Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Senseonics Holdings, Inc. | tm2035417-2_8k.htm |
Exhibit 99.1
 |
SENSEONICS CONFIDENTIAL l NOVEMBER 2020 EVERSENSE®CGMSYSTEM The world’s first and only long-term CGM Tim Goodnow, PhD President and CEO Credit Suisse 29th Annual Virtual Healthcare Conference November 15, 2020 |
 |
FORWARD-LOOKING STATEMENTS This presentation contains forward-looking statements, as defined in the Private Securities Litigation Reform Act of 1995, including statements regarding managements plans, objectives and goals for future operations, including preliminary targets for future financial performance and cash projections of Senseonics Holdings, Inc. (the “Company”). Such forward-looking statements may be identified by the use of words such as “believe,” expect,” anticipate, “plan,” “estimate,” “project,” “target” or other similar expressions. These forward-looking statements are based on management’s current expectations and projections about future events, and such statements are, by their nature subject to uncertainties. Additionally, the preliminary projections of key financial targets and cash projections have been developed based on the model for the partnership with Ascensia, as reviewed and approved by the Senseonics Board of Directors. The Company expects to further refine such projections following the completion of the Company’s 2021 budgeting process and the development of more formal financial guidance that it intends to disclose along with its full year 2020 financial results in early 2021. This more formal guidance and actual performance could vary materially from the preliminary projections included in this presentation. The risks and uncertainties that may cause actual results, levels of activity, performance or achievements to differ materially from the Company’s current expectations include uncertainties related to the timing of the submission of the application for regulatory approval of the up to 180-day product and the up to 365-day product to the U.S. Federal Drug Administration (“FDA”), the receipt of approval from the FDA with respect to the up to 180-day and the up to 365-day product, and the timing of any such approvals; the timing of the initiation of Ascensia’s commercial activities, including the timing of U.S. commercial launch of the up to 180-day product and the up to 365-day product; the success of the transition of commercial activities to Ascensia, the risks attendant to the launch and commercialization of a new product in a competitive market, the availability of capital to fund the Company’s continued operations and execute its business plan, and such other factors that are more fully described in the “Risk Factors” section of the Company’s Annual Report on Form 10-K for the year ended December 31, 2019, as filed with the Securities and Exchange Commission (“SEC”) on March 16, 2020, the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2020, as filed with the SEC on November 9, 2020, and its other filings with the SEC. Statements made