Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ORASURE TECHNOLOGIES INC | osur-8k_20200825.htm |

OraSure Technologies Summary of Recent CoVID-19 Communications August 25, 2020 Exhibit 99.1

Forward-Looking Statements Disclaimer This presentation contains certain forward-looking statements, including with respect to expected revenues and earnings/loss per share. Forward-looking statements are not guarantees of future performance or results. Known and unknown factors that could cause actual performance or results to be materially different from those expressed or implied in these statements include, but are not limited to: ability to successfully manage and integrate acquisitions of other companies in a manner that complements or leverages our existing business, or otherwise expands or enhances our portfolio of products and our end-to-end service offerings, and the diversion of management’s attention from our ongoing business and regular business responsibilities to effect such integration; the expected economic benefits of acquisitions (and increased returns for our stockholders), including that the anticipated synergies, revenue enhancement strategies and other benefits from the acquisitions may not be fully realized or may take longer to realize than expected and our actual integration costs may exceed our estimates; impact of increased or different risks arising from the acquisition of companies located in foreign countries; ability to market and sell products, whether through our internal, direct sales force or third parties; impact of significant customer concentration in the genomics business; failure of distributors or other customers to meet purchase forecasts, historic purchase levels or minimum purchase requirements for our products; ability to manufacture products in accordance with applicable specifications, performance standards and quality requirements; ability to obtain, and timing and cost of obtaining, necessary regulatory approvals for new products or new indications or applications for existing products; ability to comply with applicable regulatory requirements; ability to effectively resolve warning letters, audit observations and other findings or comments from the U.S. Food and Drug Administration (“FDA”) or other regulators; the impact of the novel coronavirus (“COVID-19”) pandemic on our business and our ability to successfully develop new products, validate the expanded use of existing collector products and commercialize such products for COVID-19 testing; changes in relationships, including disputes or disagreements, with strategic partners or other parties and reliance on strategic partners for the performance of critical activities under collaborative arrangements; ability to meet increased demand for the Company’s products; impact of replacing distributors; inventory levels at distributors and other customers; ability of the Company to achieve its financial and strategic objectives and continue to increase its revenues, including the ability to expand international sales; ability to identify, complete, integrate and realize the full benefits of future acquisitions; impact of competitors, competing products and technology changes; reduction or deferral of public funding available to customers; competition from new or better technology or lower cost products; ability to develop, commercialize and market new products; market acceptance of oral fluid or urine testing, collection or other products; market acceptance and uptake of microbiome informatics, microbial genetics technology and related analytics services; changes in market acceptance of products based on product performance or other factors, including changes in testing guidelines, algorithms or other recommendations by the Centers for Disease Control and Prevention (“CDC”) or other agencies; ability to fund research and development and other products and operations; ability to obtain and maintain new or existing product distribution channels; reliance on sole supply sources for critical products and components; availability of related products produced by third parties or products required for use of our products; impact of contracting with the U.S. government; impact of negative economic conditions; ability to maintain sustained profitability; ability to utilize net operating loss carry forwards or other deferred tax assets; volatility of the Company’s stock price; uncertainty relating to patent protection and potential patent infringement claims; uncertainty and costs of litigation relating to patents and other intellectual property; availability of licenses to patents or other technology; ability to enter into international manufacturing agreements; obstacles to international marketing and manufacturing of products; ability to sell products internationally, including the impact of changes in international funding sources and testing algorithms; adverse movements in foreign currency exchange rates; loss or impairment of sources of capital; ability to attract and retain qualified personnel; exposure to product liability and other types of litigation; changes in international, federal or state laws and regulations; customer consolidations and inventory practices; equipment failures and ability to obtain needed raw materials and components; the impact of terrorist attacks and civil unrest; and general political, business and economic conditions. These and other factors that could affect the Company’s results are discussed more fully in the Company’s Securities and Exchange Commission (“SEC”) filings, including our registration statements, Annual Report on Form 10-K for the year ended December 31, 2019, Quarterly Reports on Form 10-Q for the quarters ended March 31, 2020 and June 30, 2020, and other filings with the SEC. Although forward-looking statements help to provide information about future prospects, readers should keep in mind that forward-looking statements may not be reliable. The forward-looking statements are made as of the date of this presentation and OraSure Technologies undertakes no duty to update these statements.
