Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Spero Therapeutics, Inc. | d54756dex992.htm |
| 8-K - 8-K - Spero Therapeutics, Inc. | d54756d8k.htm |

ADAPT-PO Phase 3 Topline Data Conference Call September 8, 2020 Exhibit 99.1

Forward-looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, the initiation, timing and submission to the U.S. Food and Drug Administration (FDA) of a New Drug Application (NDA) for tebipenem HBr and the potential approval of tebipenem HBr by the FDA; future commercialization, the potential number of patients who could be treated by tebipenem HBr and market demand for tebipenem HBr generally; expected broad access across payer channels for tebipenem HBr; the expected pricing of tebipenem HBr and the anticipated shift from intravenous to oral administration. In some cases, forward-looking statements can be identified by terms such as “may,” “will,” “should,” “expect,” “plan,” “aim,” “anticipate,” “could,” “intent,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions. All statements other than statements of historical facts contained in this presentation are forward-looking statements. The Company may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various factors, including: the Company’s ability to timely complete related Phase 1 trials for NDA submission, taking into account the possible effects of the COVID-19 pandemic; the Company’s need for additional funding; the lengthy, expensive, and uncertain process of clinical drug development; the Company’s reliance on third parties to manufacture, develop, and commercialize its product candidates, if approved; the ability to develop and commercialize the Company’s product candidates, if approved; the potential impact of the COVID-19 pandemic; the Company’s ability to retain key personnel and to manage its growth; and other factors discussed in the “Risk Factors” section of the Company’s periodic reports filed with the U.S. Securities and Exchange Commission (SEC), and risks described in other filings the Company may make with the SEC in the future. The forward-looking statements included in this presentation represent the Company’s views as of the date of this presentation. The Company anticipates that subsequent events and developments will cause its views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this presentation.

ADAPT-PO Phase 3 Results Tebipenem HBr Building Spero as the leader in the field of antibiotic drug development Trial met primary endpoint of non-inferiority of oral tebipenem HBr versus IV ertapenem for the treatment of complicated urinary tract infections and acute pyelonephritis
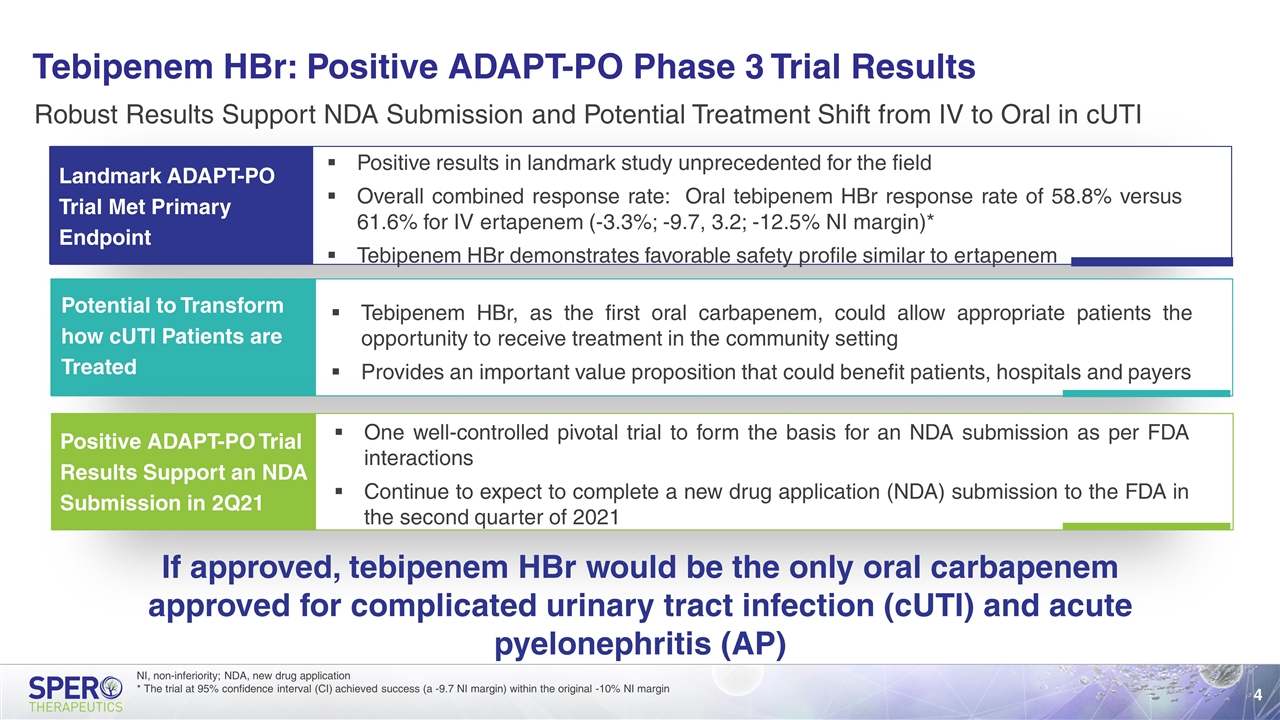
Tebipenem HBr: Positive ADAPT-PO Phase 3 Trial Results Robust Results Support NDA Submission and Potential Treatment Shift from IV to Oral in cUTI Landmark ADAPT-PO Trial Met Primary Endpoint Positive ADAPT-PO Trial Results Support an NDA Submission in 2Q21 One well-controlled pivotal trial to form the basis for an NDA submission as per FDA interactions Continue to expect to complete a new drug