in this presentation speak only as of the date of this presentation and should not be relied upon as of any subsequent date, and the Company assumes no obligation to update any such forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. |
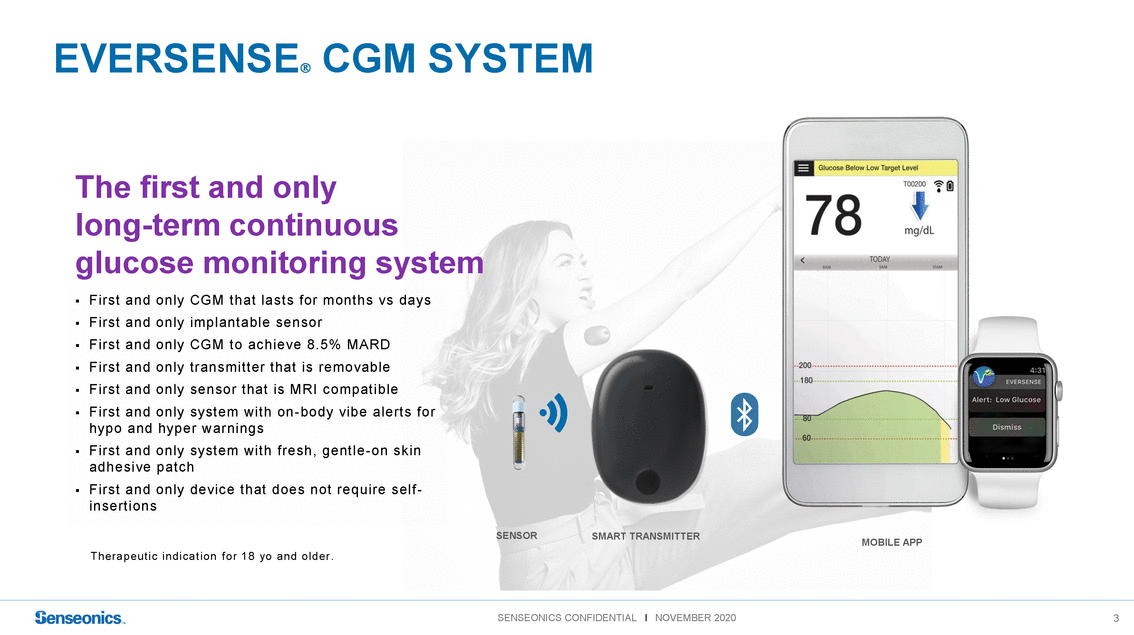 |
EVERSENSE CGMSYSTEM The first and only long-term continuous glucose monitoring system ▪ Firs t and only CGM t hat las t s f or mont hs vs days ▪ Firs t and only implant able s ens or ▪ Firs t and only CGM t o ac hieve 8. 5% MARD ▪ Firs t and only t rans mit t er t hat is removable ▪ Firs t and only s ens or t hat is MRI c ompat ible ▪ Firs t and only s ys t em wit h on -body vibe alert s f or hypo and hyper warnings ▪ Firs t and only s ys t em wit h f res h, gent le -on s k in adhes ive pat c h ▪ Firs t and only devic e t hat does not require s elf - ins ert ions T h e r a p e u t i c i n d i c a t i o n f o r 1 8 yo a n d o l d e r . SENSORSMART TRANSMITTER MOBILE APP |
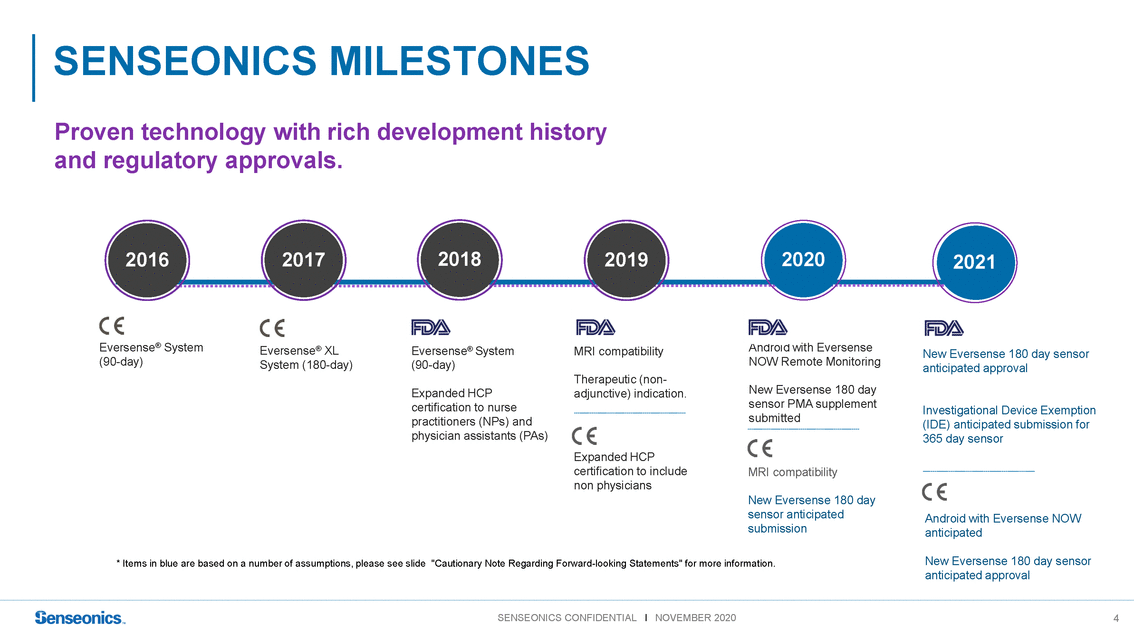 |
Proven technology with rich development history and regulatory approvals. 201620172018201920202021 Eversense® System (90-day) Eversense® XL System (180-day) Eversense® System (90-day) Expanded HCP MRI compatibility Therapeutic (non-adjunctive) indication. Android with Eversense NOW Remote Monitoring New Eversense 180 day New Eversense 180 day sensor anticipated approval certification to nurse practitioners (NPs) and physician assistants (PAs) Expanded HCP certification to include non physicians sensor PMA supplement