Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - SYNLOGIC, INC. | d55896dex992.htm |
| EX-99.1 - EX-99.1 - SYNLOGIC, INC. | d55896dex991.htm |
| 8-K - 8-K - SYNLOGIC, INC. | sybx-8k_20201104.htm |
© 2020 SYNLOGIC. QUARTERLY RESULTS. ALL RIGHTS RESERVED. | 1 © 2020 SYNLOGIC. ALL RIGHTS RESERVED. | 1 Bringing the Transformative Power of Synthetic Biology to Medicine Q3 Financial Results & Business Update 5 November 2020 Exhibit 99.3

Forward Looking Statements This presentation contains “forward-looking statements” that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in this presentation regarding strategy, future operations, future financial position, future revenue, projected expenses, prospects, plans and objectives of management are forward- looking statements. In addition, when or if used in this presentation, the words “may,” “could,” “should,” “anticipate,” “believe,” “estimate,” “expect,” “intend,” “plan,” “predict” and similar expressions and their variants may identify forward-looking statements. Examples of forward-looking statements include, but are not limited to, the approach we are taking to discover and develop novel therapeutics using synthetic biology; statements regarding the potential of our platform to develop therapeutics to address a wide range of diseases, including: metabolic diseases, inflammatory and immune disorders, and cancer; the future clinical development of Synthetic Biotic medicines; the potential of our technology to treat phenylketonuria and cancer; the expected timing of our anticipated clinical trial initiations and availability of clinical data; the benefit of orphan drug and fast track status; the adequacy of our capital to support our future operations and our ability to successfully initiate and complete clinical trials; the results of our collaborations; and the difficulty in predicting the time and cost of development of our product candidates. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors, including, without limitation: the uncertainties inherent in the preclinical development process; our ability to protect our intellectual property rights; and legislative, regulatory, political and economic developments, as well as those risks identified under the heading “Risk Factors” in our filings with the SEC. The foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors included in our quarterly report on Form 10-Q filed with the SEC on May 8, 2020, and in any subsequent filings we make with the SEC. The forward-looking statements contained in this presentation reflect our current views with respect to future events. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements in the future, we specifically disclaim any obligation to do so. These forward-looking statements should not be relied upon as representing our view as of any date subsequent to the date hereof.

© 2020 SYNLOGIC. QU©AR2T0E1R9LYSYRNELSOUGLTICS.. AALLLL RRIIGGHHTTSS RREESSEERRVVEEDD.. || 33 Opening Remarks Dr. Aoife Brennan MB CHB President & CEO
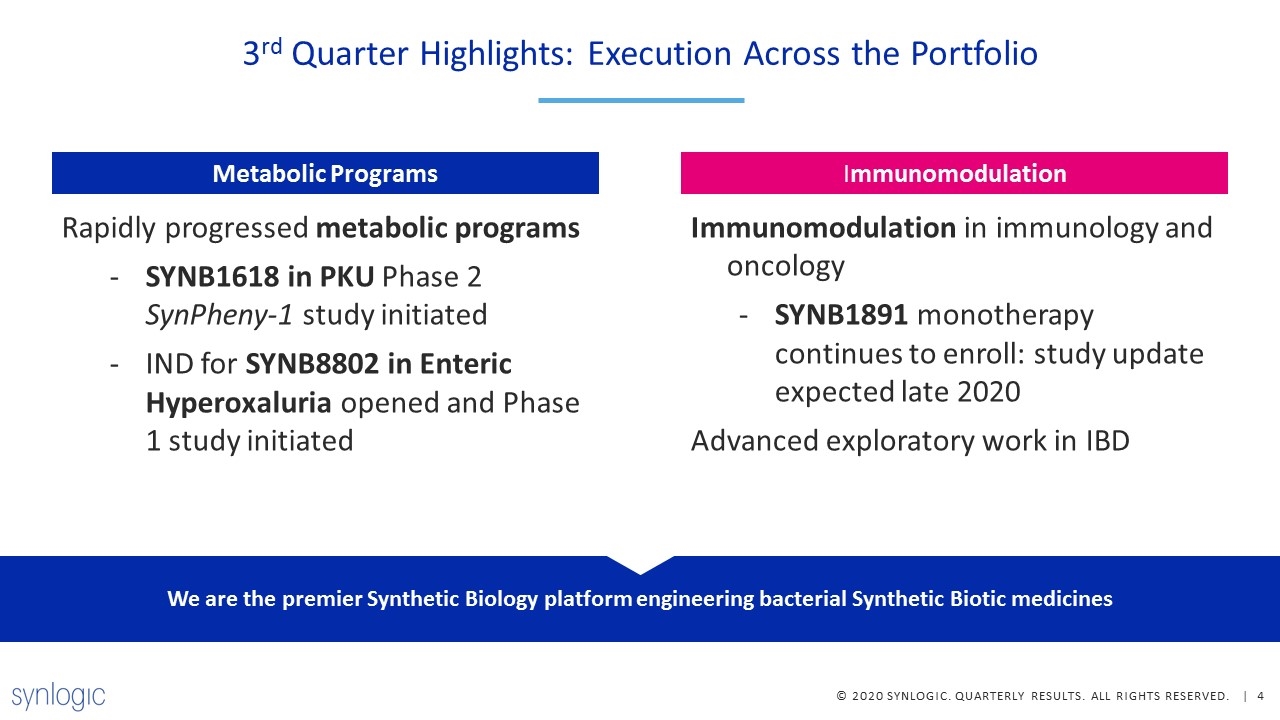