application (NDA) submission to the FDA in the second quarter of 2021 If approved, tebipenem HBr would be the only oral carbapenem approved for complicated urinary tract infection (cUTI) and acute pyelonephritis (AP) Potential to Transform how cUTI Patients are Treated Positive results in landmark study unprecedented for the field Overall combined response rate: Oral tebipenem HBr response rate of 58.8% versus 61.6% for IV ertapenem (-3.3%; -9.7, 3.2; -12.5% NI margin)* Tebipenem HBr demonstrates favorable safety profile similar to ertapenem Tebipenem HBr, as the first oral carbapenem, could allow appropriate patients the opportunity to receive treatment in the community setting Provides an important value proposition that could benefit patients, hospitals and payers NI, non-inferiority; NDA, new drug application * The trial at 95% confidence interval (CI) achieved success (a -9.7 NI margin) within the original -10% NI margin
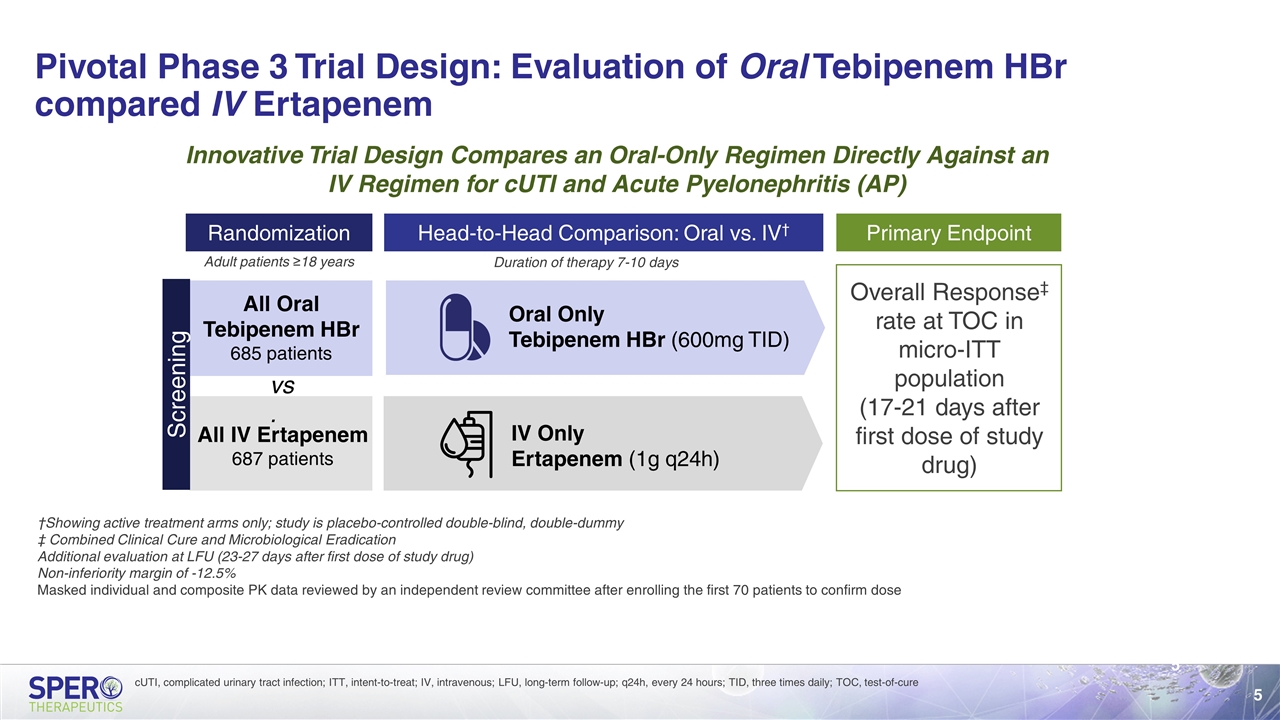
Pivotal Phase 3 Trial Design: Evaluation of Oral Tebipenem HBr compared IV Ertapenem Randomization All Oral Tebipenem HBr 685 patients All IV Ertapenem 687 patients Primary Endpoint Head-to-Head Comparison: Oral vs. IV† Oral Only Tebipenem HBr (600mg TID) IV Only Ertapenem (1g q24h) Duration of therapy 7-10 days Adult patients ≥18 years Innovative Trial Design Compares an Oral-Only Regimen Directly Against an IV Regimen for cUTI and Acute Pyelonephritis (AP) †Showing active treatment arms only; study is placebo-controlled double-blind, double-dummy ‡ Combined Clinical Cure and Microbiological Eradication Additional evaluation at LFU (23-27 days after first dose of study drug) Non-inferiority margin of -12.5% Masked individual and composite PK data reviewed by an independent review committee after enrolling the first 70 patients to confirm dose Screening Overall Response‡ rate at TOC in micro-ITT population (17-21 days after first dose of study drug) vs. cUTI, complicated urinary tract infection; ITT, intent-to-treat; IV, intravenous; LFU, long-term follow-up; q24h, every 24 hours; TID, three times daily; TOC, test-of-cure
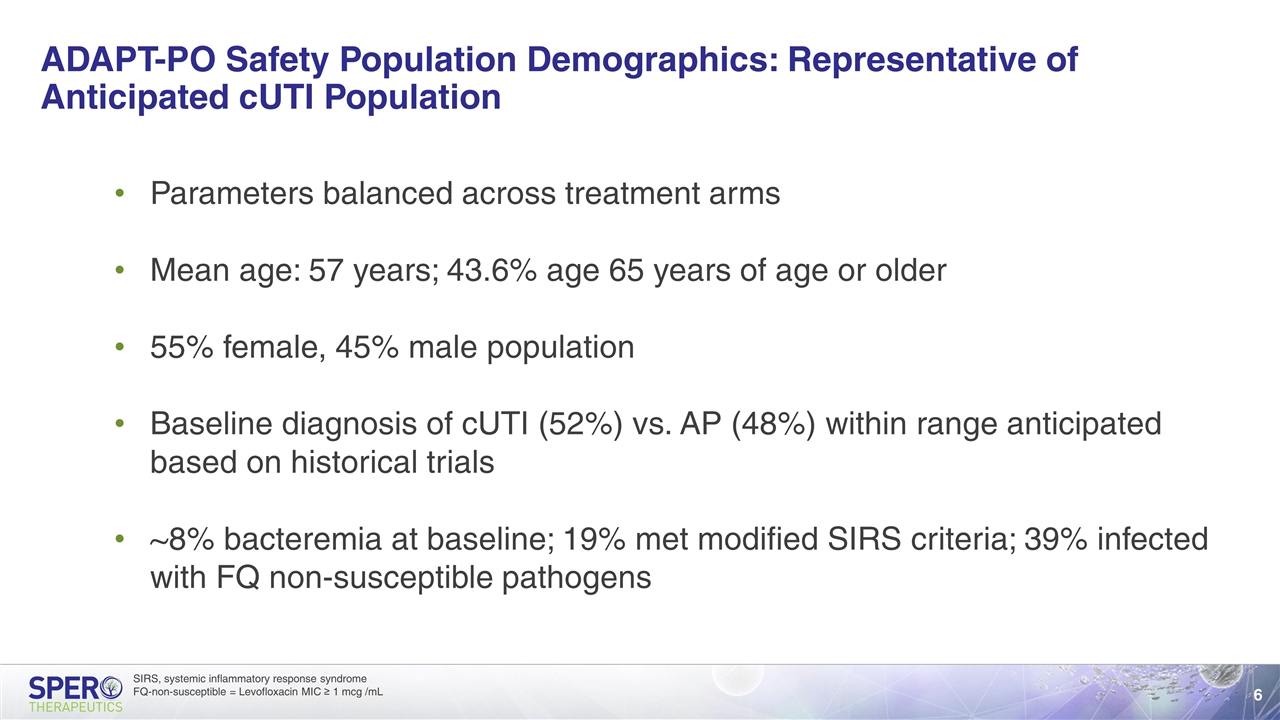
ADAPT-PO Safety Population Demographics: Representative of Anticipated cUTI Population Parameters balanced across treatment arms Mean age: 57 years; 43.6% age 65 years of age or older 55% female, 45% male population Baseline diagnosis of cUTI (52%) vs. AP (48%) within range anticipated based on historical trials ~8% bacteremia at baseline; 19% met modified SIRS criteria; 39% infected with FQ non-susceptible pathogens SIRS, systemic inflammatory response syndrome FQ-non-susceptible = Levofloxacin MIC ≥ 1 mcg /mL
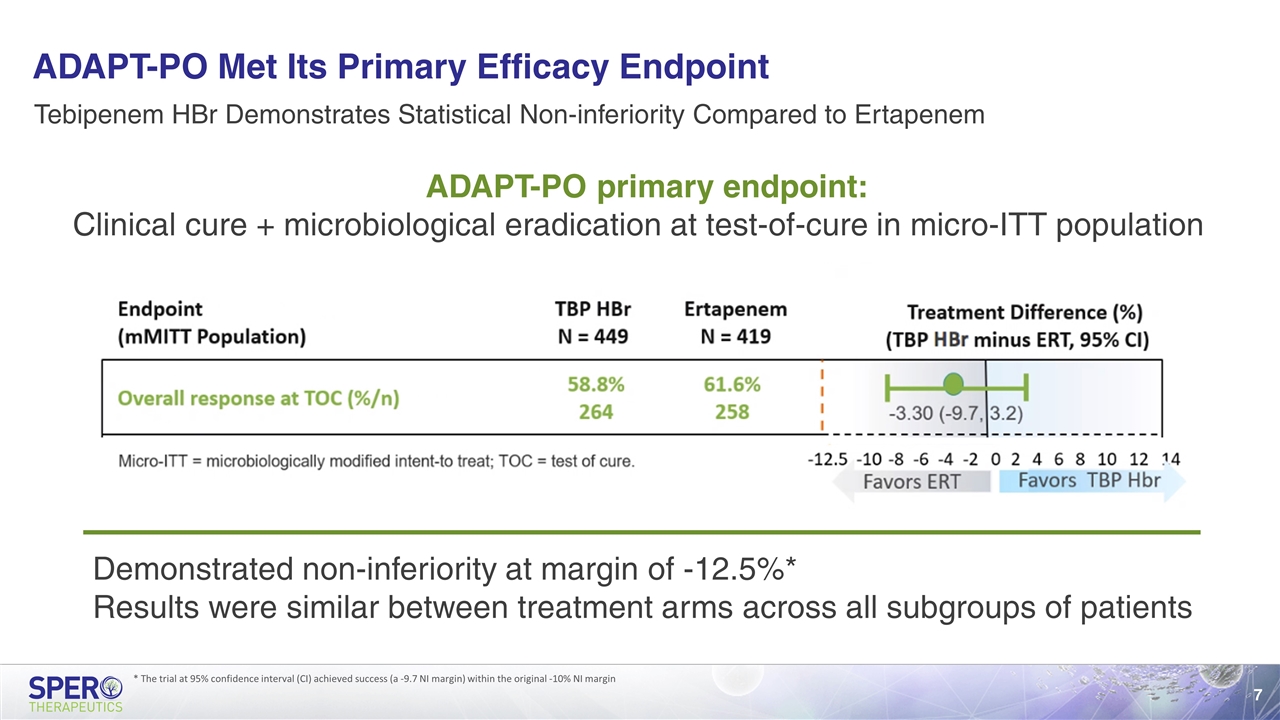
ADAPT-PO Met Its Primary Efficacy Endpoint ADAPT-PO primary endpoint: Clinical cure + microbiological eradication at test-of-cure in micro-ITT population Tebipenem HBr Demonstrates Statistical Non-inferiority Compared to Ertapenem Demonstrated non-inferiority at margin of -12.5%* Results were similar between treatment arms across all subgroups of patients * The trial at 95% confidence interval (CI) achieved success (a -9.7 NI margin) within the original -10% NI margin
