Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Axsome Therapeutics, Inc. | axsm-20201105xex99d1.htm |
| 8-K - 8-K - Axsome Therapeutics, Inc. | axsm-20201105x8k.htm |
Exhibit 99.2
| NASDAQ: AXSM 3Q 2020 Financial Results and Business Update November 5, 2020 © Axsome Therapeutics, Inc. |
| Certain information contained in this presentation may include “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. In particular, the Company’s statements regarding trends and potential future results are examples of such forward-looking statements. The forward-looking statements include risks and uncertainties, including, but not limited to, the success, timing and cost of our ongoing clinical trials and anticipated clinical trials for our current product candidates, including statements regarding the timing of initiation, pace of enrollment and completion of the trials (including our ability to fully fund our disclosed clinical trials, which assumes no material changes to our currently projected expenses), futility analyses and receipt of interim results, which are not necessarily indicative of the final results of our ongoing clinical trials and the number or type of studies or nature of results necessary to support the filing of a new drug application for any of our current product candidates; our ability to fund additional clinical trials to continue the advancement of our product candidates; the timing of and our ability to obtain and maintain U.S. Food and Drug Administration (“FDA”) or other regulatory authority approval of, or other action with respect to, our product candidates (including, but not limited to, FDA’s agreement with the Company’s discontinuation of the bupropion