Attached files
| file | filename |
|---|---|
| 8-K - 8-K - GENOCEA BIOSCIENCES, INC. | gnca-20201109.htm |

GEN-009 Part B SITC data presentation November 9, 2020

This presentation contains “forward-looking” statements that are within the meaning of federal securities laws and are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include information concerning our possible or assumed future results of operations, business strategies, clinical trials and pre- clinical studies, regulatory approval of our product candidates, liquidity position and capital needs, financing plans, industry environment, potential growth opportunities, potential market opportunities and the effects of competition. Forward-looking statements include all statements that are not historical facts and can be identified by terms such as “anticipates,” “believes,” “expects,” “could,” “seeks,” “estimates,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would” or similar expressions and the negatives of those terms. Forward-looking statements represent our management’s beliefs and assumptions only as of the date of this presentation. Our operations involve risks and uncertainties, many of which are outside our control, and any one of which, or combination of which, could materially affect our results of operations and whether the forward-looking statements ultimately prove to be correct. Factors that may materially affect Disclaimer our results of operations include, among other things, our ability to progress product candidates in preclinical and clinical trials, the ability of ATLAS™ to identify promising oncology vaccine and immunotherapy product candidates, the scope, rate and progress of our preclinical and clinical trials and other research and development activities, anticipated timing of IND applications and new clinical trials, the amount of funds that we may require to conduct our clinical trials for our product candidates, the timing of, and ability to, obtain and maintain necessary regulatory approvals for our product candidates, and those