Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - FIVE PRIME THERAPEUTICS, INC. | d907944dex991.htm |
| 8-K - 8-K - FIVE PRIME THERAPEUTICS, INC. | d907944d8k.htm |

Exhibit 99.2 Bemarituzumab Targeted Therapy for FGFR2b+ Tumors November 10, 2020 © 2020 Five Prime Therapeutics, Inc.Exhibit 99.2 Bemarituzumab Targeted Therapy for FGFR2b+ Tumors November 10, 2020 © 2020 Five Prime Therapeutics, Inc.

Forward-Looking Statements Disclaimer This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as may, will, expect, plan, anticipate and similar expressions (as well as other words or expressions referencing future events or circumstances) are intended to identify forward-looking statements. These forward-looking statements reflect Five Prime's current beliefs and expectations. Each of these forward-looking statements involves risks and uncertainties. Actual results may differ from these forward-looking statements. Forward-looking statements contained in this presentation include statements about (i) the timing of initiation, progress and scope of clinical trials for bemarituzumab; (ii) the potential use of bemarituzumab, including in combination with other products, to treat patients; (iii) the potential development of bemarituzumab in indications in addition to gastric and gastroesophageal cancer; (iv) the timing of the presentation of data for our product candidates; and (v) the extent of protein overexpression and gene amplification in certain patient populations. Many factors may cause differences between current expectations and actual results, including unexpected safety or efficacy data observed during preclinical or clinical studies, changes in expected or existing competition, failure of our collaborators to support or advance collaborations or product candidates, changes in the regulatory, pricing or reimbursement environment and unexpected litigation or other disputes. In addition, while we expect the COVID-19 pandemic to adversely affect our business operations and financial results, the extent of the impact on our ability to advance our manufacturing, clinical development and regulatory efforts and business and corporate development and other objectives and the value of and market for our common stock will depend on future developments that are highly uncertain, and we cannot predict with confidence the ultimate duration of the pandemic, travel restrictions, quarantines, social distancing and business closure requirements in the U.S. and in other countries and the effectiveness of actions taken globally to contain and treat COVID-19. Other factors that may cause our actual results to differ from current expectations are discussed in Five Prime's filings with the U.S. Securities and Exchange Commission, including the Risk Factors sections contained therein. Except as required by law, we assume no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available. © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 2Forward-Looking Statements Disclaimer This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as may, will, expect, plan, anticipate and similar expressions (as well as other words or expressions referencing future events or circumstances) are intended to identify forward-looking statements. These forward-looking statements reflect Five Prime's current beliefs and expectations. Each of these forward-looking statements involves risks and uncertainties. Actual results may differ from these forward-looking statements. Forward-looking statements contained in this presentation include statements about (i) the timing of initiation, progress and scope of clinical trials for bemarituzumab; (ii) the potential use of bemarituzumab, including in combination with other products, to treat patients; (iii) the potential development of bemarituzumab in indications in addition to gastric and gastroesophageal cancer; (iv) the timing of the presentation of data for our product candidates; and (v) the extent of protein overexpression and gene amplification in certain patient populations. Many factors may cause differences between current expectations and actual results, including unexpected safety or efficacy data observed during preclinical or clinical studies, changes in expected or existing competition, failure of our collaborators to support or advance collaborations or product candidates, changes in the regulatory, pricing or reimbursement environment and unexpected litigation or other disputes. In addition, while we expect the COVID-19 pandemic to adversely affect our business operations and financial results, the extent of the impact on our ability to advance our manufacturing, clinical development and regulatory efforts and business and corporate development and other objectives and the value of and market for our common stock will depend on future developments that are highly uncertain, and we cannot predict with confidence the ultimate duration of the pandemic, travel restrictions, quarantines, social distancing and business closure requirements in the U.S. and in other countries and the effectiveness of actions taken globally to contain and treat COVID-19. Other factors that may cause our actual results to differ from current expectations are discussed in Five Prime's filings with the U.S. Securities and Exchange Commission, including the Risk Factors sections contained therein. Except as required by law, we assume no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available. © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 2