treatment arm of the ADVANCE-1 study in accordance with the independent data monitoring committee’s recommendations); the Company’s ability to obtain additional capital necessary to fund its operations; the Company’s ability to generate revenues in the future; the potential for the MOMENTUM clinical trial to provide a basis for approval of AXS-07 for the acute treatment of migraine in adults with or without aura, pursuant to our special protocol assessment; the potential for the ASCEND clinical trial, combined with the GEMINI clinical trial results, to provide a basis for approval of AXS-05 for the treatment of major depressive disorder and accelerate its development timeline and commercial path to patients; the Company’s ability to successfully defend its intellectual property or obtain the necessary licenses at a cost acceptable to the Company, if at all; the successful implementation of the Company’s research and development programs and collaborations; the enforceability of the Company’s license agreements; the acceptance by the market of the Company’s product candidates, if approved; the Company’s anticipated capital requirements, including the Company’s anticipated cash runway; and other factors, including general economic conditions and regulatory developments, not within the Company’s control. These factors could cause actual results and developments to be materially different from those expressed in or implied by such statements. Forward-looking statements are not guarantees of future performance, and actual results may differ materially from those projected. The forward-looking statements are made only as of the date of this presentation and the Company undertakes no obligation to publicly update such forward-looking statements to reflect subsequent events or circumstance. