tumor types Demonstrate preliminary clinical activity of FLX475 as monotherapy Demonstrate preliminary clinical activity of FLX475 in combination with pembrolizumab in checkpoint-naïve cancers Demonstrate preliminary clinical activity of FLX475 in combination with pembrolizumab in checkpoint-experienced cancers Expand at least one cohort to Stage 2
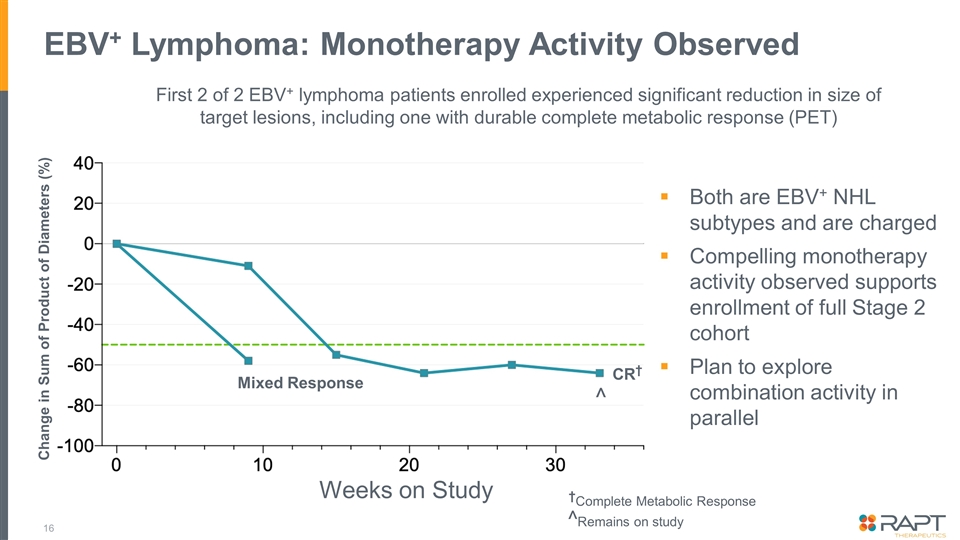
EBV+ Lymphoma: Monotherapy Activity Observed Change in Sum of Product of Diameters (%) CR† †Complete Metabolic Response Mixed Response ^Remains on study First 2 of 2 EBV+ lymphoma patients enrolled experienced significant reduction in size of target lesions, including one with durable complete metabolic response (PET) ^ Weeks on Study Both are EBV+ NHL subtypes and are charged Compelling monotherapy activity observed supports enrollment of full Stage 2 cohort Plan to explore combination activity in parallel
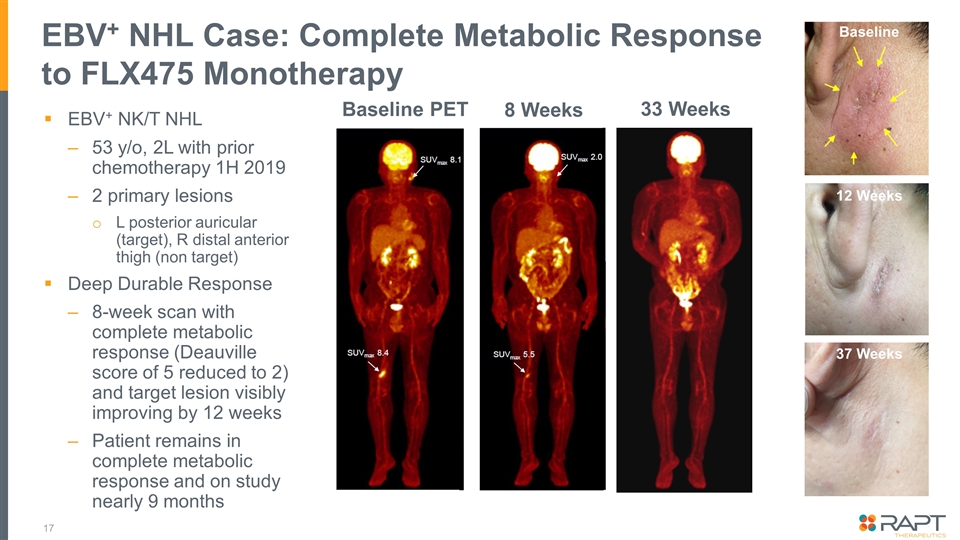
EBV+ NHL Case: Complete Metabolic Response to FLX475 Monotherapy EBV+ NK/T NHL 53 y/o, 2L with prior chemotherapy 1H 2019 2 primary lesions L posterior auricular (target), R distal anterior