Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Lipocine Inc. | tm2033940d2_8k.htm |
| EX-99.2 - EXHIBIT 99.2 - Lipocine Inc. | tm2033940d2_ex99-2.htm |

TLANDO™, Oral Testosterone Replacement Therapy without Dose Titration Requirement in Hypogonadal Men Anthony DelConte, MD 1,2 ; Nachiappan Chidambaram, PhD 1 ; Kongnara Papangkorn, PhD 1 ; Benjamin J. Bruno, PharmD, PhD 1 ; Kilyoung Kim, PhD 1 ; Mahesh V. Patel, PhD 1 1 Lipocine Inc., Salt Lake City, UT; 2 Saint Joseph’s University, Philadelphia, PA 1 SMSNA 2020 *Dr. DelConte is a medical director and paid consultant at Lipocine, Inc, ** Drs Chidambaram, Papangkorn, Bruno, Kim, and Patel are employees of Lipocine, Inc Exhibit 99.1
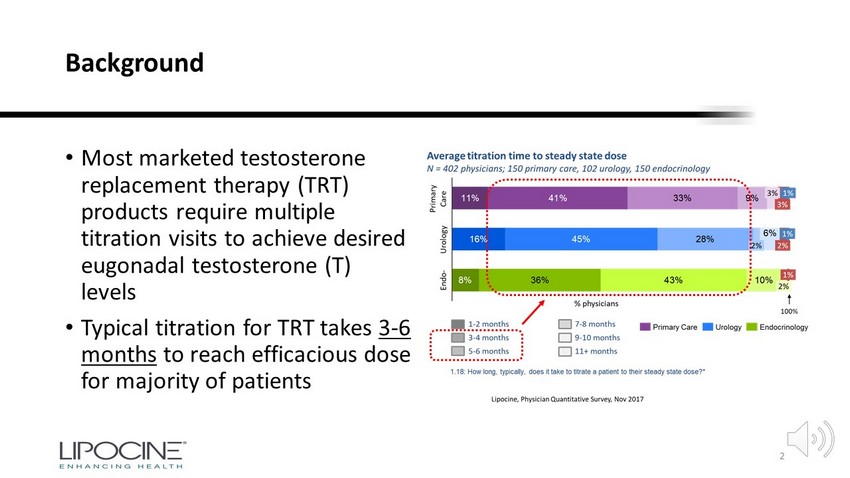
Background • Most marketed testosterone replacement therapy (TRT) products require multiple titration visits to achieve desired eugonadal testosterone (T) levels • Typical titration for TRT takes 3 - 6 months to reach efficacious dose for majority of patients 2
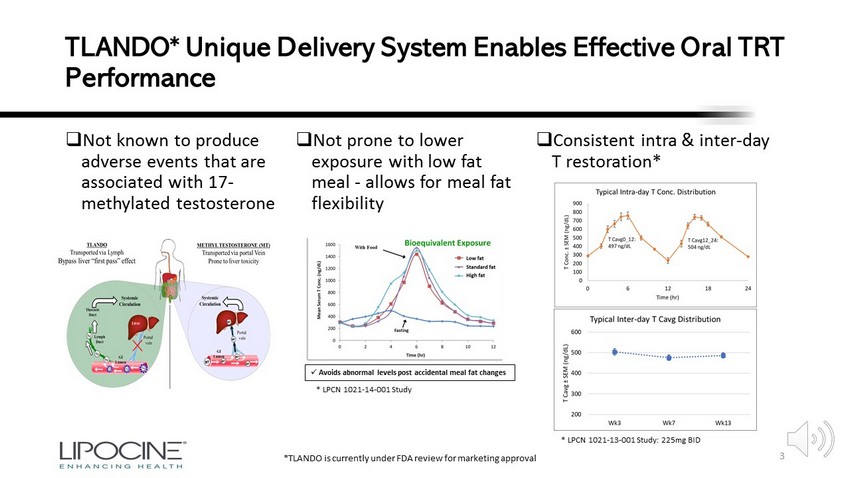
TLANDO* Unique Delivery System Enables Effective Oral TRT Performance □ Not known to produce adverse events that are associated with 17 - methylated testosterone □ Consistent intra & inter - day T restoration* * LPCN 1021 - 14 - 001 Study * LPCN 1021 - 13 - 001 Study: 225mg BID 3 □ Not prone to lower exposure with low fat meal - allows for meal fat flexibility x Avoids abnormal levels post accidental meal fat changes *TLANDO is currently under FDA review for marketing approval
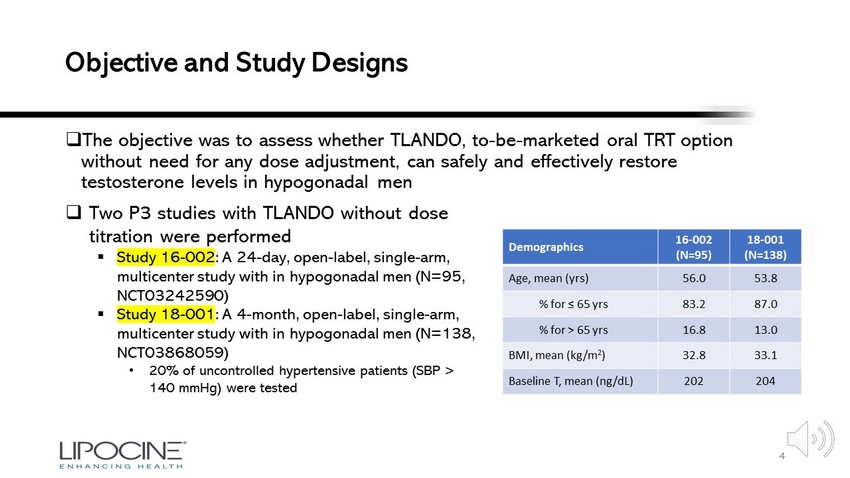