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation. In addition, these projections, assumptions and estimates are necessarily subject to a high degree of uncertainty and risk. 2 Forward-Looking Statements & Safe Harbor © Axsome Therapeutics, Inc. FLS |
| 3 © Axsome Therapeutics, Inc. Axsome Therapeutics 1Q 2020 Financial Results and Business Update Agenda Introduction Mark Jacobson, Chief Operating Officer Business Update Herriot Tabuteau, MD, Chief Executive Officer Commercialization Update Lori Englebert, Sr VP, Commercial & Business Development Financial Results Nick Pizzie, Chief Financial Officer Q&A Presenters Cedric O’Gorman, MD, Sr VP Clinical Development & Medical Affairs Concluding Remarks Herriot Tabuteau, MD, Chief Executive Officer |
| • COMET Phase 3 long-term safety trial of AXS-05 in MDD, and MOVEMENT Phase 3 long-term safety trial of AXS-07 in migraine completed • NDA submissions for AXS-05 in depression expected in January 2021, and for AXS-07 in migraine expected in 1Q 2021 • Launch readiness activities progressing with buildout of Digital-Centric Commercialization (DCC™) platform • Efficacy results from three Phase 2 open-label efficacy trials of AXS-05 in TRD, antidepressant unresponsive MDD, and suicidal ideation, on track for 4Q 2020 • Efficacy results from MOVEMENT Phase 3 open-label trial of AXS-07 in migraine expected in 4Q 2020 • Phase 3 trial of AXS-05 in Alzheimer’s disease agitation on track for initiation in 4Q 2020 • Phase 3 trial of AXS-12 in narcolepsy on track for initiation in 1Q 2021 • FDA meeting for AXS-14 in fibromyalgia scheduled for 1Q 2021 4 Axsome Therapeutics 3Q 2020 © Axsome Therapeutics, Inc. Corporate |