thigh (non target) Deep Durable Response 8-week scan with complete metabolic response (Deauville score of 5 reduced to 2) and target lesion visibly improving by 12 weeks Patient remains in complete metabolic response and on study nearly 9 months 8 Weeks 12 Weeks Baseline PET 33 Weeks 12 Weeks Baseline 37 Weeks
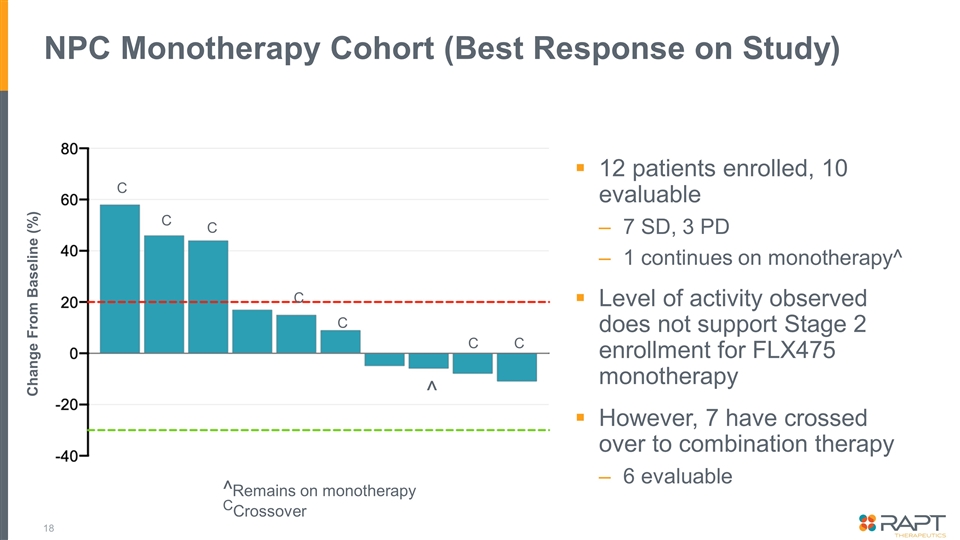
NPC Monotherapy Cohort (Best Response on Study) 12 patients enrolled, 10 evaluable 7 SD, 3 PD 1 continues on monotherapy^ Level of activity observed does not support Stage 2 enrollment for FLX475 monotherapy However, 7 have crossed over to combination therapy 6 evaluable ^ C Change From Baseline (%) C ^Remains on monotherapy CCrossover C C C C C
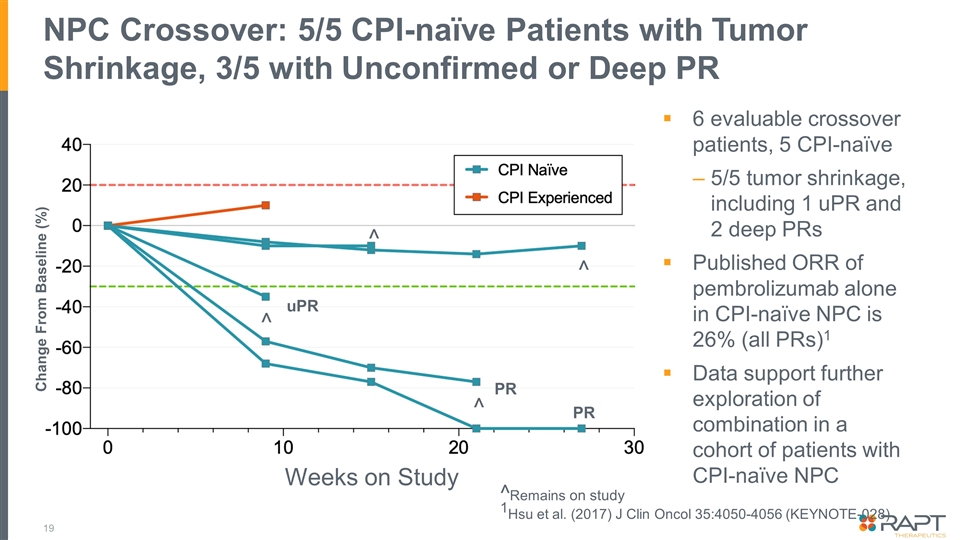
NPC Crossover: 5/5 CPI-naïve Patients with Tumor Shrinkage, 3/5 with Unconfirmed or Deep PR 19 6 evaluable crossover patients, 5 CPI-naïve 5/5 tumor shrinkage, including 1 uPR and 2 deep PRs Published ORR of pembrolizumab alone in CPI-naïve NPC is 26% (all PRs)1 Data support further exploration of combination in a cohort of patients with CPI-naïve NPC Change From Baseline (%) Weeks on Study PR PR ^ ^ ^ ^Remains on study 1Hsu et al. (2017) J Clin Oncol 35:4050-4056 (KEYNOTE-028) ^ uPR