Objective and Study Designs □ The objective was to assess whether TLANDO, to - be - marketed oral TRT option without need for any dose adjustment, can safely and effectively restore testosterone levels in hypogonadal men 4 □ Two P3 studies with TLANDO without dose titration were performed ▪ Study 16 - 002 : A 24 - day, open - label, single - arm, multicenter study with in hypogonadal men (N=95, NCT03242590) ▪ Study 18 - 001 : A 4 - month, open - label, single - arm, multicenter study with in hypogonadal men (N=138, NCT03868059) • 20% of uncontrolled hypertensive patients (SBP > 140 mmHg) were tested Demographics 16 - 002 (N=95) 18 - 001 (N=138) Age, mean ( yrs ) 56.0 53.8 % for ≤ 65 yrs 83.2 87.0 % for > 65 yrs 16.8 13.0 BMI, mean (kg/m 2 ) 32.8 33.1 Baseline T, mean (ng/dL) 202 204

TLANDO Efficacy & Tolerability Daily dose 450mg without requiring dose adjustment □ Efficacy* ▪ 81% (95% CI: 72 – 88%) ▪ Mean T Cavg : 478 ng/dL □ Adverse Events ▪ No death, no drug - related SAEs ▪ Mild to moderate Adverse Reaction ≥ 2% (STUDY 18 - 001) Overall (N=138) n (%) Hematocrit increased/Polycythemia 6 (4.3) Adverse Reaction ≥ 2% (STUDY 16 - 002) Overall (N=95) n (%) Blood prolactin increased 4 (4.2) 5 Nighttime peak Daytime peak * PK measured at efficacy day (Day 24) in Study 16 - 002

Summary & Conclusions □ Safety and efficacy of TLANDO™ (Oral Testosterone Undecanoate) was confirmed □ TLANDO™ is a novel oral TRT option without requiring dose titration □ Start of therapy with the effective dose without delay □ Devoid of accidental T transference and pulmonary oil micro - embolism risks □ No burden of additional dose adjustment visits/invasive samplings □ Potential to improve compliance and persistence rates 6