| Commercial Update 5 © Axsome Therapeutics, Inc. Lori Englebert SVP, COMMERCIAL & BUSINESS DEVELOPMENT AXSOME THERAPEUTICS, INC. |
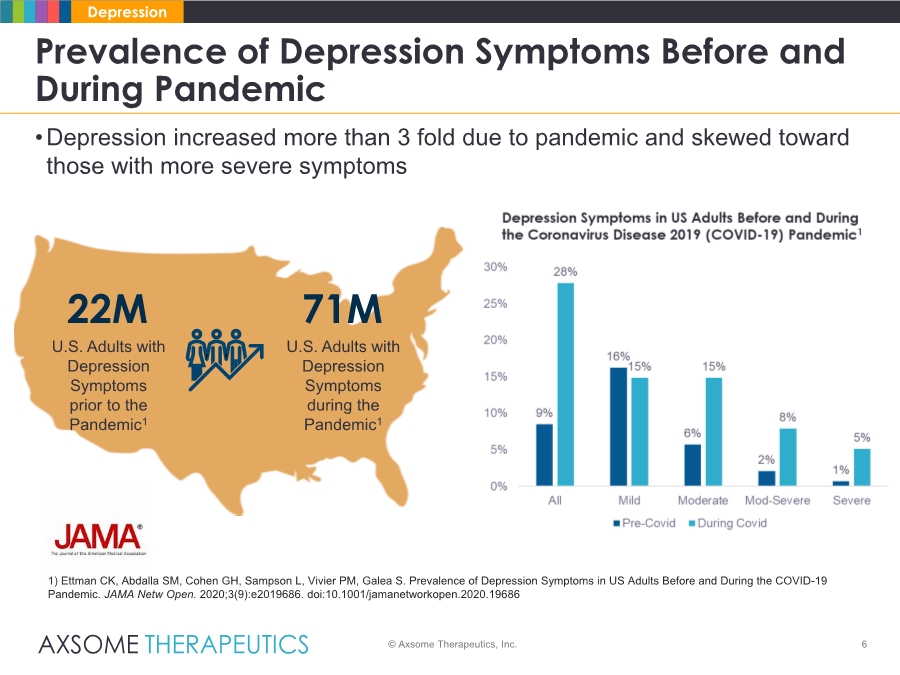
| •Depression increased more than 3 fold due to pandemic and skewed toward those with more severe symptoms 6 Prevalence of Depression Symptoms Before and During Pandemic © Axsome Therapeutics, Inc. Depression 1) Ettman CK, Abdalla SM, Cohen GH, Sampson L, Vivier PM, Galea S. Prevalence of Depression Symptoms in US Adults Before and During the COVID-19 Pandemic. JAMA Netw Open. 2020;3(9):e2019686. doi:10.1001/jamanetworkopen.2020.19686 22M U.S. Adults with Depression Symptoms prior to the Pandemic1 71M U.S. Adults with Depression Symptoms during the Pandemic1 |
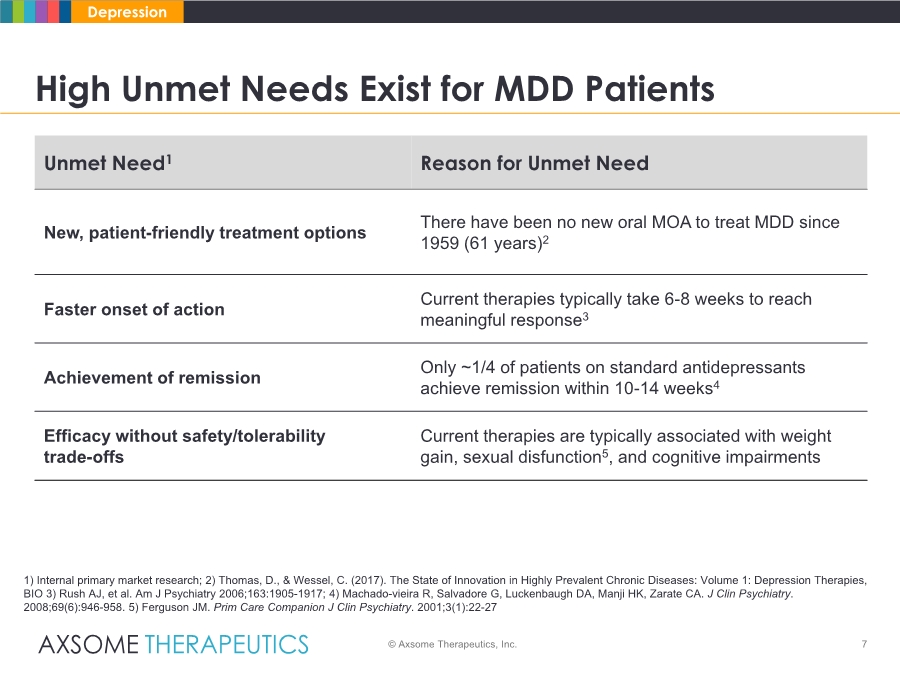
| 7 High Unmet Needs Exist for MDD Patients © Axsome Therapeutics, Inc. Depression 1) Internal primary market research; 2) Thomas, D., & Wessel, C. (2017). The State of Innovation in Highly Prevalent Chronic Diseases: Volume 1: Depression Therapies, BIO 3) Rush AJ, et al. Am J Psychiatry 2006;163:1905-1917; 4) Machado-vieira R, Salvadore G, Luckenbaugh DA, Manji HK, Zarate CA. J Clin Psychiatry. 2008;69(6):946-958. 5) Ferguson JM. Prim Care Companion J Clin Psychiatry. 2001;3(1):22-27 Unmet Need1 Reason for Unmet Need New, patient-friendly treatment options There have been no new oral MOA to treat MDD since 1959 (61 years)2 Faster onset of action Current therapies typically take 6-8 weeks to reach meaningful response3 Achievement of remission Only ~1/4 of patients on standard antidepressants achieve remission within 10-14 weeks4 Efficacy without safety/tolerability trade-offs Current therapies are typically associated with weight gain, sexual disfunction5, and cognitive impairments |