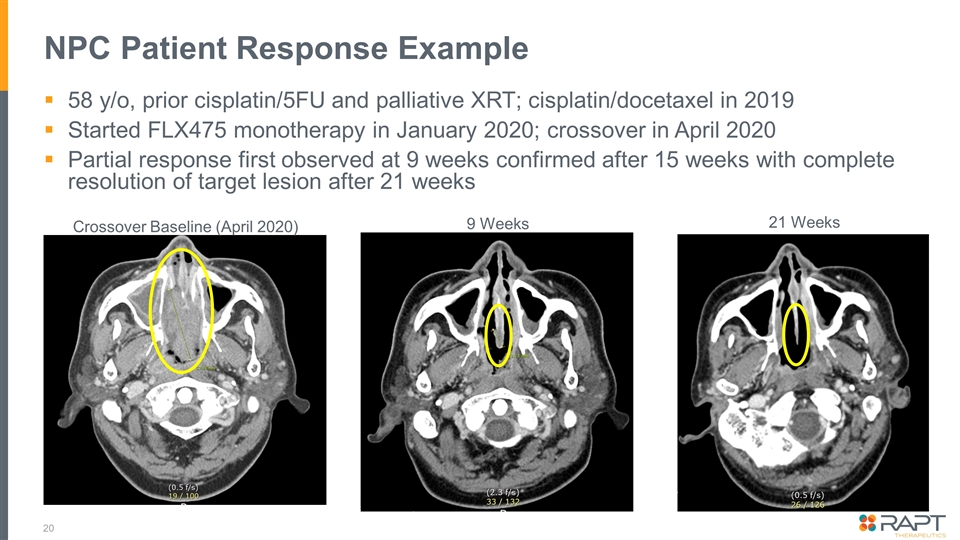
NPC Patient Response Example 58 y/o, prior cisplatin/5FU and palliative XRT; cisplatin/docetaxel in 2019 Started FLX475 monotherapy in January 2020; crossover in April 2020 Partial response first observed at 9 weeks confirmed after 15 weeks with complete resolution of target lesion after 21 weeks Crossover Baseline (April 2020) 9 Weeks 21 Weeks
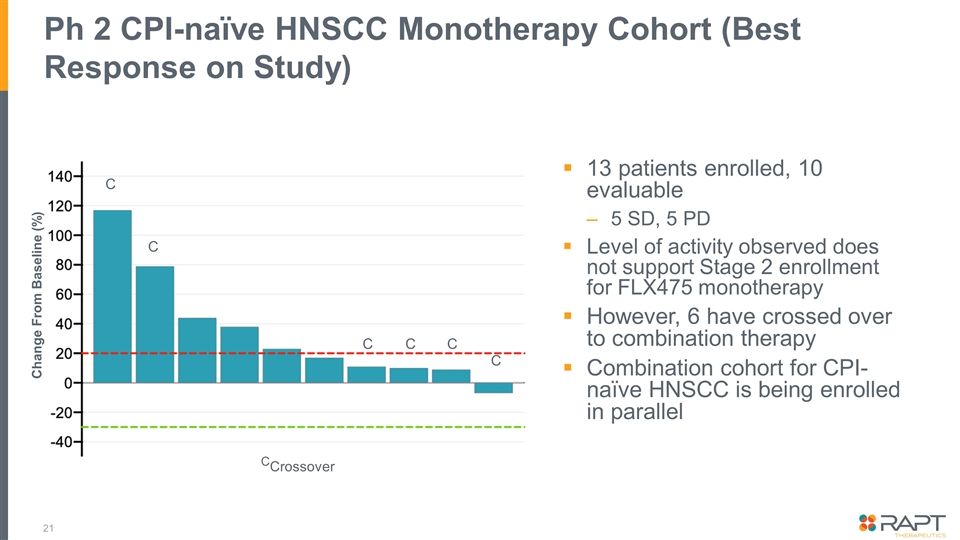
Ph 2 CPI-naïve HNSCC Monotherapy Cohort (Best Response on Study) 13 patients enrolled, 10 evaluable 5 SD, 5 PD Level of activity observed does not support Stage 2 enrollment for FLX475 monotherapy However, 6 have crossed over to combination therapy Combination cohort for CPI-naïve HNSCC is being enrolled in parallel Change From Baseline (%) C C C C C C CCrossover
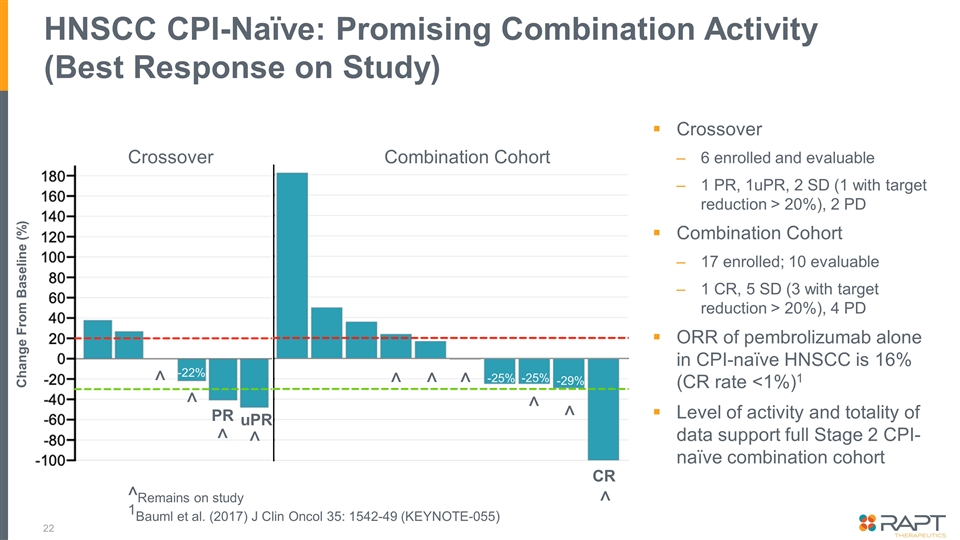
HNSCC CPI-Naïve: Promising Combination Activity (Best Response on Study) Crossover 6 enrolled and evaluable 1 PR, 1uPR, 2 SD (1 with target reduction > 20%), 2 PD Combination Cohort 17 enrolled; 10 