submitted MRI compatibility New Eversense 180 day sensor anticipated submission Investigational Device Exemption (IDE) anticipated submission for 365 day sensor Android with Eversense NOW anticipated * Items in blue are based on a number of assumptions, please see slide "Cautionary Note Regarding Forward-looking Statements" for more information. New Eversense 180 day sensor anticipated approval |
 |
[LOGO] |
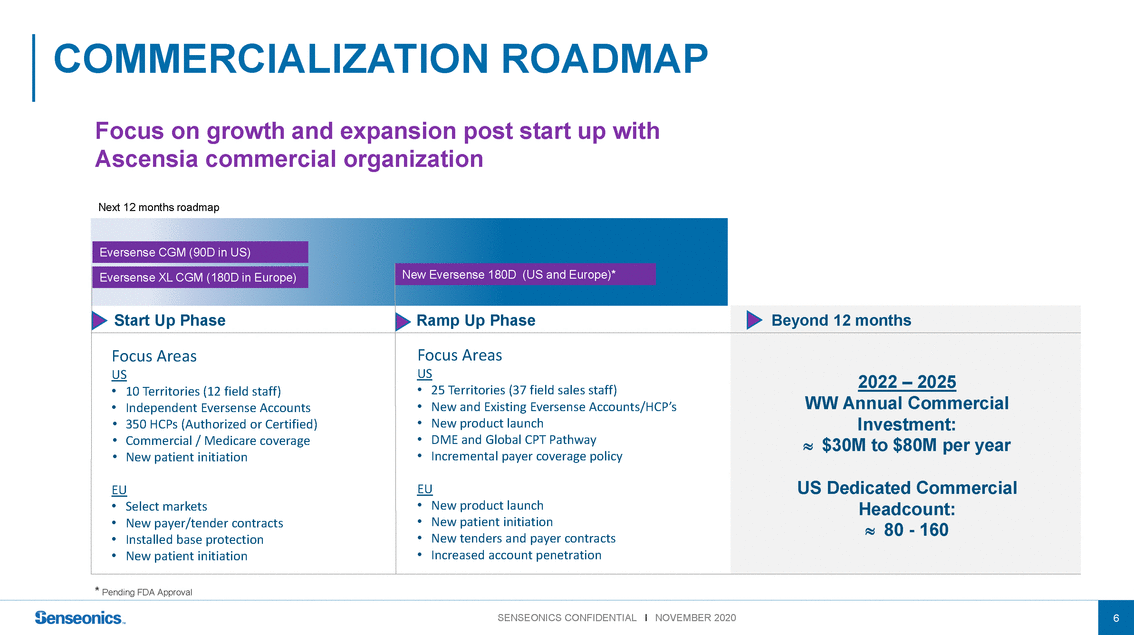 |
Focus on growth and expansion post start up with Ascensia commercial organization Next 12 months roadmap Eversense CGM (90D in US) Eversense XL CGM (180D in Europe) New Eversense 180D (US and Europe)* Start Up PhaseRamp Up Phase Beyond 12 months Focus Areas US • 10 Territories (12 field staff) • Independent Eversense Accounts • 350 HCPs (Authorized or Certified) • Commercial / Medicare coverage • New patient initiation EU • Select markets • New payer/tender contracts • Installed base protection • New patient initiation * Pending FDA Approval Focus Areas US • 25 Territories (37 field sales staff) • New and Existing Eversense Accounts/HCP’s • New product launch • DME and Global CPT Pathway • Incremental payer coverage policy EU • New product launch • New patient initiation • New tenders and payer contracts • Increased account penetration 2022 – 2025 WW Annual Commercial Investment: $30M to $80M per year US Dedicated Commercial Headcount: 80 - 160 |
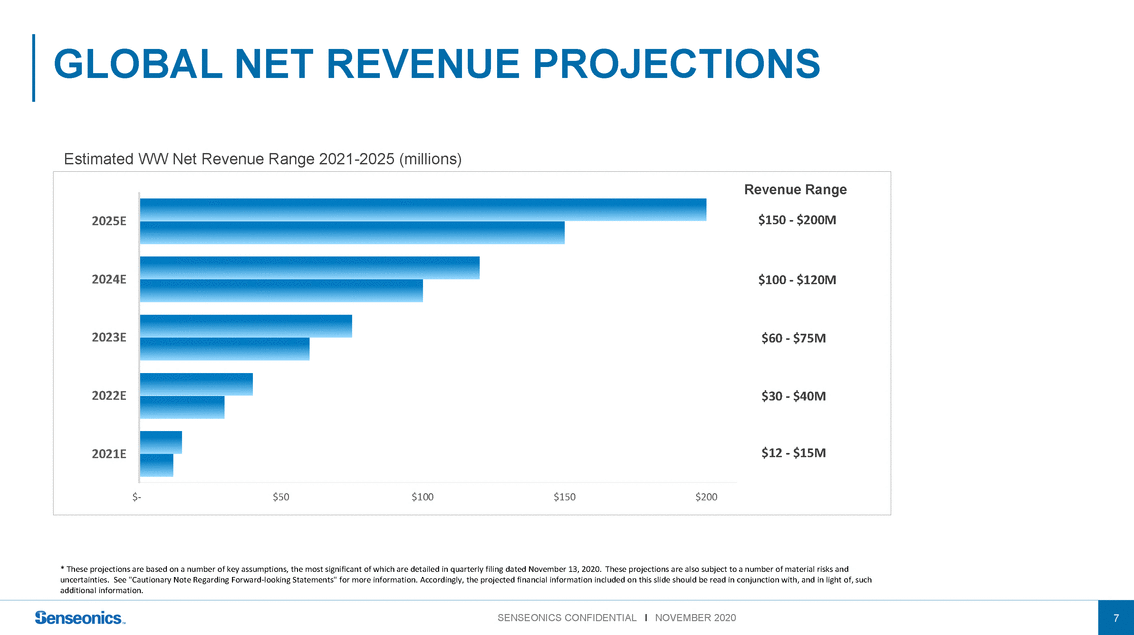 |