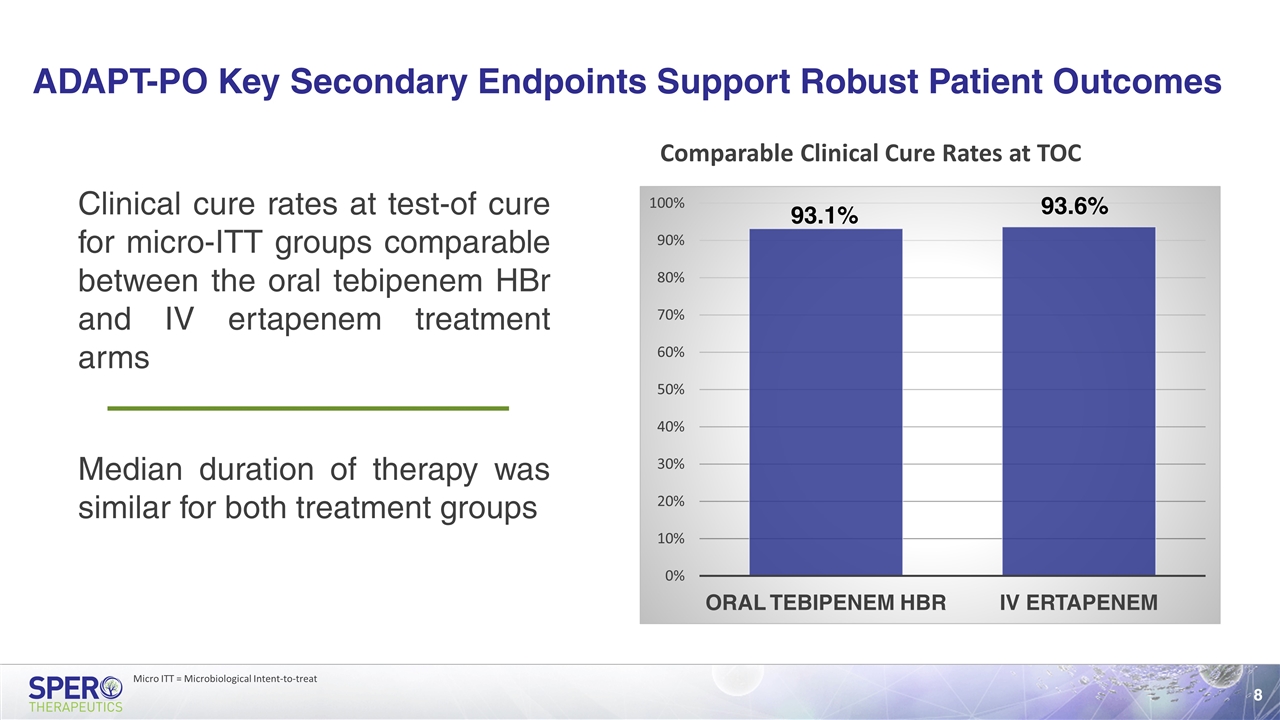
ADAPT-PO Key Secondary Endpoints Support Robust Patient Outcomes Clinical cure rates at test-of cure for micro-ITT groups comparable between the oral tebipenem HBr and IV ertapenem treatment arms Comparable Clinical Cure Rates at TOC Median duration of therapy was similar for both treatment groups Micro ITT = Microbiological Intent-to-treat
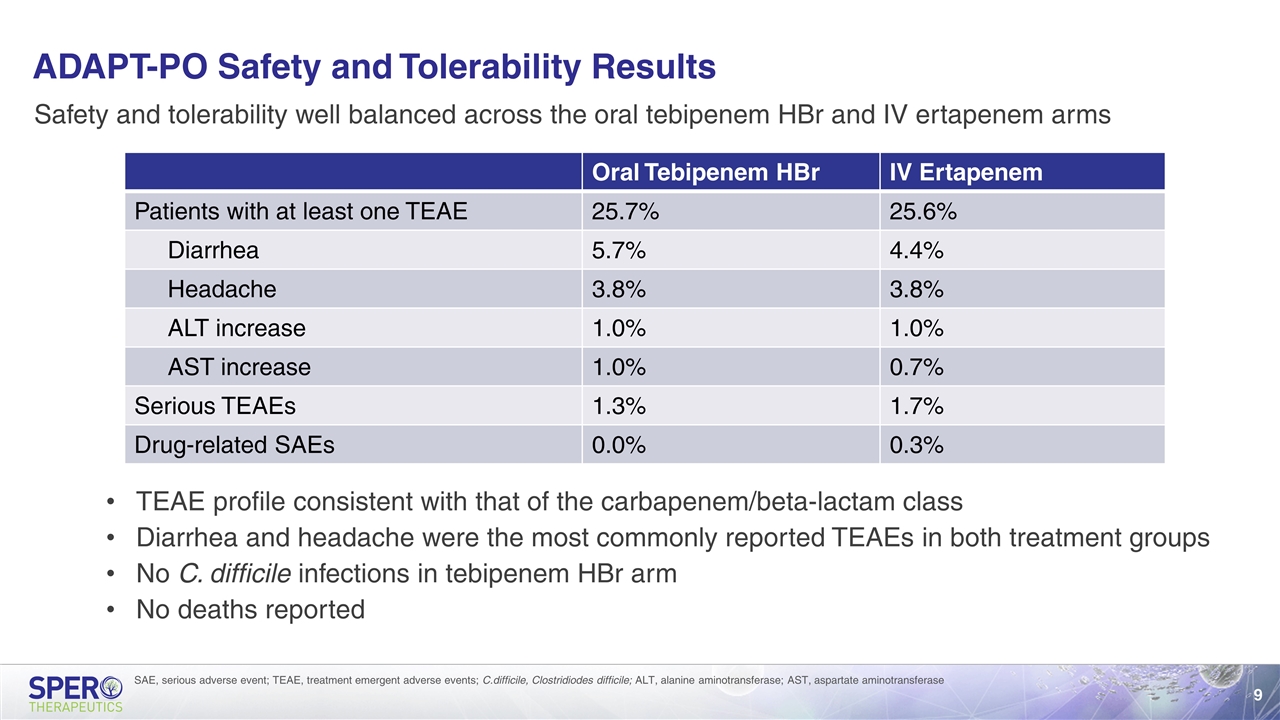
ADAPT-PO Safety and Tolerability Results Safety and tolerability well balanced across the oral tebipenem HBr and IV ertapenem arms TEAE profile consistent with that of the carbapenem/beta-lactam class Diarrhea and headache were the most commonly reported TEAEs in both treatment groups No C. difficile infections in tebipenem HBr arm No deaths reported SAE, serious adverse event; TEAE, treatment emergent adverse events; C.difficile, Clostridiodes difficile; ALT, alanine aminotransferase; AST, aspartate aminotransferase Oral Tebipenem HBr IV Ertapenem Patients with at least one TEAE 25.7% 25.6% Diarrhea 5.7% 4.4% Headache 3.8% 3.8% ALT increase 1.0% 1.0% AST increase 1.0% 0.7% Serious TEAEs 1.3% 1.7% Drug-related SAEs 0.0% 0.3%
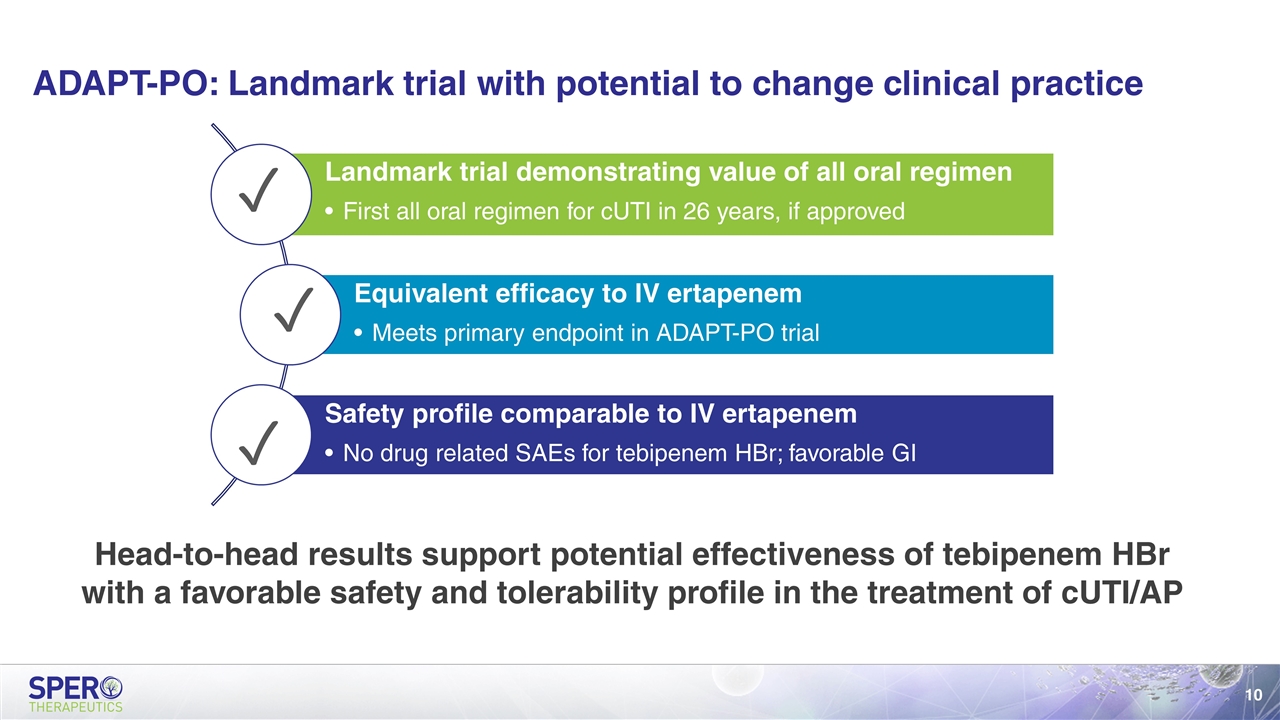