evaluable 1 CR, 5 SD (3 with target reduction > 20%), 4 PD ORR of pembrolizumab alone in CPI-naïve HNSCC is 16% (CR rate <1%)1 Level of activity and totality of data support full Stage 2 CPI-naïve combination cohort ^Remains on study 1Bauml et al. (2017) J Clin Oncol 35: 1542-49 (KEYNOTE-055) Change From Baseline (%) CR ^ ^ ^ ^ ^ -29% -25% -25% -22% Crossover Combination Cohort ^ ^ uPR ^ ^ ^ PR

Phase 2 Safety To date, no new significant safety findings vs Phase 1 No evidence of increased severity or frequency of AEs in combination therapy vs either FLX475 or pembrolizumab given alone Asymptomatic and reversible QTc prolongation continues to be the primary FLX475-related finding Serious adverse events potentially related to study treatment in the Phase 2 patients being described today (44 patients, 4 cohorts) 1 QTc prolongation (asymptomatic) in a patient on monotherapy 1 episode of colitis and concurrent renal insufficiency in one patient on combination therapy
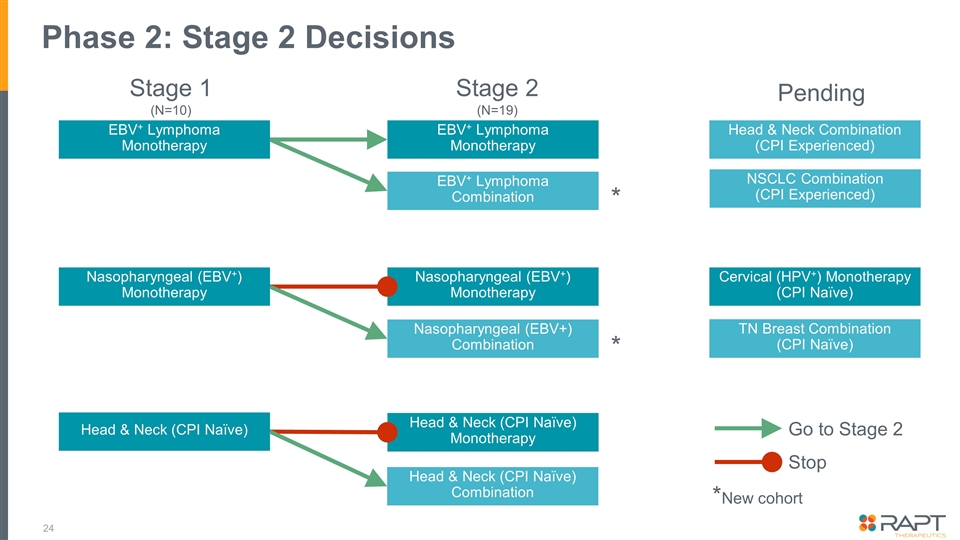