Estimated WW Net Revenue Range 2021-2025 (millions) Revenue Range 2025E$150 - $200M 2024E 2023E 2022E 2021E $100 - $120M $60 - $75M $30 - $40M $12 - $15M $-$50$100$150$200 * These projections are based on a number of key assumptions, the most significant of which are detailed in quarterly filing dated November 13, 2020. These projections are also subject to a number of material risks and uncertainties. See "Cautionary Note Regarding Forward-looking Statements" for more information. Accordingly, the projected financial information included on this slide should be read in conjunction with, and in light of, such additional information. |
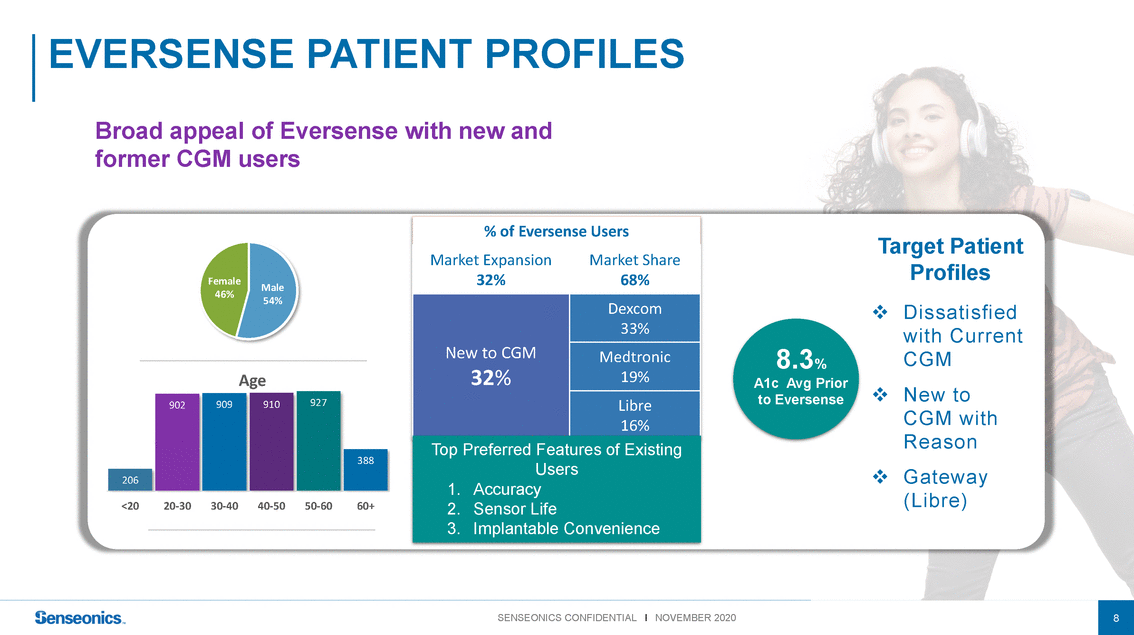 |
EVERSENSEPATIENT PROFILES Broad appeal of Eversense with new and former CGM users % of Eversense Users Target Patient Female 46% Male 54% Market Expansion 32% New to CGM Market Share 68% Dexcom 33% Medtronic 8.3% Profiles ❖Dissatisfied with Current CGM Age 902909910927 32% 19% Libre 16% A1c Avg Prior to Eversense ❖New to CGM with 206 388 Top Preferred Features of Existing Users 1. Accuracy Reason ❖Gateway <2020-3030-4040-5050-6060+ 2. Sensor Life 3. Implantable Convenience (Libre) SENSEONICS CONFIDENTIAL l NOVEMBER 20208 |
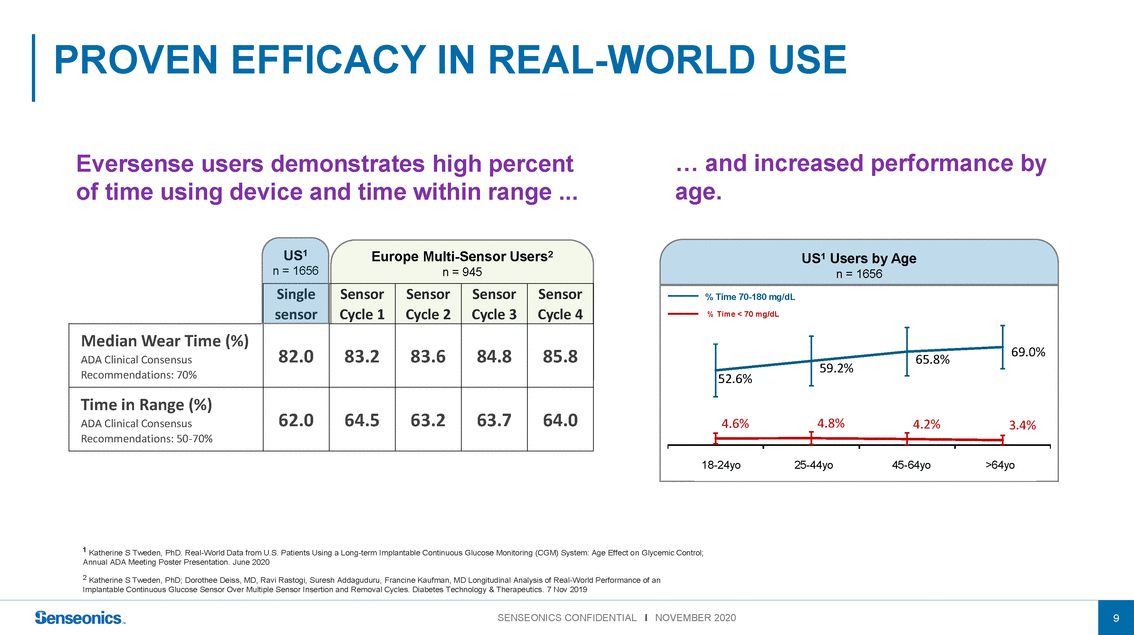 |
Eversense users demonstrates high percent of time using device and time within range ... … and increased performance by age. US1 n = 1656 Europe Multi-Sensor Users2 n = 945 US1 Users by Age n = 1656 Median Wear Time (%) Single sensor Sensor Cycle 1 Sensor Cycle 2 Sensor Cycle 3 Sensor Cycle 4 % Time 70-180 mg/dL % Time < 70 mg/dL 69.0% ADA