Three Distinct COVID-19 Opportunities: Current Progress+ OraQuick Coronavirus Rapid Antigen Self-test Would use a nasal sample easily collected from the lower nostril to maximize accuracy and convenience Currently in clinical testing with human subjects Expected launch in Q4 2020 following EUA Initial launch will be Professional Test for symptomatic individuals; second phase will be Prescription Self-test; third phase for Over-The-Counter use in asymptomatic individuals. EUA for first two phases expected in Q4 2020 COVID-19 ELISA Antibody Test Potential to be the first COVID-19 antibody test to use oral fluid samples Expected launch in Q4 2020 following EUA Oral Fluid Collection Devices for COVID-19 Molecular Testing Sample collection devices already included in four* customer EUAs (CRL; Biocerna; P23 Labs; and Phosphorous), with more expected $8.5M in revenue recorded in 2Q 2020 +As of 8/5/20 *As of 8/1/20.
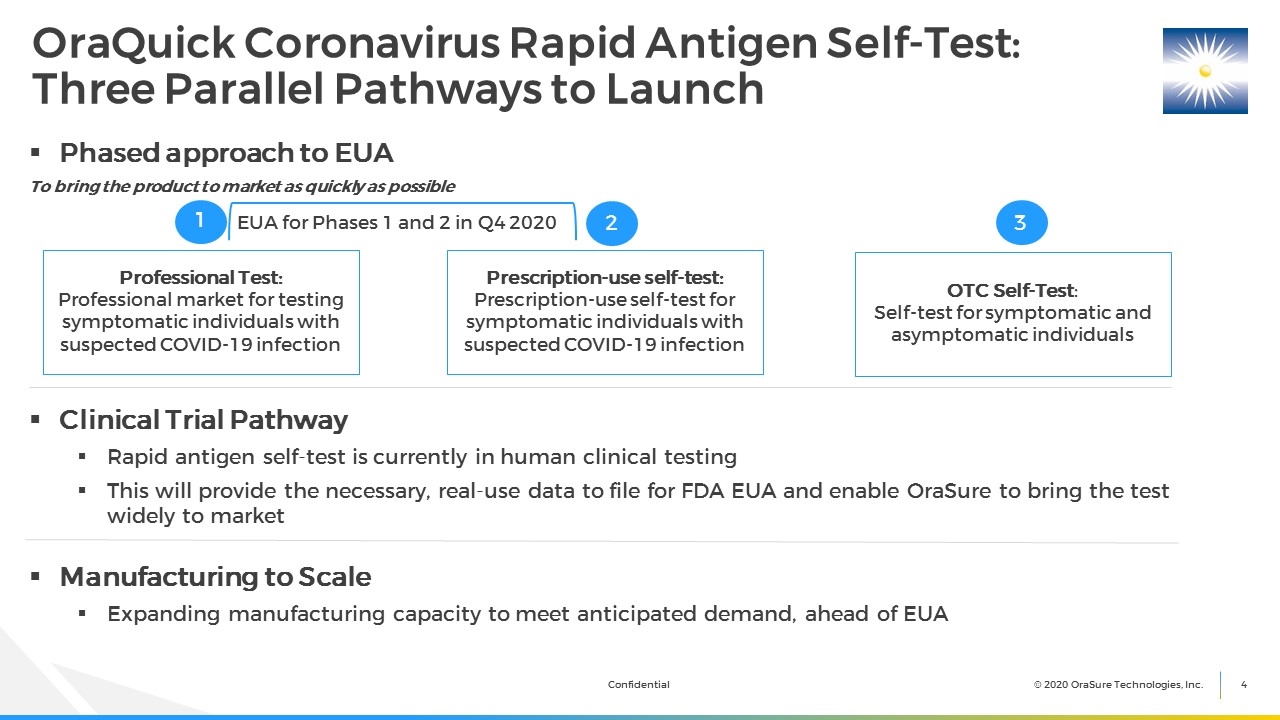