listed in our Annual Report on Form 10-K for the fiscal year ended December 31, 2019 and other filings with the Securities and Exchange Commission (“SEC”). Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. You may get copies of our Annual Report on Form 10-K, Quarterly Report on Form 10-Q and our other SEC filings for free by visiting EDGAR on the SEC website at http://www.sec.gov. | CONFIDENTIAL 2

Today’s summary Compelling early signal persists with longer follow-up Clear anti-tumor activity “Targets matter” Highly differentiated immunogenicity results Powerful readthrough to GEN-011 ATLAS enables unique breadth and specificity 3
Clinical trial design (Part B) • Combination of GEN-009 and standard-of-care PD-1-based regimens in patients with advanced disease • Tumor types: Melanoma, NSCLC, SCCHN, Urothelial, RCC • Objectives: safety, immunogenicity, efficacy • Vaccination contribution to be determined after CPI response established
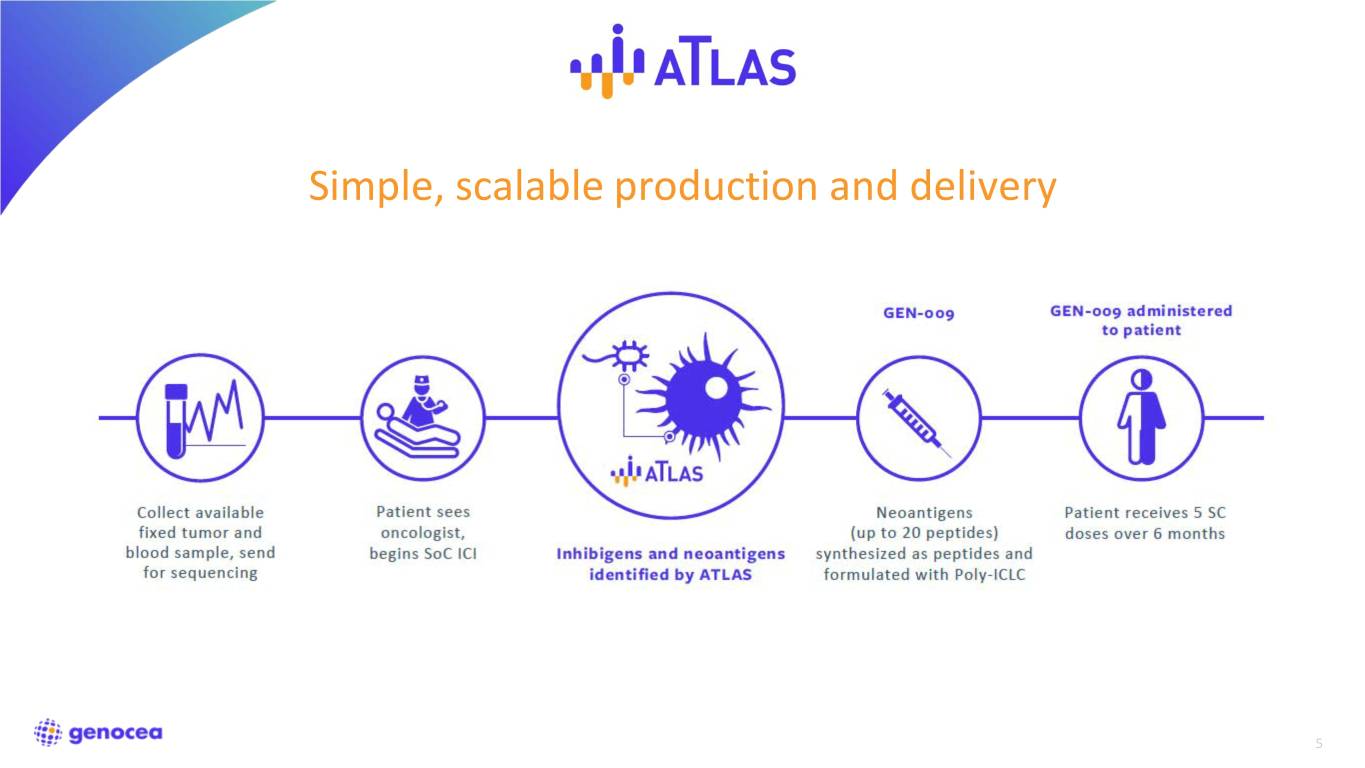
Simple, scalable production and delivery 5