ADAPT-PO: Landmark trial with potential to change clinical practice Head-to-head results support potential effectiveness of tebipenem HBr with a favorable safety and tolerability profile in the treatment of cUTI/AP ✓ ✓ ✓ Landmark trial demonstrating value of all oral regimen First all oral regimen for cUTI in 26 years, if approved Equivalent efficacy to IV ertapenem Meets primary endpoint in ADAPT-PO trial Safety profile comparable to IV ertapenem No drug related SAEs for tebipenem HBr; favorable GI
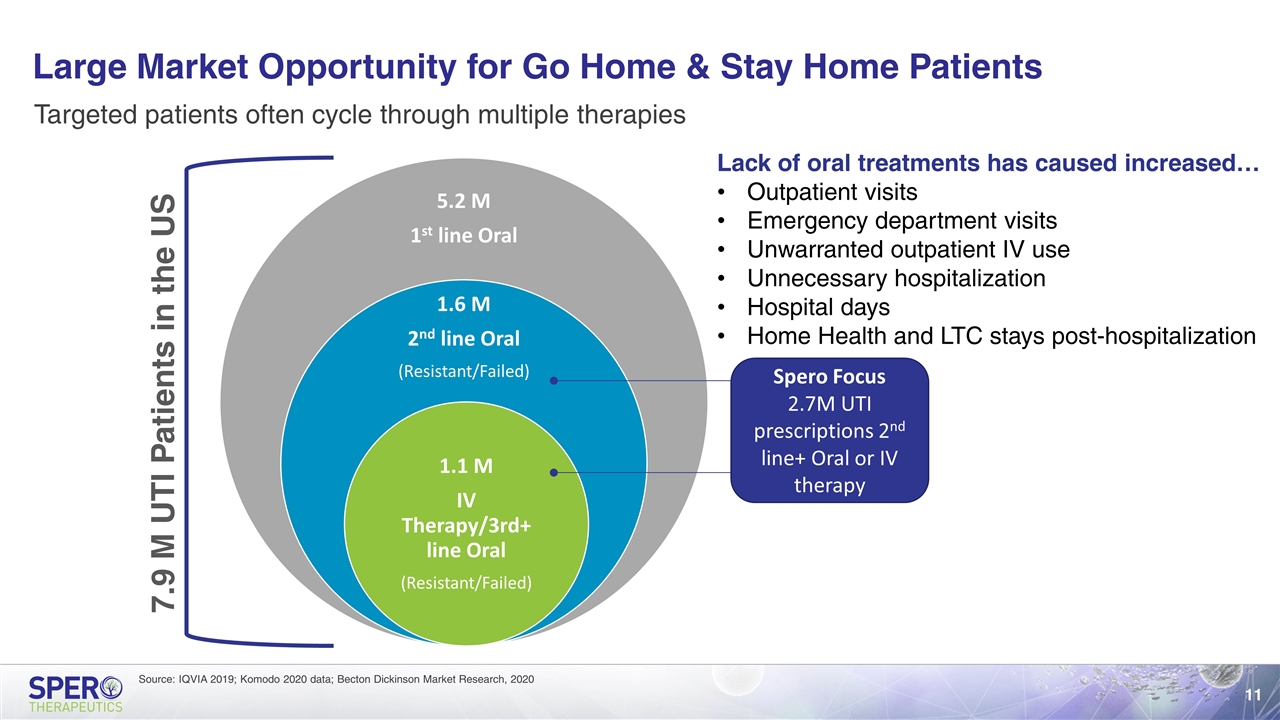
Large Market Opportunity for Go Home & Stay Home Patients Source: IQVIA 2019; Komodo 2020 data; Becton Dickinson Market Research, 2020 Targeted patients often cycle through multiple therapies 7.9 M UTI Patients in the US Spero Focus 2.7M UTI prescriptions 2nd line+ Oral or IV therapy Lack of oral treatments has caused increased… Outpatient visits Emergency department visits Unwarranted outpatient IV use Unnecessary hospitalization Hospital days Home Health and LTC stays post-hospitalization 5.2 M 1 st line Oral 1.6 M 2 nd line Oral (Resistant/Failed) 1.1 M IV Therapy/3rd+ line Oral (Resistant/Failed)
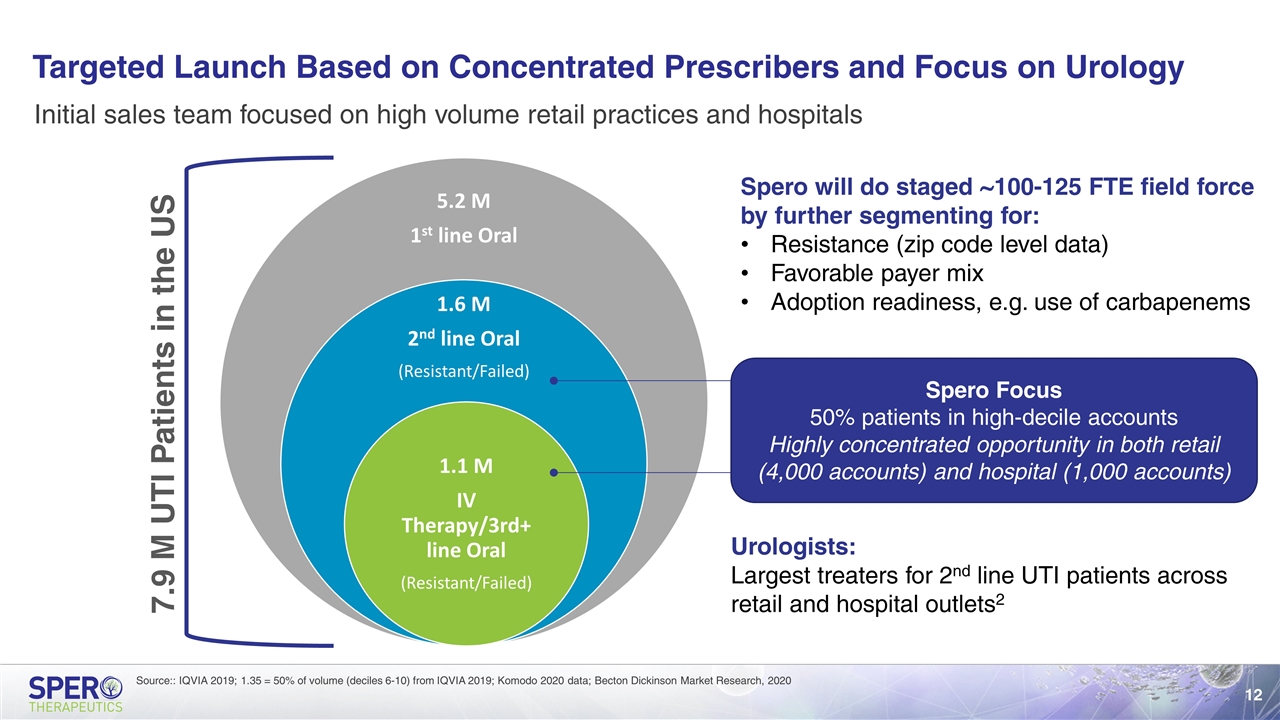
Targeted Launch Based on Concentrated Prescribers and Focus on Urology Source:: IQVIA 2019; 1.35 = 50% of volume (deciles 6-10) from IQVIA 2019; Komodo 2020 data; Becton Dickinson Market Research, 2020 Initial sales team focused on high volume retail practices and hospitals 7.9 M UTI Patients in the US Spero Focus 50% patients in high-decile accounts Highly concentrated opportunity in both retail (4,000 accounts) and hospital (1,000 accounts) Spero will do staged ~100-125 FTE field force by further segmenting for: Resistance (zip code level data) Favorable payer