OraQuick Coronavirus Rapid Antigen Self-Test: Three Parallel Pathways to Launch Phased approach to EUA To bring the product to market as quickly as possible Clinical Trial Pathway Rapid antigen self-test is currently in human clinical testing This will provide the necessary, real-use data to file for FDA EUA and enable OraSure to bring the test widely to market Manufacturing to Scale Expanding manufacturing capacity to meet anticipated demand, ahead of EUA 1 2 3 Professional Test: Professional market for testing symptomatic individuals with suspected COVID-19 infection Prescription-use self-test: Prescription-use self-test for symptomatic individuals with suspected COVID-19 infection OTC Self-Test: Self-test for symptomatic and asymptomatic individuals EUA for Phases 1 and 2 in Q4 2020
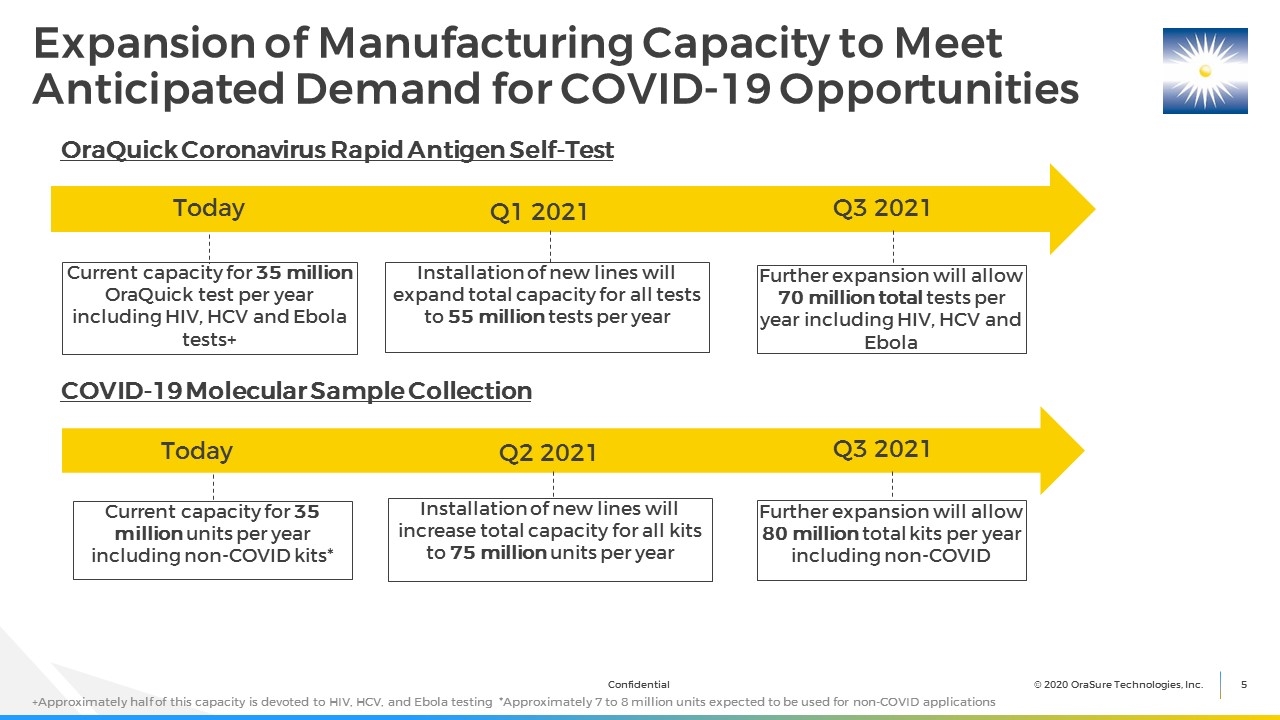
Expansion of Manufacturing Capacity to Meet Anticipated Demand for COVID-19 Opportunities Current capacity for 35 million OraQuick test per year including HIV, HCV and Ebola tests+ Installation of new lines will expand total capacity for all tests to 55 million tests per year Further expansion will allow 70 million total tests per year including HIV, HCV and Ebola Today Q1 2021 Q3 