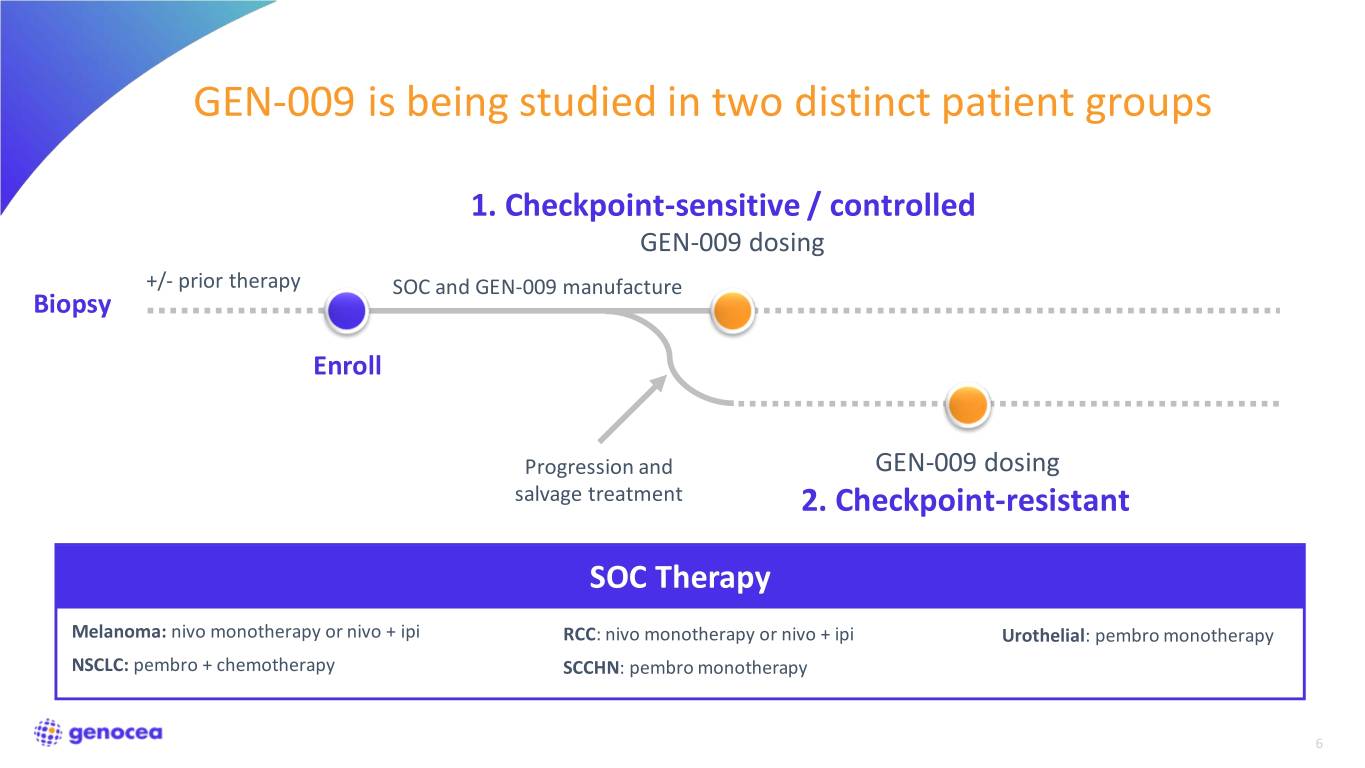
GEN-009 is being studied in two distinct patient groups 1. Checkpoint-sensitive / controlled GEN-009 dosing +/- prior therapy SOC and GEN-009 manufacture Biopsy Enroll Progression and GEN-009 dosing salvage treatment 2. Checkpoint-resistant SOC Therapy Melanoma: nivo monotherapy or nivo + ipi RCC: nivo monotherapy or nivo + ipi Urothelial: pembro monotherapy NSCLC: pembro + chemotherapy SCCHN: pembro monotherapy 6
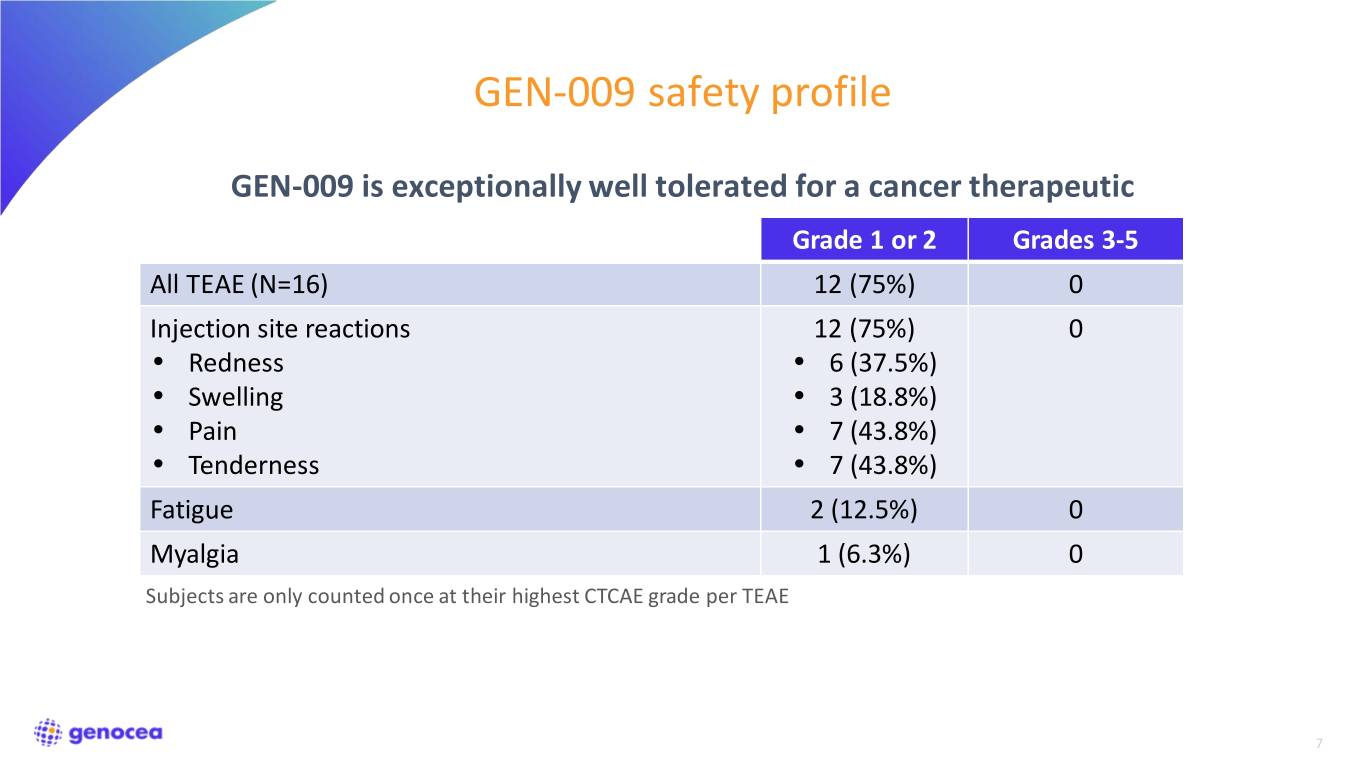
GEN-009 safety profile GEN-009 is exceptionally well tolerated for a cancer therapeutic Grade 1 or 2 Grades 3-5 All TEAE (N=16) 12 (75%) 0 Injection site reactions 12 (75%) 0 • Redness • 6 (37.5%) • Swelling • 3 (18.8%) • Pain • 7 (43.8%) • Tenderness • 7 (43.8%) Fatigue 2 (12.5%) 0 Myalgia 1 (6.3%) 0 Subjects are only counted once at their highest CTCAE grade per TEAE 7
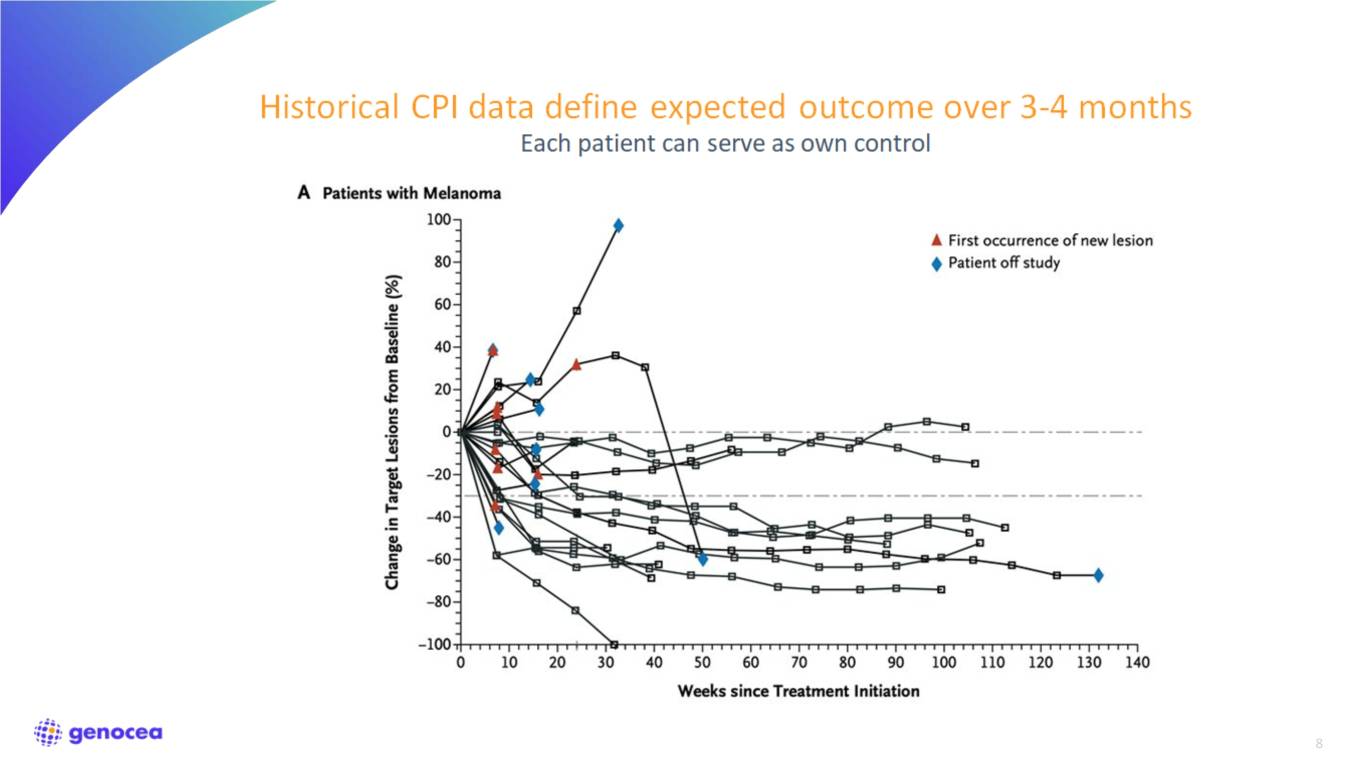
8
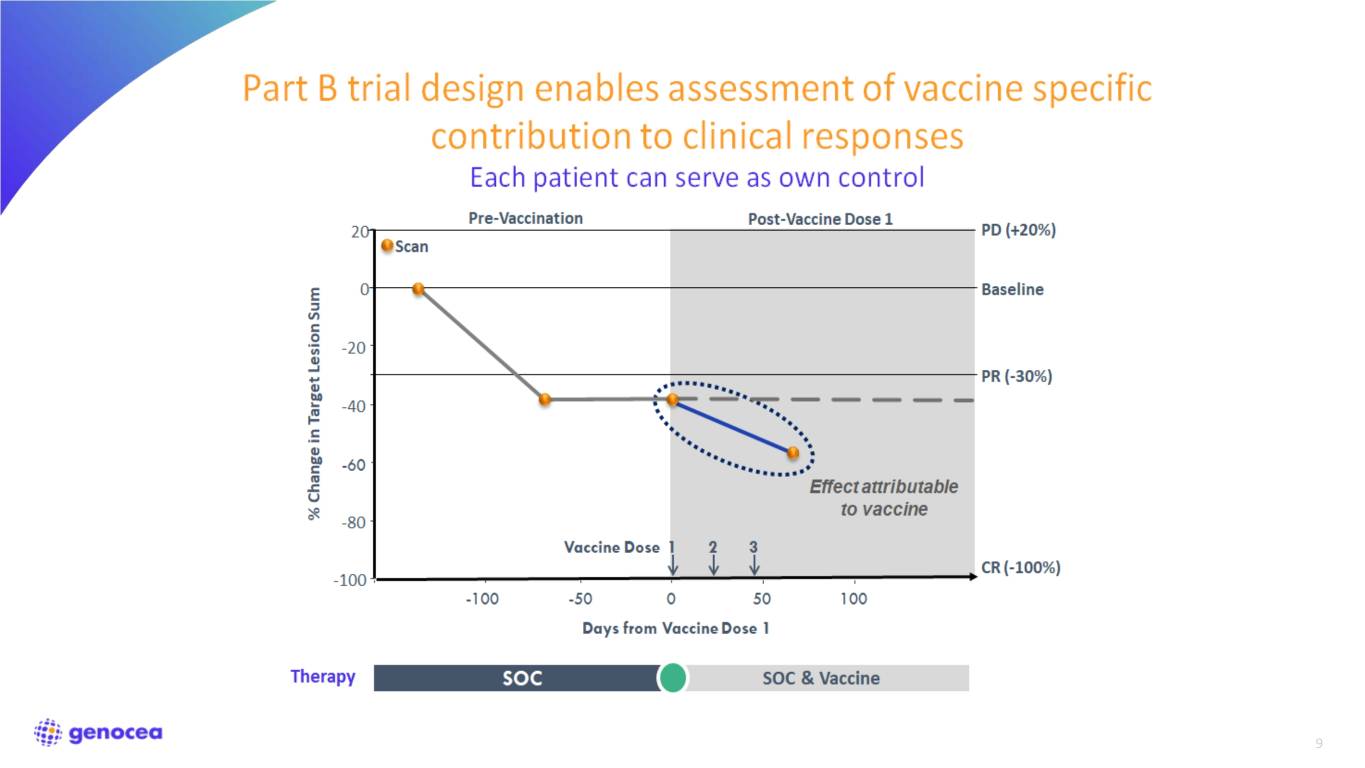
9
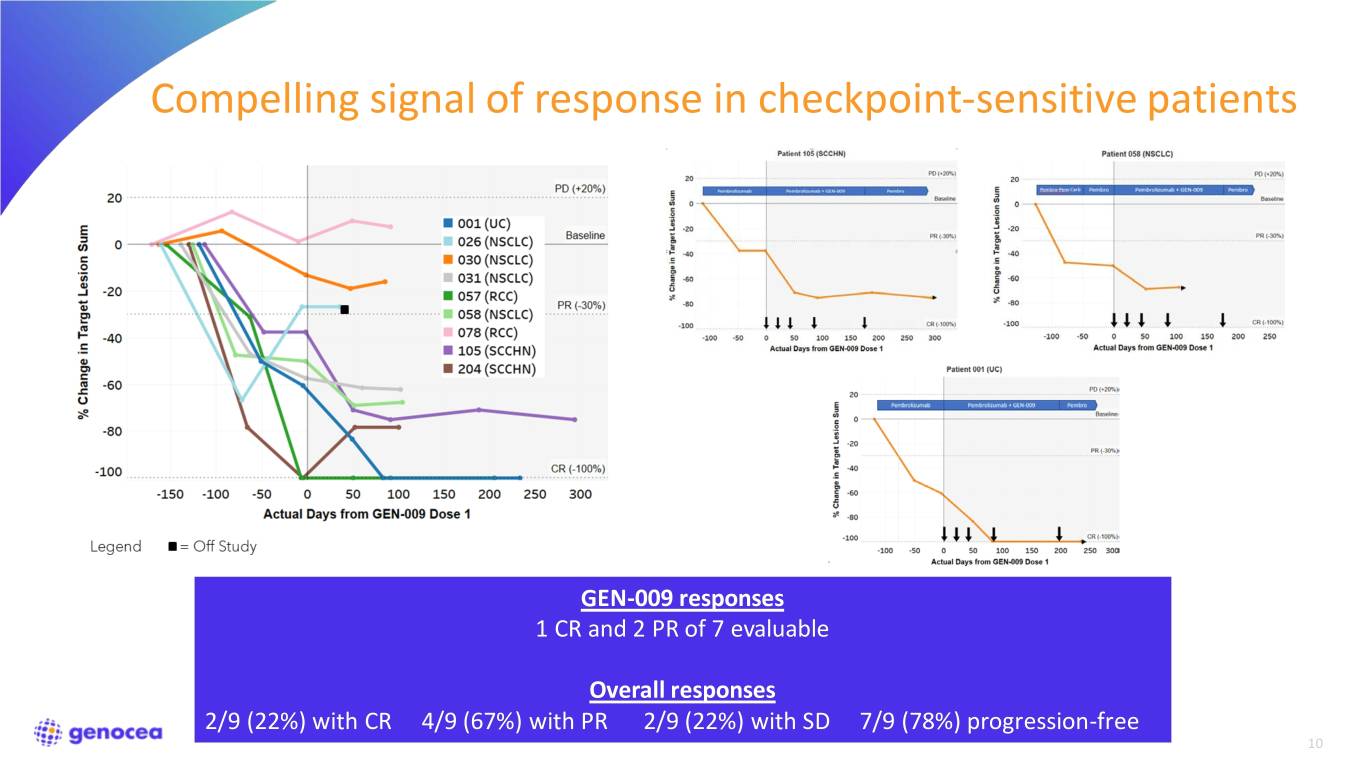