Rapidly progressed metabolic programs SYNB1618 in PKU Phase 2 SynPheny-1 study initiated IND for SYNB8802 in Enteric Hyperoxaluria opened and Phase 1 study initiated Metabolic Programs Immunomodulation in immunology and oncology SYNB1891 monotherapy continues to enroll: study update expected late 2020 Advanced exploratory work in IBD Immunomodulation 3rd Quarter Highlights: Execution Across the Portfolio We are the premier Synthetic Biology platform engineering bacterial Synthetic Biotic medicines
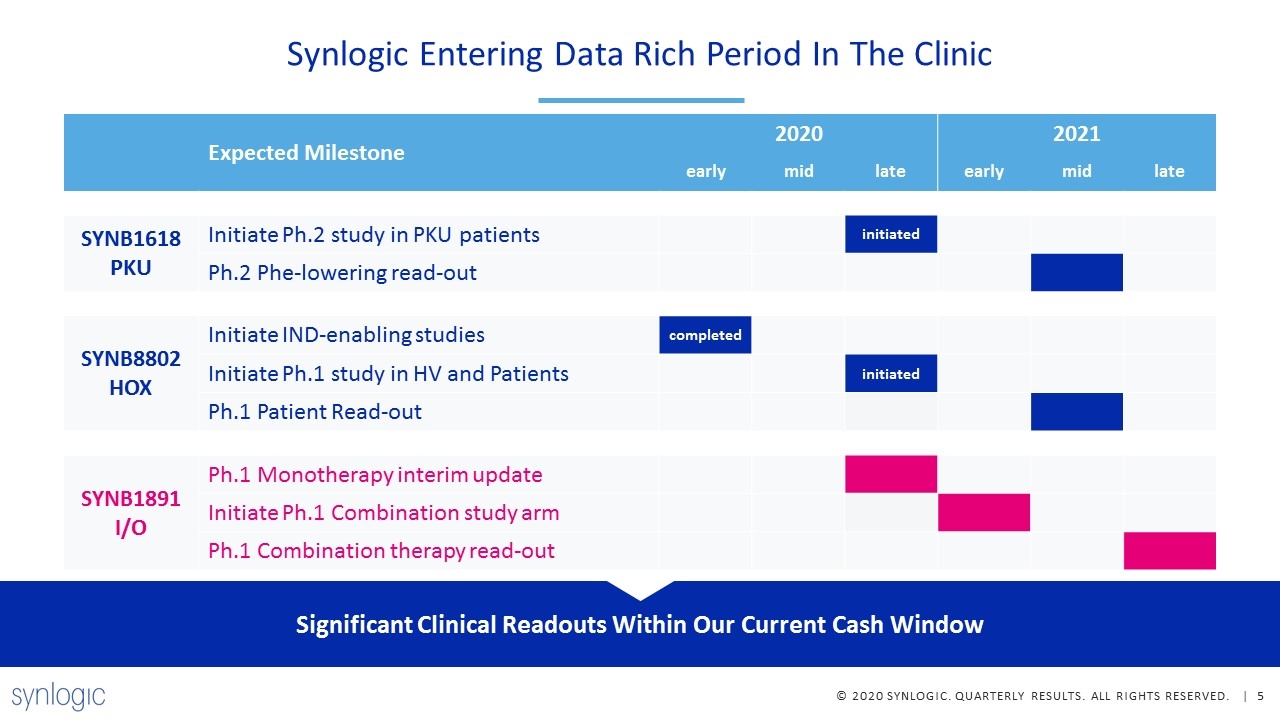
Synlogic Entering Data Rich Period In The Clinic Expected Milestone 2020 2021 early mid late early mid late SYNB1618 PKU Initiate Ph.2 study in PKU patients initiated Ph.2 Phe-lowering read-out SYNB8802 HOX Initiate IND-enabling studies completed Initiate Ph.1 study in HV and Patients initiated Ph.1 Patient Read-out SYNB1891 I/O Ph.1 Monotherapy interim update Initiate Ph.1 Combination study arm Ph.1 Combination therapy read-out Significant Clinical Readouts Within Our Current Cash Window