2021 OraQuick Coronavirus Rapid Antigen Self-Test +Approximately half of this capacity is devoted to HIV, HCV, and Ebola testing *Approximately 7 to 8 million units expected to be used for non-COVID applications Current capacity for 35 million units per year including non-COVID kits* Installation of new lines will increase total capacity for all kits to 75 million units per year Today Q2 2021 COVID-19 Molecular Sample Collection Further expansion will allow 80 million total kits per year including non-COVID Q3 2021
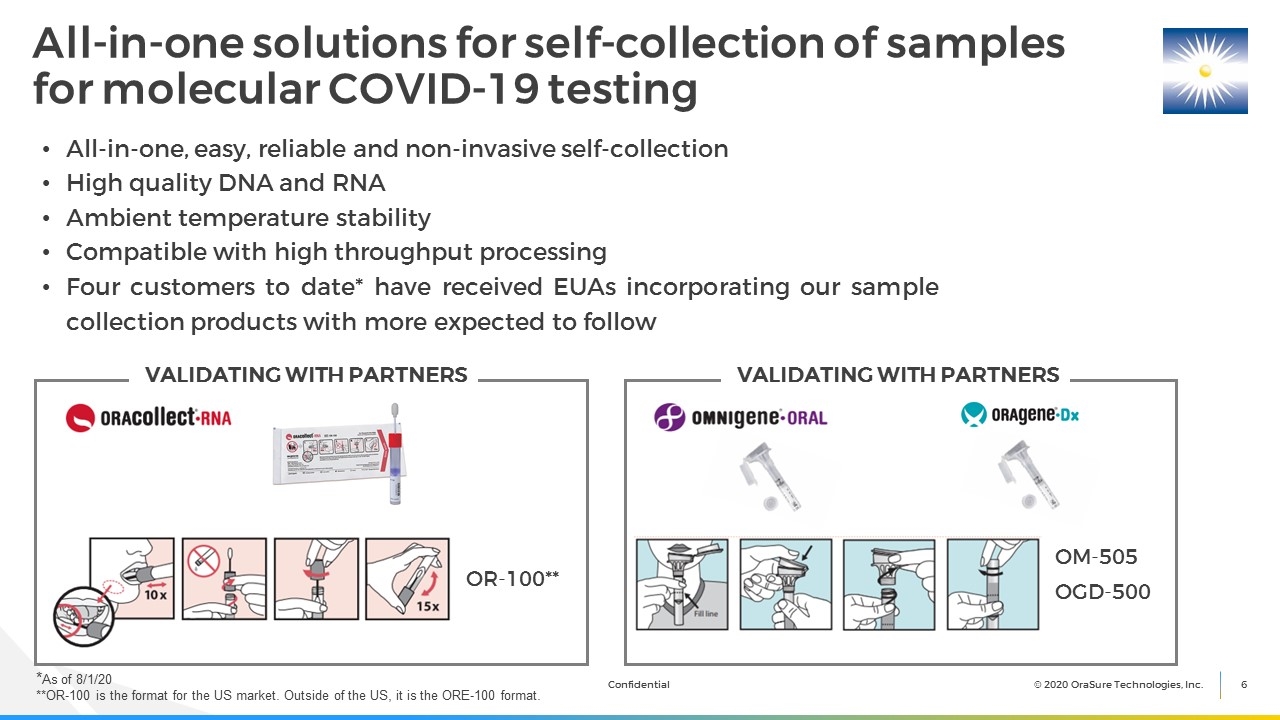
All-in-one, easy, reliable and non-invasive self-collection High quality DNA and RNA Ambient temperature stability Compatible with high throughput processing Four customers to date* have received EUAs incorporating our sample collection products with more expected to follow All-in-one solutions for self-collection of samples for molecular COVID-19 testing OM-505 OR-100** OGD-500 VALIDATING WITH PARTNERS VALIDATING WITH PARTNERS *As of 8/1/20 **OR-100 is the format for the US market. Outside of the US, it is the ORE-100 format.