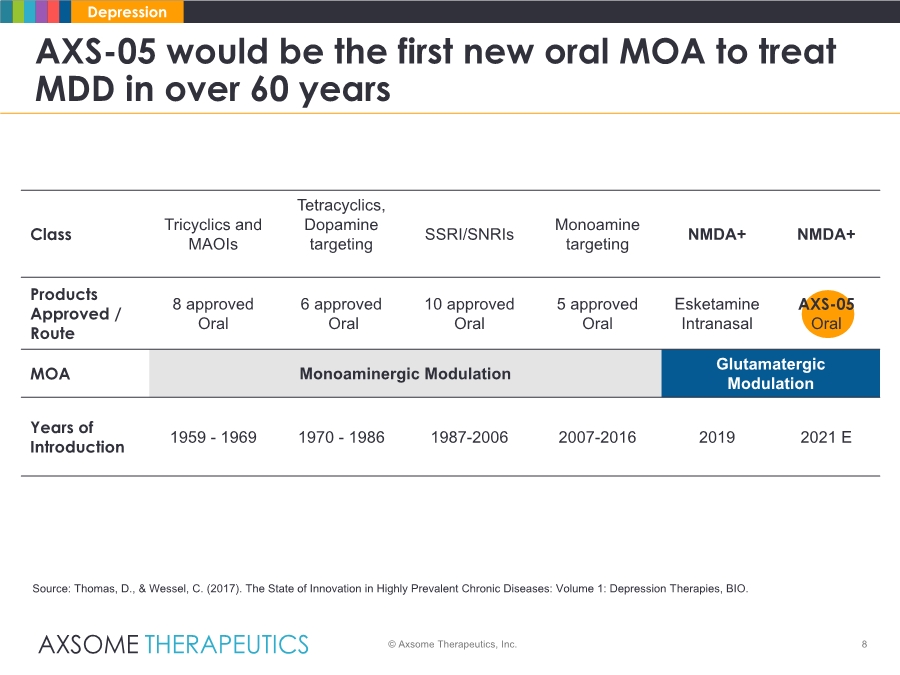
| 8 AXS-05 would be the first new oral MOA to treat MDD in over 60 years © Axsome Therapeutics, Inc. Depression Source: Thomas, D., & Wessel, C. (2017). The State of Innovation in Highly Prevalent Chronic Diseases: Volume 1: Depression Therapies, BIO. Class Tricyclics and MAOIs Tetracyclics, Dopamine targeting SSRI/SNRIs Monoamine targeting NMDA+ NMDA+ Products Approved / Route 8 approved Oral 6 approved Oral 10 approved Oral 5 approved Oral Esketamine Intranasal AXS-05 Oral MOA Monoaminergic Modulation Glutamatergic Modulation Years of Introduction 1959 - 1969 1970 - 1986 1987-2006 2007-2016 2019 2021 E |
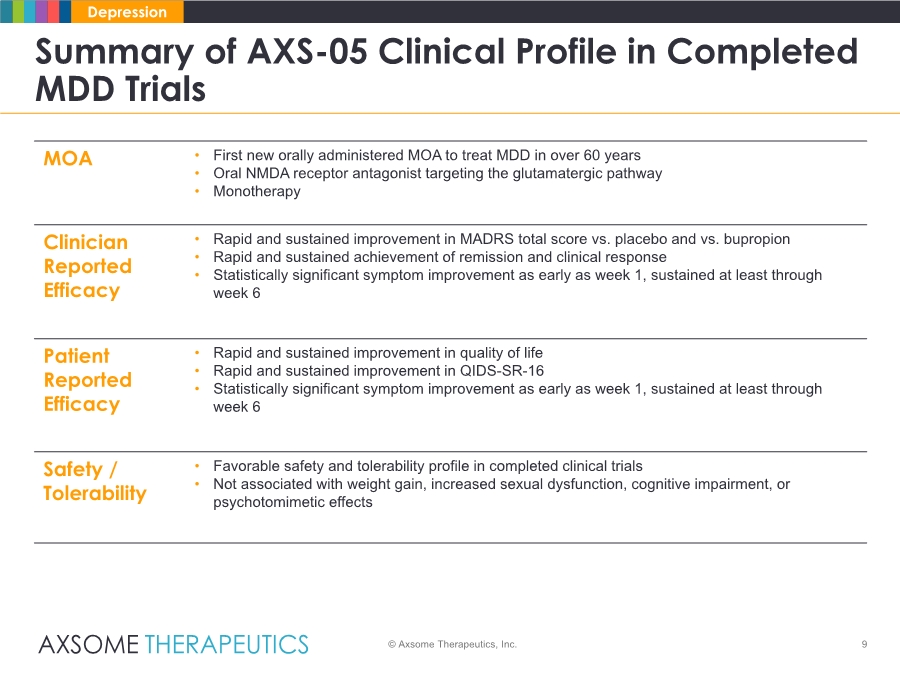
| 9 Summary of AXS-05 Clinical Profile in Completed MDD Trials © Axsome Therapeutics, Inc. Depression MOA • First new orally administered MOA to treat MDD in over 60 years • Oral NMDA receptor antagonist targeting the glutamatergic pathway • Monotherapy Clinician Reported Efficacy • Rapid and sustained improvement in MADRS total score vs. placebo and vs. bupropion • Rapid and sustained achievement of remission and clinical response • Statistically significant symptom improvement as early as week 1, sustained at least through week 6 Patient Reported Efficacy • Rapid and sustained improvement in quality of life • Rapid and sustained improvement in QIDS-SR-16 • Statistically significant symptom improvement as early as week 1, sustained at least through week 6 Safety / Tolerability • Favorable safety and tolerability profile in completed clinical trials • Not associated with weight gain, increased sexual dysfunction, cognitive impairment, or psychotomimetic effects |