Compelling signal of response in checkpoint-sensitive patients GEN-009 responses 1 CR and 2 PR of 7 evaluable Overall responses 2/9 (22%) with CR 4/9 (67%) with PR 2/9 (22%) with SD 7/9 (78%) progression-free 10
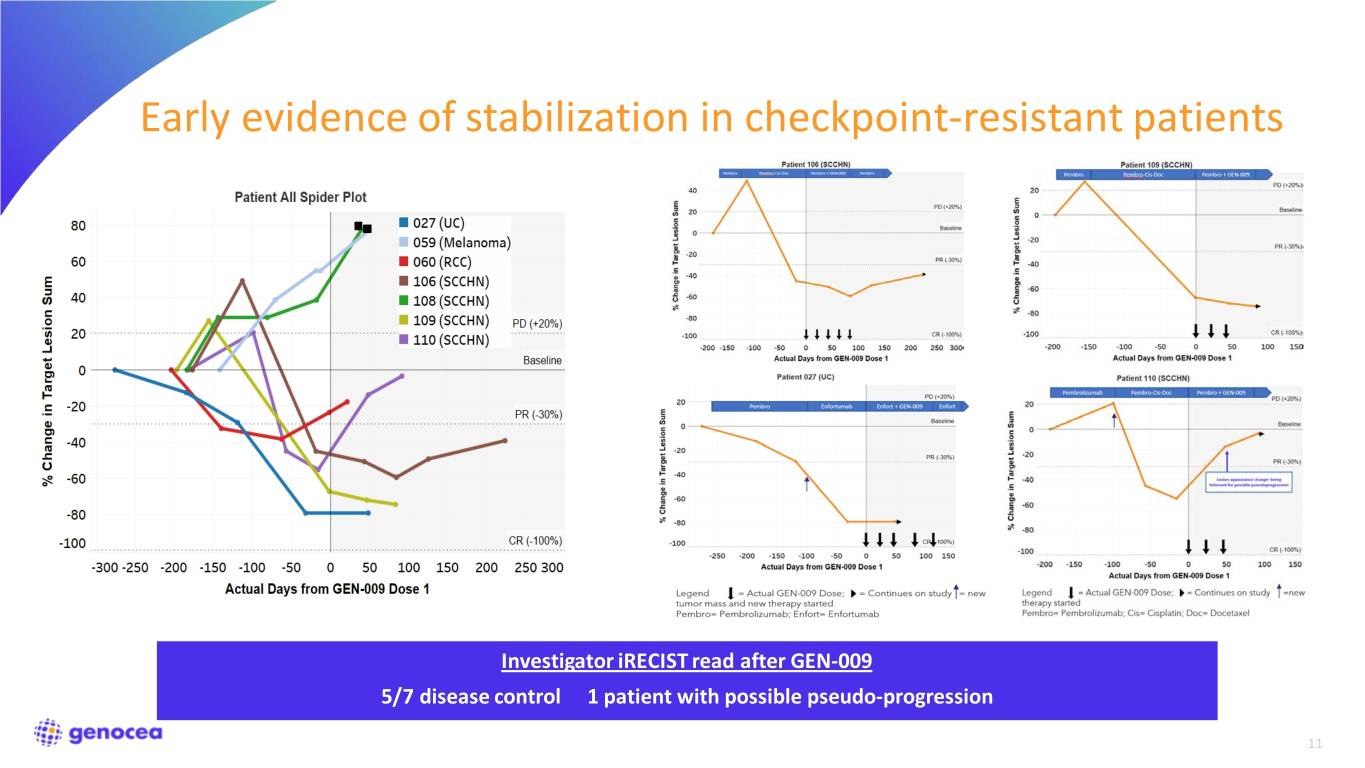
Early evidence of stabilization in checkpoint-resistant patients Investigator iRECIST read after GEN-009 5/7 disease control 1 patient with possible pseudo-progression 11
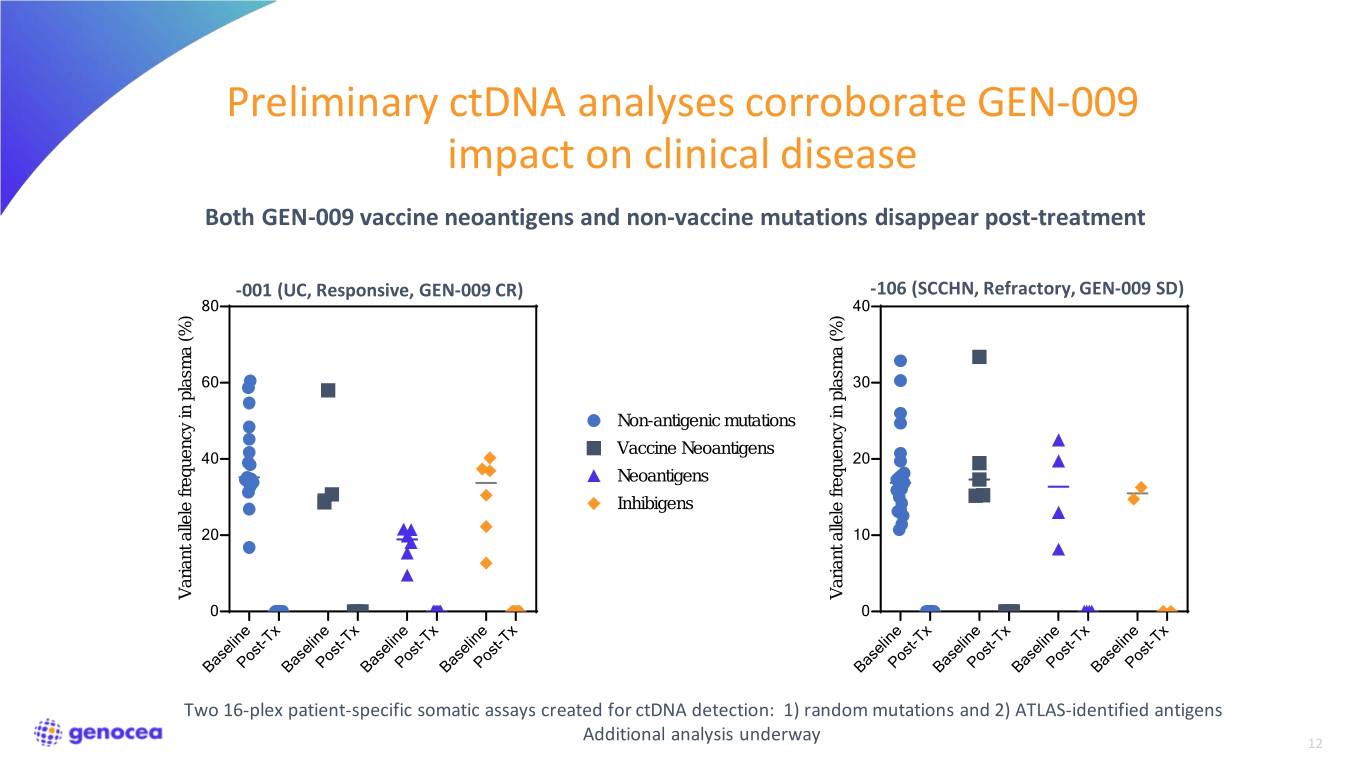
Preliminary ctDNA analyses corroborate GEN-009 impact on clinical disease Both GEN-009 vaccine neoantigens and non-vaccine mutations disappear post-treatment -001 (UC, Responsive, GEN-009 CR) -106 (SCCHN, Refractory, GEN-009 SD) 80 40 60 30 Non-antigenic mutations Vaccine Neoantigens 40 20 Neoantigens Inhibigens 20 10 Variant allele frequency in plasma (%) plasma inVariant allele frequency Variant allele frequency in plasma (%) plasma inVariant allele frequency 0 0 BaselinePost-Tx BaselinePost-Tx BaselinePost-Tx BaselinePost-Tx BaselinePost-Tx BaselinePost-Tx BaselinePost-Tx BaselinePost-Tx