Executive Summary: Bemarituzumab Value Proposition targeting FGFR2b+ advanced gastric and First-in-class gastroesophageal junction (GC/GEJ) cancers antibody FIGHT Phase 2 Results PFS, OS, ORR PFS HR=0.68 (95% CI: 0.44-1.04; p=0.073) statistically OS HR=0.58 (95% CI: 0.35-0.95; p=0.027) 1 significant ORR improved -13.1% (CI: -29.0%, 2.8%; p=0.106) in newly diagnosed/front-line non-HER2+ advanced ~30% FGFR2b+ GC/GEJ cancers Significant unmet 2 >200,000 patients worldwide need in gastric 1 2 Statistical significance (at 2-sided alpha 0.20) for PFS, OS and ORR was pre-specified and tested sequentially; Company estimates © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 3Executive Summary: Bemarituzumab Value Proposition targeting FGFR2b+ advanced gastric and First-in-class gastroesophageal junction (GC/GEJ) cancers antibody FIGHT Phase 2 Results PFS, OS, ORR PFS HR=0.68 (95% CI: 0.44-1.04; p=0.073) statistically OS HR=0.58 (95% CI: 0.35-0.95; p=0.027) 1 significant ORR improved -13.1% (CI: -29.0%, 2.8%; p=0.106) in newly diagnosed/front-line non-HER2+ advanced ~30% FGFR2b+ GC/GEJ cancers Significant unmet 2 >200,000 patients worldwide need in gastric 1 2 Statistical significance (at 2-sided alpha 0.20) for PFS, OS and ORR was pre-specified and tested sequentially; Company estimates © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 3

Gastric Cancer Has Global Impact and a Poor Prognosis 1 Gastric Cancer Incidence 1 million+ cases globally 1M+ each year rd 3 most common cause 1 of cancer death globally 3rd After lung and breast cancer 5th Most 1 Common Cancer After lung, breast, prostate 5th and colon cancer Unmet Medical Need 2 No new FDA approved frontline therapy for 10+ years Chemotherapy is the standard of care for the ~80-85% of 3 patients whose tumors are HER2- 1 Bray F, Ferlay J, Soerjomataram I, et al: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2 3 2018;68(6):394-424. doi:10.3322/caac.21492 Herceptin approved for HER2-positive metastatic stomach cancer, October 20, 2010 Wagner et. al. © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 4Gastric Cancer Has Global Impact and a Poor Prognosis 1 Gastric Cancer Incidence 1 million+ cases globally 1M+ each year rd 3 most common cause 1 of cancer death globally 3rd After lung and breast cancer 5th Most 1 Common Cancer After lung, breast, prostate 5th and colon cancer Unmet Medical Need 2 No new FDA approved frontline therapy for 10+ years Chemotherapy is the standard of care for the ~80-85% of 3 patients whose tumors are HER2- 1 Bray F, Ferlay J, Soerjomataram I, et al: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2 3 2018;68(6):394-424. doi:10.3322/caac.21492 Herceptin approved for HER2-positive metastatic stomach cancer, October 20, 2010 Wagner et. al. © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 4
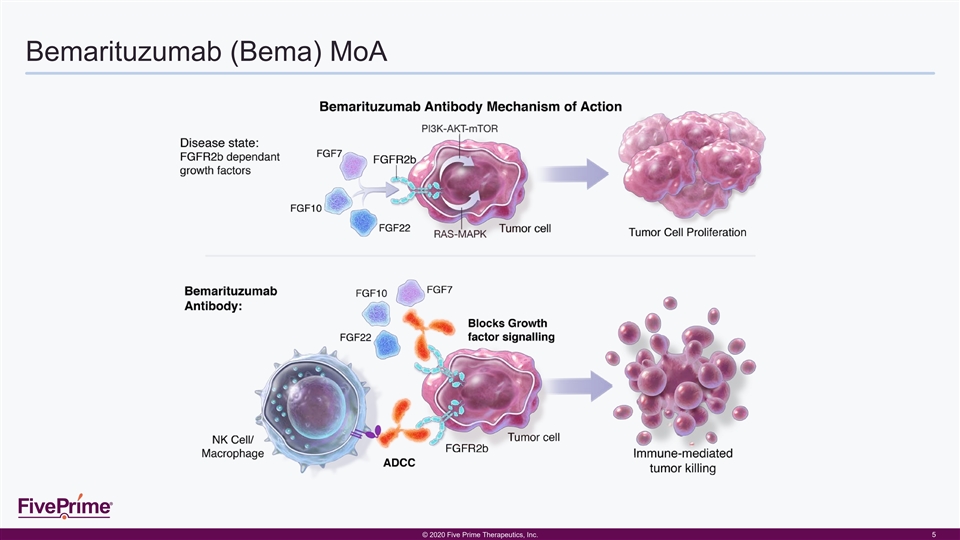
Bemarituzumab (Bema) MoA © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 5Bemarituzumab (Bema) MoA © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 5