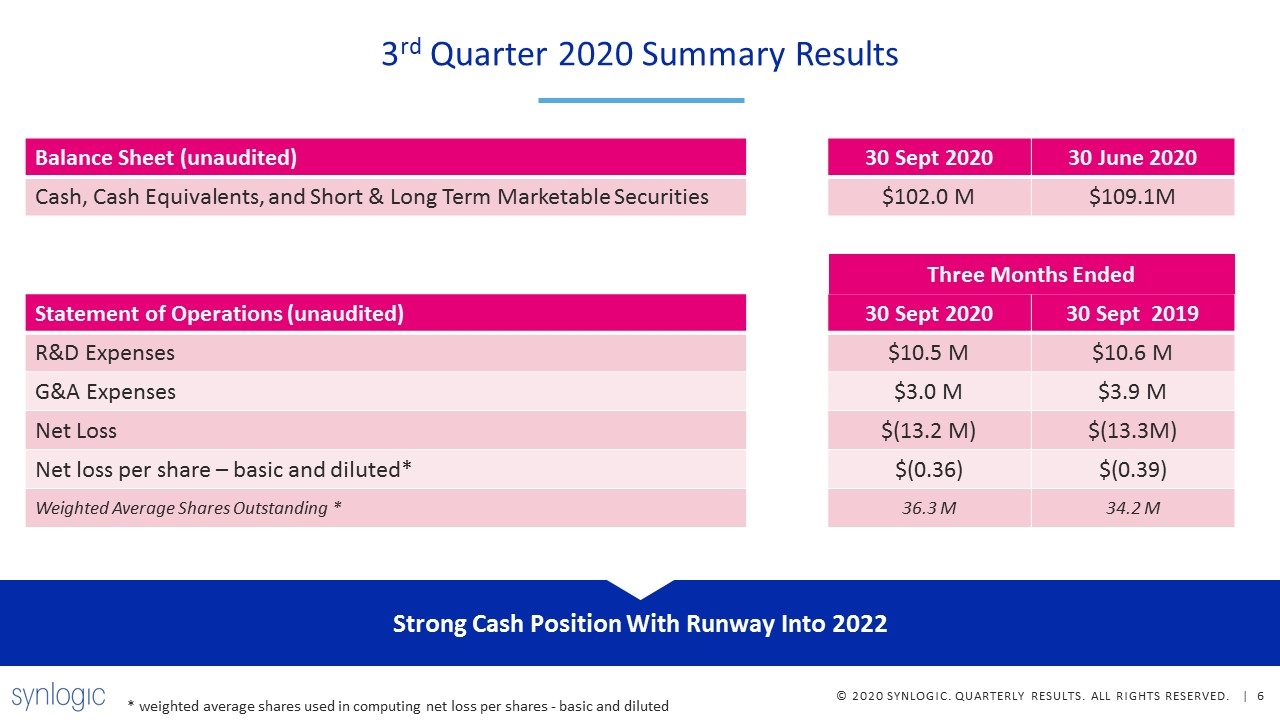
Strong Cash Position With Runway Into 2022 Balance Sheet (unaudited) 30 Sept 2020 30 June 2020 Cash, Cash Equivalents, and Short & Long Term Marketable Securities $102.0 M $109.1M Statement of Operations (unaudited) 30 Sept 2020 30 Sept 2019 R&D Expenses $10.5 M $10.6 M G&A Expenses $3.0 M $3.9 M Net Loss $(13.2 M) $(13.3M) Net loss per share – basic and diluted* $(0.36) $(0.39) Weighted Average Shares Outstanding * 36.3 M 34.2 M Three Months Ended * weighted average shares used in computing net loss per shares - basic and diluted 3rd Quarter 2020 Summary Results

© 2020 SYNLOGIC. QUARTERLY RESULTS. ALL RIGHTS RESERVED. | 9 Progress in Metabolic Programs Dr. Richard Riese, MD, PhD Chief Medical Officer
Phenylketonuria (PKU) Emerging treatment options will continue to leave many patients behind SYNB1618 demonstrates potential to lower Phe in PKU patients Phase 2 Phe -lowering trial initiated
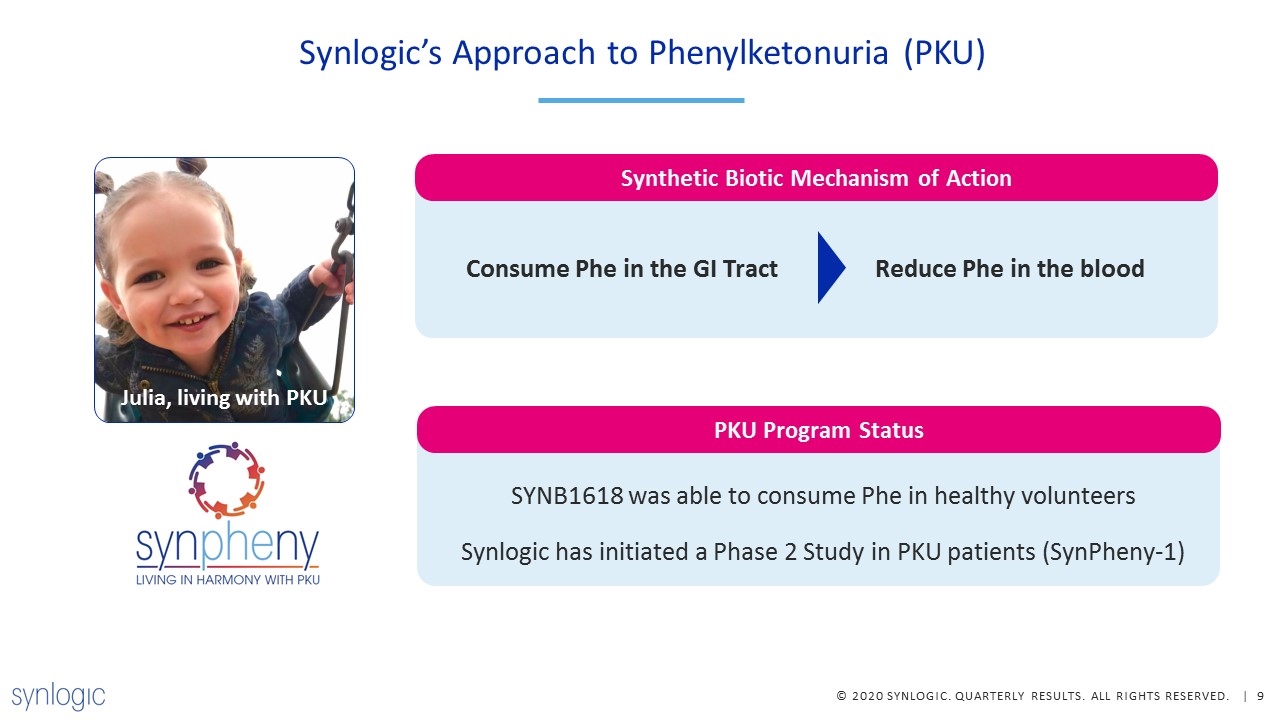