| 10 Preparing For AXS-05 Launch: Digital Centric CommercializationTM (DCC) Platform © Axsome Therapeutics, Inc. DCC™ Using digital to redefine patient care by providing meaningful, optimized customer engagements |
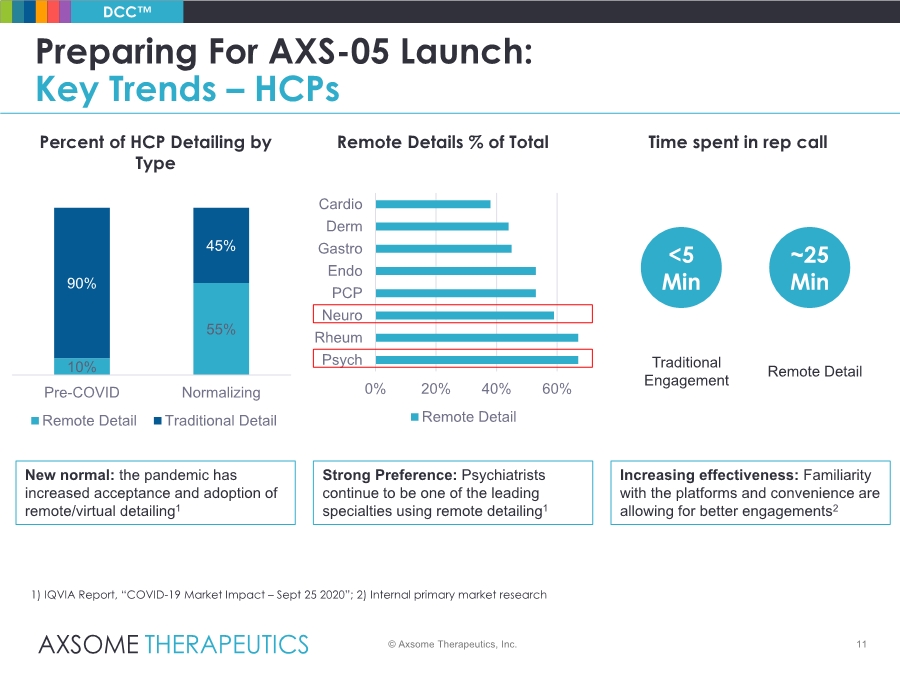
| 11 Preparing For AXS-05 Launch: Key Trends – HCPs © Axsome Therapeutics, Inc. DCC™ 1) IQVIA Report, “COVID-19 Market Impact – Sept 25 2020”; 2) Internal primary market research New normal: the pandemic has increased acceptance and adoption of remote/virtual detailing1 Strong Preference: Psychiatrists continue to be one of the leading specialties using remote detailing1 Increasing effectiveness: Familiarity with the platforms and convenience are allowing for better engagements2 Traditional Engagement Remote Detail <5 Min ~25 Min Time spent in rep call 10% 55% 90% 45% Pre-COVID Normalizing Remote Detail Traditional Detail 0% 20% 40% 60% Psych Rheum Neuro PCP Endo Gastro Derm Cardio Remote Detail Percent of HCP Detailing by Type Remote Details % of Total |
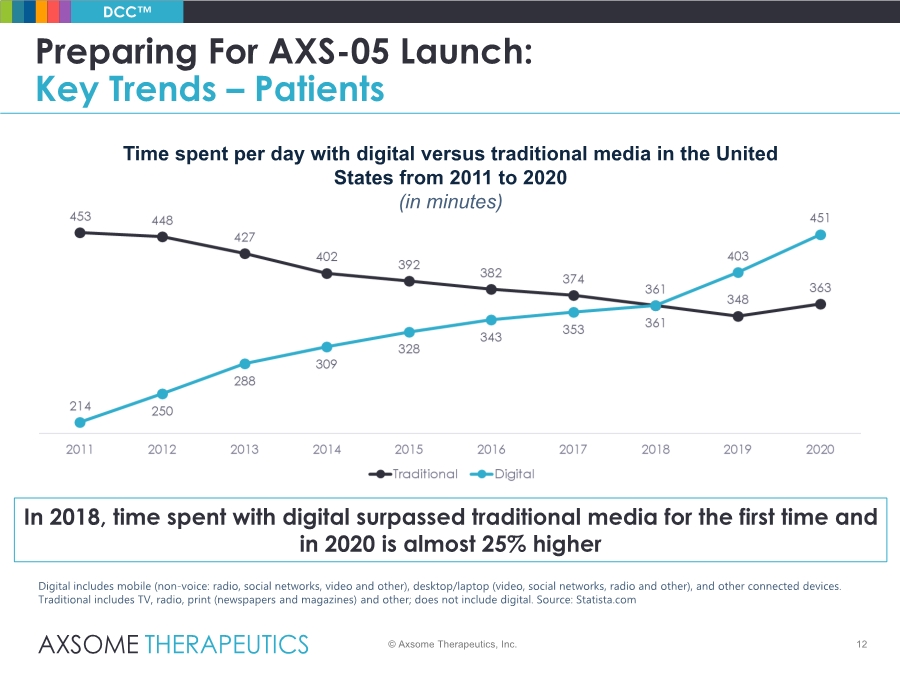
| 12 Preparing For AXS-05 Launch: Key Trends – Patients © Axsome Therapeutics, Inc. DCC™ Time spent per day with digital versus traditional media in the United States from 2011 to 2020 (in minutes) Digital includes mobile (non-voice: radio, social networks, video and other), desktop/laptop (video, social networks, radio and other), and other connected devices. Traditional includes TV, radio, print (newspapers and magazines) and other; does not include digital. Source: Statista.com In 2018, time spent with digital surpassed traditional media for the first time and in 2020 is almost 25% higher |