Overview of Bemarituzumab Clinical Development Study Objective(s) Results FGFR2b Selected Number of patients Phase 1 Monotherapy Tolerability, safety and preliminary efficacy of monotherapy ORR 18% in late-line FGFR2b+ Gastric / Mixed 1 (n=79) in late-line patients GEJ Phase 1 Monotherapy Tolerability, safety and PK profile in late-line Japanese PK is comparable to non-Japanese in Japan No patients with GEJ patients (n=6) Tolerability, safety and PK of bemarituzumab + mFOLFOX6 Phase 1 Combo No evidence of overlapping toxicity or in any GI cancer No (Bema + Chemo) Safety effect on PK Evaluate ability to achieve C PK by Day 15 with new trough (n=12) All pts achieved target C by Day 8 trough dosing schedule (Q2W + a single Day 8 dose) Phase 2 Evaluate bemarituzumab + mFOLFOX6 vs placebo + Primary Endpoint: PFS FIGHT Randomized Yes mFOLFOX6 in FGFR2b+/non-HER2+ front-line gastric / Secondary Endpoints: OS, ORR (n=155) GEJ cancers 1 Catenacci, et al JCO 2020 © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 6Overview of Bemarituzumab Clinical Development Study Objective(s) Results FGFR2b Selected Number of patients Phase 1 Monotherapy Tolerability, safety and preliminary efficacy of monotherapy ORR 18% in late-line FGFR2b+ Gastric / Mixed 1 (n=79) in late-line patients GEJ Phase 1 Monotherapy Tolerability, safety and PK profile in late-line Japanese PK is comparable to non-Japanese in Japan No patients with GEJ patients (n=6) Tolerability, safety and PK of bemarituzumab + mFOLFOX6 Phase 1 Combo No evidence of overlapping toxicity or in any GI cancer No (Bema + Chemo) Safety effect on PK Evaluate ability to achieve C PK by Day 15 with new trough (n=12) All pts achieved target C by Day 8 trough dosing schedule (Q2W + a single Day 8 dose) Phase 2 Evaluate bemarituzumab + mFOLFOX6 vs placebo + Primary Endpoint: PFS FIGHT Randomized Yes mFOLFOX6 in FGFR2b+/non-HER2+ front-line gastric / Secondary Endpoints: OS, ORR (n=155) GEJ cancers 1 Catenacci, et al JCO 2020 © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 6

FIGHT: Phase 2 Randomized Trial in Frontline FGFR2b+, non HER2+ Gastric/GEJ Cancers fiveprime.com | NASDAQ: FPRX © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 7FIGHT: Phase 2 Randomized Trial in Frontline FGFR2b+, non HER2+ Gastric/GEJ Cancers fiveprime.com | NASDAQ: FPRX © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 7

FIGHT Trial Design Patients Selected for FGFR2b+ Tumors (~30% of all non-HER2+ Gastric/GEJ Cancer) Key Eligibility Criteria • No prior therapy for unresectable Bema + mFOLFOX6 locally advanced or metastatic Primary endpoint (n = 77) gastric or GEJ adenocarcinoma Progression-Free • RECIST v1.1 evaluable disease Survival R VS • FGFR2b overexpression and/or 1:1 Secondary endpoints FGFR2 gene amplification Overall Survival Placebo + mFOLFOX6 • Non-HER2+ (n = 78) Response Rate Stratification Factors • Geographic region • Single dose of mFOLFOX during screening • Prior adjuvant or neo-adjuvant chemotherapy © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 8FIGHT Trial Design Patients Selected for FGFR2b+ Tumors (~30% of all non-HER2+ Gastric/GEJ Cancer) Key Eligibility Criteria • No prior therapy for unresectable Bema + mFOLFOX6 locally advanced or metastatic Primary endpoint (n = 77) gastric or GEJ adenocarcinoma Progression-Free • RECIST v1.1 evaluable disease Survival R VS • FGFR2b overexpression and/or 1:1 Secondary endpoints FGFR2 gene amplification Overall Survival Placebo + mFOLFOX6 • Non-HER2+ (n = 78) Response Rate Stratification Factors • Geographic region • Single dose of mFOLFOX during screening • Prior adjuvant or neo-adjuvant chemotherapy © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 8