Synlogic’s Approach to Phenylketonuria (PKU) Julia, living with PKU SYNB1618 was able to consume Phe in healthy volunteers Synlogic has initiated a Phase 2 Study in PKU patients (SynPheny-1) PKU Program Status Synthetic Biotic Mechanism of Action Consume Phe in the GI Tract Reduce Phe in the blood
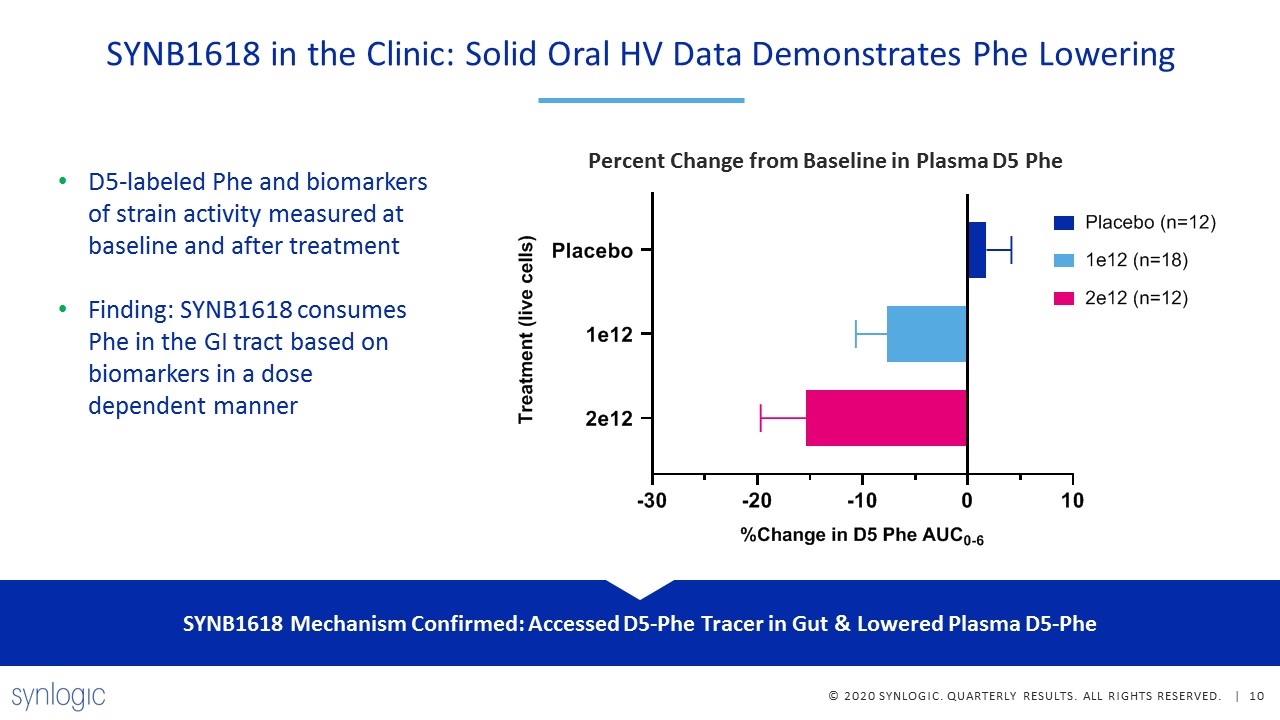
SYNB1618 in the Clinic: Solid Oral HV Data Demonstrates Phe Lowering D5-labeled Phe and biomarkers of strain activity measured at baseline and after treatment Finding: SYNB1618 consumes Phe in the GI tract based on biomarkers in a dose dependent manner SYNB1618 Mechanism Confirmed: Accessed D5-Phe Tracer in Gut & Lowered Plasma D5-Phe Percent Change from Baseline in Plasma D5 Phe
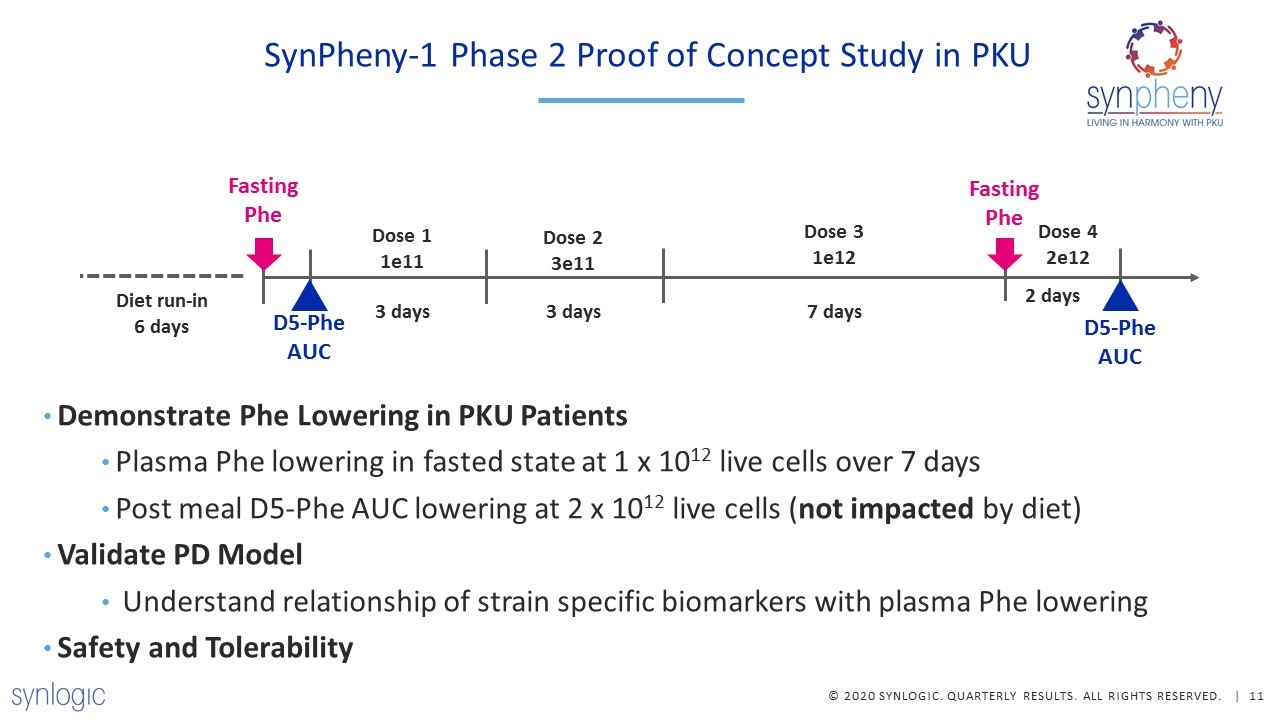
SynPheny-1 Phase 2 Proof of Concept Study in PKU Dose 2 3e11 3 days Dose 1 1e11 Dose 3 1e12 Dose 4 2e12 7 days 2 days Diet run-in 6 days D5-Phe AUC Fasting Phe Fasting Phe 3 days D5-Phe AUC Demonstrate Phe Lowering in PKU Patients Plasma Phe lowering in fasted state at 1 x 1012 live cells