| 13 Preparing For AXS-05 Launch: Initial Engagement Launch Strategy © Axsome Therapeutics, Inc. DCC™ • Extensive use of remote detailing, with traditional as needed • Focused targeting of psychiatrists and mental health focused PCPs • Primarily digital engagement • Omnichannel approach • Focused targeting |
| 14 Preparing For AXS-05 Launch: Digital Centric CommercializationTM (DCC) Platform © Axsome Therapeutics, Inc. DCC™ Using digital to redefine patient care by providing meaningful, optimized customer engagements |
| Financial Update 15 © Axsome Therapeutics, Inc. Nick Pizzie CHIEF FINANCIAL OFFICER AXSOME THERAPEUTICS, INC. |
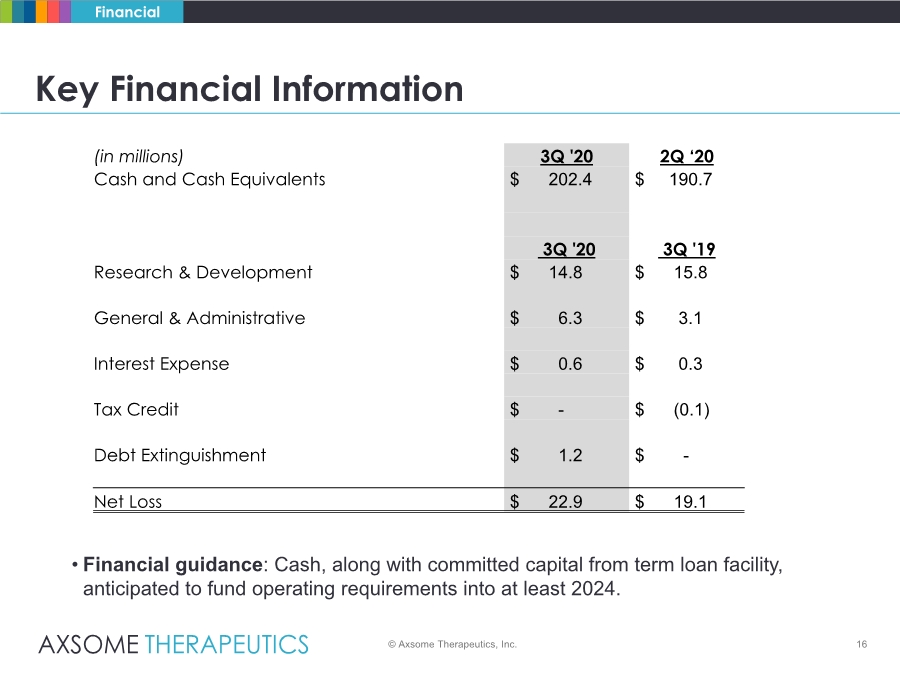
| 16 Key Financial Information © Axsome Therapeutics, Inc. Financial • Financial guidance: Cash, along with committed capital from term loan facility, anticipated to fund operating requirements into at least 2024. (in millions) 3Q '20 2Q ‘20 Cash and Cash Equivalents $ 202.4 $ 190.7 3Q '20 3Q '19 Research & Development $ 14.8 $ 15.8 General & Administrative $ 6.3 $ 3.1 Interest Expense $ 0.6 $ 0.3 Tax Credit $ - $ (0.1) Debt Extinguishment $ 1.2 $ - Net Loss $ 22.9 $ 19.1 |
| 17 Q&A © Axsome Therapeutics, Inc. |
| Concluding Remarks 18 © Axsome Therapeutics, Inc. Herriot Tabuteau, MD CHIEF EXECUTIVE OFFICER AXSOME THERAPEUTICS, INC. |
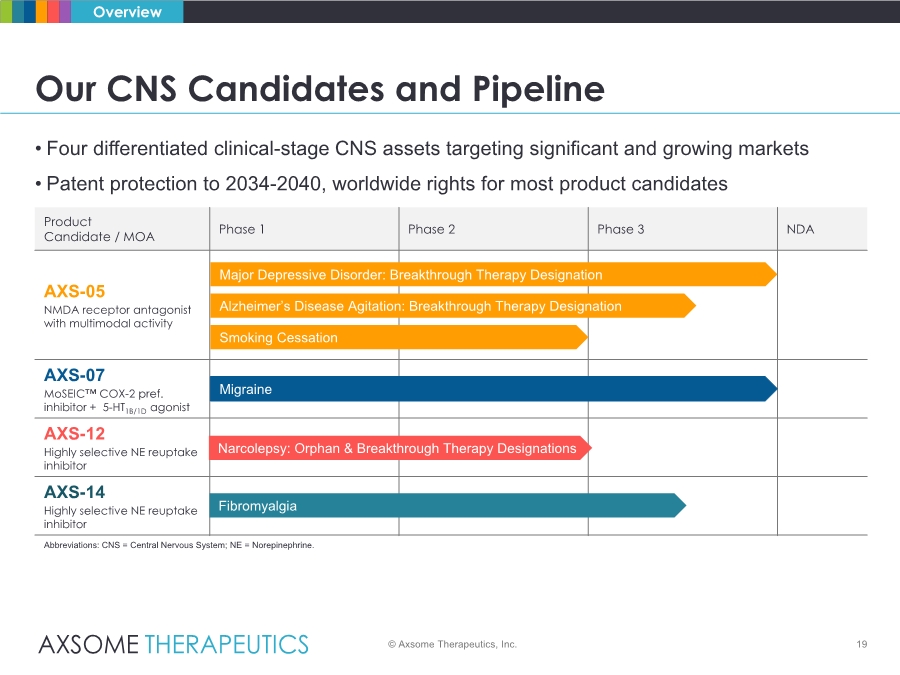
| • Four differentiated clinical-stage CNS assets targeting significant and growing markets • Patent protection to 2034-2040, worldwide rights for most product candidates Product Candidate / MOA Phase 1 Phase 2 Phase 3 NDA AXS-05 NMDA receptor antagonist with multimodal activity AXS-07 MoSEIC™ COX-2 