Phase 2: Stage 2 Decisions Nasopharyngeal (EBV+) Monotherapy Nasopharyngeal (EBV+) Monotherapy Nasopharyngeal (EBV+) Combination * Go to Stage 2 Stop *New cohort Stage 1 (N=10) Stage 2 (N=19) EBV+ Lymphoma Monotherapy EBV+ Lymphoma Monotherapy EBV+ Lymphoma Combination * Pending NSCLC Combination (CPI Experienced) Cervical (HPV+) Monotherapy (CPI Naïve) TN Breast Combination (CPI Naïve) Head & Neck Combination (CPI Experienced) Head & Neck (CPI Naïve) Head & Neck (CPI Naïve) Monotherapy Head & Neck (CPI Naïve) Combination
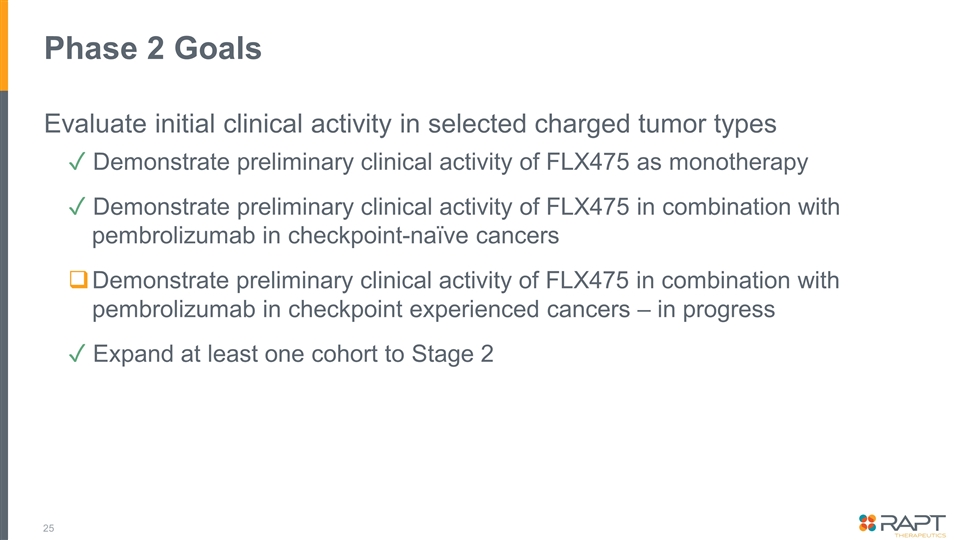
Phase 2 Goals Evaluate initial clinical activity in selected charged tumor types ✓ Demonstrate preliminary clinical activity of FLX475 as monotherapy ✓ Demonstrate preliminary clinical activity of FLX475 in combination with pembrolizumab in checkpoint-naïve cancers Demonstrate preliminary clinical activity of FLX475 in combination with pembrolizumab in checkpoint experienced cancers – in progress ✓ Expand at least one cohort to Stage 2
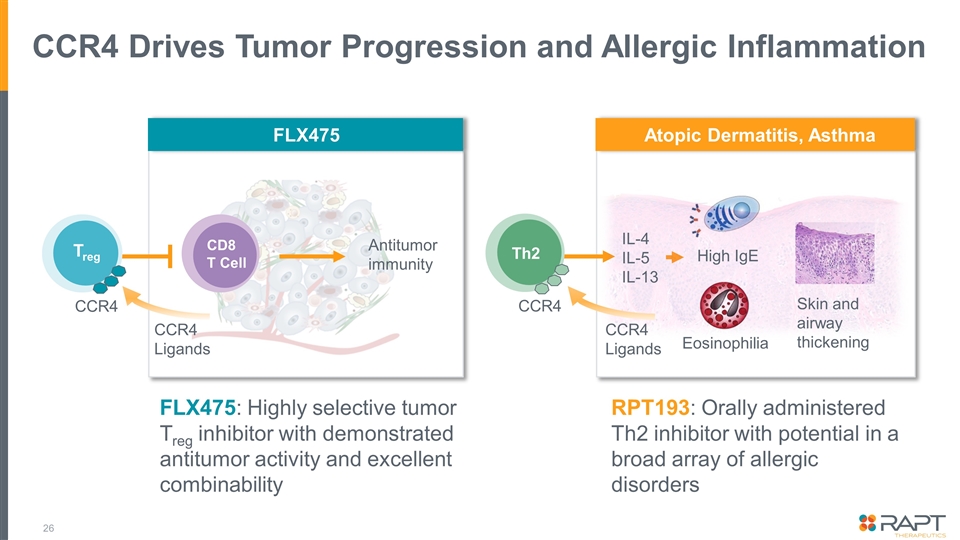
CCR4 Drives Tumor Progression and Allergic Inflammation FLX475: Highly selective tumor Treg inhibitor with demonstrated antitumor activity and excellent combinability CCR4 Treg CCR4 Th2 Eosinophilia Skin and airway thickening High IgE IL-4 IL-5 IL-13 FLX475 Atopic Dermatitis, Asthma CCR4 Ligands CD8 T Cell Antitumor immunity CCR4 Ligands RPT193: Orally administered Th2 inhibitor with potential in a broad array of allergic disorders

Next Catalysts FLX475: next Phase 2 update expected in 2H 2021 including additional data from Stage 1 cohorts as well as Stage 2 expansions RPT193: data release now expected in 1H 2021
DISCOVERY Oral Drugs Targeting Critical Immune Drivers of Disease FLX475 (Oncology): Selectively targets immunosuppressive tumor Treg Proof of concept established in Phase 2 with multiple expansions underway Next comprehensive Phase 2 update 2H 2021 RPT193 (Allergic Disease): Oral agent targets inflammatory Th2 cells Robust PK/PD with excellent safety in Ph1 study Phase 1b PoC in atopic dermatitis ongoing – data readout in 1H 2021 GCN2 (Oncology): Turns on an antitumor metabolic switch in TME HPK1 (Oncology): Unlocks T cell activation to tumor antigens CLINICAL Proprietary discovery engine Diversified pipeline Large market opportunities Multiple near-term clinical readouts Strategic collaborations

Thank You