Two 16-plex patient-specific somatic assays created for ctDNA detection: 1) random mutations and 2) ATLAS-identified antigens Additional analysis underway 12
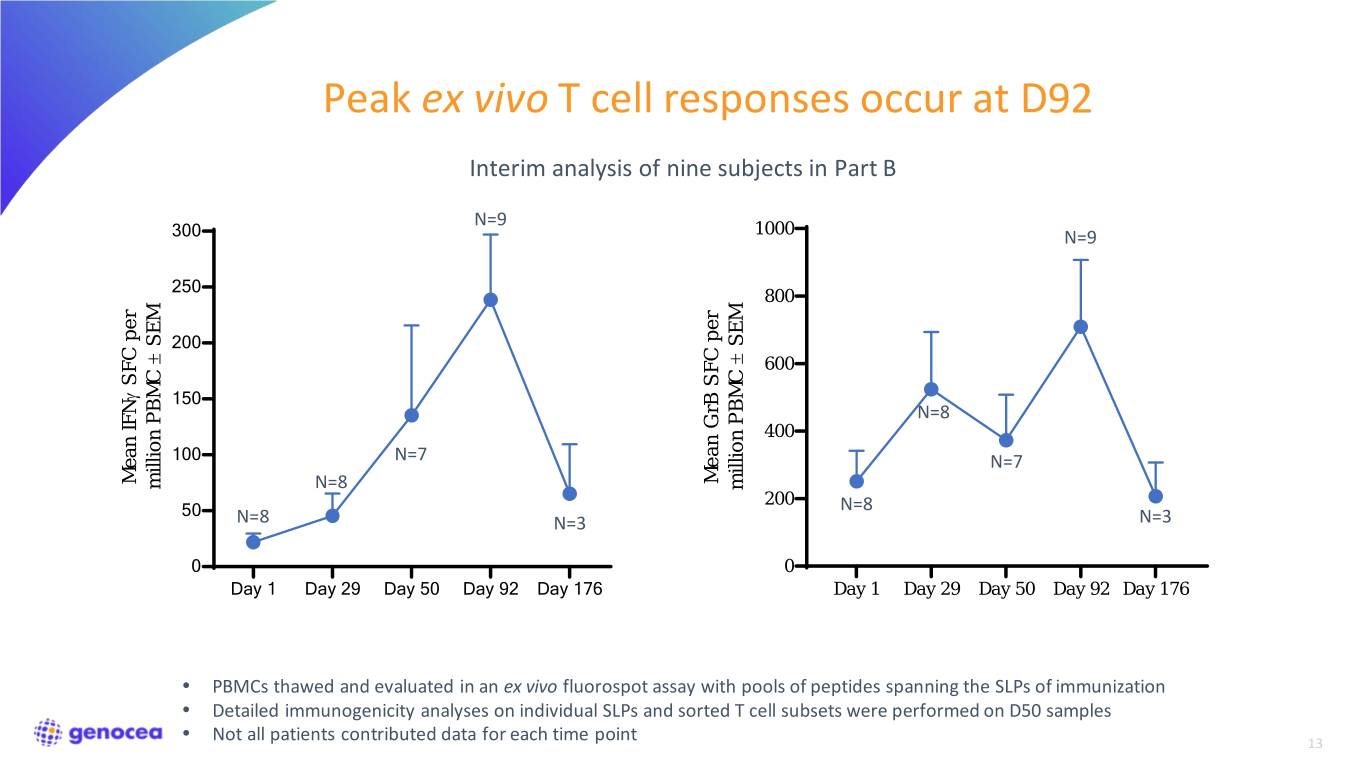
Peak ex vivo T cell responses occur at D92 Interim analysis of nine subjects in Part B N=9 300 1000 N=9 250 800 SEM SEM 200 ± ± 600 SFC per γ 150 N=8 400 100 N=7 N=7 Mean GrB SFC per GrBMean SFC Mean IFN Mean million PBMC million N=8 PBMC million 200 50 N=8 N=8 N=3 N=3 0 0 Day 1 Day 29 Day 50 Day 92 Day 176 Day 1 Day 29 Day 50 Day 92 Day 176 • PBMCs thawed and evaluated in an ex vivo fluorospot assay with pools of peptides spanning the SLPs of immunization • Detailed immunogenicity analyses on individual SLPs and sorted T cell subsets were performed on D50 samples • Not all patients contributed data for each time point 13
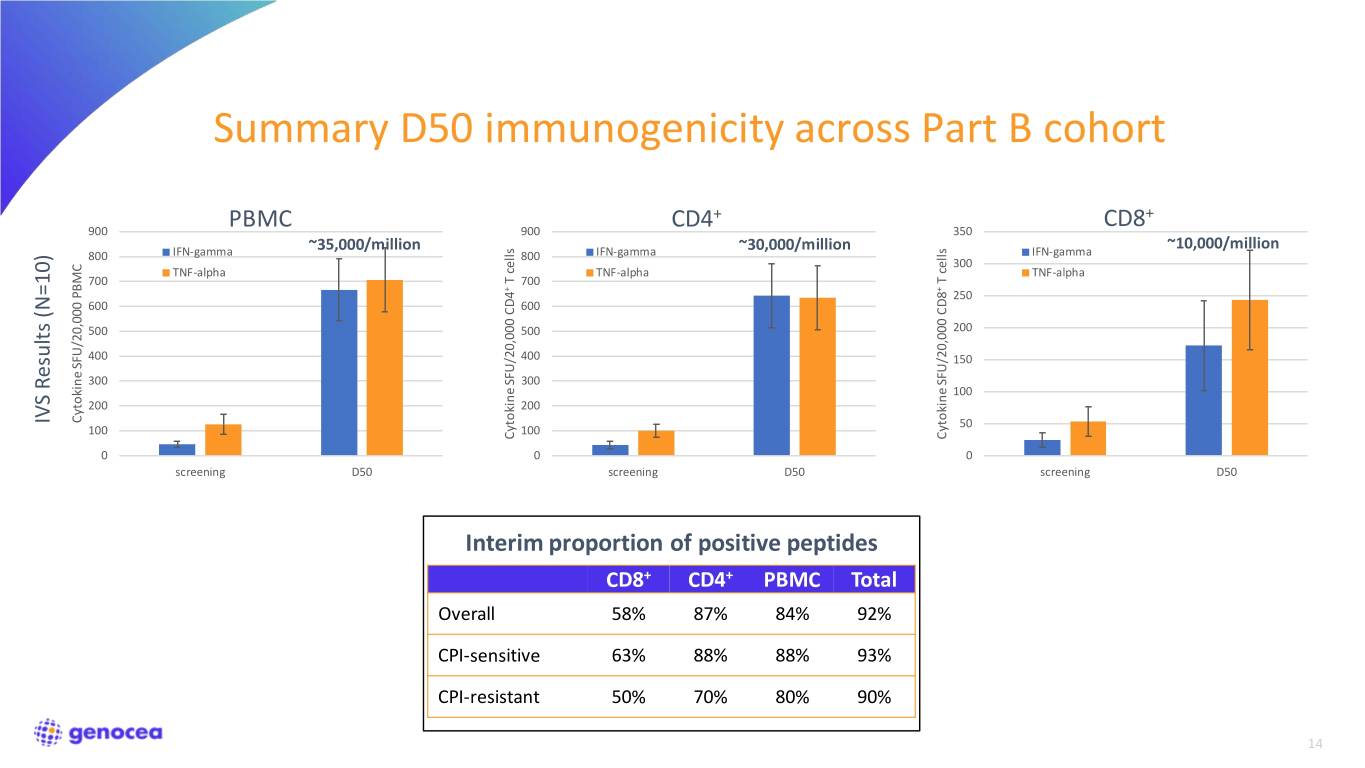