Favor Placebo 1 Primary and Secondary Efficacy Endpoints with Statistical Significance Hazard Ratio MEDIAN PFS/OS (MON) HR (95% CI) ENDPOINT RESPONSE RATE DIFFERENCE IN ORR (95% CI) 0 0.25 0.5 0.75 1 1.25 1.5 1.75 2 Bema: 9.5 0.68 (0.44, 1.04) PFS Placebo: 7.4 p=0.073 Bema: NR 0.58 (0.35, 0.95) OS Placebo: 12.9 p=0.027 2 Bema: 36 (46.8%) -13.1% (-29.0%, 2.8%) ORR Placebo: 26 (33.3%) p=0.106 • All results are primary analysis on ITT -0.4 -0.3 -0.2 -0.1 0 0.1 0.2 0.3 0.4 • NR = Not reached Difference in ORR 1 2 Statistical significance (at 2-sided alpha 0.20) for PFS, OS and ORR was pre-specified and tested sequentially; Difference in ORR is calculated by (Placebo – Bema) © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 9 Favor BemaFavor Placebo 1 Primary and Secondary Efficacy Endpoints with Statistical Significance Hazard Ratio MEDIAN PFS/OS (MON) HR (95% CI) ENDPOINT RESPONSE RATE DIFFERENCE IN ORR (95% CI) 0 0.25 0.5 0.75 1 1.25 1.5 1.75 2 Bema: 9.5 0.68 (0.44, 1.04) PFS Placebo: 7.4 p=0.073 Bema: NR 0.58 (0.35, 0.95) OS Placebo: 12.9 p=0.027 2 Bema: 36 (46.8%) -13.1% (-29.0%, 2.8%) ORR Placebo: 26 (33.3%) p=0.106 • All results are primary analysis on ITT -0.4 -0.3 -0.2 -0.1 0 0.1 0.2 0.3 0.4 • NR = Not reached Difference in ORR 1 2 Statistical significance (at 2-sided alpha 0.20) for PFS, OS and ORR was pre-specified and tested sequentially; Difference in ORR is calculated by (Placebo – Bema) © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 9 Favor Bema
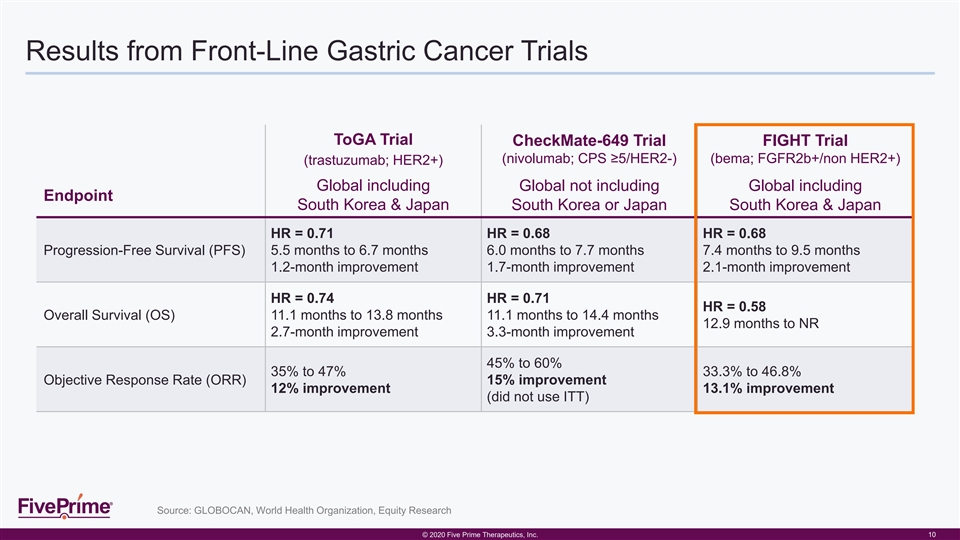
Results from Front-Line Gastric Cancer Trials ToGA Trial CheckMate-649 Trial FIGHT Trial (nivolumab; CPS ≥5/HER2-) (bema; FGFR2b+/non HER2+) (trastuzumab; HER2+) Global including Global not including Global including Endpoint South Korea & Japan South Korea or Japan South Korea & Japan HR = 0.71 HR = 0.68 HR = 0.68 Progression-Free Survival (PFS) 5.5 months to 6.7 months 6.0 months to 7.7 months 7.4 months to 9.5 months 1.2-month improvement 1.7-month improvement 2.1-month improvement HR = 0.74 HR = 0.71 HR = 0.58 Overall Survival (OS) 11.1 months to 13.8 months 11.1 months to 14.4 months 12.9 months to NR 2.7-month improvement 3.3-month improvement 45% to 60% 35% to 47% 33.3% to 46.8% Objective Response Rate (ORR) 15% improvement 12% improvement 13.1% improvement (did not use ITT) Source: GLOBOCAN, World Health Organization, Equity Research © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 10Results from Front-Line Gastric Cancer Trials ToGA Trial CheckMate-649 Trial FIGHT Trial (nivolumab; CPS ≥5/HER2-) (bema; FGFR2b+/non HER2+) (trastuzumab; HER2+) Global including Global not including Global including Endpoint South Korea & Japan South Korea or Japan South Korea & Japan HR = 0.71 HR = 0.68 HR = 0.68 Progression-Free Survival (PFS) 5.5 months to 6.7 months 6.0 months to 7.7 months 7.4 months to 9.5 months 1.2-month improvement 1.7-month improvement 2.1-month improvement HR = 0.74 HR = 0.71 HR = 0.58 Overall Survival (OS) 11.1 months to 13.8 months 11.1 months to 14.4 months 12.9 months to NR 2.7-month improvement 3.3-month improvement 45% to 60% 35% to 47% 33.3% to 46.8% Objective Response Rate (ORR) 15% improvement 12% improvement 13.1% improvement (did not use ITT) Source: GLOBOCAN, World Health Organization, Equity Research © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 10