Clinical Consensus Recommendations: 70% Time in Range (%) 82.083.283.684.885.8 52.6% 59.2%65.8% ADA Clinical Consensus 18 - <2525 - <4545 - <65≥65 (N=140)(N=686)(N=704)(N=88) 62.064.563.263.764.0 4.6%4.8%4.2%3.4% 18-24yo25-44yo45-64yo>64yo 1 Katherine S Tweden, PhD. Real-World Data from U.S. Patients Using a Long-term Implantable Continuous Glucose Monitoring (CGM) System: Age Effect on Glycemic Control; Annual ADA Meeting Poster Presentation. June 2020 2 Katherine S Tweden, PhD; Dorothee Deiss, MD, Ravi Rastogi, Suresh Addaguduru, Francine Kaufman, MD Longitudinal Analysis of Real-World Performance of an Implantable Continuous Glucose Sensor Over Multiple Sensor Insertion and Removal Cycles. Diabetes Technology & Therapeutics. 7 Nov 2019 9 |
 |
Commercial and Medicare coverage to 200 million covered lives since mid-2018 launch ~263M Lives are covered by the Top 25 US Health Insurers SENSEONICS CONFIDENTIAL l NOVEMBER 202010 |
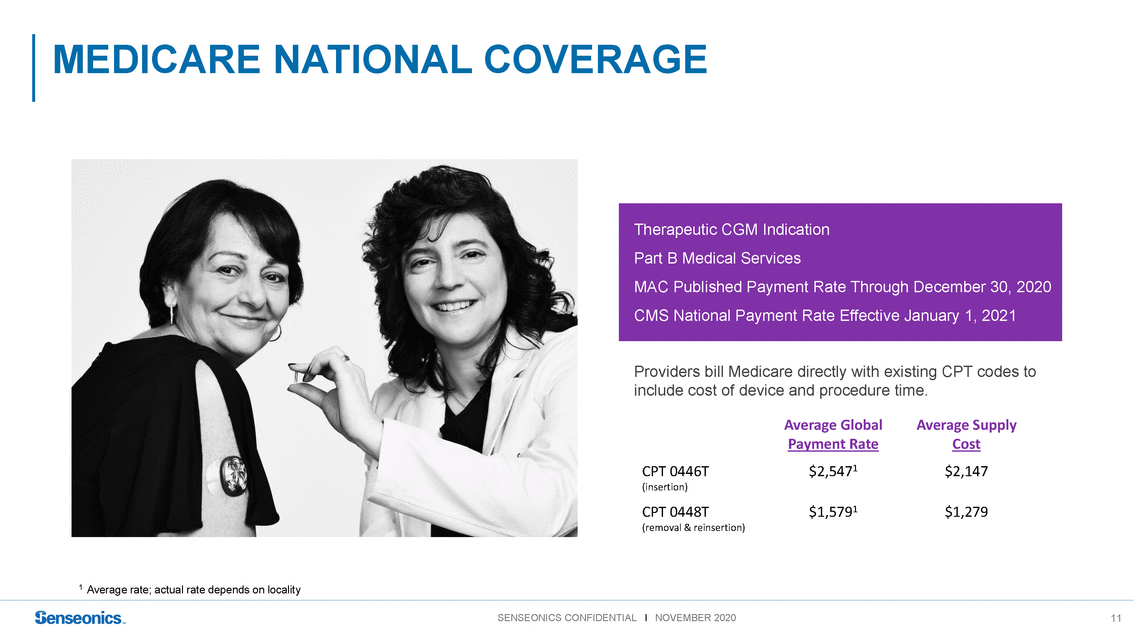 |
Therapeutic CGM Indication Part B Medical Services MAC Published Payment Rate Through December 30, 2020 CMS National Payment Rate Effective January 1, 2021 Providers bill Medicare directly with existing CPT codes to include cost of device and procedure time. Average Global Payment Rate Average Supply Cost 1 Average rate; actual rate depends on locality CPT 0446T (insertion) CPT 0448T (removal & reinsertion) $2,5471$2,147 $1,5791$1,279 SENSEONICS CONFIDENTIAL l NOVEMBER 202011 |
 |
SENSEONICS CONFIDENTIAL l NOVEMBER 2020 EVERSENSE PIPELINE 12 |