over 7 days Post meal D5-Phe AUC lowering at 2 x 1012 live cells (not impacted by diet) Validate PD Model Understand relationship of strain specific biomarkers with plasma Phe lowering Safety and Tolerability
Dose ramp to improve tolerability & compliance Patient-Centered Clinical Trial Design & Execution Directly informed by patient feedback on executing trials in the COVID era Flexible design allowing home-based or office-based visits Rigorous & personalized diet control to ensure consistent Phe intake, including 6-day run-in
Enteric Hyperoxaluria Enteric Hyperoxaluria results in significant kidney damage with no available treatment options SYNB8802 has the potential to meaningfully lower urinary oxalate levels SYNB8802 Phase 1 clinical study initiated ahead of schedule
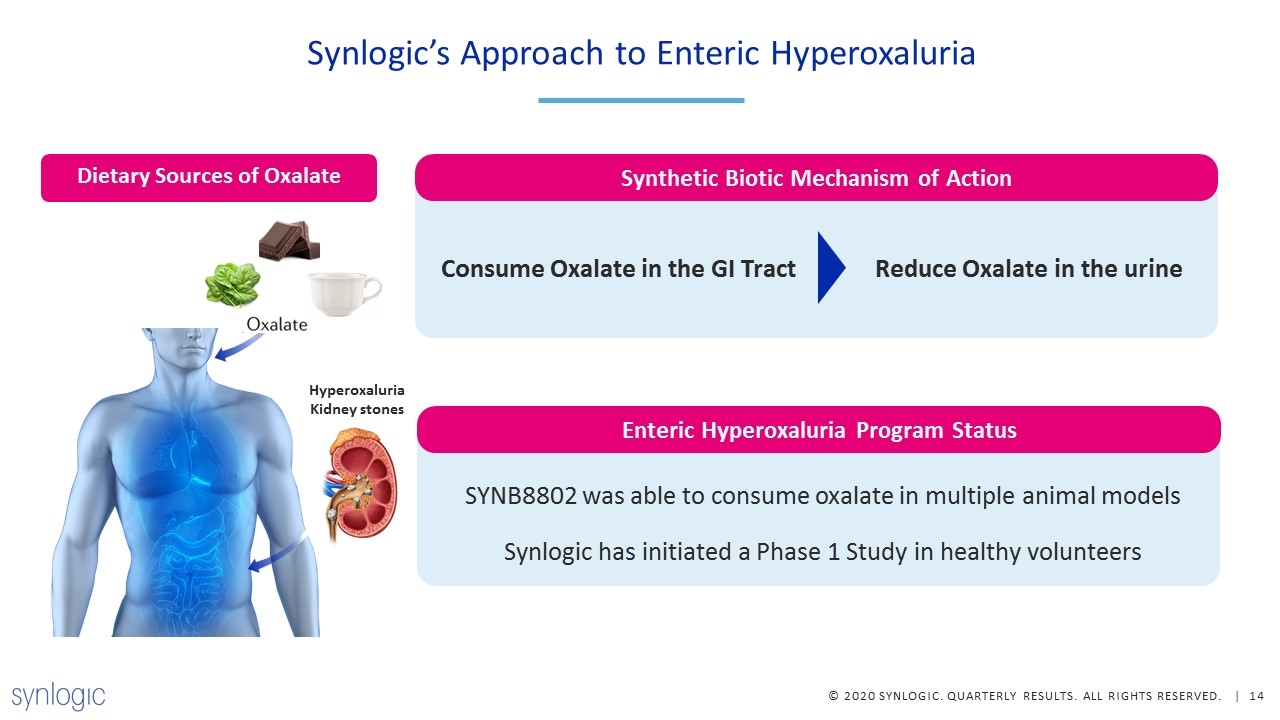
Synlogic’s Approach to Enteric Hyperoxaluria SYNB8802 was able to consume oxalate in multiple animal models Synlogic has initiated a Phase 1 Study in healthy volunteers Enteric Hyperoxaluria Program