Summary of Treatment-Emergent Adverse Events (TEAEs) Bema (N = 76) Placebo (N = 77) Total (N = 153) All TEAE 76 (100.0%) 76 (98.7%) 152 (99.3%) Grade ≥ 3 63 (82.9%) 57 (74.0%) 120 (78.4%) Leading to death (Grade 5) 5 (6.6%) 4 (5.2%) 9 (5.9%) SAE 24 (31.6%) 28 (36.4%) 52 (34.0%) SAE related to any study drug 11 (14.5%) 15 (19.5%) 26 (17.0%) Related to any study drug 72 (94.7%) 73 (94.8%) 145 (94.8%) Related to any study drug with Grade ≥ 3 57 (75.0%) 47 (61.0%) 104 (68.0%) Leading to any component of mFOLFOX6 discontinuation 35 (46.1%) 28 (36.4%) 63 (41.2%) Leading to bema/placebo discontinuation 26 (34.2%) 4 (5.2%) 30 (19.6%) Leading to bema/placebo reduction 9 (11.8%) 7 (9.1%) 16 (10.5%) • Corneal and stomatitis adverse events were more frequent in the bemarituzumab + mFOLFOX6 arm • No retinal detachment or hyperphosphatemia were reported in the bemarituzumab + mFOLFOX6 arm © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 11Summary of Treatment-Emergent Adverse Events (TEAEs) Bema (N = 76) Placebo (N = 77) Total (N = 153) All TEAE 76 (100.0%) 76 (98.7%) 152 (99.3%) Grade ≥ 3 63 (82.9%) 57 (74.0%) 120 (78.4%) Leading to death (Grade 5) 5 (6.6%) 4 (5.2%) 9 (5.9%) SAE 24 (31.6%) 28 (36.4%) 52 (34.0%) SAE related to any study drug 11 (14.5%) 15 (19.5%) 26 (17.0%) Related to any study drug 72 (94.7%) 73 (94.8%) 145 (94.8%) Related to any study drug with Grade ≥ 3 57 (75.0%) 47 (61.0%) 104 (68.0%) Leading to any component of mFOLFOX6 discontinuation 35 (46.1%) 28 (36.4%) 63 (41.2%) Leading to bema/placebo discontinuation 26 (34.2%) 4 (5.2%) 30 (19.6%) Leading to bema/placebo reduction 9 (11.8%) 7 (9.1%) 16 (10.5%) • Corneal and stomatitis adverse events were more frequent in the bemarituzumab + mFOLFOX6 arm • No retinal detachment or hyperphosphatemia were reported in the bemarituzumab + mFOLFOX6 arm © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 11