 |
Uniquely differentiated long-term technology solution with multiple product configuration ONE SENSOR TWO PRODUCTS ALL PATIENT SEGMENTS ✓NO sensor change for 1 year ✓NO daily calibration required ✓NO transmitter on body For Type 1 and Type 2 Patients On Demand and Continuous Monitoring No transmitter wear On body * Product vision subject to a number of assumptions and regulatory approval see “Cautionary Note Regarding Forward-looking Statements.” |
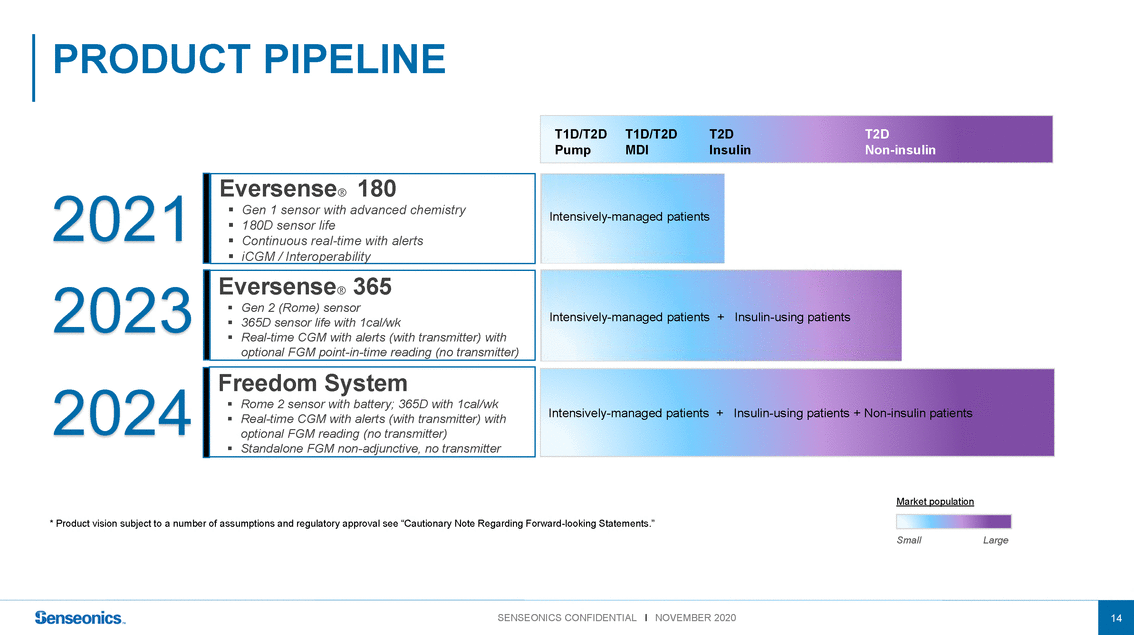 |
2021 2023 2024 Eversense 180 ▪ Gen 1 sensor with advanced chemistry ▪ 180D sensor life ▪ Continuous real-time with alerts ▪ iCGM / Interoperability Eversense 365 ▪ Gen 2 (Rome) sensor ▪ 365D sensor life with 1cal/wk ▪ Real-time CGM with alerts (with transmitter) with optional FGM point-in-time reading (no transmitter) Freedom System ▪ Rome 2 sensor with battery; 365D with 1cal/wk ▪ Real-time CGM with alerts (with transmitter) with optional FGM reading (no transmitter) ▪ Standalone FGM non-adjunctive, no transmitter Intensively-managed patients Intensively-managed patients +Insulin-using patients Intensively-managed patients +Insulin-using patients + Non-insulin patients Market population * Product vision subject to a number of assumptions and regulatory approval see “Cautionary Note Regarding Forward-looking Statements.” SmallLarge |
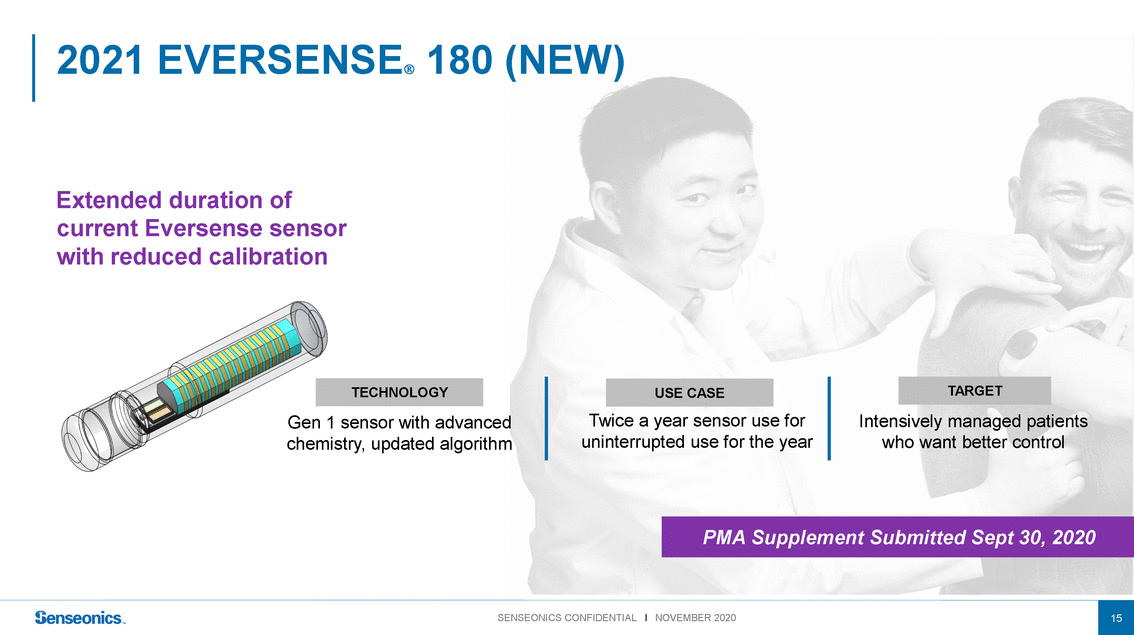 |