Status Synthetic Biotic Mechanism of Action Consume Oxalate in the GI Tract Reduce Oxalate in the urine Dietary Sources of Oxalate Hyperoxaluria Kidney stones
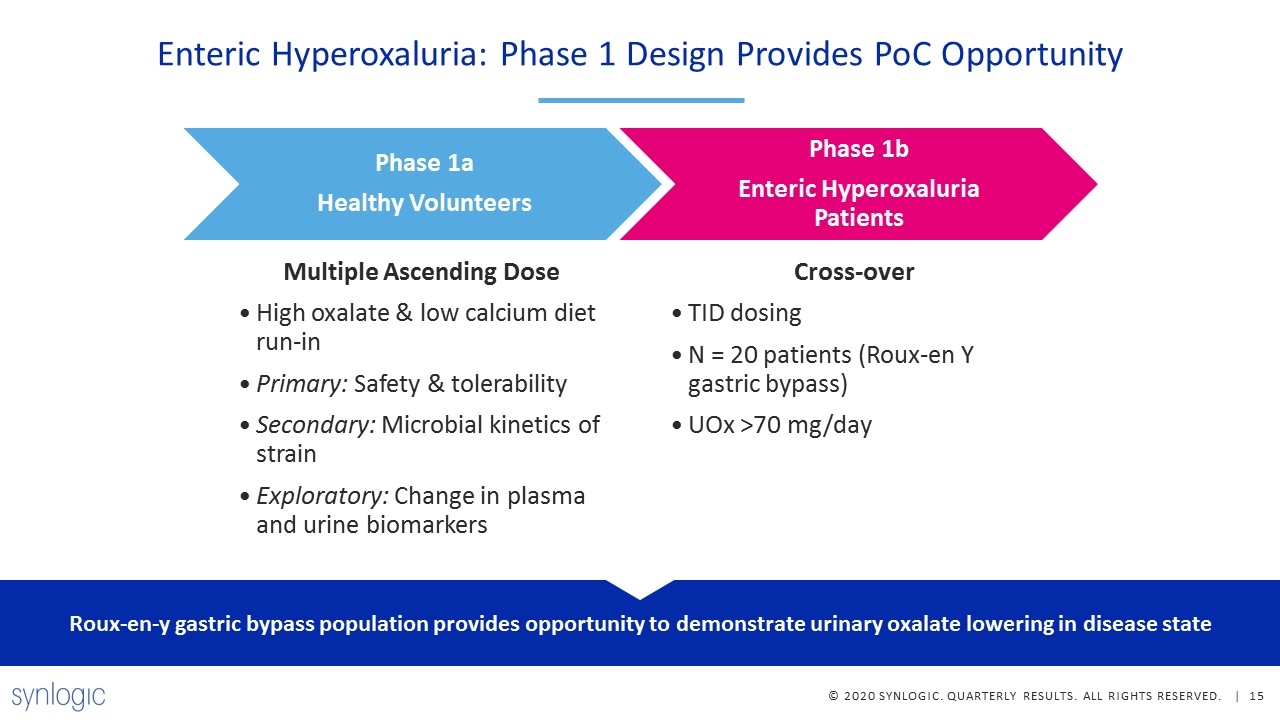
Enteric Hyperoxaluria: Phase 1 Design Provides PoC Opportunity Roux-en-y gastric bypass population provides opportunity to demonstrate urinary oxalate lowering in disease state Phase 1a Healthy Volunteers Multiple Ascending Dose High oxalate & low calcium diet run-in Primary: Safety & tolerability Secondary: Microbial kinetics of strain Exploratory: Change in plasma and urine biomarkers Phase 1b Enteric Hyperoxaluria Patients Cross-over TID dosing N = 20 patients (Roux-en Y gastric bypass) UOx >70 mg/day

© 2020 SYNLOGIC. QU©AR2TE1R9LYSYRNELSOUGLTICS.. AALLLL RRIIGGHHTTSS RREESSEERRVVEEDD.. || 1414 Concluding Remarks Dr. Aoife Brennan MD CHB President & CEO

Available For Questions Aoife Brennan, MD CHB President & CEO Richard Riese, MD PhD CMO Gregg Beloff, JD MBA Interim CFO Antoine Awad, COO David Hava, PhD CSO