Summary D50 immunogenicity across Part B cohort + + 900 PBMC 900 CD4 350 CD8 ~35,000/million ~30,000/million ~10,000/million 800 IFN-gamma 800 IFN-gamma IFN-gamma 300 TNF-alpha TNF-alpha TNF-alpha 700 cells T 700 cells T + + 250 600 600 500 500 200 400 400 150 300 300 100 200 200 Cytokine SFU/20,000 PBMC IVS Results (N=10) Results IVS 50 100 100 Cytokine SFU/20,000 CD4 Cytokine SFU/20,000 CD8 0 0 0 screening D50 screening D50 screening D50 Interim proportion of positive peptides CD8+ CD4+ PBMC Total Overall 58% 87% 84% 92% CPI-sensitive 63% 88% 88% 93% CPI-resistant 50% 70% 80% 90% 14
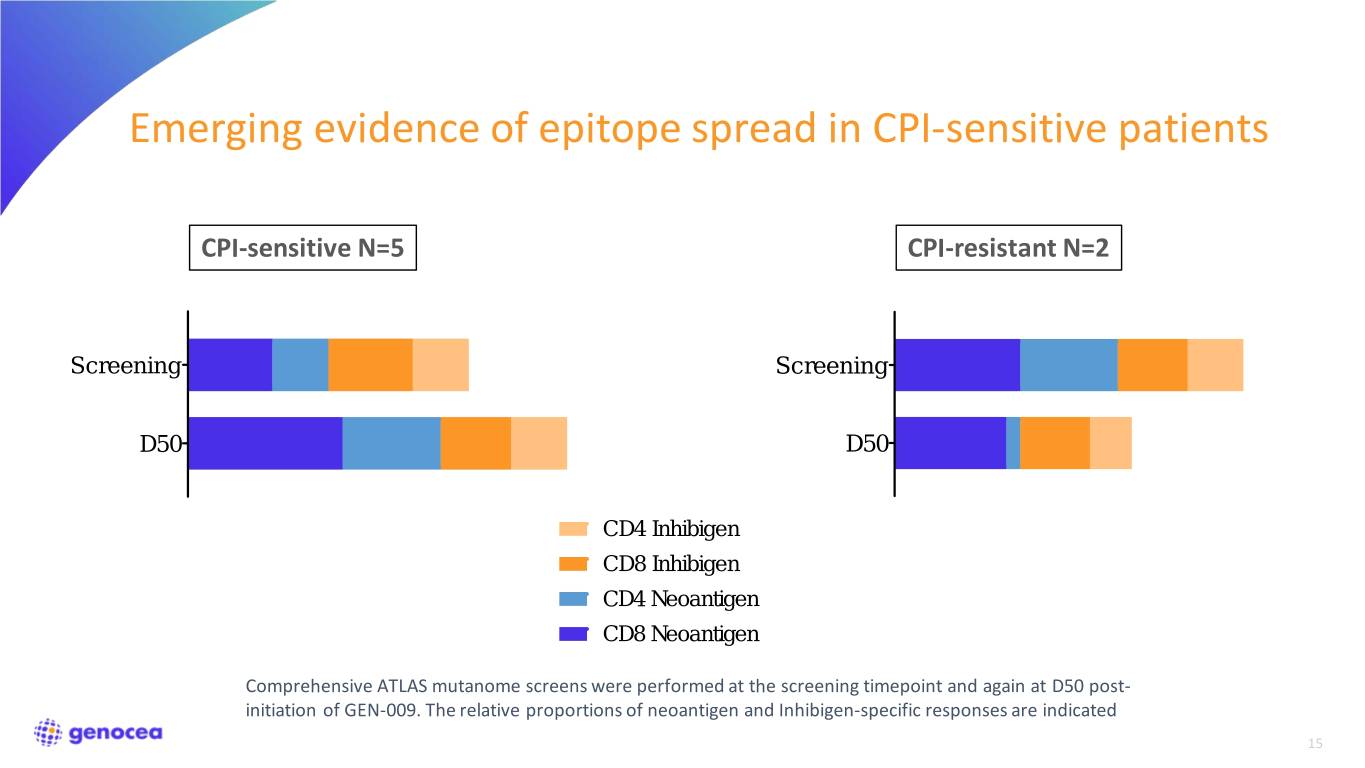
Emerging evidence of epitope spread in CPI-sensitive patients CPI-sensitive N=5 CPI-resistant N=2 Screening Screening D50 D50 CD4 Inhibigen CD8 Inhibigen CD4 Neoantigen CD8 Neoantigen Comprehensive ATLAS mutanome screens were performed at the screening timepoint and again at D50 post- initiation of GEN-009. The relative proportions of neoantigen and Inhibigen-specific responses are indicated 15
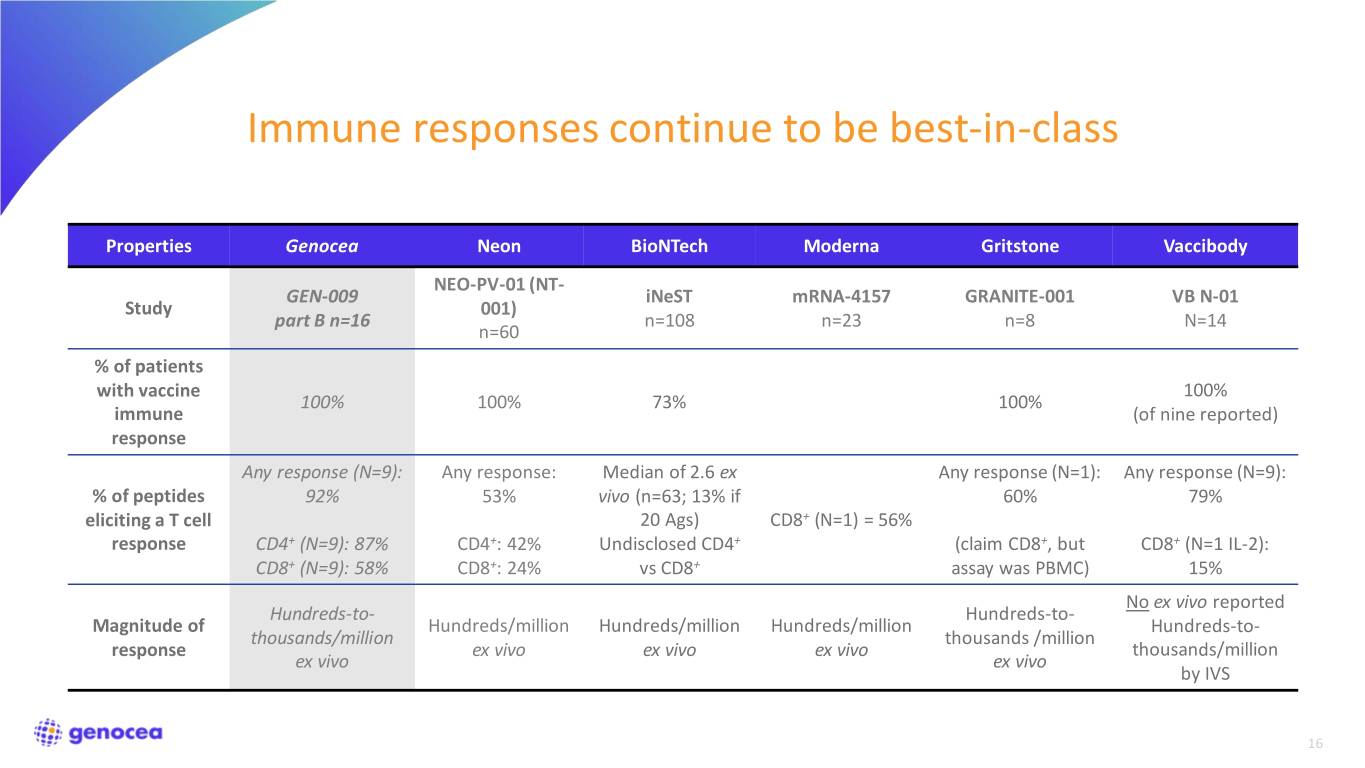