pref. inhibitor + 5-HT1B/1D agonist AXS-12 Highly selective NE reuptake inhibitor AXS-14 Highly selective NE reuptake inhibitor Abbreviations: CNS = Central Nervous System; NE = Norepinephrine. Migraine Major Depressive Disorder: Breakthrough Therapy Designation Alzheimer’s Disease Agitation: Breakthrough Therapy Designation Smoking Cessation Narcolepsy: Orphan & Breakthrough Therapy Designations 19 Our CNS Candidates and Pipeline Overview © Axsome Therapeutics, Inc. Fibromyalgia |
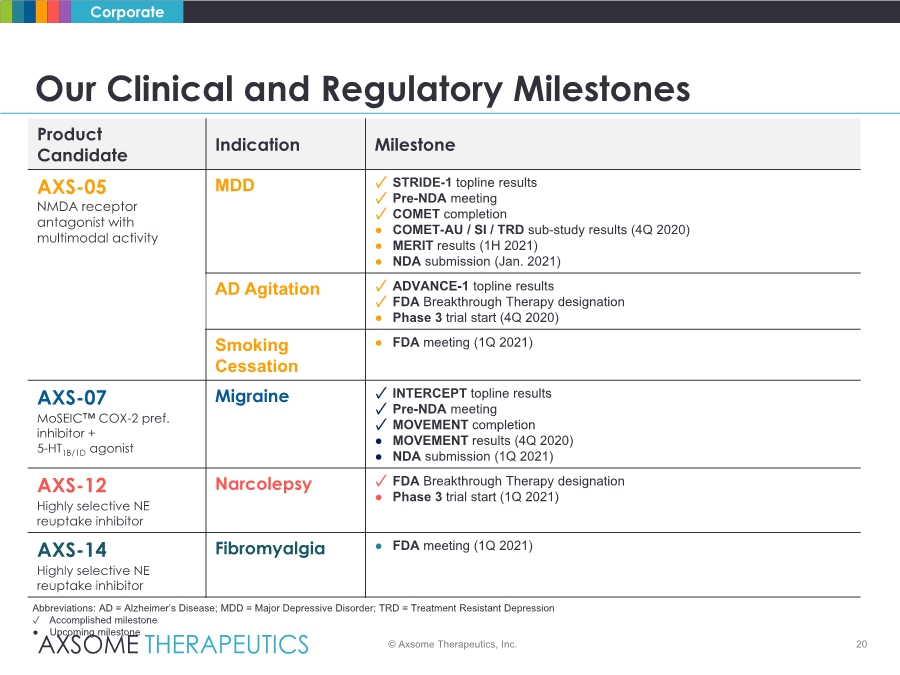
| 20 Our Clinical and Regulatory Milestones Corporate Product Candidate Indication Milestone AXS-05 NMDA receptor antagonist with multimodal activity MDD ✓ STRIDE-1 topline results ✓ Pre-NDA meeting ✓ COMET completion ● COMET-AU / SI / TRD sub-study results (4Q 2020) ● MERIT results (1H 2021) ● NDA submission (Jan. 2021) AD Agitation ✓ ADVANCE-1 topline results ✓ FDA Breakthrough Therapy designation ● Phase 3 trial start (4Q 2020) Smoking Cessation ● FDA meeting (1Q 2021) AXS-07 MoSEIC™ COX-2 pref. inhibitor + 5-HT1B/1D agonist Migraine ✓ INTERCEPT topline results ✓ Pre-NDA meeting ✓ MOVEMENT completion ● MOVEMENT results (4Q 2020) ● NDA submission (1Q 2021) AXS-12 Highly selective NE reuptake inhibitor Narcolepsy ✓ FDA Breakthrough Therapy designation ● Phase 3 trial start (1Q 2021) AXS-14 Highly selective NE reuptake inhibitor Fibromyalgia ● FDA meeting (1Q 2021) Abbreviations: AD = Alzheimer’s Disease; MDD = Major Depressive Disorder; TRD = Treatment Resistant Depression ✓ Accomplished milestone ● Upcoming milestone © Axsome Therapeutics, Inc. |
| For more information, please contact Mark Jacobson Chief Operating Officer 212-332-3243 mjacobson@Axsome.com axsome.com |