2021 EVERSENSE 180 (NEW) Extended duration of current Eversense sensor with reduced calibration TECHNOLOGY Gen 1 sensor with advanced chemistry, updated algorithm USE CASE Twice a year sensor use for uninterrupted use for the year TARGET Intensively managed patients who want better control PMA Supplement Submitted Sept 30, 2020 SENSEONICS CONFIDENTIAL l NOVEMBER 202015 |
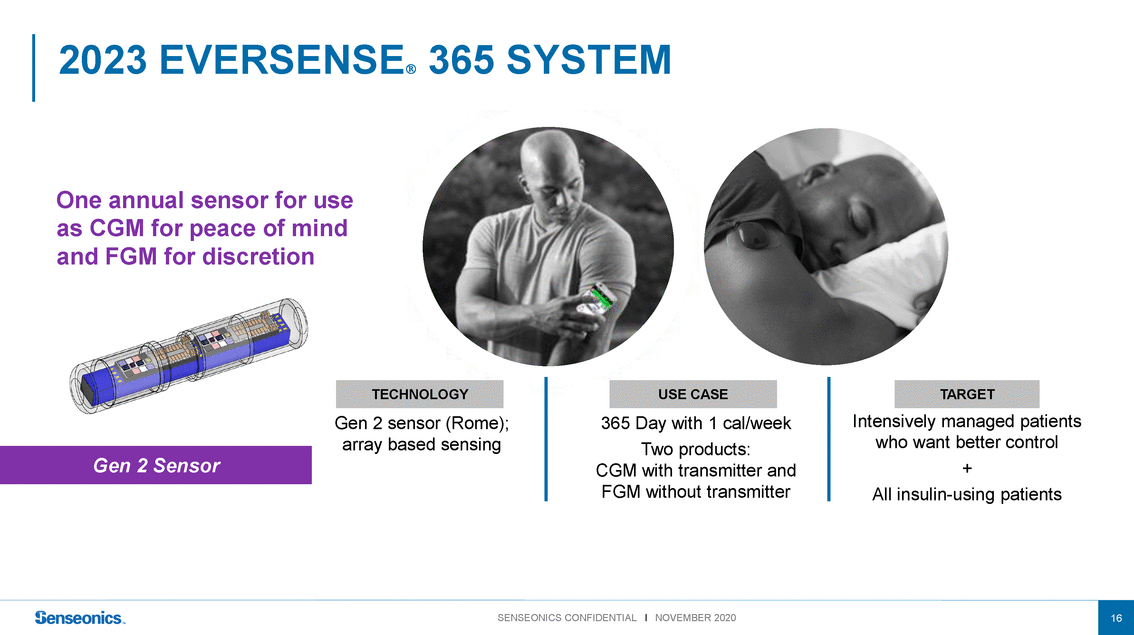 |
One annual sensor for use as CGM for peace of mind and FGM for discretion Gen 2 Sensor TECHNOLOGY Gen 2 sensor (Rome); array based sensing USE CASE 365 Day with 1 cal/week Two products: CGM with transmitter and FGM without transmitter TARGET Intensively managed patients who want better control + All insulin-using patients |
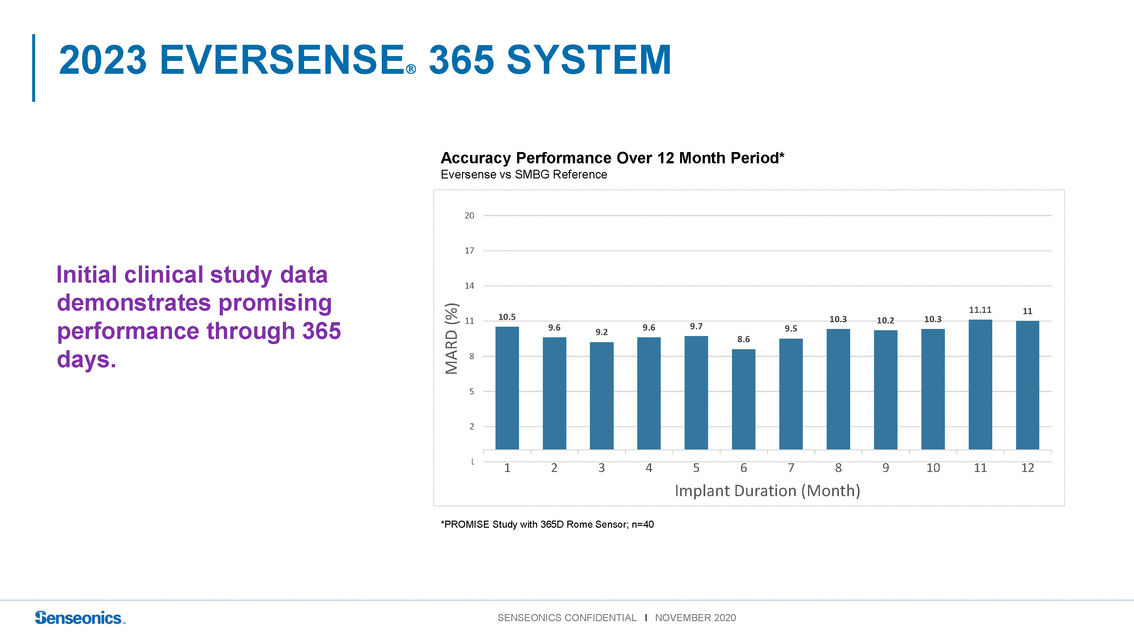 |
Accuracy Performance Over 12 Month Period* Eversense vs SMBG Reference 20 Initial clinical study data demonstrates promising performance through 365 days. 17 14 1110.5 8 9.69.29.69.7 8.6 9.5 10.310.210.3 11.1111 MARD (%) 2 - Implant Duration (Month) *PROMISE Study with 365D Rome Sensor; n=40 |
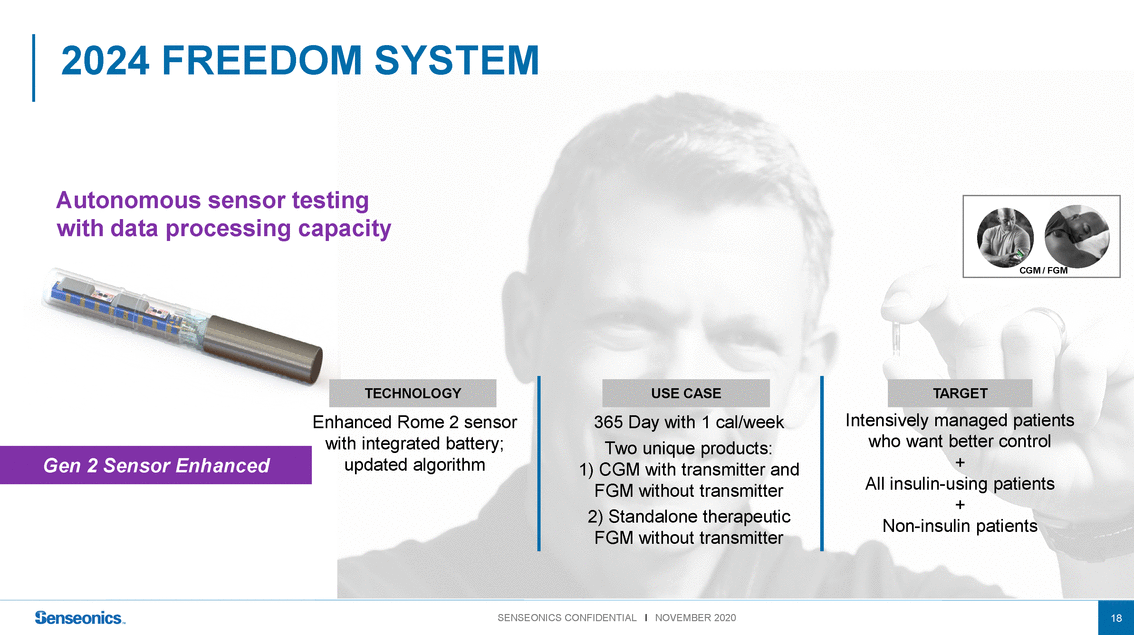 |
2024 FREEDOM SYSTEM Autonomous sensor testing with data processing capacity CGM / FGM Gen 2 Sensor Enhanced TECHNOLOGY Enhanced Rome 2 sensor with integrated battery; updated algorithm USE CASE 365 Day with 1 cal/week Two unique products: 1) CGM with transmitter and FGM without transmitter 2) Standalone therapeutic FGM without transmitter TARGET Intensively managed patients who want better control + All insulin-using patients + Non-insulin patients SENSEONICS CONFIDENTIAL l NOVEMBER 202018 |
 |
SENSEONICS CONFIDENTIAL l NOVEMBER 2020 THANKYOU |