mix Adoption readiness, e.g. use of carbapenems Urologists: Largest treaters for 2nd line UTI patients across retail and hospital outlets2 5.2 M 1 st line Oral 1.6 M 2 nd line Oral (Resistant/Failed) 1.1 M IV Therapy/3rd+ line Oral (Resistant/Failed)
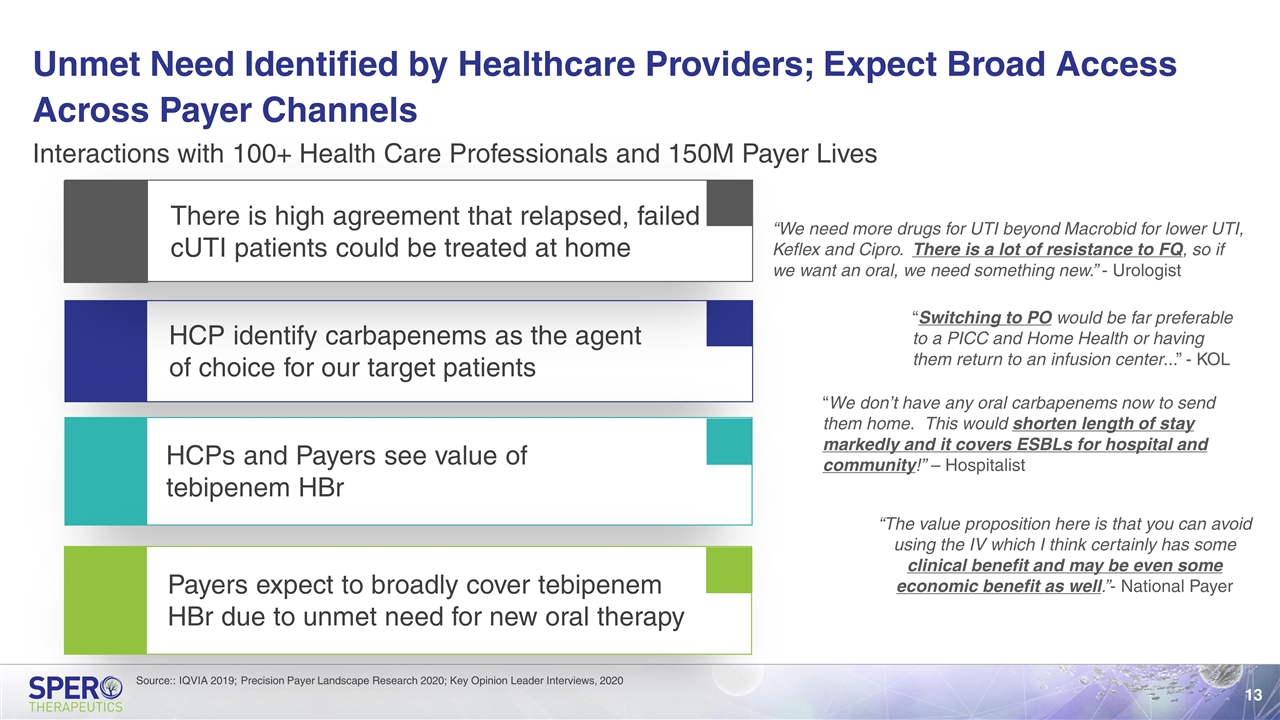
Unmet Need Identified by Healthcare Providers; Expect Broad Access Across Payer Channels HCP identify carbapenems as the agent of choice for our target patients HCPs and Payers see value of tebipenem HBr Interactions with 100+ Health Care Professionals and 150M Payer Lives There is high agreement that relapsed, failed cUTI patients could be treated at home Payers expect to broadly cover tebipenem HBr due to unmet need for new oral therapy “We need more drugs for UTI beyond Macrobid for lower UTI, Keflex and Cipro. There is a lot of resistance to FQ, so if we want an oral, we need something new.” - Urologist “Switching to PO would be far preferable to a PICC and Home Health or having them return to an infusion center...” - KOL “We don’t have any oral carbapenems now to send them home. This would shorten length of stay markedly and it covers ESBLs for hospital and community!” – Hospitalist “The value proposition here is that you can avoid using the IV which I think certainly has some clinical benefit and may be even some economic benefit as well.”- National Payer Source:: IQVIA 2019; Precision Payer Landscape Research 2020; Key Opinion Leader Interviews, 2020
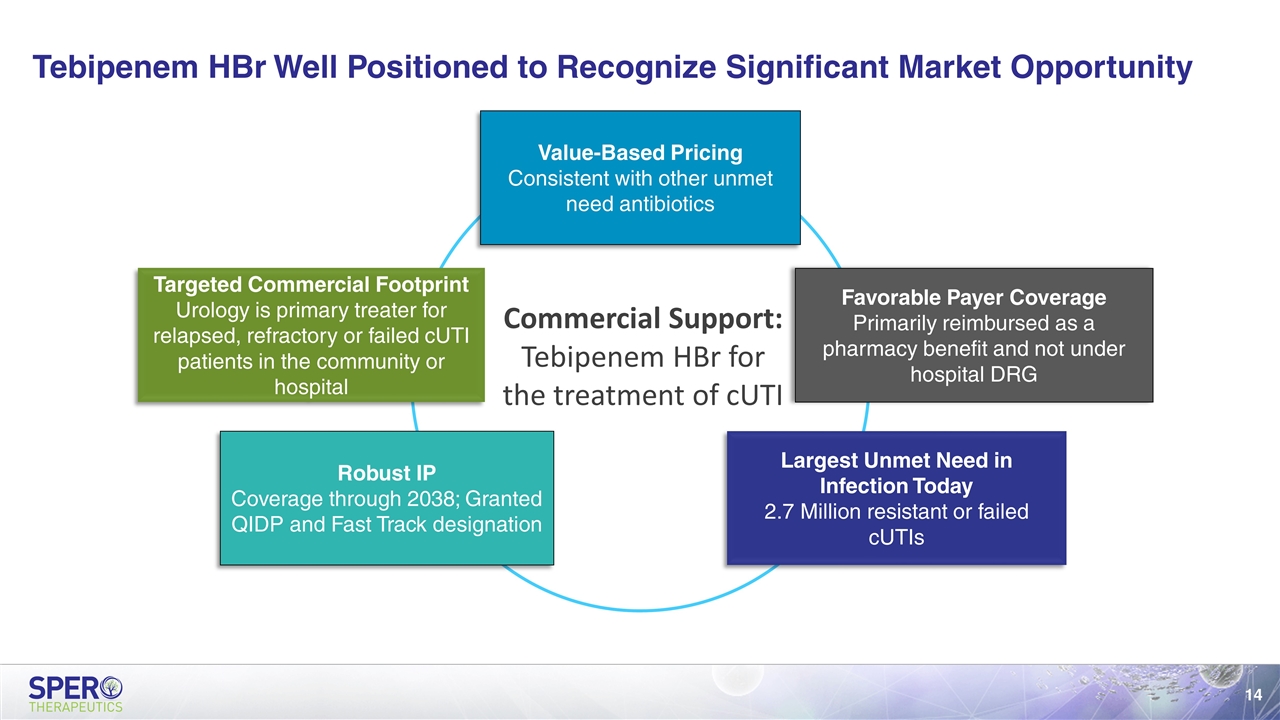