Immune responses continue to be best-in-class Properties Genocea Neon BioNTech Moderna Gritstone Vaccibody NEO-PV-01 (NT- GEN-009 iNeST mRNA-4157 GRANITE-001 VB N-01 Study 001) part B n=16 n=108 n=23 n=8 N=14 n=60 % of patients with vaccine 100% 100% 100% 73% 100% immune (of nine reported) response Any response (N=9): Any response: Median of 2.6 ex Any response (N=1): Any response (N=9): % of peptides 92% 53% vivo (n=63; 13% if 60% 79% eliciting a T cell 20 Ags) CD8+ (N=1) = 56% response CD4+ (N=9): 87% CD4+: 42% Undisclosed CD4+ (claim CD8+, but CD8+ (N=1 IL-2): CD8+ (N=9): 58% CD8+: 24% vs CD8+ assay was PBMC) 15% No ex vivo reported Hundreds-to- Hundreds-to- Magnitude of Hundreds/million Hundreds/million Hundreds/million Hundreds-to- thousands/million thousands /million response ex vivo ex vivo ex vivo thousands/million ex vivo ex vivo by IVS 16

GEN-009: Delivering on the promise of neoantigen therapies Compelling early signal persists with longer follow-up Clear anti-tumor activity “Targets matter” Highly differentiated immunogenicity results Powerful readthrough to GEN-011 ATLAS enables unique breadth and specificity 17