Summary of FIGHT Phase 2 Top-Line Results Primary endpoint Secondary efficacy endpoints st 1 secondary endpoint OS: Bema is superior to Primary endpoint PFS: Bema is superior to placebo placebo 1 • HR = 0.68 (95% CI: 0.44, 1.04; p=0.073 ) 1 • HR = 0.58 (95% CI: 0.35, 0.95; p=0.027 ) • Median PFS (months): 9.5 vs. 7.4 • Median OS (months): NR vs. 12.9 nd 2 secondary endpoint ORR: Bema is superior to placebo 1 • Improvement in ORR = 13.1% (p=0.106 ) • ORR: 46.8% vs. 33.3% Preliminary Safety Summary: • Overall incidence of AEs and SAEs were similar in the 2 arms • Expected: corneal and stomatitis adverse events were more frequent in the bemarituzumab + mFOLFOX6 arm • No adverse events of retinal detachment or hyperphosphatemia identified in the bemarituzumab + mFOLFOX6 arm 1 Statistical significance (at 2-sided alpha 0.20) for PFS, OS and ORR was pre-specified and tested sequentially © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 12Summary of FIGHT Phase 2 Top-Line Results Primary endpoint Secondary efficacy endpoints st 1 secondary endpoint OS: Bema is superior to Primary endpoint PFS: Bema is superior to placebo placebo 1 • HR = 0.68 (95% CI: 0.44, 1.04; p=0.073 ) 1 • HR = 0.58 (95% CI: 0.35, 0.95; p=0.027 ) • Median PFS (months): 9.5 vs. 7.4 • Median OS (months): NR vs. 12.9 nd 2 secondary endpoint ORR: Bema is superior to placebo 1 • Improvement in ORR = 13.1% (p=0.106 ) • ORR: 46.8% vs. 33.3% Preliminary Safety Summary: • Overall incidence of AEs and SAEs were similar in the 2 arms • Expected: corneal and stomatitis adverse events were more frequent in the bemarituzumab + mFOLFOX6 arm • No adverse events of retinal detachment or hyperphosphatemia identified in the bemarituzumab + mFOLFOX6 arm 1 Statistical significance (at 2-sided alpha 0.20) for PFS, OS and ORR was pre-specified and tested sequentially © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 12

Potential Development Opportunity in Other FGFR2b+ Tumors FGFR2b+ Rate Estimated Global 1 Tumor Type Global Prevalence (2+ / 3+ IHC Cutoff) FGFR2b+ Population (Study source) 31% NSCLC-Squamous 543,141 ~150,000 (Ventana data, n=100) 13% TNBC 825,012 ~100,000 (Five Prime data, n=97) 40% Ovarian cancer 762,663 ~300,000 (Ventana data, n=25) 4% Pancreatic cancer 282,574 ~10,000 (Five Prime data, n=100) 22% Intrahepatic-cholangiocarcinoma 52,576 ~10,000 (Ventana data, n=125) 1 GLOBOCAN, World Health Organization, Equity Research © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 13Potential Development Opportunity in Other FGFR2b+ Tumors FGFR2b+ Rate Estimated Global 1 Tumor Type Global Prevalence (2+ / 3+ IHC Cutoff) FGFR2b+ Population (Study source) 31% NSCLC-Squamous 543,141 ~150,000 (Ventana data, n=100) 13% TNBC 825,012 ~100,000 (Five Prime data, n=97) 40% Ovarian cancer 762,663 ~300,000 (Ventana data, n=25) 4% Pancreatic cancer 282,574 ~10,000 (Five Prime data, n=100) 22% Intrahepatic-cholangiocarcinoma 52,576 ~10,000 (Ventana data, n=125) 1 GLOBOCAN, World Health Organization, Equity Research © © 2 202 020 0 F Fiiv ve e P Pr riim me e T The her ra ape peu ut tiic cs s, , I In nc c. . 13

Bemarituzumab Targeted Therapy for FGFR2b+ Tumors November 10, 2020 © 2020 Five Prime Therapeutics, Inc.Bemarituzumab Targeted Therapy for FGFR2b+ Tumors November 10, 2020 © 2020 Five Prime Therapeutics, Inc.