Tebipenem HBr Well Positioned to Recognize Significant Market Opportunity Value-Based Pricing Consistent with other unmet need antibiotics Favorable Payer Coverage Primarily reimbursed as a pharmacy benefit and not under hospital DRG Largest Unmet Need in Infection Today 2.7 Million resistant or failed cUTIs Robust IP Coverage through 2038; Granted QIDP and Fast Track designation Targeted Commercial Footprint Urology is primary treater for relapsed, refractory or failed cUTI patients in the community or hospital Commercial Support: Tebipenem HBr for the treatment of cUTI

Next Steps for Tebipenem HBr Complete NDA package - Meeting with FDA and completing Phase 1 trials Exploring lifecycle management opportunities – Microbiological surveillance and clinical studies Manufacturing readiness – Process validation and launch planning Launch readiness – Market development work, pricing research, distribution strategy, key hires
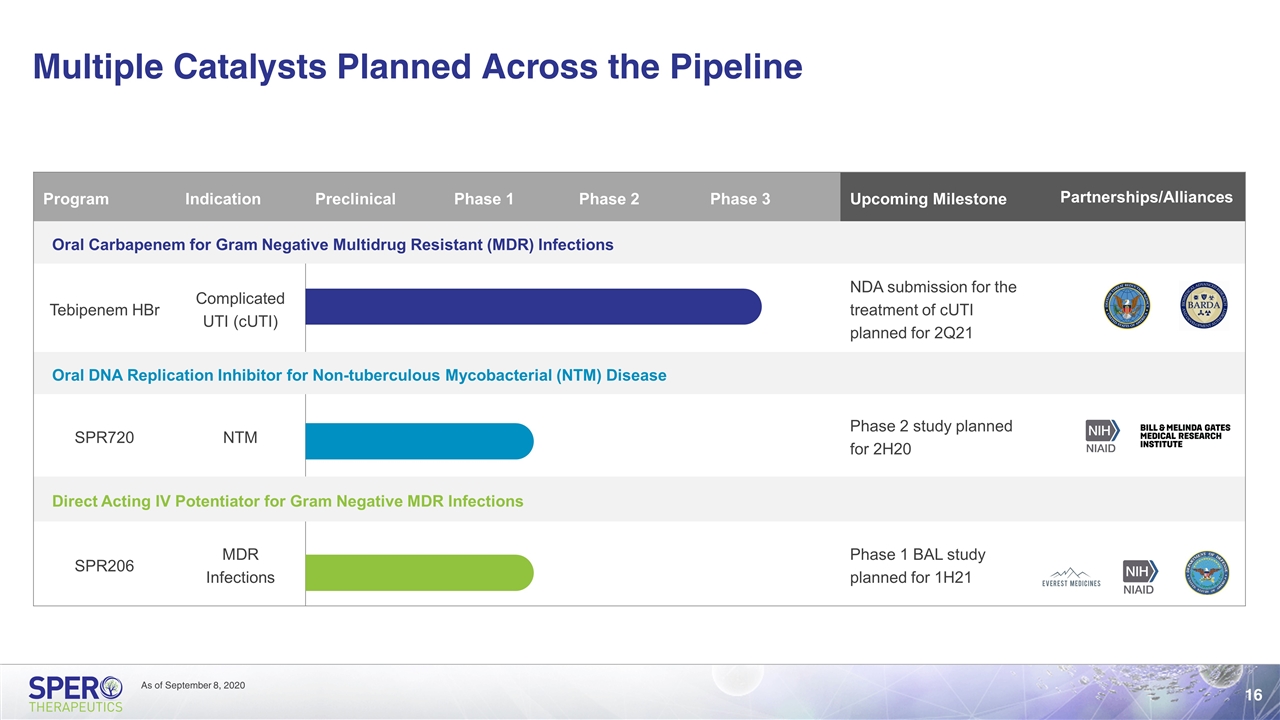
Multiple Catalysts Planned Across the Pipeline Program Indication Preclinical Phase 1 Phase 2 Phase 3 Upcoming Milestone Partnerships/Alliances Partnerships/Alliances Oral Carbapenem for Gram Negative Multidrug Resistant (MDR) Infections Tebipenem HBr Complicated UTI (cUTI) NDA submission for the treatment of cUTI planned for 2Q21 Oral DNA Replication Inhibitor for Non-tuberculous Mycobacterial (NTM) Disease SPR720 NTM Phase 2 study planned for 2H20 Direct Acting IV Potentiator for Gram Negative MDR Infections SPR206 MDR Infections Phase 1 BAL study planned for 1H21 As of September 8, 2020
